
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 25 April 2023
Sec. Sport and Exercise Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1109789
This article is part of the Research TopicNutrition in Sports: A Focused Lens on Dietary SupplementsView all 5 articles
Objective: Sarcopenia is a typical age-related disorder characterized by loss of muscle mass, strength, and physical function. Resistance training has a noticeable effect on sarcopenia, but there is no consensus on whether nutritional supplements can boost this effect. We conducted a meta-analysis of relevant literature to investigate the therapeutic effect of resistance training combined with nutrition intervention on sarcopenia compared with resistance training alone.
Methods: Cochrane Library, PubMed, Web of Science, Embase, Sinomed, CNKI, VIP, and Wanfang Data were searched for relevant studies on resistance training combined with nutritional intervention for aging adults with sarcopenia. The retrieval period ranged from the inception of the databases to May 24, 2022. Literature screening and information extraction were performed by two researchers. The Physiotherapy Evidence Database (PEDro) scale was adopted for literature quality evaluation and Stata 15.0 software for analysis.
Results: Twelve clinical trials were included, involving 713 older adults diagnosed with sarcopenia, of whom 361 were assigned to the experimental group and 352 to the control group. Compared with the control group, grip strength of the experimental group was substantially elevated [WMD = 1.87, 95% CI (0.01, 3.74), P = 0.049]. Subgroup analysis demonstrated that vitamin D and protein increased grip strength and gait speed. There were no significant improvement in grip strength and gait speed in the protein and vitamin D free subgroup.
Conclusions: This meta-analysis demonstrated that resistance training combined with additional nutritional supplementation, especially compound nutritional supplements that included protein and vitamin D, might further enhance grip strength rather than muscle mass in older adults with sarcopenia.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022346734.
Sarcopenia is recognized as a complex syndrome that tends to occur in older adults. It is a phenomenon of aging accompanied by progressive and systemic skeletal muscle decline, and it has been defined as loss of muscle mass, muscle strength, and physical function (1). Hereditary factors, lack of exercise, malnutrition, increased inflammation, age-related declines in hormone concentrations, and low vitamin D levels are currently regarded as risk factors for sarcopenia (2, 3). Estimated morbidity in both males and females over 60 years is 10% (4). Sarcopenia causes frailty, handicap, reduced quality of life, and even death in older adults (5).
Muscle mass and strength decline with age after middle age, and the development and progression of age-related primary sarcopenia may involve protein synthesis, proteolysis, muscle fat content, and neuromuscular integrity (6). The pathogenesis may be multifactorial. First, aging causes the disfunction of satellite cells, which play an important role in muscle regeneration, leading to a decline in the regeneration capacity and number of muscle fibers (7, 8). Various myocyte growth factors can also regulate the function of satellite cells to increase or reduce muscle fibers (9). Second, the activity of the IGF-1/PI3K/mTOR system, which plays an important role in muscle hypertrophy, decreases with aging (10). Furthermore, amyotrophy-related factors in skeletal muscles increase with aging (11), resulting in less muscle protein synthesis (MPS) and more muscle protein breakdown (MPB). Third, chronic inflammation induced by various factors in aging compromises muscle strength and function by enhancing the infiltration of macrophage into skeletal muscle, reducing muscle mass, and increasing the accumulation of ectopic fat (12). Fourth, the renin-angiotensin system (RAS) promotes muscle protein degradation through: (i) direct oxidative stress via angiotensin II type 1 receptors; (ii) decreased anabolic hormones via indirect induction; (iii) induction of proinflammatory cytokines; and (iv) enhanced muscle protein degradation via increased myostatin (9). Fifth, some relevant studies have reported that sex hormones (e. g., serum testosterone and estrogen) decreasing with age may be associated with sarcopenia (13, 14). Sixth, excessive mitochondrial reactive oxygen species (ROS) accumulate in skeletal muscle cells, and the accumulation of single-strand breaks in the telomeric region may accelerate telomere erosion, leading to cellular senescence (15). Besides, the generation of ROS causes inflammatory markers to overexpress and induces alterations in transcription factors and gene expression, which can affect protein equilibrium (16) and reduce muscle protein.
Skeletal muscle mass is determined by a complex balance between MPS and MPB, and resistance exercise is known as an effective stimulus to MPS promotion (17), and a recognized non-drug therapy that has produced satisfactory effects on the prevention and treatment of sarcopenia (18–22). However, whether nutritional supplementation can enhance the effect of resistance exercise remains uncertain. Although recommended by both the European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS) (1, 23), the “International Conference on Sarcopenia and Frailty Research” (ICFSR) indicated a low level of evidence (24). Some studies have reported that exercise training combined with dietary supplements has increased benefits, but some other research has conflicting findings (25). Currently, there is no systematic review and meta-analysis on nutritional supplementation combined with resistance exercises in older sarcopenic individuals. Therefore, the present study aimed to compare the therapeutic effect of resistance training combined with nutrition intervention vs. resistance training alone on muscular strength, quality, and function in elderly patients with sarcopenia.
