
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 04 April 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1075877
This article is part of the Research Topic Nutrition and Sustainable Development Goal 14: Life Below Water View all 10 articles
Background and aims: Gout, the most prevalent inflammatory arthritis, has undesirable effects on the quality of life. Omega-3 polyunsaturated fatty acids (n-3 PUFA) has a strong link with anti-inflammatory impacts. However, whether the harmful effects of seafood in relation to gout may vary owing to different levels of n-3 PUFA in seafood is still unclear. It was the goal of this study to examine the relationship between n-3 PUFA poor/rich seafood consumption and gout.
Methods: Between 2007 and 2016, five NHANES cycles were performed, with 12,505 subjects having complete data for gout and two 24-h dietary intake interviews. The 24-h dietary recalls were utilized to evaluate dietary habits. Gout was defined based on questionnaires. Weighted logistic regression models were conducted to investigate the association between n-3 PUFA poor/rich seafood consumption and gout. Moreover, subgroup analysis was utilized to estimate the stability of results. Covariates including age, gender, race/ethnicity, income, education, body mass index, chronic kidney disease, diabetes mellitus, hypertension, smoking status, and drinking status were stratified in different models.
Results: In the fully adjusted model, each unit of increase of n-3 PUFA poor seafood intake was associated with an 8.7% increased risk of gout (OR = 1.087, 95% CI: 1.039, 1.138, P < 0.001), whereas, no correlation was found between n-3 PUFA rich seafood consumption and gout. It also provided a proof-of-concept regarding the potential for n-3 PUFA rich seafood to counteract harmful effects of purines in relation to gout. A dose-response analysis showed that there was a non-linear relationship between n-3 PUFA rich seafood intake and the risk of gout in the female group.
Conclusion: Findings suggest that n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not.
Gout is an inflammatory crystal arthritis, the most important pathological characteristic of which is monosodium urate deposition in the joints. The incidence of gout has been stably increasing to 0.58–2.89 per 1,000 person-years worldwide with the development of the world economy and lifestyle changes (1). Additionally, gout episodes have undesirable effects on the quality of life (2).
Recent studies have revealed a strong link between omega-3 polyunsaturated fatty acids (n-3 PUFA) and anti-inflammatory impacts (3–5). Numerous systematic reviews and meta-analysis have noted that n-3 PUFAs exhibited anti-inflammatory effects on multiple non-communicable diseases, including systemic inflammatory response syndrome (3), colorectal cancer (4), type 2 diabetic mellitus (5), bipolar disorder (6), mental disorders (7), polycystic ovary syndrome (8), heart failure (9), hypertension (10), and rheumatoid arthritis (11). With respect to gout, experiments in vitro noted that n-3 PUFAs could inhibit the NLRP3 inflammasome in monocytes via downstream effects of a two-signal initiation system (12, 13), including suppression of nuclear factor kappa-B (NF-κB) via Toll-like receptor 4 and Toll-like receptor 2 (14) and assembly of the inflammasome and activation of caspase-1 (15). Clinically, a case control study showed that low serum n-3 PUFA levels were connected with frequent gout flares (16). Although it is well established that most seafood, which typically contains large quantities of purines, leads to increased risk of gout (17, 18), whether the harmful effects of seafood in relation to gout may vary owing to different levels of n-3 PUFA in seafood is still unclear. According to the US Department of Agriculture (USDA) Dietary Research Nutrition Database, the seafood component is divided into: seafood that are high in n-3 PUFA and seafood that are low in n-3 PUFA. Whether n-3 PUFA poor seafood or n-3 PUFA rich seafood can affect gout remained to be elucidated.
Hence, the preliminary aim of this study was to determine the relationship between n-3 PUFA poor/rich seafood consumption and gout in US adults using data from the National Health and Nutrition Examination Survey (NHANES). Our study was the first large-scale cross-sectional study evaluating n-3 PUFA poor/rich seafood consumption and gout, which could shed new light on gout management.
