- 1Shenzhen Cadre and Talent Health Institute (Shenzhen Talent Institute), Shenzhen, Guangdong, China
- 2Shenzhen Birth Cohort Study Center, Department of Science and Education, Nanshan Maternity and Child Healthcare Hospital of Shenzhen, Shenzhen, Guangdong, China
- 3Department of Nutrition, School of Medicine, Jinan University, Guangzhou, Guangdong, China
- 4Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, IA, United States
- 5Department of Mathematics and Physics, School of Biomedical Engineering, Southern Medical University, Guangzhou, Guangdong, China
Background: Although assisted reproductive technology (ART) plays a critical role in reducing infertility, ART pregnant women are reported at higher risk of preterm birth (PTB). Besides, women undergoing ART encounter a higher risk of developing gestational diabetes mellitus (GDM). However, existing studies on the combined effect of ART treatment and GDM on PTB risk are sparse.
Methods: This population-based retrospective cohort study used nationwide birth certificate data from the US National Vital Statistics System 2015-2019. All mothers who had a singleton live birth without pre-pregnancy diabetes were included. Multivariable logistic regression models were used to estimate the odds ratio (OR) of PTB.
Results: We finally included 18,140,241 American mother-infant pairs. The overall rate of PTB was 7.92% (n = 1,436,328). The PTB rate for non-ART mothers without GDM, ART mothers without GDM, non-ART mothers with GDM, and ART mothers with GDM were 7.67, 10.90, 11.23, and 14.81%, respectively. The incidence of GDM in ART mothers (10.48%) was significantly higher than in non-ART mothers (6.26%). After adjusting for potential confounders, compared with non-ART mothers without GDM, the PTB risk was significantly increased for ART mothers without GDM (AOR: 1.47, 95% CI 1.44-1.50), non-ART mothers with GDM (AOR:1.35, 95% CI 1.34-1.36) and ART mothers with GDM (AOR: 1.82, 95% CI 1.74-1.90) respectively, showing an increasing tendency. This phenomenon was stable among mothers in all groups of mothers older than 25 years.
Conclusion: To prevent PTB, effective approaches for the prevention of GDM are crucial to mothers who conceived through ART.
Introduction
Preterm birth (PTB) is defined as delivery before 37 weeks of gestation. According to WHO, approximately 15 million infants (11%) are born preterm annually worldwide, of which more than 1 million children die before the age of 5 years due to PTB and its complications (1). Unfortunately, to date, few interventions are efficient to reduce PTB. Assisted reproductive technology (ART) is a relatively mature procedure to address infertility, its demand is increasing worldwide (2). To date, more than 8 million babies have been born after ART globally (3). According to the Centers for Disease Control and Prevention (CDC), in 2019, 2.1% of all infants born in the United States were conceived through ART (4). The PTB rate was higher among infants conceived with ART (24.4%) than among all infants born in the total birth population (10.2%). Aside from the direct role of multifetal pregnancies, even among ART singletons, the rate of PTB was higher among ART-conceived infants (15.4%) than among all infants (8.5%). Similar results were found in a meta-analysis of 50 cohort studies and indicated that singleton pregnancies created with ART experienced a significantly increased risk of PTB (RR 1.71, 95% CI 1.59–1.83) (4). Although ART may help infertile couples achieve pregnancy, the attendant risk of PTB can bring an extra burden to the family. However, the reasons for the greater increase in PTB among ART pregnancies remain obscure. This ART-PTB association may be fully or partially confounded by maternal health during pregnancy.
Gestational diabetes mellitus (GDM) is the most common pregnancy complication defined as glucose intolerance first identified during pregnancy (5), that is affecting 7–25% of pregnancies worldwide (6). It is well recognized now that GDM is a critical risk factor for spontaneous and indicated PTB (< 37 weeks of gestation) (7–9). Despite advances in the care of women with GDM, the odds of PTB were 30% higher than in those without diabetes (10). Maternal hyperglycemic states during pregnancy with alterations in insulin and glucose metabolism can result in an adverse intrauterine environment for the developing fetus (11). Moreover, some early delivery in GDM women may be recommended because the baby is large. Several research reported that pregnant women with a history of fertility problems, particularly from ovulation disorders and tubal blockage, are at increased risk of GDM, reaching an approximate prevalence of 11-40% in women undergoing ART (12–15). A study by Szymanska et al. suggested that IVF patients may develop GDM earlier in pregnancy because of higher first trimester fasting glucose levels (16). Although previous studies have suggested an association of PTB with GDM and ART separately, rarely studies have investigated the PTB risk in ART women combined with GDM.
