- 1Clinical Medical College of Acupuncture Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2School of Physical Education and Health, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Science and Technology Division, Guangdong Food and Drug Vocational College, Guangzhou, China
Introduction: Numerous studies have confirmed the effects of low carbohydrate diet (LChD) on metabolism and chronic diseases. However, there were no bibliometric studies on LChD. This study was conducted through a bibliometric analysis to investigate the current status, hotspots and frontiers trends.
Methods: We searched all research publications related to LChD from 2002 to 2021 on the Web of Scientific Core Collection (WoSCC). CiteSpace and VOSviewer software was used to analyze countries/regions, institutions, journals, authors, references, and keywords.
Results: A total of 6938 papers were included, with an increasing trend of annual publication. LChD categories mainly included nutrition, endocrinology, and neurosciences which reflected the interdisciplinary characteristics. USA was with the largest number and the world science center in LChD field. Universities were main research institutions and five of the top 10 institutions were from USA. Eric Heath Kossoff had 101 publications and ranked first. Nutrients was the leading journal. “A randomized trial of a low-carbohydrate diet for obesity” and “Obesity” were considered to be the most co-cited and cited reference respectively. The hotspots of LChD are four aspects, “ketogenic diet”, “metabolism disease”, “cardiovascular disease” and “cancer”. We summarized that “oxidative stress”, “gut microbiota”, and “inflammation factors” are becoming frontiers trends of LChD research in the future and deserve further study.
Discussion: Over the past 20 years research on LChD has gained great attention. To better explore LChD field, multilevel mechanism studies will be required in the future.
Introduction
Obesity-related complications affect almost all body systems and are significant risk factors for coronary heart disease, type 2 diabetes, cancers such as endometrial, breast, prostate, and skin cancers, as well as several other chronic non-communicable diseases. Diet therapy methods, theories, and applications are constantly updated as a result of ongoing research on the metabolism of the organism in normal and disease states (1). It has been demonstrated that consuming a diet high in carbohydrate increases the risk of developing metabolic and chronic diseases, and that lowering carbohydrate intake decreases the incidence of morbidity (2). The effects of LChD on health have garnered a lot of attention recently. There are many types of low carbon diet prescriptions according on the carbohydrate intake ratio. The American Diabetes Association recommended a conventional 2,000 calorie daily diet with < 130 g of carbohydrates (3). The other study suggested consuming < 40% of one's daily calories from carbohydrates (4). Anyway, the two prescriptions LChD above are powered by glucose first and then switch to ketone bodies after fasting. Moreover, the ketogenic diet (KD) is another LChD that calls for a very low carbohydrate intake (< 10%). KD, a sort of LChD, was initially used to cure epilepsy (5). Atkins, an American, wrote about an LChD in his 1972 book “Dr. Atkins' New Diet Revolution,” in which the intake of carbohydrate was rigorously limited while the intake of protein and fat is raised (6). Currently modified Atkins diet, a easier KD, has showed very similar effects with KD (7). A LChD can lower excess body weight (8, 9), as well as the risk of diabetes, cancer, cardiovascular disease, and internal inflammatory responses brought on by obesity (10, 11). Of fact, some research has indicated that LChD can also produce negative health effects, such as gastric dysfunction (12), atherosclerosis (13), physical fatigue (14), etc.
Studies on LChD are becoming increasingly popular in recent years as a response of the academic community's intense interest in the disease's favorable health effects (15–17). Most of studies, nevertheless, have concentrated on how LChD affects certain disease locations. We require a thorough understanding of the development process and research trends in this subject given the rapid proliferation of research on LChD. However, there are no bibliometric and visual analysis article on LChD.
Bibliometrics, a mathematical and statistical tool for quantitatively analyzing all knowledge (18), has been used to assess distributions, collaboration, citation, keywords, hotspots, and frontiers trends (19). CiteSpace and VOSviewer are software for visualization for bibliometrics analysis (20, 21). These two software generate network maps that allow researchers to intuitively analyze the current status within the field, and determine the research hotspots and frontiers trends (22). Therefore, this study employs CiteSpace and VOSviewer software to analyze the publications on LChD from 2002 to 2021, to evaluate and analysis the research hotspots and frontiers trends. This has been the first study to use bibliometric strategies in the field of LChD. The study is expected to help researchers extract potential information for further research in the field of LChD research and offer them helpful advice in choosing ground-breaking subject matter by answering the following questions:
(i) Which countries, institutions, journals, authors, and references are the current status of research in the field of LChD ?
(ii) What are the current hotspots and major categories of LChD?
(iii) Where are in the future frontiers trends of LChD ?
Materials and methods
Data acquisition and search strategy
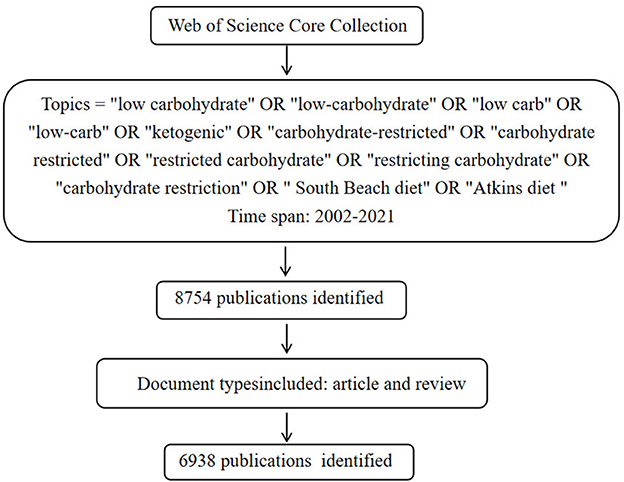
In this study, WoSCC was selected as the data source. As a high-quality digital literature resource database, WoSCC has been accepted by many researchers, and considered as the most suitable database for literature analysis (23). All publications were retrieved from the Science Citation Index Expanded (SCI-E) of the WoSCC database on November 12, 2022. We completed the search within the same day to avoid any bias caused by database updates. The following methods were conducted for search publications: topic words = (“low carbohydrate” OR “low-carbohydrate” OR “low carb” OR “low-carb” OR “ketogenic” OR “carbohydrate-restricted” OR “carbohydrate restricted” OR “restricted carbohydrate” OR “restricting carbohydrate” OR “carbohydrate restriction” OR “South Beach diet” OR “Atkins diet”). In order to more accurately analyze the current status, hotspots and frontiers trends of LChD, the publications from 2002 to 2021 were selected. Time span = January 1, 2002–December 31, 2021. To ensure the representativeness of the included studies, the types of publications were limited to “articles” and “reviews” (24). No languages limitation to avoid bias in the geographical distribution of publications. The content of literature records were “full records and cited references,” downloaded and saved in plain text document format.
Statistical analysis
We used the CiteSpace (6.1.R3) and VOSviewer (1.6.18) for a bibliometric analysis of 6,938 publications on LChD from 2002 to 2021. The java-based program CiteSpace does bibliometric analysis of publications using distribution network maps, co-citation network maps, dual maps of journal overlay, and keyword burst citation maps (25). Nodes and links are included in the visual network diagram produced by CiteSpace. Every node is a factor, such as an author, an institution, or a country (26). Links between different nodes show a network of relationships involving co-operation, co-citation, or co-occurrence (27) A wider line indicates a more effective collaboration. The higher the centrality, the larger the circle is in terms of centrality. When a node has a purple circle around it, it has a high centrality score and is therefore an important node in the field (22). VOSviewer was used to form keyword co-occurrence of overlay visualization. The colors represent the years (28). The size of the node is proportional to the frequency of keyword occurrences (29). Data was managed, charts were made, and all data tables were created using Microsoft Excel 2021 software.
Results
Annual output and categories
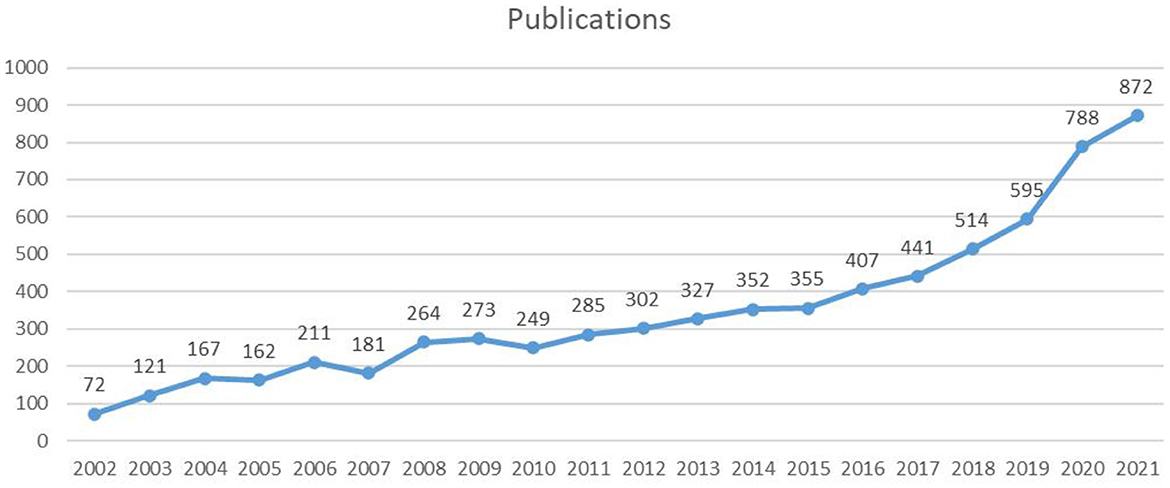
A total of 6,938 publications including 5,350 articles and 1,588 reviews, related to LChD from 2002 to 2021 were retrieved by searching the WoSCC database. The flowchart was shown in Figure 1. The annual publications reflected the activities in the field and the attention given to certain areas of research (30). As seen in Figure 2, the number of annual publications on LChD showed an overall upward trend in spite of fluctuation slightly in some years over the past 20 years. It indicates that LChD research is becoming a research of great interest to scholars and has attracted great interest from scholars in recent years.
