- 1College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha, China
- 2State Key Laboratory of Utilization of Woody Oil Resource, Hunan Academy of Forestry, Changsha, China
Lilii Bulbus, an edible Chinese herbal medicine, has a long history in medicine. However, research on effectively boiling Lilii Bulbus is rare. To make the more nutritious Lilii Bulbus soup, the optimized boiling process, using an alternate heating mode by decoction pot carrying a mixture of water and Chinese liquor at the ration of 9:1, was established in this study. Compared to the soup prepared by the daily process, the polysaccharide amount improved by 54%, and the total heavy metals decreased by 33.5% using the optimized boiling process. In addition, the total saponins at 34.3 μg/g were determined in the soup prepared by the optimized process. Meanwhile, the colchicine content in the boiled Lilii Bulbus soup was undetectable using the optimized process. This research performs an optimized boiling process for making Lilii Bulbus soup, and provides a reference for generating high commercial value from Lilii Bulbus soup in the future.
Introduction
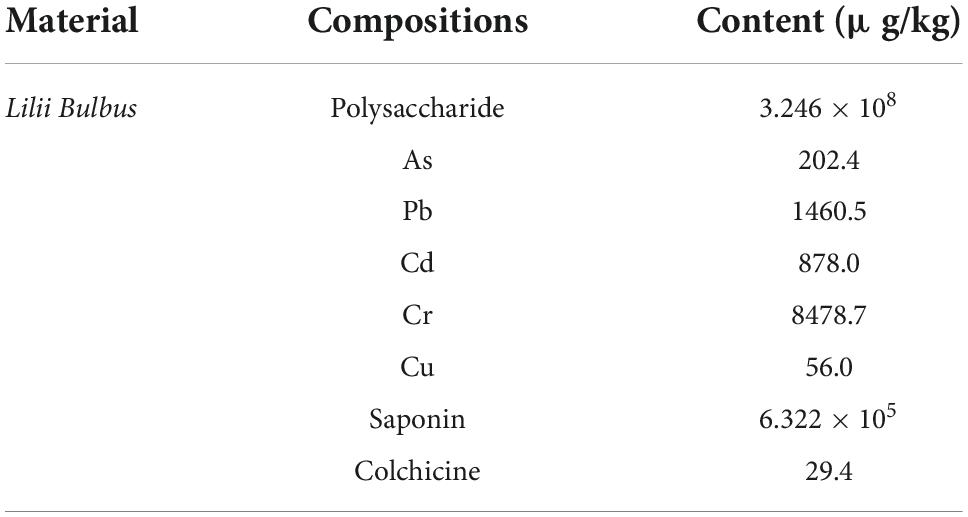
Lilii Bulbus, an edible Chinese herbal medicine recognized by the China Food and Drug Administration (CFDA), is the fleshy bulb scale of Lilium brownii F.E. Brown var. viridulum Baker from the family Liliaceae. Lilii Bulbus contains many active components, including polysaccharides, steroidal saponins, and alkaloids, which perform clinical functions, such as relieving cough and asthma, reducing blood sugar, and improving immunity (1). Among these, polysaccharides are one of the major components of Lilii Bulbus because they exhibit anti-tumor, blood lipid reduction, anti-viral, and anti-mutation functionalities, and also improve cellular immune activity (2, 3). Lilii Bulbus is also particularly rich in steroidal saponins which exhibit anti-inflammatory, antioxidant, antidepressant, and anti-tumor effects (4, 5).
Lilii Bulbus is cultivated in China and has a long history of being consumed locally as food (6). In particular, Lilii Bulbus cultivated in Hunan, Jiangsu, Zhejiang, and Gansu is of high quality and is beneficial to humans. At present, boiling is the most common method used to cook Lilii Bulbus in China, such as in its frequent use in Cantonese slow-cooked soups (7). However, the structure of the nutritional components is subject to change by the cooking method, resulting in their loss and destruction (8, 9).
Presently, the food chain is readily contaminated because of the bioaccumulation, lack of degradability, and excessive presence of heavy metals (10). Long-term intake and accumulation of such heavy metals in the blood can be very harmful to human health (11–13). Previously, the National Poisons Unit in London conducted a pilot survey to investigate the frequency of occurrence and the severity of adverse effects and toxicity from exposure to traditional medicines and food supplements. In this survey, five confirmed cases of heavy metal poisoning resulting from the use of contaminated traditional herbal remedies were found (14). Exogenous contaminants, such as the previously discussed heavy metals, are inevitably found in Lilii Bulbus because it grows underground (15). In previous studies, cadmium (Cd) content exceeded the safe values in Lilii Bulbus samples from Zhejiang (16). More recently, the contents of Cd, copper (Cu), and mercury in 60 commercial Chinese medicinal materials, including Lilii Bulbus, were reported to exceed the limits of the “Green Trade Standards of Importing and Exporting Medicinal Plants and Preparations” (WM2-2001). Cd, mercury, and Cu content in these herbal medicines exceeded safe limits by 38.8, 8.3, and 1.7%, respectively (17). Thus, it is obvious that in relation to functional nutritional content and heavy metal contamination, the boiling methods used in the preparation of soup need further investigation.
Numerous studies have been conducted on the characteristics of the nutrients in soups in relation to different boiling processes. For example, Song et al. (18) investigated the effects of different boiling times on Bian-Que Triple-Bean Soup. In this study, the total content of phenolics, saponins, tannins, and monomeric anthocyanins in the soup were determined, and the best boiling time that also retained the active ingredients was found. Additional auxiliary materials are also commonly added during cooking and can have a desirable effect on the active elements discussed previously. Liquor or vinegar is often used to process medicinal materials in the pharmacopoeia (1). For example, liquor has been used to reduce the bitter taste of Angelica dahurica (19). Silva et al. (20) found that vinegar can be used to improve the preservation capability of pickled jurubeba fruits. However, the researchers have focused on the desirable qualities of Lilii Bulbus (21, 22); research on effectively boiling Lilii Bulbus is rare.
To prepare more nutritious Lilii Bulbus soup based on the retention of active components (polysaccharides and saponins) and the reduction of harmful substances (heavy metals and colchicine), this study aimed to establish a more suitable boiling process by optimizing the heating mode and boiling solvent used. These conditions were varied in a series of boiling processes which were compared to the daily process. Thus, this research will provide an optimized boiling process for making Lilii Bulbus soup in daily life and also offers the reference for generating a higher commercial value for this soup.
Materials and methods
Preparation of materials
Lilii Bulbus was purchased from Beijing QianCao Chinese Herbal Pieces Co., Ltd. Chinese liquor (38% concentration) was purchased from Beijing Shunxin Agriculture Co., Ltd. White vinegar was purchased from Changsha Xianglian Sauce and Food Co., Ltd. The total polysaccharide were measured by the Total Polysaccharide Determination Kit (KeMing, China). The decoction pot was purchased from Joyoung Co., Ltd. The digital electric heater was purchased from Shanghai LiChen Instrument Technology Co., Ltd.