This meta-analysis was conducted according to the Cochrane Handbook for the Systematic Review of Interventions (http://training.cochrane.org/handbook) and the Preferred Reporting Items for Systematic Review and Meta-Analyses. PMID: 19622552 (26). This study protocol was approved on PROSPERO (ID: CRD42022346734).
Interventional study.
The inclusion criteria were as follows. Participants: (i) aged 60 or above; (ii) diagnosed with sarcopenia. Since there is no unified definition of sarcopenia, we accept different definitions of “sarcopenia” in different studies; and (iii) have no major physical disability and can perform activities freely to ensure that they can perform resistance training normally. Intervention: (i) The experimental group performed resistance exercise combined with nutritional intervention as the experimental design, and took no specific drugs; The control group only performed resistance exercise and they were given placebo or isocaloric products with the same diet habit as usual. (ii) Intervention time ≥ 8 weeks, all participants had twice resistance exercise per week, and each time was not <20 min. The experimental group received resistance exercise plus intensive nutritional supplementation once or twice per day. (iii) The resistance exercise was designed as a progressive full-body workout with resistance equipment such as resistance bands or fitness facilities. Nutritional supplements included but were not limited to, protein, ω-3 fatty acids, vitamin D, and other substances that may be beneficial for improving muscle mass and strength.
The exclusion criteria were as follows: (i) Research disease was not related to sarcopenia; (ii) Research data was not accessible. (iii) Publication types were single-arm studies, reviews, pathological studies, animal experiments, dissertations or conference papers, etc.
According to the definition of sarcopenia, grip strength and chair stand test were used to evaluate the muscle strength of upper and lower limbs; total lean mass, appendicular skeletal muscle mass (ASM), and ASM index were used to assess muscle mass; timed up & go (TUG) and gait speed were used to evaluate muscle performance. Besides, body mass, body mass index (BMI), and total fat mass were used as secondary outcome indicators to observe the therapeutic effects of the two intervention methods on the elderly with sarcopenia.
A computer search was performed in Cochrane Library, PubMed, Web of Science, Embase, CNKI, and Wanfang Data, for research on the application effect of resistance training combined with nutritional intervention against sarcopenia. The retrieval period was from the inception of the databases to May 24, 2022. English key search terms included “sarcopenia”, “sarcopenias”, “training, resistance”, “strength training”, “nutrition therapy”, and “therapy, nutrition”. The Chinese terms used for search included “肌少症”, “骨骼肌减少症”, “抗阻训练”, and “营养”. Detailed search strategies are available in Supplementary Table 1.
Two researchers (ZS and TP) were responsible for the literature screening strictly following inclusion and exclusion criteria, and Endnote software version X9 was employed to manage the literature. The retrieved literature was initially imported into the software and the repeated literature was removed. Eligible studies were preliminarily selected by titles or abstracts, of which the full texts were downloaded. After reading the full texts, the original studies that met the requirements of this systematic review were included. Literature information was extracted; cross-validation was performed; and measurement units were unified. If there was any discrepancy, a third researcher (ZZ) was consulted to assist in the final decision. The extracted information mainly included title, first author, year of publication, country, type of study, diagnostic criteria for sarcopenia, sample size and gender of the experimental group and control group, intervention method, intervention time, and outcome indicators.
Two researchers (ZS and TP) assessed the quality of the included studies using the Physiotherapy Evidence Database (PEDro) scale (27). The PEDro scale consists of 11 items, including eligibility criteria, random allocation, concealed allocation, similar measures between groups at baseline, instructor blinding, assessor blinding, participant blinding, more than 85% dropout rate, intention-to-treat analysis, statistical comparison between groups, and ≥1 key outcome estimated. Each item is worth 1 point, except for criterion number 1. A higher total score indicates better methodological quality. Hence, a study with a PEDro score of 6 or higher was rated as high quality (6–8:good; 9–10:excellent), whereas a study with a score of 5 or lower was considered low quality (4–5: acceptable; < 4: poor) (28).
