- 1Chongqing Academy of Animal Sciences, Chongqing, China
- 2Southwest University, Chongqing, China
- 3National Center of Technology Innovation for Pigs, Chongqing, China
Mulberry leaf is an important medicinal food plant, which is rich in polyphenol compounds. Mulberry leaf polyphenols (MLP) possess significant lipid-lowering and antioxidant effects, and healthcare functions. In this study, the polyphenol content of mulberry leaf ethanol extract was measured using HPLC. The analysis of mulberry leaf extract resulted in the identification of 14 compounds, of which Chlorogenic acid and Quercitrin were the highest. A high-fat diet (HFD)-induced obese mouse model was developed and treated with MLP for 12 weeks to explore their effect on lipid metabolism in HFD-induced obese mice. The results showed that the MLP could inhibit the weight gain and fat cell volume increase in the HFD-induced obese mice in a dose-dependent manner. Further analysis revealed that the MLP decelerated the fatty acid composition in the adipose tissues of HFD-induced obese mice, and significantly increased the polyunsaturated-to-saturated fatty acid (PUFA/SFA) ratio. The real-time quantitative PCR (RT-qPCR) results indicated that the MLP significantly inhibited the down regulation of uncoupling protein (UCP) 1 (UCP1), UCP3, and PR domain zinc finger protein 16 (PRDM16) caused by the HFD. These beneficial effects of MLP on HFD-induced obese mice might be attributed to their ability to change the fatty acid composition of adipose tissue and increase the expression of thermogenesis genes. Overall, the study results suggested that the MLP could serve as potential lipid-lowering and weight-loss functional food and healthcare products.
Introduction
Mulberry leaf, the dried leaf of Morus alba L., is widely acknowledged as a traditional Chinese herbal medicine (1). Mulberry leaf is rich in vitamins, minerals, and active ingredients, such as alkaloids, flavonoids, polysaccharides, and polyphenols, with the potential benefits of lowering blood sugar and blood lipids and antiaging, anticancer, and antibacterial effects (2, 3). Of the active ingredients, polyphenols are the fundamental compounds in mulberry leaves, contributing to their antioxidant effects (4).
Obesity has emerged as a global pandemic, eroding human health; thus, making it a major public health concern (5). Obesity is a chronic metabolic disease that disrupts physical health, such as the normal functions of the digestive and endocrine systems, and is often associated with various diseases, such as cardiovascular diseases, dyslipidemia, hyperlipidemia, and hypertension (6, 7). Obesity is mostly caused by high-calorie food intake, lack of physical exercise, and incorrect eating habits. Although the most effective approach to reducing obesity is to control diet and increase exercise (8), the application of weight loss drugs is the most favored method of weight loss for obese people (9). Therefore, finding effective drugs without apparent side effects, especially from natural products, has become the area of research interest in the field of weight loss (10).
Numerous studies have revealed that plant polyphenols may contribute to the prevention of obesity and metabolic syndrome. Chlorogenic acid and caffeine combination regulated the mRNA and protein levels of lipid metabolism-related genes in mice liver, and then inhibited fat synthesis and promoted lipid oxidation to achieve a slimming effect (11). Rutin improves the impaired insulin tolerance in SAMP8 mice fed a high-fat diet (HFD) (12). Quercetin and resveratrol in plant polyphenols are good SIRT1 agonists that can enhance PGC-1α and promote oxidative metabolism and mitochondrial genesis (13).
Mulberry leaf extract contains chlorogenic acid and its isomers, rutin, quercetin, resveratrol, quercetin, p-hydroxycinnamic acid, and caffeic acid (14). The role of MLP in anti-obesity has received increasing attention. A previous study reported that mulberry leaf ethanol extract decreases weight by acting as a melanin-concentrating hormone-1 antagonist in diet-induced obese mice (15). Mulberry leaf ethanol extract inhibited adipogenesis and the expression of adipogenesis-related factors in 3T3-L1 adipocytes (16). Therefore, MLP could be considered as potential weight loss and healthcare drugs. The anti-obesity mechanisms of MLP are multifaceted, which are not completely clear at present, and need to be further studied.
The mulberry leaf is also a high-quality domestic animal feed (17). Fatty acid composition is one of the effective indicators of the nutritional value of food (18). Will there be changes in the composition of the tissues of animals eating MLP? Is this change beneficial to the health of humans who use these animal products as food? With these questions in mind, the present study sought to explore the effects of MLP on HFD-induced obese mice and to analyze their potential value in improving animal products by evaluating their effects on fatty acid composition.
Materials and methods
Instrumentation and materials
The instruments used were AGILENGT 1260 High-efficiency liquid chromatography (Agilent Technologies, CA, United States), GC-MS 7890B-5977A (Agilent Technologies, CA, United States), and Milli-Q ultra-pure water purification system (Millipore, MA, United States). Gallic acid, gentisic acid, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, epicatechin, rutin, hyperoside, benzoic acid, quercitrin, quercetin, kaempferol, and resveratrol were purchased from Beijing Solabao Technology Co., Ltd. (Beijing, China); with puritygreater than 98%. Quality spectrometer pure methanol (swedish Oceanpak, GOT, Sweden), mass spectrometry pure acetamin and methampite (Fisher, MFL, United States), and other reagents were all of analytical grade.
Mulberry leaf polyphenol extraction
Approximately 1 kg of mulberry leaf powder was weighed and mixed with 70% ethanol according to the material concentration of 0.04 g/ml. The solution was subjected to ultrasonic extract for 60 min at 400 W and filtered with suction. The filtrate was concentrated under reduced pressure at 55°C and stored at 4°C. MLP samples were diluted 10 times in pure methanol before high-performance liquid chromatography (HPLC) analysis.
High-performance liquid chromatography conditions
The mulberry polyphenol appraisal and detection methods used by Shen et al. (19) were modified. HPLC analysis was performed on an Alltima C18 column (4.6 mm × 250 mm, 5 μm). The flow phase A was acetylene and B was 0.2% acetic acid solution. The elution procedures were 0–10 min, 5% A; 10–40 min, 5–25% A; 40–45 min, 25–35% A; and 45–50 min, 35–50% A. The flow velocity was 1 ml/min, the running time was 55 min, the column temperature was 30°C, the amount of inlet was 5 μl, and the detection wavelength was 280 nm.