The different boiling processes of Lilii Bulbus
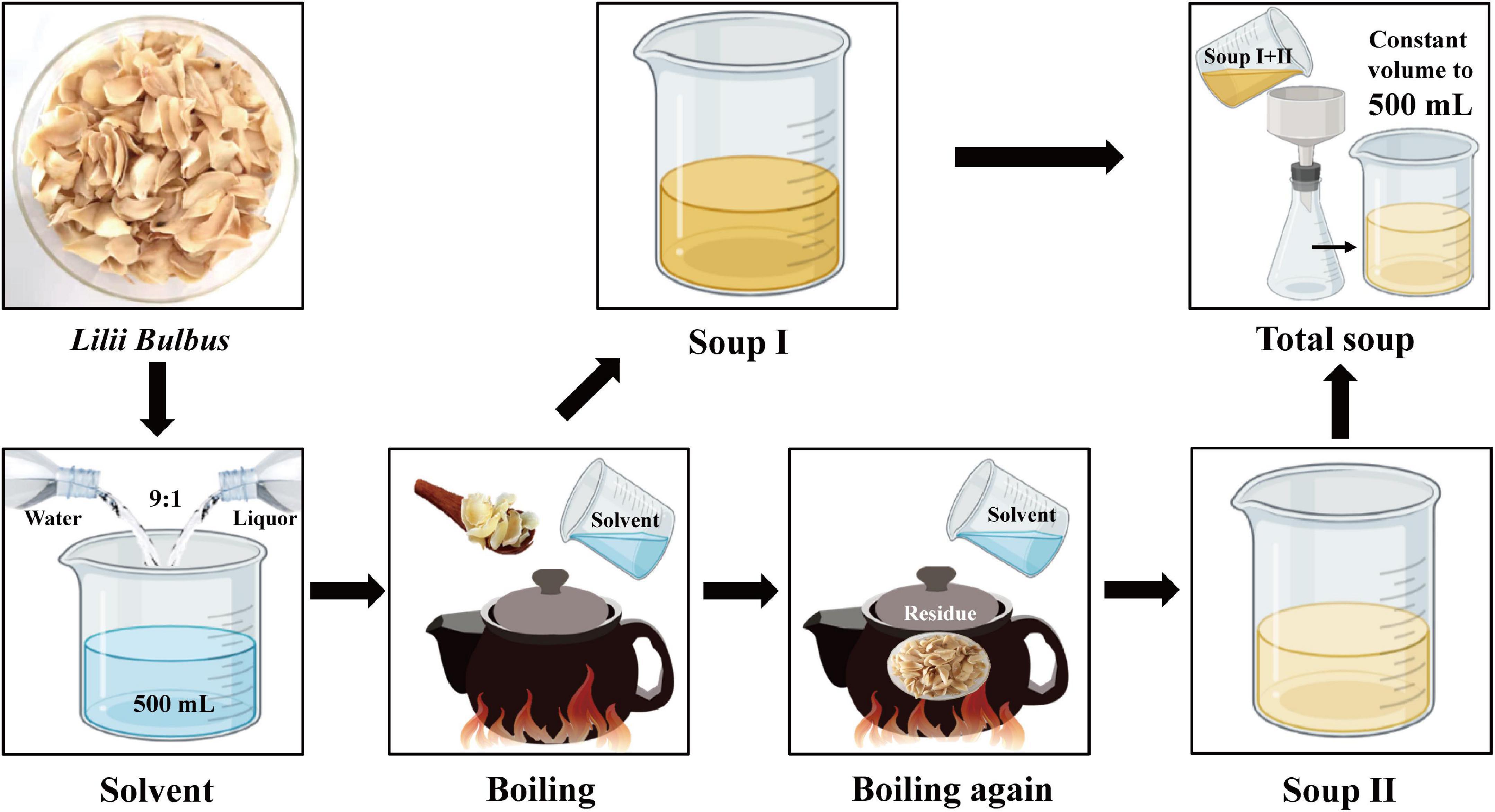
Fifty gram Lilii Bulbus were weighed out and soaked in 500 mL solvent with a 1:10 material to liquid ratio for 30 min.
Daily process: Pure water was used as the solvent, and a decoction pot was used to boil the Lilii Bulbus. The high heat mode was applied to boil for 30 min, and then the gentle fire mode was applied to boil for 20 min. The obtained soup was filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same manner to obtain the second soup. The final soup was a mixture of the two supernatants.
Process 1: Pure water was used as solvent, and a digital electric heater was used to boil Lilii Bulbus. The 100°C mode was applied to boil the soup for 30 min. The boiled soup was filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same process to obtain the second soup. The final soup was a mixture of the two supernatants.
Process 2: Using a mixture of water and Chinese liquor in a 9:1 ratio as the solvent, the digital electric heater was used to boil Lilii Bulbus. The 100°C mode was applied to boil for 30 min. The boiled soup was filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same manner to obtain the second soup. The final soup was a mixture of the two supernatants.
Process 3: Using a mixture of water and Chinese liquor in a 9:1 ratio as the solvent, a decoction pot was used for boiling Lilii Bulbus. The high heat mode was applied to boil the soup for 30 min, and was then switched to the gentle fire mode to boil for 20 min. The boiled soup was filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same manner to obtain the second soup. The final soup was a mixture of the two supernatants.
Process 4: A mixture of water and white vinegar in a 9:1 ratio was used as the solvent, and a digital electric heater was used for boiling Lilii Bulbus. The 100°C mode was applied to boil the soup for 30 min. The boiled soup was then filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same process to obtain the second soup. The final soup was a mixture of the two supernatants.
Process 5: A mixture of water and white vinegar in a 9:1 ratio was used as the solvent, and a decoction pot was used to boil the Lilii Bulbus. The high heat mode was applied to boil the soup for 30 min, after which the gentle fire mode was used to boil it for another 20 min. The boiled soup was filtered to obtain the supernatant, 500 mL of solvent was added again, and Lilii Bulbus was boiled in the same manner to obtain the second soup. The final soup was a mixture of the two supernatants.
The boiled soup was kept at a constant volume of 500 mL and filtered using a 0.22 μm membrane for analysis in all processes.
Extraction and determination of polysaccharide content
The total polysaccharide content of Lilii Bulbus was extracted in accordance with a previously reported method (23), which involved extracting the polysaccharide with water and precipitating it using alcohol. The amount obtained was tabulated using the phenol-sulfuric acid method. 1 mL of soup was used for this procedure to which 1 mL of water was added to dissolve the polysaccharide, 200 μL of which was absorbed. 100 μL of Reagent 1 and 0.5 mL concentrated sulfuric acid were added to the product, which was kept in a water bath at 90°C for 20 min. Running water was used to cool the product after this step. The absorbance value A was determined at a wavelength of 490 nm. Polysaccharide content was determined according to the manufacturer’s instructions.
Analysis of heavy metal content
A boiling tube containing 1 mL of the soup and 10 mL of acid mixture (GR grade HNO3: GR grade HClO4 = 4:1) was held at 25°C for approximately 16 h. It was then heated to 95°C and held at that temperature for 2 h using a water bath, and was removed when the digested sample was transparent and had a volume of approximately 1 mL. The cooled volume was fixed at 25 mL using pure water before filtering. The digested samples were analyzed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
Extraction and determination of saponins content
The total saponin content of Lilii Bulbus and its presence in the soup was determined using vanillin-perchloric acid colorimetry, which has been reported previously (24). A 5 mg/mL vanillin-glacial acetic acid test solution and a 0.2 mg/mL oleanolic acid standard solution were prepared. The solvent of the soup was completely volatilized at 80°C, after which 50 μL vanillin-glacial acetic acid and 200 μL perchloric acid were added to it. It was then placed in a water bath at 70°C for 15 min, and then cooled with a cold water bath for another 5 min. Thereafter, 650 μL glacial acetic acid was added and the mixture was shaken well and allowed to stand for 10 min. The absorbance value A was determined at a wavelength of 540 nm.
