- 1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Department of Community Nutrition, School of Nutrition and Food Science, Tabriz University of Medical Sciences, Tabriz, Iran
- 3Department of Food Science and Technology, North Tehran Branch, Islamic Azad University, Tehran, Iran
- 4Cardiovascular Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 5Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
- 6Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
Several meta-analyses have revealed that n-3 PUFAs can lower blood pressure, but the findings are conflicting. In this regard, the present umbrella meta-analysis aimed was performed to clarify whether n-3 PUFAs have effects on blood pressure. PubMed, Scopus, Embase, Web of Science, and Google Scholar were used as international databases from inception to May 2022. To examine the effects of n-3 PUFA supplementation on blood pressure, a random-effects model was applied. The leave-one-out method was performed for the sensitivity analysis. The pooled estimate of 10 meta-analyses with 20 effect sizes revealed significant reductions in both systolic (ES = −1.19 mmHg; 95% CI: −1.76, −0.62, p < 0.001) and diastolic blood pressure (ES = −0.91 mmHg, 95% CI: −1.35, −0.47; p < 0.001) following n-3 PUFAs supplementation. In studies with a sample size of ≤ 400 participants and a mean age over 45, SBP and DBP were found to be substantially reduced. Overall, this umbrella meta-analysis indicates that n-3 PUFAs supplementation might play a role in improving DBP and SBP.
Introduction
Hypertension is among the most common health conditions worldwide affecting over 1 billion people and accounting for 9.4 million deaths annually (1). Elevated blood pressure is responsible for heart attacks, arrhythmias, stroke and premature death (2). Research shows that some recommendations such as healthy dietary habits, and smoking cessation can reduce blood pressure (BP), thereby decreasing hypertension and CVD risks (3).
Omega-3 polyunsaturated fatty acids (PUFAs) mainly consist of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and alpha-linolenic acid (ALA) (4). Diets deficient in n-3 PUFAs from seafood were classified as the sixth most significant dietary risk factor −1.5 million deaths and 33 million disability-adjusted life years worldwide were attributed to this (5). According to most national and international guidelines, adults should consume at least one serving of oily fish per week (≥ 250 mg/day of EPA and DHA) (6, 7). Despite this, an estimated 20% of the world’s population consumes less than 250 mg of EPA and DHA per day (8). Although the role of PUFAs in hypertension is still subject to an scientific debate, they have been shown to have multiple beneficial effects in cardiovascular disease (9). Additionally, they demonstrated that they had anti-inflammatory (10) and antithrombotic (11) properties, improved endothelial dysfunction (12) and positively affected resting heart rate (HR), HR variability (13, 14), heart rhythm (15), and cardiac remodeling (16). In terms of their antihypertensive properties, n-3 PUFAs were reported to modulate ion channels in blood vessels, thereby causing vasodilation (17). The red blood cell membrane omega-3 content of humans is associated with smaller brachial artery diameter (an independent predictor of cardiovascular events) and increased vasodilatory function (18). Omega-3 content in red blood cell membranes of humans is associated with smaller brachial arteries (an indicator of cardiovascular events) and increased vasodilation (18). Generally, n-3 PUFAs were shown to improve arterial compliance and reduce pulse wave velocity. Some benefits of omega-3-PUFAs may be mediated through downstream effects on BP (8, 19, 20). According to a report by Averna et al. (21) high-dose omega-3 fatty acids (Icosapent ethyl) may be considered a benefit to managing TG-rich lipoproteins. By TG-lowering, the risk of cardiovascular events decreases. In recent years, several systematic reviews and meta-analyses of randomized controlled trials have been performed investigating the effects of n-3 PUFAs supplementation on BP (22, 23), while In some studies, controversial results have been reported (24–26). Therefore, the present umbrella meta-analysis was performed to assess the explicit impact of n-3 PUFA supplementation on systolic BP (SBP) and diastolic BP (DBP) providing valid and authentic evidence.
Methods
The current umbrella of meta-analysis was performed based on the PRISMA. We registered our study protocol in PROSPERO (CRD42022311888).
Search strategy of literature
Searches were conducted in PubMed, Scopus, Embase, Web of Science, and Google Scholar databases to find relevant studies published inception to May 2022 The following search strategy using MeSH terms and keywords was applied; [“omega-3 fatty acid” (Mesh) OR “omega-3 fatty acid” (tiab) OR “fish oil” (tiab) OR “omega-3 polyunsaturated fatty acids” (tiab) OR “EPA” (Mesh) OR “DHA” (Mesh) OR “DHA” (tiab) OR “EPA” (tiab) AND “hypertension” (Mesh) OR “hypertension” (tiab) OR “HTN” (tiab) OR “blood pressure” (tiab) OR “BP” (tiab) OR “hypertens” (tiab) AND “systematic review” (Publication Type) OR “meta-analysis” (tiab)]. Additionally, we enhanced sensitivity of the search strategy by using the wildcard term “*”. To ensure no publications were missing, reference lists of relevant studies were manually screened. In this umbrella of meta-analysis, English-language articles were eligible.
Inclusion and exclusion criteria
A PICO analysis was conducted to summarize the results of the current umbrella meta-analysis. The PICO criteria were: population/patients (P: adults over 18 years’ old who received omega-3 PUFAs); intervention (I: omega-3 PUFAs therapy); comparison (C: control or placebo group); outcomes (O: BP including SBP and DBP). Our study included meta-analyses that assessed the effects of n-3 PUFA supplementation on BP (SBP and DBP) along with their effect sizes (ES) and corresponding confidence intervals (CI). In vivo, in vitro, and ex vivo studies, observational studies, quasi-experimental studies, case reports, and controlled clinical trials were excluded.
