- 1Department of Food Sciences, Government College University, Faisalabad, Pakistan
- 2Department of Economics, Kebri Dehar University, Kebri Dehar, Ethiopia
- 3Division of Research and Development, Lovely Professional University, Phagwara, Punjab, India
The amount of food waste throughout the world has become quite alarming and is contributing to lower food resources. The study aimed to extract and characterize the polyphenols from corn silks at immature and mature stages through conventional and green extraction techniques. Purposely, corn silks, which are some of the by-products of corn, (Zea mays L.) were collected and subjected to proximate analysis including moisture, ash, protein, fiber, and minerals. Secondly, the antioxidants from both immature and mature corn silks were extracted by techniques involving supercritical and ultrasound extraction alongside conventional extraction. The results displayed a promising quantity of protein and fiber along with calcium, magnesium, sodium potassium, and copper. Among the extraction techniques, supercritical extraction at 3,000 Pa acquired the highest total phenolic contents (TPC), total flavonoids (TF), 2, 2-diphenylpicrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP) activities as 128.08 ± 3.74 mg GAE/100 g, 86.73 ± 2.75 mg CE/100 g, 106.73 ± 5.10%, and 73.52 ± 2.33 μM Fe + 2/g, respectively, followed by the ultrasound and conventional extraction techniques. Between the immature and mature corn silks, the highest antioxidant activity was displayed by immature corn silks.
Introduction
Agro-industrial waste poses huge environmental and financial hurdles at the point of disposal. However, they hold rich phytochemistry with special reference to polyphenols that may prove effectual against various lifestyle-related ailments. In this context, waste valorization is among the leading strategies to ensure food security by minimizing loss and recycling beneficial components that may be further utilized as therapeutic agents (1). Among the various waste-generating crops, corn (Zea mays L.) has vital importance primarily due to its higher consumption and rich phytochemistry. Parts of the corn (Zea mays L.) plant hold promising nutritional and therapeutic potential owing to its strong polyphenol contents (2, 3). Amongst the different waste products of corn, the bundles of silky, hair-like, long, and yellow in color strands, which can be found on the top of the immature or mature corn, are called corn silks and they hold significant importance. Corn silk is usually treated as waste; however, recent investigations unveiled its nutritional and functional profile with special reference to fiber, minerals, and antioxidants (4). Research has also found that the stage of maturity also plays a pivotal role in its antioxidant profile (5). Conventionally, these hair-like strands are utilized as a diuretic agent and are vital for providing ease for the passage of kidney stones or urinary bladder stones (6). Furthermore, Chinese people have been utilizing these corn silks for medical purposes and treating several ailments usually related to the kidneys (7). The therapeutic potential of corn silk has been attributed to its high polyphenolic contents with special reference to phenolic acids like ferulic acid, caffeic acid, and gallic acid. Moreover, the demand for natural antioxidants has been on the increasing side because of negative perceptions associated with synthetic antioxidants that are available in the market (8). All around the world, plants and their waste products are a potent source of natural antioxidants and help in combating many ailments (9–11).
Currently, green extraction technologies have been adapted widely for the extraction of high-value-added compounds from agro-industrial wastes (12). The utilization of novel technologies for polyphenol extraction can improve the extraction yield and therapeutic potential. Moreover, they are rated as affordable, safe, effective, and ecologically friendly alternatives, enabling the clean label status (13). Polyphenol extraction yield depends on the technique applied and novel green extraction technologies like ultrasound-assisted extraction, supercritical fluid extraction, microwave-assisted extraction, and pulse electric field extraction are considered the most efficient owing to their higher yield, extraction time, costs, and environmental impact. However, the success of the applied technique depends on several factors including the nature of raw material, chemical constituents, and processing parameters (14). The green technologies utilized for extraction help in efficient structural elucidation along with less solvent and time required for the completion of the procedures. Supercritical fluid extraction (SFE) uses CO2 to separate the components while ultrasonication uses the frequencies from sound waves to extract the required components. Considering the abovementioned facts, it is envisaged that there would be conversations regarding the impact of the different extraction techniques on the polyphenol extraction of corn silk and their structural elucidation. This study aimed to elucidate the nutritional composition of corn silk and its antioxidant profiling through different green extraction techniques like supercritical fluid extraction and ultrasonication alongside side conventional extraction for comparison.
Materials and methods
Procurement of raw material
For the current research, the corn was procured from the Ayub Agriculture Research Institute (AARI), Faisalabad, with a plant voucher number (GCBFA-3347), while the chemicals utilized for the HPLC standards and analytical procedures were procured from Merck (Germany) and Sigma-Aldrich (Japan).
Raw material handling
The corn silks were removed from the corn fruits and washed thoroughly with distilled water to remove any impurities. After washing the corn silks, they were dried in a hot air oven at 50 to 60°C for approximately 48 h. Finally, the oven-dried sample of corn silks was ground into a powder form with the help of a small laboratory grinder (Panasonic, Japan, Model MJ-W176P) and stored at an optimum temperature for further use in the research.
Proximate analysis
The proximate analysis of the corn silks was carried out by the determination of the moisture content, protein, lipid (fat), ash, and dietary fiber by following the protocol of AACC (15). However, the nitrogen free extract (NFE) was calculated at the end through the subtraction method.
Mineral analysis
The corn silk powder sample was then evaluated for its mineral content consisting of minerals like Na (sodium), K (potassium), Ca (calcium), Zn (zinc), Fe (iron), and Mg (magnesium) following the guidelines of AOAC (16). The first two minerals were determined by Flame Photometer-410 (Sherwood Scientific Ltd., Cambridge) while the remaining were estimated by Atomic Absorption Spectrophotometer (Varian AA240, Australia).
Preparation of extracts
Experimental design
We utilized the following conditions for the different extraction techniques owing to the experimental techniques that were already approved appropriately in a preliminary trial. Moreover, the extraction of polyphenols from waste materials had already been carried out in our laboratories and the following conditions had been proven suitable so we used them in our study.
Conventional solvent extraction
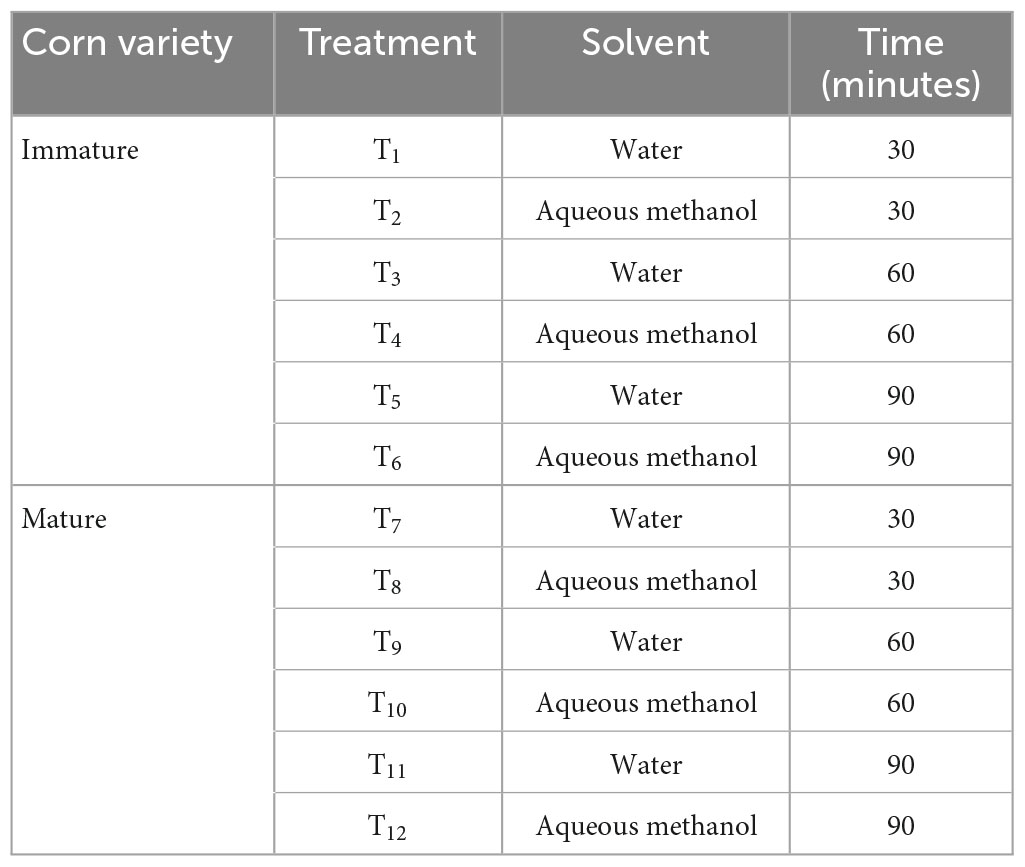
The extracts of the corn silk powder were prepared by the maceration technique using the method proposed by Rusak et al. (17). Two different solvents namely aqueous methanol and water were utilized with three different time intervals (20, 30, and 40 min), temperatures (30, 40, and 50°C), and solvent-to-sample ratios (40:60, 60:40, and 80:20). The extracts obtained as a result of the technique used were further treated for concentration and was turned to powder form using the freeze dry technique and then stored at an optimum temperature (Table 1A).
