
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 March 2023
Sec. Clinical Nutrition
Volume 10 - 2023 | https://doi.org/10.3389/fnut.2023.1032048
Background: Although the association of zinc (Zn) with cardiovascular disease (CVD) has been studied, no consensus has been reached on this relationship, particularly dietary Zn intake. The purpose of this study was to assess the effect of dietary Zn intake on the risk of CVD and to analyze whether this effect varied according to zinc consumption using representative data from China.
Methods: 11,470 adults from the China Health and Nutrition Survey (CHNS) were eventually enrolled. The dietary information was collected by the 3 day 24-h dietary recalls combined with dietary weighting method. CVD was defined as participants with self-reported physician-diagnosed apoplexy and/or myocardial infarction during the follow-up. Cox regression was used to calculate the hazard ratios (HRs) of CVD with 95% confidence intervals. Restricted cubic spline function plus Cox regression was used to visualize the influence trend of dietary Zn intake on new-onset CVD and to test whether this trend is linear. 2-segment Cox regression was established to address the nonlinear trend.
Results: 431 participants developed CVD, including 262 strokes and 197 myocardial infarctions. Compared with the lowest quintile (Q1), the adjusted hazard ratios and 95% confidence interval (CI) of CVD in Q2 to Q5 of dietary Zn intake were 0.72 (0.54, 0.97), 0.59 (0.42, 0.81), 0.50 (0.34, 0.72) and 0.44 (0.27, 0.71), respectively. The influence trend of dietary Zn intake on new-onset CVD was nonlinear and L-shaped. When dietary Zn intake <13.66 mg/day, increased dietary Zn intake was significantly associated with decreased risk of developing CVD (HR = 0.87, 95% CI: 0.82–0.92, p-value <0.0001).
Conclusion: An L-shaped trend was observed between dietary Zn intake and the risk of developing CVD, indicating that dietary Zn intake should be improved moderately, but not excessively, for the benefit of cardiovascular disease.
Cardiovascular disease (CVD) refers to the assembly of diseases caused by the heart and blood vessels. Common CVD includes ischemic heart disease (myocardial infarction), ischemic stroke (apoplexy), arteriovenous disease and other conditions. Epidemiological evidence suggested that prevalent cases of CVD was 523 million, including 330 million in China (1, 2). According to the World Health Organization, 17.9 million people die of CVD each year, accounting for an estimated 32% of the total global deaths. 85% of cardiovascular deaths are due to heart attacks and strokes, and more than three quarters of cardiovascular deaths occur in developing countries. CVD remains the single largest cause of mortality worldwide and the leading cause of reduced quality of life (3). China has the highest cardiovascular mortality rate, and the disease burden due to CVD is increasingly severe and even higher than the global average (4). In addition, CVD has a strong genetic basis and is influenced by environmental factors. To date, the easiest way to prevent or reduce the occurrence of CVD is through changes in lifestyle factors, especially diet and exercise.
As an important cofactor of more than 2,000 transcription factors and more than 300 enzymes, zinc (Zn) is essential for regulating cell metabolism, proliferation and differentiation, as well as maintaining normal physiological functions of human body (5). Although foods such as lean meat, seafood and nuts are rich in zinc, about 17% of the world’s population is at risk of insufficient Zn intake (6, 7). Zn insufficient can lead to CVD, hypertension, diabetes, Alzheimer’s disease, and other chronic diseases (8–10). In recent decades, the role of Zn in CVD has become a research hotspot. Nevertheless, researchers have not come to an agreement on the effect of Zn, especially dietary Zn, on CVD (11). In addition, it is unclear whether different doses of dietary Zn intake have different effects on CVD.
As the most populous country in the world, it is particularly important to study the impact of Zn consumption on risk of CVD in China. However, to our knowledge, there is no study to analyze the relationship between dietary Zn intake and the incidence of CVD using large sample data in China. In order to figure out the relationship between dietary Zn intake and new-onset CVD in Chinese adults and analyze whether this relationship is affected by Zn intake, we conducted this study.
The data for this study came from the China Health and Nutrition Survey (CHNS), a longitudinal follow-up survey based on community population. The survey included socio-economic status, health services, nutritional and dietary status. Please refer to relevant literature or official website1 for specific survey population and sampling methods (12). Since CHNS has provided more accurate dietary data since 2004 and can be obtained on the official website of CHNS, the subjects in this study were adults over 18 years old who participated in the survey from 2004 to 2015. Participants who have received at least two rounds of research surveys, and have complete sociodemographic indicators, socioeconomic indicators, lifestyle, anthropometric indicators, physical health status indicators and three consecutive 24-h diet recall data were considered valid subjects, and the first survey round was considered as baseline. The exclusion criteria include: (1) Age < 18, (2) Pregnant or lactating women, (3) Participants with CVD at baseline, (4) Exceeding the energy intake limit (Male: > 6,000 kcal or < 800 kcal, Female: > 4,000 or < 600 kcal), systolic blood pressure (SBP) < 40 mmHg or > 300 mmHg, diastolic blood pressure (DBP) < 30 mmHg or > 200 mmHg, body mass index (BMI) < 14 kg/ m2 or > 45 kg/ m2 or other unreliable observations. Finally, 11,470 participants were included in the final analysis (Figure 1). In addition, the number of people with complete data was 13,946 at baseline, so, the response rate was 11,470/13946 = 82.25%.
Three consecutive 24-h dietary recalls were used to assess dietary intake of participating individuals and household food consumption over the same 3-day period was calculated by subtracting the ending inventory from the starting inventory, combined weighing and measuring methods (the consumption of edible oil and condiments is obtained by the household food consumption). The accuracy of dietary data can be ensured by comparing each individual’s average daily dietary intake, calculated from household surveys with her dietary intake based on 24-h recall data. Food consumption data were converted into the nutrient intake and total energy using Chinese Food Composition Tables (FCTs). The cumulative average dietary intake from baseline to the latest year before the year of the first cardiovascular event or the end of follow-up was used for analysis, because it can better reflect long-term dietary intake and minimize within-person variation.
The outcome variable was whether CVD occurred during the follow-up period. The on-site investigators asked the participants whether they had myocardial infarction or stroke through interviews: “Has a doctor from a public hospital at or above the county level ever given you a diagnosis of myocardial infarction (no, yes, unknown) Or “Has a doctor from a public hospital at or above the county level ever given you a diagnosis of stroke (no, yes, unknown). New-onset CVD was defined as a self-reported physician diagnosis of myocardial infarction or stroke during follow-up and a certificate of stroke and/or myocardial infarction diagnosis should be provided. For participants who provided inconsistent answers during the follow-up, the first recorded stroke event was adopted to limit recall bias. The follow-up time was the date of the first discovery of CVD or the end of follow-up (2015) minus the date of first entering into the cohort.
