
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 18 May 2023
Sec. Nutritional Epidemiology
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1103330
Background: Observational studies have reported inconsistent associations between micronutrient levels and the risk of coronary artery disease (CAD) in diabetic patients. We aim to explore the causal association between genetically predicted concentrations of micronutrients (phosphorus, magnesium, selenium, iron, zinc, and copper) and CAD in patients with diabetes.
Methods: Single nucleotide polymorphisms (SNPs) connected to serum micronutrient levels were extracted from the corresponding published genome-wide association studies (GWASs). Summary-level statistics for CAD in diabetic patients were obtained from a GWAS of 15,666 patients with diabetes. The primary analysis was carried out with the inverse variance weighted approach, and sensitivity analyses using other statistical methods were further employed to assess the robustness of the results.
Results: Genetically predicted selenium level was causally associated with a higher risk of CAD in diabetic patients (odds ratio [OR]: 1.25; 95% confidence interval [CI]: 1.10–1.42; p = 5.01 × 10−4). While, genetically predicted iron concentrations in patients with diabetes were inversely associated with the risk of CAD (OR: 0.82; 95% CI: 0.75–0.90; p = 2.16 × 10−5). The association pattern kept robust in most sensitivity analyses. Nominally significant associations were observed for magnesium and copper with the risk of CAD in patients with diabetes. No consistent evidence was found for the causal associations between phosphorus and zinc levels, and the risk of CAD in patients with diabetes.
Conclusion: We provide consistent evidence for the causal effect of increased selenium and decreased iron levels on CAD in patients with diabetes, highlighting the necessity of micronutrient monitoring and application in these patients.
Coronary artery disease (CAD) remains the leading cause of death worldwide, especially in patients with diabetes (1, 2). Since CAD is responsible for more than 50% of diabetes-related mortality, it dictates the prognosis for diabetic patients (3). Therefore, the 2019 European Society of Cardiology (ESC) guidelines have clarified the importance of preventing CAD in patients with diabetes (4).
Growing evidence from observational studies and randomized controlled trials (RCTs) indicated that essential micronutrients may play a critical role in the development of CAD in people with diabetes, but the results were inconsistent (5–7). For example, a meta-analysis including 40 prospective cohort studies with over 1 million individuals has shown that increasing dietary magnesium intake was associated with a reduced risk of diabetes and all-cause mortality, but not CAD or total cardiovascular diseases (CVDs) (8). Observational studies found the negative or no association between selenium biomarkers and CAD (9, 10), however, the RCTs revealed that decreased heart disease mortality among individuals with diabetes was related to increased selenium concentration (11). On the one hand, as observational studies based on reports of participants were subjected to confounding factors, which might be inaccurate leading to biased results (12). On the other hand, due to the limits of the sample size, the evidence from RCTs may not be powerful enough to evaluate the causal effect of micronutrients on the risk of CAD in diabetic patients (13).
Mendelian randomization (MR) approach can be applied to explore the potential causal association between exposure and disease by using genetic variants as instrumental variables (IVs) (14). The constraints of observational studies are successfully resolved by the random assignment of genotype at conception and the non-influence of genetic variations by potential confounding variables (15). In the current study, a two-sample MR analysis was conducted to investigate the causal associations between the risk of CAD in diabetic patients and circulating concentrations of six systematically selected micronutrients, including phosphorus, magnesium, selenium, iron, zinc, and copper.
A two-sample MR analysis was designed to estimate the causal relationship between genetically determined circulating micronutrient concentrations and the risk of CAD in diabetic patients (Figure 1). The following three core assumptions should be met by the single nucleotide polymorphisms (SNPs) chosen as IVs for circulating concentrations of micronutrients: (1) IVs should be closely related to the circulating concentrations of micronutrients, (2) IVs should be independent of any potential confounders, and (3) IVs should be associated with the risk of CAD in patients with diabetes only through the concentrations of micronutrients.
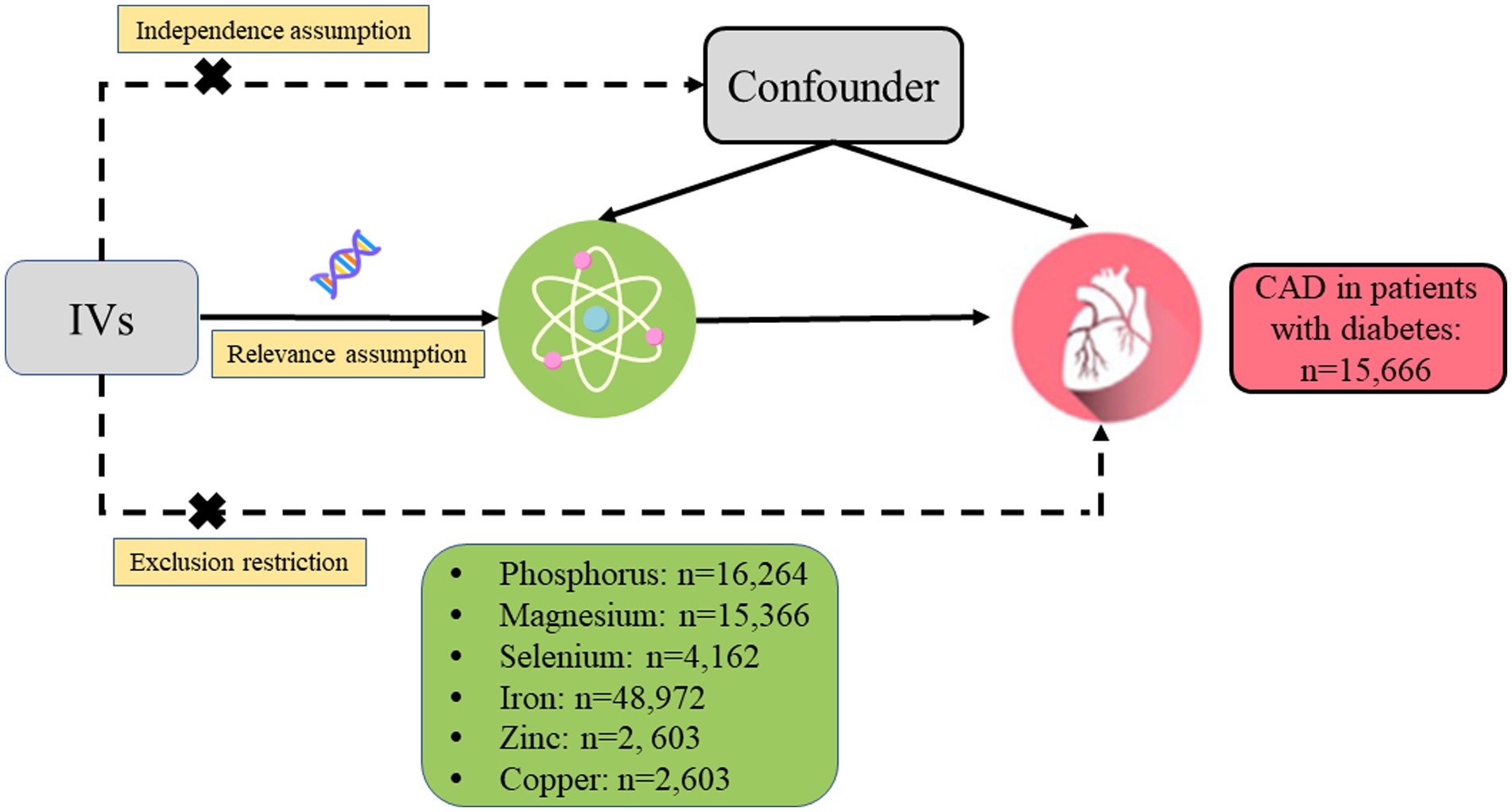
