- 1Department of Infectious Diseases, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 2Department of Clinical Nutrition, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 3Endoscopy Center, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, China
- 4Health Management Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, China
- 5Clinical Research Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 6Big Data Center, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 7Center for Digestive Disease, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, Guangdong, China
- 8Department of Infectious Diseases, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, China
Background: Obesity is a common and highly convincing risk factor for many cancers, including liver cancer. Sex disparities in the body composition and regulatory mechanisms involved in energy homeostasis may contribute to the difference in the incidence of cancer. However, evidence on the gender-specific association between body composition and liver cancer incidence is limited. We performed this study to investigate the linear and non-linear associations of body composition with liver cancer risk by gender.
Materials and methods: This prospective analysis included 4,75,659 participants free of cancer, based on the UK Biobank. We used Cox proportional hazard models to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) after adjusting for potential confounders. Restricted cubic spline was performed to investigate the potential non-linear associations.
Results: During a median follow-up, 275 cases (174 male patients and 101 female patients) of liver cancer were identified. Male patients in the highest body fat percentage group are more likely to develop liver cancer (HR = 1.89, 95% CI: 1.17–3.03) compared with those in the lowest group. The one-unit increase of whole-body fat mass, arm fat mass, and trunk fat mass was associated with 1.03-, 1.14-, and 1.05-fold increased risk of liver cancer in male subjects, respectively. U-shaped associations of body composition with liver cancer risk were observed in the female subjects. Both high and low levels of whole-body fat-free mass, particularly in the arm and trunk, were associated with an increased risk of liver cancer.
Conclusion: This study found a gender-specific association between body composition and liver cancer risk and provided evidence for individualized weight management for the prevention of liver cancer.
1. Introduction
Liver cancer is the sixth most frequently occurring cancer in the world and the second most common cause of cancer mortality. Data from the GLOBOCAN database showed that liver cancer was responsible for over 8,41,000 new cases and 78,200 deaths in 2020 (1). Many risk factors for liver cancer, including hepatitis B virus (HBV) or hepatitis C virus (HCV), alcohol, aflatoxin B1, and metabolic associated fatty liver disease (obesity, type II diabetes), have been identified (2).
The prevalence of obesity-related liver cancer has been increasing in recent years and has become the second biggest cause of liver cancer globally. A recent report by the World Cancer Research Fund (WCRF) showed that a high body mass index (BMI) is associated with a higher risk of liver cancer (3). One meta-analysis of 26 prospective studies indicated that excess body weight or obesity was associated with an increased risk of primary liver cancer (4).
Obesity, as a modifiable carcinogenic factor, has been demonstrated with remarkable gender disparities in the incidence and the cumulative risk of obesity-related liver cancer (5–9). A prospective study of more than 9,00,000 adults indicated that men with a BMI of 35 kg/m2 exhibited a dramatic 4.52-fold increase in relative risk of death from liver cancer, while a modest 1.68-fold increase was observed in women (10). A cohort study of 5.24 million adults in the UK has further confirmed the significant modulation of hepatocellular cancer (HCC) incidence by gender (11). Deregulated signaling of sex hormones is considered to be one of the drivers of sexual dimorphism.
Most previous studies have focused on the association between conventional measure indicators of obesity such as body mass index (BMI) and waist circumference (WC). However, conventional measure indicators tend to be conservative compared to others, and they may not adequately predict body composition and fat distribution. Evidence indicated that body fat percentage (BFP) and fat mass (FM)/fat-free mass (FFM) also had associations with liver cancer as well as obesity-related biomarkers. A cohort study of 4,37,393 participants found that BFP was associated with an increased risk of liver cancer (12). Due to its inadequate adjustment for important confounders, such as complications, and lack of analysis for fat distribution, further studies are still required.
The UK Biobank is a large population-based prospective study that includes more than 0.5 million individuals aged 37–73 years from the UK between 2006 and 2010. This dataset has collected a wide range of information on sociodemographic factors, lifestyle, and anthropometric measurements, as well as clinical diagnoses (13). Based on the UK Biobank, our study aimed to investigate the gender-specific relationship between body composition and the risk of liver cancer and further assess the potential non-linear associations.
2. Materials and methods
2.1. Study design and data collection
Data were collected from the UK Biobank (application number 51671, approved August 2019). The study protocol and information about data access are available online, and the details of the recruitment have been published elsewhere (11). For this analysis, participants were excluded if they had any cancer diagnoses (except for non-melanoma skin cancer ICD-10 C44) prior to baseline assessment or had missing data on the measure of body composition (n = 26,868). All the participants had follow-up from the date of the recruitment until the earliest date of liver cancer diagnosis, the date of death, and the date of loss to follow-up or end of follow-up for cancer incidence. Finally, a total of 4,75,659 participants were included in this analysis, and 275 cases were diagnosed with liver cancer (the flowchart of study selection is shown in Supplementary Figure S1). Information on cancer diagnoses in the UK Biobank is provided by the Health and Social Care Information Centre for participants in England and Wales and the NHS Central Register for participants in Scotland. The cancer registration codes were used from the International Statistical Classification of Diseases Tenth Modification (ICD-10). The UK Biobank was approved by the Northwest Multi-Center Research Ethics Committee (MREC), the Patient Information Advisory Group (PIAG), and the Community Health Index Advisory Group (CHIAG).