Considering the increasing usage of ART and growing awareness of ART pregnant women at high risk of developing GDM, we aim to use a large population-based study to explore the combined effect of ART treatment and GDM on the risk of preterm singleton birth.
Materials and methods
Study design and data sources
The data were from the US National Vital Statistics System (NVSS), an extensive data archive accessible to the public, which was conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC). NVSS collects and publishes nationwide data on births in all the 50 US states and the District of Columbia from birth certificates according to Federal law. In this population-based study, we used birth data from Jan 1, 2015, to Dec 31, 2019 (NVSS 2015-2019), including all mothers (n = 18,140,241) who had a live singleton birth without prepregnancy diabetes, excluding women with incomplete data on ART treatment, GDM and gestational age at birth. The screening process is shown in Supplementary Figure 1. This study was approved as exempt from review by the Institutional Review Board at Jinan University due to the use of de-identified data from the open database. The conduct and reporting of this study followed the reporting guidelines in the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Exposure measurement and outcome ascertainment
Information on ART treatment (in vitro fertilization (IVF), gamete intrafallopian transfer (GIFT), zygote intra-fallopian transfer (ZIFT)), GDM, and gestational age were collected directly from the medical record by the facility worksheet. According to WHO, PTB was further subdivided into extremely preterm (< 28 weeks), very preterm (28-31 weeks), and moderate preterm (32-36 weeks) (17).
Covariates measures
Information on maternal age (<25, 25-29, 30-34, 35-39, > 40 years), maternal race/ethnicity (Hispanic, non-Hispanic black, non-Hispanic whites, and others), maternal education (lower than high school, high school, higher than high school, and unknown), marital status (married, unmarried, and unknown), pre-pregnancy body mass index (underweight < 18.5 kg/m2, normal weight 18.5-24.9 kg/m2, overweight 25.0-29.9 kg/m2, obesity ≥ 30.0 kg/m2), Smoking before or during pregnancy (yes, no) were collected using the mother’s worksheet. Information on parity (1, 2, 3, ≥ 4), pre-pregnancy hypertension (yes, no), previous preterm birth (yes, no, nulliparous), initiation of prenatal care (no prenatal care, 1st–3rd month, 4th–6th month, 7th–final month, or missing), gestational hypertension or preeclampsia or eclampsia (yes, no) and infant sex (male or female) were collected using the facility worksheet.
Statistical analysis
Baseline characteristics are presented as numbers and proportions. Comparisons between categorical variables were tested using chi-square tests. This retrospective cohort is divided into four groups according to ART and GDM: non-ART mothers without GDM, ART mothers without GDM, non-ART mothers with GDM, and ART mothers with GDM. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs) for PTB, after adjusting for potential confounders, including maternal age, race/ethnicity, education, marital status, parity, pre-pregnancy body mass index, hypertension before pregnancy, previous history of PTB, smoking before pregnancy, smoking during pregnancy, initiation of prenatal care, gestational hypertension or preeclampsia & eclampsia and infant sex. We further conducted secondary analyses stratified by maternal age (< 25, 25-34, ≥ 35 years old). P for interaction was calculated on the basis of multivariable logistic regression models with multiplicative interaction term (age/pre-pregnancy body mass index *exposure groups). All analyses were performed with R statistical software (version 3.6.4) and GraphPad Prism 8 (GraphPad Software, San Diego, CA). A two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 details the baseline characteristics of the study population, according to ART and GDM categories. Among all the participants, ART mothers account for 0.80% (n = 145,557), GDM mothers account for 6.21% (n = 1,125,651). ART mothers were more likely to be elder, non-Hispanic white, higher educated, married primiparous, and earlier initiation of prenatal care and had gestational hypertension or preeclampsia or eclampsia, while less smoking before or during pregnancy than non-ART mothers without GDM. Mothers with GDM were more likely to be elder, non-Hispanic white, obesity and hypertension before pregnancy, and earlier initiation of prenatal care than non-ART mothers without GDM. We also found the incidence of GDM in ART mothers (10.48%) was higher than in non-ART mothers (6.17%) (Supplementary Table 1). The PTB rate for non-ART mothers without GDM, ART mothers without GDM, non-ART mothers with GDM, and ART mothers with GDM were 7.67, 10.90, 11.23, and 14.81%, respectively, showing an increasing tendency (Table 1). Supplementary Table 2 shows the characteristics of the study population according to PTB. Comparison between any two or more groups was performed by the Chi-square test (Supplementary Table 3). We found that ART mothers with GDM had the highest rate of PTB compared to the other three groups.