Detecting colchicine in the prepared soup
The presence of colchicine in soup was determined by High-Performance Liquid Chromatography (HPLC) under the following working conditions: Column: GL C18 (150 mm × 4.6 mm, 5 μm). Mobile phase: methanol: water (40:60, V: V) and isocratic elution. Flow rate: 1.0 mL/min. Determination wavelength: 254 nm. Column temperature: 30°C. Injection volume: 10 μL.
Statistical analyses
The raw data were processed using Microsoft Excel (Microsoft, USA). Statistical analyses and charting were performed using OriginPro 2021 (OriginLab, USA). Pairwise comparisons were analyzed using Student’s t-test. Differences were considered statistically significant at p < 0.05. All reported results were the average of three identical experimental runs.
Results and discussion
Effects of different boiling processes on total polysaccharides content in Lilii Bulbus soup
Polysaccharides are important active components of traditional Chinese medicine. To evaluate the boiling effects of different boiling processes, the total polysaccharide content in Lilii Bulbus and its soup under different boiling processes was analyzed. As shown in Figure 1 and Table 1, an 18.6 mg/g concentration of polysaccharide was present in the boiled soup prepared by the daily process, accounting for 5.7% of total polysaccharide content in Lilii Bulbus. This indicates that there is still considerable potential to further optimize the boiling of Lilii Bulbus soup. Therefore, we optimized the solvent and heating methods to obtain a higher polysaccharide content in the soup of Lilii Bulbus. The best polysaccharide extraction was achieved by process 4, in which the polysaccharide content of the soup was 31.4 mg/g (p < 0.05), which is approximately twice that of the soup prepared by daily processing. It should be noted that white vinegar was added as an additive solvent and an electric heater was used to maintain boiling in this process. In addition, the polysaccharide content of the soup prepared by process 3 reached 28.7 mg/g (p < 0.05). Furthermore, we found that the extraction of polysaccharides in soups prepared by the boiling processes that used an additive (processes 2, 3, 4, and 5) was generally better than when using only pure water (daily process and process 1), regardless of the type of additive used.
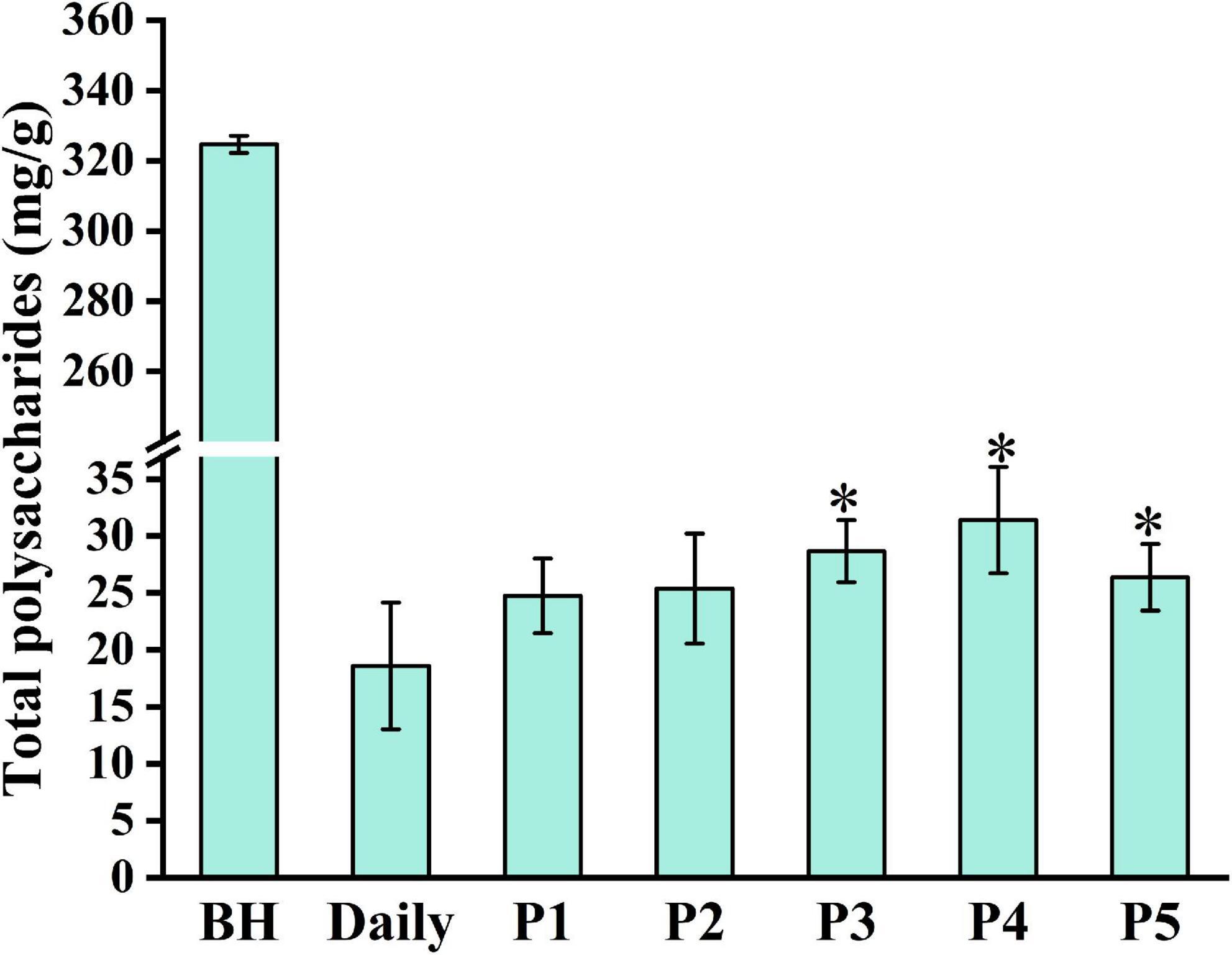
Figure 1. Total polysaccharide content in Lilii Bulbus and its soup. BH, Lilii Bulbus; Daily, Daily process; P1, Process 1; P2, Process 2; P3, Process 3; P4, Process 4; P5, Process 5. Significant differences between Daily and other processes (Student’s t-test): *P < 0.05.
Modern pharmacological studies show that polysaccharides from the traditional Chinese medicine have anti-tumor, immunity enhancing, anti-inflammatory, and anti-oxidation effects as well as helping to regulate the intestinal microenvironment (25–29). It is shown that polysaccharide can easily be depolymerized during the extraction process, and starch can easily be gelatinized and denaturalized (30). For example, garlic, like Lili Bulbus, is a plant of Liliaceae, which is also rich in polysaccharides. Lu et al. (31) found that high temperatures promote the degradation of high-MW polysaccharides into low-MW oligosaccharides and monosaccharides during black garlic processing. This phenomenon may explain why the boiled soup contains fewer polysaccharides than Lilii Bulbus itself. Moreover, various polysaccharides coexist in natural materials (32). Different extracting solvents, such as aqueous solutions with different concentrations of acid or alkali and temperatures, are used to separate polysaccharides with special properties (33–36). In addition to the water-soluble polysaccharides that are often studied, the alcohol-soluble polysaccharides in the traditional Chinese medicine are rarely studied (37, 38). The Chinese liquor was added to the solvent of process 2 and process 3, and some alcohol-soluble polysaccharides may be extracted. This may be why the content of polysaccharides in the soup prepared by process 2 and process 3 is higher than that of the daily process.
Effects of different boiling processes on heavy metal content in Lilii Bulbus soup
Heavy metals with significant biological toxicity, such as Cd, lead (Pb), chromium (Cr), and arsenic (As), can harm humans (39, 40). Herein, five common heavy metals in Lilii Bulbus itself and its soup, including As, Pb, Cd, Cr, and Cu, were analyzed.