Figure 1. Design of the current two-sample Mendelian randomization study. Three core assumptions were as follows: (α) Relevance assumption; (β) Independence assumption; (γ) Exclusion restriction. IVs, instrumental variables; CAD, coronary artery disease.
First, SNPs were obtained from recently published genome-wide association studies (GWASs) that independently affect these nutrient concentrations at the genome-wide significance level (p < 5 × 10−8). Then, the linkage disequilibrium tests were performed based on the European 1000 Genomes Project reference panel (r2 < 0.01). If two SNPs were in linkage disequilibrium, the one with smaller value of p would be kept. Considering palindromic SNPs, those with minor allele frequency larger than 0.42 were regarded as not inferable and removed. Specifically, SNPs linked to serum phosphorus levels were extracted from a large GWAS meta-analysis including 16,264 participants of European ancestry (16). Six SNPs that achieved genome-wide significance in the joint analysis of the discovery (n = 15,366 participants) and replication (n = 8,463 participants) cohorts from European descent were utilized as genetic IVs for serum magnesium concentration (17). The GWAS meta-analysis of log-transformed toenail selenium concentrations and standardized residuals of log-transformed blood selenium concentrations, which included up to 4,162 individuals in four United States studies, provided the genetic summary data for serum selenium levels (18). The genetic association with serum iron levels was derived from the Genetics of Iron Status consortium, with up to 48,972 participants (19). The GWAS meta-analysis employing two cohorts from Australia and the United Kingdom yielded the SNPs chosen as genetic IVs for zinc and copper concentrations (20).
The summary statistics for CAD in patients with diabetes were extracted from the recently published GWAS, including 15,666 patients of European ancestry with diabetes (3,968 CAD cases and 11,696 controls) from the United Kingdom Biobank (21). The average age at diabetes diagnosis was 52.4 ± 12.2 for CAD cases (Male: 2,936; 74.0%) and it was 51.2 ± 12.6 for controls (Male: 7,037; 60.2%). The average age at visit was 62.7 ± 5.6 and 60.2 ± 7.0 for individuals with or without CAD, respectively.
All of the studies in our analyses have obtained relevant ethics review approvals, and all the participants included in the original studies provided written informed permission. All the data used in the current study had been publicly available.
The multiplicative random-effects inverse-variance weighted (IVW) method was employed as the primary analysis to evaluate the effect of genetically predicted micronutrient concentrations on the risk of CAD in diabetic individuals. Specifically, the causal estimate for each SNP was generated using the Wald estimator, and the corresponding standard error was calculated using the Delta method. Subsequently, the overall estimate was calculated by meta-analyzing all the estimates by the IVW method (22).
To further validate the accuracy of the findings, the Maximum likelihood (22), Weighted median (23), MR-Egger regression (24), and Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) methods were applied in follow-up sensitivity analyses (25). For instance, the Maximum likelihood method could provide a greater empirical power of estimates as it assumed that the genetic association between risk factors and outcomes follows a bivariate normal distribution (22). The Weighted median method could still produce reliable estimates even if ≤50% of the weight comes from the ineffective SNPs (23). Intercept tests could be used in the MR-Egger regression to assess the potential horizontal pleiotropy (24). The MR-PRESSO method was conducted to identify potential outliers and, after eliminating them, to provide relatively unbiased causal estimates (25). In addition, scatter plots and leave-one-out analyses were performed to depict the associations of genetically determined micronutrient levels with CAD in patients with diabetes. However, sensitivity analyses and leave-one-out analyses could not be performed as the number of SNPs for zinc and copper was less than three. Cochran’s Q statistics and corresponding value of p were calculated to assess the degree of heterogeneity in the IVW analyses (26). Considering the Bonferroni adjustment for multiple tests, a value of p of <0.008 (0.05/6 exposures) was deemed statistically significant. The value of ps between 0.008 and 0.05 were considered to indicate suggested associations. All the statistical analyses were conducted by R Software (version 4.1.1.; R Foundation for Statistical Computing, Vienna, Austria), the R package TwoSampleMR,1 and MR-PRESSO.2
Two to seven SNPs genetically determining the serum phosphorus levels were identified as IVs for serum phosphorus, magnesium, selenium, iron, zinc, and copper levels, respectively (Table 1). In the MR analysis, all F-statistic values of the genetic tools were above the suggested threshold of 10 (Table 1).