Evaluation of methodological quality
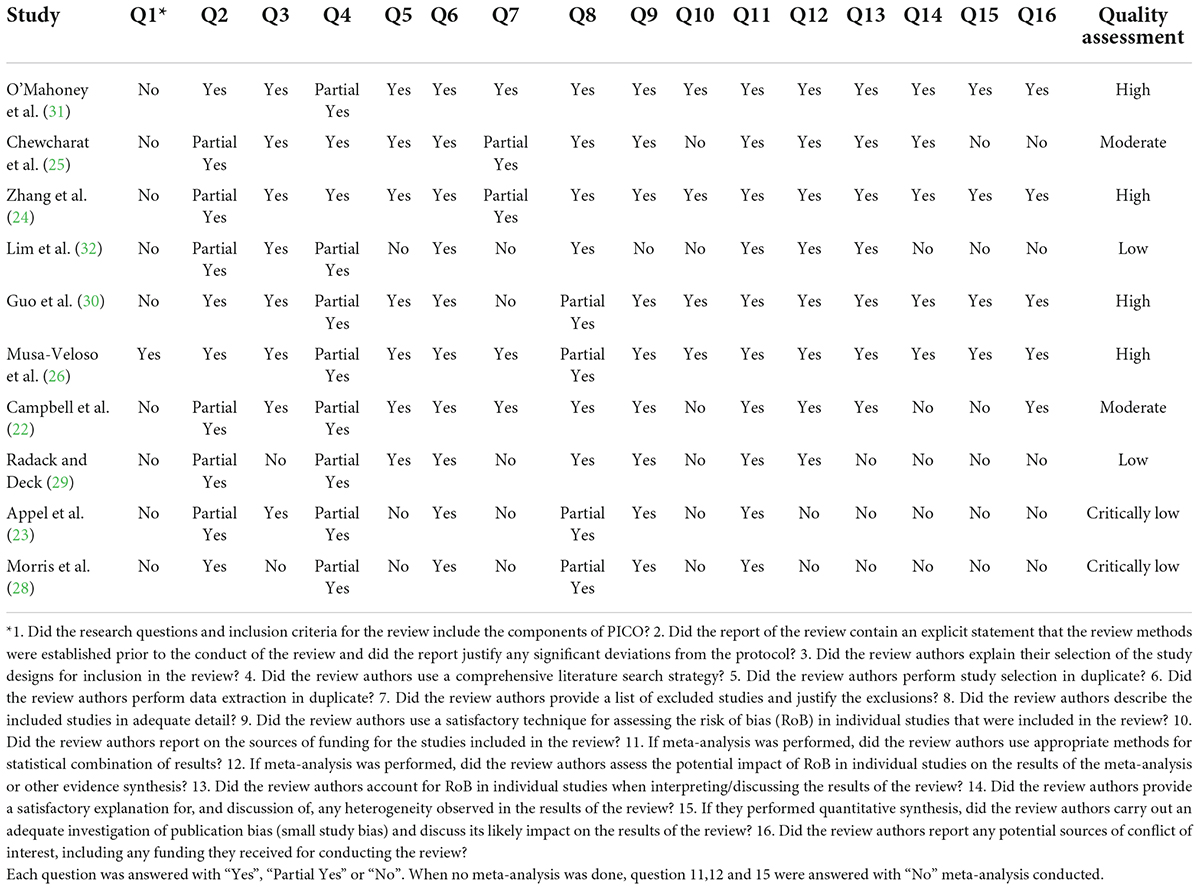
In terms of their methodology, two independent reviewers (VM and MV) performed AMSTAR2 (Assessing the Methodological Quality of Systematic Reviews) questionnaires to evaluate the quality of meta-analyses (27). The questionnaire consists of 16 questions that ask reviewers to answer “Yes” or “Partial Yes” or “No” or “No Meta-analysis.” Four categories were developed for the AMSTAR 2 checklist: “Critical low quality,” “low quality,” “moderate quality,” and “high quality.
Study selection and data extraction
Based on eligibility criteria, articles were screened by two independent reviewers (MV and VM). After reviewing based on their titles and abstracts, irrelevant studies were removed. Then, by evaluating the full text of the relevant articles, eligible studies for the umbrella of meta-analysis were identified. Finally, any disagreement was resolved by discussion and consulting with other researchers (PD). The first author’s name, the publication year, the sample size, the study location, supplementation dosages, and duration, as well as the ESs and CIs for SBP and DBP, are extracted from the selected meta-analyses.
Synthesis of data and statistical analysis
ESs and CIs were applied to estimate overall effect sizes. Cochran’s Q test and I2 statistics were used to determine heterogeneity. When I2-value > 50% or the Q-test had p < 0.1, we considered between-study heterogeneity significant. The random-effects model was chosen when there was significant between-study heterogeneity. By performing subgroup analysis based on predefined variables including sample size, duration of intervention, health condition, the dose of supplementation, and mean age, potential sources of heterogeneity were detected. Sensitivity analysis was conducted to investigate the impact of one single study omission on the pooled effect size. Tests Egger’s and Begg’s were used to assessing a small-study effect. The publication bias was assessed by visual inspection of the funnel plots, and if any publication bias was detected, then a trim and fill test was carried out subsequently. In order to run the meta-analysis, we used STATA (version 16, Stata Corporation, College Station, TX, United States). In this study, p < 0.05 was considered statistically significant.