Ethics statement
A total of 40 C57BL/6 healthy male mice (23.95 ± 0.81) g were procured from the Hunan SJA Laboratory Animal Co., Ltd., Chengdu, China. All the procedures and animal care were carried out in accordance with the requirements of the British Animal (Scientific Procedures) Act 1986. The experimental protocol was approved by the Animal Care and Ethics Committee of Chongqing Academy of Animal Sciences (No. cqaa2020007).
Animals and treatment
All the experimental animals were housed in a dedicated animal room at (22 ± 3)°C with a 12-h light/12-h dark cycle and allowed to eat and drink freely. After 1 week of adaptation, the experimental animals were randomly divided into 4 groups, with 10 animals in each group. The NC group was fed with a normal diet; the HFD group was fed with special feed containing 40% fat; the HFD-H group was fed with special feed containing 40% fat and gavage of 200 μl of highly concentrated mulberry leaf polyphenol extract (the liquid from 1 kg mulberry leaves was concentrated after suction filtration and diluted in 500 ml water); the HFD-L group was fed with special feed containing 40% fat and gavage of 200 μl of low concentrated mulberry leaf polyphenol extract (half of the high concentration mulberry leaf extract). Gavage was administered at 10 a.m. every day, and normal saline was administered to the NC and HFD groups only.
Organ index and Lee’s index
The unit of organ weight and body weight is g, and the unit of body length is cm.
Blood test and tissue sectioning
The concentration of total cholesterol (TC) and triglyceride (TG) in the serum was determined using a kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The fasting blood glucose concentrations were measured using a blood glucose meter (Accu-Chek Active, Roche, Ireland) (20). After sectioning, the adipose tissue was fixed with 4% paraformaldehyde, dehydrated by ethanol, infiltrated and embedded in paraffin, and stained with hematoxylin and eosin (H&E) to analyze the cell size.
Fatty acid composition analysis
The fatty acid composition test was performed according to the previously reported method (21). Gas chromatography-mass spectrometry (GC-MS 7890B-5977A, Agilent, United States) was used to detect the fatty acid composition. Then, 100 mg of adipose tissue was homogenized, 2 ml of n-hexane added, and it was shaken for 30 min at 50°C. Next, 3 ml of methanol solution (0.4 moL/L) was added and then shaken for 30 min at 50°C. Lastly, 1 ml of water was mixed with 2 ml of n-hexane and shaken for 20 min at (22 ± 3)°C. Afterward, the solution was allowed to stand for stratification, and the upper layer was separated for gas injection detection. Chromatographic column: DB-23 (30 m × 320 μm × 0.25 μm), the carrier gas was helium, inlet temperature was 250°C; the split ratio was 1/5; injection volume was 1 μl, detector temperature was 230°C, the column oven temperature was 50°C for 1 min, 25°C/min to 175°C, 4°C/min to 230°C for 24.75 min.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from mouse fat using TRIzol reagent (Invitrogen, Guangzhou, China). After reverse transcription, the mRNA level was detected using an RT-qPCR SYBR premixed Dalian kit (TaKaRa, Dalian, China) and a real-time PCR detection system (Bio-Rad, Richmond, CA, United States). ACTB was used as the mRNA internal control. The primer sequences are listed in Supplementary Table 1.
Statistical analysis
All the data were expressed as mean ± standard deviation (SD). The analysis of variance (ANOVA) and significance of differences among the means were tested using a one-way ANOVA test and SPSS 20.0 software (SPSS Inc., NY, United States). P ≤ 0.05 was considered significant.
Results
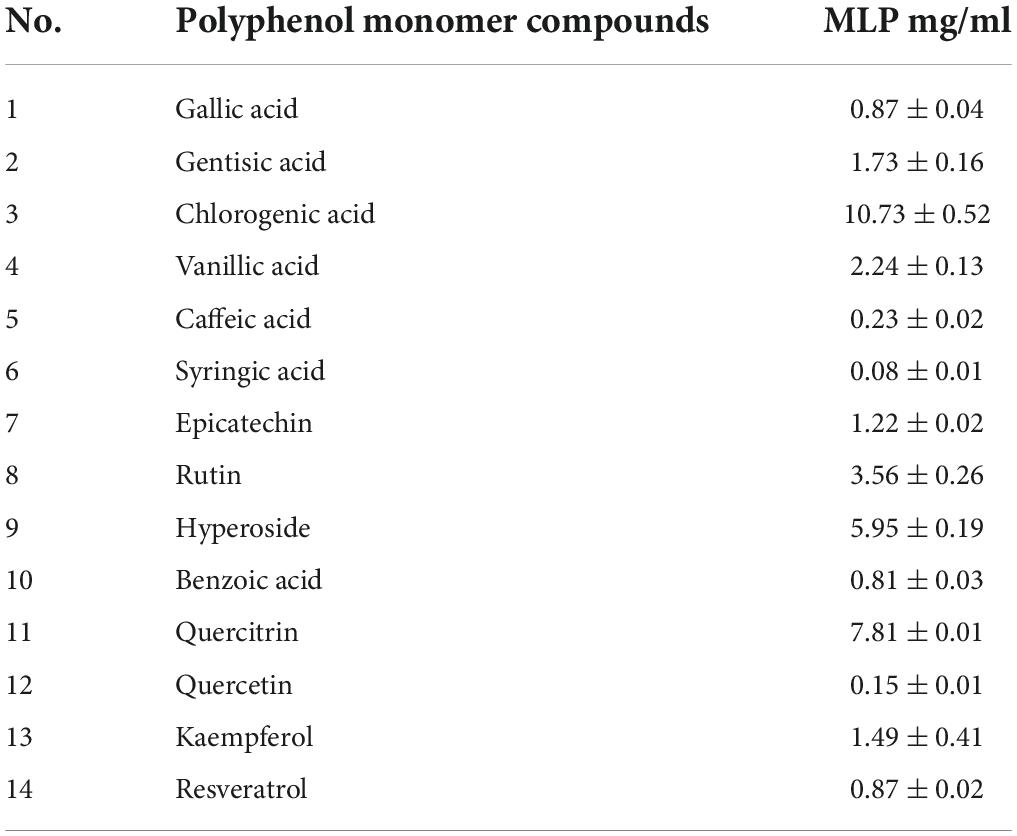
Analysis of polyphenol monomer compounds in mulberry leaf polyphenols
The HPLC method was used to qualify and quantitatively analyze MLP. The following compounds were identified in mulberry leaf extract: dragon gallic acid, gentisic acid, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, epicatechin, rutin, hyperoside, benzoic acid, quercitrin, quercetin, kaempferol, and resveratrol (14) polyphenol monomer compounds (Table 1).