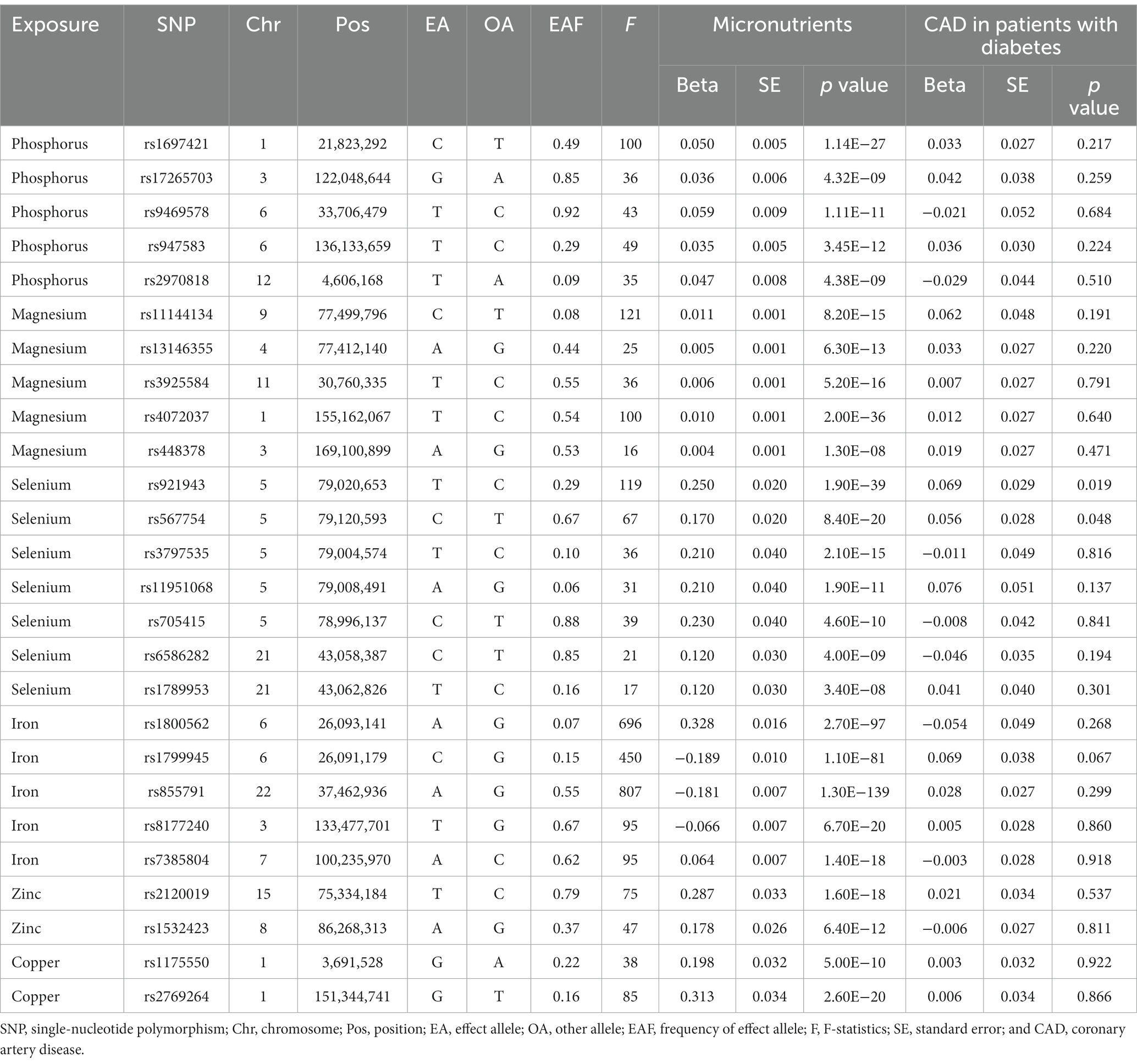
Table 1. Characteristics of the single-nucleotide polymorphisms associated with serum micronutrients levels and coronary artery disease in patients with diabetes.
The primary findings of MR studies of genetically predicted circulation concentrations of micronutrients with the risk of CAD in individuals with diabetes were displayed in Figure 2. The random-effects IVW results indicated that genetically predisposition to one standard deviation increase in concentrations of serum copper, selenium, and magnesium was linked to 2% (odds ratio [OR], 1.02; 95% CI, 1.02–1.02 p = 3.75 × 10−49), 25% (OR, 1.25; 95% CI, 1.10–1.42; p = 5.01 × 10−4), and 41% (OR, 1.41; 95% CI, 1.14–1.73; p = 1.25 × 10−3) higher risk of CAD in diabetic patients, respectively (Figure 2). An 18% (OR, 0.82; 95% CI, 0.75–0.90, p = 2.16 × 10−5) reduced risk of CAD was observed in patients with diabetes when the genetically predicted serum iron content increase by one standard deviation (Figure 2). There was minimal proof that circulating concentrations of phosphorus and zinc were associated with the risk of CAD in patients with diabetes (Figure 2). The scatter plots also visually depicted the associations between micronutrients and CAD in diabetic patients (Supplementary Figures 1–6).