2.2. Anthropometry and body composition
At the baseline interview, trained personnel collected data on body composition and size using a standard protocol. The Tanita BC-418MA hepatocellular cancer (HCC) body composition analyzer (Tanita, Tokyo, Japan) was used to measure the FM (kg) and FFM (kg). Dual-energy X-ray absorptiometry (DXA) was also used to evaluate the body composition of 5,170 participants. Standing height was measured using the Seca 202 device (Seca, Hamburg, Germany). The Wessex non-stretchable sprung tape measure (Wessex, United Kingdom) was used to measure the waist/hip circumference, while the waist-to-hip ratio was calculated by dividing waist circumference (cm) by hip circumference (cm).
2.3. Data analysis
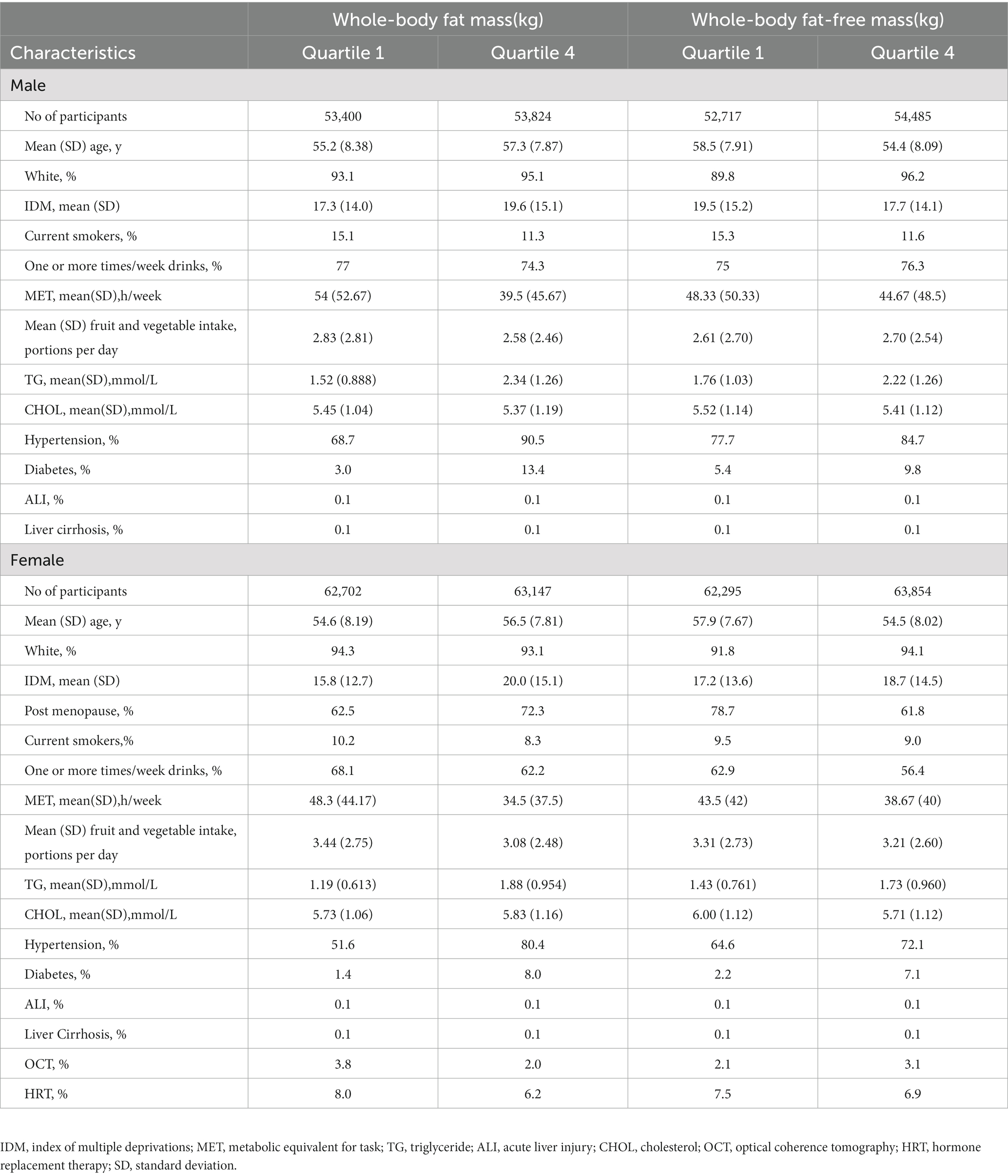
Baseline characteristics were compared between the first (lowest) and the fourth (highest) quartiles of both whole-body fat mass (WBFM) and whole-body fat-free mass (WBFFM). We used Cox regression models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association of body composition and liver cancer risk.
