
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 16 September 2022
Sec. Nutrition and Sustainable Diets
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.996541
This article is part of the Research TopicHealthy Foods and Dietary Patterns in Modern ConsumerView all 15 articles
Growing numbers of Americans are adopting vegetarian or vegan diets. While risk for some chronic conditions may be lower when following these diets, concern remains over the ability to consume adequate amounts of various nutrients, notably, protein. Knowing that serum creatinine is a reliable marker of muscle mass, this study examined the relationships between serum creatinine, lean body mass (LBM), handgrip strength, and protein intake in healthy vegetarian (n = 55) and omnivorous (n = 27) adults. Significantly higher protein intakes (+31%), LBM (+7%), serum creatinine (+12%) and handgrip strength (+14%) were observed for the omnivore participants compared to vegetarian participants. Positive correlations (p < 0.001) were noted between creatinine and LBM (R2 = 0.42), creatinine and handgrip strength (R2 = 0.41), protein intake and LBM (R2 = 0.29), and handgrip strength and LBM (R2 = 0.69). These data show that serum creatinine concentrations were lower in vegetarian women and men in comparison to their omnivorous counterparts and that serum creatinine concentrations correlate with LBM and strength in healthy adults, regardless of diet.
In recent years vegetarian diets have increased in popularity. A 2020 Harris Poll reported that 4% of men and 7% of women surveyed over 18 years of age in the United States identified as vegetarians, with half of those identifying as vegan (1). While these diets are often, but not exclusively, associated with decreased risk for cancer (2–4), cardiometabolic diseases (3–5), diabetes (6), and obesity (7–9), the possibility of micronutrient inadequacies, including iron, vitamin B12, and vitamin D remain a concern. Furthermore, although energy intakes tend to be similar, protein intake is often significantly lower for vegetarians when compared to omnivores (10–14). This can be concerning as protein intakes are directly linked to muscle mass and strength (15–17). Following ingestion, protein is digested to component amino acids, which are rapidly absorbed. The increase in plasma amino acid concentrations, particularly the essential amino acid leucine, stimulates muscle protein synthesis (18, 19). In comparison to animal proteins, plant proteins contain lower amounts of leucine (20); moreover, plant protein is 10–20% less bioavailable due to antinutrient factors present in these foods (11, 21). Clinical trials have demonstrated that the rate of muscle protein synthesis is less for plant-based verse animal-based protein (22, 23). Indeed, decreased lean body mass (LBM) and reduced handgrip strength has been reported in vegetarians (24, 25). Since handgrip strength is an indicator of health-related quality of life irrespective of age and gender (26–28), it is important to consider this quality in vegetarians.
Serum creatinine, a normal waste product of muscle metabolism, has been shown to be a reliable marker of muscle mass since it is continually produced and filtered through the kidney (29, 30). However, many factors can impact serum creatinine levels, most notably declining kidney function. At the nephron, creatinine is filtered through the glomerulus and is not reabsorbed by the tubule; hence, elevations in serum creatinine can be utilized as a proxy for renal function. Since serum creatinine is generated from muscle, concentrations are impacted by age, sex, and body size. Equations have been developed using serum creatinine to estimate the glomerulus filtration rate (GFR) while controlling for these common confounding factors, and currently, the CKD–EPI Collaboration (Chronic Kidney Disease Epidemiology Collaboration) equation is considered the most accurate GFR measurement for clinical use (29), and GFR values <90 ml/min/1.73 m2 indicate declining kidney function. However, serum creatinine is influenced by other factors as well including medications, chronic illness, nutritional status and diet (31), all of which need to be considered when utilizing this measure.