Supercritical fluid extraction (SFE)
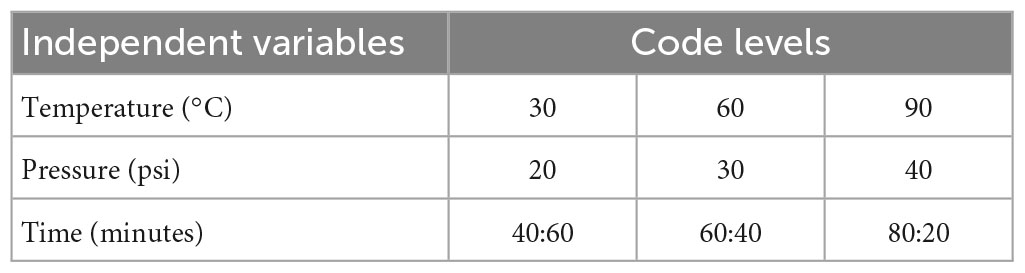
The supercritical fluid extraction of polyphenols from the corn silk was carried out using three different factors including time, pressure, and temperature of 20, 30, and 40 min; 2,000, 3,000, and 4,000 Pa; and 30, 40, and 50°C, respectively (18), as shown in Table 1B.
Ultrasonic-assisted extraction (UAE)
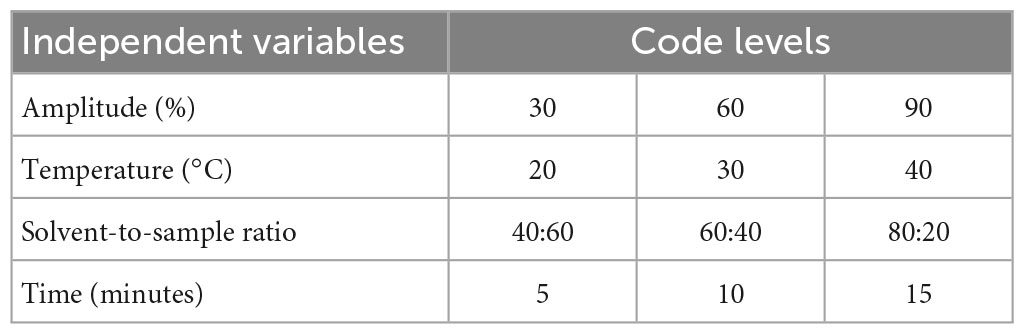
The ultrasonic-assisted extraction of the corn silk was performed by the protocol given by Albu et al. (19) with some variations. The solvents utilized for the purpose of extraction were water and aqueous methanol at different time intervals (20, 30, and 40 min), temperatures (30, 40, and 50°C), solvent-to-sample ratio (40:60, 60:40, and 80:20), and amplitude (20, 30, and 40%) (Table 1C).
Phytochemical screening assays
Phytochemical screening of the extracts obtained from different techniques was carried out by performing various antioxidant assays including total phenolic content (TPC), total flavonoid content (TFC), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis, 3-ethylbenzothiazoline-6-sulfonic acid (ABTS), and ferric-reducing antioxidant power (FRAP) estimation.
Determination of total phenolics contents (TPC)
To determine the antioxidant potential of the extracts, the TPC was estimated through the assay against the standard reagents. For this purpose, an equal amount of the FC agent and the sample of corn silk was mixed with 500 μL of water (distilled) and allowed to rest for approximately 5 min after, 7% Na2CO3 (4.5 ml) was incorporated and allowed to rest for another 90 min. Finally, the assay was used to measure the absorbance through the spectrophotometer (IRMECO, U2020) at 760 nm. The TPC was then estimated as the gallic acid equivalent (mg gallic acid/g) using the method proposed by Sengul (20).
Determination of total flavonoid (TF)
One of the important polyphenol classes includes flavonoids, which are the largest class and can provide therapeutic benefits. In the assay to determine the TFC, a mixture consisting of distilled water, NaNO2, and 10 percent AlCl3 in the ratio of 0.1, 4, 0.3, and 5%, respectively, was prepared. The solution was allowed to rest for 6 min and then 1.0M NaOH was added. The absorbance was then estimated at 430 nm following the protocol of Ghasemzadeh and Jaafar (21).
Antioxidant potential
DPPH radical scavenging assay
The free radical scavenging ability was determined by the DPPH (1,1-diphenyl-2-picrylhydrazyl) assay. The estimation is carried out by taking the sample and the DPPH solution at 0.12 mM concentration added in a test tube in the ratio of 4:1, respectively. The solution was allowed to rest in a dark place for approximately 30 min. Later on, the absorbance of the solution was estimated at 520 nm using the UV/visible spectrophotometer against the standard control and the blank (22, 23).
ABTS (2,2′-azino-bis, 3-ethylbenzothiazoline-6-sulfonic acid) assay
The ABTS (2,2′-azino-bis, 3-ethylbenzothiazoline-6-sulfonic acid) assay of the corn silk extracts was carried out by the procedure proposed by Hossain et al. (24). To prepare the ABTS radicals, ABTS solution of 7 mM concentration was incorporated with 5 ml of potassium per sulfate solution of 2.45 mM concentration to make a total of 10 ml volume. This prepared mixture was transferred to an opaque bottle and allowed to rest for approximately 16 h in a dark and cool place to achieve stable oxidization. After the mixture had rested, it was diluted with ethanol and then adjusted to provide 0.7 absorbance at 734 nm. In addition, 10 μL of corn silk extract was added to 1 ml of the ABTS solution and then mixed thoroughly. After mixing, the solution was then subjected to a spectrophotometer to measure the absorbance at 734 nm after making it rest for 30 min. The antioxidant capacity of the extracts was determined using Trolox standard curve recorded in μmol Trolox/g sample extract.
Ferric-reducing antioxidant power (FRAP)
Another assay performed for the determination of the antioxidant capacity of the extract was the FRAP. In this assay, the metal ion chelating power is the parameter that helps in assessing the antioxidant potential of the compound under experiment. For this purpose, 0.5 ml of the experimental sample was added to 125 ml of the phosphate buffer with a concentration of 0.2 M and pH of 6.6, and potassium ferricyanide solution with a concentration of 1% was altogether placed in a water bath at a temperature of 50°C for 15 min. In the sample, 125 ml of trichloroacetic acid (10%) and distilled water were incorporated with 0.25 ml of ferric chloride (1%) and allowed to rest for 10 min. After the resting period, the absorbance was calculated at 700 nm (25).