LChD publications in the past 20 years can be divided into 2 stages. The initial stage (2002–2010) was a steady growth period. The average number of publications was 188 publications every year, with the lowest number of publications being 72 publications in 2002 and the highest number being 273 publications in 2009. In 1927, a low carbohydrate ketogenic diet had been reported for epilepsy (31). As an early study in 1948, LChD was used to control of dental caries (32). Since 2002, LChD was contributed to a variety of areas, including obesity (33), diabetes (34), and cardiovascular disease (35). Although the number of papers varied at this stage, the overall trend was one of consistent growth. The second stage (2011–2021) was a sustained growth period. The average number of publications annually was 476 publications. The number of publications reached 872 in 2021. Nutrition has a significant role in daily life, and it is crucial for the advancement of social development to support research on diet and health. LChD research has gained popularity as a nutritional approach and is rapidly developing into a research hotspot.
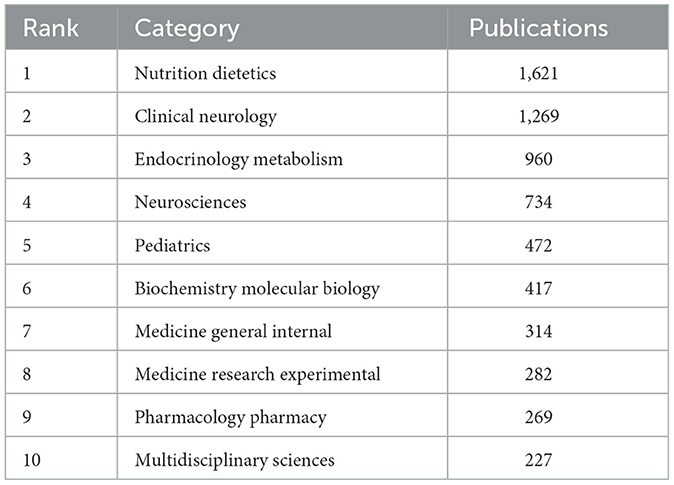
The categories refer to the disciplines covered by the dissertation research. At top 10 categories (Table 1), Nutrition Dietetics had 1,621 publications and ranked first, followed by Clinical Neurology (1,269 publications), Endocrinology Metabolism (960 publications), Neurosciences (734 publications) and Pediatrics (472 publications). LChD research mainly covered the fields of nutrition, endocrinology, and neurosciences, reflecting the multidisciplinary nature and comprehensive knowledge.
Analysis of countries/regions
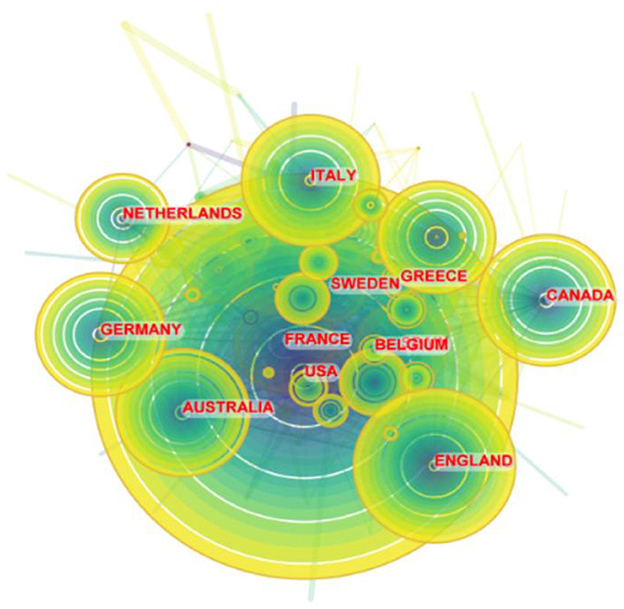
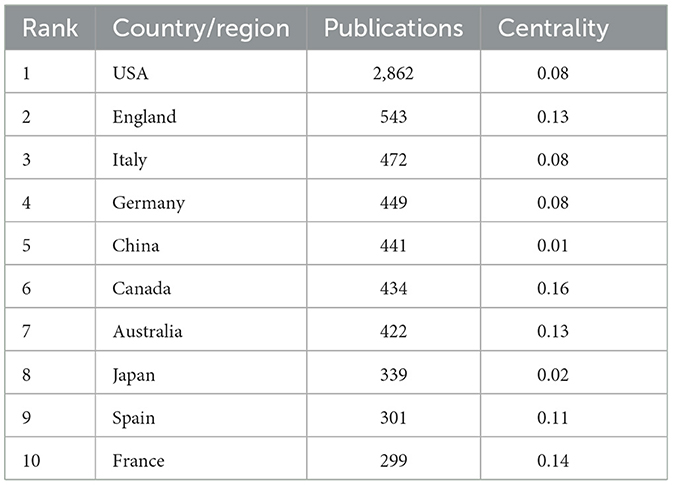
In total, 112 countries/regions participated in 6,938 publications on LChD from 2002 to 2021. CiteSpace generated the countries/regions distribution map, and 112 nodes and 880 links were shown in the map (Figure 3). Table 2 presented the top 10 countries/regions published in LChD research field. USA had the highest number of publications, 2,862 papers, accounting for 41.25%. The Yuasa phenomenon states that the nation whose research output accounts for more than 25% of all scientific output at any given moment can be referred to as the world center of science during that time (36). As the leader in LChD research, USA published far more than a quarter of the total publications and was the world science center in the field of LChD. England (543 publications), Italy (472 publications), China (449 publications), and Germany (441 publications) followed closely behind. In terms of centrality, Canada (0.16) ranked first, followed by, Spain (0.14), Australia (0.13), England (0.13), France (0.11) and, which maintain close cooperation relationships. Countries/regions with centrality played an important role in LChD research. Germany, Canada, Australia and France each had < 450 publications, but their research roles were important. In terms of publications, China had 449 papers, but the centrality was only 0.01. It demonstrated that despite having a high publications number, China had few connections and little influence over the network map. The level of LChD research in China therefore was raised effectively by deepening the field's research, advancing cross-disciplinary and cross-field collaboration, and enhancing researchers' capacity for creative thinking and global communication.
Analysis of institutions
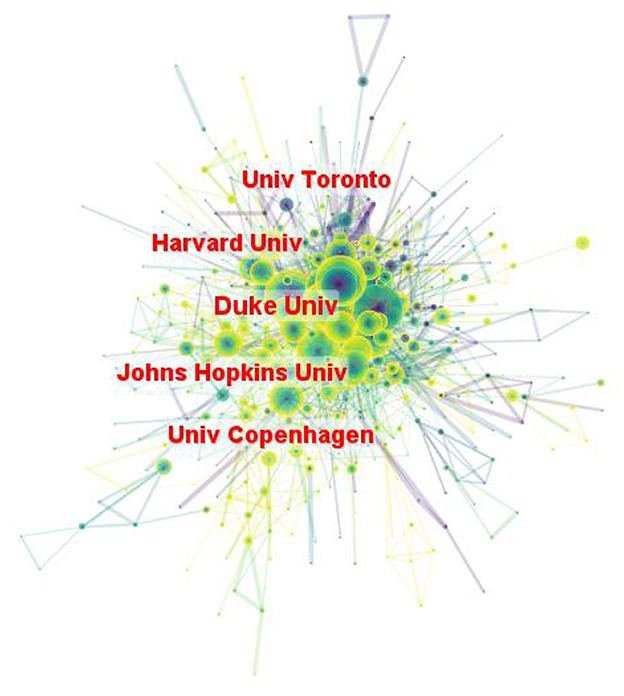
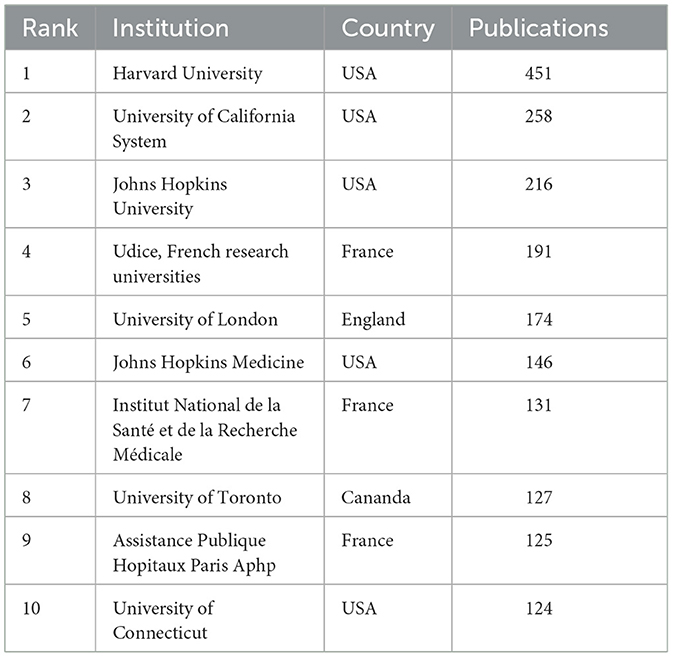
A total of 604 institutions provided research in the field of LChD. CiteSpace generated the institutions distribution map with 604 nodes and 2,103 links (Figure 4). The institutions with large numbers of publications have been identified as influential institutions (37). Table 3 listed the top 10 institutions in publications, and they were the most influential institutions in LChD research. Universities were major institutions for LChD research. Harvard University ranking first, had 451 papers, followed by University of California System (258 publications), Johns Hopkins University (216 publications), Udice French research universities (191 publications), and University of London (174 publications). Five of the top 10 institutions were from USA, which further confirmed US predominance in the field of LChD research. Duke University, Harvard University, Johns Hopkins University and University of Toronto had close collaboration relationships.