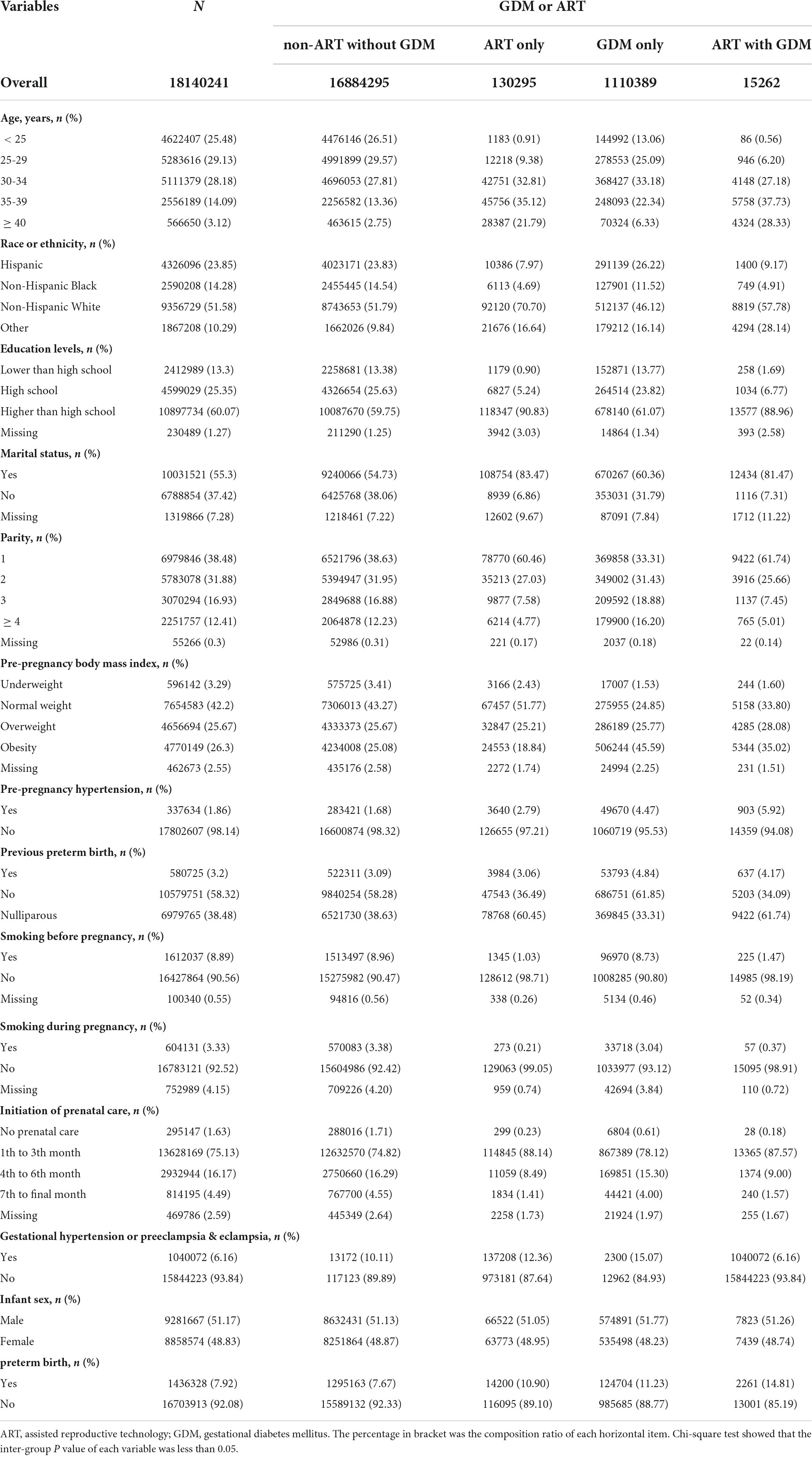
Table 1. Characteristics of the study population, according to received assisted reproductive technology and/or developed gestational diabetes mellitus.