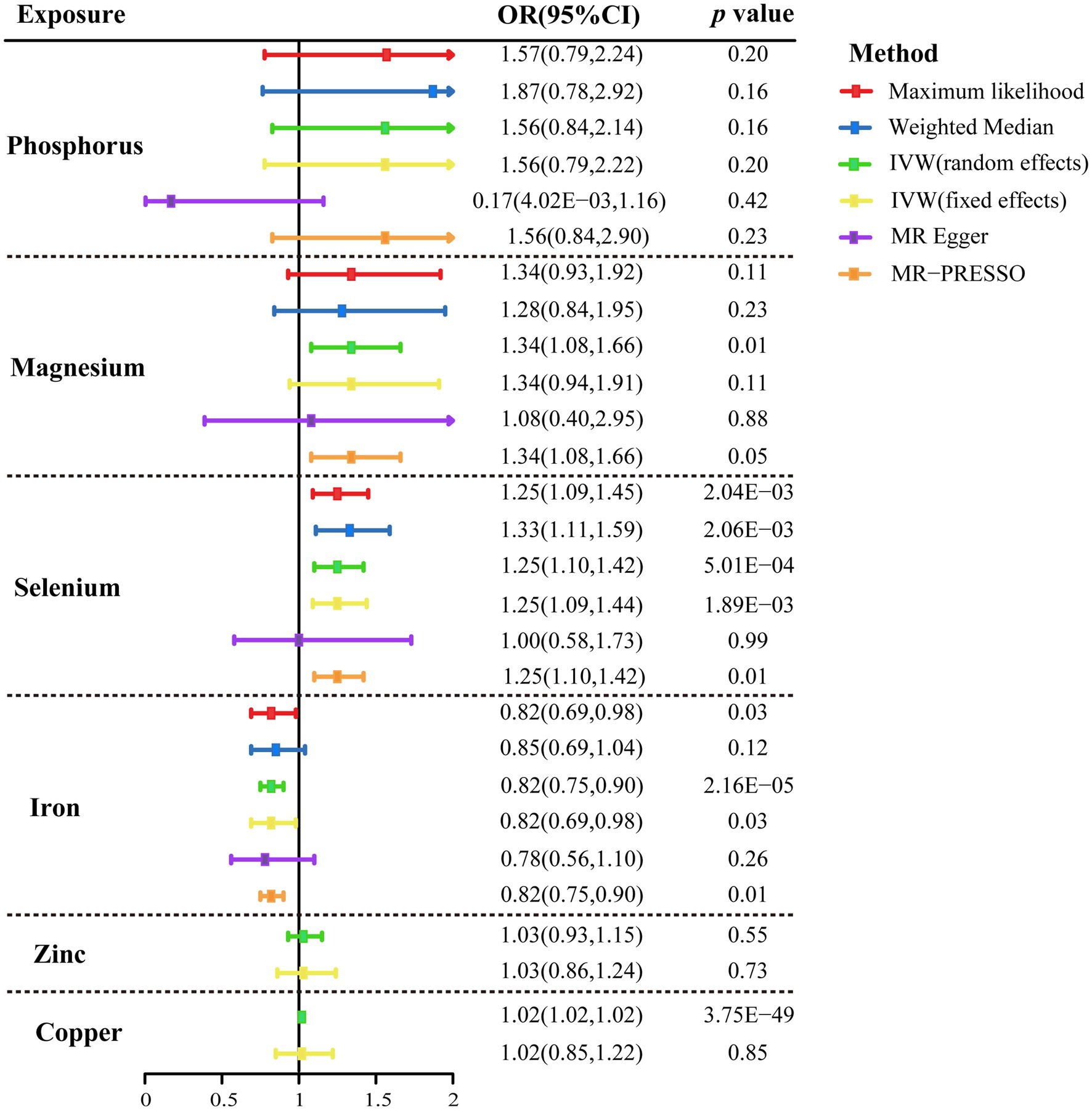
Figure 2. Mendelian randomization association of genetically predicted serum micronutrients levels with coronary artery disease in patients with diabetes using different statistical models. OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted method; MR, Mendelian randomization; and MR-PRESSO, MR-pleiotropy residual sum and outlier.
The association patterns of phosphorus, selenium, and iron based on sensitivity analyses were consistent with the IVW MR analyses, but not magnesium (Figure 2). In addition, stable correlations were found in the MR-PRESSO analysis of serum phosphorus (OR, 1.56; 95% CI, 0.84–2.90; p = 0.23), serum magnesium (OR, 1.34; 95% CI, 1.08–1.66; p = 0.05), serum selenium (OR, 1.25; 95% CI, 1.10–1.42; p = 0.01), and serum iron (OR, 0.82; 95% CI, 0.75–0.90; p = 0.01) with no outliers were revealed (Figure 2). Between the estimates of chosen SNPs, no evidence of heterogeneity for the relationships between micronutrients and CAD in diabetic patients was observed, and neither the MR-Egger intercept test nor the Cochrane’s Q test indicated any possible directional pleiotropy (all p > 0.05; Table 2). Leave-one-out analyses suggested that no single SNP significantly influenced the effect of serum micronutrient levels on CAD in diabetic patients (Supplementary Figures 7–10).