We treated FM/FFM as a catalog or continuous variables to evaluate their relationship with the subsequent risk of liver cancer. To control potential confounding effects, we stratified the analyses by age in the basic Cox regression model and further adjusted for ethnicity (white/non-white), height, education (the highest qualification achieved), index of multiple deprivations, alcohol consumption (daily or almost daily, one or two times a week, one to three times a month, special occasions only, three or four times a week, and never or unknown), smoking status (never, current, and previous), physical activity, portions of fruit and vegetable intake, menopause status (even on hormone replacement therapy), family history of cancer, and previous comorbidities (diabetes/hepatitis/liver cirrhosis/hypertension diagnosis) in the multivariable-adjusted model (model 2). In addition, we used a restricted cubic spline with four knots at the 5th, 35th, 65th, and 95th percentiles to investigate possible non-linearity in each FM/FFM–liver cancer association, in which the median was set as the reference.
2.4. Sensitivity analysis
We performed two sensitivity analyses to test the robustness of the results. First, we excluded cancer diagnosed during the first 2 years of follow-up to minimize reverse causality. Second, we used the complete-case analysis to verify the influence of missing data. Data analysis was conducted using the R software (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a p-value of <0.05.
2.5. Patient and public involvement statement
There was no public involvement in the study; we used publicly available or privately held data for the analysis.
3. Results
3.1. Baseline characteristics
This study included 4,75,659 participants with a median follow-up of 6.6 years. Over this period, 275 incident liver cancer cases (174 male patients and 101 female patients) were recorded. As the whole-body fat mass and whole-body fat-free mass level quartiles increased, the participants tended to be less physically active and had a higher rate of hypertension and diabetes. The participants with lower WBFM or WBFFM tended to be current smokers. The distribution of the study population characteristics by quartiles of WBFM/WBFFM is presented in Table 1.
3.2. Body composition and incidence of liver cancer
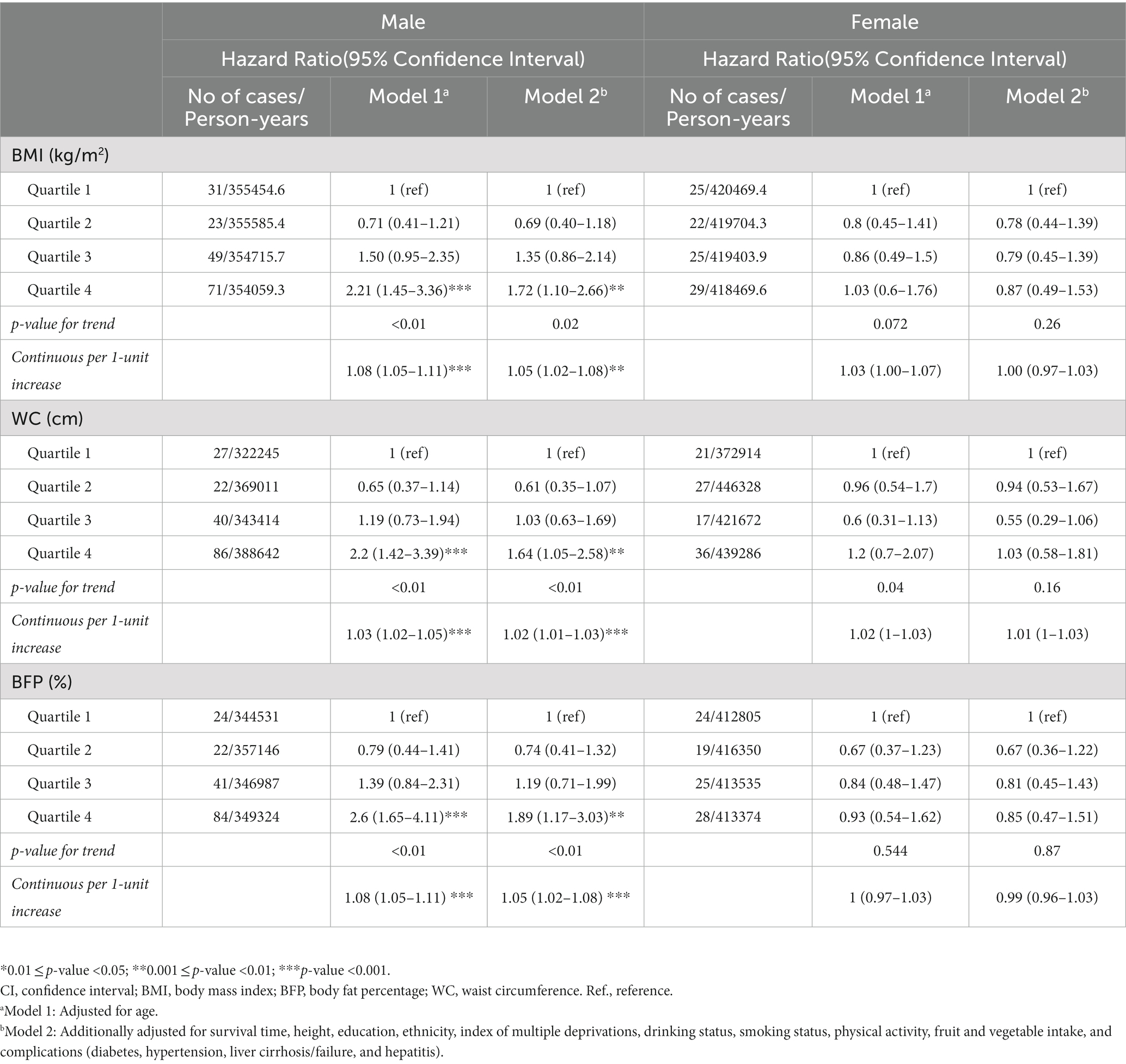
The associations between body composition and liver cancer were likely to vary from sex, and the results are shown in Table 2. For male patients, WBFM was associated with an increased risk of liver cancer in male individuals. After adjustment for potential confounders, the liver cancer risk in the highest quartile increased 1.69 times greater (adjusted HR = 1.69, 95% CI: 0.99–2.88) compared with those in the lowest quartile. In addition, per 1 SD increase in WBFM was associated with a 3% increased risk of liver cancer. While in female participants, as the WBFFM increased, the risk of liver cancer decreased in the second quartile (adjusted HR: 0.37, 95% CI: 0.19–0.71) compared with that in the lowest quartile. While, in the third quartile (adjusted HR: 0.52, 95% CI: 0.28–0.99) and the highest quartile (adjusted HR: 0.63, 95% CI: 0.29–1.37), the risk was increased compared with that in the second quartile.