Several investigators have reported lower serum creatinine concentrations in vegetarian populations in comparison to omnivores (32, 33). However, a comparison between serum creatinine, LBM, and strength based on diet adherence in a healthy adult population has not been reported. Therefore, the purpose of this investigation was to examine the relationship between serum creatinine, LBM, and handgrip strength in healthy, non-athlete vegetarian and omnivore women and men. We hypothesized that serum creatinine would be positively associated with LBM and handgrip strength in both genders, with omnivores exhibiting higher levels of creatinine, LBM, and handgrip strength than vegetarians.
This study is a secondary analysis of a cross-sectional data set gathered from healthy, non-obese adults (body mass index [BMI] >18.5 and <30 kg/m2) to examine associations between indicators of bone health, bone mineral density, and diet adherence (vegetarian vs. omnivore) (34). Participants (27 omnivores and 55 vegetarians), aged 19–50 y, were recruited from the Phoenix metropolitan area using university list serves, connections with local vegetarian groups and farmers markets, and social media. Participants were healthy and free of chronic diseases by self-report; not taking prescription medications with the exception of oral contraceptives; not competitive athletes or training for an endurance event; and, if female, not recently pregnant. These exclusion criteria were designed to control covariables known to impact bone mineral density, but they were also relevant to this secondary analysis with a focus on lean body mass. Participants were classified as “vegetarian” if they reported never eating meat, fish, or poultry over the preceding year. “Omnivores” were classified as eating at least 3 servings of meat, fish, and/or poultry per week over the preceding year. Omnivores who reported less than 3 servings of flesh foods weekly [e.g., flexitarians or “meat-avoiders” (35)] were excluded from participation. All participants provided written consent and the study was approved by the Institutional Review Board at Arizona State University.
A 24-h diet recall was conducted by a trained nutrition professional using the multiple pass method, and diet data were analyzed using the Food Processor software (version 7.71; ESHA Research, Salem, OR, USA). Physical activity levels were estimated by validated questionnaire and reported as metabolic equivalents (METS) (36). A venous blood sample was collected following a 12-h fast, and serum was extracted for the creatinine analyses (Jaffé method, COBAS C311, Roche Diagnostics International Ltd, Switzerland). Glomerular filtration rate (GFR) was calculated using the CKD-EPI equation (29). Height was measured using a stadiometer, and body mass was recorded using a calibrated scale (model TBF-300A, Tanita Corporation, Tokyo, Japan). Dual energy X-ray absorptiometry (DEXA) (GE Lunar iDXA, Chicago, IL, USA) was used to measure LBM by a trained X-ray technician. Dominant arm handgrip strength was measured in triplicate while seated with the elbow flexed at 90 degrees and a neutral wrist position using a handheld dynamometer (Takei Scientific Instruments, Niigata-City, Japan). Three consecutive measures were taken, and the mean score was used for analyses.
For this secondary analysis, relationships between dietary protein, serum creatinine, strength, and LBM in omnivore vs. vegetarian participants were examined by gender. Data not normally distributed based on the Kolmogorov–Smirnov test were transformed prior to analyses (LBM only). Following a significant multivariate analysis, univariate analyses were used to assess differences between means while controlling for covariates. Pearson correlations were used to identify relationship between variables, and multiple regression analyses were utilized to determine the predictive value of variables for LBM and handgrip strength. Statistical analyses were performed using SPSS version 24 (IBM, Armonk, NY, USA), and p ≤ 0.05 was considered significant. Data are reported as mean ± SD.
The study sample (n = 82) was composed of 55 vegetarians (67%) and 58 women (71%) (Table 1). Gender, BMI, energy intake, and physical activity did not vary between the diet groups; however, the vegetarian group was older on average than the omnivorous group (32.5 ± 8.8 and 27.2 ± 6.7 y respectively; p = 0.008). In subgroup analyses, the vegetarian men were less active than their omnivorous counterparts (41.1 ± 25.6 and 74.9 ± 49.0 METS respectively; p = 0.036); hence, age and physical activity were controlled in the remaining analyses.