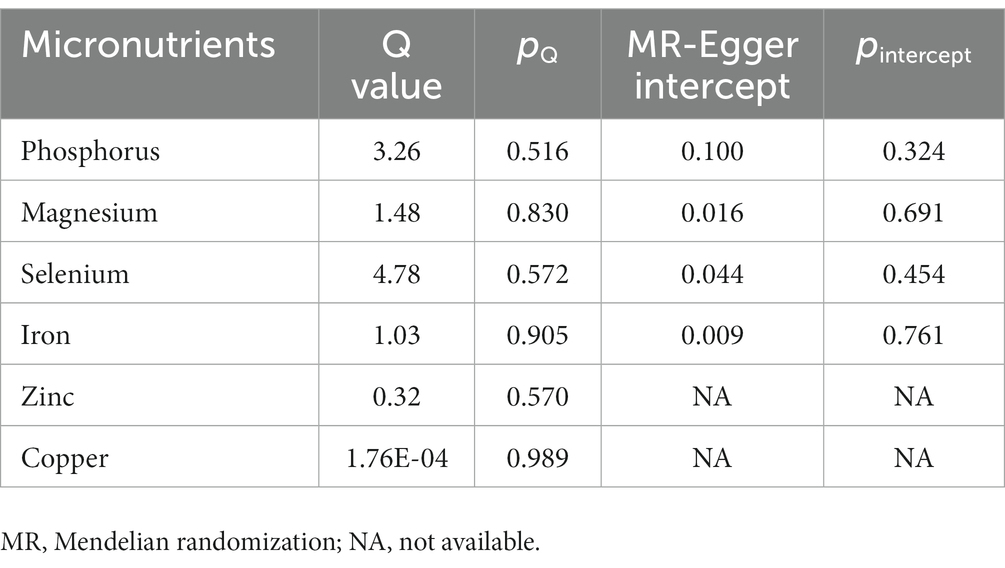
Table 2. Heterogeneity and pleiotropy tests for the associations of micronutrients with coronary artery disease in patients with diabetes.
In this comprehensive MR analysis, genetic data from the largest published GWAS were leveraged to evaluate the relationship between genetic susceptibility to six micronutrients and the risk of CAD in diabetic patients. We provided consistent evidence that circulating selenium concentrations were genetically expected to be related with a higher risk of CAD, whereas iron concentrations were associated with a lower risk of CAD in patients with diabetes. The association pattern remained consistent when repeated in the majority of supplementary analyses. However, there was limited evidence to link the risk of CAD in diabetic patients with circulating levels of magnesium, phosphorus, zinc, and copper.
According to the previous observational studies and RCTs, the association between selenium and CAD in patients with diabetes was inconsistent (11, 27, 28). A prospective study involving 3,897 diabetes in the Dongfeng-Tongji cohort suggested an inverse association between plasma levels of selenium and risk of cardiovascular diseases (CVDs) in patients with diabetes (29). Selenium supplementation was not sufficient however, to reduce CAD mortality, according to the findings from a meta-analysis that included 16 RCTs (30). Additionally, previous observational studies have reported no difference in circulating selenium concentrations between diabetic patients with and without CAD (27). A positive association of selenium with diabetes was found in previous observational studies (31–33), several RCTs (34–36), and a MR study (37). Numerous in vitro and animal investigations have revealed the mechanism for how selenium increases the risk of diabetes and CAD. As a member of the glutathione peroxidase (GPx) family, selenium serves as the center of redox (38). Transgenic animal models have found increased GPx1 expression interferes with insulin signaling by removing hydrogen peroxide, leading to the development of insulin resistance, hyperglycemia, and obesity (39, 40). Selenoprotein P (SelP), a selenium-supply protein, is hypothesized to raise the risk of diabetes by promoting insulin resistance and dysregulating glucose metabolism (41). In addition, Selk, a selenoprotein of the endoplasmic reticulum membrane, contribute to foam cell formation and atherogenesis by stabilizing expression of CD36 in macrophages during inflammation (42, 43). According to the results of our MR investigation, selenium may be associated in a directionally consistent manner with CAD in patients with diabetes. Given that diabetes is a known risk factor for CAD, this conclusion might seem intuitive. However, considering the majority of the individuals covered with this research were of European origin, the generalizability of our findings to other groups needs to be further investigated. The inverse association between levels of selenium and CVDs risk in Asian diabetic may due to the difference of dietary structure, lifestyle and genetic predisposition.
An inverse association between iron concentration and CAD in diabetic patients was observed in our MR analysis. A two-sample MR approach examining serum iron status for CAD risk in the general population revealed that serum iron concentration was linked to a lower chance of developing CAD (OR, 0.94; 95%CI, 0.88–1.00; p = 0.039), which is consistent with our findings in the diabetic population (44). In addition, a recent two-sample MR study based on the data from United Kingdom Biobank discovered that high levels of iron status were protective against coronary atherosclerosis in the male population (45). Furthermore, a meta-analysis of prospective studies involving 156,427 participants showed a negative association between serum iron and risk of coronary heart disease after excluding the study by Morrisson et al. (risk ratio [RR], 0.80; 95%CI, 0.73–0.87) (46). Numerous observational studies have also demonstrated the protective effect of iron on CAD in diabetic individuals (47, 48). An inverse correlation between iron reserves and cardiovascular disease in patients with diabetes was reported by a cross-sectional and prospective observational study encompassing 38,671 people and 821 diabetes patients (OR, 0.81; 95%CI, 0.68–0.96; p = 0.018) (48). Similarly, the results of an observational study including 424 consecutive men with type 2 diabetes mellitus showed high ferritin levels may reduce cardiovascular risk in men with diabetes (49). Several plausible mechanisms have been hypothesized to elucidate the protective effect of high iron load on CAD. For instance, an animal study found that a high-iron diet attenuates atherosclerosis in mice lacking apolipoprotein E (50). Similar, another recent animal study suggested that iron overload could diminish atherosclerosis in apolipoprotein E knockout mice by interfering with hepatic CD36 and fatty acid binding proteins-mediated fatty acid uptake and transport (51). Furthermore, it has been proven that ferritin, a natural antioxidant, may reduce the risk of CAD in patients with diabetes by compensating for chronic systemic inflammation in diabetes (52, 53).