Table 2. Associations between whole-body fat-free mass/whole-body fat mass and risk of liver cancer.
3.3. Distribution of body composition and liver cancer incidence
The association between liver cancer risk and the distribution of body composition is presented in Figure 1. Arm fat mass in male patients showed a 14% per 1 SD increased risk of liver cancer (HR = 1.14, 95% CI, 1.05–1.24), followed by trunk fat mass (HR = 1.05, 95% CI, 1.02–1.08). There was no evidence of the linearity between body distribution with the risk of liver cancer for female patients.
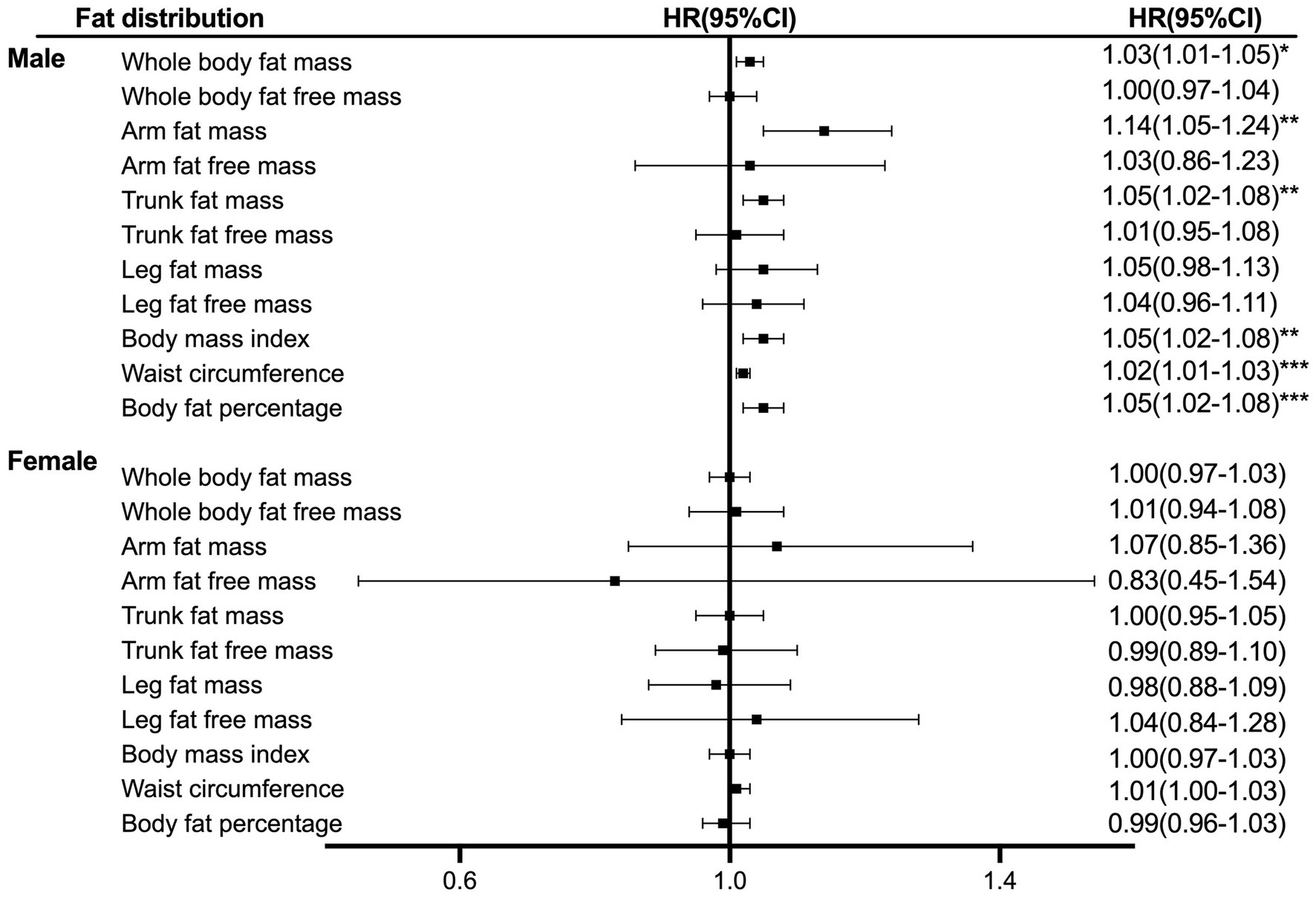
Figure 1. Hazard ratio per SD increase in liver cancer risk across anthropometric indices. The analyses were stratified age, survival time, education, ethnicity, index of multiple deprivations, drinking status, smoking status, physical activity, fruit and vegetable intake, and complications (diabetes, hypertension, liver cirrhosis/failure, and hepatitis).