All variables tested were significantly impacted by diet type (Table 2). Significantly higher protein intakes (+31%), LBM (+7%), serum creatinine (+12%) and handgrip strength (+14%) were observed for the omnivore participants compared to vegetarian participants (p>0.05; Table 2). Additionally, the diet related differences for serum creatinine and handgrip strength were observed within gender groups. There were no significant relationships between serum creatinine and energy intake (r = −0.131; p = 0.289) or alcohol intake (r = −0.010; p = 0.934) among the participants. The average GFR measure was raised for the vegetarian participants relative to the omnivorous participants (+8%; p = 0.002), and this diet related difference was also observed within genders.
With LBM as the criterion variable, multiple regression analyses identified gender and serum creatinine as significant predictor variables (p < 0.05) in a model including age (p = 0.396), diet group (p = 0.316) and dietary protein (p = 0.111) [F(5, 65) = 32.094, p < 0.001, R2 = 0.712] (Table 3). With handgrip strength as the criterion variable, gender, diet group, and LBM, were predictor variables (p < 0.05) in a model also including age (p = 0.614) and dietary protein (p = 0.423) [F(5, 65) = 44.816, p < 0.001, R2 = 0.775] (Table 3). Correlations between serum creatinine, LBM and handgrip strength, dietary protein and LBM, and handgrip strength and LBM were significant in the study sample (p ≤ 0.001; Figure 1), and these significant results were retained after controlling for GFR.
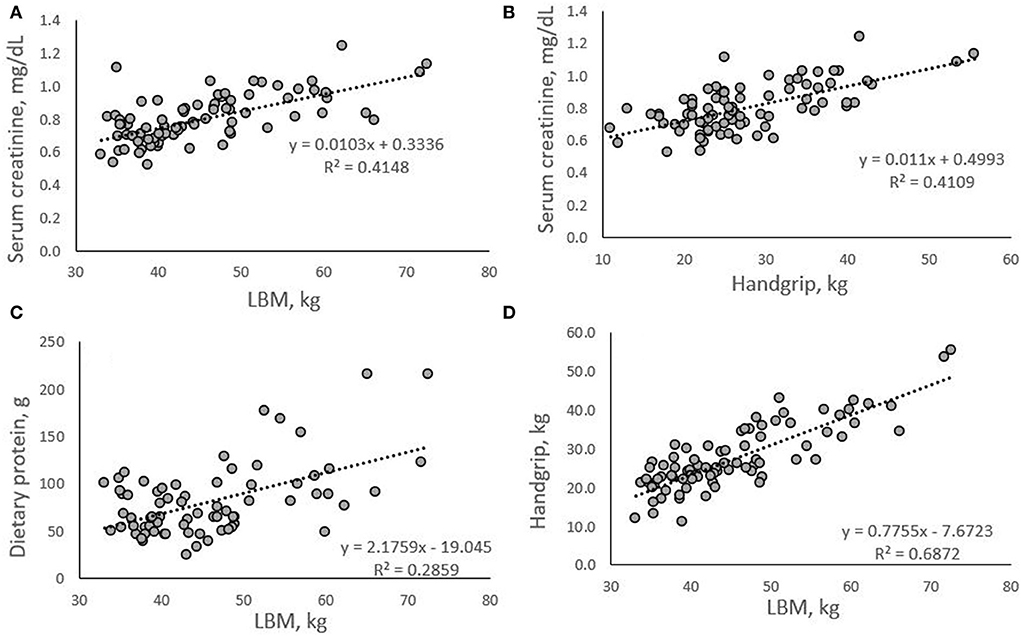
Figure 1. Correlations between serum creatinine, LBM (A), and handgrip (B); dietary protein and LBM (C); and handgrip and LBM (D). All correlations were significant (p < 0.001).