In the current MR study, we observed a nominally significant association between genetically predicted concentrations of magnesium and the risk of CAD in patients with diabetes, but the other four statistical models were not statistically significant. As a result, we preclude the presence of a stable causal association between serum magnesium concentration and outcome. We also observed a significant correlation between genetically predicted concentrations of copper and the risk of CAD in patients with diabetes in the main analysis; however, because there are only two genetic instruments for copper, we are unable to perform sensitivity analysis to assess the stability of the results. Meanwhile, the results of IVW (fixed effects) suggested no causal connection between copper and CAD in patients with diabetes. Thus, we are unable to tell whether there is a possible causal relationship between copper and the outcome. The results of the current MR study showed that little evidence approved the causal effects of genetically predicted concentrations of phosphorus and zinc on CAD risk in diabetic patients. There is a scarcity of observational epidemiology research on these micronutrient concentrations and the incidence of CAD in diabetic patients, and the results from the few available observational studies of the general population are inconclusive (12, 54–57). Thus, our results from the current MR study may imply that serum phosphorus and zinc levels should not be regarded as independent risk factors for CAD in patients with diabetes.
The design of MR study, which avoids biases frequently seen in standard observational studies and provides the non-biased causal connection between exposure and outcome, is the main merit (58). Besides, our MR study uses summary-level data from the large genetic consortium to date, which allows us to more accurately formulate our study hypothesis. Meanwhile, the statistical power in the current investigation is ensured by the estimated effects (F-statistics) of each instrumental variable exceeding the threshold. Moreover, sensitivity analyses based on multiple statistical models combined with leave-one-out analyses were employed to detect the stability of the main results, which offered additional reliable evidence.
It is crucial to acknowledge several potential limitations when interpreting our results. First, although MR-PRESSO analysis and MR-Egger intercept tests did not reveal any evidence of pleiotropy that might have influenced our results, potential horizontal pleiotropy cannot be completely excluded. Second, the current study was based on summary-level data and lacked subgroup-specific analyses, as there are no corresponding sex- or age-specific data sets in the consortium. Third, the majority of the individuals in our MR research were of European origin, which may restrict the generalizability of the primary findings to other groups. Therefore, the corresponding results should be cautious to make the conclusion.
The current study provides genetic evidence for the possible causal effects of increased selenium and decreased iron levels on the increased risk of CAD in patients with diabetes. Diet, supplements, or other methods to modify circulating selenium and iron concentrations may be effective strategies to prevent CAD in patients with diabetes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
MT, NH, TH, and HC designed the study and wrote the analysis plan. NH and TH undertook analyses. MT and TH wrote the first draft of the manuscript with critical revisions from NH, JY, and HC. MT, TH, JY, HC, and NH interpreted the results in the study and gave final approval of the version to be published. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, China (Grant No. 2022E10026), National Natural Science Foundation of China (82200489), the Major Project of Science and Technology Innovation 2025 in Ningbo, China (Grant No. 2021Z134), the Key research and development project of Zhejiang Province, China (Grant No. 2021C03096), and Public Science and Technology Projects of Ningbo (202002N3175).
The authors thank all the investigators of CHARGE Consortium, GEFOS consortium, United Kingdom Biobank study, MAGIC, and GISC for providing the data publicly.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1103330/full#supplementary-material
1. Virani, SS, Alonso, A, Aparicio, HJ, Benjamin, EJ, Bittencourt, MS, Callaway, CW, et al. Heart disease and stroke Statistics-2021 update: a report from the American Heart Association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
2. Newman, JD, Schwartzbard, AZ, Weintraub, HS, Goldberg, IJ, and Berger, JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. (2017) 70:883–93. doi: 10.1016/j.jacc.2017.07.001
3. Low Wang, CC, Hess, CN, Hiatt, WR, and Goldfine, AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. (2016) 133:2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194
4. Cosentino, F, Grant, PJ, Aboyans, V, Bailey, CJ, Ceriello, A, Delgado, V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. doi: 10.1093/eurheartj/ehz486
5. Dong, H, Hu, P, Wang, J, Zhang, Y, and Lu, N. Associations of serum calcium, magnesium levels, and their ratio with apolipoproteins in Chinese adults with coronary artery disease: a cross-sectional study. Biol Trace Elem Res. (2021) 200:4221–9. doi: 10.1007/s12011-021-03015-3