Potential subjects in the present study were selected from 2007 to 2016 cycle of NHANES. NHANES is a periodic, nationally representative health study conducted by the Nation Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) aimed at evaluating individuals’ health and nutritional status. NHANES was approved by the NCHS Ethics Review Board. All subjects provided written informed consent. All data in this study are publicly accessible at http://www.cdc.gov/nchs/nhanes/.
Participants aged <20 years old (n = 21,387), without complete information about dietary data (n = 6,489), missing data on gout (n = 1,131), or missing baseline condition (n = 9,076) were excluded, leaving a total of 12,505 subjects for the present analysis (Figure 1).
A total of 24-h dietary recalls were utilized to calculate individual dietary intakes, which have been examined by the Nutrition Methodology Working Group (19). To ensure precise food recall and alleviate the respondent burden, eligibility criteria included subjects who had both two valid 24-h dietary recalls.
Definition of seafood was founded on the USDA Dietary Research Nutrition Database. Seafood high in n-3 PUFA included anchovy, herring, mackerel, salmon, sardine, shark, trout, and bluefin and albacore tuna. Seafood low in n-3 PUFA included catfish, clams, cod, crabs, crayfish, croaker, eel, flounder, haddock, lobster, mussels, octopus, oyster, perch, pollock, scallop, shrimp, snapper, tilapia, tuna (other than bluefin and albacore), and turtle.
Similar to previous NHANES reports (20), all participants were asked “Has a doctor or other health professional ever told you that you had gout?” and then classified as non-gout participants and gout participants.
According the current articles, the following variables were collected during the household interview, including age, gender, race/ethnicity, education level, and family poverty income ratio (PIR). Race/ethnicity was categorized into Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other race (including multi-racial) based on NHANES classification (21). Education level was classified as less than high school, high school graduate/General Education Development (GED); some college/Associate of Arts (AA) degree and college graduate or more (22). PIR was utilized to evaluate family income. PIR was categorized into three strata (<1, 1–3, and ≥3) and defined as poor, near poor, and not poor, respectively (23).
Body mass index (BMI) was calculated as the weight divided by the square of height (kg/m2). BMI <18.5 was considered as underweight, 18.5–24.9 as normal, 25–30 as overweight, and ≥30 as obese (24).
Smoking behavior was classified as never smoker, former smoker, and current smoker based on their answers to questionnaire about smoking more than 100 cigarettes in their life and whether they had quit smoking (25).
Drinking behavior was categorized as mild drinking (≤1 drink per day for women or ≤2 drinks per day for men on average over the past 12 months), moderate drinking (1–3 drinks per day for women or 2–4 drinks per day for men on average over the past 12 months), and heavy drinking (≥4 drinks per day for women or ≥5 drinks per day for men on average over the past 12 months) (26).
We used the Chronic Kidney Disease Epidemiology Collaboration formulate to assess estimated glomerular filtration rate (eGFR). Chronic kidney disease (CKD) was defined as eGFR <60 ml/min/1.73 m2 or urine albumin creatinine ratio (UACR) >30 mg/g (27).
Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg based on the average of three measurements of all subjects’ blood pressure (28).
Diabetes mellitus (DM) was defined as self-reported diabetes, the use of diabetes medication or insulin, glycohemoglobin (HbA1c) >6.5%, fasting glucose ≥7.0 mmol/L, random blood glucose ≥11.1 mmol/L, or 2-h oral glucose tolerance test (OGTT) blood glucose (mmol/L) ≥11.1 (29). Impaired fasting glucose (IFG) was defined as fasting plasma glucose concentration ≥5.6 and ≤6.9 mmol/L (30). Impaired glucose tolerance (IGT) was defined as a plasma glucose level ≥7.8 and ≤11.0 mmol/L at 2 h after OGTT (30).