Arsenic
As is the major pollutant in soil and water, and easily finds its way into the food chain through plants (41). The change of As content in the soup corresponding to the different boiling treatments is shown in Figure 2A. Compared to Lilii Bulbus, the Lilii Bulbus soup prepared by process 1 exhibited the best removal of As. The As content in the soup prepared by process 1 was 4.6 μg/kg, which was 19.3% lower than that in the soup prepared by daily process (5.7 μg/kg). Process 2 and process 3 exhibited 5.5 and 5.9 μg/kg of As, respectively. The results showed no significant improvement on the daily process. In addition, the addition of white vinegar showed no advantage regarding the removal of As.
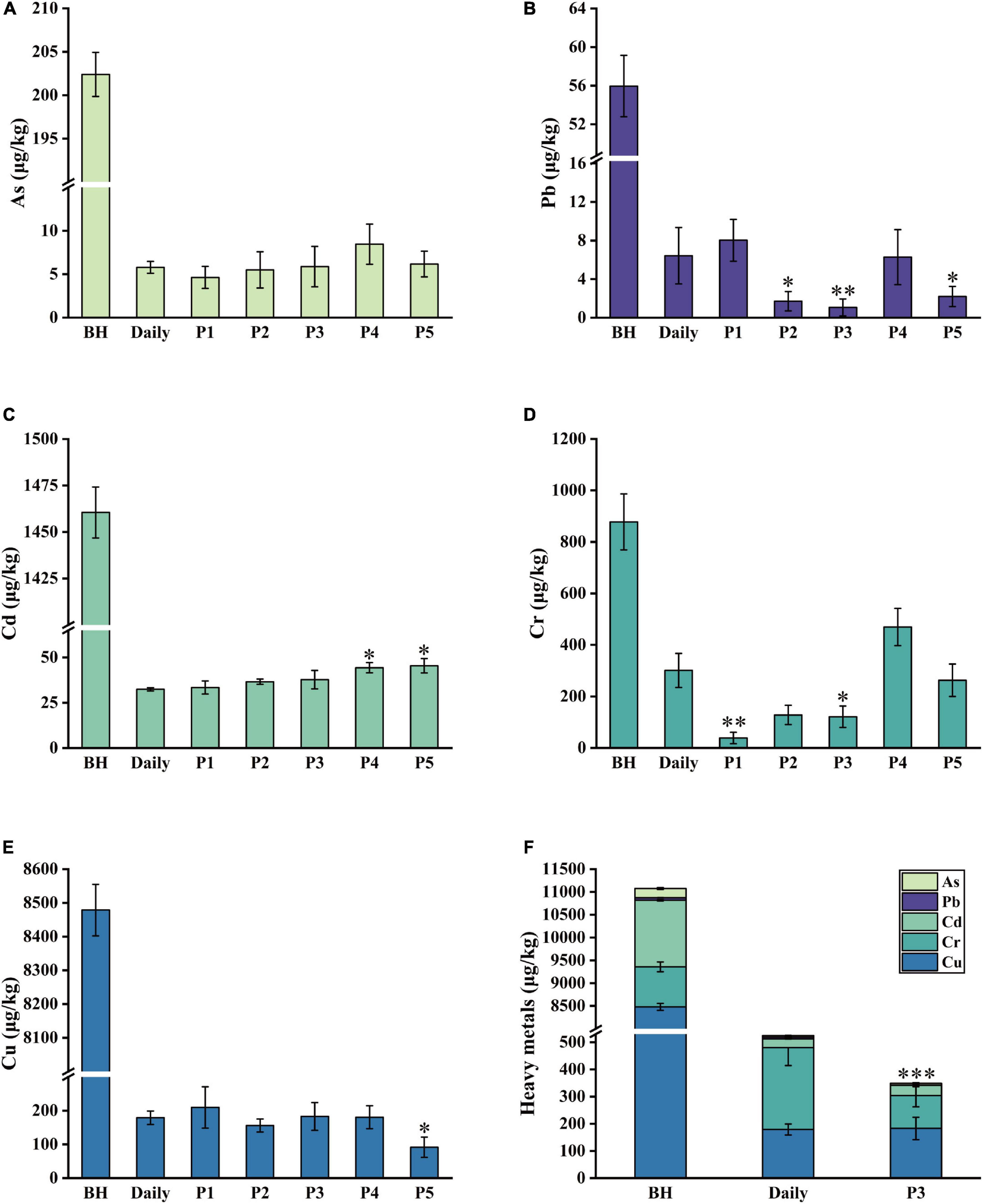
Figure 2. Five heavy metals content in Lilii Bulbus and its soup. (A) As. (B) Pb. (C) Cd. (D) Cr. (E) Cu. (F) Total heavy metals content in the soup prepared by optimized process. BH, Lilii Bulbus; Daily, Daily process; P1, Process 1; P2, Process 2; P3, Process 3; P4, Process 4; P5, Process 5. Significant differences between Daily and other processes (Student’s t-test): *P < 0.05, **P < 0.01, ***P < 0.001.
Lead
As shown in Figure 2B, Pb removal from the soup was also significantly affected by the different boiling processes. In particular, boiling process 3 performed the best, and reduced the Pb content in the soup significantly to 1.1 μg/kg, which was 98.1% lower than that of the daily process (56.0 μg/kg, p < 0.01). The Pb content in the soup prepared by process 2 was 1.7 μg/kg, which is 97% lower than that of the daily process (p < 0.05). Thus, process 2 was second only to process 3. In addition, process 5 had a desirable impact on the removal of Pb from boiled soup. As shown in Figure 2B, under boiling process with different solvents, the Pb content of the boiled soup prepared by continuous boiling at 100°C was higher than that of the soup prepared by boiling via high heat and gentle fire.
Cadmium
As shown in Figure 2C, the Cd content in Lilii Bulbus was 1460.5 μg/kg and in the soup prepared by the daily process it was 32.5 μg/kg. The Cd removal effects of process 1 (33.5 μg/kg), 2 (36.7 μg/kg), and 3 (37.8 μg/kg) were similar to that of the daily process, with no significant difference between them. This demonstrates that the Cd content was not removed more efficiently by the different boiling processes. The Cd content in the soup prepared by 100°C continuous boiling was similar to that of the soup prepared by high heat mode and gentle fire mode, regardless of the type of solvent used (Figure 2C). Thus, optimization of the heating method and boiling time may have little effect on Cd removal. Nevertheless, compared with that in Lilii Bulbus (878.0 μg/kg, Table 1), the Cd content in the boiled soup was decreased by more than 96.8%.
Chromium
There was a clear change observed in the Cr content compared to the soup prepared by the daily process. As shown in Figure 2D, process 1 showed the best removal of Cr. The Cr content in the resulting soup was found to be 38.7 μg/kg, which was 87.1% lower than that of the daily process (301.1 μg/kg, p < 0.01). Process 3 was the second most effective and the Cr content in the soup made by process 3 was found to be 121.2 μg/kg, 59.7% lower than that in the soup prepared by daily processing (p < 0.05). These results are consistent with the trend observed for As removal.
Copper
As shown in Figure 2E, the minimum Cu content, 91.5 μg/kg, was demonstrated in the soup prepared by process 5. This was 48.9% lower than that in the soup prepared by daily process (179.0 μg/kg, p < 0.05), and the optimized effect was remarkable. The subsequent most effective process was process 2 (156.2 μg/kg). However, it achieved a Cu content only 12.8% lower than that in the daily process. The effects of processes 3 and 4 were similar to those of the daily process, and the difference was not significant.