6. Rayman, MP. Selenium and human health. Lancet. (2012) 379:1256–68. doi: 10.1016/S0140-6736(11)61452-9
7. Cebi, A, Kaya, Y, Gungor, H, Demir, H, Yoruk, IH, Soylemez, N, et al. Trace elements, heavy metals and vitamin levels in patients with coronary artery disease. Int J Med Sci. (2011) 8:456–60. doi: 10.7150/ijms.8.456
8. Fang, X, Wang, K, Han, D, He, X, Wei, J, Zhao, L, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med. (2016) 14:210. doi: 10.1186/s12916-016-0742-z
9. Eaton, CB, Abdul Baki, AR, Waring, ME, Roberts, MB, and Lu, B. The association of low selenium and renal insufficiency with coronary heart disease and all-cause mortality: NHANES III follow-up study. Atherosclerosis. (2010) 212:689–94. doi: 10.1016/j.atherosclerosis.2010.07.008
10. Flores-Mateo, G, Navas-Acien, A, Pastor-Barriuso, R, and Guallar, E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. (2006) 84:762–73. doi: 10.1093/ajcn/84.4.762
11. Qiu, Z, Geng, T, Wan, Z, Lu, Q, Guo, J, Liu, L, et al. Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes. Am J Clin Nutr. (2022) 115:53–60. doi: 10.1093/ajcn/nqab241
12. Eshak, ES, Iso, H, Yamagishi, K, Maruyama, K, Umesawa, M, and Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem. (2018) 56:126–32. doi: 10.1016/j.jnutbio.2018.02.008
13. Hamedifard, Z, Farrokhian, A, Reiner, Ž, Bahmani, F, Asemi, Z, Ghotbi, M, et al. The effects of combined magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis. (2020) 19:112. doi: 10.1186/s12944-020-01298-4
14. Thomas, DC, and Conti, DV. Commentary: the concept of 'Mendelian Randomization'. Int J Epidemiol. (2004) 33:21–5. doi: 10.1093/ije/dyh048
15. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
16. Kestenbaum, B, Glazer, NL, Köttgen, A, Felix, JF, Hwang, SJ, Liu, Y, et al. Common genetic variants associate with serum phosphorus concentration. J Am Soc Nephrol. (2010) 21:1223–32. doi: 10.1681/ASN.2009111104
17. Meyer, TE, Verwoert, GC, Hwang, SJ, Glazer, NL, Smith, AV, van Rooij, FJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. (2010) 6:e1001045. doi: 10.1371/journal.pgen.1001045
18. Cornelis, MC, Fornage, M, Foy, M, Xun, P, Gladyshev, VN, Morris, S, et al. Genome-wide association study of selenium concentrations. Hum Mol Genet. (2015) 24:1469–77. doi: 10.1093/hmg/ddu546
19. Benyamin, B, Esko, T, Ried, JS, Radhakrishnan, A, Vermeulen, SH, Traglia, M, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun. (2014) 5:4926. doi: 10.1038/ncomms5926
20. Evans, DM, Zhu, G, Dy, V, Heath, AC, Madden, PA, Kemp, JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet. (2013) 22:3998–4006. doi: 10.1093/hmg/ddt239
21. Fall, T, Gustafsson, S, Orho-Melander, M, and Ingelsson, E. Genome-wide association study of coronary artery disease among individuals with diabetes: the UK biobank. Diabetologia. (2018) 61:2174–9. doi: 10.1007/s00125-018-4686-z
22. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
23. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
24. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
25. Verbanck, M, Chen, CY, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
26. Greco, MF, Minelli, C, Sheehan, NA, and Thompson, JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
27. Sotiropoulos, A, Papadodima, SA, Papazafiropoulou, AK, Ioannidis, A, Kokkinari, A, Apostolou, O, et al. Serum selenium levels do not differ in type 2 diabetic subjects with and without coronary artery disease. BMC Res Notes. (2011) 4:270. doi: 10.1186/1756-0500-4-270
28. Wang, W, Wang, X, Cao, S, Duan, Y, Xu, C, Gan, D, et al. Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: a prospective cohort study. Front Nutr. (2022) 9:849727. doi: 10.3389/fnut.2022.849727
29. Long, T, Wang, R, Wang, J, Wang, F, Xu, Y, Wei, Y, et al. Plasma metals and cardiovascular disease in patients with type 2 diabetes. Environ Int. (2019) 129:497–506. doi: 10.1016/j.envint.2019.05.038
30. Ju, W, Li, X, Li, Z, Wu, GR, Fu, XF, Yang, XM, et al. The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. (2017) 44:8–16. doi: 10.1016/j.jtemb.2017.04.009
31. Shao, R, Su, L, Li, L, Wu, J, He, X, Mao, D, et al. Higher selenium was associated with higher risk of diabetes: consistent evidence from longitudinal and cross-sectional studies based on nail and serum selenium measures. Sci Total Environ. (2022) 840:156618. doi: 10.1016/j.scitotenv.2022.156618
32. Cheng, Z, Li, Y, Young, JL, Cheng, N, Yang, C, Papandonatos, GD, et al. Long-term association of serum selenium levels and the diabetes risk: findings from a case-control study nested in the prospective Jinchang cohort. Sci Total Environ. (2022) 818:151848. doi: 10.1016/j.scitotenv.2021.151848
33. Dias, JPV, Costa Sobrinho, PS, Pimenta, AM, Hermsdorff, HHM, Bressan, J, and Nobre, LN. Dietary selenium intake and Type-2 diabetes: a cross-sectional population-based study on CUME project. Front Nutr. (2021) 8:678648. doi: 10.3389/fnut.2021.678648
34. Thompson, PA, Ashbeck, EL, Roe, DJ, Fales, L, Buckmeier, J, Wang, F, et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. (2016) 108:djw152. doi: 10.1093/jnci/djw152
35. Kohler, LN, Florea, A, Kelley, CP, Chow, S, Hsu, P, Batai, K, et al. Higher plasma selenium concentrations are associated with increased odds of prevalent type 2 diabetes. J Nutr. (2018) 148:1333–40. doi: 10.1093/jn/nxy099