Effects of assisted reproductive technology and gestational diabetes mellitus on preterm birth
We used a multivariable logistic regression model to assess the effects of ART and GDM on PTB (Table 2). After adjusting for confounding factors, compared with non-ART mothers without GDM, the overall PTB risk was significantly increased for ART mothers without GDM, non-ART mothers with GDM, and ART mothers with GDM respectively, and ART mothers with GDM had the highest PTB risk. Furthermore, subgroup analysis by gestational age (extremely preterm <28 weeks, very preterm 28-31 weeks, and moderate preterm 32-36 weeks) was also performed. We also found that ART mothers with GDM had the highest risk ratios for moderate and very PTB. The OR values kept increasing as the severity of PTB increased in ART mothers without GDM. However, having GDM only was mainly associated with an increased the risk of moderately PTB, unexpectedly a lower risk of being severely preterm.
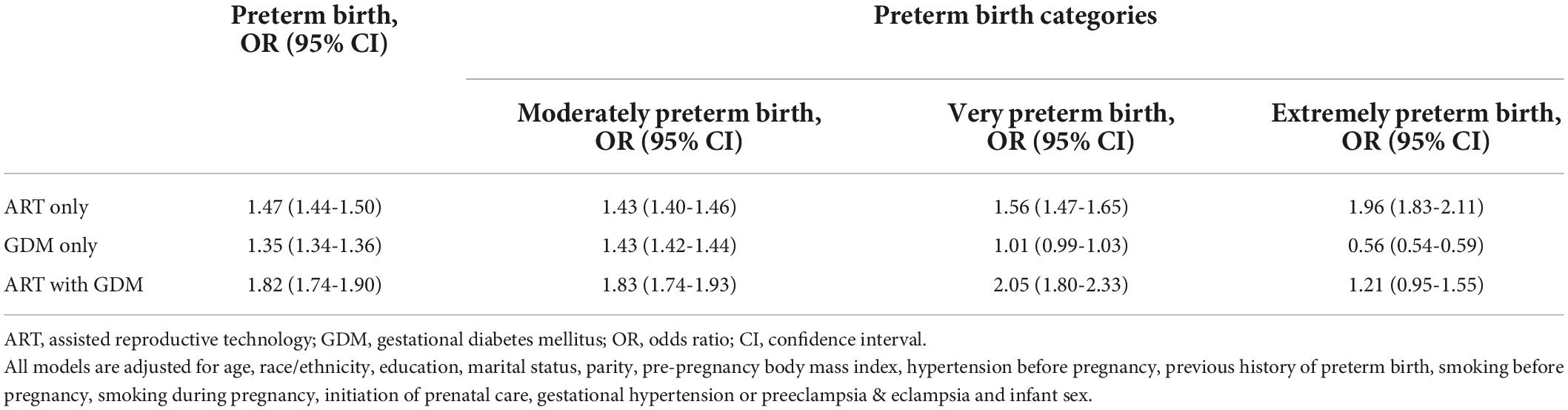
Table 2. Effects of assisted reproductive technology and/or gestational diabetes mellitus on preterm birth.
Subgroup analysis by maternal age and prepregnancy body mass index
Considering maternal age as an important confounding factor, we conducted secondary analyses stratified by maternal age (< 25, 25-34, ≥ 35 years old) (Table 3). We found that ART mothers with GDM had the significantly highest rate of PTB in all groups of mothers older than 25 years. Scarified analysis by prepregnancy BMI (underweight < 18.5 kg/m2, normal weight 18.5-24.9 kg/m2, overweight 25.0-29.9 kg/m2, obesity ≥ 30.0 kg/m2) also showed the same tendency that ART mothers with GDM had the highest rate of PTB in those with prepregnancy normal weigh, overweight and obesity groups (Table 4).