HPLC quantification of bioactive compounds
The different preparations of corn silk extracts were then subjected to HPLC quantification to determine the comparative profusion of the bioactive compounds present. Ferulic acid which was found in abundance in corn silks has proven to be a potent antioxidant with health benefits. Purposely, the HPLC quantification was carried out for the approximation of ferulic acid content in corn silk extracts obtained through the extraction techniques mentioned above. Reverse phase HPLC (PerkinElmer, Series 200, USA) with C18 column was utilized. The mobile phase consisted of methanol/H2O, 65:35 (v/v), with a sample size of 1 ml, and at a per-minute flow rate of 1.0 ml. For the estimation procedure, ultraviolet detection was carried out at 282 nm. The comparison with the peak time and height of the ferulic acid standard was provided with the calculations (26).
Statistical analysis
The data for each parameter was statistically analyzed to determine the level of significance using a software package (MATLAB). Analysis of variance technique (ANOVA) was applied for the estimation of significance (p ≤ 0.05) between the various factors. Purposely, a one-factor or two-factor factorial design was applied as per the nature of the variables. Moreover, the comparison of the extraction techniques and maturity vs. immaturity two-factor factorial design was applied. Duncan’s multiple ranges (DMR) test was utilized to estimate the level of significance between the groups.
Results and discussion
Nutritional composition of corn silk
Proximate and mineral analysis
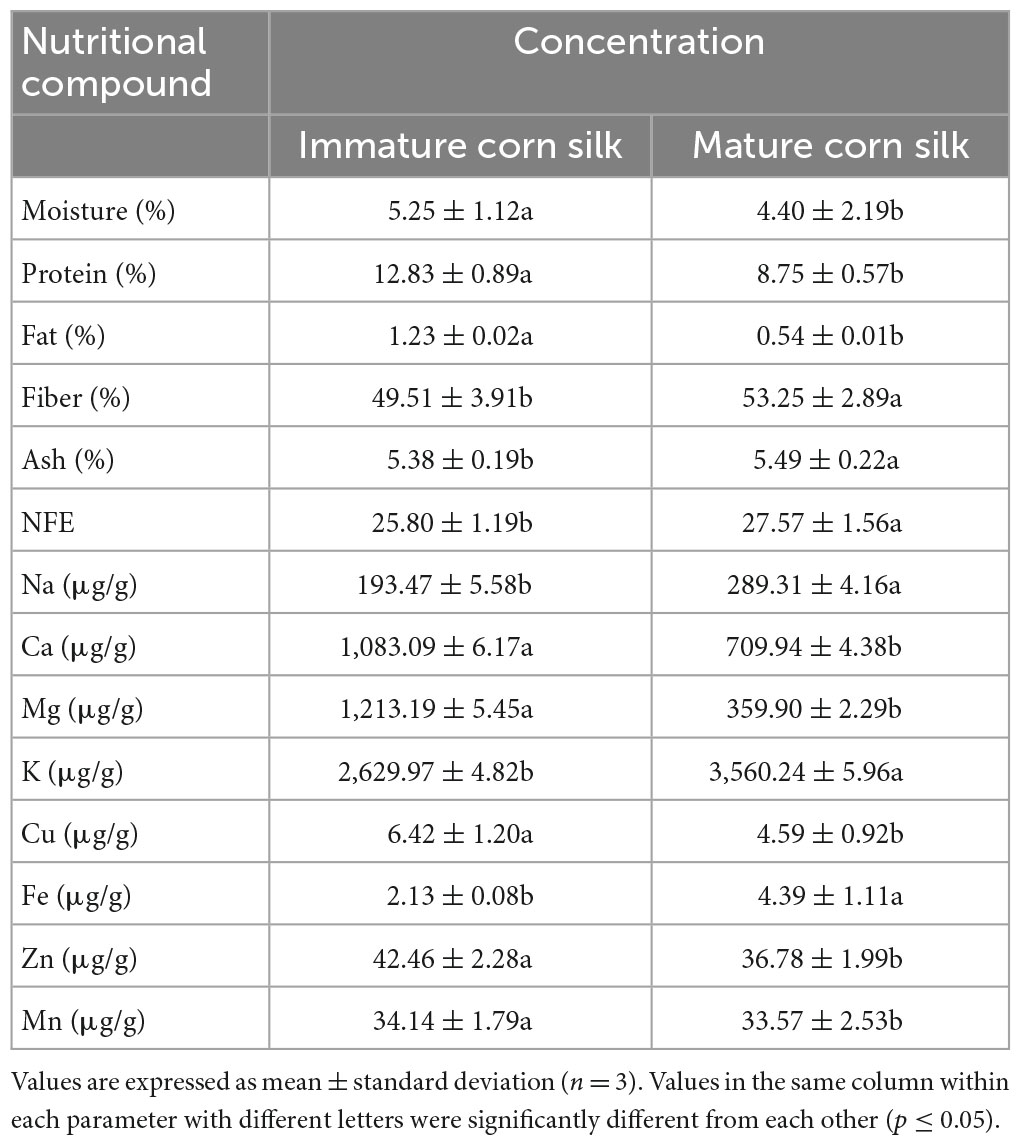
The mean values for moisture, protein, fat, fiber, total ash, and NFE (nitrogen-free extract) of immature and mature corn silk were found to be 5.25 ± 1.12 and 4.40 ± 2.19, 12.83 ± 0.89 and 8.75 ± 0.57, 1.23 ± 0.02 and 0.54 ± 0.01, 49.51 ± 3.91 and 53.25 ± 2.89, 5.38 ± 0.19 and 5.49 ± 0.22, and 25.80 ± 1.19 and 27.57 ± 1.56%, respectively. Likewise, the values observed for minerals in immature and mature corn silk were sodium (Na) 193.47 ± 5.58 and 289.31 ± 4.16, potassium (K) 2,629.97 ± 4.82 and 3,560.24 ± 5.96, calcium (Ca) 1,083.09 ± 6.17 and 709.94 ± 4.38, magnesium (Mg) 1,213.19 ± 5.45 and 359.90 ± 2.29, zinc (Zn) 42.46 ± 2.28 and 36.78 ± 1.99, manganese (Mn) 34.14 ± 1.79 and 33.57 ± 2.53, and iron (Fe) 2.13 ± 0.08 and 4.39 ± 1.11 μg/g, respectively (Table 2).
In vitro characterization
The grand mean obtained for the parameters of the in vitro characterization including TPC, TFC, radical scavenging assay (DPPH), ferric-reducing antioxidant potential (FRAP), and ABTS assay for the mature and immature corn silk extracts showed that both the extraction techniques [(p ≤ 0.05)] and stage of maturity of corn silk [(p ≤ 0.05)] showed significant differences in the antioxidant profile, whereas their interaction showed non-significant differences [(p 0.0250)]. Moreover, the effect of the time, temperature, type of solvent used, pressure, and amplitude was found to be quite significant on the TPC, TFC, DPPH, FRAP, and ABTS of the obtained extracts. The peak recovery was presented by the supercritical fluid extraction followed by the ultrasound, and the least was found to be by the conventional extraction. The peak values were obtained in the supercritical fluid extraction with the parameters including a pressure of 4,000 Pa, temperature of 50°C, and time interval of 40 min, whereas in the ultrasonic extraction, the highest recovery point was with the following parameters: a temperature of 50°C, a time interval of 40 min, ethanol of 80%, and an amplitude of 40%. Similarly, in the conventional extraction technique, the parameters included 50°C temperature, 40 min time interval, and 80% ethanolic solution, which showed better recovery as compared to other solvents utilized (Table 3).