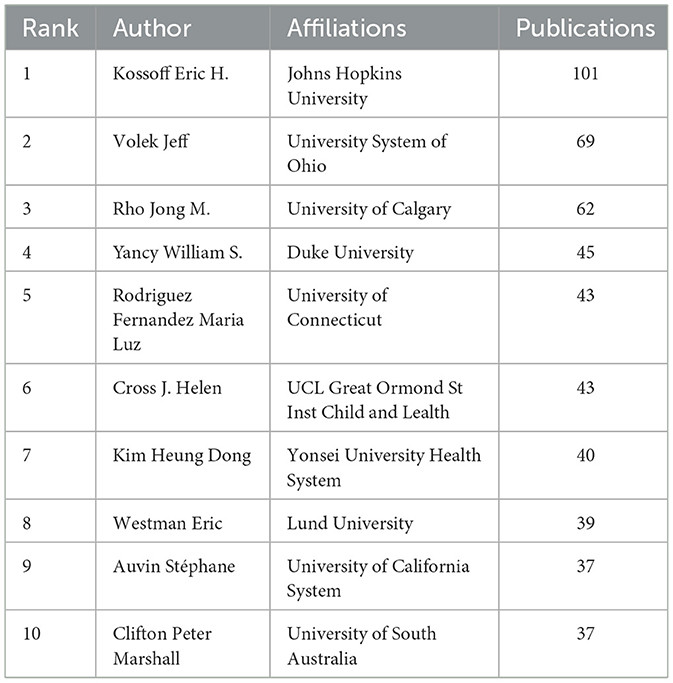
Analysis of authors
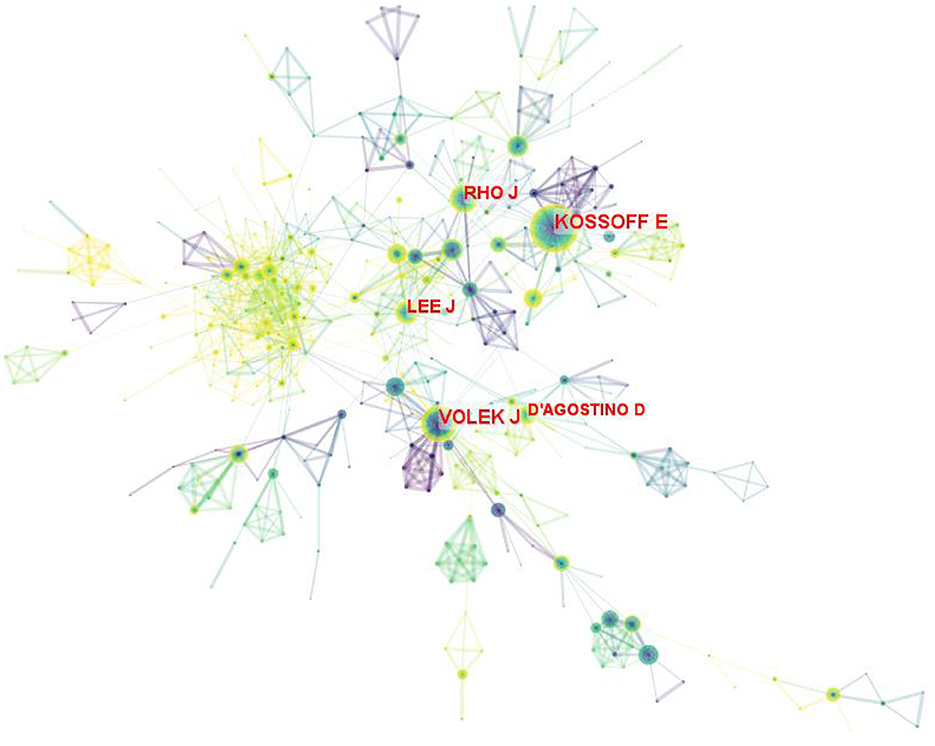
In total of 890 authors participated in 6,938 publications on LChD from 2002 to 2021. CiteSpace generated the institutions distribution map with 890 nodes and 1965 links (Figure 5). The top 10 authors participating in the LChD research are shown in Table 4. The most productive authors were Eric Heath Kossoff (101 publications), Jeff Scott Volek (69 publications), Jong M. Rho (62 publications), William S. Yancy (45 publications), and Maria Luz Fernandez (43 publications). Eric Heath Kossoff ranked first in the number of publications devoted to the study of the effects of a high-fat, low-carb ketogenic diet on neurological disorders. He demonstrated that a high-fat, low-carb ketogenic diet reduced the number of seizures in refractory epilepsy and reported no cardiovascular or cerebrovascular events (38, 39). In addition ketogenic diets are being applied to a range of neurological disorders from autism to Alzheimer's disease (40). Jeff Scott Volek was the second position of papers. He reported that in individuals with atherosclerotic dyslipidemia, a 12-week carbohydrate restriction diet improved postprandial vascular function more than a low-fat diet (41). An study revealed that LChD (10%) not only decreased lipid deposition but avoided the buildup of plasma and aortic oxidation, decreased inflammatory cytokines within the artery wall, and prevented atherosclerosis (42). Jong M. Rho was in the third place in terms of number of publications. In addition to a high-fat, low-carbon-water ketogenic diet that improves epilepsy (43), he emphasized that a ketogenic diet enhances mitochondrial function and reduces autistic behavior in humans and rodent models of autism spectrum disorder (44, 45). The authors' collaboration displayed a geographical concentration and general decentralization.
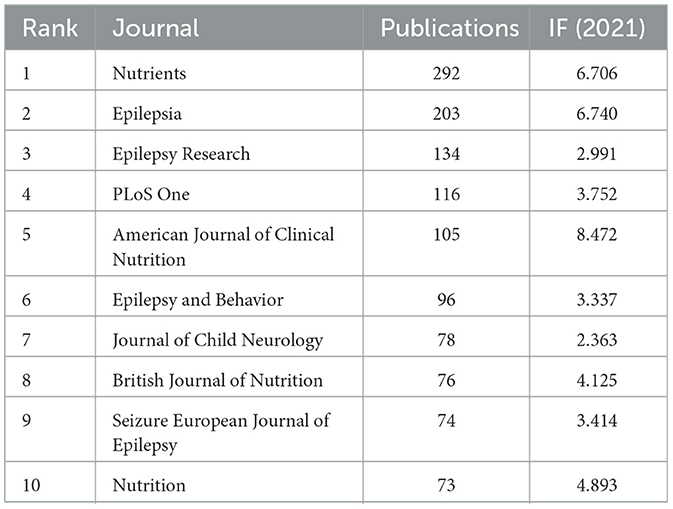
Analysis of journals
Researchers can accurately understand the core journals in a topic by analyzing its source journals, which also serves as a reliable resource for further field research (46). A total of 1,545 academic journals published 6,938 publications in the field of research on LChD from 2002 to 2021. As shown in Table 5, the top 10 journals accounted for 17.93% of the total publications. The most productive journals were Nutrients (292 publications), Epilepsia (203 publications), Epilepsy Research (134 publications), PLoS One (116 publications), and American Journal of Clinical Nutrition (105 publications). Of the top 10 journals, eight journals' IF more than 3.0. With a maximum of 8.472, the top 2 journals had an IF >6.0. This shows that high IF journals are open to publishing LChD research.