Based on the above content analysis, five heavy metals were found to accumulate in Lilii Bulbus at different levels (Table 1). Among them, Cu was the most abundant in Lilii Bulbus, which may be because Cu is an essential nutrient for plants (42). Researchers have also used boiling to bring about a significant reduction in pesticide residues in pepper fruit (43). Here, our results showed that boiling is also effective for the removal of heavy metals. Korfali et al. (44) investigated the changes of heavy metal content in a variety of local herbs. In this study, it was found that the contents of Cr, Pb, and As in the boiled decoction are lower than those in the soaked decoction, while it was only boiled with pure water for 5 min. Arpadjan et al. (45) also showed that the contents of As, Cd, and Pb in the herbal decoction are significantly lower than those in the herbs themselves.
The As, Pb, Cd, Cr, and Cu contents in the soup prepared by processes 2 and 3 were significantly lower than those of Lilii Bulbus and the soup prepared by the daily process. In particular, process 3 reduced the total heavy metal content in the boiled soup by 33.5% (Figure 2F). This suggests that the addition of Chinese liquor for boiling is beneficial for heavy metal removal, and a similar trend may be observed in general herbal medicine. Ajaiyeoba et al. (46) found that boiling in water or alcohol is the most commonly used method to prepare drug decoctions. Yi et al. (47) also found that the main components, including chlorogenic acid and syringoside, in the alcohol extract of Saussurea laniceps are higher than those in the water extract, and the alcohol extract was also more effective in pharmacological tests. In addition, the study showed that processing with rice wine can significantly reduce the cytotoxicities and enhance the anti-inflammatory effects of Herba Siegesbeckiae (48).
According to the first edition of general rule 0212 of the Chinese Pharmacopoeia (2020 Edition), the contents of Pb, Cd, As, Hg, and Cu in medicinal materials and decoction pieces (plants) should not exceed 5, 1, 2, 0.2, and 20 mg/kg, respectively (1). The Joint FAO/WHO Expert Committee for Food Additives (JECFA) reported that the weekly allowable intake of Pb, As, Cd, and Hg was 25 μg/kg body weight (BW) (49), 15 μg/kg BW (50), 7 μg/kg BW (51), and 5 μg/kg BW (49), respectively, and the maximum allowable intake for healthy adults weighing 60 kg was 14 μg/d for Pb, 129 μg/d for As, 60 μg/d for Cd, and 43 μg/d for Hg (52). The heavy metal content in Lilii Bulbus soup prepared using processes 2 and 3 was demonstrated to be far below these limits, respectively. Moreover, the polysaccharide content in the soup prepared using process 3 was higher than that in the soup prepared using process 2. Process 3 was thus found to be the most optimized process with the most desirable effects in our study. The procedure of process 3 is shown in Figure 3.
Effects of the optimized process on total saponins content in Lilii Bulbus soup
Saponins in Lilii Bulbus are steroidal saponins, including spirostanol saponins (53), isospirostanol saponins (54), deformed spirostanol saponins (55), and furostanol saponins (56), which perform a variety of pharmacological functions (57, 58). To evaluate the effect of process 3 on the total saponins, the saponin content in Lilii Bulbus soup prepared by process 3 was determined. As shown in Figure 4, the total saponin content in the soup obtained was only 34.3 μg/g, whereas the total saponin content in Lilii Bulbus itself was 632.2 μg/g (Table 1). Thus, nearly 94.6% of the saponins could not be retained in the soup after boiling, indicating that process 3 could be further optimized for saponin retention, even though it was the most effective in terms of the extraction of polysaccharides and the removal of heavy metals.
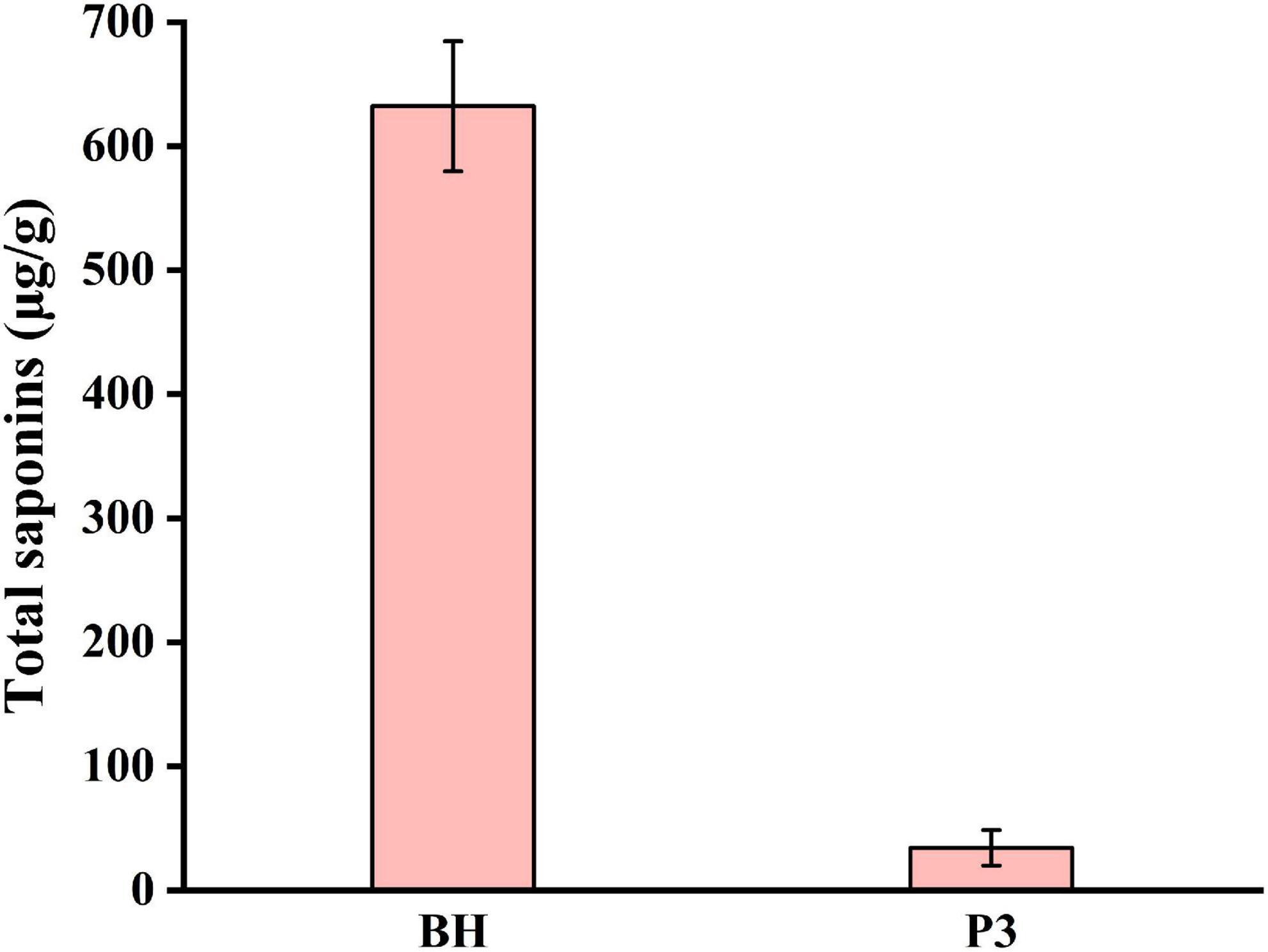
Figure 4. Total saponins content in the Lilii Bulbus soup prepared by process 3. BH: Lilii Bulbus, P3, Process 3.