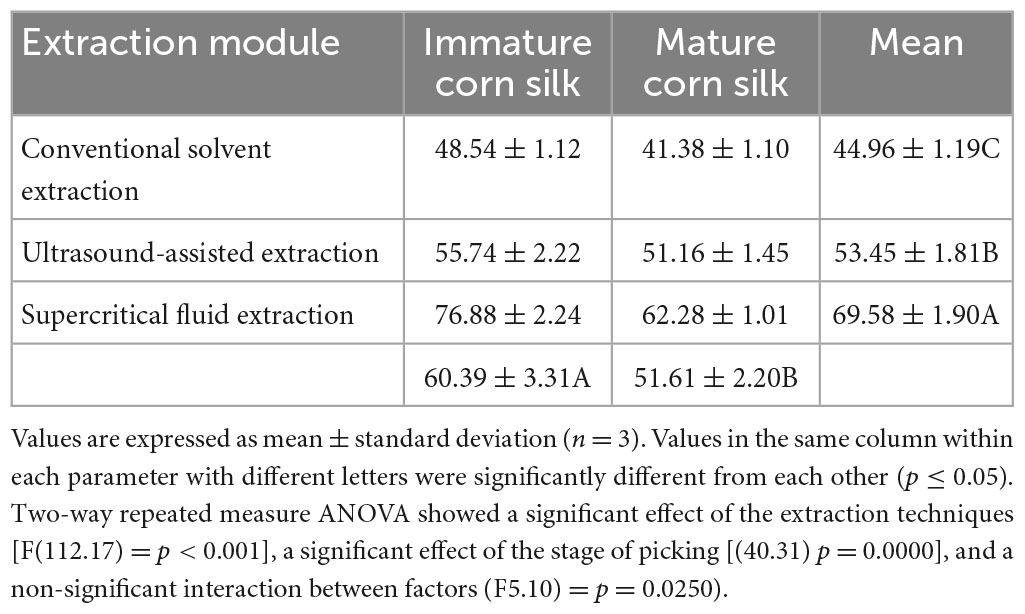
Table 3. Comparison of extraction techniques on the collective antioxidant profile of polyphenols from immature and mature corn silk polyphenols.
Antioxidant potential of mature and immature corn silk extracts
The below tables represent the effect of treatments on TPC, TFC, DPPH, FRAP, and ABTS assays of the mature and immature corn silk extracts, respectively. The treatment with the highest TPC value was the supercritical fluid extract, while lower values were observed in the ultrasonicated extraction technique (Table 4). Further decreased values were found to be for the conventionally extracted extracts. A similar trend was observed for the TFC, which showed the peak values for the supercritical fluid extraction to be the same as the former. Also seen in the DPPH (Table 5) and FRAP (Table 6) was the same trend as the TPC, showing maximum extraction in the supercritical fluid technique (T9S2), followed by the ultrasound (T6S1 and T6S2) and conventional (T3S1 and T3S2) techniques. The highest mean values for the TPC of the mature and immature corn silk extracts displayed by the supercritical fluid extraction presented the values to be 143.8917 ± 2.32 (immature) and 112.2672 ± 2.07 (mature) mg/GAE 100 g for the extract T9S2. Similar results were obtained for TFCs (Table 7) for the extract T9S2 to be 97.0109 ± 1.99 (immature) and 76.45491 ± 2.22 (mature) mg CE/100 g. The values of DPPH, FRAP, and ABTS (Table 8) showed the same trend followed by the TPC and TFC as mentioned earlier. The values of DPPH were found to be 117.0109 ± 4.52% (immature) and 96.45491 ± 2.57% (mature), that of FRAP were noted to be 80.64263 ± 2.11 (immature) and 66.41158 ± 1.90 (mature) μM (Fe + 2/g), while that of ABTS were presented as 1.206477 ± 0.228 (immature) and 1.200152 ± 0.902 (mature) μM TE/g.
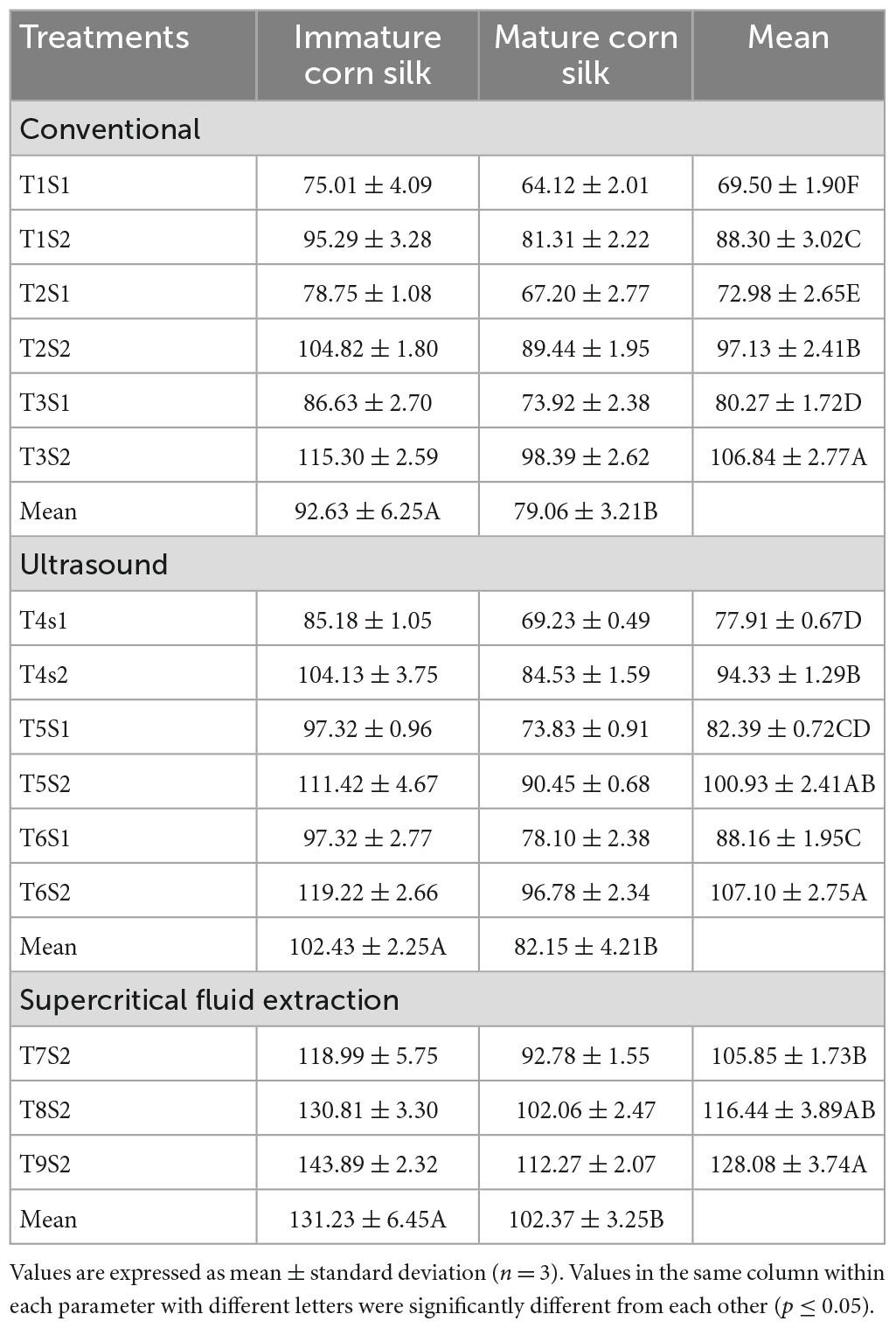
Table 4. Comparison of total phenolic contents (mg/100 g gallic acid equivalent) of mature and immature corn silk extracted through different extraction technologies.