All statistical analyses were performed following CDC guidelines for analysis of NHANES data. A suitable sample weight was utilized to calculate all statistical analyses in order that the data corresponded to the non-institutionalized civilian population. Continuous variables were represented by mean and standard deviation (SD), whereas categorical variables were expressed by counts and weighted percentages. One-way ANOVA test (for normally distributed continuous variables), Kruskal–Wallis H-test (for non-normally distributed continuous variables) and Chi-square test (for categorical variables) were utilized to measure differences among different groups.
Weighted logistic regression analysis was set up to estimate the relationship between n-3 PUFA poor/rich seafood consumption and gout. To further examine the covariable effect on this correlation, we employed Model 1 (unadjusted), Model 2 (age, gender, and race/ethnicity were adjusted), and Model 3 (all covariates in Table 1 were adjusted). In addition, restricted cubic spline models were utilized to investigated their dose-response relationship in Model 3. Finally, we further explored the heterogenicity in different groups with interaction terms. P < 0.05 with effective confidence interval (CI) was of statistical significance. All analyses were constructed with R version 4.0.41 (The R foundation).
As illustrated in Figure 1, a total of 12,505 subjects were enrolled in this study. Table 1 summarized the population characteristics overall and between two groups (adults with/without gout). In the whole research population, the median age at baseline was 46.29 years, 48.33% were females and 51.17% were males. The overall prevalence of gout among US adults was 4.31%. Participants with gout were more likely be older, male, non-Hispanic White, obese, alcoholic, former smokers (P < 0.01). Additionally, they suffered from CKD, DM, hypertension, and had higher n-3 PUFA poor seafood intake (P < 0.05). There was no difference in income and education levels (P > 0.05).
Results of weighted logistic regression analysis for the association between n-3 PUFA poor/rich seafood consumption and gout are shown in Table 2. Weighted logistic regression analysis confirmed that higher n-3 PUFA poor seafood intake was associated with an increased risk of gout (Model 1, OR = 1.098, 95% CI: 1.053, 1.145, P < 0.001; Model 2, OR = 1.096, 95% CI: 1.048, 1.146, P < 0.001; Model 3, OR = 1.087, 95% CI: 1.039, 1.138, P < 0.001). In model 3, which adjusted for all confounding variables, the results still revealed that each unit of increase of n-3 PUFA poor seafood intake was associated with an 8.7% increased risk of gout. With respect to n-3 PUFA rich seafood intake, the connection with gout was only significant in model 1(OR = 1.112, 95% CI: 1.017, 1.216, P = 0.019), but become non-significant after stepwise adjusting for confounding variables (Models 2 and 3). In dose-response relationships, n-3 PUFA rich seafood consumption was non-linearly related with gout in the female group. We found an inverted L-shaped association. The prevalence of gout reached a plateau when the n-3 PUFA poor seafood intake was lower than 1.207 ounce/day in the female group. Figure 2 depicts the dose response relationship. We also conducted subgroup analyses stratified by age, gender, race/ethnicity, income, education, BMI, CKD, DM, hypertension, smoking, and drinking status to evaluate the association between n-3 PUFA rich seafood intake and gout (Figure 3). This association was similar among participants aged >37 and <56 years old. Relatively stronger relationships were also observed among male, non-Hispanic White, other race and patients with underweight, normal weight, overweight, IFG, non-DM, mild drinking, and moderate drinking. Additionally, statistically significant ORs were only present in people without CKD, hypertension and with a lower household PIR and current smoking. Besides, we also explored the heterogenicity among each subgroup with interaction terms and no significant difference was revealed among age, gender, race/ethnicity, income, education, CKD, DM, hypertension, smoking status, and drinking status (P for interaction >0.05 for all), revealing that the magnitude of this relationship was the same for the subjects separated into different subgroups.