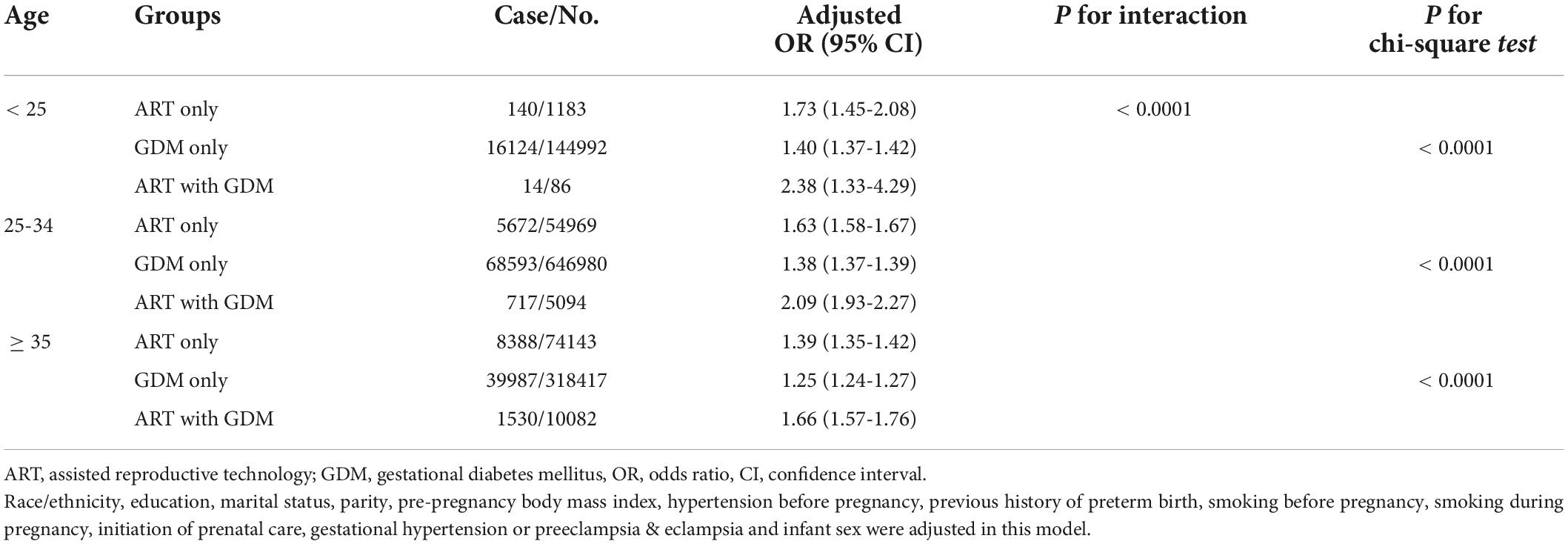
Table 3. Effects of assisted reproductive technology and/or gestational diabetes on preterm birth according to age.
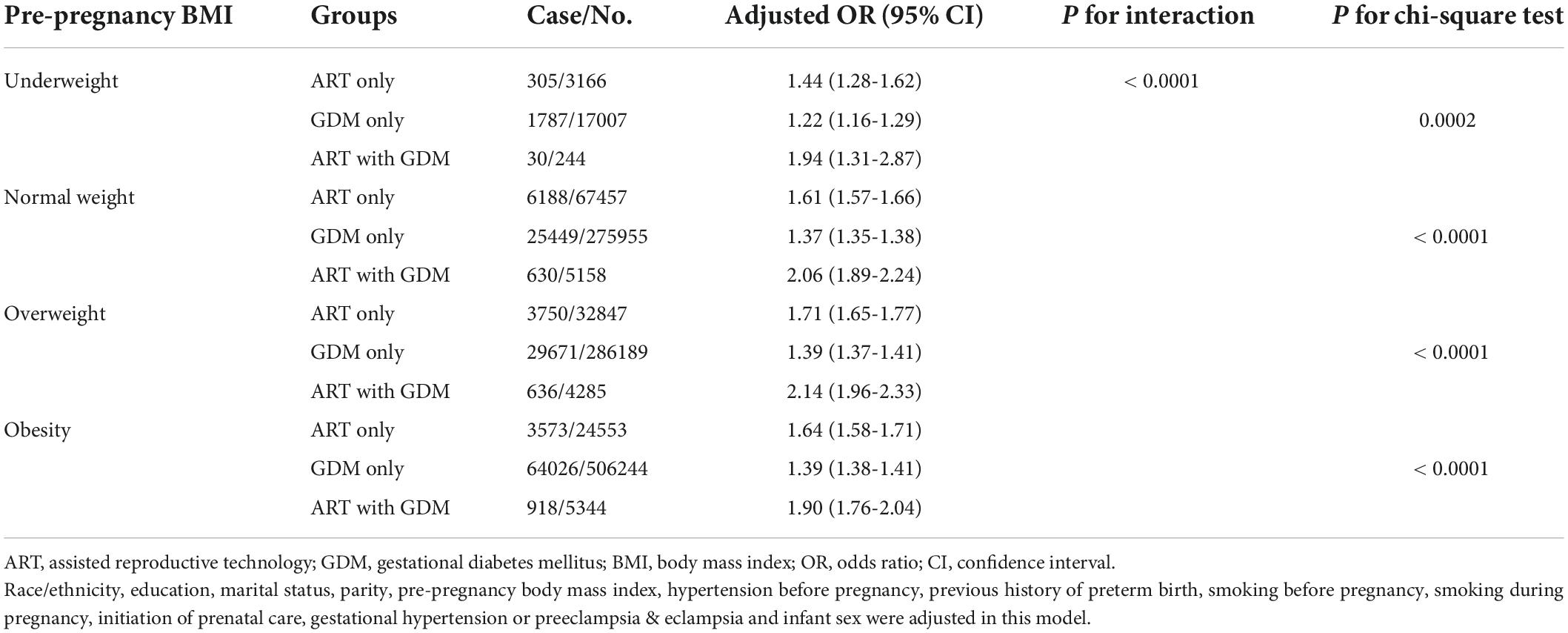
Table 4. Effects of assisted reproductive technology and/or gestational diabetes on preterm birth according to pre-pregnancy body mass index.
Discussion
In the present study based on the 2015-2019 NVSS singleton livebirths databases, we found that either GDM or ART use was associated with an increased risk of PTB, especially ART mothers with GDM were at maximum risk of PTB. Moreover, ART mothers had a higher incidence of GDM than non-ART mothers. Subgroup analysis further found that ART-GDM mothers had the highest risk of mid-to-late PTB, indicating the timing of PTB at most risk. Moreover, ART-GDM mothers had the highest rate of PTB in all groups of mothers older than 25 years. Therefore, ART pregnancies need to pay special attention to GDM for preventing PTB.
In this study, we found that after adjustment for potential confounding factors, ART mothers with GDM had the highest risk of PTB compared to the other three groups. This is an important finding, as most of the previous studies report separate estimates of the causal effect of ART and GDM on PTB, very few studies have investigated the PTB risk in ART women combined with GDM. Although it is clear that both ART and GDM are independent risk factors for PTB (7–9, 18), little is known about the risk of PTB among ART-GDM pregnancies. A similar result was found in the previous study (Kouhkan et al. (19)), compared to spontaneously conceived pregnancies, ART-GDM pregnancies are delivered 2 weeks earlier and those conceived by ART and SC-GDM are delivered 1 week earlier (20). But obvious limitations of their study include a single-center study in Tehran and with small sample size, consisting of only 260 ART and 314 SC, 135 and 152 women were GDM women, respectively. The GDM rates in this cohort are significantly higher than the general population, so the biases may affect the representativeness of their findings. Consequently, we consider our results representative of a more accurate estimate, and PTB prevention efforts should have an increased focus on ART-GDM pregnancies.