Figure 6 illustrated the dual- map overlay of journals that produced literature linked to the topic of LChD. On the map, the right labels represented the disciplines of the journals that published the cited papers, while the left labels represented the fields of the citing journals. Citation links can show the in and out of the citation dataset. Figure 6 showed 5 reference pathways. Three yellow pathways indicate articles published in molecular/biological/immunology journals mainly citing journals in the molecular/biological/genetics field. Two green pathways suggest that articles published in medicine/clinical journals mainly cite journals in the molecular/biology/genetics/health/nursing/medicine fields. One red pathway shows that the publications from neurology/sports/ophthalmology mainly cite journals in the in molecular/biology/genetics field.
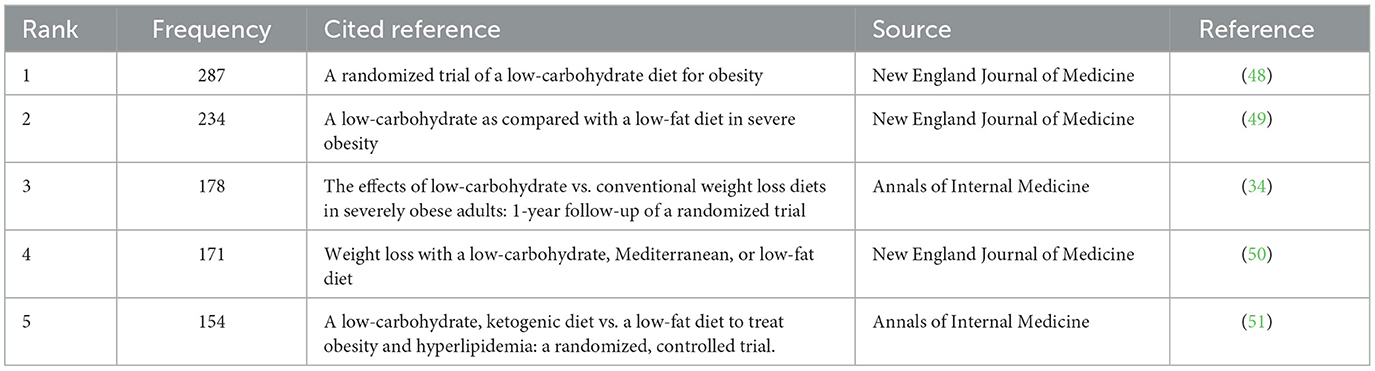
Analysis of co-cited references
The co-cited reference analysis is one of the important indicators in bibliometric research and is usually used to explore research priorities in specific academic fields (47). CiteSpace generated the co-cited reference map, and 1,693 nodes and 9,455 links were shown in the map (Figure 7). The top 5 co-cited references in terms of frequency were in Table 6. Analysis of co-cited references provided basic data for LChD research. Noteworthy were three publications from the New England Journal of Medicine and two from the Annals of Internal Medicine, both of which have significant academic influence. The five references were all clinical trials. In most co-cited reference, obese people were given Atkins diet, and lost more weight in the first 6 months (48). Additionally, high density lipoprotein cholesterol levels increased and triglyceride levels decreased more in Atkins diet participants than in control group, indicating that Atkins diet had a higher impact on the risk factors for coronary heart disease. The second-most co-cited reference reported that patients who received a carbohydrate-restricted diet with 30 g per day or less, lost more weight than control group did and had relative improvements in their insulin sensitivity and triglyceride levels (49). The third most co-cited reference of 132 obese people on who were restricted carbohydrate intake to < 30 g per day showed more beneficial effects than those on conventional diets at 1 year; the effects of restricted carbohydrate on atherogenic dyslipidemia and glycemic control remained more favorable (34). Diet therapies were given to moderately obese subjects, and a low-carbohydrate diet and a Mediterranean diet were found to have beneficial effects on lipids and blood glucose, respectively (50). Individualized dietary regimens tailored to individual preferences and metabolism are recommended. A low-carbohydrate, ketogenic diet exhibited higher participant retention and more weight loss compared to low-fat diets in the literature with the sixth greatest co-citation frequency (51).
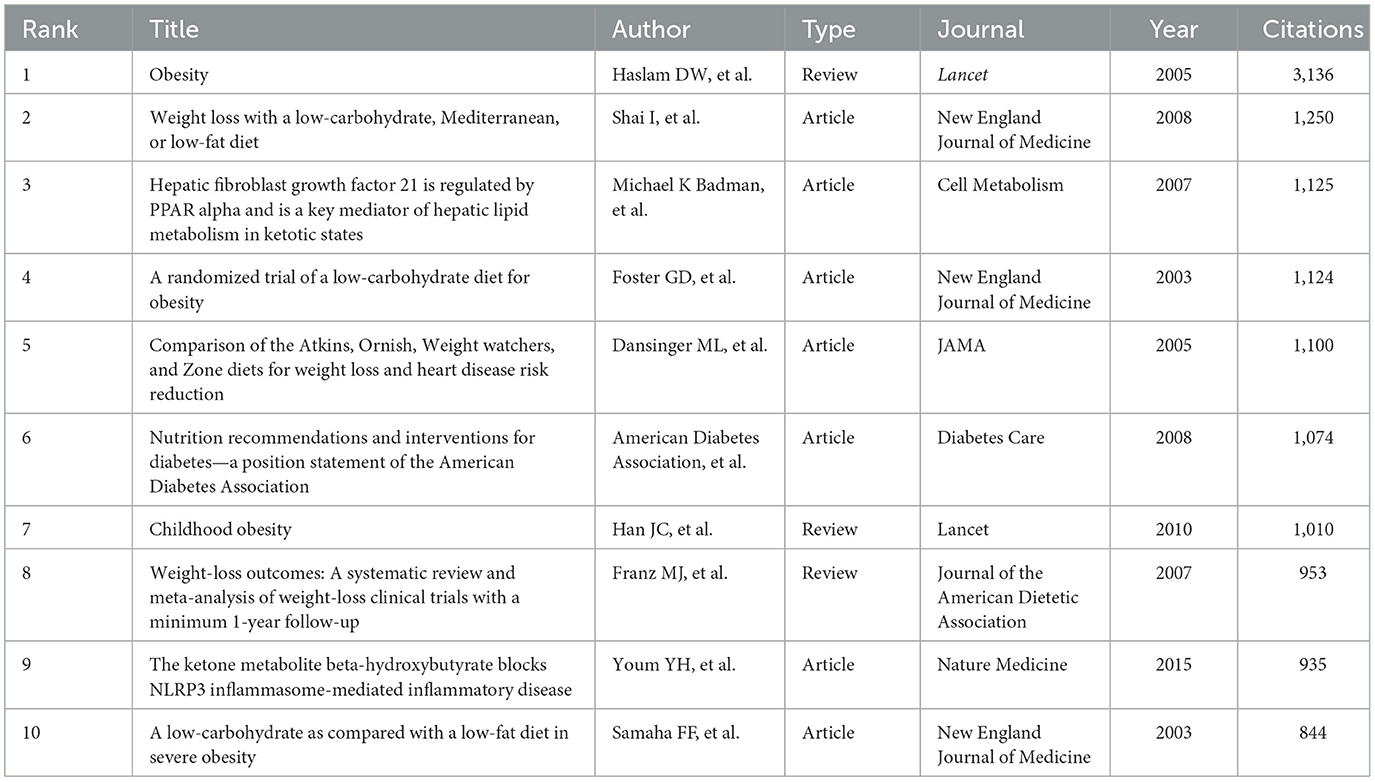
References analysis
High cited references lay the foundation and accelerate the development of research in the field (23). The top 10 cited references were listed in Table 7. Of the top 10 references, 7 references were articles and 3 were reviews. Three references were published in the New England Journal of Medicine and two were published in Lancet. “Obesity” published by Haslam et al. in 2005, was cited 3,136 times, and ranked first. Shai et al. published in 2008 in New England Journal of Medicine of “Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet” was cited 1,250 times, and ranked second. Foster et al. published in 2003 in “A randomized trial of a low-carbohydrate diet for obesity” in New England Journal of Medicinewas cited 1,124 times, and ranked third.