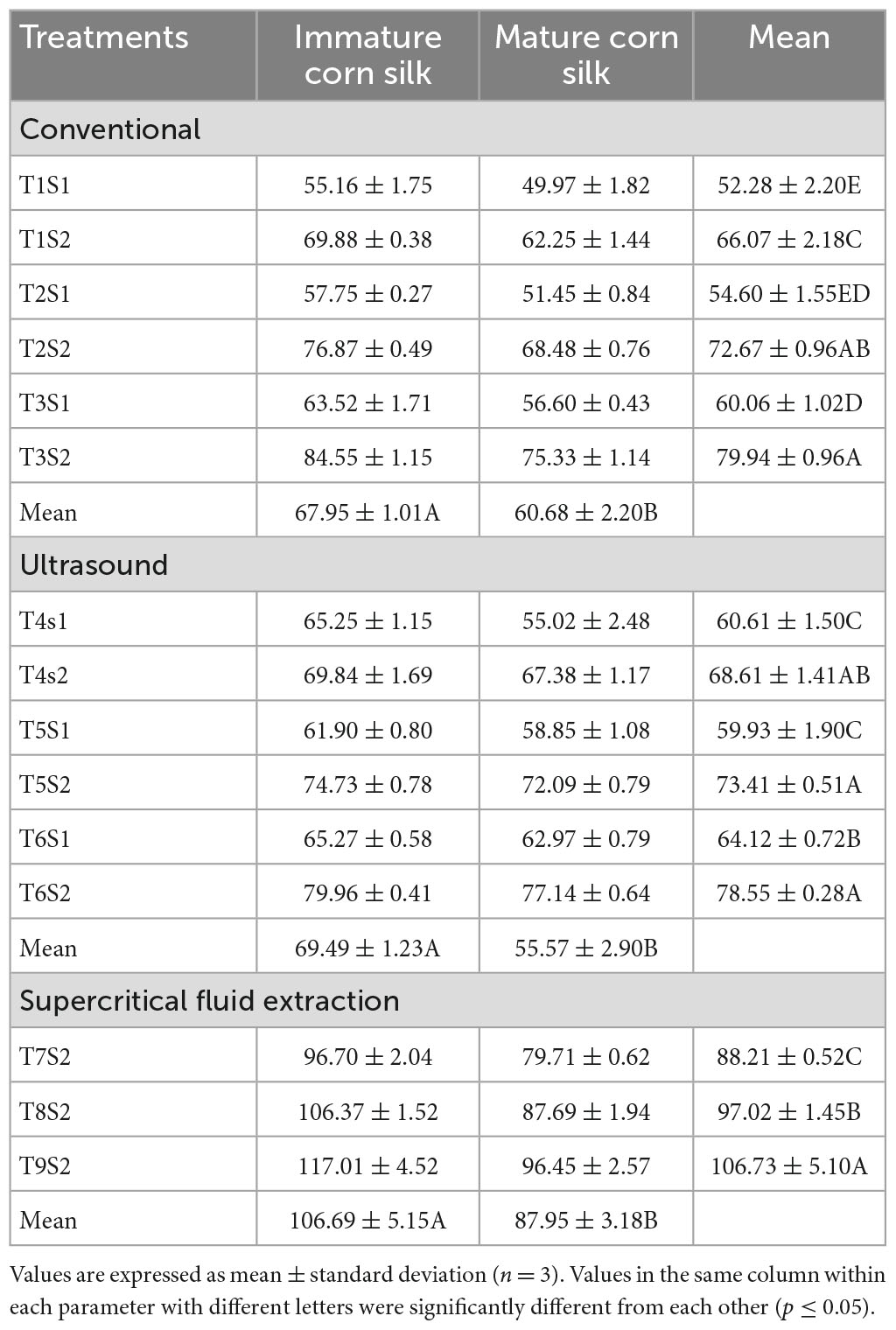
Table 5. Comparison of DPPH activity (%) of mature and immature corn silk extracted through different extraction technologies.
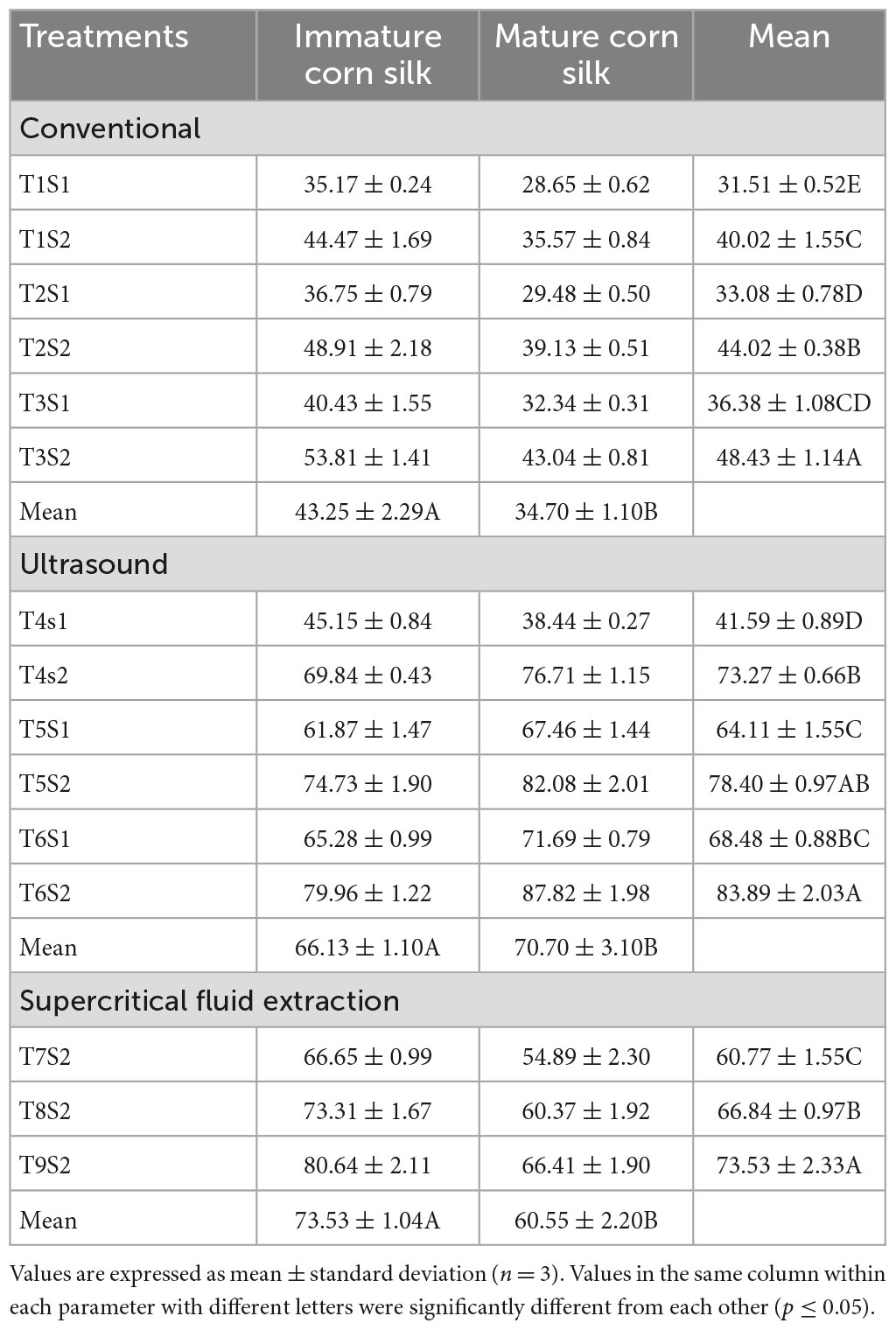
Table 6. Comparison of FRAP (μM Fe + 2/g) of mature and immature corn silk extracted through different extraction technologies.