Evidence continues to accumulate that women undergoing ART often encounter major risk factors for GDM, such as advanced maternal age, obesity, multiple pregnancies, and polycystic ovary syndrome (PCOS), suggesting a link between GDM and ART (13–15). A recent meta-analysis by Mohammadi et al. including 48 studies with 91,487 pregnancies conceived through ART and 2,525,234 spontaneously conceived indicates that the use of ART treatment is associated with a 1.51-fold increase in GDM (21). This was also confirmed in our study that ART women were at higher risk of GDM, reflected in 10.49% GDM rate in ART mothers with singleton pregnancies and 6.17% GDM rate in non-ART group. We further tried to understand the reasons behind this observation. Similarly, compared to the non-ART mothers, ART mothers were observed for older age, and higher prepregnancy BMI, which are known to promote insulin resistance, and insulin resistance increases the risk for developing GDM. Another pivotal factor that must be mentioned is PCOS, the most common cause of female infertility, and 62% of the women with persistent PCOS underwent ART treatment. Previous researchers revealed that women with PCOS had a more than twofold increased odds of GDM compared with women without PCOS (22, 23). Even though studies that specifically excluded PCOS women still reported ART mothers had a higher risk of GDM than non-ART mothers (15). Kouhkan et al. performed a nested case-control study including 270 ART women with singleton pregnancies (consisting of 135 GDM and 135 non-GDM women) also showed that the route of progesterone administration (OR 2.28, 95% CI 1.27–4.09), previous ovarian hyper-stimulation syndrome (OHSS) risk (OR 2.40, 95% CI 1.34–4.31) and history of PCOS (OR 2.76, 95% CI 1.26–6.06) seem to be putative risk factors for GDM in women whose pregnancies conceived by ART (19). Given the complexity of ART, multiple factors affect PTB risk. Besides, many of these risk factors for ART and GDM are also risk factors for PTB. Together, these findings suggest that ART population is at higher risk for developing of GDM, ART and GDM might exert a combined effect on the risk of PTB.
Moreover, we stratified the outcomes into subcategories of PTB: moderately preterm (32–36 weeks), very preterm (28-31 weeks), and extremely preterm (<28 weeks). Notably, ART-GDM mothers had the highest risk of PTB from 28 to less than 37 weeks (mid-to-late gestation) compared to the other three groups after adjustment for potential confounding factors. In more detail, compared to non-ART mothers without GDM, ART-only mothers had a high risk of PTB in early, middle, and late pregnancy, and the ratio increased with increasing severity of PTB. Furthermore, using data from the Quebec Pregnancy Cohort (QPC) included 57,624 pregnancies, recent results from Gorgui et al. (24) report similar findings. They also observed a trend across PTB categories that the use of ovarian stimulators or ART or both were associated with an increased risk of late (AOR:1.61, 95%CI 1.03–2.51), moderate (AOR:1.59, 95%CI 0.94–2.68) and extremely (AOR: 2.39, 95%CI 1.30–4.39) PTB (24). Since the inclusion of ovarian stimulators as a variable, the PTB risk was slightly higher than those observed in our study. Nonetheless, we discovered that GDM-only mothers were more likely to have a moderate PTB. Disentangling this question is not an easy feat. The majority of recent research has found that GDM is linked to rapid fetal growth (25), putting big newborns at risk of premature birth. Maternal insulin resistance, in addition to the consequences of maternal hyperglycemia, causes excessive fetal growth by increasing the placental transfer of additional growth substrates such as amino acids and lipids (26). Chronic fetal hyperinsulinism causes increased fetal substrate absorption, which raises tissue oxygen demand. This causes relative fetal hypoxia, which raises the risk of iatrogenic PTB or fetal mortality within the womb (27). In addition, GDM mothers are more likely to have pre-eclampsia, premature placental aging, placental abruption, and other complications in late pregnancy, resulting in late-PTB. Surprisingly, having GDM only was associated with a lower risk of being severely preterm. However, the evidence for the association between GDM and early PTB is inconsistent and often hampered by small sample size. A study from Medical Center’s (SUMC) birthing center yielded similar results as ours, which comprised 334,415 deliveries between 1991 and 2018 and found that diabetes mellitus had an inverse connection with the risk of early PTB (28). But Geurtsen et al. indicated that maternal early-pregnancy non-fasting glucose levels were not associated with PTB or delivery complications (29). GDM is generally diagnosed at 24-28 weeks of gestation, and the onset of GDM typically occurs in the second trimester of pregnancy, when progesterone levels are high (30). Besides, fetal insulin is a key fetal growth factor, which is inactive until the onset of corticoid action in the second trimester that why glycogen storage does not occur before the 27th week (31). In addition, threatened miscarriage and stillbirth are frequent during the first trimester, but not being considered together with extremely PTB and may introduce some bias. To date, the mechanisms underlying the association of GDM on fetal during the first trimester is unclear and require further study. Furthermore, since maternal age is an important factor affecting PTB, we found that ART-GDM mothers had the highest rate of PTB in over 25 age groups, suggesting no effect of age on the primary outcome. Overall, women whose pregnancies conceived through ART should be monitored for GDM, which directly and indirectly increases the risk of mid-to-late PTB.