There are two reasons that may lead to a low extraction rate of saponins in the soup. Firstly, saponins are steroid or triterpenoid glycosidic compounds that are not highly water-soluble, therefore, the saponins leached from the tissue would be minimal. Secondly, saponins are often lost when the raw materials are processed. For example, significant decreases in the total saponin content and the content of the three major saponin components were observed following soaking and canning of dry beans (59). Similar losses have been noted in the saponin content of moth beans that have undergone pressure-cooking (60). This phenomenon is similar to our results. Choi et al. (61) studied the contents of crude saponin in decoctions of Platycodi Radix prepared using different extraction processes. In this study, it was found that the crude saponin content in the decoctions increase with increasing extraction time, volume of water, extraction temperature, and number of repetitions for extraction.
Effects of the optimized process on colchicine content in Lilii Bulbus soup
Colchicine is a botanical alkaloid originally extracted from the seeds and bulbs of Colchicum autumnale L and is also found in Lilii Bulbus. The colchicine content in the soup prepared by process 3 was determined to evaluate its specific leaching effect. As shown in Figure 5, colchicine was not detected in the soup. In contrast, the colchicine content of Lilii Bulbus was 29.4 μg/kg (Table 1). This demonstrates that colchicine in Lilii Bulbus will not be extracted after boiling according to process 3, and there are no concerns about colchicine dosing when consuming the Lilii Bulbus soup.
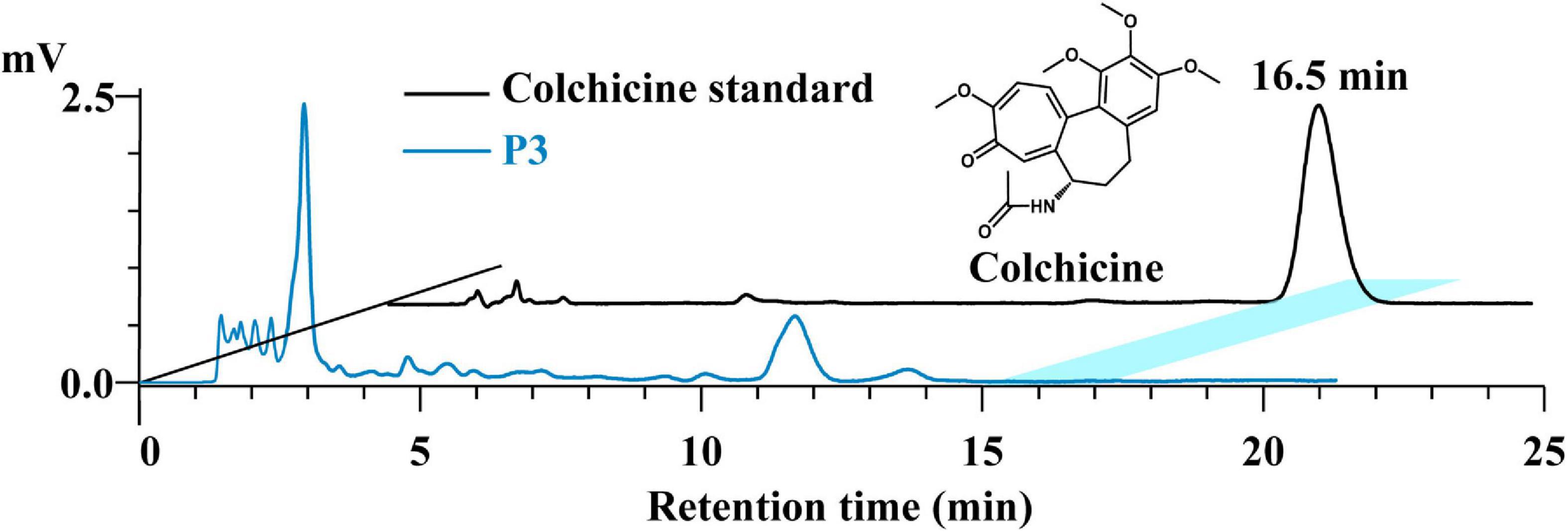
Figure 5. HPLC analysis of the colchicine in the Lilii Bulbus soup prepared by process 3. P3, Process 3.
Colchicine has been used to treat and prevent gout attacks and, in the past decade, the properties of colchicine was explored for use in the secondary prevention of cardiovascular diseases (62). Colchicine is most commonly administered orally, formulated in tablets of 0.5 or 0.6 mg. Intravenous administration is also possible in the form of a 0.5 mg/mL solution. However, gastrointestinal side effects occur in up to 80% of the patients receiving full therapeutic doses of colchicine (63). Therefore, to take in as little colchicine as possible when the dosage is uncertain, its retention should be minimized in the Lilii Bulbus soup. Here, our result was consistent with our view. In fact, colchicine may combine with the acidic components of plants and be found as salts in them because of its polarity and alkalinity, and the Lilii Bulbus must accordingly be alkalified before extraction to allow colchicine to exist as a free alkali (64, 65). Our boiling process does not include the alkalization step, and this may be the reason why we failed to detect colchicine in the boiled soup.
Conclusion
In this study, we optimized the boiling process for making Lilii Bulbus soup, focusing on the heating method and the boiling solvent. The best effects on boiling polysaccharide of Lilii Bulbus and removing five heavy metals were shown under process 3, resulting in 28.7 mg/L of polysaccharide and 0.349 mg/kg of five heavy metals in total in the boiled soup. Compared to the soup prepared by the daily process, the polysaccharide amount improved by 54%, and the total heavy metals decreased by 33.5%. In addition, 34.3 μg/g of total saponins was determined in the soup prepared by the optimized boiling process 3. Meanwhile, the colchicine content in boiled Lilii Bulbus soup was undetectable using the optimized process. This study explored the characteristics of Lilii Bulbus soup under different boiling processes, focusing on the retention of polysaccharides and removal of heavy metals. More factors, including color and phytochemical composition, can be considered to comprehensively improve the quality of soup. Together, this research provides a reference for making commercial Lilii Bulbus soup via optimizing the boiling process of Lilii Bulbus.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
GH: conceptualization, methodology, data curation, and writing-original draft. ZL: conceptualization, methodology, and supervision. HC: validation and formal analysis. YW: formal analysis. WH: investigation. XL: data curation. YT: writing-reviewing and editing and funding acquisition. HL: funding acquisition, writing-reviewing and editing, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (Nos. 2019YFC1604903 and 2019YFC1604905), the Training Program for Excellent Young Innovators of Changsha, China (No. kq2106049), and the Foundation of State Key Laboratory of Utilization of Woody Oil Resource (No. GZKF202119).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. China Pharmacopoeia Committee of Ministry of Health of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Beijing: China Medical Science Press (2020).