3.4. Other anthropometric measures and liver cancer incidence
The risk of liver cancer was associated with BMI, WC, and BFP (Table 3). For male patients, a significant positive association with liver cancer incidence was observed per 1 SD increase in BMI (adjusted HR 1.05, 95% CI: 1.02–1.08), WC (adjusted HR 1.02, 95% CI:1.01–1.03), and BFP (adjusted HR 1.05, 95% CI: 1.02–1.08).
3.5. Non-linear relationship between measures and the risk of liver cancer
We further evaluated the non-linear relationship between anthropometric indices markers and the risk of liver cancer (Figure 2; Supplementary Figures S2–S4). The liver cancer risk showed a U-shape relation with the markers including BMI, WC, WBFFM, arm fat-free mass, and trunk fat-free mass levels in female patients (p-overall <0.01, p-non-linear <0.01). Both higher and lower of these markers were associated with an increased risk of liver cancer. The nadir for incidence of liver cancer risk was estimated to be at a BMI of 27.3 kg/m2, a WC of 83 cm, a whole-body fat-free mass of 44 kg, an arm fat-free mass of 4.5 kg, and a trunk fat-free mass of 24.8 kg.
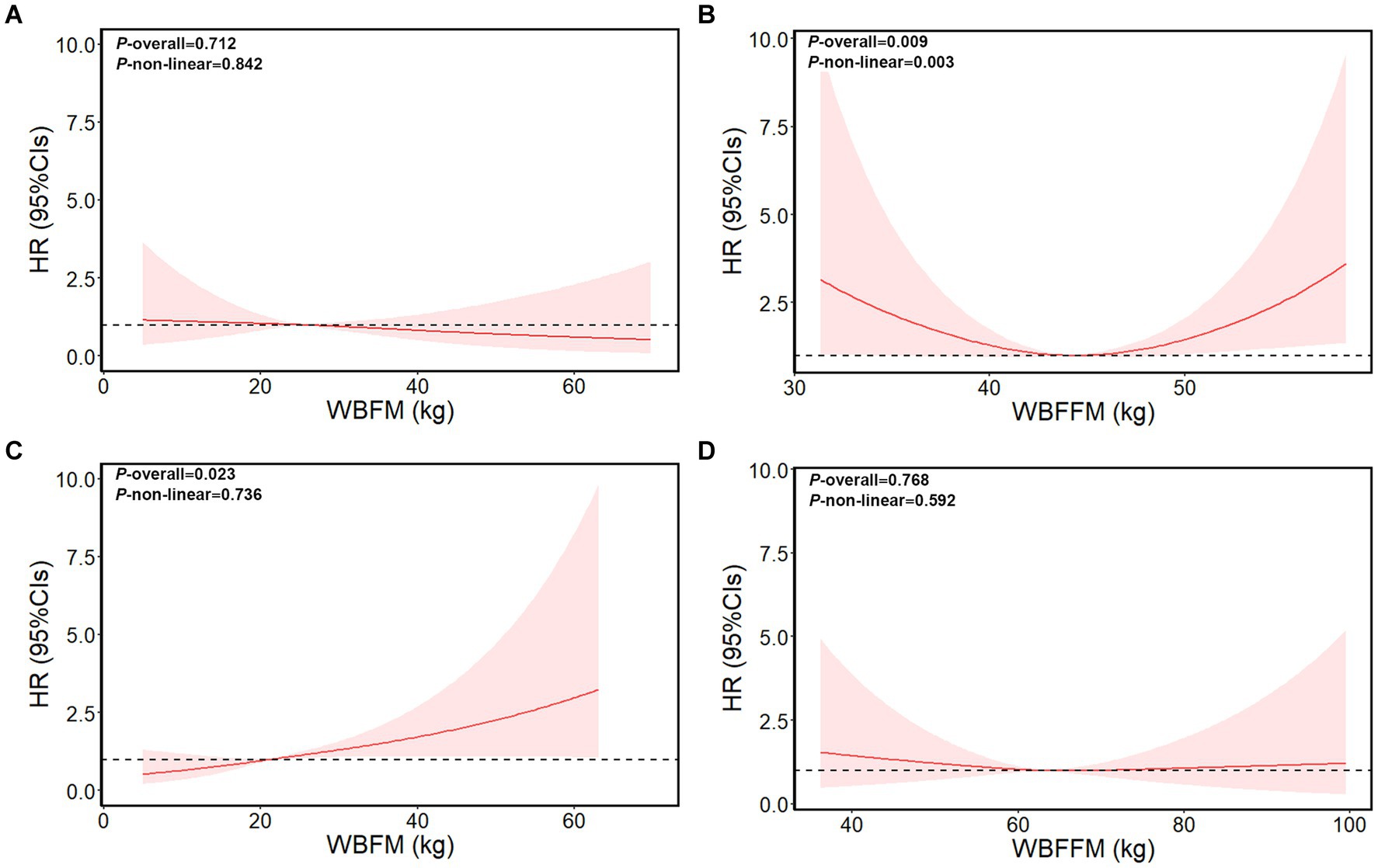
Figure 2. Dose–response relationship of whole-body fat mass/whole-body fat-free mass with liver cancer risk in female patients and male patients. (A) Whole-body fat mass of female patients. (B) Whole-body fat-free mass of female patients. (C) Whole-body fat mass of male patients. (D) Whole-body fat-free mass of male patients. The model adjusted for age, survival time, height, education, ethnicity, index of multiple deprivations, drinking status, smoking status, physical activity, fruit and vegetable intake, and complications (diabetes, hypertension, liver cirrhosis/failure, and hepatitis). WBFM: whole-body fat mass, WBFFM: whole-body fat-free mass.
3.6. Sensitivity analyses
Appendix Supplementry Figure S5 shows the sensitivity analyses. By lagging the exposure for a time window of 2 years, the positive association between FM/FFM was stable. The result was unchanged even using the complete-case analysis to verify the influence of missing data.
4. Discussion
Based on this prospective cohort study of nearly half a million participants, we observed that BMI, WC, BFP, and FM distributed in the arm and the trunk were associated with an increased risk of liver cancer in male patients. However, in female participants, BMI, WC, and FFM distributed in the arm and the trunk had a U-shape relationship with liver cancer incidence. Collectively, these findings indicated a sexually dimorphic association between conventional measure indicators and body composition markers with liver cancer risk.
Prior studies showed that obesity contributes to the increased risk of liver cancer (3, 10, 14, 15). One meta-analysis, including 28 prospective cohort studies, reported that the incidence of liver cancer increased by approximately 36 and 77% in overweight adults and obese adults, respectively (16). A cohort study conducted in the UK found that 10% or more of liver cancer could be attributable to excess weight (7). Consistent with these studies, our data suggested a positive association of FM, as well as the BFP with increased risk of liver cancer in male patients, and also found the same effect with BMI and WC. However, we did not observe a linear relationship in female patients.