The demographic, socioeconomic and lifestyle information of the participants was obtained through face-to-face interviews conducted by trained field investigators using well-designed questionnaires, including age, sex, race, marital status, residence, marital status, education level, occupational activity level, smoking status, alcohol intake. Weight and height were obtained by measuring to an accuracy of 0.1 cm in height and 0.1 kg in weight, after removing shoes and heavier clothing. Body mass index (BMI) was calculated by dividing body weight by the square of height (kg/m2). In a quiet environment, the participants sat quietly for more than 5 minutes. Then, after removing their heavy clothing, their blood pressure was measured three times, 10 min apart, by a trained health nurse using a calibrated mercury sphygmomanometer according to standard procedures, and the average of the three blood pressure measurements was used for the final analysis. Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or taking antihypertensive medication according to the guidelines for preventing and treating hypertension in China (2010), or providing a self-report of diagnosis by a physician in public hospitals above the county level. Diabetes was defined based on self-report of diagnosis by a physician in public hospitals above the county level, or using oral medicine, injection of insulin to control blood glucose.
SAS version 9.2 (SAS Institute, Cary, North Carolina, United States) and R version 4.0.5 were used for statistical analysis. Participants were categorized into quintile groups (< 7.87 mg/day, 7.87–9.63 mg/day, 9.63–11.38 mg/day, 11.38–13.56 mg/day, ≥ 13.56 mg/day) according to their Zn intake levels. Continuous variables that did not meet the normal distribution were represented by the median and interquartile range, while categorical values were expressed as numbers (percentage). Differences between groups were evaluated by using Kruskal–Wallis H test, and the chi-square test for the categorical variables.
Four Cox proportional hazards regression models were used to calculate the risk of CVD incidence with 95% confidence intervals. Model 1 was non-adjusted; Model 2 was adjusted for age, gender, race and energy intake. Model 3 was additionally adjusted for residence, marital status, education and activity level, smoking and drinking status, body mass index, hypertension and diabetes based on model 2; Model 4 was further adjusted for d intake of dietary fiber, niacin, vitamin C, vitamin E, calcium, iron, selenium, magnesium, copper, manganese based on model 3.
Restricted cubic spline (RCS) functions, with 4 knots, combined with Cox proportional hazards models were used to visualize the relationship between dietary Zn intake and the risk of developing CVD, after adjusting the variables of model 4. When non-linearity is detected, that the inflection point is calculated by the recursion algorithm, and a two-segment Cox proportional risk model was performed on both sides of the inflection point. Previous studies have observed associations between nutritional intake and health outcomes influenced by age, sex, BMI, smoking status, alcohol consumption, hypertension, and diabetes. Therefore, stratified analyses assessed whether these factors altered the association (13, 14). The test level was α = 0.05, two-tailed.
There was no collinearity between independent variables (Supplementary Table 1). A total of 11,470 adults subjects were eventually enrolled in this study, which consisted of 5,349 males and 6,121 females. Among the entire participants, the average Zn intake was 10.90 (SD, 3.91) mg/day, 40.44% had Zn intake lower than recommended nutrient intake (RNI, 12.50 mg/day for males and 7.50 mg/day for females aged 18 years and above), and 22.71% had Zn intake lower than estimated average requirement (EAR, 10.40 mg/day for males and 6.1 mg/day for females aged 18 years and above) (15). More details of Zn intake were shown in Table 1.
The baseline characteristics of the study population according to the intake of Zn were shown in Table 2. Participants with higher intake of dietary Zn were more likely to be younger, male, never married, smokers, and drinkers, had higher education levels, had a higher intake of energy, dietary fiber, niacin, vitamin C, vitamin E, calcium, iron, selenium, magnesium, copper, manganese, and less likely to be Han race, divorced, separated or widowed, light physically active, had lower education levels, had hypertension and diabetes. The result of comparing the indicators between non-CVD and new-onset CVD were shown in Supplementary Table 2.
431 participants developed new-onset CVD after 77,470 person-years of follow-up (mean follow-up time was 6.75 years) including 262 apoplexies and 197 myocardial infarctions. Table 3 showed that Zn intake had a negative impact on the incidence risk of CVD in the four Cox proportional hazards models. After controlling for age, gender, race, residence, marital status, education level, activity level, smoking status, drinking status, hypertension status, diabetes status, BMI, and the intake of energy, dietary fiber, niacin, vitamin C, vitamin E, calcium, iron, selenium, magnesium, copper, manganese, we found that the adjusted hazard ratios (HRs) and 95% confidence interval (CI) of CVD in Q2 to Q5 of the Zn intake were 0.72(0.54, 0.97), 0.59 (0.42, 0.81), 0.50 (0.34, 0.72) and 0.44 (0.27, 0.71), respectively, compared with Q1, and p-value for the trend was 0.0026. Similar results were obtained for apoplexy but not myocardial infarction. Furthermore, to avoid the influence of food sources on the relationship between single nutrition and disease, we also estimated the associations between Zn intake from animal and Zn intake from all other sources. Both Zn intake, Zn intake from meat and Zn intake from other sources were all associated with a reduced risk of CVD, as shown in Supplementary Table 3.