Effect of mulberry leaf polyphenols on body weight in obese mice
The weights of the HFD-induced mice were significantly higher than those of the control group from the second week (Figures 1A,C). The gavage of MLP did not affect the feeding and drinking habit of mice (Figure 1B). However, the gavage of MLP significantly reduced the weight gain (Figure 1A), fasting blood glucose (Figure 1D), and Lee’s index (Figure 1E) of the HFD-induced mice.
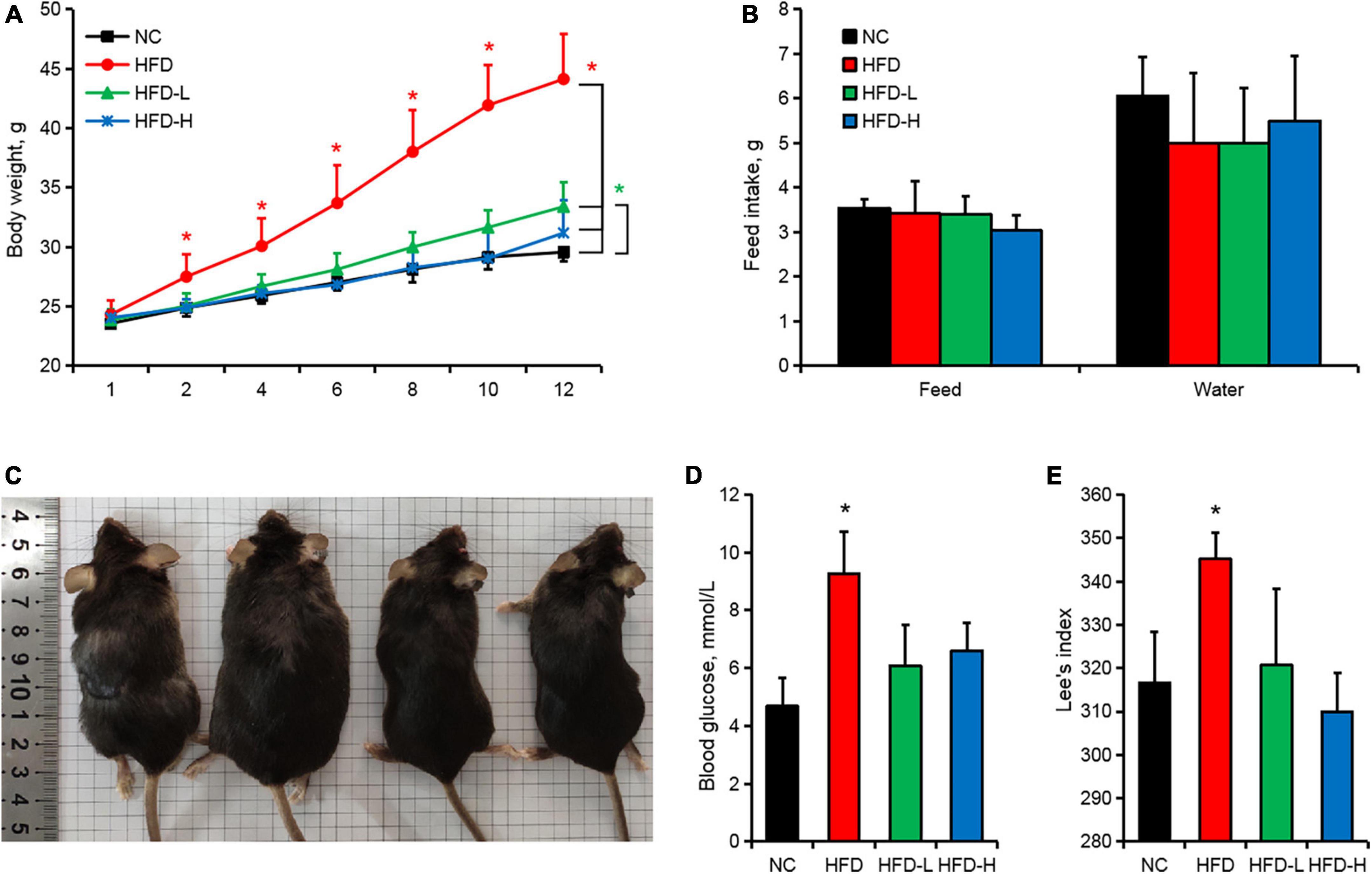
Figure 1. Mulberry leaf polyphenols (MLP) alleviated the weight gain induced by the high-fat diet (HFD) in mice. (A) Mouse body weight change curve. (B) The average daily food intake and water intake of the mice throughout the whole period. (C) Images of mouse body shapes after 12 weeks of different treatments. (D) Fasting blood glucose of mice fasted for 12 h. (E) The Lee’s index of mice after 12 weeks of different treatments. *P ≤ 0.05, n = 10.
Effects of mulberry leaf polyphenols on organ development in high-fat diet-induced obese mice
In addition to inducing obesity in mice, the HFD significantly increased the weight of the liver (Figure 2B) and kidneys (Figure 2F) in mice, but it had no significant effect on the weight of the heart (Figure 2A), gastrocnemius muscle (Gas muscle, Figure 2C), lungs (Figure 2E), and spleen (Figure 2G). Additionally, the HFD significantly increased the liver index of mice and significantly reduced the heart index, gastrocnemius index, and lung index (Figures 2D,H).