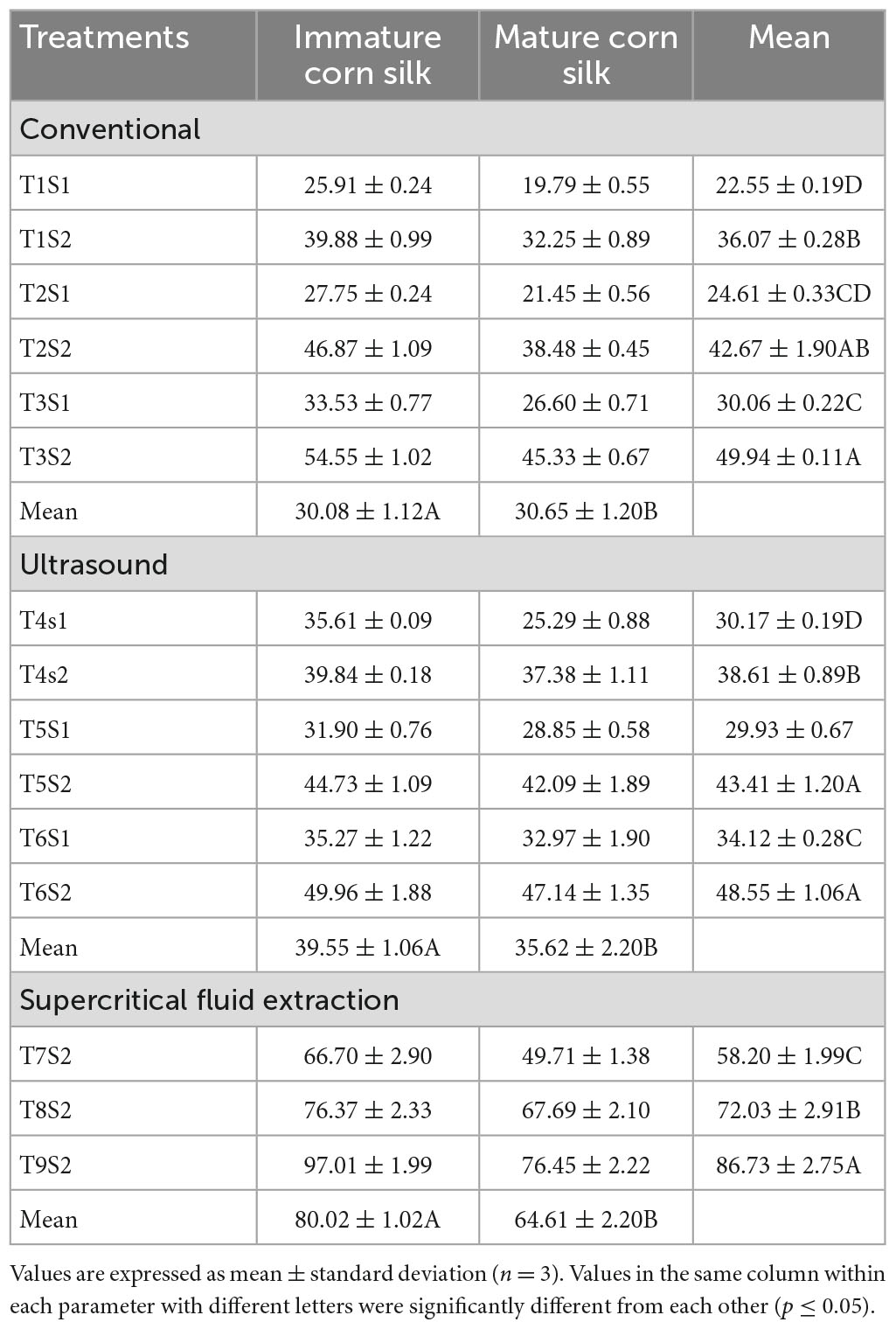
Table 7. Comparison of total flavonoid contents (mg CE/100 g) of mature and immature corn silk extracted through different extraction technologies.
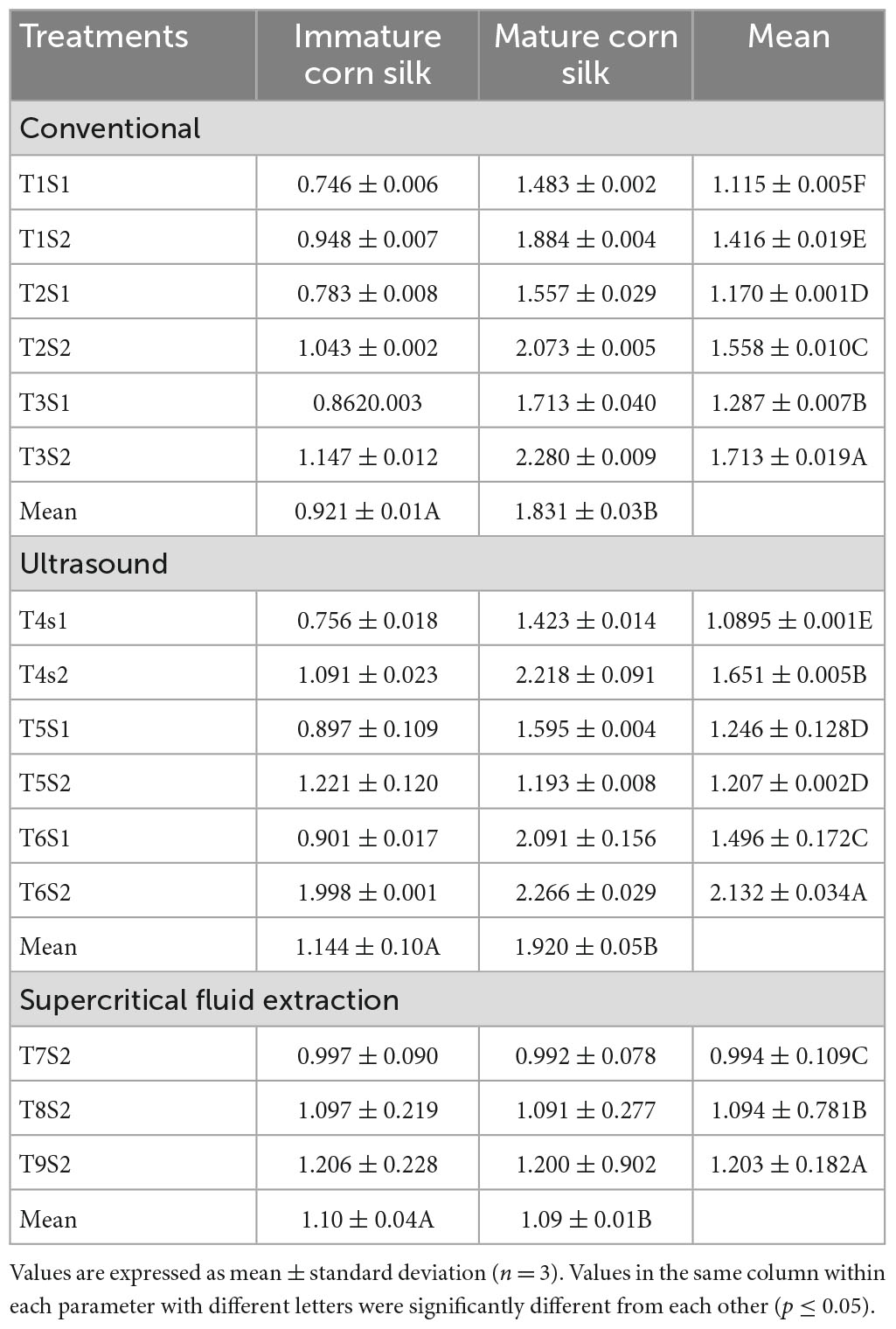
Table 8. Comparison of ABTS (μM TE/g) of mature and immature corn silk extracted through different extraction technologies.
HPLC quantification of ferulic acid in corn silk
The statistical analysis of corn silk bioactive components displayed considerable differences in the ferulic acid content of the extracts obtained through different techniques. The varying solvents i.e., methanol and water, and their respective ratios showed ferulic acid as having the highest recovery in aqueous methanolic extract among all the techniques. Among the extraction techniques, supercritical fluid extraction showed the highest quantity of ferulic acid, compared to the conventional and UAE techniques (Figure 1).