Stata 15.0 software was adopted to perform statistical analysis of the included literature, such as the tests for heterogeneity, publication bias analysis, and sensitivity analysis. The extracted data in the study were all continuous variables with unified measurement units. Effect sizes were combined using mean difference (MD), and 95% confidence intervals (CIs) were calculated. Heterogeneity was assessed using the Q statistic and I2 tests. If P > 0.1 and I2 ≤ 50%, the heterogeneity between studies was acceptable, and a fixed effect model was adopted for meta-analysis. If P ≤ 0.1 or I2 > 50%, heterogeneity between studies was high, and a random effect model was employed for meta-analysis. Publication bias of the included studies was verified by running the “metabias” command, and a P < 0.05 were considered statistically significant.
A total of 730 articles were retrieved from the described databases, of which 29 articles were from Cochrane Library, 36 from PubMed, 266 from Web of Science, 339 from Embase, 5 from Sinomed, 14 from CNKI, 38 from Wanfang Data, and 3 from the VIP database. After the retrieved literature was imported into EndNote X9, 146 repeated literature were excluded. After reading the titles and abstracts, 441 irrelevant works of literature were removed. According to full-text reading, 131 unqualified works of literature were removed, Finally, 12 articles were included (29–40). The literature screening process is shown in Figure 1.
A total of 12 studies involving 713 older adults diagnosed with sarcopenia were included. The included studies were published in English. The basic characteristics of the included literature are shown in Table 1.
Notably, we extracted participants' baseline nutrition absorbed from their daily diets. Four studies (29–31, 37) did not provide baseline dietary intake, but one reported mini nutritional assessment scores and nutritional status (29). However, the nutritional status of the participants at enrollment in the other three studies was unknown due to the lack of baseline data. If most of them had an insufficient nutritional intake, it would amplify the effect of nutritional supplementation.
Protein was reported in three studies (33, 34, 36) amino acid powder supplements in one study (31), collagen peptides in one study (37), catechins in one study (30), leucine and whey protein in one study (29), and mixed nutritional supplements containing vitamin D and proteins in five studies (32, 35, 38–40).
The PEDro scores are shown in Table 1, and the detailed scores are presented in Supplementary Table 2. The scores for study quality ranged from 5 to 9. One study (36) scored 5 because it was quasi-experimental intervention research, and the rest of the studies were all randomized controlled trials with scores >6, indicating high quality.
Muscle strength was assessed by indicators of grip strength and chair stand test. The grip strength and chair stand test were combined using the weighted MD (WMD).
Eight studies reported grip strength, with 280 cases in the experimental group and 266 cases in the control group. A random-effects model (I2=68.8%, P = 0.002) was used for meta-analysis, and the analysis results revealed that the difference in grip strength between the experimental group and the control group was statistically significant [WMD = 1.87, 95% CI (0.01, 3.74), P = 0.049], as shown in Figure 2.
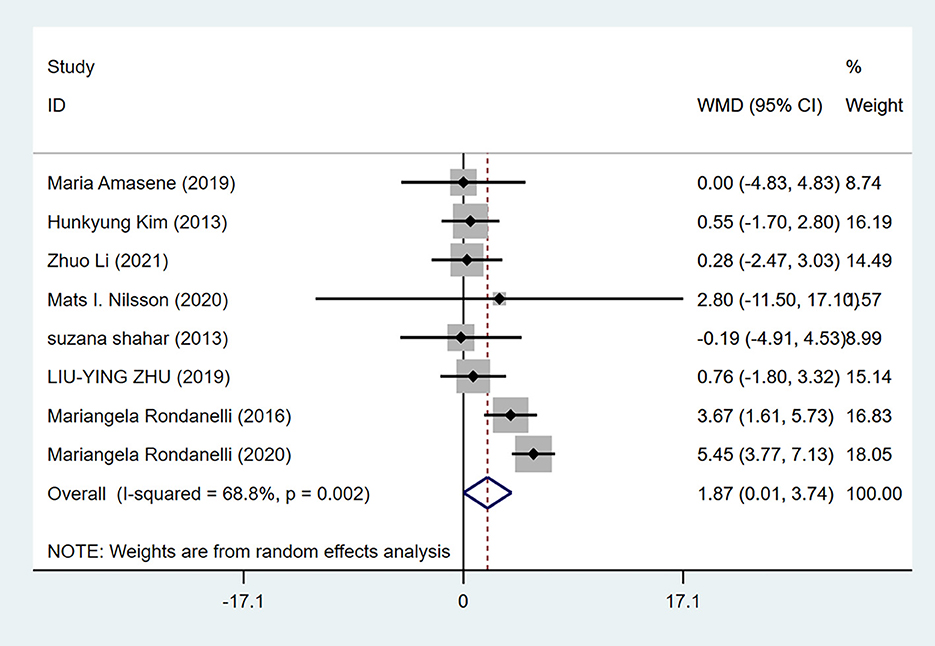
Figure 2. Forest plot for grip strength of resistance training combined with nutritional supplementation vs. resistance training alone. Overall estimates were obtained from forest plots of the meta-analysis using the random-effects model. Diamond icons and horizontal bars represent the overall estimate and 95% CI. WMD, weighted mean difference; CI, confidence interval.