Analysis of keywords
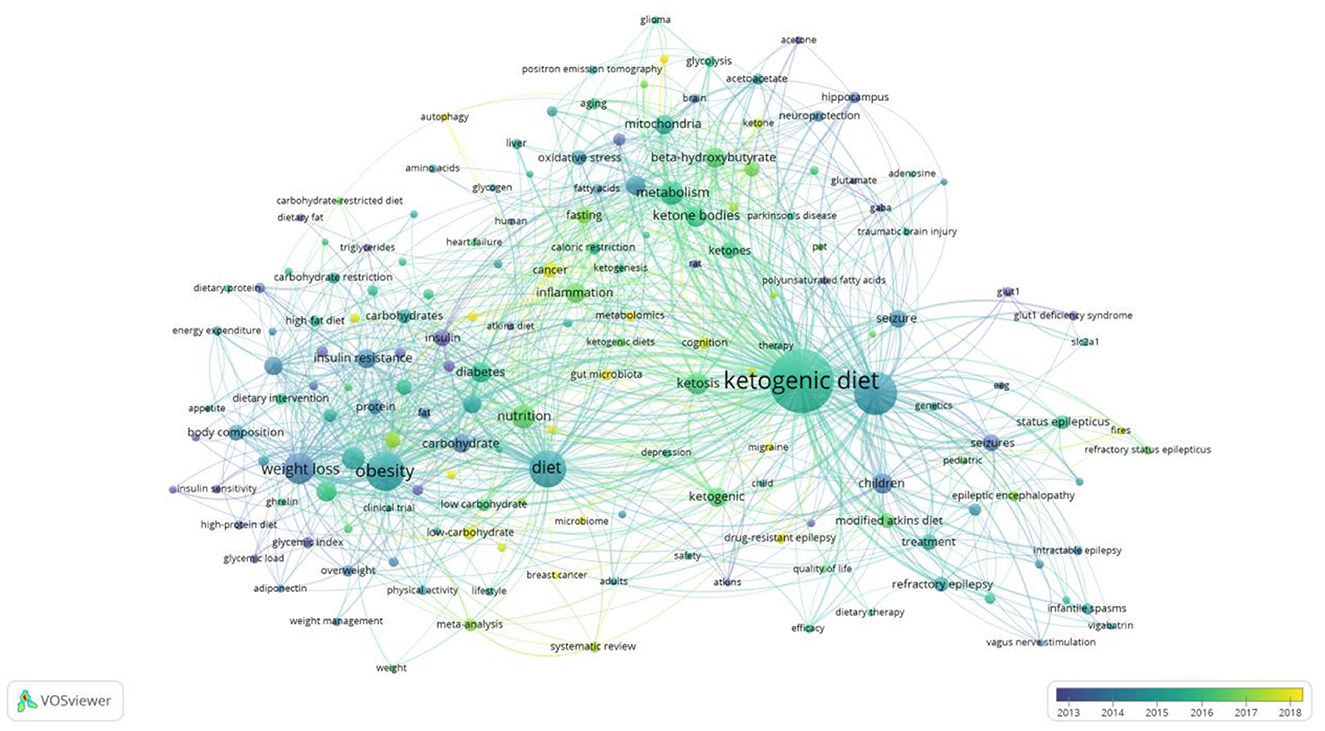
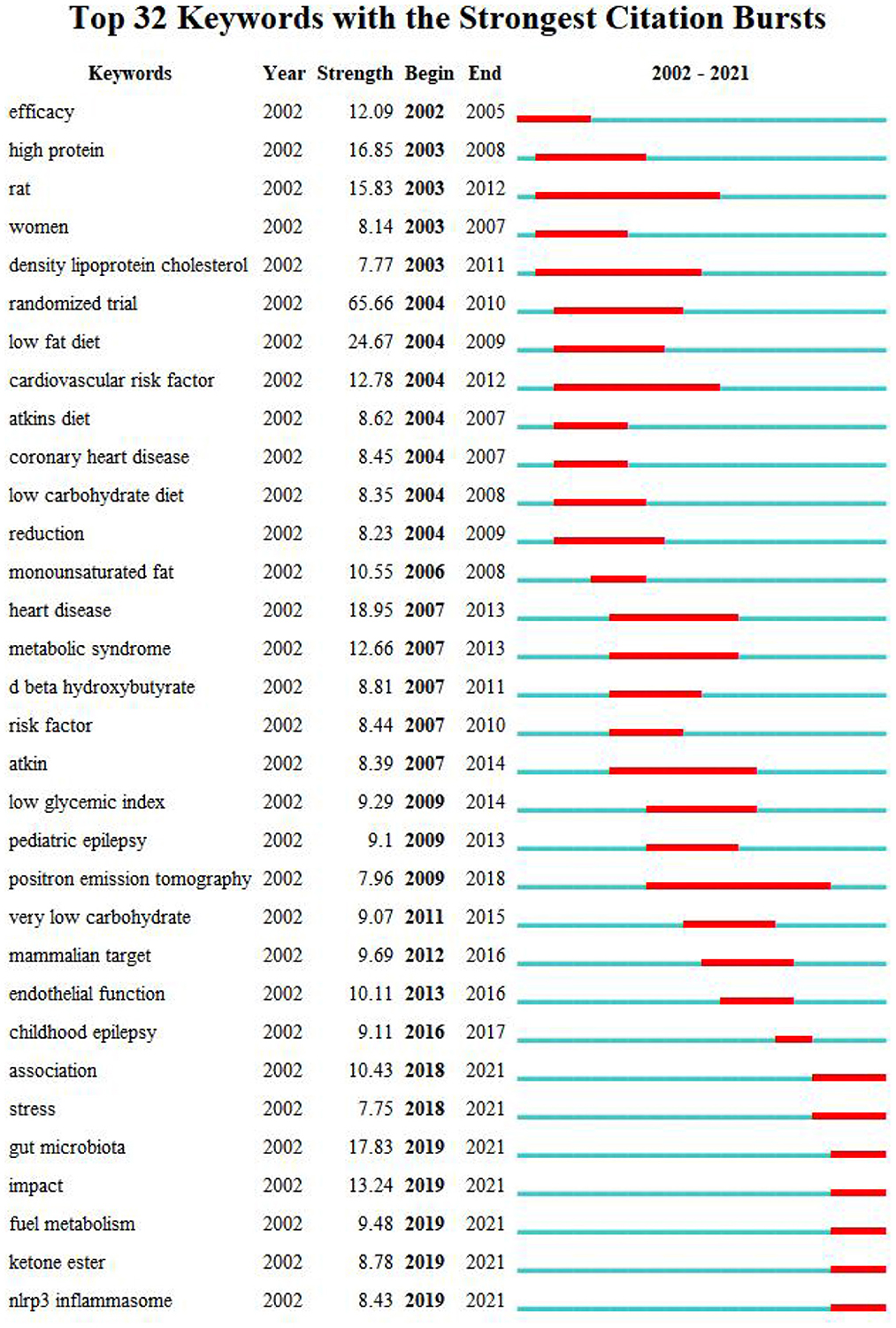
The map of keywords can present the main research objects and the hot topics and frontiers trends. In this study, VOSviewer software performed the keyword co-occurrence of overlay visualization (Figure 8). A total of 9,750 keywords, 172 keywords met the thresholds when the minimum number of occurrences of a keywords was 20. From Figure 8, we found that the keywords research hotspots were categorized into “ketogenic diet,” “metabolism disease,” “cardiovascular disease” and “cancer.” Bursts keywords were frequently used at a period time, reflecting the frontiers trends. We used CiteSpace software to map the top 32 keywords with the strongest citation bursts from 2002 to 2021 (Figure 9). We summarized that “oxidative stress,” “gut microbiota,” and “inflammation factors” are becoming frontiers trends of LChD research in the future.
Discussion
We performed a bibliometric analysis of the publications from WoSCC on LChD from 2002 to 2021 using CiteSpace and VOSviewer software. We then summarized the current status, hotspots and frontiers trends in this field.
A total of 6,938 publications including 5,350 articles and 1,588 reviews, related to LChD from 2002 to 2021 were retrieved by searching WOSCC database. The number of annual publications on LChD showed an overall upward trend in spite of fluctuation slightly in some years. LChD research mainly involved the categories of nutrition, endocrinology, and neurosciences, reflecting the multidisciplinary nature and comprehensive knowledge about LChD research. USA was with the largest number and the world science center in LChD field, and Australia, Canada, England, France and Germany maintained close cooperation relationships. Universities were major institutions for LChD research. Five of the top 10 institutions were from USA, which further confirmed US predominance in the field of LChD research. Duke University, Harvard University, Johns Hopkins University and University of Toronto had close collaboration relationships. The most productive authors were Eric Heath Kossoff, Jeff Scott Volek, Jong M. Rho, William S. Yancy, and Maria Luz Fernandez. The authors' collaboration showed a geographical concentration and general decentralization. The most productive journals were Nutrients, Epilepsia, Epilepsy Research, PLoS One, and American Journal of Clinical Nutrition. “A randomized trial of a low-carbohydrate diet for obesity” and “Obesity” were considered to be the most co-cited and cited reference respectively.
Based on the keywords the keyword co-occurrence of overlay visualization, we can explore the hotspots. From Figure 8, we summarized and analyzed four hotspots in LChD field. Here, we further analyzed the following aspects according to the application field of LChD: ketogenic diet, metabolism disease, cardiovascular disease and cancer.
(i) Ketogenic diet: The KD is a type of low-carb diet, characterized by high fat and very low carbohydrate. KD works on the basis of the biological principle of starvation, using fat as the main energy source of the body (52). KD initially achieved satisfactory results in pediatric refractory epilepsy and obesity (53). In recent years, research on KD has been extended to metabolic (54), cardiovascular (55), cancer (56), neurological (57), and respiratory (58) diseases with positive results, especially in hyperglycemia (59), hyperlipidemia (60), and insulin resistance (61). Of course there are some shortcomings. The long-term efficacy, such as weight regain (62), cardiovascular events (63), and bone metabolism (64), cannot be ignored. Therefore, the use of KD for disease treatment needs to be individualized according to different diseases and patients' data.