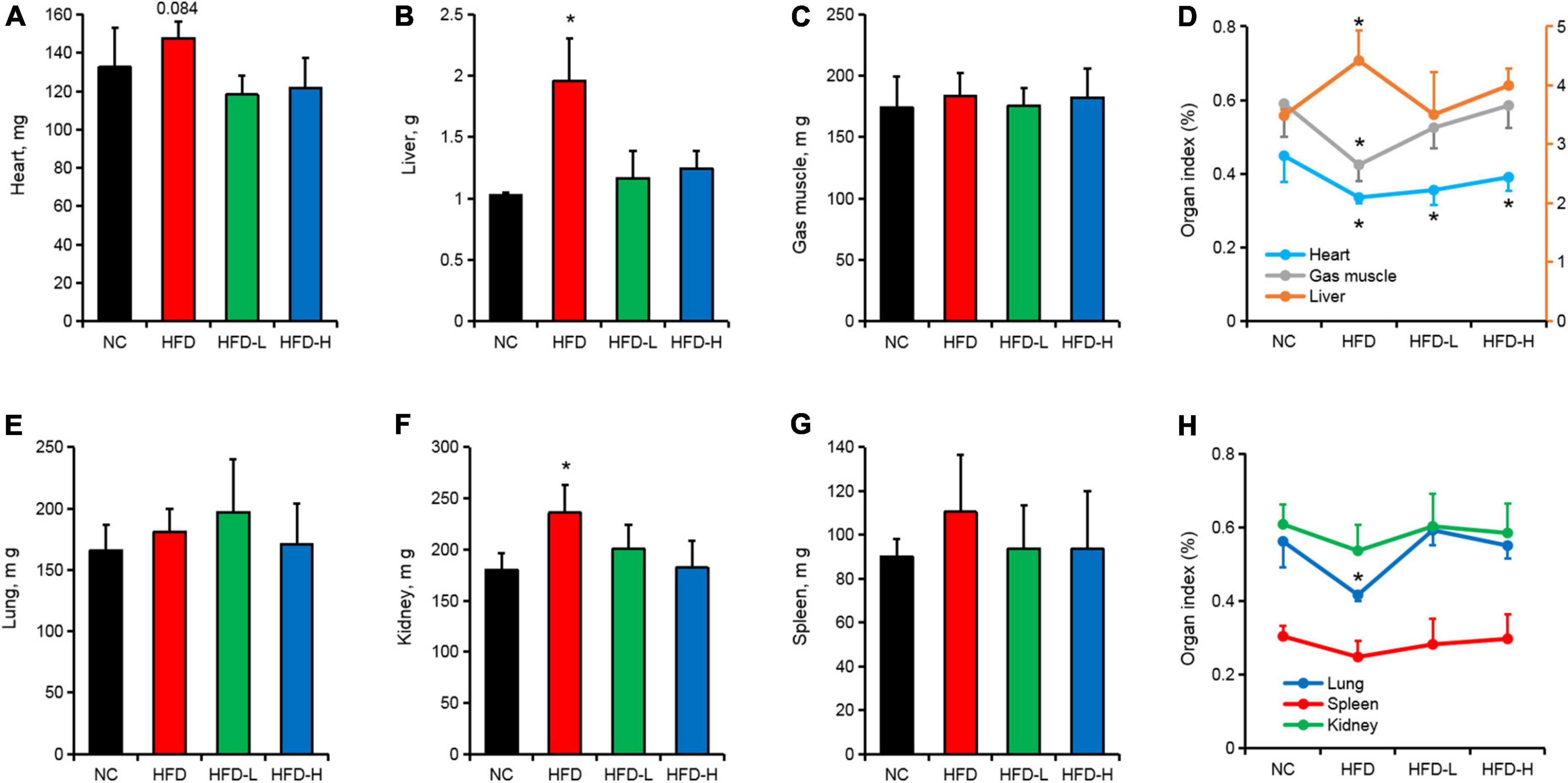
Figure 2. Effect of mulberry leaf polyphenols (MLP) on the organs of obese mice. (A–C) The weight of the heart (A), liver (B), and gastrocnemius (C) of mice after 12 weeks of MLP and high-fat diet (HFD) treatments. (D) Mice heart, liver, and gastrocnemius organ index after 12 weeks of MLP and HFD treatments. (E–G) The weight of the lungs (E), kidney (F), and spleen (G) of mice after 12 weeks of MLP and HFD treatments. (H) Mice lung, kidney, and spleen organ index after 12 weeks of MLP and HFD treatments. *P ≤ 0.05, n = 6.
Effect of mulberry leaf polyphenols on the adipose tissue of high-fat diet-induced obese mice
High-fat diet significantly increased the weight of scapular, inguinal, and gonadal fats in the mice, while low-dose and high-dose MLP significantly inhibited fat gain in different parts of the mice (Figures 3A–C,E). For the fatty organ index, only a high concentration of MLP significantly alleviated the increase in fatty organ index induced by the HFD (Figure 3D). Moreover, the H&E staining of adipose tissue and fat cell size determination results showed that a high concentration of MLP significantly inhibited the increase in fat cell volume in different parts of the mice induced by the HFD. In contrast, low-concentration MLP significantly inhibited the inguinal and gonadal fats (Figures 3F,G).
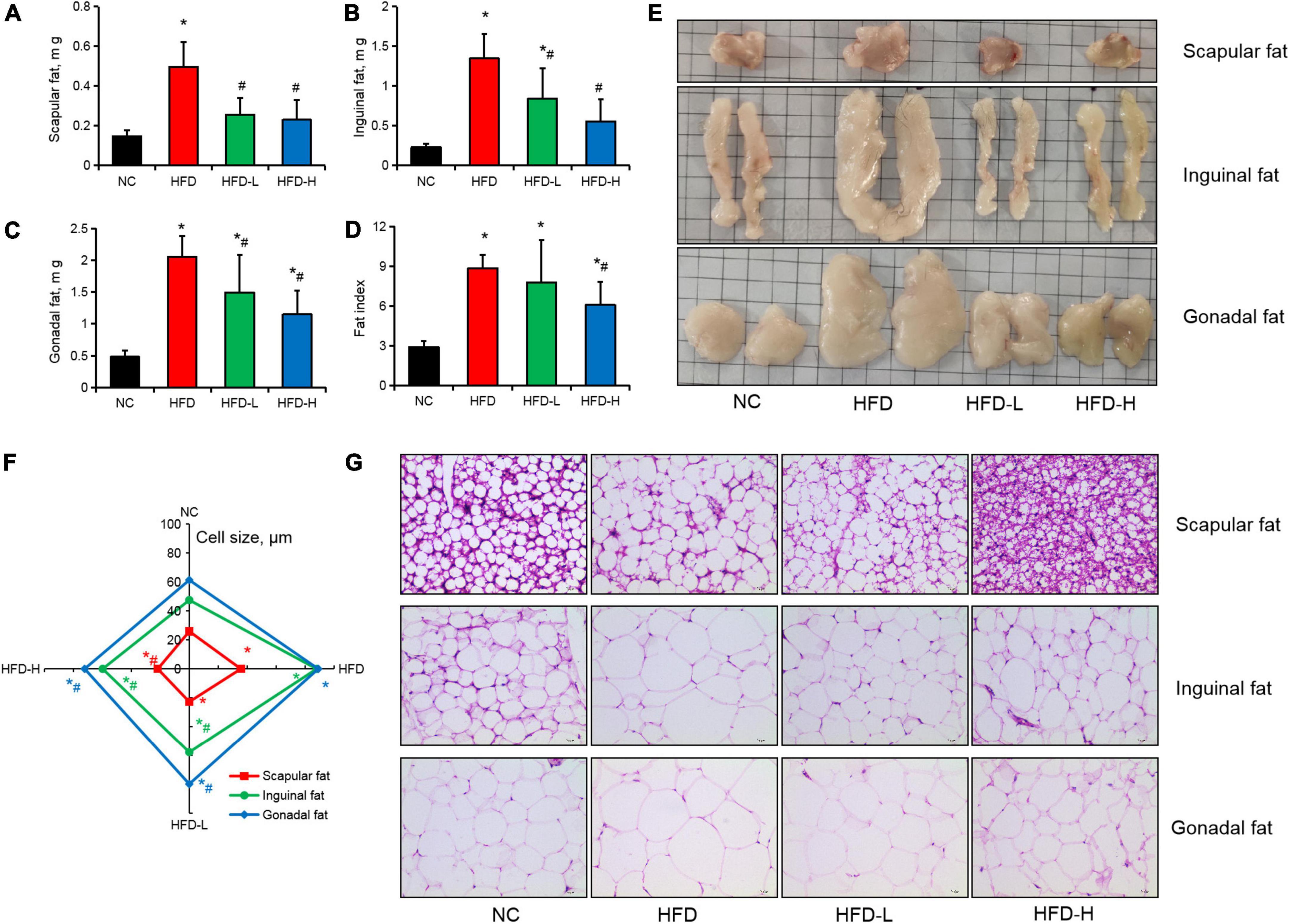
Figure 3. Mulberry leaf polyphenols (MLP) reduced fat gain in obese mice. (A–C) The scapular (A), inguinal (B), and gonadal (C) fat weights of mice after 12 weeks of MLP and high-fat diet (HFD) treatment. (D) The fat index after 12 weeks of MLP and HFD treatment (contains scapular, inguinal, and