Five studies reported chair stand test, with 132 cases in the experimental group and 132 in the control group. A random-effects model (I2=86.6%, P = 0.000) was adopted for meta-analysis, and the results indicated no significant difference in chair stand test between the experimental group and the control group [WMD = −2.03, 95% CI (−5.82, 1.76), P = 0.295] as shown in Supplementary Figure 1.
Muscle mass was assessed by total lean body mass, ASM, and ASM index. Since the measurement of related indicators used two different instruments, either dual-energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA), we combined the results using standardized MD (SMD).
Six studies reported lean body mass, with 229 cases in the experimental group and 214 cases in the control group. A fixed-effects model (I2 = 0.0%, P = 0.913) was employed to calculate the pooled effect sizes, and the results indicated no significant difference in lean body mass between the experimental group and the control group [SMD = 0.10, 95% CI (−0.14, 0.34), P = 0.422] as shown in Supplementary Figure 2.
Five studies reported ASM, with 179 cases in the experimental group and 169 in the control group. A fixed-effects model (I2 = 0.0%, P = 0.928) was employed to calculate the pooled effect sizes, and the results indicated no significant difference in ASM between the experimental group and the control group [SMD = 0.15, 95% CI (−0.06, 0.36), P = 0.168] as presented in Supplementary Figure 3.
Six studies reported the intervention effects of resistance training combined with nutritional supplementation and resistance training alone on old adults with sarcopenia, with 229 cases in the experimental group and 214 cases in the control group. A fixed-effects model (I2 = 0.0%, P = 0.807) was employed to calculate the pooled effect sizes, and the results indicated no significant difference in ASM index between the experimental group and the control group [SMD = 0.16, 95% CI (−0.03, 0.35), P = 0.096] as presented in Supplementary Figure 4.
Muscle performance was assessed using TUG and gait speed. As studies with different gait speeds adopted different measures, we pooled the data using SMD.
Four studies reported TUG, with 112 cases in the experimental group and 115 in the control group. A random-effects model (I2 = 81.4%, P = 0.001) was adopted to calculate the pooled effect sizes, and the results indicated no significant difference in TUG test between the experimental group and the control group [WMD = −0.81, 95% CI (−2.50, 0.88), P = 0.348] as shown in Supplementary Figure 5.
Seven studies gait speeds, with 197 cases in the experimental group and 204 cases in the control group. A fixed-effects model (I2 = 23.2%, P = 0.252) was employed to combine the effect sizes, and the results indicated no significant difference in gait speed between the experimental group and the control group [SMD = 0.17, 95% CI (−0.03, 0.36), P = 0.098] as presented in Supplementary Figure 6.
For older adults with sarcopenia, there were no statistically significant differences in body mass, BMI, and fat mass between the experimental group and the control group (Supplementary Figures 7–9), as shown in Supplementary Table 3.
Subgroup analyses were performed according to the presence of protein and vitamin D in nutrients, and the results revealed an increase of grip strength in the protein and vitamin D subgroup [WMD = 2.72, 95% CI (0.38, 5.05), P = 0.022], whereas grip strength and gait speed were not statistically significant in the protein and vitamin D free subgroup [WMD = 0.35, 95% CI (−1.52, 2.22), P = 0.713] (Supplementary Figure 10). Gait speed was improved in the protein and vitamin D subgroup [SMD = 0.36, 95% CI (0.08, 0.63), P = 0.011], while there was no significant improvement in gait speed in the protein and vitamin D free subgroup [SMD = −0.04, 95% CI (−0.32, 0.25), P = 0.795], (Supplementary Figure 11). No significant differences were observed in skeletal muscle mass, TUG, and chair stand test between the two subgroups (Supplementary Figures 12–14).
Subgroup analysis was performed according to different diagnostic criteria. AWGS diagnostic criteria was used in two studies (32, 38), EWGSOP diagnostic criteria in three studies (35, 37, 40), and EWGSOP2 in one study (29). The findings suggested that the grip strength of the EWGSOP subgroup was significantly improved [WMD = 5.41, 95% CI (3.74, 7.09), P = 0.000], and there was no difference in the AWGS subgroup [WMD = 0.54, 95% CI (−1.34, 2.41), P = 0.574] (Supplementary Figure 15). EWGSOP2 trial findings revealed that protein supplementation did not further improve grip strength in sarcopenic patients after resistance training, and there was no difference between the EWGSOP and AWGS subgroups in skeletal muscle mass index (Supplementary Figure 16). There were insufficient data for subgroup analyses of the remaining indicators.