Results
Study selection and study characteristics
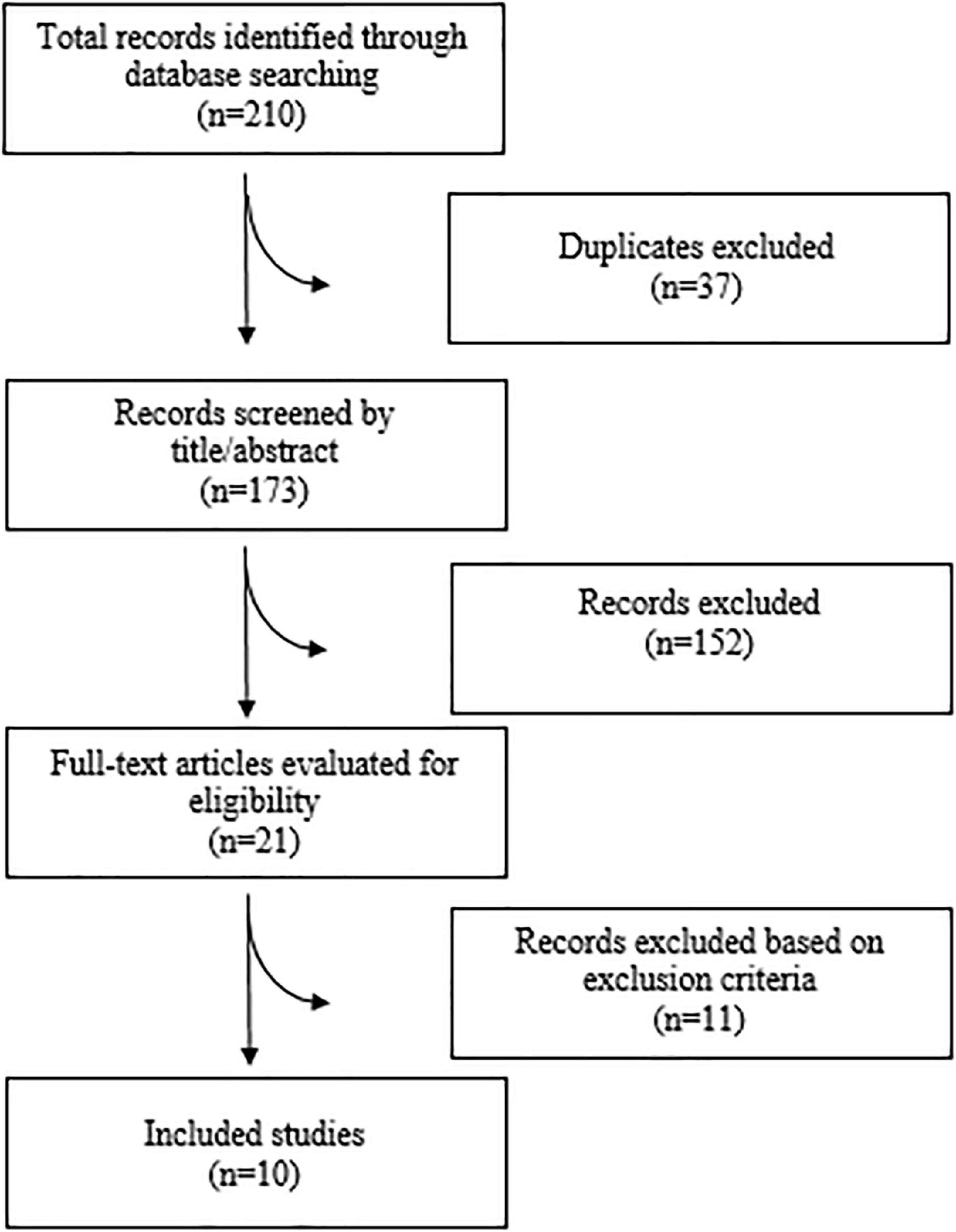
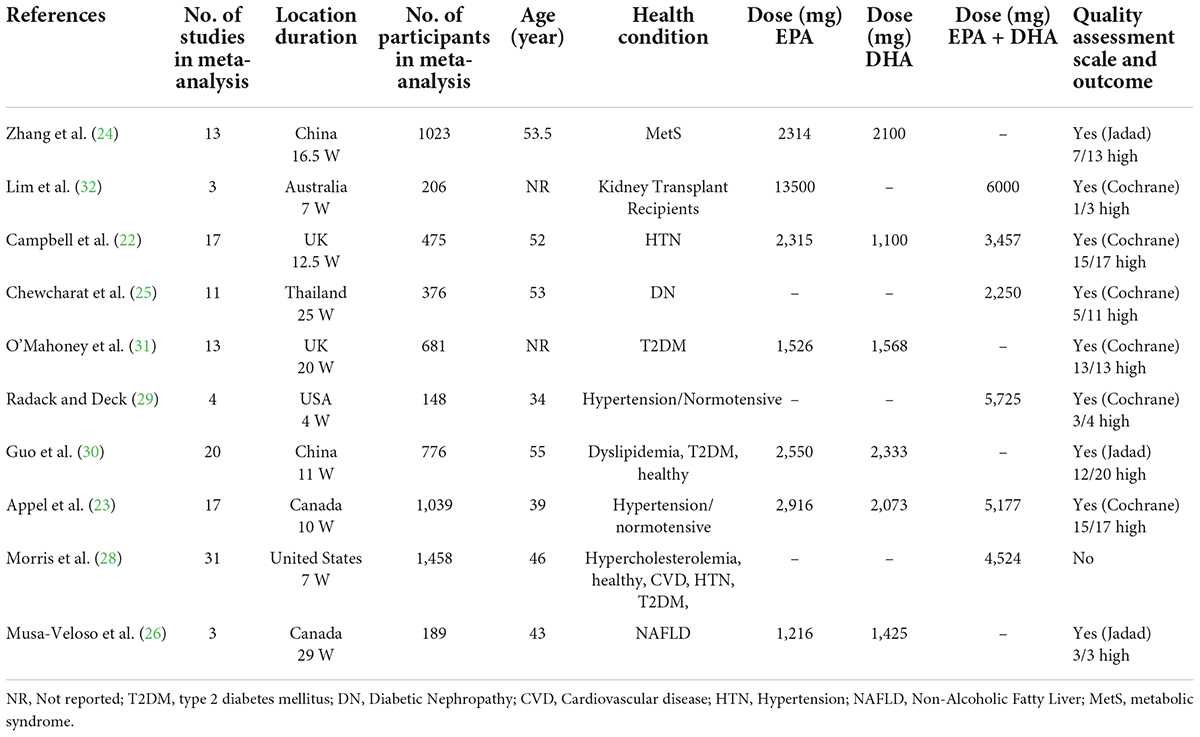
A total of 210 articles were found after searching the electronic databases. 173 articles were carefully screened with titles and abstracts, after removing 37 duplicate articles, of which 21 articles were selected for full-text evaluation. Finally, 10 meta-analyses were included in the current umbrella meta-analysis. Figure 1 illustrates the flow chart of PRISMA studies. The characteristics of the meta-analyses that were qualified are summarized in Table 1. All studies were carried out between 1989 and 2021, and the participants ranged in age from 34 to 55 years. There was an average amount of 2.2–6 g/day of administered n-3 PUFAs across studies. The duration of n-3 PUFA supplementation ranged from 4 to 29 weeks. The location of the studies performed were as follows: three in United States (25, 28, 29), two in China (24, 30), two in United Kingdom (22, 31), two in Canada (23, 26), and one in Australia (32). The quality of the randomized controlled trials (RCTs) included in the current study is presented in Table 1.
Methodological quality
Results of the quality assessment of the qualified studies using the AMSTAR2 questionnaire are presented in Table 2.