36. Stranges, S, Marshall, JR, Natarajan, R, Donahue, RP, Trevisan, M, Combs, GF, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. (2007) 147:217–23. doi: 10.7326/0003-4819-147-4-200708210-00175
37. Rath, AA, Lam, HS, and Schooling, CM. Effects of selenium on coronary artery disease, type 2 diabetes and their risk factors: a Mendelian randomization study. Eur J Clin Nutr. (2021) 75:1668–78. doi: 10.1038/s41430-021-00882-w
38. Rayman, MP. The importance of selenium to human health. Lancet. (2000) 356:233–41. doi: 10.1016/S0140-6736(00)02490-9
39. Wang, XD, Vatamaniuk, MZ, Wang, SK, Roneker, CA, Simmons, RA, and Lei, XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. (2008) 51:1515–24. doi: 10.1007/s00125-008-1055-3
40. Steinbrenner, H, Speckmann, B, Pinto, A, and Sies, H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. (2011) 48:40–5. doi: 10.3164/jcbn.11-002FR
41. Misu, H, Takamura, T, Takayama, H, Hayashi, H, Matsuzawa-Nagata, N, Kurita, S, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. (2010) 12:483–95. doi: 10.1016/j.cmet.2010.09.015
42. Lu, C, Qiu, F, Zhou, H, Peng, Y, Hao, W, Xu, J, et al. Identification and characterization of selenoprotein K: an antioxidant in cardiomyocytes. FEBS Lett. (2006) 580:5189–97. doi: 10.1016/j.febslet.2006.08.065
43. Meiler, S, Baumer, Y, Huang, Z, Hoffmann, FW, Fredericks, GJ, Rose, AH, et al. Selenoprotein K is required for palmitoylation of CD36 in macrophages: implications in foam cell formation and atherogenesis. J Leukoc Biol. (2013) 93:771–80. doi: 10.1189/jlb.1212647
44. Gill, D, Del Greco, MF, Walker, AP, Srai, SKS, Laffan, MA, and Minelli, C. The effect of Iron status on risk of coronary artery disease: a Mendelian randomization study-brief report. Arterioscler Thromb Vasc Biol. (2017) 37:1788–92. doi: 10.1161/ATVBAHA.117.309757
45. Yang, F, Bao, Q, Wang, Z, Ma, M, Shen, J, Ye, F, et al. Sex-specific genetically predicted Iron status in relation to 12 vascular diseases: a Mendelian randomization study in the UK biobank. Biomed Res Int. (2020) 2020:1–8. doi: 10.1155/2020/6246041
46. Das De, S, Krishna, S, and Jethwa, A. Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis. (2015) 238:296–303. doi: 10.1016/j.atherosclerosis.2014.12.018
47. Ponikowska, B, Suchocki, T, Paleczny, B, Olesinska, M, Powierza, S, Borodulin-Nadzieja, L, et al. Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care. (2013) 36:4147–56. doi: 10.2337/dc13-0528
48. Suárez-Ortegón, MF, McLachlan, S, Price, AH, Fernández-Balsells, M, Franch-Nadal, J, Mata-Cases, M, et al. Decreased iron stores are associated with cardiovascular disease in patients with type 2 diabetes both cross-sectionally and longitudinally. Atherosclerosis. (2018) 272:193–9. doi: 10.1016/j.atherosclerosis.2018.03.028
49. Hermans, MP, Ahn, SA, Amoussou-Guenou, KD, Balde, NM, and Rousseau, MF. Do high ferritin levels confer lower cardiovascular risk in men with type 2 diabetes? Diabet Med. (2010) 27:417–22. doi: 10.1111/j.1464-5491.2010.02979.x
50. Kirk, EA, Heinecke, JW, and LeBoeuf, RC. Iron overload diminishes atherosclerosis in apoE-deficient mice. J Clin Invest. (2001) 107:1545–53. doi: 10.1172/JCI7664
51. Xiao, L, Luo, G, Li, H, Yao, P, and Tang, Y. Dietary iron overload mitigates atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice: role of dysregulated hepatic fatty acid metabolism. Biochim Biophys Acta Mol Cell Biol Lipids. (2021) 1866:159004. doi: 10.1016/j.bbalip.2021.159004
52. Balla, G, Jacob, HS, Balla, J, Rosenberg, M, Nath, K, Apple, F, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. (1992) 267:18148–53. doi: 10.1016/S0021-9258(19)37165-0
53. Lecube, A, Hernández, C, Pelegrí, D, and Simó, R. Factors accounting for high ferritin levels in obesity. Int J Obes. (2008) 32:1665–9. doi: 10.1038/ijo.2008.154
54. Chen, Q, Zhang, Y, Ding, D, Li, D, Yang, Y, Li, Q, et al. Associations between serum calcium, phosphorus and mortality among patients with coronary heart disease. Eur J Nutr. (2018) 57:2457–67. doi: 10.1007/s00394-017-1518-8
55. Bai, W, Li, J, and Liu, J. Serum phosphorus, cardiovascular and all-cause mortality in the general population: a meta-analysis. Clin Chim Acta. (2016) 461:76–82. doi: 10.1016/j.cca.2016.07.020
56. Taylor, EN, Rimm, EB, Stampfer, MJ, and Curhan, GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. (2011) 161:956–62. doi: 10.1016/j.ahj.2011.02.012
57. Milton, AH, Vashum, KP, McEvoy, M, Hussain, S, McElduff, P, Byles, J, et al. Prospective study of dietary zinc intake and risk of cardiovascular disease in women. Nutrients. (2018) 10:38. doi: 10.3390/nu10010038
Keywords: micronutrient, coronary artery disease, diabetes, causal association, selenium, iron
Citation: Tian M, Hu T, Ying J, Cui H and Huangfu N (2023) Increased selenium and decreased iron levels in relation to risk of coronary artery disease in patients with diabetes. Front. Nutr. 10:1103330. doi: 10.3389/fnut.2023.1103330
Received: 29 November 2022; Accepted: 03 May 2023;
Published: 18 May 2023.
Edited by:
Xiaohua Liang, Children‘s Hospital of Chongqing Medical University, ChinaReviewed by:
Bojana B. Vidovic, University of Belgrade, SerbiaCopyright © 2023 Tian, Hu, Ying, Cui and Huangfu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Huangfu, bmluZ2h1YW5nZnVAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.