Publication bias of the included studies was detected using Egger's test, and a P > 0.05 indicated the absence of publication bias. There was no publication bias in each index of the included literature (Table 2).
There are some debates over whether additional nutritional supplementation has better therapeutic effects for older sarcopenic individuals engaging in resistance exercise, and what nutritional supplements should be taken. As a result, the present study aimed to compare the beneficial effects of resistance training combined with nutritional supplementation vs. resistance training alone on older patients with sarcopenia, thereby providing comprehensive evidence for resource optimization and the improvement of the quality of life of older sarcopenia patients.
The current research analyzed 12 studies, including 11 randomized control trials and 1 quasi clinical trial, and the findings indicated that resistance training combined with extra nutritional supplements produced little effect on muscle mass, body mass, BMI, and fat mass in older patients with sarcopenia. After additional nutritional supplements were provided, especially complex supplements containing protein and vitamin D, grip strength and gait speed might be further improved in older adults with sarcopenia after resistance training. On the contrary, no improvement in lower limb strength assessed by the chair stand test was observed. According to a previous review, although some suggestive evidence revealed that a higher intake of nutritional supplements was associated with increased muscle strength, the finding was controversial with a lack of data on muscle strength (41). Hence, the present study can only conclude that additional nutritional supplements combined with resistance training can improve grip strength to some extent, whereas whether it can improve overall muscle strength needs to be further investigated.
Nutrients currently beneficial for sarcopenia management include protein, leucine, methyl β-hydroxyβ-butyrate, vitamin D, omega-3 fatty acids, and other antioxidants (42), all of which were reported in the included RCTs. Furthermore, one of the included RCTs focued on catechins. Most nutrients are not supplemented alone, and they are usually combined with other nutrients to form a compound nutrient. Protein was mostly reported in the included studies, with supplies ranging from a minimum of 13.53 g to a maximum of 35 g each time. Some studies have verified that older adults need protein intake of 1.6–1.8 g/kg/day to maintain muscle mass and function (43), which exceeds the recommended dietary allowance (RDA) of 0.8 g/kg/day (44). One of the possible reasons is that the anabolic resistance in older adults may inhibit the stimulating effects of strength exercise and protein intake on protein synthesis (45), so a higher protein intake is needed to maintain the synthesis. Notably, given the differences in participants' health statuses and dietary habits, for older people who have enough dietary protein intake each day, extra protein intake provides no more benefits, instead, its satiety properties may generate a “protein paradox,” which means that an excessive increase in protein intake may come at the expense of other important nutrients due to energy redistribution (46). A meta-analysis has indicated negative effects of whey protein supplementation at the same dose range (or even higher) as compared to control (47), and long-term high-protein diets may also affect colon health (48). The failure to improve muscle mass or performance may also be due to improper nutrition intake. In a case-control study, researchers used a specialized application program to provide health education and personalized nutrition and exercise guidance for older sarcopenia patients and observed improvements in muscle mass (49). Hence, simply increasing protein intake is not an optimal option, and daily dietary intake should be taken into account and evaluated carefully.
In terms of the duration of resistance exercise and nutritional supplementation, 3 months was reported in two studies, 12 weeks in eight studies, 8 weeks in one study, and 16 weeks in one study. The frequency of resistance training varied from 2 to 5 times per week. Taaffe et al. (50) have suggested that moderate-intensity training once or twice a week is sufficient to improve muscle mass (50). The frequency of nutritional supplementation ranged from 2 times a day to 3 times a week. Due to slower protein turnover in older adults, improvements in strength and physical performance are often observed before significant changes in muscle mass (51). This may be another reason why we did not observe a significant improvement in muscle mass. In this 16-week study (33), the researchers found that resistance training plus milk protein supplementation resulted in a significant increase in the lean body mass. Hence, appropriately extending the intervention duration without increasing frequency may improve muscle mass.
Protein and vitamin D were the most common combinations of compound nutrients, implying that the addition of vitamin D to a management regimen for sarcopenia may contribute to the recovery of functions of the patients (52). Subgroup analyses were performed for the studies containing both substances, and the findings indicated significant improvements in grip strength and gait speed in older patients with sarcopenia, which has important implications for clinical practice. However, the fact that this effect may be achieved due to other nutrients in the mixture can not be ignored. A previous meta-analysis indicated that vitamin D plus protein enhanced grip strength, but not gait speed, in patients with sarcopenia (53). Most of the RCTs included in that study did not perform resistance training, so the regime of protein and vitamin D supplements after resistance training may achieve better results.