gonadal fats). (E) Images of adipose tissue in different parts of a mouse. (F) The size distribution of fat cells in different parts of the adipose tissues in mice. (G) H&E staining of scapular, inguinal, and gonadal fats. *P ≤ 0.05, compared with the NC group; #P ≤ 0.05, compared with the HFD group, n = 6.
Effect of mulberry leaf polyphenols on the fatty acid composition of adipose tissues in mice
The low concentration of MLP had no significant effect on the fat organ index and scapular fossa fat cell morphology (Figures 3D,F). Therefore, we analyzed the fatty acids in the adipose tissues of mice in the NC, HFD, and HFD-H groups and found that the fatty acid composition of the three groups was clustered separately (Figure 4A). Further analysis indicated that the HFD significantly promoted SFA and MFA content, but significantly reduced the PUFA content and PUFA/SFA ratio (Figures 4B–F). Compared with the HFD group, a high concentration of MLP significantly reduced the SFA content and increased the PUFA/SFA ratio (Figures 4B,F).
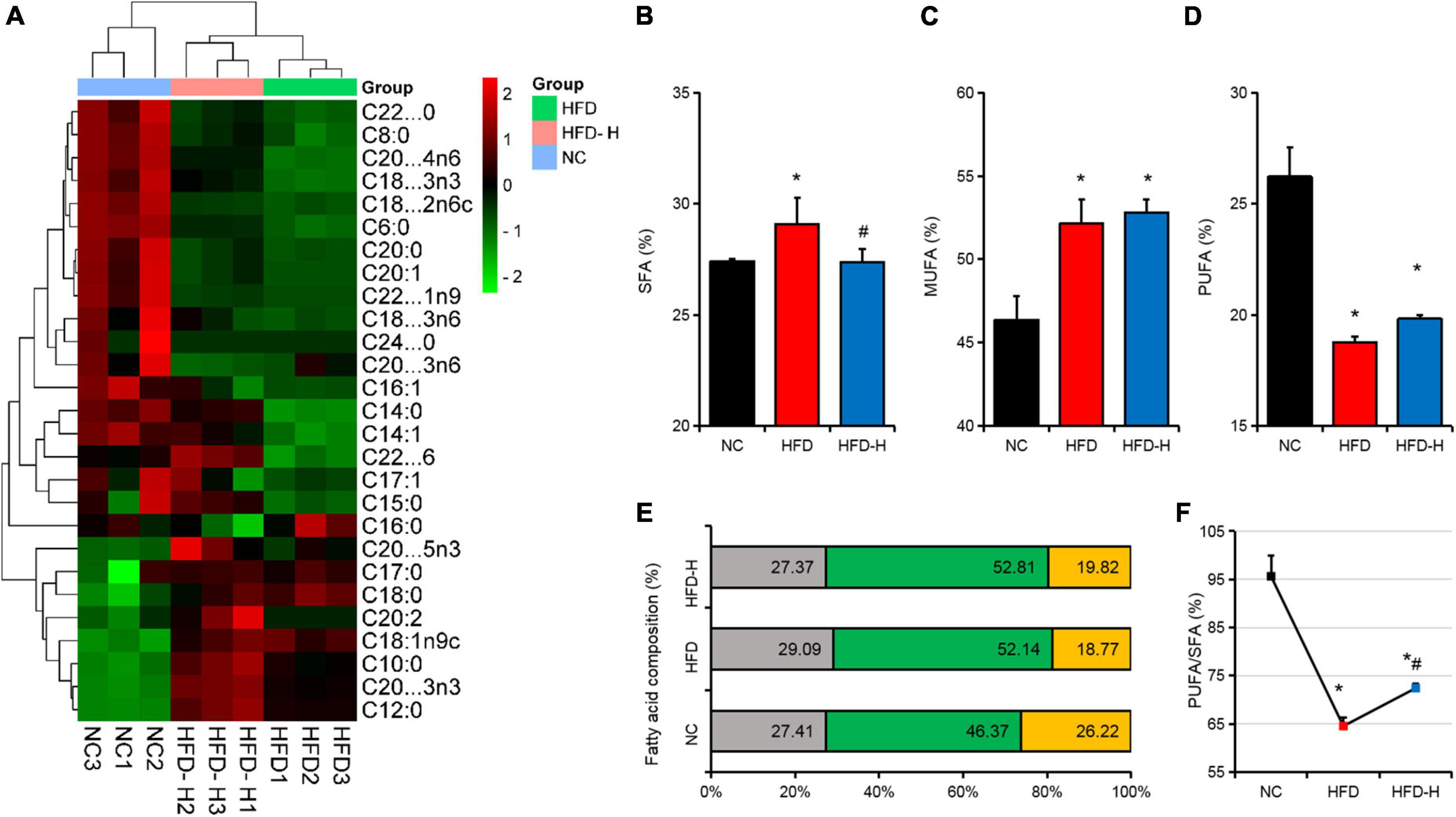
Figure 4. Mulberry leaf polyphenols (MLP) changed the fatty acid composition of the adipose tissues in obese mice. (A) The heat map shows the fatty acid composition of the adipose tissues in mice with different treatments. (B–D) The composition of saturated fatty acids (SFA, B), monounsaturated fatty acids (MUFA, C), and polyunsaturated fatty acids (PUFA, D) in mice adipose tissues. (E) SFA, MUFA, and PUFA composition distribution stacking diagram of mouse adipose tissues. (F) Mice adipose tissue PUFA/SFA ratio. *P ≤ 0.05, compared with the NC group; #P ≤ 0.05, compared with the high-fat diet (HFD) group. n = 3.