(ii) Metabolism disease: In this study, obesity and diabetes are common metabolic diseases. By consuming less carbohydrates, restricting the body's usage of exogenous glucose, and boosting lipolysis and fatty acid oxidation to meet the body's energy needs, low carbohydrate nutrition enable individuals to lose weight (65). The studies have shown that LChD are efficient in assisting obese individuals reduce their weight (16, 66). In addition, a low-carbohydrate-high-fat diet can reduce the risk factors for obesity-related diseases while improving obesity and have good prospects for healthy weight loss (67). According to type 2 diabetes epidemiological report, a rise in carbohydrates is largely responsible for the increased calories in patients. Glycemic management begins with dietary carbohydrate restriction (68). One study has shown that patients who have dietary carbohydrate restriction maintains lower levels of glycosylated hemoglobin after a year (69). Research already have proven to the positive effects of LChD on the metabolic diseases of diabetes, obesity, and hypertension. Along with the changes in lifestyle and work style currently occurring, the current research on dietary nutrition and metabolism diseases is going to become a popular topic.
(iii) Cardiovascular disease: The risk of cardiovascular disease rises with a high carbohydrate diet (70). The risk of cardiovascular disease can be decreased by a low carbohydrate nutrition (71). Blood pressure and cardiovascular disease morbidity and mortality are known to be strongly causally correlated. Several studies have shown that LChD can improve blood pressure by lowering diastolic and systolic pressure (72, 73). Increased levels of triglycerides, total cholesterol, and low-density lipoprotein cholesterol are crucial contributors to the development of atherosclerotic cardiovascular disease. Excessive levels of high-density lipoprotein cholesterol have a preventive impact, but elevated levels of total cholesterol and triglycerides are significant risk factors for atherosclerotic cardiovascular disease. A number of lipids, including triacylglycerol, total cholesterol, low density lipoprotein cholesterol, and high density lipoprotein cholesterol, are improved by LChD (74, 75). A reasonable LChD program is good for cardiovascular health since it has a long-term positive impact on the prevention of cardiovascular disease.
(iv) Cancer: Nutrition is receiving increasing attention in oncology clinical research (76). Proper diet can prevent and treat cancer and reduce the incidence of cancer (77). Seyfried et al. (78) found that the rate of tumor growth is directly proportional to blood sugar levels. Reducing carbohydrate intake, especially KD, can make blood sugar at a low level and effectively inhibit tumor cell proliferation (79). Low-carb diet and KD can improve the quality of life, physical performance, body composition and metabolic health of cancer patients (80). Low-carb diet and KD may create an unfavorable metabolic environment for cancer cells. Therefore, LChD or/and standard therapy, enhance the potential of anti-tumor effects and improve quality of life (11).
Burst keywords can explore the future development trends. Therefore, we summarized the burst keywords into three aspects, and considered them to be frontiers trends of LChD field and anticipated to occur frequently in the future years.
(i) Oxidative stress: Oxidative stress is a negative effect produced by free radicals in the body, and it is considered to be an important pathogenic factor, such as diabetes mellitus, obesity, heart disease, and cancer (81). The metabolite of the carbohydrate is glucose. The intake of excessive carbohydrates produces more glucose and increases the oxidative pressure on mitochondria, which increases the production of excessive reactive oxygen species, leading to the occurrence of disease (82). Low-carbohydrate intake reduces to reduce the occurrence of oxidative stress in the body, thus reducing the incidence of disease (83). A review showed that the low-carbon ketogenic diet-mediated reduction in glucose levels and enhanced electron transport in the mitochondria further disrupt the energy metabolism of tumor cells, thus adversely affecting tumor cell proliferation (83).
(ii) Gut microbiota: Gut flora can regulate body metabolism and participate in the occurrence of diseases through a variety of mechanisms. With increasing research on gut microbiota, the dietary pattern was identified as one of the main drivers of gut microbiota change. Recently, carbon aquatic ketone diet has been shown to effectively treat neurological diseases (84), tumor (85, 86), metabolic diseases (87), inflammatory bowel disease (88), etc. Its effect source is related to the participation of intestinal flora in neurodevelopment (84), various pathways to hinder tumor cell growth (89, 90), inhibit the growth of bifidobacterium and reduce the inflammatory factor (89). The current research on intestinal microbiota has explained the action mechanism of low-carbon ketogenic diet to some extent, but the number of studies is too small, requiring further exploration in the future to provide a more solid theoretical basis for the application of low-carbon ketogenic diet.
(iii) Inflammation factors: With the development of life science and technology, the current literature also further explains the disease treatment and prevention of LChD from the inflammation level. Tumor necrosis factor-a, interleukin-6, lipocalin, and C-reactive protein are inflammation factors produced by adipose tissue (91– 94). Dietary habits can influence immune function and have anti-inflammatory effects (95). The diets of 9.6% energy from carbohydrate (96) and carbohydrate < 40 g/day (97) enhanced lipocalin and lowered C-reactive protein levels in obese patients. According to Jonasson et al. (98), type 2 diabetic individuals who took LChD of 20% energy from carbohydrate, had lower serum levels of interleukin-1 receptor and interleukin-6. An further experiment revealed that LChD lessens lipid deposition, avoids the buildup of plasma and aortic oxidation, lowers inflammatory cytokines in the arterial wall, and inhibits atherosclerosis (99). There will be a spectrum of levels at which research on LChD is conducted, with more inflammation factors becoming increasingly prevalent in the future.
Limitations
To the best of our knowledge, the present study is the first bibliometric analysis to assess LChD. However, it has many limitations. First, considering that the data difference and incompleteness of other database data, we only analyzed publications from the WoSCC. Next, to better present the analysis result and to ensure the quality of the included literature, we included only articles and reviews published in English. This may lead to some screening bias.
Conclusion
We searched all research publications related to LChD on the Web of Scientific Core Collection (WoSCC). CiteSpace software was used to analyze countries/regions, institutions, journals, authors, references, and keywords. LChD is a popular diet, attracting attention from scholars. The hotspots of LChD are three aspects, “metabolism disease,” “cardiovascular disease,” and “risk factor.” We summarize that “research on prevention and treatment,” “research on diet,” and “research on molecular level” are becoming frontiers trends of LChD research in the future directions and deserve further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HP and GL: conceptualization. XH: methodology, writing-original draft preparation, and writing-review and editing. CL and LZ: software. LZ: investigation, data curation, and supervision. GL and XH: resources. GL and CL: visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Natural Science Foundation of Guangdong Province, China (No. 2021A1515011506) and Department of Education of Guangdong Province, China (No. 2021KTSCX237). This study was also supported by Guangdong Food and Drug Vocational College.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rinonapoli G, Pace V, Ruggiero C, Ceccarini P, Bisaccia M, Meccariello L, et al. Obesity and bone: a complex relationship. Int J Mol Sci. (2021) 22:13662. doi: 10.3390/ijms222413662
2. Gower BA, Goss AM. A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. J Nutr. (2015) 145:177S–83S. doi: 10.3945/jn.114.195065
3. Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab. (2008) 5:9. doi: 10.1186/1743-7075-5-9
4. York LW, Puthalapattu S, Wu GY. Nonalcoholic fatty liver disease and low-carbohydrate diets. Annu Rev Nutr. (2009) 29:365–79. doi: 10.1146/annurev-nutr-070208-114232
5. Diamond DM, Alabdulgader AA, de Lorgeril M, Harcombe Z, Kendrick M, Malhotra A, et al. Dietary recommendations for familial hypercholesterolaemia: an evidence-free zone. BMJ Evid Based Med. (2021) 6:295–301. doi: 10.1136/bmjebm-2020-111412