This investigation revealed that serum creatinine is significantly reduced in vegetarian women and men compared to their meat-eating counterparts. Moreover, serum creatinine was a strong, independent predictor of LBM. The vegetarian men and women tended to consume less protein than the omnivore men and women and, importantly, protein intake and LBM were directly correlated. Moreover, vegetarians in this study had significantly lower handgrip strength than their omnivore counterparts. These findings may have important physiological relevance, as LBM and handgrip strength are related to quality of life (26–28). Moreover, Srikanthan et al. reported a negative association between muscle mass and all-cause mortality in older Americans (37). Kang et al. found a direct correlation between decreased handgrip strength and impaired mobility, increased pain and greater discomfort in both Korean men and women, with exacerbated effects as age increased, thus leading to a poorer quality of life (26). Lower levels of physical activity are associated with both a lower quality of life and with reduced handgrip strength; hence, physical activity needs to be controlled in these types of investigations (38). Herein, adults who participated in competitive sports were excluded, and the diet related differences in handgrip strength by gender remained significant controlling for age and physical activity level.
In several large cross-sectional trials, serum creatinine concentrations were significantly reduced in vegetarian populations in comparison to omnivores (32, 33). Utilizing a randomized controlled study design, Dinu et al. reported a significant reduction in serum creatinine in adults at medium-to-low risk for cardiovascular disease randomized to a hypocaloric vegetarian diet vs. a hypocaloric Mediterranean diet for 3 months (−0.04 and +0.01 mg/dL respectively, p < 0.001) (39). The only dietary differences between groups were for protein and fiber; however, correlation analyses showed no significant associations between these dietary changes and change in serum creatinine. Losses in total body mass was similar between groups; however, 26% more fat mass was lost in the omnivore group in comparison to the vegetarian group suggesting a greater degree of LBM loss in the latter group, which may have contributed to the significant reduction in serum creatinine noted for this group (38). In a case report, a 65-y old male with type 2 diabetes and stage 3 kidney disease, adopted a vegetarian diet, and after four months serum creatinine concentrations fell 38% from 1.6 to 1.03 mg/dL, a reduction attributed to the loss of lean body mass (40). Kochlik et al. followed serum creatinine change in adults adhering to a meatless diet for 6 days but fed 160 g cooked chicken (e.g., approximately two servings meat equating to 35 g protein) on day 4 (41). Creatinine concentrations did not fluctuate on trial days 3, 4, 5 and 6, suggesting the dependence of plasma creatinine on muscle metabolism and not meat ingestion (41). However, Mayersohn et al. reported a 52% rise in serum creatinine 2 h after consumption of the equivalent of 225 g cooked meat (42). Animal products, primarily meat, fish, and poultry, are the sole dietary source of creatine, which is partially converted to creatinine based on the cooking method (43).
Creatinine is generated at a constant rate from the spontaneous, non-enzymatic cyclization of creatine in muscle cells (30). Due to its steady production, and the fact it is freely filtered through the glomerulus, serum creatinine is used clinically as an indicator of kidney function, and high serum concentrations of creatinine (>1.4 mg/dL) are a marker of compromised kidney function (44). In fact, acute elevations in serum creatinine are considered a potent risk factor for adverse outcomes in general in the inpatient setting (43). However, less attention is directed at creatinine concentrations in the low-normal range. Low serum creatinine concentrations can indicate acute illness, severe liver disease, and the loss of muscle mass, such as in malnutrition, muscular dystrophy, or sarcopenia in older adults (45–49). Also, serum creatinine is linked to hydration status, and concentrations are lower in dehydrated states (50, 51).
In the present report, participants were young, healthy adults, screened for chronic disease, underweight and medical conditions. Thus, this report suggests that low-normal serum creatinine concentrations were linked to low LBM, reduced strength, and vegetarian diet adherence in healthy, young adults. Participants were likely well-hydrated when tested since less than 5% of the adults had serum sodium concentrations >146 mEq/L. The vegetarian men and women averaged creatinine concentrations below the mid-point of the reference ranges of 0.7–1.2 mg/dL for men and 0.5–1.0 mg/dL for women (52). In the men, serum creatinine was below 0.95 mg/dL in 81% of vegetarians vs. 13% of omnivores (p = 0.002). Although not statistically significant, serum creatinine was below 0.75 mg/dL in 67% of vegetarian women vs. 42% of omnivorous women (p = 0.094).