Figure 2. The dose-response relationship between n-3 PUFA poor seafood intake and gout. Adjusted for age, gender, race/ethnicity, income, education, BMI, CKD, DM, hypertension, smoking status, and drinking status. Solid lines hazard ratio, blue or pink area 95% confidence interval. n-3 PUFA, omega-3 polyunsaturated fatty acid; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus.
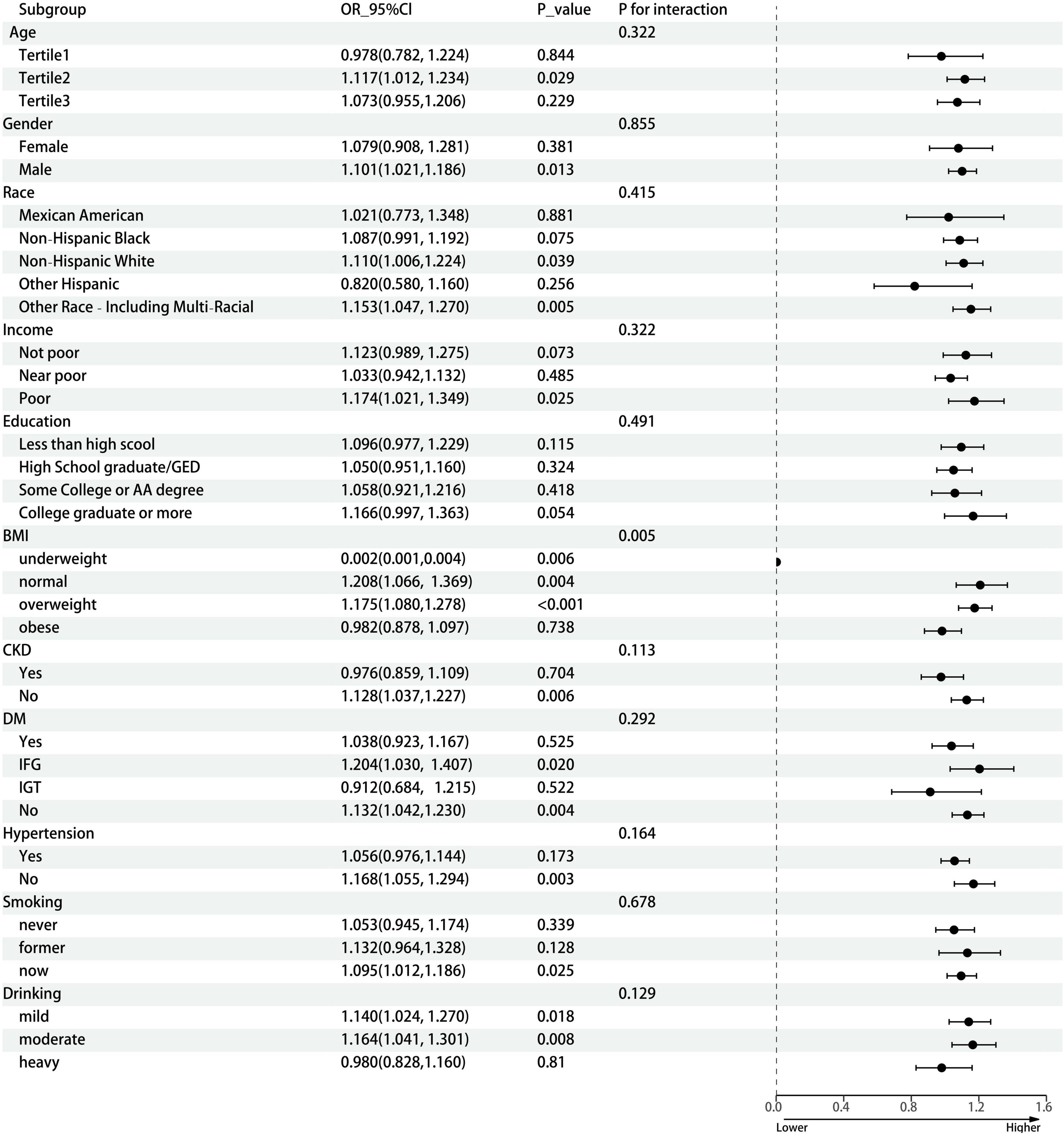
Figure 3. Subgroup analysis between n-3 PUFA poor seafood intake and gout. The subgroup analysis was adjusted for age, gender, race/ethnicity, income, education, BMI, CKD, DM, hypertension, smoking status, and drinking status. Age (years old), Tertile 1, 20–37, Tertile 2, 37–56, Tertile 3, 56–80; n-3 PUFA, omega-3 polyunsaturated fatty acid; OR, odds ratio; CI, confidence interval; GED, General Education Development; AA degree, Associate of Arts; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
In this nationwide cross-sectional study with 12,505 adults, a significant positive connection of n-3 PUFA poor seafood intake with gout was uncovered, revealing that higher consumption of n-3 PUFA poor seafood may contribute to an increased risk of gout. This connection remained statistically significant after we adjusted for all confounders, including age, gender, race/ethnicity, income, education, BMI, CKD, DM, hypertension, smoking status, and drinking status. Correlated subgroup analyses stratified by different variables revealed that this positive connection was not influenced, suggesting that this connection could be appropriated for different population.
It was worth noting that there was a lack of research on the effect of n-3 PUFA poor/rich seafood consumption on gout. It is well established that purine-rich foods consumption, which comprised most seafood, brings about an increased risk of gout (17). Interestingly, our study demonstrated that only n-3 PUFA poor seafood consumption was associated with increased risk of gout with statistical significance. The results of our study also provided a proof-of-concept regarding the potential for n-3 PUFA rich seafood to counteract harmful effects of purines in relation to gout. Emerging evidence has shown that n-3 PUFAs could exert protective anti-inflammatory effects. A meta-analysis revealed that supplementation of n-3 PUFAs in adults can improve inflammatory biomarkers, such as serum C-reactive protein, tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) (31).