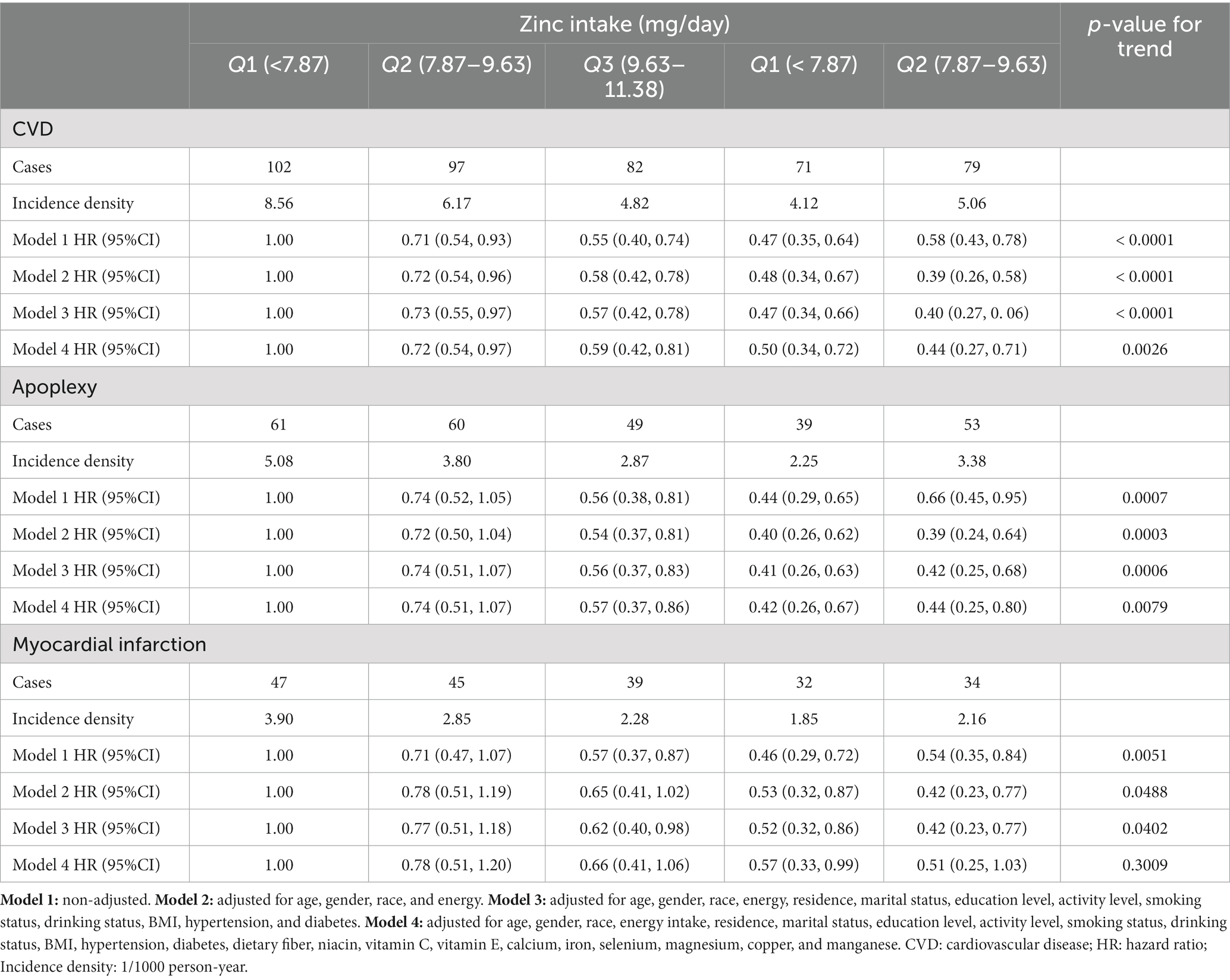
Table 3. HR (95% CI) for CVD, apoplexy, and myocardial infarction according to quintiles of dietary zinc intake.
Several sensitivity analyses were performed to assess the robustness of the relationship. Firstly, the results did not change substantially after further adjustments for the intake of cereals, potatoes, vegetables, fruits, nuts, meat, poultry, fish and shrimp, milk and eggs (Supplementary Table 4). Secondly, based on RNI, Zn intake was divided into two groups. The risk of CVD was reduced in the group with Zn intake ≥ RNI, which supported the above results (Supplementary Table 5). Thirdly, based on EAR, Zn intake was divided into two groups. The risk of CVD was reduced in the group with Zn intake ≥ EAR (Supplementary Table 6). Fourthly, to avoid the effects of dietary habits change, individuals with prior diabetes were excluded, and the results were consistent in the primary analysis (Supplementary Table 7). Fifthly, after imputation for missing values by multiple imputation, the associations remained consistent with previous results (Supplementary Table 8).
Fully adjusted Cox proportional hazards regression model combined with restricted cubic spline function, with 4 knots was conducted to clarify the relationship between the intake of dietary Zn, animal-derived-Zn, Zn from other sources and the risk of new-onset CVDs, and an L-shaped (p-value for nonlinearity was 0.0217, 0.0038, 0.0158, respectively) trend were found (Figure 2). A Cox proportional hazards model and a two-stage Cox proportional hazards model were used to evaluate the relationship between dietary Zn intake and the risk of new-onset CVD, respectively (Table 4). According to the results of the log likelihood ratio, it suggested that the Cox proportional risk model with 2-segment was more suitable to fit this relationship (p-value = 0.0150). Furthermore, we analyzed a threshold effect of Zn intake on the risk of new-onset CVD (Table 4). The inflection point of this L-shaped curve for dietary Zn intake was 13.66 mg/day. When dietary Zn intake <13.66 mg/day, the risk of developing CVD was significantly lower with the increment of Zn intake (HR = 0.87, 95% CI: 0.82–0.92, p-value <0.0001). But there was no significant association between Zn intake on the risk of new-onset CVD when dietary Zn intake ≥13.66 mg/day (HR = 0.96, 95% CI: 0.90–1.03, p-value = 0.2763).
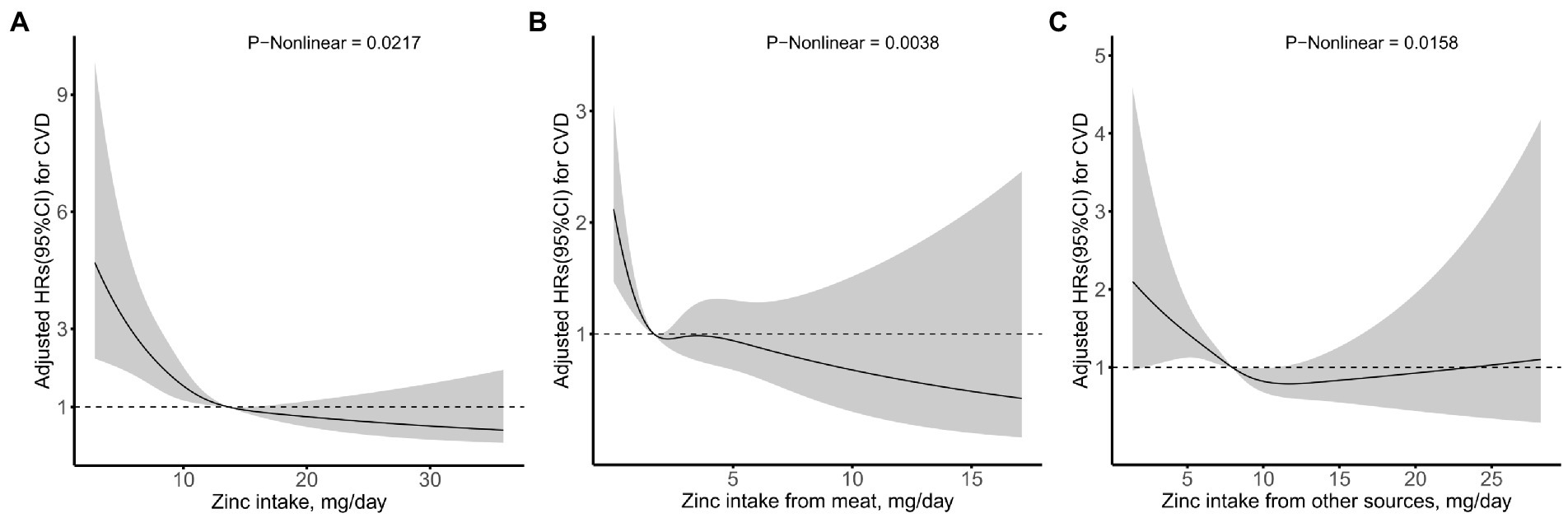
Figure 2. Relation of zinc intake from (A) total, (B) meat, and (C) other sources with risk of new-onset CVD*. *Adjusted for age, gender, race, energy intake, residence, marital status, education level, activity level, smoking status, drinking status, BMI, hypertension, diabetes, dietary fiber, niacin, vitamin C, vitamin E, calcium, iron, selenium, magnesium, copper, and manganese.