Impact of n-3 PUFAs on diastolic blood pressure
Overall, ten included meta-analyses with 20 effect sizes comprising a total of 6,334 subjects have examined the impact of n-3 PUFAs supplementation on DBP (Figure 2A). It was found that DBP was significantly reduced after n-3 PUFAs supplementation (ES = −0.91 mmHg, 95% CI: −1.35, −0.47; p < 0.001), with high heterogeneity between-studies (I2 = 70.8%, p < 0.001). N-3 PUFA supplementation > 4,500 mg/day to hypertension subjects > 45 years of age and a sample size of ≤ 400 significantly reduced DBP (Table 3).
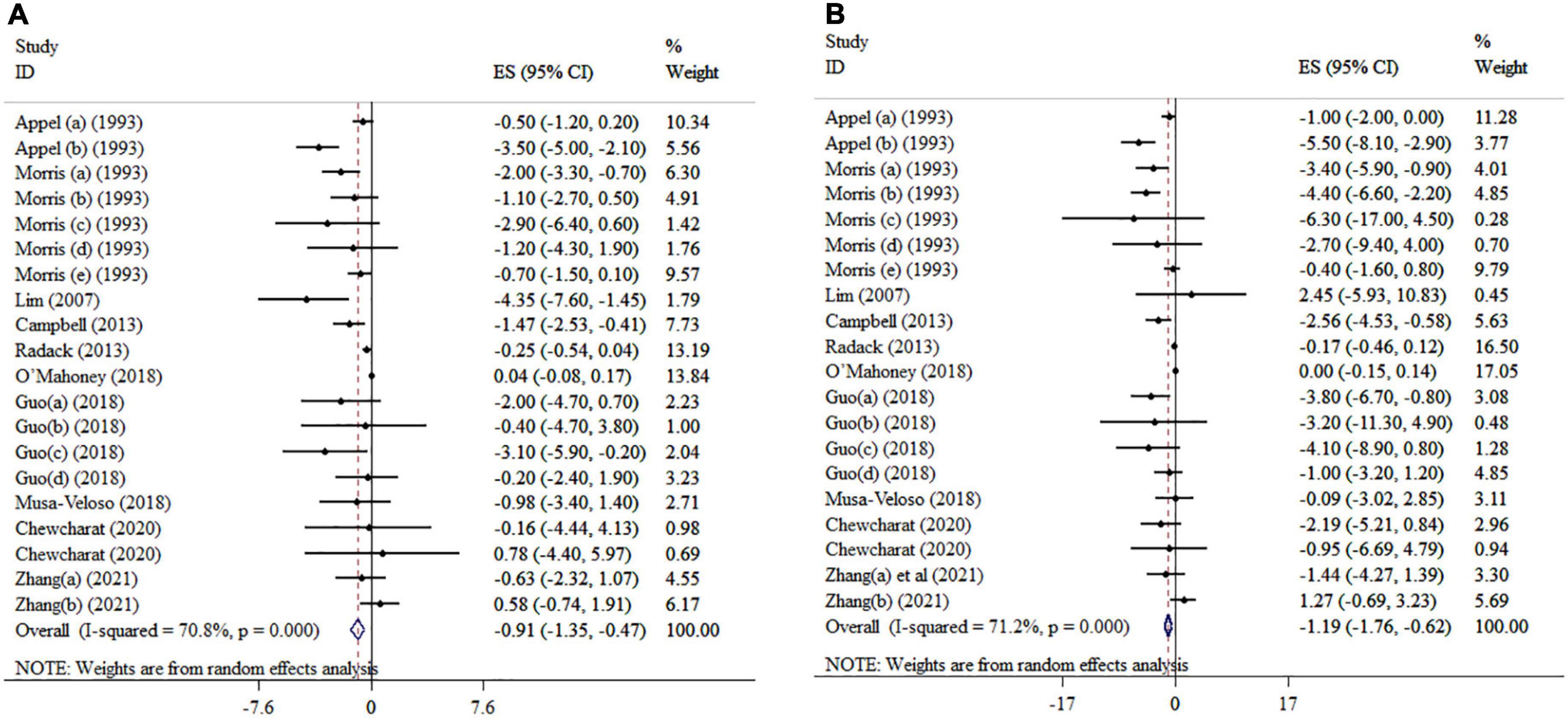
Figure 2. Mean difference and 95% CIs presented in forest plots of the studies on the effects of n-3 PUFA supplementation on DBP (A), and SBP (B) levels (a, b, c, d, etc. indicates a separate effect size for different diseases in each study).