Several hypotheses could be put forward to explain the anti-inflammatory effects of n-3 PUFAs. First, n-3 PUFAs may involve in the regulation of immunological and inflammatory responses via the gut microbiome (32–34). Gut microbiota can influence the metabolism and absorption of n-3 PUFAs, in the meantime, n-3 PUFAs can also affect the diversity and abundance of the gut microbiome. For example, n-3 PUFAs possibly suppressed the Firmicutes/Bacteroidetes ratio and the levels of Coprococcus and Faecalibacterium and increased the abundance of butyrate-producing bacterial genera, thus further reducing the inflammatory processes (35–37).
Second, n-3 PUFAs are important mediators in membrane phospholipid fatty acid composition of inflammatory cells (38). n-3 PUFAs could vary the composition of membrane phospholipid fatty acid via a variety of general mechanisms, resulting in affecting inflammatory cell function. To begin with, the physical properties of the membrane, such as membrane fluidity and raft structure could be altered by n-3 PUFAs (38). Then, n-3 PUFAs could exert influences on cell signaling pathways. Certain n-3 PUFAs have been shown to have anti-inflammatory effects through inhibition of the principal inflammatory cytokine via extracellular inflammatory stimuli (39) and G-protein coupled receptors (40). An in vivo experiment revealed that peroxisome proliferator-activated receptor γ, an anti-inflammatory factor, was induced by n-3 PUFAs in dendritic cells (41). Furthermore, n-3 PUFAs could alter the production in the pattern of the lipid mediators. Animal studies have shown that n-3 PUFAs may modify the fatty acid of eicosanoid, a key mediators of inflammation, resulting in producing several anti-inflammatory and inflammation resolving mediators, such as resolvins and protectins (39). Finally, with respect to T cell signaling, n-3 PUFAs were found to disturb membrane-cytoskeletal structure and function in CD4+ T lymphocytes (33).