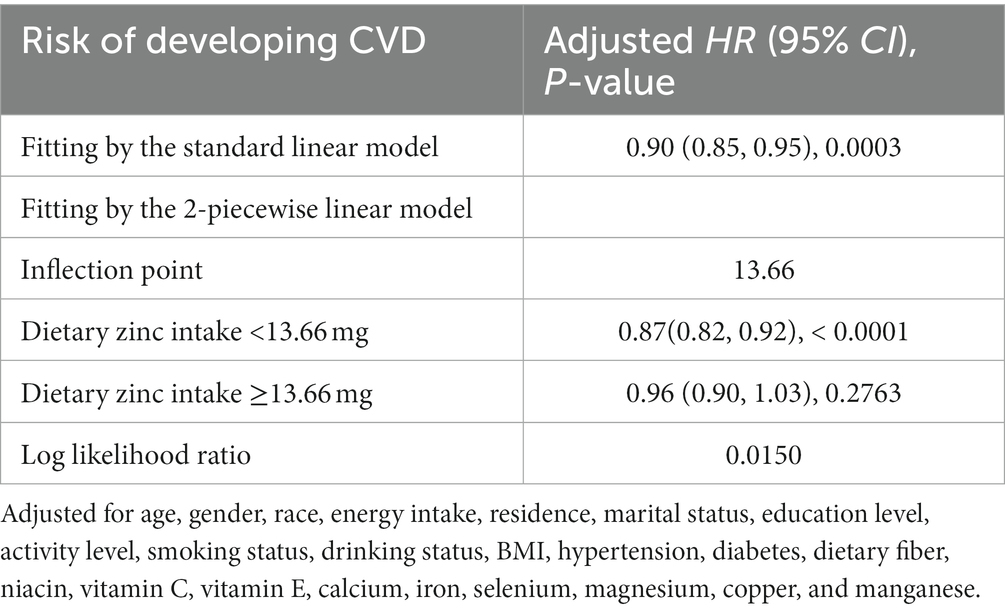
Table 4. Threshold effect analyses of dietary zinc intake on the risk of new-onset CVD using 2-piecewise regression models.
We further performed a tentative analysis to assess whether there is any other potential factor that might affect the L-shaped relationship between the intake of the Zn and the newly diagnosed cardiovascular disease, as shown in Figure 3. The influence trends of dietary Zn intake on CVD remained similar in most subgroups by age, gender, BMI, cigarette smoking, alcohol drinking, and hypertension status. p-value for interaction for hypertension is 0.0331, suggesting that there is an interaction between Zn and hypertension. It is worth noting that the small number of diabetes cases may lead to non-clinical significance of the results in the diabetes subgroup.
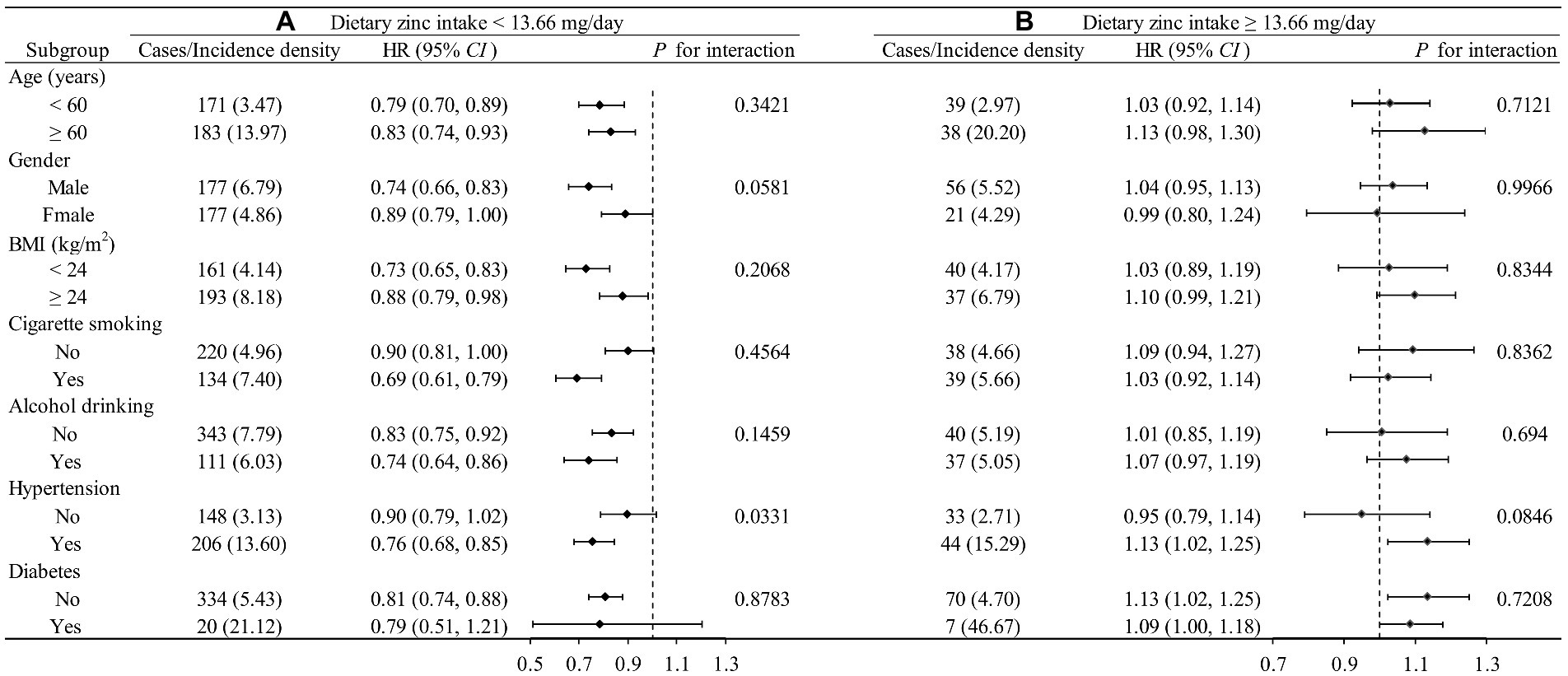
Figure 3. Stratified analysis by potential effect modifiers for the association between dietary zinc intake and new-onset CVD in various subgroups divided by 13.662 mg/day. (A) Dietary zinc intake <13.662 mg/day. (B) Dietary zinc intake ≥3.662 mg/day. Incident rate is presented as per 1,000 person-years of follow-up. Adjusted, if not stratified, age, gender, race, energy intake, residence, marital status, education level, activity level, smoking status, drinking status, BMI, hypertension, diabetes, dietary fiber, niacin, vitamin C, vitamin E, calcium, iron, selenium, magnesium, copper, and manganese; Incidence density: 1/1000 person-year.