The effect of mulberry leaf polyphenols on the expression of characteristic genes in the adipose tissues of obese mice
The HFD significantly promoted the serum TC and TG levels. The MLP significantly inhibited the serum TC level induced by the HFD but did not significantly inhibit the serum TG level (Figures 5A,B). The expression of genes related to fat deposition and adipose tissue browning in the adipose tissues was detected using RT-qPCR. The results indicate that HFD significantly promoted the expression of PPARγ and C/EBPα, but significantly inhibited the expression of browning-related genes, such as UCP1, UCP3, and PRDM16 (Figures 5C,D). Compared with the HFD group, high concentrations of MLP significantly inhibited the expression of FASN and significantly promoted the expression of UCP1, UCP3, and PRDM16 (Figures 5C,D).
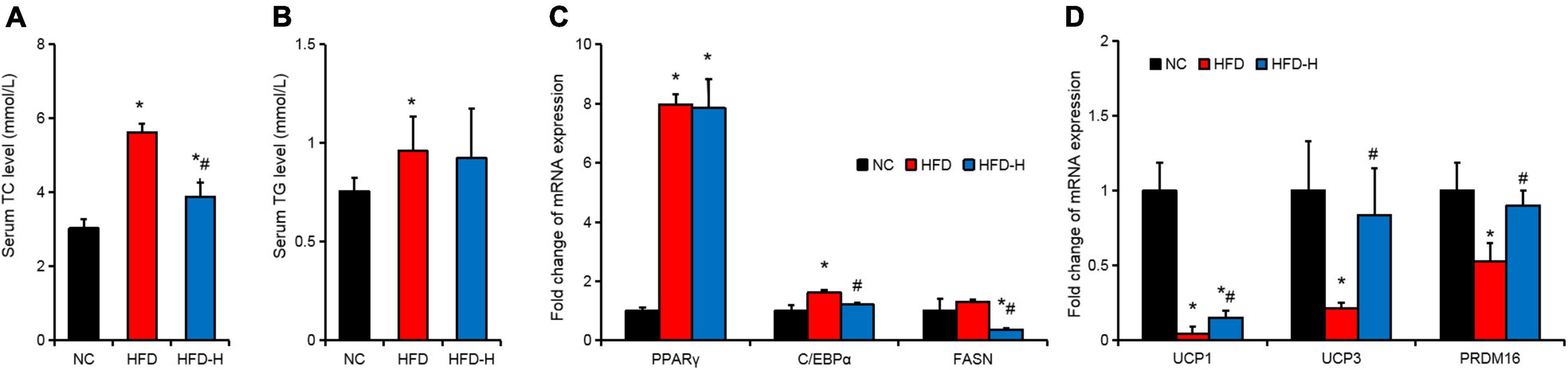
Figure 5. Mulberry leaf polyphenols (MLP) promoted the expression of thermogenesis genes in the adipose tissues of obese mice. (A,B) Mice serum total cholesterol (TC, A) and triglyceride (TG, B) content of mice with different treatments. (C) Expression of genes related to fat deposition in the adipose tissues of mice with different treatments. (D) The expression of browning-related genes in the adipose tissues of mice with different treatments. *P ≤ 0.05, compared with the NC group; #P ≤ 0.05, compared with the high-fat diet (HFD) group. n = 3.
Discussion
Obesity has become a worldwide public health concern. The imbalance in energy intake and output is the primary cause of obesity, leading to excessive body fat accumulation (22). Moreover, factors, such as genetics, physiology, nutrition, and environment, may also contribute to the occurrence of obesity (23). Obesity often leads to various chronic diseases, such as hypertension, dyslipidemia, type 2 diabetes, cardiovascular disease, insomnia, and apnea (7). According to reports, the global rate of overweight and obesity has increased to 40%, affecting more than 2 billion people (24). Therefore, obesity intervention has become a key concern of the whole society. The increasing changes in lifestyle, increased work pressure, and the safety and tolerance of drug treatments have influenced increasingly more people to opt for functional foods with anti-obesity effects (25). Phytochemical ingredients are recognized as safe because they have no side effects within a certain concentration range, and hence, are favored by most researchers and consumers. ML Pare one of the natural products with potential effects of lipid-lowering, antioxidant, and weight loss (26). In this study, the effects of MLP on fat deposition in obese mice were assessed.
In recent years, many polyphenols have been extracted from various plants, such as apples, tea, grapes, and blueberries (27–29). Polyphenols are characterized by antioxidant, antibacterial, and antiviral activities. Regarding the treatment of metabolic diseases, MLP have the characteristic effects of lowering blood sugar, lowering blood pressure, and preventing cardiovascular diseases (30). Our study results proved that both low and high concentrations of MLP could significantly reduce the level of fasting blood glucose in HFD-induced obese mice. Additionally, they could reduce serum triglycerides and total cholesterol levels in obese mice.
Stimulating the body’s heat production and accelerating energy consumption are effective approaches to losing weight (31). Studies have reported that the anti-obesity effect of polyphenols might be attributed to the heat production stimulation in the body (25). Previous studies have found that green tea extract could increase and prolong sympathetic nerve stimulation and heat production, and effectively reduce body weight and fat deposition in the body organs (32). The results of the present study indicated that MLP could significantly upregulate the expression of brown fat thermogenesis-related genes, such as UCP1, UCP3, and PRDM16 (33, 34).