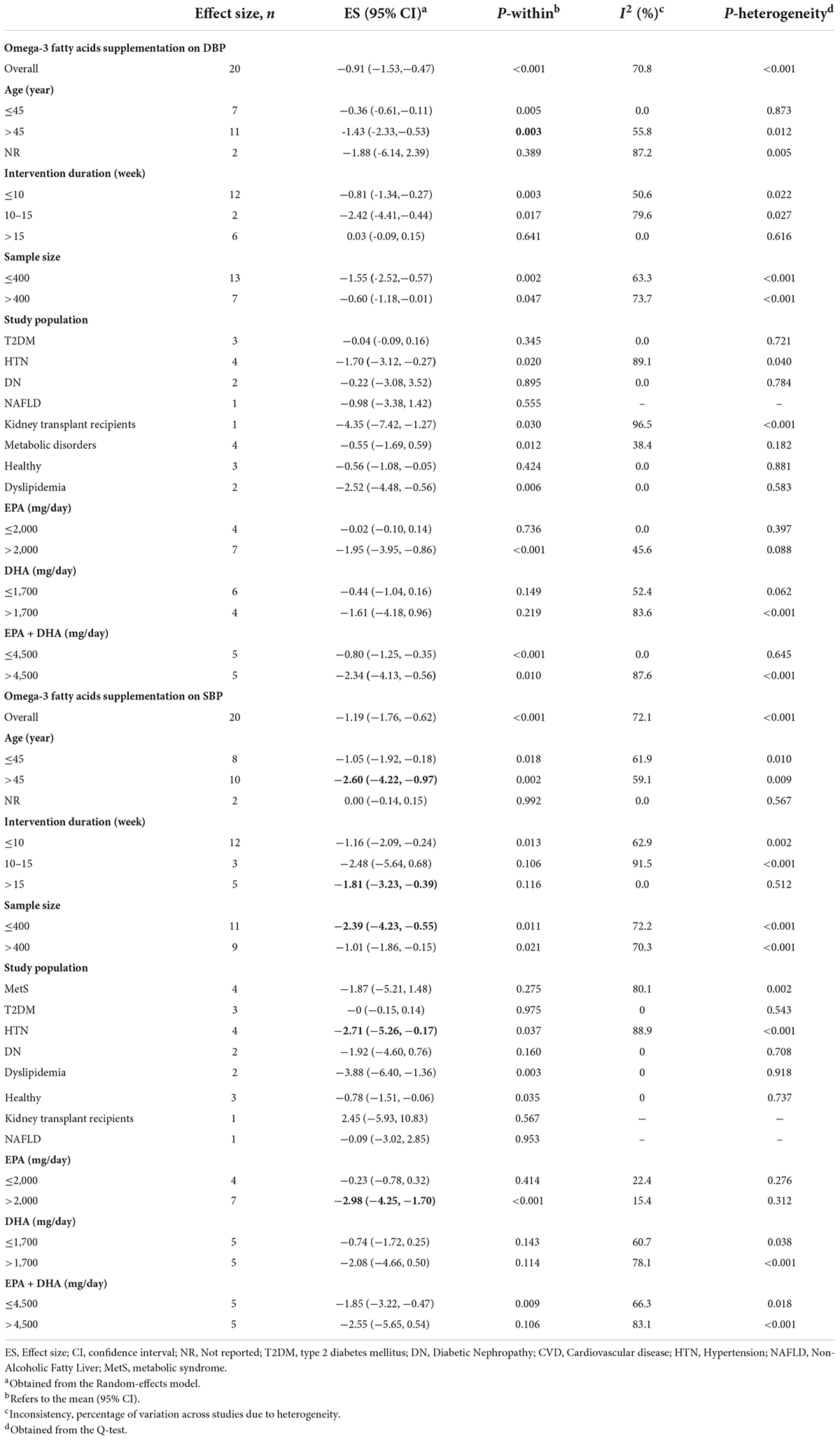
Table 3. Subgroup analyses for the effects of omega-3 fatty acids supplementation on blood pressure.
Impact of n-3 PUFAs on systolic blood pressure
The effect of n-3 PUFAs supplementation on SBP was investigated in ten meta-analyses with 20 effect sizes, including 6,734 subjects. The pooled estimate revealed that in subjects who consumed n-3 PUFAs supplements, SBP significantly was reduced (ES = −1.19 mmHg; 95% CI: −1.76, −0.62, p < 0.001) (Figure 2B). There was detected meaningful heterogeneity between the studies (I2 = 71.2%, p < 0.001). Subgroup analysis showed that EPA supplementation (> 2,000 mg/day) in intervention duration of > 15-weeks, a mean age > 45 years, and a sample size of ≤ 400 participants contributes to a more pronounced influence in decreasing SBP (Table 3).
Sensitivity analysis and publication bias
Sensitivity analysis indicated that the effect of n-3 PUFAs supplementation on DBP and SBP levels was not significant (Supplementary File 1).
Egger’s test, unlike Begg’s, showed a significant small-study effect for DBP (p = < 0.001 and 0.626, respectively), and SBP (p = < 0.001 and 0.948, respectively). In addition, publication bias was detected through a visual assessment of the funnel plots (Figure 3). Trim and fill adjustment did not alter the overall effect size.
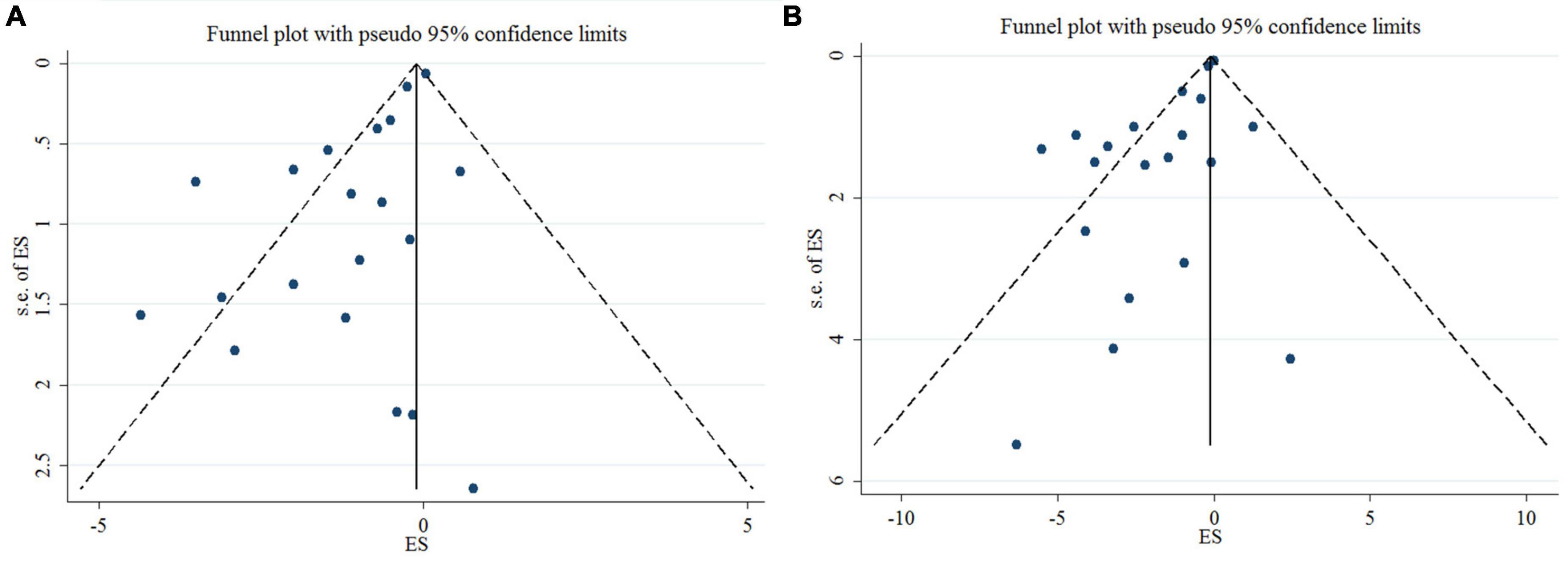
Figure 3. Mean difference and 95% CIs presented in funnel plots; and publication bias of the studies the effects of n-3 PUFA supplementation on DBP (A), and SBP (B) levels.