Our study is the first relatively large-scale, retrospective cohort study to explore the effect of dietary Zn on new-onset CVD among Chinese adults. A nonlinear, L-shaped trend between dietary Zn and CVD, with an inflection point at about 13.66 mg/day was discovered. Higher dietary Zn intake, Zn intake from meat and Zn intake from other sources were all significantly associated with lower cardiovascular incidence, within a certain range. Both the sensitivity and stratified analyses illustrated that this relationship remained robust.
To our knowledge, the association between Zn and CVD in humans was first studied in 1988 (16). In this study, however, no association was found between regular use of Zn supplements and risk of developing CVD among people aged 65 years and older. Subsequent studies on dietary Zn intake and CVD have shown conflicting results.
Most of the previous studies have indicated that Zn plays a protective role in CVD. Dietary Zn has been found to be a protective nutrient against coronary artery disease in both Indians and Australians (17–19). According to the NHANES, after adjusting for covariates including demographics, comorbidities, renal function, and serum phosphorus levels, an increase in dietary Zn intake was independently associated with a lower probability of severe abdominal aortic calcification (AAC) (an increase of 1 mg daily dietary zinc intake was associated with an 8% lower probability of severe AAC) (20). Multiracial cohort studies of atherosclerosis supported this conclusion (7). The study found that a high-Zn diet was significantly associated with a lower risk of coronary artery calcification (CAC) progression in both men (HR = 0.697, 95%CI: 0.553–0.878; p-value = 0.002) and women (HR = 0.675, 95% CI: 0.496–0.919, p-value = 0.012, both groups were compared with the extreme group). In addition, the dietary intake of Zn increased by 1 mg, carotid artery intima-media thickness (cIMT) decreased by 3.36 μm (13). The risk of atherosclerosis, a fundamental process in CVD, has also been demonstrated to decrease with the increasing of Zn intake (21). In chronic kidney disease (CKD) patients, the beneficial effect of Zn intake or supplementation on cardiovascular disease risk factors remained significant (22). The dietary antioxidant index based on dietary Zn and other antioxidant nutrients was associated with decreased odds ratio of CVD (23). Furthermore, some studies demonstrated that higher dietary Zn intake is beneficial to reduce cardiovascular mortality (14, 24). In Iowa women, cardiovascular mortality decreased as dietary Zn intake increased (14). Compared with the lowest quintile Zn intake, the 2nd, 3rd, 4th, and 5th quintile Zn intake groups had a 39, 41, 43 and 63% lower risk of CVD death, respectively (p-value for trend = 0.07). When the effect of Zn supplementation on cardiovascular mortality was further investigated, no significant association was found. One possible explanation is that micronutrient supplementation may have a different effect than the same micronutrient in food. Some experts believe that Zn plays an important role in the maintaining cardiovascular health, and impaired zinc balance adversely affects cardiovascular dysfunction (25, 26).
Nevertheless, some literature supporting that the adverse effects of high Zn on CVD. For example, Milton et al. (27) concluded that in women, high dietary Zn intake may lead to higher CVD incidence rate, and Marcia et al. (28) found that Zn intake from red meat, but not from other sources, was associated with a higher risk of cardiovascular disease. Differences in study population, study outcomes, dietary Zn intake levels and sample size may contribute to the inconsistent conclusions. However, no significant association between Zn and CVD mortality was found in adults from Jiangsu, China (29). Compared with the first quartile of dietary Zn intake (mean: 10.4 mg/day), the adjusted HRs (95% CI) of CVD mortality in quartile 2 (mean: 11.7 mg/day), quartile 3 (mean: 12.3 mg/day), and quartile 4 (mean: 13.7 mg/day) were 0.97 (0.39, 2.42), 1.96 (0.91, 4.24), and 1.29 (0.51, 3.27), respectively. One possible explanation for the inconsistency with our results is that the average intake of Zn was higher than that we reported in our study, besides, different outcomes may play a role.