Compared to previous studies, the present study is more targeted. Choi et al. (54) have published a study on the effects of resistance training combined with nutritional intervention vs. resistance training alone on healthy older adults, and they found that additional nutritional supplements did not improve muscle mass, strength, or performance in older adults engaging in resistance exercise. In contrast, the present study centered on older individuals with sarcopenia and found different results. The reason for the difference may be that older patients with sarcopenia were in poorer health and could gain more benefits from nutritional supplements. The meta-analysis published by Luo et al. (55) investigated the effect of nutritional supplements combined with exercise on elderly individuals with sarcopenia, while the present study focused on resistance exercise to minimize the impact of different forms of exercise on the study population. Furthermore, we included more high-quality original studies than previous studies.
An additional strength of this study is that it is the first meta-analysis comparing the effects of resistance training combined with nutritional supplementation vs. resistance training alone on elderly patients with sarcopenia. Besides, we performed a subgroup analysis according to nutritional combinations containing protein and vitamin D as well as different diagnostic criteria to conclude more meaningful results. To explore whether additional nutritional supplementation would improve the therapeutic effect of resistance training on older adults with sarcopenia, muscle strength, muscle mass, and muscle performance were assessed using multiple indicators.
The current study also has several limitations. First, the current study covers several types of nutrients, while the effect of a specific nutrient is not apparent. The reason is that, apart from protein-related studies, there are relatively few trials conducted to elucidate the effect of other kinds of nutrients. Subsequent trials focusing on a specific nutrient are necessary. Second, the improved effects on grip strength and gait speed cannot exclude the influence of additional nutrients. Thirdly, due to the lack of an international unified standard for the diagnosis of sarcopenia, the diagnostic criteria in half of the included studies were developed by their researchers themselves. Hence, the amount of muscle loss varied among the included populations. Fourth, the time, intensity, frequency, and nutrient supply of resistance training in the experiments were not consistent, which might have a certain impact on the research results. Finally, there are still few trials performed using the plan of several nutrients combined with resistance training for older patients with sarcopenia, and the number of participants in the study is also very limited. In one of the included studies, patients with sarcopenia were analyzed as a subgroup, and the number of these patients was only 7 (35), which might affect the reliability of the results.
The results of this study indicate that nutritional supplementation combined with resistance training does improve some aspects of muscle condition in older patients with sarcopenia, providing a basis for future nutritional supplementation for older sarcopenic patients. Furthermore, complex nutritional supplements rich in protein and vitamin D can be used for sarcopenic patients engaging in resistance training to further improve their muscle strength and performance.
Combining nutritional supplementation with resistance training can improve grip strength in older patients with sarcopenia. Especially, complex nutritional supplements rich in protein and vitamin D can help the patients ameliorate their gait speed to some extent. Our results underpin the recommendation to combine nutritional supplementation with resistance training in older patients with sarcopenia. Reasonable and appropriate nutritional supplementation is advised to enhance the therapeutic effect of resistance training on older patients with sarcopenia. More high-quality, large-scale RCTs are desired to verify these findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
ZS and ZZ contributed to conception and design of the study and organized the database. TP performed the statistical analysis. ZS wrote the first draft of the manuscript. TP and ZZ wrote sections of the manuscript. XT reviewed and edited the manuscript. YY made a critical review, commentary, and revision of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by State Administration of Traditional Chinese Medicine Key Discipline Fund for Geriatric Diseases.