6. Astrup A, Meinert Larsen T, Harper A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. (2004) 9437:897–9. doi: 10.1016/S0140-6736(04)16986-9
7. Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. (2008) 49:37–41. doi: 10.1111/j.1528-1167.2008.01831.x
8. Atallah R, Filion KB, Wakil SM, Genest J, Joseph L, Poirier P, et al. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: a systematic review of randomized controlled trials. Circ Cardiovasc Qual Outcomes. (2014) 7:815–27. doi: 10.1161/CIRCOUTCOMES.113.000723
9. Muscogiuri G, Barrea L, Laudisio D, Pugliese G, Salzano C, Savastano S, et al. The management of very low-calorie ketogenic diet in obesity outpatient clinic: a practical guide. J Transl Med. (2019) 1:356. doi: 10.1186/s12967-019-2104-z
10. Adam-Perrot A, Clifton P, Brouns F. Low-carbohydrate diets: nutritional and physiological aspects. Obes Rev. (2006) 7:49–58. doi: 10.1111/j.1467-789X.2006.00222.x
11. Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer: where do we stand? Mol Metab. (2020) 33:102–21. doi: 10.1016/j.molmet.2019.06.026
12. Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic diet and microbiota: friends or enemies? Genes. (2019) 10:534. doi: 10.3390/genes10070534
13. Jovanovski E, Zurbau A, Vuksan V. Carbohydrates and endothelial function: is a low-carbohydrate diet or a low-glycemic index diet favorable for vascular health? Clin Nutr Res. (2015) 4:69–75. doi: 10.7762/cnr.2015.4.2.69
14. Burke LM, Sharma AP, Heikura IA, Forbes SF, Holloway M, McKay AK, et al. Crisis of confidence averted: Impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS ONE. (2020) 6:e0234027. doi: 10.1371/journal.pone.0234027
15. Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. (2018) 72:311–25. doi: 10.1038/s41430-017-0019-4
16. Ebbeling CB, Feldman HA, Klein GL, Wong JM, Bielak L, Steltz SK, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial [published correction appears in BMJ. 2020 Nov 3;371:m4264]. BMJ. (2018) 363:k4583. doi: 10.1136/bmj.k4583
17. Hite AH, Berkowitz VG, Berkowitz K. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract. (2011) 26:300–8. doi: 10.1177/0884533611405791
18. Oelrich B, Peters R, Jung K. A bibliometric evaluation of publications in urological journals among European Union countries between 2000 and 2005. Eur Urol. (2007) 52:1238–48. doi: 10.1016/j.eururo.2007.06.050
19. Aydinoglu AU, Taşkin Z. Origins of life research: a bibliometric approach. Orig Life Evol Biosph. (2018) 48:55–71. doi: 10.1007/s11084-017-9543-4
20. Galetsi P, Katsaliaki K. Big data analytics in health: an overview and bibliometric study of research activity. Health Info Libr J. (2020) 37:5–25. doi: 10.1111/hir.12286
21. Blake H, Bermingham F, Johnson G, Tabner A. Mitigating the psychological impact of COVID-19 on healthcare workers: a digital learning package. Int J Environ Res Public Health. (2020) 9:2997. doi: 10.3390/ijerph17092997
22. Li W, Weng L, Xiang Q, Fan T. Trends in research on traditional Chinese health exercises for improving cognitive function: a bibliometric analysis of the literature from 2001 to 2020. Front Public Health. (2022) 9:794836. doi: 10.3389/fpubh.2021.794836
23. You Y, Li W, Liu J, Li X, Fu Y, Ma X. Bibliometric review to explore emerging high-intensity interval training in health promotion: a new century picture. Front Public Health. (2021) 9:697633. doi: 10.3389/fpubh.2021.697633
24. Lu C, Li X, Yang K. Trends in shared decision-making studies from 2009 to 2018: a bibliometric analysis. Front Public Health. (2019) 7:384. doi: 10.3389/fpubh.2019.00384
25. Waqas A, Teoh SH, Lapão LV, Messina LA, Correia JC. Harnessing telemedicine for the provision of health care: bibliometric and scientometric analysis. J Med Internet Res. (2020) 22:e18835. doi: 10.2196/18835
26. Zheng KY, Dai GY, Lan Y, Wang XQ. Trends of repetitive transcranial magnetic stimulation from 2009 to 2018: a bibliometric analysis. Front Neurosci. (2020) 14:106. doi: 10.3389/fnins.2020.00106
27. Wang XQ, Peng MS, Weng LM, Zheng YL, Zhang ZJ, Chen PJ. Bibliometric study of the comorbidity of pain and depression research. Neural Plast. (2019) 2019:1657498. doi: 10.1155/2019/1657498
28. Murdayanti Y, Khan MNAA. The development of internet financial reporting publications: a concise of bibliometric analysis. Heliyon. (2021) 12:e08551. doi: 10.1016/j.heliyon.2021.e08551
29. Leal Filho W, Ternova L, Parasnis SA, Kovaleva M, Nagy GJ. Climate change and zoonoses: a review of concepts, definitions, and bibliometrics. Int J Environ Res Public Health. (2022) 2:893. doi: 10.3390/ijerph19020893
32. Pankey LD. Control of dental caries by a low carbohydrate diet. J Fla State Dent Soc. (1948) 19:5–7.
33. Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. The effects of low-carbohydrate vs. conventional weight loss diets in severely obese adults: 1-year follow-up of a randomized trial. Ann Intern Med. (2004) 140:778–85. doi: 10.7326/0003-4819-140-10-200405180-00007
34. Kamuren ZT, Sanders R, Watkins JB. Low-carbohydrate diet and oxidative stress in diabetic and nondiabetic rats. J Biochem Mol Toxicol. (2006) 20:259–69. doi: 10.1002/jbt.20142
35. Focardi M, Dick GM, Picchi A, Zhang C, Chilian WM. Restoration of coronary endothelial function in obese Zucker rats by a low-carbohydrate diet. Am J Physiol Heart Circ Physiol. (2007) 292:H2093–9. doi: 10.1152/ajpheart.01202.2006
36. Shi GJ, Geng Y. Science is flat: new explanation of Yuasa phenomenon. Stud Sci Educ. (2012) 30:1770–80.
37. Dang Q, Luo Z, Ouyang C, Wang L. First systematic review on health communication using the CiteSpace software in China: exploring its research hotspots and frontiers. Int J Environ Res Public Health. (2021) 18:13008. doi: 10.3390/ijerph182413008
38. McDonald TJW, Ratchford EV, Henry-Barron BJ, Kossoff EH, Cervenka MC. Impact of the modified Atkins diet on cardiovascular health in adults with epilepsy. Epilepsy Behav. (2018) 79:82–6. doi: 10.1016/j.yebeh.2017.10.035
39. Cervenka MC, Patton K, Eloyan A, Henry B, Kossoff EH. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci. (2016) 3:131–7. doi: 10.1179/1476830514Y.0000000162
40. deCampo DM, Kossoff EH. Ketogenic dietary therapies for epilepsy and beyond. Curr Opin Clin Nutr Metab Care. (2019) 4:264–8. doi: 10.1097/MCO.0000000000000565
41. Volek JS, Ballard KD, Silvestre R, Judelson DA, Quann EE, Forsythe CE, et al. Effects of dietary carbohydrate restriction vs. low-fat diet on flow-mediated dilation. Metabolism. (2009) 58:1769–77. doi: 10.1016/j.metabol.2009.06.005
42. Leite JO, DeOgburn R, Ratliff J, Su R, Smyth JA, Volek JS, et al. Low-carbohydrate diets reduce lipid accumulation and arterial inflammation in guinea pigs fed a high-cholesterol diet. Atherosclerosis. (2010) 209:442–8. doi: 10.1016/j.atherosclerosis.2009.10.005
43. Chun KC, Ma SC, Oh H, Rho JM, Kim DY. Ketogenic diet-induced extension of longevity in epileptic Kcna1-null mice is influenced by gender and age at treatment onset. Epilepsy Res. (2018) 140:53–5. doi: 10.1016/j.eplepsyres.2017.11.005
44. Cheng N, Rho JM, Masino SA. A metabolic dysfunction underlying autism spectrum disorder and potential treatment approaches. Front Mol Neurosci. (2017) 10:34. doi: 10.3389/fnmol.2017.00034
45. Ahn Y, Sabouny R, Villa BR, Yee NC, Mychasiuk R, Uddin GM, et al. Aberrant mitochondrial morphology and function in the BTBR mouse model of autism is improved by 2 weeks of ketogenic diet. Int J Mol Sci. (2020) 9:3266. doi: 10.3390/ijms21093266
46. Shi D, Xie C, Wang J, Xiong L. Changes in the structures and directions of heavy metal-contaminated soil remediation research from 1999 to 2020: a bibliometric and scientometric study. Int J Environ Res Public Health. (2021) 18:7358. doi: 10.3390/ijerph18147358
47. Trujillo CM, Long TM. Document co-citation analysis to enhance transdisciplinary research. Sci Adv. (2018) 4:e1701130. doi: 10.1126/sciadv.1701130
48. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. (2003) 348:2082–90. doi: 10.1056/NEJMoa022207
49. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. (2003) 348:2074–81. doi: 10.1056/NEJMoa022637
50. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet [published correction appears in N Engl J Med. 2009 Dec 31;361(27):2681]. N Engl J Med. (2008) 359:229–41. doi: 10.1056/NEJMoa0708681
51. Yancy WS, Olsen MK, Guyton JR, Bakst RP, Westman EC, A. low-carbohydrate, ketogenic diet vs. a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. (2004) 140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006
52. Dhamija R, Eckert S, Wirrell E. Ketogenic diet. Can J Neurol Sci. (2013) 2:158–67. doi: 10.1017/S0317167100013676
53. Vasquez A, Farias-Moeller R, Sánchez-Fernández I, Abend NS, Amengual-Gual M, Anderson A, et al. Super-refractory status epilepticus in children: a retrospective cohort study. Pediatr Crit Care Med. (2021) 12:e613–25. doi: 10.1097/PCC.0000000000002786