Our study provided a new idea for assessing the dose–response relationship between dietary Zn intake and the risk of developing CVD in the general population. The effect of dietary zinc intake on cardiovascular disease was observed in an L-shaped trend. The beneficial effect of increasing dietary Zn intake on CVD appears to peak in people with adequate Zn intake levels. That is, the risk of CVD decreased with increased dietary Zn consumption in those with a dietary Zn intake of <13.66 mg/day. Although the underlying mechanism of the inverse association between Zn intake and CVD remains to be studied. Based on the available evidence, our findings are biologically plausible. Firstly, high Zn levels decrease the expression of inflammatory factors. Zn supplementation can improve phosphate induced bone/cartilage transdifferentiation and vascular calcification of vascular smooth muscle cells (VSMCs) by inhibiting NF KB pathway (30). After 6 months of Zn supplementation in the intervention group, compared with placebo, the plasma Zn concentration in the intervention group was significantly increased, while the concentration of inflammatory related factors such as high-sensitivity C-reactive protein (hsCRP) was significantly decreased, reported by a clinical randomized placebo-controlled trial (31). The team further conducted cell experiment and the results supported the conclusions of the above tests (31). Secondly, Zn is a cofactor of copper Zn superoxide dismutase and participates in the regulation of multiple antioxidant enzymes. Impaired superoxide dismutase function causes oxidative stress (32). Thirdly, NO has the functions of inhibiting platelet aggregation, inhibiting smooth muscle cell (SMC) proliferation and inhibiting endothelial cell apoptosis. Zn can affect the pathogenesis of CVD by regulating the production and release of NO (33). Fourthly, Zn is key to the proper removal of reactive oxygen species and nitrogen (34). Therefore, Zn deficiency will increase the production of reactive oxygen species, promote apoptosis of endothelial cells and vascular smooth muscle cells, activate proinflammatory cytokines, and amplifie the oxidation of low-density lipoproteins, thereby exacerbating oxidative stress and ultimately leading to CVDs (35–37). Fifthly, in vitro experiments, endothelial cells cultured in Zn-deficient medium lead to decreased integrity and increased permeability of endothelial cells, and Zn deficiency also affects the severity of apoptosis. In turn, Zn-rich media improved their structure (38, 39). Sixthly, Proteomic studies have shown that the phenotype of VSMCS in large arteries may be altered in animal models of Zn deficiency, which may lead to vascular diseases (40). Studies of mice fed a Zn-deficient diet found that inadequate Zn intake increased lipoprotein concentrations, increased remodeling of VSMCS, increased inflammation, and induced atherosclerotic plaque formation (34, 41).
Whereas, the beneficial effect of Zn intake on CVD became statistically insignificant, when dietary Zn intake ≥13.66 mg/day. Ting Yin et al. also found that there was an L-shaped association between Zn and the prevalence of CVD risk in American adults, but the inflection point of Zn was 6.61 mg/day, while our study showed that the inflection point of Zn was 13.66, which was closer to the high recommended intake of zinc (12.5 mg) (42). One possible explanation for the different inflection points of dietary Zn was the difference between the two studies (cross-sectional study vs. cohort study). Another possible explanation could be attributed in part to differences in the sources of zinc between the two studies. Ting Yin et al. studied a Western population with an animal-based diet, while we studied an Asian population with a plant-based diet. The animal source of Zn had higher bioavailability than plant source because of the possible presence of phytate, which inhibits Zn absorption in the intestine (43). In addition, higher intake of meat including processed meat, unprocessed red meat, or poultry was significantly associated with increased risk of incident CVD (44). Therefore, it seems reasonable that the inflection point of the relationship between zinc and CVD in the Western population with a diet dominated by animals is lower than that in the Chinese population with high phytic acid content of corn, cereals, rice, legumes and other foods as the staple food (45).
Laura M Pompano and Erick Boy reviewed 27 articles and concluded that compared with high-dose and short-term Zn supplementation, low-dose and long-term Zn supplementation improved more cardiovascular risk factors (46). One plausible explanation is that the body’s absorption of Zn is limited. When the intake of Zn exceeds the body’s absorption capacity, the protective effect of Zn on CVD will not be further enhanced even if the intake of Zn is increased. Besides, although Zn has antioxidant and anti-inflammatory effects, too high plasma Zn concentration can have the opposite effect, such as inhibiting lymphocyte function and causing abnormal expression of proinflammatory cytokines (47). Excessive intake of Zn leads to elevated systemic blood pressure through oxidative stress, and it has been reported that excessive clearance of ROS or RNS and their derivatives by antioxidant supplementation may disrupt cellular signaling pathways and may increase the risk of chronic disease (48, 49).
Several limitations need to be acknowledged. Firstly, although the dietary assessment was a 24-h dietary recall and was conducted in every survey wave (usually 2 years), which only reflects the short-term dietary situation, and the changes in nutritional intake during different seasons of the year may be overlooked. The accuracy of 24-h dietary recall method for assessing nutrition intake has been verified and the average intake over 3 days can offer a relatively valid estimate of usual diet, as has been shown in earlier research using the CHNS (50–52). In addition, the method of cumulative dietary nutrient intake was adopted to minimize dietary measurement errors and minimize within-individual variation. Therefore, it may not cause fatal to the significance of the study. Secondly, there is a lack of information on Zn supplementation in this survey. Whereas the proportion of Chinese individuals using dietary supplements was quite low, therefore, we speculated that our results may not change substantially due to the use of dietary supplements. Thirdly, apoplexy and myocardial infarction cases in this database are based on self-reports, and only these two diseases were used to define CVD in this study.
To sum up, this is the first research revealed an L-shaped trend between dietary Zn intake and the risk of developing CVD in Chinese adults. That is, increasing dietary Zn intake significantly reduced the risk of developing CVD in subjects with dietary Zn < 13.66 mg/day, and the beneficial effect of Zn on CVD became insignificant in the presence of dietary Zn ≥ 13.66 mg/day. These findings indicated that raising dietary zinc to a certain level can help reduce the risk of cerebrovascular diseases, especially stroke, which not only provides a theoretical basis for the prevention of cardiovascular diseases but also emphasizes the importance of adequate, but not excessive, dietary zinc intake.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cpc.unc.edu/projects/china.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of the National Institute of Nutrition and Food Safety of China (Beijing) and the University of North Carolina (Chapel Hill, NC, United States). The patients/participants provided their written informed consent to participate in this study.
HQ, YZ, and HZ contributed to the design and conduct of the research. HZ and SW carried out data analysis and the initial draft of the paper. XG and YZ conducted the data collection and advised on statistical analysis. All authors reviewed and edited the draft and approved the final version of the manuscript.
This research was funded by the Natural Science Foundation of Heilongjiang Province of China (ZD2022H006), Postdoctoral Science Foundation of Heilongjiang Province of China (LBH-Q21047), Research Innovation Team of Metabolic Disease Prevention and Treatment, the first affiliated hospital of Jiamusi University (202303), Scientific and Technological Innovation Team of Jiamusi University (cxtd202101), North Medicine and Functional Food Characteristic Subject Project in Heilongjiang Province (No. HLJTSXK-2022-03), and Gout Etiology and Functional Food Research Innovation Team.