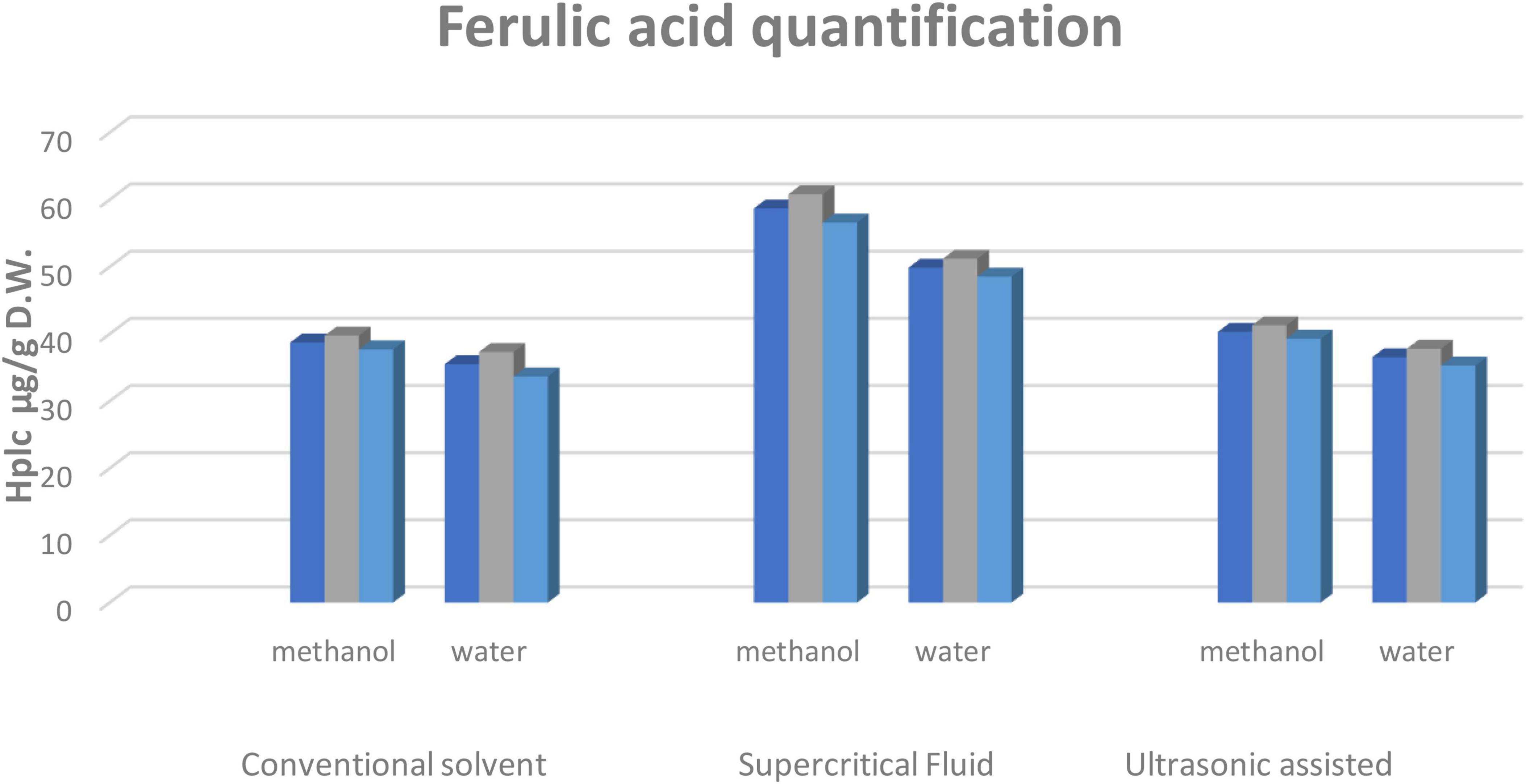
Figure 1. HPLC quantification of ferulic acid from corn silks. Values for ferulic acid characterized through reverse phase HPLC in μg/g. Two solvents and three extraction techniques were applied. Values are mean ± SEM (n = 03) and the level of significance was determined at (p ≤ 0.05).
Discussion
This research might be the first to highlight the impact of different extraction techniques (supercritical fluid, ultrasonication, and conventional) and their parameters of extraction and isolation of corn silk polyphenols. Moreover, their maturity and immaturity were also discussed according to the different extraction techniques in the light of the study. The results of the current study found an ethanolic solution for extraction to be much more effective, compared to the other solvents. Similar results, as in the study conducted by Humadi and Istudor (27), were obtained for the extracts obtained from a methanolic solution, which displayed lower TPCs in both the mature and the immature corn silk extracts when compared to the aqueous extracts, noting the values to be 40.38 + 1.10 μg/g of TAE lower than 42.71 + 0.87 μg/g of TAE. The extraction procedure involved in this ongoing study utilized different solvents and techniques as in the study conducted by Das et al. (28). The use of different solvents led to greater extractions of the required polyphenols, which elevated the antioxidant potential of the corn silk extracts (29, 30). Moreover, the affinity of the phenols for similar polarity solvents plays an important role in extraction processes (31). The possibility of having lower antioxidants might be due to the limitations of utilizing the whole corn silks due to hygienic issues; however, the yellow color imparting corn silks providing the flavones and the flavonols can be of great importance. The polyphenols which present as antioxidants also play a vital role in chelating metals and scavenging free radicals (32–34). On the other hand, there can still be several factors like fertilization, soil conditions, size, maturity, season, etc., which may affect the antioxidant potential of corn silk. According to earlier studies (35, 36), the ethanolic method is greatly used for extraction purposes. The extraction of the polyphenols and the flavonoids depended on the different extraction conditions including extraction time and extraction temperature, as well as the pressure and amplitude in some of the extraction techniques (34, 37, 38). Insufficient time for extraction may yield lower concentrations of flavonoids; thus, it is best to have an optimum temperature and time for carrying out the proper extraction procedure. In a study carried out earlier on the optimum condition for the extraction of corn silks, it was found that 5.32 mg of the total flavonoids was extracted from 1 g of corn silk (39). The different polarities of different solvents allowed for the diffusion of various constituents of the material in the plant and thus helped with the extraction yield as well. In a previous investigation, the percentage of the radical scavenging activity (DPPH) was observed to rise with larger concentrations of the extract in all samples. The methanolic extract had the greatest DPPH activity in the research, followed by the ethanolic and aqueous extracts. The values noted were 140.89 μg/ml, 143.55 μg/ml, and 195.21 μg/ml, respectively.
Conclusion and recommendations
With the advancement of technology, the extraction from several plant varieties has become quite easier than in the earlier days. The wide use of microwave-assisted, ultrasound, and supercritical fluid extraction is now taking the place of the conventional techniques used in older days. Utilizing plant sources for their beneficial antioxidants has been carried out around the world by several researchers and scientists. Our study comprising of the extraction of polyphenols from the corn silk has provided new insight into the beneficial aspects of the rarely used part, the corn silk of the corn scientifically known as the Zea mays. The study of the antioxidant potential of these corn hairs or the corn silk has displayed a potent scavenging free radical effect, chelating of the catalytic metal ions, and also exerting a therapeutic effect against the oxidative stress/damage to the cellular macromolecules. The chemical examination of the different varieties of extracts showed the presence of polyphenols and flavonoids, imposing the antioxidant characteristic of the plant material thereof. The greater scavenging power of the corn silk may be possible because of the hydroxyl group that exists in the phenolic compounds. Thus, these seldomly utilized parts of the corn (Zea mays L.) named corn silk can be of great importance due to their antioxidant potential. From a future perspective, extensive characterization of the by-products through GC-MR spectroscopy can be carried out for elucidating the potential polyphenolics for further utilization.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NF: conceptualization and writing—original draft. AI: supervision, writing—original draft and review, and editing. MUA: validation and methodology. MSA: formal analysis, investigation, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Government College University Faisalabad, Pakistan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Usman I, Imran A, Arshad MU, Saeed F, Afzaal M, Sana S, et al. Valorization of mustard and sesame oilseed cakes for food application through eco-innovative technologies. Food Sci Nutr. (2023). doi: 10.1002/fsn3.3214
2. Naqvi S, Ramessar K, Farré G, Sabalza M, Miralpeix B, Twyman RM, et al. High-value products from transgenic maize. Biotechnol Adv. (2011) 29:40–53. doi: 10.1016/j.biotechadv.2010.08.009
3. Voca N, Varga B, Kricka T, Curic D, Jurisic V, Matin A. Progress in ethanol production from corn kernel by applying cooking pre-treatment. Bioresource Technol. (2009) 100:2712–8. doi: 10.1016/j.biortech.2008.12.030
4. Singh J, Inbaraj BS, Kaur S, Rasane P, Nanda V. Phytochemical analysis and characterization of corn silk (Zea mays, G5417). Agronomy. (2022) 12:777. doi: 10.3390/agronomy12040777
5. Žilić S, Janković M, Basić Z, Vančetović J, Maksimović V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): comparison with medicinal herbs. J Cereal Sci. (2016) 69:363–70. doi: 10.1016/j.jcs.2016.05.003
6. Maksimović ZA, Kovačević N. Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia. (2003) 74:144–7. doi: 10.1016/S0367-326X
7. Zhao W, Yin Y, Yu Z, Liu J, Chen F. Comparison of anti-diabetic effects of polysaccharides from corn silk on normal and hyperglycemia rats. Int J Biol Macromol. (2012) 50:1133–7. doi: 10.1016/j.ijbiomac.2012.02.004
8. Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, et al. Natural antioxidants from residual sources. Food Chem. (2001) 72:145–71. doi: 10.1016/S0308-8146(00)00223-5
9. Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radical Biol Med. (2011) 51:978–92. doi: 10.1016/j.freeradbiomed.2011.05.004
10. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. doi: 10.1016/j.biocel.2006.07.001
11. Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radical Biol Med. (2012) 52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010
12. Freitas LC, Barbosa JR, da Costa ALC, Bezerra FWF, Pinto RHH, de Carvalho Junior RN. From waste to sustainable industry: how can agro-industrial wastes help in the development of new products? Resources Conservation Recycling. (2021) 169:105466. doi: 10.3390/ma12040553