2. Hou R, Chen J, Yue C, Li X, Liu J, Gao Z, et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr Polym. (2016) 142:73–81. doi: 10.1016/j.carbpol.2016.01.032
3. Zhang T, Gao J, Jin ZY, Xu XM, Chen HQ. Protective effects of polysaccharides from Lilium lancifolium on streptozotocin-induced diabetic mice. Int J Biol Macromol. (2014) 65:436–40. doi: 10.1016/j.ijbiomac.2014.01.063
4. Kwon OK, Lee MY, Yuk JE, Oh SR, Chin YW, Lee HK, et al. Anti-inflammatory effects of methanol extracts of the root of Lilium lancifolium on LPS-stimulated Raw264.7 cells. J Ethnopharmacol. (2010) 130:28–34. doi: 10.1016/j.jep.2010.04.002
5. Mimaki Y, Sashida Y. Steroidal saponins and alkaloids from the bulbs of Lilium brownii var. colchesteri. Chem Pharm Bull. (1990) 38:3055–9. doi: 10.1248/cpb.38.3055
6. Mu D, Yi MF, Xia Y editors. Production and cultivation of lilies in China. Int Soc Hortic Sci. (2014) 1027:97–103.
7. Liu YJ, Liu Q, Li P, Xing DK, Hu HG, Li L, et al. Plants traditionally used to make cantonese slow-cooked soup in China. J Ethnobiol Ethnomed. (2018) 14:4. doi: 10.1186/s13002-018-0206-y
8. Meng Q, Zhou JW, Gao D, Xu EB, Guo MM, Liu DH. Desorption of nutrients and flavor compounds formation during the cooking of bone soup. Food Control. (2022) 132:108408. doi: 10.1016/j.foodcont.2021.108408
9. Zhao C, Liu YY, Lai SS, Cao H, Guan Y, San Cheang W, et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci Technol. (2019) 85:55–66. doi: 10.1016/j.tifs.2019.01.004
10. Nkwunonwo UC, Odika PO, Onyia NI. A review of the health implications of heavy metals in food chain in Nigeria. Sci World J. (2020) 2020:6594109. doi: 10.1155/2020/6594109
11. Sherwood CL, Liguori AE, Olsen CE, Lantz RC, Burgess JL, Boitano S. Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PLoS One. (2013) 8:e82970–e. doi: 10.1371/journal.pone.0082970
12. Kumar A, Kumar A, Mms CP, Chaturvedi AK, Shabnam AA, Subrahmanyam G, et al. Lead Toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches. Int J Env Res Public Health. (2020) 17:2179. doi: 10.3390/ijerph17072179
13. Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol. (2006) 1:22. doi: 10.1186/1745-6673-1-22
14. Perharic L, Shaw D, Colbridge M, House I, Leon C, Murray V. Toxicological problems resulting from exposure to traditional remedies and food supplements. Drug Saf. (1994) 11:284–94. doi: 10.2165/00002018-199411040-00006
15. Wu J, Chen Y, Ma J, Cao J, Jiang Y. Sustainable strategies for the agricultural development of Shaanxi province based on the risk assessment of heavy metal pollution. Foods. (2022) 11:1409. doi: 10.3390/foods11101409
16. Gong WQ, Du YQ, Li LW. Thinking on exploring a way to clarify cadmium excessive in edible Lilium. Agric Technol. (2015) 35:34–5.
17. Wang ZZ, Wang HB, Wang HJ, Li QC, Li Y. Heavy metal pollution and potential health risks of commercially available Chinese herbal medicines. Sci Total Environ. (2019) 653:748–57. doi: 10.1016/j.scitotenv.2018.10.388
18. Song Y, Xu B, Cai W. Active substances and in vitro anti-diabetic effects of a traditional folk remedy Bian-Que Triple-Bean Soup as affected by the boiling time. Food Funct. (2013) 4:635–43. doi: 10.1039/C3FO30303A
19. Yu M, Li T, Raza A, Wang L, Song H, Zhang Y, et al. Sensory-guided identification of bitter compounds in Hangbaizhi (Angelica Dahurica). Food Res Int. (2020) 129:108880. doi: 10.1016/j.foodres.2019.108880
20. Silva MB, Rodrigues LFOS, Rossi TC, Vieira MCS, Minatel IO, Lima GPP. Effects of boiling and oil or vinegar on pickled jurubeba (Solanum paniculatum L.) fruit. Afr J Biotechnol. (2016) 15:125–33. doi: 10.5897/ajb2015.15077
21. Zhou J, An RF, Huang XF. Genus Lilium: A review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. (2021) 270:113852. doi: 10.1016/j.jep.2021.113852
22. Qin K, Cai H, Zheng L, Zhang M, Zhang X, Gu J, et al. Development of HPLC fingerprint for quality assessment of Bulbus Lilii. Nat Prod Commun. (2013) 8:1934578X1300801028. doi: 10.1177/1934578X1300801028
23. Chen L, Huang GL. Extraction, characterization and antioxidant activities of pumpkin polysaccharide. Int J Biol Macromol. (2018) 118:770–4. doi: 10.1016/j.ijbiomac.2018.06.148
24. Lin J, Li XC, Han L, Li F, Lu WB, Bai Y, et al. Folium Sennae protects against hydroxyl radical-induced DNA damage via antioxidant mechanism: An in vitro study. Bot Stud. (2014) 55:16. doi: 10.1186/1999-3110-55-16
25. Long XS, Hu X, Liu SC, Pan C, Chen SJ, Li LH, et al. Insights on preparation, structure and activities of Gracilaria lemaneiformis polysaccharide. Food Chem X. (2021) 12:100153. doi: 10.1016/j.fochx.2021.100153
26. Niu XY, Shang HQ, Chen SY, Chen RC, Huang J, Miao YQ, et al. Effects of Pinus massoniana pollen polysaccharides on intestinal microenvironment and colitis in mice. Food Funct. (2021) 12:252–66. doi: 10.1039/D0FO02190C
27. Wen LR, Sheng ZL, Wang JP, Jiang YM, Yang B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. (2022) 373:131374. doi: 10.1016/j.foodchem.2021.131374
28. Lin YY, Pi JJ, Jin PY, Liu YT, Mai XM, Li PF, et al. Enzyme and microwave co-assisted extraction, structural characterization and antioxidant activity of polysaccharides from purple-heart radish. Food Chem. (2022) 372:131274. doi: 10.1016/j.foodchem.2021.131274
29. Guo R, Chen M, Ding Y, Yang P, Wang M, Zhang H, et al. Polysaccharides as potential anti-tumor biomacromolecules —a review. Front Nutr. (2022) 9:838179. doi: 10.3389/fnut.2022.838179
30. Han HP, Xie HC. A study on the extraction and purification process of lily polysaccharide and its anti-tumor effect. Afr J Tradit Complement Altern Med. (2013) 10:485–9. doi: 10.4314/ajtcam.v10i6.16
31. Lu X, Li N, Qiao X, Qiu Z, Liu P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT Food Sci. Technol. (2018) 95:223–9. doi: 10.1016/j.lwt.2018.04.059
32. Liu J, Willför S, Xu C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact Carbohydr Diet Fibre. (2015) 5:31–61. doi: 10.1016/j.bcdf.2014.12.001
33. Capek P, Hr̆íbalová V, Švandová E, Ebringerová A, Sasinková V, Masarová J. Characterization of immunomodulatory polysaccharides from Salvia officinalis L. Int J Biol Macromol. (2003) 33:113–9. doi: 10.1016/S0141-8130(03)00075-8
34. Colodel C, Vriesmann LC, de Oliveira Petkowicz CL. Cell wall polysaccharides from Ponkan mandarin (Citrus reticulata Blanco cv. Ponkan) peel. Carbohydr Polym. (2018) 195:120–7. doi: 10.1016/j.carbpol.2018.04.066
35. Jackson MG. Review article: The alkali treatment of straws. Anim Feed Sci Technol. (1977) 2:105–30. doi: 10.1016/0377-8401(77)90013-X
36. Ruthes AC, Smiderle FR, Iacomini M. D-glucans from edible mushrooms: A review on the extraction, purification and chemical characterization approaches. Carbohydr Polym. (2015) 117:753–61. doi: 10.1016/j.carbpol.2014.10.051