We would like to thank the researchers and study participants for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1109789/full#supplementary-material
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
2. Liao X, Wu M, Hao Y, Deng H. Exploring the preventive effect and mechanism of senile sarcopenia based on “gut-muscle axis”. Front. Bioeng. Biotechnol. (2020) 8:590869. doi: 10.3389/fbioe.2020.590869
3. Lenchik L, Boutin RD. Sarcopenia: beyond muscle atrophy and into the new frontiers of opportunistic imaging, precision medicine, and machine learning. Semin Musculoskelet Radiol. (2018) 22:307–22. doi: 10.1055/s-0038-1641573
4. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
7. Shang M, Cappellesso F, Amorim R, Serneels J, Virga F, Eelen G, et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. (2020) 587:626–31. doi: 10.1038/s41586-020-2857-9
8. Yamakawa H, Kusumoto D, Hashimoto H, Yuasa S. Stem cell aging in skeletal muscle regeneration and disease. Int J Mol Sci. (2020) 21:830. doi: 10.3390/ijms21051830
9. Nishikawa H, Fukunishi S, Asai A, Yokohama K, Nishiguchi S, Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int J Mol Med. (2021) 48:4989. doi: 10.3892/ijmm.2021.4989
10. Parkington JD. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. (1985) 97:243–8. doi: 10.1152/japplphysiol.01383.2003
11. Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. (2005) 21:253–63. doi: 10.1152/physiolgenomics.00249.2004
12. Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, et al. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol. (2013) 115:937–48. doi: 10.1152/japplphysiol.00019.2013
13. Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. World J. Men's Health. (2018) 36:192–8. doi: 10.5534/wjmh.180001
14. Collins BC, Laakkonen EK, Lowe DA. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone. (2019) 123:137–44. doi: 10.1016/j.bone.2019.03.033
15. Picca A, Calvani R. Molecular mechanism and pathogenesis of sarcopenia: an overview. Int J Mol Sci. (2021) 22:3032. doi: 10.3390/ijms22063032
16. Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. (2017) 8:1045. doi: 10.3389/fphys.2017.01045
17. McKendry J, Currier BS, Lim C, McLeod JC, Thomas ACQ, Phillips SM. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients. (2020) 12:2057. doi: 10.3390/nu12072057
18. Jankowski C. Resistance exercise for muscular strength in older adults: a meta-analysis. Yearbook of Sports Medicine. (2011) 9:407–10. doi: 10.1016/j.yspm.2011.03.064
19. Chen N, He X, Feng Y, Ainsworth BE, Liu Y. Effects of resistance training in healthy older people with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Eur Rev Aging Phys Act. (2021) 18:23. doi: 10.1186/s11556-021-00277-7
20. Kirk B, Mooney K, Amirabdollahian F, Khaiyat O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University - Sarcopenia Aging Trial (LHU-SAT). Front Physiol. (2019) 10:445. doi: 10.3389/fphys.2019.00445
21. Ahtiainen JP, Walker S, Peltonen H, Holviala J, Sillanpaa E, Karavirta L, et al. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr). (2016) 38:10. doi: 10.1007/s11357-015-9870-1
22. Churchward-Venne TA, Tieland M, Verdijk LB, Leenders M, Dirks ML, de Groot LC, et al. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J Am Med Dir Assoc. (2015) 16:400–11. doi: 10.1016/j.jamda.2015.01.071
23. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-7 e2. doi: 10.1016/j.jamda.2019.12.012
24. Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging. (2018) 22:1148–61. doi: 10.1007/s12603-018-1139-9
25. Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. (2015) 10:859–69. doi: 10.2147/CIA.S55842
26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
27. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. doi: 10.1093/ptj/83.8.713
28. Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro). Aust J Physiother. (2002) 48:43–9. doi: 10.1016/S0004-9514(14)60281-6
29. Amasene M, Besga A, Echeverria I, Urquiza M, Ruiz JR, Rodriguez-Larrad A, et al. Effects of leucine-enriched whey protein supplementation on physical function in post-hospitalized older adults participating in 12-weeks of resistance training program: a randomized controlled trial. Nutrients. (2019) 11:2337. doi: 10.3390/nu11102337
30. Kim H, Suzuki T, Saito K, Yoshida H, Kojima N, Kim M, et al. Effects of exercise and tea catechins on muscle mass, strength and walking ability in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. Geriatr Gerontol Int. (2013) 13:458–65. doi: 10.1111/j.1447-0594.2012.00923.x
31. Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. (2012) 60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x
32. Forbes SC, Candow DG, Ostojic SM, Roberts MD, Chilibeck PD. Meta-analysis examining the importance of creatine ingestion strategies on lean tissue mass and strength in older adults. Nutrients. (2021) 13:1912. doi: 10.3390/nu13061912