Previous research indicates that creatinine can be used reliably to estimate muscle mass (30, 53) and data from the present analysis support this contention (Figure 1A). Additionally, we show a positive relationship with large effect size between serum creatinine and handgrip strength, with serum creatinine explaining 41% of variance in handgrip strength (R2 = 0.4109; Figure 1B). It is known that dietary protein, and specifically the amino acid leucine, leads to the upregulation and activation of anabolic signaling systems responsible for muscle protein synthesis (18, 19). We have previously shown that in vegetarians and vegans who averaged less than the protein recommended dietary allowance (RDA) value of 0.8 g/kg/d, an increase in protein intake by 18 g per day significantly increased muscular strength in the absence of a training program (54). This novel finding suggested that increasing protein intake above the RDA when intake is low has beneficial effects on strength levels in vegetarians. It is well documented that inadequate protein intakes are associated with muscle protein breakdown, leading to catabolism and functional decline (55).
Some factors presented limitations in this study. Data were analyzed from a cross-sectional trial; therefore, causation cannot be determined. Additionally, this was a secondary data analysis, thus the study and data collection procedures were not initially designed for this specific outcome variable. There are many confounding variables that need to be considered as discussed, and although many of these variables were measured and/or controlled in this investigation, others were not. Gender significantly impacted the outcome variables and must be carefully considered when interpreting these data. Furthermore, flexitarians (e.g., meat-avoiders) were excluded from the study; hence, it is not known how occasional meat consumption may impact the outcome measures. This is an important consideration for a future trial. The renal function of participants was estimated using the calculated GFR; however, the gold standard measurement of renal function involves the injection of a tracer and its clearance by the kidneys (56). A single 24-h recall was used to estimate protein intakes. Research suggests that two to three 24-h records are needed for reasonable accuracy in estimating dietary protein (57). Future studies might include randomized controlled trials examining the impact of dietary protein on the outcome measures herein while addressing the limitations encountered in this report.
This study shows that both men and women who follow an omnivore diet have significantly greater dietary protein intake, serum creatinine levels, and grip strength compared to vegetarian men and women. Additionally, the data show that creatinine is positively correlated to LBM and handgrip strength, and that dietary protein is positively correlated to LBM. These data offer the possibility that in healthy adults, serum creatinine may be a complementary indicator of muscle mass and strength and can be utilized by practitioners and coaches, particularly for advising vegetarian clients. However, more work is warranted regarding the relevance of serum creatinine concentrations in the low-normal range in terms of LBM and functional strength, and it is important to consider gender, altered kidney function and physical activity in these assessments.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board at Arizona State University. The patients/participants provided their written informed consent to participate in this study.
EB and CJ designed the secondary research, analyzed the data, wrote the manuscript, and had primary responsibility for the final content. JK and CJ designed the original research. EB conducted the secondary research and drafted the first version of the manuscript. All authors read and approved the final manuscript and provided critical comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stahler C,. How Many People Are Vegan? How Many Eat Vegan When Eating Out? Asks the Vegetarian Resource Group. (2020). Available online at: https://www.vrg.org/journal/vj2020issue4/2020_issue4_poll_results.php (accessed July 14, 2022).
2. Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Epidemiol Prev Biomark. (2009) 22:286–94. doi: 10.1158/1055-9965.EPI-12-1060
3. Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. (2017) 57:3640–9. doi: 10.1080/10408398.2016.1138447
4. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. (2012) 60:233–40. doi: 10.1159/000337301
5. Kahleova H, Levin S, Barnard ND. Vegetarian dietary patterns and cardiovascular disease. Prog Cardiovasc Dis. (2018) 61:54–61. doi: 10.1016/j.pcad.2018.05.002
6. Lee Y, Park K. Adherence to a vegetarian diet and diabetes risk: a systematic review and meta-analysis of observational studies. Nutrients. (2017) 9:603. doi: 10.3390/nu9060603
7. Viguiliouk E, Kendall CW, Kahleová H, Rahelić D, Salas-Salvadó J, Choo VL, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. (2019) 38:1133–45. doi: 10.1016/j.clnu.2018.05.032
8. Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J Gen Intern Med. (2016) 31:109–16. doi: 10.1007/s11606-015-3390-7
9. Turner-McGrievy GM, Barnard ND, Scialli AR. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity (Silver Spring). (2007) 15:2276–81. doi: 10.1038/oby.2007.270
10. Schüpbach R, Wegmüller R, Berguerand C, Bui M, Herter-Aeberli I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr. (2017) 56:283–93. doi: 10.1007/s00394-015-1079-7
11. Kniskern MA, Johnston CS. Protein dietary reference intakes may be inadequate for vegetarians if low amounts of animal protein are consumed. Nutrition. (2011) 27:727–30. doi: 10.1016/j.nut.2010.08.024
12. Lesser S. The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications. Nutr Bull. (2013) 38:421–8. https://doi.org/10.1111/nbu.12063
13. Phillips SM, Van Loon LJC. Dietary protein for athletes: From requirements to optimum adaptation. J Sport Sci. (2011) 29:29–38. doi: 10.1080/02640414.2011.619204
14. Neufingerl N, Eilander A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients. (2021) 14:29. doi: 10.3390/nu14010029 PMID
15. Gregorio L, Brindisi J, Kleppinger A, Sullivan R, Mangano KM, Bihuniak JD, Kenny AM, Kerstetter JE, Insogna KL. Adequate dietary protein is associated with better physical performance among post-menopausal women 60-90 years. J Nutr Health Aging. (2014) 18:155–60. doi: 10.1007/s12603-013-0391-2
16. Mishra S, Goldman JD, Sahyoun NR, Moshfegh AJ. Association between dietary protein intake and grip strength among adults aged 51 years and over: What we eat in America, national health and nutrition examination survey 2011–2014. PLoS ONE. (2018) 13:e0191368. doi: 10.1371/journal.pone.0191368
17. Tong TYN, Key TJ, Sobiecki JG, Bradbury KE. Anthropometric and physiologic characteristics in white and British Indian vegetarians and non-vegetarians in the UK Biobank. Am J Clin Nutr. (2018) 107:909–20. doi: 10.1093/ajcn/nqy042
18. Stokes T, Hector AJ, Morton RW, McGlory C, Phillips SM. Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients. (2018) 10:180. doi: 10.3390/nu10020180
19. Tipton KD, Phillips SM. Dietary protein for muscle hypertrophy. Nestle Nutr Inst Workshop Ser. (2013) 76:73–84. doi: 10.1159/000350259
20. Dietrich S, Trefflich I, Ueland PM, Menzel J, Penczynski KJ, Abraham K, et al. Amino acid intake and plasma concentrations and their interplay with gut microbiota in vegans and omnivores in Germany. Eur J Nutr. (2022) 61:2103–14. doi: 10.1007/s00394-021-02790-y
21. Ciuris C, Lynch HM, Wharton C, Johnston CS. A comparison of dietary protein digestibility, based on DIAAS scoring, in vegetarian and non-vegetarian athletes. Nutrients. (2019) 11:3016. doi: 10.3390/nu11123016
22. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. (2007) 85:1031–40. doi: 10.1093/ajcn/85.4.1031
23. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. (2007) 86:373–81. doi: 10.1093/ajcn/86.2.373
24. Aubertin-Leheudre M, Adlercreutz H. Relationship between animal protein intake and muscle mass index in healthy women. Br J Nutr. (2009) 102:1803–10. doi: 10.1017/S0007114509991310
25. Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, et al. Effect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur J Nutr. (2016) 55:207–17. doi: 10.1007/s00394-015-0838-9
26. Kang SY, Lim J, Park HS. Relationship between low handgrip strength and quality of life in Korean men and women. Qual Life Res. (2018) 27:2571–80. doi: 10.1007/s11136-018-1920-6
27. Marques A, Gomez-Baya D, Peralta M, Frasquilho D, Santos T, Martins J, et al. The effect of muscular strength on depression symptoms in adults: a systematic review and meta-analysis. Int J Environ Res Public Health. (2020) 17:5674. doi: 10.3390/ijerph17165674
28. Lee MR, Jung SM, Bang H, Kim HS, Kim YB. The association between muscular strength and depression in Korean adults: a cross-sectional analysis of the sixth Korea National Health and Nutrition Examination Survey (KNHANES VI) 2014. BMC Public Health. (2018) 18:1123. doi: 10.1186/s12889-018-6030-4
29. Cusumano AM, Tzanno-Martins C, Rosa-Diez GJ. The glomerular filtration rate: from the diagnosis of kidney function to a public health tool. Front Med (Lausanne). (2021) 8:769335. doi: 10.3389/fmed.2021.769335
30. Kashani K, Rosner MH, Ostermann M. Creatinine: From physiology to clinical application. Eur J Intern Med. (2020) 72:9–14. doi: 10.1016/j.ejim.2019.10.025
31. Thongprayoon C, Cheungpasitporn W, Kashani K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in critically ill patients. J Thorac Dis. (2016) 8:E305–11. doi: 10.21037/jtd.2016.03.62
32. Tong TYN, Perez-Cornago A, Bradbury KE, Key TJ. Biomarker concentrations in white and british indian vegetarians and non-vegetarians in the UK biobank. J Nutr. (2021) 151:3168–79. doi: 10.1093/jn/nxab192
33. Xu K, Cui X, Wang B, Tang Q, Cai J, Shen X. Healthy adult vegetarians have better renal function than matched omnivores: a cross-sectional study in China. BMC Nephrol. (2020) 21:268. doi: 10.1186/s12882-020-01918-2
34. Knurick JR, Johnston CS, Wherry SJ, Aguayo I. Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients. (2015) 7:3416-26. doi: 10.3390/nu7053416
35. Dagevos H, Voordouw J. Sustainability and meat consumption: is reduction realistic? Sustain Sci Pract Policy. (2013) 9:2. doi: 10.1080/15487733.2013.11908115
36. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. (1985) 10:141–6.
37. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. (2014) 127:547-53. doi: 10.1016/j.amjmed.2014.02.007
38. Haider S, Luger E, Kapan A, Titze S, Lackinger C, Schindler KE et al. Associations between daily physical activity, handgrip strength, muscle mass, physical performance and quality of life in prefrail and frail community-dwelling older adults. Qual Life Res. (2016) 25:3129–38. doi: 10.1007/s11136-016-1349-8
39. Dinu M, Colombini B, Pagliai G, Giangrandi I, Cesari F, Gori A, et al. Effects of vegetarian vs. mediterranean diet on kidney function: findings from the CARDIVEG study. Eur J Clin Invest. (2021) 51:e13576. doi: 10.1111/eci.13576
40. Campbell TM, Liebman SE. Plant-based dietary approach to stage 3 chronic kidney disease with hyperphosphataemia. BMJ Case Rep. (2019) 12:e232080. doi: 10.1136/bcr-2019-232080