The results of this study revealed that MLP could change the fatty acid composition of adipose tissues in obese mice. Obesity leads to increased fat deposition and affects the composition of fatty acids in tissues (35). Studies have reported that in children and adolescents, 16:0 and 18:0 are positively correlated with obesity, while 20:4n-6 and 22:6n-3 are negatively correlated with obesity (36). In this study, the MLP downregulated 16:0 and 18:0 in obese mice and upregulated the 20:4n-6 and 22:6n-3 content. The fatty acid composition of food also affects the occurrence of obesity, while a higher SFA is not conducive to body health (37–39). This study found that MLP could downregulate the SFA content of obese mice. The PUFA/SFA ratio is also closely related to health, with an approximate ratio of 1 (40). The current results show that the PUFA/SFA ratio of obese mice was only 65%, and MLP significantly increased the PUFA/SFA ratio of obese mice. Therefore, using MLP to feed domestic animals might improve the quality of livestock products by changing their fatty acid composition (41).
Conclusion
The results of this study show that MLP could alleviate HFD-induced obesity in mice fat deposition reduction. Moreover, MLP could reduce the expression of fat synthesis-related genes and increase the expression of browning-related genes in the adipose tissues of HFD-induced obese mice. In addition, this study found that MLP may also affect the health of obese mice by changing the fatty acid composition of adipose tissue. Overall, MLP could be potential weight-loss drugs. This study provides a reference for developing animal feed additives using MLP.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This animal study was reviewed and approved by the Animal Care and Ethics Committee of Chongqing Academy of Animal Sciences.
Author contributions
RL: conceptualization, methodology, software, investigation, formal analysis, funding acquisition, and writing—original draft and editing. QZ: software, data curation, detection, methodology, and writing—review and editing. XW: data curation, methodology, software, and validation. HW: conceptualization, funding acquisition, resources, supervision, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by The Key R&D Project in Agriculture and Animal Husbandry of Rongchang (Grant cstc2019ngzx0006) and General Project of Chongqing Natural Science Foundation (Grant cstc2019jcyj-msxmx0090).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.979058/full#supplementary-material
Abbreviations
MLP, mulberry leaf polyphenols; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; PPARγ, peroxisome proliferative activated receptor, gamma; C/EBPα, CCAAT/enhancer-binding protein alpha; FASN, fatty acid synthase; UCP1, uncoupling protein 1; UCP3, uncoupling protein 3; PRDM 16, PR domain-containing 16.
References
1. Guo XX, Liu J, Zhang HJ, Ji BP, Zhou F. Addition of mulberry leaf (Morus Alba L.) to a diet formula impeded its hypoglycemic effect and exacerbated dyslipidemia in high-fructose- and high-fat-induced cd-1 mice. Emir J Food Agric. (2017) 29:532–8. doi: 10.9755/ejfa.2017-05-1067
2. Li XJ, Yu JP, Du GD. Study on the optimization of process for extraction and separation of flavonoids and polysaccharides from mulberry leaves. Food Sci. (2005) 26:159–62.
3. Ying Z, Han X, Li J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. (2011) 127:1273–9. doi: 10.1016/j.foodchem.2011.01.083
4. Wei FM, Chen C, Chao L, Xiong FU. Antioxidant and hypoglycemic activities of mulberry leaves extract, tea polyphenols and their compounds. Sci Technol Food Ind. (2018) 39:299–305.
5. Curtin C, Bandini LG, Perrin EC, Tybor DJ, Must A. Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: a chart review. BMC Pediatr. (2005) 5:48. doi: 10.1186/1471-2431-5-48
6. Alwan IA, Badri M, Al-Ghamdi M, Aljarbou A, Tamim H. Prevalence of self-reported cardiovascular risk factors among Saudi physicians: a comparative study. Int J Health Sci. (2013) 7:3–13.
7. Satin L, Butler P, Ha J, Sherman A. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol Aspects Med. (2015) 42:61–77. doi: 10.1016/j.mam.2015.01.003
8. Fenwick P, Jeejeebhoy K, Dhaliwal R, Royall D, Brauer P, Tremblay A, et al. Lifestyle genomics and the metabolic syndrome: a review of genetic variants that influence response to diet and exercise interventions. Crit Rev Food Sci Nutr. (2019) 59:2028–39. doi: 10.1080/10408398.2018.1437022
9. Bray G, Ryan D. Evidence-based weight loss interventions: individualized treatment options to maximize patient outcomes. Diabetes Obes Metab. (2020) 23:1–13. doi: 10.101111/dom.14200
10. Wu ZC, Boger DL. The quest for supernatural products: the impact of total synthesis in complex natural products medicinal chemistry. Nat Prod Rep. (2020) 37:1511–31. doi: 10.1039/d0np00060d
11. Zhu YP, Yang L, Lin L, Gan S, Zheng G. Effect of caffeine and chlorogenic acid on body weight, lipid accumulation and the expression of lipid metabolism-related genes in high-fat diet-fed mice. Food Sci. (2017) 38:163–7. doi: 10.7506/spkx1002-6630-201709026
12. Cai L, LvM Rui Y, Qin L, Wan Z. Rutin improves adipose tissue dysfunction induced by high-fat diet in samp8 mice. Acta Nutriment Sin. (2018) 40:583–6. doi: 10.3969/j.issn.0512-7955.2018.06.012
13. Bird JK, Raederstorff D, Weber P, Steinert RE. Cardiovascular and antiobesity effects of resveratrol mediated through the gut microbiota. Adv Nutr. (2017) 8:839–49. doi: 10.3945/an.117.016568
14. Lim HH, Lee SO, Kim SY, Yang SJ, Lim Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp Biol Med. (2013) 238:1160–9. doi: 10.1177/1535370213498982
15. Oh KS, Ryu SY, Lee S, Seo HW, Oh BK, Kim YS, et al. Melaninconcentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J Ethnopharmacol. (2009) 122:216–20. doi: 10.1016/j.jep.2009.01.020
16. Yang SJ, Park NY, Lim Y. Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1adipocytes. Nutr Res Pract. (2014) 8:613–7. doi: 10.4162/nrp.2014.8.6.613
17. Neamat-Allah A, Mahmoud E, Mahsoub Y. Effects of dietary white mulberry leaves on hemato-biochemical alterations, immunosuppression and oxidative stress induced by Aeromonas hydrophila in Oreochromis Niloticus. Fish Shellfish Immunol. (2021) 108:147–56. doi: 10.1016/j.fsi.2020.11.028
18. Alfaia C, Castro M, Martins S, Portugal A, Alves S, Fontes C, et al. Effect of slaughter season on fatty acid composition, conjugated linoleic acid isomers and nutritional value of intramuscular fat in barros-pdo veal. Meat Sci. (2007) 75:44–52. doi: 10.1016/j.meatsci.2006.06.013
19. Shen W, Liao S, Lin G. Antioxidant activity and synergistic effect of polyphenol monomer substances in mulberry leaves. Sci Seric. (2015) 41:342–8. doi: 10.13441/j.cnki.cykx.2015.02.021
20. Gan M, Shen L, Wang S, Guo Z, Zheng T, Tan Y, et al. Genistein inhibits high fat diet-induced obesity through miR-222 by targeting BTG2 and adipor1. Food Funct. (2020) 11:2418–26. doi: 10.1039/c9fo00861f
21. Gan M, Shen L, Fan Y, Guo Z, Liu B, Chen L, et al. High altitude adaptability and meat quality in tibetan pigs: a reference for local pork processing and genetic improvement. Animals. (2019) 9:1080. doi: 10.3390/ani9121080
22. Yang Y, Harmon C. Recent developments in our understanding of melanocortin system in the regulation of food intake. Obes Rev. (2003) 4:239–48.