The data of this study are from the China Health and Nutrition Survey (CHNS). We are grateful to all the agencies that funded the CHNS survey.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1032048/full#supplementary-material
1. Roth, GA, Mensah, GA, Johnson, CO, Addolorato, G, Ammirati, E, Baddour, LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. The Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Chinese Circulation Journal. (2022) 37:553–578. doi: 10.3969/j.issn.1000-3614.2022.06.001
3. Sacco, RL, Roth, GA, Reddy, KS, Arnett, DK, Bonita, R, Gaziano, TA, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and world heart federation. Circulation. (2016) 133:e674–90. doi: 10.1161/cir.0000000000000395
4. Yuan, W, Xin-xi, C, Ya-bing, H, Chen-jie, X, Zhi, C, Shu, L, et al. Study on Chinese and global cardiovascular diseases burden in 1990 and 2017. Chin J Prev Contr Chron Dis. (2020) 28:10–3+9. doi: 10.16386/j.cjpccd.issn.1004-6194.2020.01.003
5. Mammadova-Bach, E, and Braun, A. Zinc homeostasis in platelet-related diseases. Int J Mol Sci. (2019) 20:5258. doi: 10.3390/ijms20215258
6. Wessells, KR, and Brown, KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. (2012) 7:e50568. doi: 10.1371/journal.pone.0050568
7. Gao, JW, Zhang, SL, Hao, QY, Huang, FF, Liu, ZY, Zhang, HF, et al. Association of dietary zinc intake with coronary artery calcium progression: the multi-ethnic study of atherosclerosis (MESA). Eur J Nutr. (2021) 60:2759–67. doi: 10.1007/s00394-020-02452-5
8. Prasad, AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. (2013) 4:176–90. doi: 10.3945/an.112.003210
9. Gać, P, Czerwińska, K, Macek, P, Jaremków, A, Mazur, G, Pawlas, K, et al. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ Toxicol Pharmacol. (2021) 82:103553. doi: 10.1016/j.etap.2020.103553
10. Mendes Garrido Abregu, F, Caniffi, C, Arranz, CT, and Tomat, AL. Impact of zinc deficiency during prenatal and/or postnatal life on cardiovascular and metabolic diseases: experimental and clinical evidence. Adv Nutr. (2022) 13:833–45. doi: 10.1093/advances/nmac012
11. Chu, A, Foster, M, and Samman, S. Zinc status and risk of cardiovascular diseases and type 2 diabetes mellitus-a systematic review of prospective cohort studies. Nutrients. (2016) 8:707. doi: 10.3390/nu8110707
12. Chang, H, Tian, G, Liu, Q, Ren, X, Ji, B, and Jun, L. Introduction of CHNS official website and data collection methods. Chin J Evid Based Cardiovasc Med. (2018) 10:1043–7. doi: 10.3969/j.issn.1674-4055.2018.09.04
13. Maugeri, A, Hruskova, J, Jakubik, J, Kunzova, S, Sochor, O, Barchitta, M, et al. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: a cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. (2019) 131:274–81. doi: 10.1016/j.freeradbiomed.2018.12.018
14. Lee, DH, Folsom, AR, and Jacobs, DR Jr. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women's health study. Am J Clin Nutr. (2005) 81:787–91. doi: 10.1093/ajcn/81.4.787
15. Chinese Nutrition Society. Chinese DRIs handbook version of 2013. Beijing: Standards Press of China (2014).
16. Hale, WE, May, FE, Thomas, RG, Moore, MT, and Stewart, RB. Effect of zinc supplementation on the development of cardiovascular disease in the elderly. J Nutr Elder. (1989) 8:49–58. doi: 10.1300/J052v08n02_05
17. Singh, RB, Niaz, MA, Rastogi, SS, Bajaj, S, Gaoli, Z, and Shoumin, Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr. (1998) 17:564–70. doi: 10.1080/07315724.1998.10718804
18. Basnet, TB, Srijana, GC, Basnet, R, Neupane, B, and Thapa, G. Causal effects of dietary calcium, zinc and iron intakes on coronary artery disease in men: G-estimation and inverse probability of treatment weighting (IPTW) analyses. Clin Nutr ESPEN. (2021) 42:73–81. doi: 10.1016/j.clnesp.2020.12.030
19. Das, A, Cumming, RG, Naganathan, V, Blyth, F, Le Couteur, DG, Handelsman, DJ, et al. Dietary and supplemental antioxidant intake and risk of major adverse cardiovascular events in older men: the concord health and ageing in men project. Nutr Metab Cardiovasc Dis. (2021) 31:1102–12. doi: 10.1016/j.numecd.2020.11.032
20. Chen, W, Eisenberg, R, Mowrey, WB, Wylie-Rosett, J, Abramowitz, MK, Bushinsky, DA, et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. (2020) 35:1171–8. doi: 10.1093/ndt/gfz134
21. Yang, YJ, Choi, BY, Chun, BY, Kweon, SS, Lee, YH, Park, PS, et al. Dietary zinc intake is inversely related to subclinical atherosclerosis measured by carotid intima-media thickness. Br J Nutr. (2010) 104:1202–11. doi: 10.1017/s0007114510001893
22. Nakatani, S, Mori, K, Shoji, T, and Emoto, M. Association of Zinc Deficiency with development of CVD events in patients with CKD. Nutrients. (2021) 13:1680. doi: 10.3390/nu13051680
23. Zujko, ME, Waśkiewicz, A, Witkowska, AM, Cicha-Mikołajczyk, A, Zujko, K, and Drygas, W. Dietary Total antioxidant capacity-a new indicator of healthy diet quality in cardiovascular diseases: a polish cross-sectional study. Nutrients. (2022) 14:3219. doi: 10.3390/nu14153219
24. Eshak, ES, Iso, H, Yamagishi, K, Maruyama, K, Umesawa, M, and Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem. (2018) 56:126–32. doi: 10.1016/j.jnutbio.2018.02.008
25. Mohammadifard, N, Humphries, KH, Gotay, C, Mena-Sánchez, G, Salas-Salvadó, J, Esmaillzadeh, A, et al. Trace minerals intake: risks and benefits for cardiovascular health. Crit Rev Food Sci Nutr. (2019) 59:1334–46. doi: 10.1080/10408398.2017.1406332
26. Tamura, Y. The role of zinc homeostasis in the prevention of diabetes mellitus and cardiovascular diseases. J Atheroscler Thromb. (2021) 28:1109–22. doi: 10.5551/jat.RV17057