Third, n-3 PUFAs may exert anti-inflammatory effects in macrophages. The balance between pro- and anti-inflammation is coordinated by macrophages. Previous animal studies have demonstrated that adding n-3 PUFA to the diet induced a decrease in macrophages (40, 42). In studies of mechanisms, the anti-inflammatory function by n-3 PUFAs has been proved through the free fatty acid receptor 4 protein in macrophages resulting in suppressing activity of the NF-κB complex (42). Otherwise, in vivo findings have been supported that n-3 PUFAs regulated inflammatory signaling in macrophages via the autophagic receptor SQSTM1/p62-bodies and NFE2L2 (43).
The major strengths of this population-based study are using a nationally representative sample, which facilitates the finding to be universal to a broader population. Nevertheless, several limitations cannot be ignored. First, there were only two 24-h dietary recalls in NHANES rather than three 24-h dietary recalls (two weekdays and one Friday), where recall bias is inevitable.
To minimize the influence of two-time recall bias, the NHANES design utilized sampling weight and multiple-pass method to ensure precise of dietary intake and subjects who had both two valid 24-h dietary recalls were included in this study. Second, we were unable to consider more purine-rich foods except alcohol consumption, which may lead to confounding bias. Third, cross-section design cannot draw causal relationship.
In summary, our results suggest that n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not and also provide a proof-of-concept regarding the potential for n-3 PUFA rich seafood to counteract harmful effects of purines in relation to gout. A dose-response analysis showed that there was a non-linear relationship between n-3 PUFA rich seafood intake and the risk of gout in the female group. Given this cross-section design, more well-designed prospective studies are warranted to validate the causal relationship between n-3 PUFA poor/rich seafood and gout.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
GZ and JW: conceptualization. GZ and DY: methodology. GZ, DY, and JW: software. GZ, LY, and JW: formal analysis. GZ, DY, LY, HS, JL, YW, and ZJ: data collection. GZ: writing—original draft preparation. JW: writing—review and editing and supervision. All authors read and agreed to the published version of the manuscript.
We thank the NHANES participants and staff for their contributions. We also thank Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database. His outstanding work, nhanesR package, and webpage, makes it easier for us to explore NHANES database.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. (2020) 16:380–90. doi: 10.1038/s41584-020-0441-1
2. Smith E, Hoy D, Cross M, Merriman T, Vos T, Buchbinder R, et al. The global burden of gout: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1470–6. doi: 10.1136/annrheumdis-2013-204647
3. Wan X, Gao X, Bi J, Tian F, Wang X. Use of N-3 PUFAs can decrease the mortality in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. Lipids Health Dis. (2015) 14:23. doi: 10.1186/s12944-015-0022-5
4. Mocellin M, Camargo C, Nunes E, Fiates G, Trindade EA. Systematic review and meta-analysis of the N-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr. (2016) 35:359–69. doi: 10.1016/j.clnu.2015.04.013
5. Lin N, Shi J, Li Y, Zhang X, Chen Y, Calder P, et al. What is the impact of N-3 PUFAs on inflammation markers in type 2 diabetic mellitus populations?: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. (2016) 15:133. doi: 10.1186/s12944-016-0303-7
6. Saunders E, Ramsden C, Sherazy M, Gelenberg A, Davis J, Rapoport S. Omega-3 and omega-6 polyunsaturated fatty acids in bipolar disorder: a review of biomarker and treatment studies. J Clin Psychiatry. (2016) 77:e1301–8. doi: 10.4088/JCP.15r09925