Body composition has shown sex differences. Women exhibit a higher tendency of deposition of fat in the form of subcutaneous adipose tissue (SAT), whereas in men, more fat tends to be deposited in the form of visceral adipose tissue (VAT) (17). VAT induces the production of not only circulating concentrations of insulin, free fatty acids (FFAs), and TG but also proinflammatory adipokines, such as leptin, tumor necrosis factor α (TNF-α), interleukin (IL)-6, and hypoxia-inducible factor, and immune cell infiltration, while SAT shows lower lipolysis activity, thus posing a lower risk of metabolic complications (18). Androgen promotes the development of liver cancer, while it should be noted that the signal transduction of estrogen and estrogen receptors might play a protective role in the initiation and progression of liver cancer (19). Estrogen has been proven to exert protective effects against HCC in the regulation of the inflammation network by restraining proinflammatory cytokines and inhibiting downstream signaling pathways.
There were few prospective studies on FFM and liver cancer risk. Epidemiological studies showed that FFM had related to the risk of malignancies, such as gastric and esophageal adenocarcinoma (20), rectal cancer (21), prostate cancer (22), and lung cancer (23). It has been believed that a greater FFM, and therefore, a greater resting metabolic rate, will protect against obesity and associated comorbidities (24). However, our study indicated that FFM, particularly in the arm and the trunk fat-free mass, had a U-shape association with cancer risks in female patients. FFM, mainly included skeletal muscle, is the main site of insulin consumption, and low muscle mass may contribute to the development of insulin resistance (IR) (25). As the muscle’s capacity to uptake the postprandial glucose is decreased, the glucose diverts from the muscles to the liver, leading to fat accumulation, which may increase the risk of carcinogenesis (26). The skeletal muscle mass is not only associated with the histological grades of steatosis and hepatocellular ballooning but also the stage of fibrosis. Patients with low muscle mass have approximately two times increased odds ratio of suffering from NASH or liver fibrosis. FFM depletion is an independent prognostic factor of liver cancer (27).
It is well known that the excess adipose tissue may result in insulin resistance, while the excess muscle also affected the sensitivity of insulin. Brochu et al. (28) showed that FFM was independently associated with changes in glucose uptake in obese female patients (29). Higher FFM demonstrated more severely impaired endothelial function and higher systemic inflammation as compared to the lower FFM (30). Further research studies are needed to test this novel concept.