23. Jimenez-Sanchez G, Silva-Zolezzi I, Hidalgo A, March S. Genomic medicine in Mexico: initial steps and the road ahead. Genome Res. (2008) 18:1191–8. doi: 10.1101/gr.065359.107
24. Alberca R, Oliveira L, Branco A, Pereira N, Sato M. Obesity as a risk factor for covid-19: an overview. Crit Rev Food Sci Nutr. (2021) 61:2262–76. doi: 10.1080/10408398.2020.1775546
25. Sheng Y, Liu J, Zheng S, Liang F, Luo Y, Huang K, et al. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. (2019) 10:4771–81. doi: 10.1039/c9fo00883g
26. Yang M, Huang C, Chan K, Yang Y, Peng C, Wang C. Mulberry leaf polyphenolspossess antiatherogenesis effect via inhibiting ldl oxidation and foam cell formation. J Agric Food Chem. (2011) 59:1985–95. doi: 10.1021/jf103661v
27. Correa-Betanzo J, Allen-Vercoe E, Mcdonald J, Schroeter K, Corredig M, Paliyath G. Stability and biological activity of wild blueberry (vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. (2014) 165:522–31. doi: 10.1016/j.foodchem.2014.05.135
28. McSweeney M, Seetharaman K. State of polyphenols in the drying process of fruits and vegetables. Crit Rev Food Sci Nutr. (2015) 55:660–9. doi: 10.1080/10408398.2012.670673
29. Gong ES, Li B, Li BX, Podio NS, Chen HY, Li T, et al. Identification of key phenolic compounds responsible for antioxidant activities of free and bound fractions of blackberry varieties extracts by boosted regression trees. J Sci Food Agric. (2022) 102:984–94. doi: 10.1002/jsfa.11432
30. Landete J. Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr. (2013) 53:706–21. doi: 10.1080/10408398.2011.555018
31. Palmer B, Clegg D. Non-shivering thermogenesis as a mechanism to facilitate sustainable weight loss. Obes Rev. (2017) 18:819–31. doi: 10.1111/obr.12563
32. Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes. (2000) 24:252–8. doi: 10.1038/sj.ijo.0801101
33. Pan M, Koh Y, Lee T, Wang B, Chen W, Nagabhushanam K, et al. Resveratrol and oxyresveratrol activate thermogenesis via different transcriptional coactivators in high-fat diet-induced obese mice. J Agric Food Chem. (2019) 67:13605–16. doi: 10.1021/acs.jafc.9b05963
34. Azzu V, Brand M. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. (2010) 35:298–307. doi: 10.1016/j.tibs.2009.11.001
35. Nankam P, Jaarsveld P, Chorell E, Smidt M, Adams K, Blüher M, et al. Circulating and adipose tissue fatty acid composition in black South African women with obesity: a cross-sectional study. Nutrients. (2020) 12:1619. doi: 10.3390/nu1261619
36. Tang J, Yan Y, Li J, Yang B, Zhao X, Wan Y, et al. Relationship between erythrocyte phospholipid fatty acid composition and obesity in children and adolescents. J Clin Lipidol. (2019) 13:70–9. doi: 10.1016/j.jacl.2018.09.013
37. Monnard C, Dulloo A. Polyunsaturated fatty acids as modulators of fat mass and lean mass in human body composition regulation and cardiometabolic health. Obes Rev. (2021) 22:13197. doi: 10.1111/obr.13197
38. Tao F, Ngadi M. Recent advances in rapid and nondestructive determination of fat content and fatty acids composition of muscle foods. Crit Rev Food Sci Nutr. (2018) 58:1565–93. doi: 10.1080/10408398.2016.1261332
39. Siri-Tarino P, Chiu S, Bergeron N, Krauss R. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Ann Rev Nutr. (2015) 35:517–43. doi: 10.1146/annurev-nutr-071714-034449
40. Gupta SV, Khosla P. Pork fat and chicken fat similarly affect plasma lipoprotein metabolism in cynomolgus monkeys fed diets with adequate levels of linoleic acid. J Nutr. (2000) 130:1217–24. doi: 10.1093/jn/130.5.1217.source:pubmed
Keywords: mulberry leaf polyphenols, mice, obesity, fatty acid composition, adipose tissue browning
Citation: Li R, Zhu Q, Wang X and Wang H (2022) Mulberry leaf polyphenols alleviated high-fat diet-induced obesity in mice. Front. Nutr. 9:979058. doi: 10.3389/fnut.2022.979058
Received: 27 June 2022; Accepted: 25 August 2022;
Published: 15 September 2022.
Edited by:
Er Sheng Gong, Gannan Medical University, ChinaReviewed by:
Qian Li, Hubei University of Technology, ChinaHong Wang, Zhongkai University of Agriculture and Engineering, China
Copyright © 2022 Li, Zhu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Wang, d2h5ZGV0aWFuQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Rui Li
Rui Li Qubo Zhu2,3†
Qubo Zhu2,3† Haiyan Wang
Haiyan Wang