7. Gao X, Su X, Han X, Wen H, Cheng C, Zhang S, et al. Unsaturated fatty acids in mental disorders: an umbrella review of meta-analyses. Adv Nutr. (2022) 13:2217–36. doi: 10.1093/advances/nmac084
8. Yuan J, Wen X, Jia M. Efficacy of Omega-3 polyunsaturated fatty acids on hormones, oxidative stress, and inflammatory parameters among polycystic ovary syndrome: a systematic review and meta-analysis. Ann Palliat Med. (2021) 10:8991–9001. doi: 10.21037/apm-21-2018
9. Zheng S, Qiu M, Wu J, Pan X, Liu X, Sun L, et al. Long-chain Omega-3 polyunsaturated fatty acids and the risk of heart failure. Ther Adv Chronic Dis. (2022) 13:20406223221081616. doi: 10.1177/20406223221081616
10. Musazadeh V, Kavyani Z, Naghshbandi B, Dehghan P, Vajdi M. The beneficial effects of Omega-3 polyunsaturated fatty acids on controlling blood pressure: an umbrella meta-analysis. Front Nutr. (2022) 9:985451. doi: 10.3389/fnut.2022.985451
11. Goldberg R, Katz JA. Meta-analysis of the analgesic effects of Omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. (2007) 129:210–23. doi: 10.1016/j.pain.2007.01.020
12. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. (2006) 440:237–41. doi: 10.1038/nature04516
13. Novak T, Babcock T, Jho D, Helton W, Espat N. NF-Kappa B inhibition by Omega -3 fatty acids modulates Lps-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. (2003) 284:L84–9. doi: 10.1152/ajplung.00077.2002
14. Zhao Y, Joshi-Barve S, Barve S, Chen L. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappab activation. J Am Coll Nutr. (2004) 23:71–8. doi: 10.1080/07315724.2004.10719345
15. Weatherill A, Lee J, Zhao L, Lemay D, Youn H, Hwang D. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. (2005) 174:5390–7. doi: 10.4049/jimmunol.174.9.5390
16. Abhishek A, Valdes A, Doherty M. Low Omega-3 fatty acid levels associate with frequent gout attacks: a case control study. Ann Rheum Dis. (2016) 75:784–5. doi: 10.1136/annrheumdis-2015-208767
17. Choi H, Atkinson K, Karlson E, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. (2004) 350:1093–103. doi: 10.1056/NEJMoa035700
18. Villegas R, Xiang Y, Elasy T, Xu W, Cai H, Cai Q, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the shanghai men’s health study. Nutr Metab Cardiovasc Dis. (2012) 22:409–16. doi: 10.1016/j.numecd.2010.07.012
19. National Center for Health Statistics. Plan and operation of the third national health and nutrition examination survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. (1994) 32:1–407.
20. Nie J, Deng M, Wang K, Liu F, Xu H, Feng Q, et al. Higher HEI-2015 scores are associated with lower risk of gout and hyperuricemia: results from the national health and nutrition examination survey 2007-2016. Front Nutr. (2022) 9:921550. doi: 10.3389/fnut.2022.921550
21. Tsai J, Homa D, Gentzke A, Mahoney M, Sharapova S, Sosnoff C, et al. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep. (2018) 67:1342–6. doi: 10.15585/mmwr.mm6748a3
22. Scholes S, Bann D. Education-related disparities in reported physical activity during leisure-time, active transportation, and work among us adults: repeated cross-sectional analysis from the national health and nutrition examination surveys, 2007 to 2016. BMC Public Health. (2018) 18:926. doi: 10.1186/s12889-018-5857-z
23. Liu Y. The relationship between lifestyle and self-reported oral health among American adults. Int Dent J. (2014) 64:46–51. doi: 10.1111/idj.12061
24. Lu N, Dubreuil M, Zhang Y, Neogi T, Rai S, Ascherio A, et al. Gout and the risk of Alzheimer’s disease: a population-based, BMI-matched cohort study. Ann Rheum Dis. (2016) 75:547–51. doi: 10.1136/annrheumdis-2014-206917