37. Yu J, Ji H, Liu A. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int J Biol Macromol. (2018) 118:2057–64. doi: 10.1016/j.ijbiomac.2018.07.073
38. Feng Y, Ji H, Dong X, Liu A. An alcohol-soluble polysaccharide from Atractylodes macrocephala Koidz induces apoptosis of Eca-109 cells. Carbohydr Polym. (2019) 226:115136. doi: 10.1016/j.carbpol.2019.115136
39. Flora SJS, Flora SJS. Arsenic-induced oxidative stress and its reversibility. Free Radical Biol Med. (2011) 51:257–81. doi: 10.1016/j.freeradbiomed.2011.04.008
40. Letelier ME, Sánchez-Jofré S, Peredo-Silva L, Cortés-Troncoso J, Aracena-Parks P. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem Biol Interact. (2010) 188:220–7. doi: 10.1016/j.cbi.2010.06.013
41. Mondal S, Pramanik K, Ghosh SK, Pal P, Ghosh PK, Ghosh A, et al. Molecular insight into arsenic uptake, transport, phytotoxicity, and defense responses in plants: A critical review. Planta. (2022) 255:87. doi: 10.1007/s00425-022-03869-4
42. Shabbir Z, Sardar A, Shabbir A, Abbas G, Shamshad S, Khalid S, et al. Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere. (2020) 259:127436. doi: 10.1016/j.chemosphere.2020.127436
43. Kim SW, Abd El Aty AM, Rahman MM, Choi JH, Lee YJ, Ko AY, et al. The effect of household processing on the decline pattern of dimethomorph in pepper fruits and leaves. Food Control. (2015) 50:118–24. doi: 10.1016/j.foodcont.2014.08.023
44. Korfali SI, Mroueh M, Al-Zein M, Salem R. Metal concentration in commonly used medicinal herbs and infusion by lebanese population: Health impact. J Food Res. (2013) 2:70–80. doi: 10.5539/jfr.v2n2p70
45. Arpadjan S, Çelik G, Taşkesen S, Güçer ş. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem Toxicol. (2008) 46:2871–5. doi: 10.1016/j.fct.2008.05.027
46. Ajaiyeoba EO, Oladepo O, Fawole OI, Bolaji OM, Akinboye DO, Ogundahunsi OAT, et al. Cultural categorization of febrile illnesses in correlation with herbal remedies used for treatment in southwestern Nigeria. J Ethnopharmacol. (2003) 85:179–85. doi: 10.1016/S0378-8741(02)00357-4
47. Yi T, Lo H, Zhao Z, Yu Z, Yang Z, Chen H. Comparison of the chemical composition and pharmacological effects of the aqueous and ethanolic extracts from a Tibetan “Snow Lotus” (Saussurea laniceps) Herb. Molecules. (2012) 17:7183–94. doi: 10.3390/molecules17067183
48. Su T, Yu H, Kwan H, Ma X, Cao H, Cheng C, et al. Comparisons of the chemical profiles, cytotoxicities and anti-inflammatory effects of raw and rice wine-processed Herba Siegesbeckiae. J Ethnopharmacol. (2014) 156:365–9. doi: 10.1016/j.jep.2014.09.038
49. Joint Fao/Who Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: Forty-fourth report of the Joint FAO/WHO expert committee on food additives. Geneva: World Health Organization (1995).
50. Joint Fao/Who Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: Sixty-first report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization (2004).
51. Joint Fao/Who Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: Seventy-third report of the Joint FAO/WHO expert committee on food additives. Geneva: World Health Organization (2011).
52. Chien MY, Yang CM, Huang CM, Chen CH. Monitoring of toxic heavy metals contamination in commonly used Chinese materia medica. SN Appl Sci. (2020) 2:1–9. doi: 10.1007/s42452-020-03227-7
53. Kintya PK, Gur’eva AS, Mashchenko NE, Shashkov AS. Structures of some lilioglycosides from the bulbs of Lilium regale. Chem Nat Compd. (1997) 33:658–62. doi: 10.1007/BF02249634
54. Zhou ZL, Feng ZC, Fu CY, Zhang HL, Xia JM. Steroidal and phenolic glycosides from the bulbs of Lilium pumilum DC and their potential Na+/K+ ATPase inhibitory activity. Molecules. (2012) 17:10494–502. doi: 10.3390/molecules170910494
55. Zhu M, Luo J, Lv H, Kong L. Determination of anti-hyperglycaemic activity in steroidal glycoside rich fraction of lily bulbs and characterization of the chemical profiles by LC-Q-TOF-MS/MS. J Funct Foods. (2014) 6:585–97. doi: 10.1016/j.jff.2013.12.002
56. Munafo JP Jr., Ramanathan A, Jimenez LS, Gianfagna TJ. Isolation and structural determination of steroidal glycosides from the bulbs of easter lily (Lilium longiflorum Thunb.). J Agric Food Chem. (2010) 58:8806–13. doi: 10.1021/jf101410d
57. Hong XX, Luo JG, Kong LY. Two new chlorophenyl glycosides from the bulbs of Lilium brownii var. viridulum. J Asian Nat Prod Res. (2012) 14:769–75. doi: 10.1080/10286020.2012.691096
58. Matsuo Y, Takaku R, Mimaki Y. Novel steroidal glycosides from the bulbs of Lilium pumilum. Molecules. (2015) 20:16255–65. doi: 10.3390/molecules200916255
59. Drumm TD, Gray JI, Hosfield GL, Uebersax MA. Lipid, saccharide, protein, phenolic acid and saponin contents of four market classes of edible dry beans as influenced by soaking and canning. J Sci Food Agric. (1990) 51:425–35. doi: 10.1002/jsfa.2740510402
60. Khokhar S, Chauhan BM. Antinutritional factors in moth bean (Vigna aconitifolia): Varietal differences and effects of methods of domestic processing and cooking. J Food Sci. (1986) 51:591–4. doi: 10.1111/j.1365-2621.1986.tb13887.x
61. Choi JS, Yoo DS, Choi YH, Yon GH, Hong KS, Lee BH, et al. Variation of saponin content in the decoctions of Platycodi Radix. Korean J Pharmacogn. (2007) 38:128–32.
62. Robinson PC, Terkeltaub R, Pillinger MH, Shah B, Karalis V, Karatza E, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. (2022) 135:32–8. doi: 10.1016/j.amjmed.2021.07.025
63. Putterman C, Ben-Chetrit E, Caraco Y, Levy M. Colchicine intoxication: Clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. (1991) 21:143–55. doi: 10.1016/0049-0172(91)90003-i
64. Cheng YH, Wen XH, Sheng CM. Analysis of colchicine in Lilium and Lilium turbid juice from different locations. Food Mach. (2007) 3:122–4. doi: 10.13652/j.issn.1003-5788.2007.03.048
Keywords: Lilii Bulbus, boiling process, total polysaccharide, heavy metal, edible Chinese herb medicine
Citation: He G, Liu Z, Chen H, Wang Y, Huang W, Lu X, Tian Y and Liu H (2022) Effects of different boiling processes on chemical compositions of Lilii Bulbus soup. Front. Nutr. 9:985105. doi: 10.3389/fnut.2022.985105
Received: 03 July 2022; Accepted: 14 September 2022;
Published: 20 October 2022.
Edited by:
Qiang Xia, Ningbo University, ChinaReviewed by:
Lingyun Zhong, Jiangxi University of Traditional Chinese Medicine, ChinaShiyi Ou, Jinan University, China
Copyright © 2022 He, Liu, Chen, Wang, Huang, Lu, Tian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Tian, dGlhbnl1bkBodW5hdS5lZHUuY24=; Huhu Liu, bGl1aHVodUBodW5hdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Guowei He1†
Guowei He1† Yun Tian
Yun Tian Huhu Liu
Huhu Liu