54. Gangitano E, Gnessi L, Lenzi A, Ray D. Chronobiology and metabolism: is ketogenic diet able to influence circadian rhythm? Front Neurosci. (2021) 15:756970. doi: 10.3389/fnins.2021.756970
55. Tzenios N, Lewis ED, Crowley DC, Chahine M, Evans M. Examining the efficacy of a very-low-carbohydrate ketogenic diet on cardiovascular health in adults with mildly elevated low-density lipoprotein cholesterol in an open-label pilot study. Metab Syndr Relat Disord. (2022) 2:94–103. doi: 10.1089/met.2021.0042
56. Shen W, He J, Hou T, Si J, Chen S. Common pathogenetic mechanisms underlying aging and tumor and means of interventions. Aging Dis. (2022) 4:1063–91. doi: 10.14336/AD.2021.1208
57. Xu Y, Jiang C, Wu J, Deng X, Zhang Y, Peng B, et al. Ketogenic diet ameliorates cognitive impairment and neuroinflammation in a mouse model of Alzheimer's disease. CNS Neurosci Ther. (2022) 4:580–92. doi: 10.1111/cns.13779
58. Melkonian SC, Daniel CR, Ye Y, Pierzynski JA, Roth JA, Wu X. Glycemic index, glycemic load, and lung cancer risk in non-hispanic whites. Cancer Epidemiol Biomarkers Prev. (2016) 3:532–9. doi: 10.1158/1055-9965.EPI-15-0765
59. Montemurro N, Perrini P, Rapone B. Clinical risk and overall survival in patients with diabetes mellitus, hyperglycemia and glioblastoma multiforme. A review of the current literature. Int J Environ Res Public Health. (2020) 22:8501. doi: 10.3390/ijerph17228501
60. Hall KD, Chung ST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. (2018) 4:308–12. doi: 10.1097/MCO.0000000000000470
61. Buehler LA, Noe D, Knapp S, Isaacs D, Pantalone KM. Ketogenic diets in the management of type 1 diabetes: safe or safety concern? Cleve Clin J Med. (2021) 10:547–55. doi: 10.3949/ccjm.88a.20121
62. Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation: a review. Nutr Res. (2020) 77:1–11. doi: 10.1016/j.nutres.2020.02.010
63. Landry MJ, Crimarco A, Gardner CD. Benefits of low carbohydrate diets: a settled question or still controversial? Curr Obes Rep. (2021) 10:409–22. doi: 10.1007/s13679-021-00451-z
64. Shu L, Beier E, Sheu T, Zhang H, Zuscik MJ, Puzas EJ, et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int. (2015) 4:313–23. doi: 10.1007/s00223-015-9954-z
65. Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, et al. Low carbohydrate nutrition and metabolism. Am J Clin Nutr. (2007) 86:276–84. doi: 10.1093/ajcn/86.2.276
66. Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JP, et al. Effect of low-fat vs. low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. (2018) 319:667–79. doi: 10.1001/jama.2018.0245
67. Brouns F. Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable? Eur J Nutr. (2018) 57:1301–12. doi: 10.1007/s00394-018-1636-y
68. Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. (2015) 31:1–13. doi: 10.1016/j.nut.2014.06.011
69. Rock CL, Flatt SW, Pakiz B, Taylor KS, Leone AF, Brelje K, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabet Care. (2014) 37:1573–80. doi: 10.2337/dc13-2900
70. Asadi Z, Shafiee M, Sadabadi F, Heidari-Bakavoli A, Moohebati M, Khorrami MS, et al. Association of dietary patterns and risk of cardiovascular disease events in the MASHAD cohort study. J Hum Nutr Diet. (2019) 32:789–801. doi: 10.1111/jhn.12669
71. Das S, McCreary J, Shamim S, Kalayjian T. Reversal of severe hypertriglyceridemia with intermittent fasting and a very-low-carbohydrate ketogenic diet: a case series. Curr Opin Endocrinol Diabetes Obes. (2020) 27:308–11. doi: 10.1097/MED.0000000000000566
72. Evans CE, Greenwood DC, Threapleton DE, Cleghorn CL, Nykjaer C, Woodhead CE, et al. Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals. J Hypertens. (2015) 33:897–911. doi: 10.1097/HJH.0000000000000515
73. Flint AJ, Hu FB, Glynn RJ, Jensen MK, Franz M, Sampson L, et al. Whole grains and incident hypertension in men. Am J Clin Nutr. (2009) 90:493–8. doi: 10.3945/ajcn.2009.27460
74. Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutr Metab Cardiovasc Dis. (2014) 24:337–43. doi: 10.1016/j.numecd.2013.12.008
75. Kim JY, Yang YH, Kim CN, Lee CE, Kim KI. Effects of very-low-carbohydrate (horsemeat- or beef-based) diets and restricted feeding on weight gain, feed and energy efficiency, as well as serum levels of cholesterol, triacylglycerol, glucose, insulin and ketone bodies in adult rats. Ann Nutr Metab. (2008) 3–4:260–7. doi: 10.1159/000189129
76. Haskins C, Cohen J, Kotecha R, Kaiser A. Low carbohydrate diets in cancer therapeutics: current evidence. Front Nutr. (2021) 8:662952. doi: 10.3389/fnut.2021.662952
77. Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME. Nutritional deterioration in cancer: the role of disease and diet. Clin Oncol. (2003) 15:443–50. doi: 10.1016/S0936-6555(03)00155-9
78. Seyfried TN, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res. (2012) 3:310–26. doi: 10.1016/j.eplepsyres.2011.06.017
79. Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. (2014) 117:125–31. doi: 10.1007/s11060-014-1362-0
80. Kämmerer U, Klement RJ, Joos FT, Sütterlin M, Reuss-Borst M. Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients. (2021) 3:1029. doi: 10.3390/nu13031029
81. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. (2017) 1:120. doi: 10.1186/s12933-017-0604-9
82. Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. (2013) 7:1157–80. doi: 10.1007/s00204-013-1034-4
83. Feng S, Wang H, Liu J, Aa J, Zhou F, Wang G. Multi-dimensional roles of ketone bodies in cancer biology: opportunities for cancer therapy. Pharmacol Res. (2019) 150:104500. doi: 10.1016/j.phrs.2019.104500
84. Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. (2018) 2:148–59. doi: 10.1016/j.biopsych.2017.06.021
85. Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. (2021) 11:2317–31.e6. doi: 10.1016/j.molcel.2021.03.037
86. Ferrere G, Alou MT, Liu P, Goubet AG, Fidelle M, Kepp O, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. (2021) 2:e145207. doi: 10.1172/jci.insight.145207
87. Abbasi J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA. (2018) 3:215–7. doi: 10.1001/jama.2017.20639
88. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. (2020) 6:1263–75.e16. doi: 10.1016/j.cell.2020.04.027
89. Lane J, Brown NI, Williams S, Plaisance EP, Fontaine KR. Ketogenic diet for cancer: critical assessment and research recommendations. Nutrients. (2021) 10:3562. doi: 10.3390/nu13103562
90. Mundi MS, Mohamed Elfadil O, Patel I, Patel J, Hurt RT. Ketogeniciet and cancer: fad or fabulous? JPEN J Parenter Enteral Nutr. (2021) S2:26–32. doi: 10.1002/jpen.2226
91. Barry JC, Simtchouk S, Durrer C, Jung ME, Little JP. Short-term exercise training alters leukocyte chemokine receptors in obese adults. Med Sci Sports Exerc. (2017) 49:1631–40. doi: 10.1249/MSS.0000000000001261
92. Pedersen BK, Fischer CP. Beneficial health effects of exercise–the role of IL-6 as a myokine. Trends Pharmacol Sci. (2007) 28:152–6. doi: 10.1016/j.tips.2007.02.002
93. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. (2009) 6:129–39. doi: 10.1016/j.mce.2009.08.018
94. Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. (2009) 10:258S–61S. doi: 10.3945/ajcn.2009.28449C
95. Romanowska-Próchnicka K, Felis-Giemza A, Olesińska M, Wojdasiewicz P, Paradowska-Gorycka A, Szukiewicz D. The role of TNF-α and Anti-TNF-α agents during preconception, pregnancy, and breastfeeding. Int J Mol Sci. (2021) 20:2922. doi: 10.3390/ijms22062922
96. Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, et al. Consuming a hypocaloric high fat low carbohydrate diet for 12 weeks lowers C-reactive protein, and raises serum adiponectin and high density lipoprotein-cholesterol in obese subjects. Metabolism. (2013) 62:1779–87. doi: 10.1016/j.metabol.2013.07.006
97. Hu T, Yao L, Reynolds K, Whelton PK, Niu T, Li S, et al. The effects of a low-carbohydrate diet vs. a low-fat diet on novel cardiovascular risk factors: a randomized controlled trial. Nutrients. (2015) 7:7978–94. doi: 10.3390/nu7095377
98. Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low-carbohydrate diet has a favorable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med. (2014) 46:182–7. doi: 10.3109/07853890.2014.894286
Keywords: bibliometric analysis, low carbohydrate diet, CiteSpace, VOSviewer, hotspots, frontiers trends
Citation: Lu G, Huang X, Lin C, Zou L and Pan H (2023) A bibliometric and visual analysis of low carbohydrate diet. Front. Nutr. 10:1085623. doi: 10.3389/fnut.2023.1085623
Received: 31 October 2022; Accepted: 06 February 2023;
Published: 23 February 2023.
Edited by:
Junrui Cheng, Ingredion Incorporated, United StatesReviewed by:
Stefan Kabisch, Charité Universitätsmedizin Berlin, GermanyJia Xiong, North Carolina State University, United States
Copyright © 2023 Lu, Huang, Lin, Zou and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huashan Pan,  cGhzNjgxMDExQDEyNi5jb20=
cGhzNjgxMDExQDEyNi5jb20=
 Gang Lu1
Gang Lu1 Huashan Pan
Huashan Pan