Discussion
The current umbrella meta-analysis of the n-3 PUFAs effect on BP indices summarized 10 meta-analyses including 131 trials. Although several studies have evaluated the effect of n-3 PUFAs on CVD risk factors, inconsistent findings have been achieved in terms of the effects of n-3 PUFAs on the BP because significant changes have not been observed consistently. Hence, we performed the present study to systematically assess the treatment effects of n-3 PUFAs on the BP in adults. Our results revealed that n-3 PUFAs supplementation can significantly decrease SBP, and DBP levels, with moderate confidence of evidence. Nevertheless, there was significant heterogeneity in the response which attributed to subject age, study population, study duration, sample size, and dosage of n-3 PUFAs. Moreover, we also perform subgroup analysis to gain further insight on the assessment of heterogeneity. Subgroup analysis revealed the effects of n-3 PUFAs were stronger in hypertensive patients, and among studies with a follow-up period of at least 10 weeks. Also, DBP and SBP were both reduced in studies lasting up to 10 weeks with daily dose of EPA > 2,000 mg/day. The quality of studies was assessed in all meta-analyses included in our study except for Morris et al. (28). Except three studies that used Jadad scale, other meta-analyses used Cochrane Collaboration’s tool to evaluate the different sources of bias in trials. Additionally, the majority of the included articles were of high risk of bias based on quality assessment.
High BP is a modifiable risk factor that can lead to cardiovascular disease (CVD), stroke, renal disease, and mortality (33, 34). In recent decades, several publications have showed that therapy with n-3 PUFAs positively effect on BP (22, 30, 35). Actually, several previous studies have reported that n-3 PUFAs may decrease CVDs risk factors by improving the inflammation (31), lipid, and glycemic profile (24, 36, 37), as well as decreasing BMI (38). In addition to above factors, the effects of n-3 PUFAs supplementation on apolipoproteins, particularly Apo-CIII has been found in recent meta-analysis (39). The findings of our study show that supplementation with n-3 PUFAs decreases DBP as well as SBP. While the magnitude of the detected effect seems small, it appears to be both clinically and statistically significant. According to previous meta-analysis, even a slight reduction in BP is helpful for CVD risk management and can efficiently decrease CVD events (40). A meta-analysis has reported that every 1 mmHg decrease in SBP leads to a nearly 2.5% sex- and age -specific reduction in vascular mortality (41). Consequently, n-3 PUFAs can be considered as an effective and without life-threatening side effects agent in control of BP.
Our results are consistent with three other meta-analyses (22, 30, 35). In the analysis of DBP, subgroup analyses indicated a stronger effect in patients with dyslipidemia, and hypertension, as well as in kidney transplant recipients. A BP lowering effect has been detected in both hypertensive and normotensive subjects, but the response appears more pronounced among hypertensive patients. Furthermore, supplementation of > 2,000 mg/d of EPA appears to be sufficient to reduce both DBP and SBP by 1.95 and 2.98 mmHg, respectively. Stratifying the meta-analysis based on the mean age of subjects revealed that n-3 PUFAs among participants with > 45 years had the most improving effect on SBP and DBP levels compared with younger participants. Long-term supplementation of n-3 PUFAs (10–15 week) led to a larger decrease in DBP levels than ≤ 10-week administration. However, given the findings of our subgroup analysis, it appears that short-term supplementation (≤ 10-week) with n-3 PUFAs, is more effective in decreasing SBP than long-term one. It should also be noted that most included meta-analysis in our review had participants with metabolic disorders, hypertension, and T2DM. Cornelissen and Fagard (42) have shown that hypertensive and normotensive participants may respond in different degrees to the same intervention. Hypertensive patients appear to be a better target population to study the hypotensive effect of n-3 PUFAs because they are more prone to changes in BP (43).
N-3 PUFAs may exert its hypotensive effects by several mechanisms. N-3 PUFAs may control the caveolae composition resulting in increased nitric oxide synthase (44). In particular, n-3 PUFAs stimulate expression and activity of endothelial nitric oxide synthase (45). The BP lowering effect of n-3 PUFAs might be ascribed to enhancements of endothelial and smooth muscle function together with lowered systemic vascular resistance (46). Also, n-3 PUFAs acts as a hypotensive agent through increasing synthesis of vasodilator mediators such as prostacyclin, and inhibiting vasoconstrictor mediators such as thromboxane which are associated with hypertension (47). Prostacyclin exerts its vasodilatory effects by activating prostacyclin receptors present on vascular smooth muscle cells and platelets, which therefore decreases peripheral vascular resistance and arterial stiffness (48). Furthermore, hypotensive property of n-3 PUFAs may be the result of its ability to significantly reduction of TC and LDL-C (31, 49). In a meta-analysis by Zhang et al. (24) significant reduction in serum TC level was observed following EPA supplementation 2 g/day. N-3 PUFAs supplementation possesses several antioxidant properties, combats intracellular reactive oxygen species, and can enhance other antioxidant defense, such as thioredoxin reductase 1, heme oxygenase-, and manganese superoxide dismutase, thus protecting endothelial cells from oxidative functional damages and regulating BP (50, 51). It has also been reported that n-3 PUFAs can counteract the release of pro-inflammatory cytokines in the myocardium and vascular endothelium, so restoring vascular reactivity and myocardial performance (50). Hence, the anti-inflammatory actions of n-3 PUFAs may be another putative way by which antihypertensive effects are exerted.
The main strength of this review was that this umbrella meta-analysis provided a comprehensive summary of the literature and its results and given the potential effect on the management and treatment of hypertension, this is a useful attempt. We revealed that there is sufficient evidence for n-3 PUFAs to elicit helpful effects on blood pressure. In the sensitivity analysis, no changes in the results were observed excluding each meta-analysis; this supports the robustness of our results. However, current study has several limitations. The analyses involved subjects with several health statuses such as T2DM, dyslipidemia, kidney transplant recipients, NAFLD, and metabolic syndrome. While this allowed more meta-analyses and subjects to be included for analysis, this could affect generalizability. However, we done random effects model in our analyses as well as explored for heterogeneity, the current findings are limited by possible residual confounding such as different background diets of participants. Finally, the proportion of n-6 PUFAs: n-3 PUFAs may play an important role in decreasing the risk factors of CVDs (52–54). However, we did not recognize any meta-analysis to involve the relationship between the ratio of n-3/n-6 PUFAs and BP.