As far as we know, there was a seldom report about the association between body composition and liver cancer in previous epidemiological studies. The main strength of our research is based on the large sample size prospective cohort with validated follow-up duration and detailed measurements. This allowed for simultaneous adjustability of potential confounders for the association of interest. In addition, we also investigated the potential non-linear relationship, which provided insight into the carcinogenicity of body composition and contributes to individualized cancer prevention.
This study had some limitations. First, participants in the UK Biobank were predominantly white individuals; therefore, the results of our study may not be generalizable to other ethnicities. Second, as an observational study, we may not exclude residual confounding effects completely and confirm the causal relationship. Third, due to a lack of histological information, the association of body composition with each subtype of liver cancer is not clear.
5. Conclusion
The available data suggested that body composition particularly in the arm and trunk tended to associate with an increased risk of liver cancer. Intentional weight loss may reduce the incidence of liver cancer in men, while limiting excessive fat-free mass gain may have benefits in reducing liver cancer in women. Our findings provided evidence for individualized weight management for the prevention of liver cancer. New body composition models and techniques for grading or predicting aspects of body composition are expected to be employed increasingly in future epidemiologic investigations of chronic disease morbidity and mortality. Further research is warranted to confirm our findings and to investigate the underlying mechanism of the gender-specified effects of FFM/FM on liver cancer development.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BX and YC designed the study, had full access to all study data, and conducted the research. YZ, JY, ZZ, CG, JF, YL, and XL performed data collection and statistical analyses. SP, AL, and BZ analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 82003408 and 82003524) and the Startup Fund for the 100 Top Talents Program, SYSU (392012).
Acknowledgments
This study was conducted using the UK Biobank Resource (application number 51671). The authors would like to thank the participants and staff of the UK Biobank cohort for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1102722/full#supplementary-material
Abbreviations
ALI, acute liver injury; BFP, body fat percentage; BMI, body mass index; CHIAG, community Health Index Advisory Group; DXA, Dual-energy X-ray absorptiometry; HBV, hepatitis B virus; HCV, hepatitis C virus; HRs, hazard ratios; HRT, hormone replacement therapy; IL-6, interleukin-6; IDM, index of multiple deprivations; IR, insulin resistance; MREC, multi-center Research Ethics Committee; MET, metabolic equivalent for task; OCT, optical coherence tomography; PIAG, patient Information Advisory Group; TG, triglyceride; WC, waist circumference; WBFM, whole-body fat mass; WBFFM, whole-body fat-free mass.
References
1. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. McGlynn, KA, Petrick, JL, and El-Serag, HB. Epidemiology of hepatocellular carcinoma. Hepatology (Baltimore, Md). (2021) 73:4–13. doi: 10.1002/hep.31288
3. Lauby-Secretan, B, Scoccianti, C, Loomis, D, Grosse, Y, Bianchini, F, Straif, K, et al. Body fatness and Cancer —viewpoint of the IARC working group. N Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
4. Chen, Y, Wang, X, Wang, J, et al. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer. (2012) 48:2137–45. doi: 10.1016/j.ejca.2012.02.063
5. Petrick, JL, Thistle, JE, Zeleniuch-Jacquotte, A, Zhang, X, Wactawski-Wende, J, van Dyke, AL, et al. Body mass index, diabetes and intrahepatic cholangiocarcinoma risk: the liver Cancer pooling project and Meta-analysis. Am J Gastroenterol. (2018) 113:1494–505. doi: 10.1038/s41395-018-0207-4
6. Yang, C, Lu, Y, Xia, H, Liu, H, Pan, D, Yang, X, et al. Excess body weight and the risk of liver Cancer: systematic review and a Meta-analysis of cohort studies. Nutr Cancer. (2020) 72:1085–97. doi: 10.1080/01635581.2019.1664602
7. Bhaskaran, K, Douglas, I, Forbes, H, dos-Santos-Silva, I, Leon, DA, and Smeeth, L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. (2014) 384:755–65. doi: 10.1016/S0140-6736(14)60892-8
8. Rahmani, J, Kord Varkaneh, H, Kontogiannis, V, Ryan, PM, Bawadi, H, Fatahi, S, et al. Waist circumference and risk of liver Cancer: a systematic review and Meta-analysis of over 2 million cohort study participants. Liver cancer. (2020) 9:6–14. doi: 10.1159/000502478