41. Kochlik B, Gerbracht C, Grune T, Weber D. The influence of dietary habits and meat consumption on plasma 3-methylhistidine-a potential marker for muscle protein turnover. Mol Nutr Food Res. (2018) 62:e1701062. doi: 10.1002/mnfr.201701062
42. Mayersohn M, Conrad KA, Achari R. The influence of a cooked meat meal on creatinine plasma concentration and creatinine clearance. Br J Clin Pharmacol. (1983) 15:227–30. doi: 10.1111/j.1365-2125.1983.tb01490.x
43. Brosnan ME, Brosnan JT. The role of dietary creatine. Amino Acids. (2016) 48:1785–91. doi: 10.1007/s00726-016-2188-1
44. Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. (2005) 5:265–70. doi: 10.1097/01.mnh.0000165894.90748.72
45. Laszczyńska O, Severo M, Mascarenhas J, Paiva JA, Azevedo A. Serum creatinine trajectories in real-world hospitalized patients: clinical context and short-term mortality. J Investig Med. (2020) 20:870–81. doi: 10.1136/jim-2019-001185
46. Beben T, Rifkin DE. GFR estimating equations and liver disease. Adv Chronic Kidney Dis. (2015) 15:337–42. doi: 10.1053/j.ackd.2015.05.003
47. Singhal S, Singh S, Upadhyay AD, Dwivedi SN, Das CJ, Mohta S, et al. Serum creatinine and cystatin C-based index can be a screening biomarker for sarcopenia in older population. Eur Geriatr Med. (2019) 19:625–30. doi: 10.1007/s41999-019-00197-x
48. Ostermann M, Kashani K, Forni LG. The two sides of creatinine: both as bad as each other? J Thorac Dis. (2016) 8:E628–E630. doi: 10.21037/jtd.2016.05.36
49. Hari P, Bagga A, Mahajan P, Lakshmy R. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatr Nephrol. (2007) 7:1757–61. doi: 10.1007/s00467-007-0535-x
50. Takuwa S, Ito Y, Ushijima K, Uchida K. Serum cystatin-C values in children by age and their fluctuation during dehydration. Pediatr Int. (2002) 2:28–31. doi: 10.1046/j.1442-200x.2002.01499.x
51. Lešnik A, Piko N, Železnik D, Bevc S. Dehydration of older patients in institutional care and the home environment. Res Gerontol Nurs. (2017) 17:260–6. doi: 10.3928/19404921-20171013-03
52. Shimada M, Dass, B, Ejaz, AA,. Assessment of elevated creatinine. BMJ Best Practice. (2020). Available online at: https://bestpractice.bmj.com/topics/en-gb/935 (accessed July 11, 2022).
53. Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin C. PLoS One. (2016) 11:e0148495. doi: 10.1371/journal.pone.0148495
54. Bartholomae E, Incollingo A, Vizcaino M, Wharton C, Johnston CS. Mung bean protein supplement improves muscular strength in healthy, underactive vegetarian adults. Nutrients. (2019) 11:2423. doi: 10.3390/nu11102423
55. Thalacker-Mercer AE, Drummond MJ. The importance of dietary protein for muscle health in inactive, hospitalized older adults. Ann N Y Acad Sci. (2014) 1328:1–9. doi: 10.1111/nyas.12509
56. Warwick J, Holness J. Measurement of glomerular filtration rate. Semin Nucl Med. (2022) 52:453–66. doi: 10.1053/j.semnuclmed.2021.12.005
Keywords: vegetarian, creatinine, lean body mass, muscle, grip strength
Citation: Bartholomae E, Knurick J and Johnston CS (2022) Serum creatinine as an indicator of lean body mass in vegetarians and omnivores. Front. Nutr. 9:996541. doi: 10.3389/fnut.2022.996541
Received: 17 July 2022; Accepted: 31 August 2022;
Published: 16 September 2022.
Edited by:
Prisco Piscitelli, Istituto Scientifico Biomedico Euro Mediterraneo (ISBEM), ItalyCopyright © 2022 Bartholomae, Knurick and Johnston. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carol S. Johnston, Y2Fyb2wuam9obnN0b25AYXN1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.