13. Tiwari BK. Ultrasound: a clean, green extraction technology. TrAC Trends Anal Chem. (2015) 71:100–9.
14. Belwal T, Pandey A, Bhatt ID, Rawal RS. Optimized microwave assisted extraction (MAE) of alkaloids and polyphenols from Berberis roots using multiple-component analysis. Sci Rep. (2020) 10:917.
15. American Association of Cereal Chemists [AACC]. Approved Methods of American Association of Cereal Chemists. 10th ed. St. Paul, MN: American Association Cereal Chemists, Inc. (2000).
16. AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International. 18th ed. Arlington, VA: AOAC Press (2006).
17. Rusak G, Komes D, Likić S, Horžić D, Kovač M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. (2008) 110:852–8. doi: 10.1016/j.foodchem.2008.02.072
18. Ahn J, Choi W, Kim S, Ha T. Anti-diabetic effect of watermelon (Citrullus vulgaris Schrad) on streptozotocin-induced diabetic mice. Food Sci Biotechnol. (2011) 20:251–4. doi: 10.1007/s10068-011-0034-5
19. Albu S, Joyce E, Paniwnyk L, Lorimer JP, Mason TJ. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrasonics Sonochem. (2004) 11:261–5. doi: 10.1016/j.ultsonch.2004.01.015
20. Sengul M, Yildiz H, Gungor N, Cetin B, Eser Z, Ercisli S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pakistan J Pharmaceut Sci. (2009) 22:102–6.
21. Ghasemzadeh A, Jaafar HZ. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Comp Alternat Med. (2013) 13:341. doi: 10.1186/1472-6882-13-341
22. Tomsone L, Kruma Z, Galoburda R. Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana). World Acad Sci Eng Technol. (2012) 64:903–8.
23. Zahnit W, Smara O, Bechki L, Souici CB, Messaoudi M, Benchikha N, et al. Phytochemical profiling, mineral elements, and biological activities of Artemisia campestris L. grown in Algeria. Horticulturae. (2022) 8:914. doi: 10.3390/horticulturae8100914
24. Hossain MB, Brunton NP, Barry-Ryan C, Martin-Diana AB, Wilkinson M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J Chem. (2008) 1:751–6.
25. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature (2008) 455:64–71. doi: 10.1038/nature07242
26. Hosoishi S, Rahman M, Murakami T, Park SH, Kuboki Y, Ogata K. Winter activity of ants in an urban area of western Japan. Sociobiology. (2019) 66:414–9.
27. Humadi SS, Istudor V. Lythrum salicaria (purple loosestrife). medicinal use, extraction and identification of its total phenolic compounds. Farmacia. (2009) 57:192–200.
28. Das K, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agents: current methods and future trends. J Med Plants Res. (2010) 4:104–11. doi: 10.5897/JMPR09.030
29. Hodzic Z, Pasalic H, Memisevic A, Srabovic M, Saletovic M, Poljakovic M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur J Sci Res. (2009) 28:471–7. doi: 10.1016/j.foodchem.2013.11.128
30. Boubekeur S, Messaoudi M, Awuchi CG, Otekunrin O, Sawicka B, Idjeri-Mecherara S, et al. Biological properties and polyphenols content of Algerian Cistus salviifolius L. aerial parts. Eur J Biol Res. (2022) 12:163–80.
31. Green RJ. Antioxidant Activity of Peanut Plant Tissues. (2004). Available online at: http://www.lib.ncsu.edu/resolver/1840.16/371
32. Michalak A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Stud. (2006) 15:523–30.
33. Kavitha A, Shanmugan S, Awuchi CG, Kanagaraj C, Ravichandran S. Synthesis and enhanced antibacterial using plant extracts with silver nanoparticles: therapeutic application. Inorganic Chem Commun. (2021) 134:109045. doi: 10.1016/j.inoche.2021.109045
34. Sarvarian M, Jafarpour A, Awuchi CG, Adeleye AO, Okpala COR. Changes in physicochemical, free radical activity, total phenolic and sensory properties of orange (Citrus sinensis L.) juice fortified with different oleaster (Elaeagnus angustifolia L.) extracts. Molecules. (2022) 27:1530. doi: 10.3390/molecules27051530
35. Li H, Liu J, Li D, Wang H. Study on separation and purification of genistein in the soybean residue using macroporous resin adsorption. Industrial Eng Chem Res. (2012) 51:44–9. doi: 10.1021/ie200057e
36. Feng X, Wang L, Tao ML, Zhou Q, Zhong ZH. Studies on antimicrobial activity of ethanolic extract of maize silk. African J Microbiol Res. (2012) 6:335–8. doi: 10.18860/al.v5i4.4182
37. Messaoudi M, Rebiai A, Sawicka B, Atanassova M, Ouakouak H, Larkem I, et al. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules. (2022) 27:11. doi: 10.3390/molecules27010011
38. Wang KJ, Zhao JL. Corn silk (Zea mays L.), a source of natural antioxidants with α-amylase, α-glucosidase, advanced glycation and diabetic nephropathy inhibitory activities. Biomed Pharmacotherapy. (2019) 110:510–7. doi: 10.1016/j.biopha.2018.11.126
Keywords: agro-industrial waste, corn silk, mature, polyphenols, green extraction technologies
Citation: Faiza N, Imran A, Arshad MU, Arshad MS and Shah MA (2023) Valorization and characterization of corn by-product polyphenols through green extraction technologies. Front. Nutr. 10:1107067. doi: 10.3389/fnut.2023.1107067
Received: 24 November 2022; Accepted: 27 March 2023;
Published: 09 May 2023.
Edited by:
Hafida Wahia, Jiangsu University, ChinaReviewed by:
Carla Pereira, Centro de Investigação de Montanha, PortugalTanase Corneliu, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureş, Romania
Copyright © 2023 Faiza, Imran, Arshad, Arshad and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Imran, YWxpaW1yYW4uZnRAZ21haWwuY29t; Mohd Asif Shah, ZHJtb2hkYXNpZnNoYWhAa2R1LmVkdS5ldA==
 Neelam Faiza
Neelam Faiza Ali Imran
Ali Imran Muhammad Umair Arshad1
Muhammad Umair Arshad1 Muhammad Sajid Arshad
Muhammad Sajid Arshad Mohd Asif Shah
Mohd Asif Shah