9. Setiawan, VW, Lim, U, Lipworth, L, Lu, SC, Shepherd, J, Ernst, T, et al. Sex and ethnic differences in the Association of Obesity with Risk of hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2016) 14:309–16. doi: 10.1016/j.cgh.2015.09.015
10. Calle, EE, Rodriguez, C, Walker-Thurmond, K, and Thun, MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
11. MacInnis, RJ, English, DR, Hopper, JL, Gertig, DM, Haydon, AM, and Giles, GG. Body size and composition and colon cancer risk in women. Int J Cancer. (2006) 118:1496–500. doi: 10.1002/ijc.21508
12. Parra-Soto, S, Cowley, ES, Rezende, LFM, Ferreccio, C, Mathers, JC, Pell, JP, et al. Associations of six adiposity-related markers with incidence and mortality from 24 cancers—findings from the UK biobank prospective cohort study. BMC Med. (2021) 19:7. doi: 10.1186/s12916-020-01848-8
13. Palmer, LJ. UK biobank: bank on it. Lancet (London, England). (2007) 369:1980–2. doi: 10.1016/S0140-6736(07)60924-6
14. Ohki, T, Tateishi, R, Sato, T, Masuzaki, R, Imamura, J, Goto, T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol. (2008) 6:459–64. doi: 10.1016/j.cgh.2008.02.012
15. Campbell, PT, Newton, CC, Freedman, ND, Koshiol, J, Alavanja, MC, Beane Freeman, LE, et al. Body mass index, waist circumference, diabetes, and risk of liver Cancer for U.S. adults. Cancer Res. (2016) 76:6076–83. doi: 10.1158/0008-5472.CAN-16-0787
16. Sohn, W, Lee, HW, Lee, S, Lim, JH, Lee, MW, Park, CH, et al. Obesity and the risk of primary liver cancer: a systematic review and meta-analysis. Clin Mol Hepatol. (2021) 27:157–74. doi: 10.3350/cmh.2020.0176
17. Fried, SK, Lee, M-J, and Karastergiou, K. Shaping fat distribution: new insights into the molecular determinants of depot-and sex-dependent adipose biology. Obesity (Silver Spring, Md). (2015) 23:1345–52. doi: 10.1002/oby.21133
18. Cheung, OKW, and Cheng, ASL. Gender differences in adipocyte metabolism and liver Cancer progression. Front Genet. (2016) 7:168. doi: 10.3389/fgene.2016.00168
19. Shi, L, Feng, Y, Lin, H, Ma, R, and Cai, X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. (2014) 12:93. doi: 10.1186/1479-5876-12-93
20. MacInnis, RJ, English, DR, Hopper, JL, and Giles, GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. (2006) 118:2628–31. doi: 10.1002/ijc.21638
21. MacInnis, RJ, English, DR, Haydon, AM, Hopper, JL, Gertig, DM, and Giles, GG. Body size and composition and risk of rectal cancer (Australia). Cancer causes & control: CCC. (2006) 17:1291–7. doi: 10.1007/s10552-006-0074-y
22. LIU, X, RYBICKI, BA, CASEY, G, and WITTE, JS. Relationship between body size and prostate cancer in a sibling based case-control study. J Urol. (2005) 174:2169–73. doi: 10.1097/01.ju.0000181207.02213.06
23. Jeong, SM, Lee, DH, and Giovannucci, EL. Predicted lean body mass, fat mass and risk of lung cancer: prospective US cohort study. Eur J Epidemiol. (2019) 34:1151–60. doi: 10.1007/s10654-019-00587-2
24. Jourdan, C, Petersen, A-K, Gieger, C, Döring, A, Illig, T, Wang-Sattler, R, et al. Body fat free mass is associated with the serum metabolite profile in a population-based study. PLoS One. (2012) 7:e40009. doi: 10.1371/journal.pone.0040009
25. Wang, C, and Bai, L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. (2012) 12:388–96. doi: 10.1111/j.1447-0594.2012.0085.x
26. Samuel, VT, and Shulman, GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. (2016) 126:12–22. doi: 10.1172/JCI77812
27. Iritani, S, Imai, K, Takai, K, Hanai, T, Ideta, T, Miyazaki, T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. (2015) 50:323–32. doi: 10.1007/s00535-014-0964-9
28. Brochu, M, Mathieu, ME, Karelis, AD, Doucet, É, Lavoie, ME, Garrel, D, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity (Silver Spring, Md). (2008) 16:1085–93. doi: 10.1038/oby.2008.23
29. Comerford, KB, Almario, RU, Kim, K, and Karakas, SE. Lean mass and insulin resistance in women with polycystic ovary syndrome. Metab Clin Exp. (2012) 61:1256–60. doi: 10.1016/j.metabol.2012.02.004
Keywords: fat-free mass, cohort study, UK biobank, liver cancer, fat mass
Citation: Pi S, Liu A, Zhu B, Zhu Y, Yuan J, Zhang Z, Gao C, Fu J, Liu Y, Liang X, Xia B and Chen Y (2023) Body composition and risk of liver cancer: a population-based prospective cohort study on gender difference. Front. Nutr. 10:1102722. doi: 10.3389/fnut.2023.1102722
Edited by:
Marilia Seelaender, University of São Paulo, BrazilReviewed by:
Lu-shan Xiao, Southern Medical University, ChinaYoshihiko Yano, Kobe University, Japan
Copyright © 2023 Pi, Liu, Zhu, Zhu, Yuan, Zhang, Gao, Fu, Liu, Liang, Xia and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Xia, eGlhYjdAbWFpbC5zeXN1LmVkdS5jbg==; YouPeng Chen, Y2hlbnlvdXBlbmdAc3lzdXNoLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Sainan Pi
Sainan Pi Anran Liu2†
Anran Liu2† Beibei Zhu
Beibei Zhu Bin Xia
Bin Xia