27. Milton, AH, Vashum, KP, McEvoy, M, Hussain, S, McElduff, P, Byles, J, et al. Prospective study of dietary zinc intake and risk of cardiovascular disease in women. Nutrients. (2018) 10:38. doi: 10.3390/nu10010038
28. de Oliveira Otto, MC, Alonso, A, Lee, DH, Delclos, GL, Bertoni, AG, Jiang, R, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. (2012) 142:526–33. doi: 10.3945/jn.111.149781
29. Shi, Z, Chu, A, Zhen, S, Taylor, AW, Dai, Y, Riley, M, et al. Association between dietary zinc intake and mortality among Chinese adults: findings from 10-year follow-up in the Jiangsu nutrition study. Eur J Nutr. (2018) 57:2839–46. doi: 10.1007/s00394-017-1551-7
30. Voelkl, J, Tuffaha, R, Luong, TTD, Zickler, D, Masyout, J, Feger, M, et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J Am Soc Nephrol. (2018) 29:1636–48. doi: 10.1681/asn.2017050492
31. Bao, B, Prasad, AS, Beck, FW, Fitzgerald, JT, Snell, D, Bao, GW, et al. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr. (2010) 91:1634–41. doi: 10.3945/ajcn.2009.28836
32. Mariani, E, Mangialasche, F, Feliziani, FT, Cecchetti, R, Malavolta, M, Bastiani, P, et al. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp Gerontol. (2008) 43:445–51. Epub 2007/12/15. doi: 10.1016/j.exger.2007.10.012
33. Zalewski, PD, Beltrame, JF, Wawer, AA, Abdo, AI, and Murgia, C. Roles for endothelial zinc homeostasis in vascular physiology and coronary artery disease. Crit Rev Food Sci Nutr. (2019) 59:3511–25. doi: 10.1080/10408398.2018.1495614
34. Choi, S, Liu, X, and Pan, Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin. (2018) 39:1120–32. doi: 10.1038/aps.2018.25
35. Valko, M, Leibfritz, D, Moncol, J, Cronin, MT, Mazur, M, and Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. Epub 2006/09/19. doi: 10.1016/j.biocel.2006.07.001
36. Cortese, MM, Suschek, CV, Wetzel, W, Kröncke, KD, and Kolb-Bachofen, V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. (2008) 44:2002–12. doi: 10.1016/j.freeradbiomed.2008.02.013
37. Li, HT, Jiao, M, Chen, J, and Liang, Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim Biophys Sin. (2010) 42:183–94. doi: 10.1093/abbs/gmq005
38. Hennig, B, Wang, Y, Ramasamy, S, and McClain, CJ. Zinc deficiency alters barrier function of cultured porcine endothelial cells. J Nutr. (1992) 122:1242–7. doi: 10.1093/jn/122.6.1242
39. Meerarani, P, Ramadass, P, Toborek, M, Bauer, HC, Bauer, H, and Hennig, B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor alpha. Am J Clin Nutr. (2000) 71:81–7. doi: 10.1093/ajcn/71.1.81
40. Beattie, JH, Gordon, MJ, Rucklidge, GJ, Reid, MD, Duncan, GJ, Horgan, GW, et al. Aorta protein networks in marginal and acute zinc deficiency. Proteomics. (2008) 8:2126–35. doi: 10.1002/pmic.200700784
41. Alcantara, EH, Shin, MY, Feldmann, J, Nixon, GF, Beattie, JH, and Kwun, IS. Long-term zinc deprivation accelerates rat vascular smooth muscle cell proliferation involving the down-regulation of JNK1/2 expression in MAPK signaling. Atherosclerosis. (2013) 228:46–52. doi: 10.1016/j.atherosclerosis.2013.01.030
42. Yin, T, Zhu, X, Xu, D, Lin, H, Lu, X, Tang, Y, et al. The association between dietary antioxidant micronutrients and cardiovascular disease in adults in the United States: a cross-sectional study. Front Nutr. (2021) 8:799095. doi: 10.3389/fnut.2021.799095
43. Lee, YA, Hwang, JY, Kim, H, Ha, EH, Park, H, Ha, M, et al. Relationships of maternal zinc intake from animal foods with fetal growth. Br J Nutr. (2011) 106:237–42. doi: 10.1017/s0007114510005878
44. Zhong, VW, Van Horn, L, Greenland, P, Carnethon, MR, Ning, H, Wilkins, JT, et al. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern Med. (2020) 180:503–12. doi: 10.1001/jamainternmed.2019.6969
45. Lönnerdal, B. Dietary factors influencing zinc absorption. J Nutr. (2000) 130:S1378–83. doi: 10.1093/jn/130.5.1378S
46. Pompano, LM, and Boy, E. Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv Nutr. (2021) 12:141–60. doi: 10.1093/advances/nmaa087
47. Foster, M, and Samman, S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. (2012) 4:676–94. doi: 10.3390/nu4070676
48. Kurutas, EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. (2016) 15:71. doi: 10.1186/s12937-016-0186-5
49. Yanagisawa, H, Miyazaki, T, Nodera, M, Miyajima, Y, Suzuki, T, Kido, T, et al. Zinc-excess intake causes the deterioration of renal function accompanied by an elevation in systemic blood pressure primarily through superoxide radical-induced oxidative stress. Int J Toxicol. (2014) 33:288–96. doi: 10.1177/1091581814532958
50. Huang, Q, Jiang, H, Zhang, B, Wang, H, Jia, X, Huang, F, et al. Threshold-effect Association of Dietary Cholesterol Intake with dyslipidemia in Chinese adults: results from the China health and nutrition survey in 2015. Nutrients. (2019) 11:2885. doi: 10.3390/nu11122885. PubMed31783560
51. Zhai, FY, Du, SF, Wang, ZH, Zhang, JG, Du, WW, and Popkin, BM. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes Rev. (2014) 15:16–26. doi: 10.1111/obr.12124
Keywords: dietary zinc, cardiovascular, cohort study, adults, CHNS
Citation: Zhang H, Wang S, Gu X, Qiu H and Zhang Y (2023) L-shaped association between dietary zinc intake and the risk of developing cardiovascular disease in Chinese adults: A cohort study. Front. Nutr. 10:1032048. doi: 10.3389/fnut.2023.1032048
Received: 30 August 2022; Accepted: 27 February 2023;
Published: 17 March 2023.
Edited by:
Firoozeh Hosseini-Esfahani, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Zahra Hajhashemy, Isfahan University of Medical Sciences, IranCopyright © 2023 Zhang, Wang, Gu, Qiu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiying Zhang, eWl5aW5nemhhbmdAaHJibXUuZWR1LmNu; Hongbin Qiu, cWl1aG9uZ2JpbkBqbXN1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.