33. Maltais ML, Perreault K, Courchesne-Loyer A, Lagacé JC, Barsalani R, Dionne IJ. Effect of resistance training and various sources of protein supplementation on body fat mass and metabolic profile in sarcopenic overweight older adult men: a pilot study. Int J Sport Nutr Exerc Metab. (2016) 26:71–7. doi: 10.1123/ijsnem.2015-0160
34. Nabuco HCG, Tomeleri CM, Fernandes RR, Sugihara Junior P, Cavalcante EF, Cunha PM, et al. Effect of whey protein supplementation combined with resistance training on body composition, muscular strength, functional capacity, and plasma-metabolism biomarkers in older women with sarcopenic obesity: a randomized, double-blind, placebo-controlled trial. Clin Nutr ESPEN. (2019) 32:88–95. doi: 10.1016/j.clnesp.2019.04.007
35. Nilsson MI, Mikhail A, Lan L, Di Carlo A, Hamilton B, Barnard K, et al. A Five-Ingredient nutritional supplement and home-based resistance exercise improve lean mass and strength in free-living elderly. Nutrients. (2020) 12:2391. doi: 10.3390/nu12082391
36. Shahar S, Kamaruddin NS, Badrasawi M, Sakian NI, Abd Manaf Z, Yassin Z, et al. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin Interv Aging. (2013) 8:1365–75. doi: 10.2147/CIA.S46826
37. Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. (2015) 114:1237–45. doi: 10.1017/S0007114515002810
38. Zhu LY, Chan R, Kwok T, Cheng KC, Ha A, Woo J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: a randomized controlled trial. Age Ageing. (2019) 48:220–8. doi: 10.1093/ageing/afy179
39. Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. (2016) 103:830–40. doi: 10.3945/ajcn.115.113357
40. Rondanelli M, Cereda E, Klersy C, Faliva MA, Peroni G, Nichetti M, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle. (2020) 11:1535–47. doi: 10.1002/jcsm.12532
41. Robinson S, Granic A, Sayer AA. Nutrition and muscle strength, as the key component of sarcopenia: an overview of current evidence. Nutrients. (2019) 11:42. doi: 10.3390/nu11122942
42. Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. (2018) 37:1121–32. doi: 10.1016/j.clnu.2017.08.016
43. Rogeri PS, Zanella R Jr, Martins GL, Garcia MDA, Leite G, Lugaresi R, et al. Strategies to prevent sarcopenia in the aging process: role of protein intake and exercise. Nutrients. (2021) 14:52. doi: 10.3390/nu14010052
44. Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: What is the optimal level of intake? Nutrients. (2016) 8:359. doi: 10.3390/nu8060359
45. Kirwan R, McCullough D, Butler T, Perez de. Heredia F, Davies IG, Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. GeroScience. (2020) 42:1547–78. doi: 10.1007/s11357-020-00272-3
46. Ispoglou T, Witard OC, Duckworth LC, Lees MJ. The efficacy of essential amino acid supplementation for augmenting dietary protein intake in older adults: implications for skeletal muscle mass, strength and function. Proc Nutr Soc. (2021) 80:230–42. doi: 10.1017/S0029665120008010
47. Huang LP, Condello G, Kuo CH. Effects of milk protein in resistance training-induced lean mass gains for older adults aged ≥ 60 y: a systematic review and meta-analysis. Nutrients. (2021) 13:815. doi: 10.3390/nu13082815
48. Lancha AH, Zanella R, Tanabe SG, Andriamihaja M, Blachier F. Dietary protein supplementation in the elderly for limiting muscle mass loss. Amino Acids. (2017) 49:33–47. doi: 10.1007/s00726-016-2355-4
49. Wang Z, Xu X, Gao S, Wu C, Song Q, Shi Z, et al. Effects of internet-based nutrition and exercise interventions on the prevention and treatment of sarcopenia in the elderly. Nutrients. (2022) 14:458. doi: 10.3390/nu14122458
51. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. (2013) 14:542–59. doi: 10.1016/j.jamda.2013.05.021
52. Cheng SH, Chen KH, Chen C, Chu WC, Kang YN. The optimal strategy of vitamin d for sarcopenia: a network meta-analysis of randomized controlled trials. Nutrients. (2021) 13:589. doi: 10.3390/nu13103589
53. Gkekas NK, Anagnostis P, Paraschou V, Stamiris D, Dellis S, Kenanidis E, et al. The effect of vitamin D plus protein supplementation on sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Maturitas. (2021) 145:56–63. doi: 10.1016/j.maturitas.2021.01.002
54. Choi M, Kim H, Bae J. Does the combination of resistance training and a nutritional intervention have a synergic effect on muscle mass, strength, and physical function in older adults? A systematic review and meta-analysis. BMC Geriatr. (2021) 21:639. doi: 10.1186/s12877-021-02491-5
Keywords: sarcopenia, nutrition, resistance training, elderly people, meta-analysis
Citation: Song Z, Pan T, Tong X, Yang Y and Zhang Z (2023) The effects of nutritional supplementation on older sarcopenic individuals who engage in resistance training: a meta-analysis. Front. Nutr. 10:1109789. doi: 10.3389/fnut.2023.1109789
Received: 28 November 2022; Accepted: 03 April 2023;
Published: 25 April 2023.
Edited by:
Hattie Wright, University of the Sunshine Coast, AustraliaReviewed by:
Kenji Nagao, Ajinomoto, JapanCopyright © 2023 Song, Pan, Tong, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze Zhang, MTMwNjY3OTYyMzJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.