25. Ssy A, Natto Z, Midle J, Gyurko R, O’Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the national health and nutrition examination surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. doi: 10.1002/jper.18-0183
26. Hicks C, Wang D, Matsushita K, Windham B, Selvin E. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. Adults: a prospective cohort study. Ann Intern Med. (2021) 174:167–74. doi: 10.7326/m20-1340
27. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100:S1–276. doi: 10.1016/j.kint.2021.05.021
28. Elliott W. Systemic hypertension. Curr Probl Cardiol. (2007) 32:201–59. doi: 10.1016/j.cpcardiol.2007.01.002
29. Qin Z, Zhao J, Li J, Yang Q, Geng J, Liao R, et al. Low lean mass is associated with lower urinary tract symptoms in us men from the 2005-2006 national health and nutrition examination survey dataset. Aging. (2021) 13:21421–34. doi: 10.18632/aging.203480
30. American Diabetes Association [ADA]. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33(Suppl. 1):S62–9. doi: 10.2337/dc10-S062
31. Kavyani Z, Musazadeh V, Fathi S, Hossein Faghfouri A, Dehghan P, Sarmadi B. Efficacy of the Omega-3 fatty acids supplementation on inflammatory biomarkers: an umbrella meta-analysis. Int Immunopharmacol. (2022) 111:109104. doi: 10.1016/j.intimp.2022.109104
32. Bäck M, Hansson G. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. (2019) 33:1536–9. doi: 10.1096/fj.201802445R
33. Hou T, McMurray D, Chapkin R. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol. (2016) 785:2–9. doi: 10.1016/j.ejphar.2015.03.091
34. Kim M, Qie Y, Park J, Kim C. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. (2016) 20:202–14. doi: 10.1016/j.chom.2016.07.001
35. Balfegó M, Canivell S, Hanzu F, Sala-Vila A, Martínez-Medina M, Murillo S, et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. (2016) 15:78. doi: 10.1186/s12944-016-0245-0
36. Andersen A, Mølbak L, Michaelsen K, Lauritzen L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J Pediatr Gastroenterol Nutr. (2011) 53:303–9. doi: 10.1097/MPG.0b013e31821d298f
37. Watson H, Mitra S, Croden F, Taylor M, Wood H, Perry S, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. (2018) 67:1974–83. doi: 10.1136/gutjnl-2017-314968
38. Yaqoob P. The nutritional significance of lipid rafts. Annu Rev Nutr. (2009) 29:257–82. doi: 10.1146/annurev-nutr-080508-141205
39. Calder P. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. (2011) 668(Suppl. 1):S50–8. doi: 10.1016/j.ejphar.2011.05.085
40. Oh D, Talukdar S, Bae E, Imamura T, Morinaga H, Fan W, et al. Gpr120 is an Omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. (2010) 142:687–98. doi: 10.1016/j.cell.2010.07.041
41. Kong W, Yen J, Vassiliou E, Adhikary S, Toscano M, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the Il-12 cytokine family. Lipids Health Dis. (2010) 9:12. doi: 10.1186/1476-511x-9-12
42. Im D. Functions of Omega-3 fatty acids and FFA4 (Gpr120) in macrophages. Eur J Pharmacol. (2016) 785:36–43. doi: 10.1016/j.ejphar.2015.03.094
Keywords: n-3 PUFA poor/rich seafood consumption, gout, inflammation, National Health and Nutrition Examination Survey, nutrition
Citation: Zeng G, You D, Ye L, Wu Y, Shi H, Lin J, Jiang Z and Wei J (2023) n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007–2016. Front. Nutr. 10:1075877. doi: 10.3389/fnut.2023.1075877
Received: 21 October 2022; Accepted: 21 March 2023;
Published: 04 April 2023.
Edited by:
Miroslava Rossenova Atanassova, Møreforsking AS, NorwayReviewed by:
Marija Takic, Institute for Medical Research, University of Belgrade, SerbiaCopyright © 2023 Zeng, You, Ye, Wu, Shi, Lin, Jiang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junping Wei, d2VpanVucGluZ0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.