Conclusion
In summary, this umbrella meta-analysis of 10 meta-analyses revealed that, compared with control, n-3 PUFAs intake was related to meaningfully greater degree of SBP, and DBP decrease, and this effect was more obvious in hypertensive subjects. Consequently, supplementation with n-3 PUFAs may be a useful adjuvant therapy in hypertensive patients.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors. All the materials used in this systematic review and meta-analysis have been fully referenced.
Author contributions
VM and PD designed research. ZK and MV conducted a systematic search. BN and ZK screened articles. ZK and VM extracted data and drew tables. VM and MV analyzed and interpreted the data. MV, VM, and ZK wrote the manuscript. PD had primary responsibility for the final content. All authors read and approved the final manuscript.
Funding
This research protocol was approved and supported by Student Research Committee, Tabriz University of Medical Sciences (Registration code: 70429).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.985451/full#supplementary-material
References
1. World Health Organization [WHO]. World Health Organization obesity and overweight fact sheet 2016. Geneva: World Health Organization (2016).
2. Kung H-C, Xiu J. Hypertension-related mortality in the United States, 2000-2013. Washington, DC: US Department of Health and Human Services (2015). p. 1–8.
3. Davis BC, Kris-Etherton PM. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am J Clin Nutr. (2003) 78:640S–6S. doi: 10.1093/ajcn/78.3.640S
4. Stanton AV, James K, Brennan MM, O’Donovan F, Buskandar F, Shortall K, et al. Omega-3 index and blood pressure responses to eating foods naturally enriched with omega-3 polyunsaturated fatty acids: A randomized controlled trial. Sci Rep. (2020) 10:15444. doi: 10.1038/s41598-020-71801-5
5. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2019) 393:1958–72.
6. EFSA Panel on Dietetic Products, Nutrition, and Allergies [NDA]. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. (2010) 8:1461.
7. You A. Dietary guidelines for Americans. US department of health and human services and US department of agriculture. Vol. 7. Washington, DC: US Department of Health and Human Services (2015).
8. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ. (2014) 348:g2272.
9. Lorente-Cebrián S, Costa AG, Navas-Carretero S, Zabala M, Martínez JA, Moreno-Aliaga MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J Physiol Biochem. (2013) 69:633–51.
10. Musazadeh V, Dehghan P, Saleh-Ghadimi S, Abbasalizad Farhangi M. Omega 3-rich Camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: A randomised placebo-controlled clinical trial. Int J Clin Pract. (2021) 75:e14744. doi: 10.1111/ijcp.14744
11. Holy EW, Forestier M, Richter EK, Akhmedov A, Leiber F, Camici GG, et al. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler Thromb Vasc Biol. (2011) 31:1772–80. doi: 10.1161/ATVBAHA.111.226118
12. Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, et al. Effect of omega-3 fatty acids supplementation on endothelial function: A meta-analysis of randomized controlled trials. Atherosclerosis. (2012) 221:536–43.
13. Christensen JH. Omega-3 polyunsaturated fatty acids and heart rate variability. Front Physiol. (2011) 2:84. doi: 10.3389/fphys.2011.00084
14. Xin W, Wei W, Li X-Y. Short-term effects of fish-oil supplementation on heart rate variability in humans: A meta-analysis of randomized controlled trials. Am Clin Nutr. (2013) 97:926–35. doi: 10.3945/ajcn.112.049833
15. Calò L, Martino A, Tota C. The anti-arrhythmic effects of n- 3 PUFAs. Int J Cardiol. (2013) 170:S21–7. doi: 10.1016/j.ijcard.2013.06.043
16. Da Cunha D, Hamlin R, Billman G, Carnes C. n-3 (omega-3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br J Pharmacol. (2007) 150:281–5.
17. Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc Natl Acad Sci U S A. (2013) 110:4816–21. doi: 10.1073/pnas.1221997110
18. Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J o Nutr. (2011) 106:974–80. doi: 10.1017/S0007114511002819
19. AbuMweis S, Jew S, Tayyem R, Agraib L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J Hum Nutr Dietetics. (2018) 31:67–84. doi: 10.1111/jhn.12493
20. Musazadeh V, Dehghan P, Khoshbaten M. Efficacy of omega-3-rich Camelina sativa on the metabolic and clinical markers in nonalcoholic fatty liver disease: A randomized, controlled trial. Eur J Gastroenterol Hepatol. (2022) 34:537–45. doi: 10.1097/MEG.0000000000002297
21. Averna M, Banach M, Bruckert E, Drexel H, Farnier M, Gaita D, et al. Practical guidance for combination lipid-modifying therapy in high-and very-high-risk patients: A statement from a European Atherosclerosis Society Task Force. Atherosclerosis. (2021) 325:99–109. doi: 10.1016/j.atherosclerosis.2021.03.039
22. Campbell F, Dickinson HO, Critchley JA, Ford GA, Bradburn M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur J Prevent Cardiol. (2013) 20:107–20.
23. Appel LJ, Miller ER, Seidler AJ, Whelton PK. Does supplementation of diet with’fish oil’reduce blood pressure?: A meta-analysis of controlled clinical trials. Arch Internal Med. (1993) 153:1429–38.
24. Zhang H-J, Gao X, Guo X-F, Li K-L, Li S, Sinclair AJ, et al. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin Nutr. (2021) 40:4538–50. doi: 10.1016/j.clnu.2021.05.025
25. Chewcharat A, Chewcharat P, Rutirapong A, Papatheodorou S. The effects of omega-3 fatty acids on diabetic nephropathy: A meta-analysis of randomized controlled trials. PLoS One. (2020) 15:e0228315. doi: 10.1371/journal.pone.0228315
26. Musa-Veloso K, Venditti C, Lee HY, Darch M, Floyd S, West S, et al. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr Rev. (2018) 76:581–602. doi: 10.1093/nutrit/nuy022
27. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008.
28. Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. (1993) 88:523–33.
29. Radack K, Deck C. The effects of omega-3 polyunsaturated fatty acids on blood pressure: A methodologic analysis of the evidence. J Am College Nutr. (1989) 8:376–85.
30. Guo X-F, Li K-L, Li J-M, Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: A meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2019) 59:3380–93. doi: 10.1080/10408398.2018.1492901
31. O’Mahoney LL, Matu J, Price OJ, Birch KM, Ajjan RA, Farrar D, et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc Diabetol. (2018) 17:1–13. doi: 10.1186/s12933-018-0740-x
32. Lim AK, Manley KJ, Roberts MA, Fraenkel MB. Fish oil treatment for kidney transplant recipients: A meta-analysis of randomized controlled trials. Transplantation. (2007) 83:831–8.
33. Zhang Y, Jiang X, Bo J, Yin L, Chen H, Wang Y, et al. Risk of stroke and coronary heart disease among various levels of blood pressure in diabetic and nondiabetic Chinese patients. J Hypertens. (2018) 36:93–100.
34. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. (2005) 365:217–23.
35. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am J Hypertens. (2014) 27:885–96.
36. Zheng T, Zhao J, Wang Y, Liu W, Wang Z, Shang Y, et al. The limited effect of omega-3 polyunsaturated fatty acids on cardiovascular risk in patients with impaired glucose metabolism: A meta-analysis. Clin Biochem. (2014) 47:369–77.
37. Xia Y, Wang Y, Cui M, Su D. Efficacy of omega-3 fatty acid supplementation on cardiovascular risk factors in patients with polycystic ovary syndrome: A systematic review and meta-analysis. Ann Palliat Med. (2021) 10:6425–37. doi: 10.21037/apm-21-1050
38. Lee C-H, Fu Y, Yang S-J, Chi C-C. Effects of omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: A systematic review and meta-analysis. Nutrients. (2020) 12:2769.
39. Sahebkar A, Simental-Mendia LE, Mikhailidis DP, Pirro M, Banach M, Sirtori CR, et al. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: A systematic review and meta-analysis of randomized controlled trials. Ann Med. (2018) 50:565–75. doi: 10.1080/07853890.2018.1511919
40. Law M, Morris J, Wald N. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. (2009) 338:b1665. doi: 10.1136/bmj.b1665
41. Collaboration PS. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360:1903–13. doi: 10.1016/s0140-6736(02)11911-8
42. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure–regulating mechanisms, and cardiovascular risk factors. Hypertension. (2005) 46:667–75. doi: 10.1161/01.HYP.0000184225.05629.51
43. MacGregor GA, Rotellar C, Markandu ND, Smith SJ, Sagnella GA. Contrasting effects of nifedipine, captopril, and propranolol in normotensive and hypertensive subjects. J Cardiovasc Pharmacol. (1982) 4:S358–62.
44. Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, et al. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. (2007) 89:169–77. doi: 10.1016/j.biochi.2006.10.009
45. Gousset-Dupont A, Robert V, Grynberg A, Lacour B, Tardivel S. The effect of n-3 PUFA on eNOS activity and expression in Ea hy 926 cells. Prostaglandins Leukot Essent Fatty Acids. (2007) 76:131–9. doi: 10.1016/j.plefa.2006.11.005
46. Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. (2000) 102:1264–9. doi: 10.1161/01.cir.102.11.1264
47. Knapp HR. Hypotensive effects of ω3 fatty acids: Mechanistic Aspects1. Health effects of omega 3 polyunsaturated fatty acids in seafoods. In: AP Simopoulos, RR Kifer, RE Martin, S Barlow editors. Proceedings of the world review of nutrition and dietetics 2nd international conference. Vol. 66. Basel: Karger (1991). p. 313–28.
48. Fetalvero KM, Martin KA, Hwa J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. (2007) 82:109–18.
49. Pei J, Zhao Y, Huang L, Zhang X, Wu Y. The effect of n-3 polyunsaturated fatty acids on plasma lipids and lipoproteins in patients with chronic renal failure—a meta-analysis of randomized controlled trials. J Renal Nutr. (2012) 22:525–32. doi: 10.1053/j.jrn.2012.04.005
50. Oppedisano F, Macrì R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines. (2020) 8:306.
51. Pertierra LR, Hughes KA, Vega GC, Olalla-Tárraga MA. High resolution spatial mapping of human footprint across Antarctica and its implications for the strategic conservation of avifauna. PLoS One. (2017) 12:e0168280. doi: 10.1371/journal.pone.0168280
52. Griffin BA. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol. (2008) 19:57–62. doi: 10.1097/MOL.0b013e3282f2e2a8
53. Takahashi M, Ando J, Shimada K, Nishizaki Y, Tani S, Ogawa T, et al. The ratio of serum n-3 to n-6 polyunsaturated fatty acids is associated with diabetes mellitus in patients with prior myocardial infarction: A multicenter cross-sectional study. BMC Cardiovasc Disord. (2017) 17:41. doi: 10.1186/s12872-017-0479-4
Keywords: n-3 PUFAs, blood pressure, systematic review, umbrella of meta-analysis, omega-3 fatty acid
Citation: Musazadeh V, Kavyani Z, Naghshbandi B, Dehghan P and Vajdi M (2022) The beneficial effects of omega-3 polyunsaturated fatty acids on controlling blood pressure: An umbrella meta-analysis. Front. Nutr. 9:985451. doi: 10.3389/fnut.2022.985451
Received: 03 July 2022; Accepted: 25 July 2022;
Published: 18 August 2022.
Edited by:
Vijaya Juturu, Lonza Capsules and Health Ingredients, United StatesReviewed by:
Philip Calder, University of Southampton, United KingdomŽeljko Reiner, University Hospital Centre Zagreb, Croatia
Copyright © 2022 Musazadeh, Kavyani, Naghshbandi, Dehghan and Vajdi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Parvin Dehghan, ZGVoZ2hhbi5udXRAZ21haWwuY29t; Mahdi Vajdi, bXYudmFqZGlAZ21haWwuY29t
 Vali Musazadeh
Vali Musazadeh Zeynab Kavyani1,2
Zeynab Kavyani1,2 Parvin Dehghan
Parvin Dehghan Mahdi Vajdi
Mahdi Vajdi