There are several strengths in this study. Firstly, this study provides the first nationally representative estimates of PTB risk in ART-GDM pregnancies and can be considered more accurate. In addition, the availability of many clinical variables and large sample size allowed us to perform a detailed adjusted analysis that accounts for many important potential confounding variables such as prepregnancy BMI and race, some of which were not accounted for in previous studies (24, 28).
We acknowledge that there are several limitations to our study. First, the different types of ART applied, such as IVF-ET, IVF-FET, ICSI-ET and ICSI-FET, may also influence PTB risk. But we could not adjust for this important confounding factor due to lacking relevant information in the database. Second, the database did not provide GDM-relevant treatment or medication information, which might vary greatly from individual to individual. As maternal blood-sugar control can affect PTB, these may be important confounding factors in the current study. Third, potential for selection bias exists because the cohort was restricted to live births. Fourth, infection and inflammation during pregnancy can also be confounding factors that we could not account for. Additionally, we also cannot distinguish between spontaneous and medically induced PTB due to lack of relevant data.
Conclusion
Our large cross-sectional study demonstrated ART pregnancies had a substantially increased risk for GDM compared with non-ART pregnancies, and ART mothers with GDM had the maximum risk of PTB, especially for mid-to-late PTB. As women receiving ART treatment were inherently at high risk of PTB by their age and comorbid conditions, this study highlights the importance of GDM prevention and controlling for the ART population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RG, JH, and CL conceived the idea. RG and KZ undertook data analysis. KZ, JZ, JH, and CL wrote draft of the manuscript. KZ, JZ, TL, SL, JL, and CL contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors were involved in data analysis, drafting the article, or revising it critically for important intellectual content, and all authors approved the final version to be published.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81903294) and Medical Scientific Research Foundation of Guangdong Province of China (A2021123), the Science and Technology Planning Project of Shenzhen Nanshan District (2020032 General), and Guangzhou Basic and Applied Basic Research Foundation (202102020120).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.977195/full#supplementary-material
References
1. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388:3027–35. doi: 10.1016/S0140-6736(16)31593-8
2. Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. (2017) 15:6. doi: 10.1186/s12958-016-0225-2
3. Banker M, Dyer S, Chambers GM, Ishihara O, Kupka M, de Mouzon J, et al. International committee for monitoring assisted reproductive technologies (ICMART): world report on assisted reproductive technologies, 2013. Fertil Steril. (2021) 116:741–56. doi: 10.1016/j.fertnstert.2021.03.039
4. Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. (2016) 105:73–85.e1–6. doi: 10.1016/j.fertnstert.2015.09.007
5. Baz B, Riveline JP, Gautier JF. Endocrinology of pregnancy: gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur J Endocrinol. (2016) 174:R43–51. doi: 10.1530/EJE-15-0378
6. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. (2016) 16:7. doi: 10.1007/s11892-015-0699-x
7. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol. (2003) 102:850–6. doi: 10.1016/s0029-7844(03)00661-6
8. Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Neonatal Med. (2010) 23:1004–8. doi: 10.3109/14767050903551392
9. Sweeting AN, Ross GP, Hyett J, Molyneaux L, Constantino M, Harding AJ, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. (2016) 39:75–81. doi: 10.2337/dc15-0433
10. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care. (2009) 32:2005–9. doi: 10.2337/dc09-0656
11. Vrachnis N, Antonakopoulos N, Iliodromiti Z, Dafopoulos K, Siristatidis C, Pappa KI, et al. Impact of maternal diabetes on epigenetic modifications leading to diseases in the offspring. Exp Diabetes Res. (2012) 2012:538474. doi: 10.1155/2012/538474
12. Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv. (2004) 59:141–54. doi: 10.1097/01.OGX.0000109523.25076.E2
13. Wang YA, Nikravan R, Smith HC, Sullivan EA. Higher prevalence of gestational diabetes mellitus following assisted reproduction technology treatment. Hum Reprod. (2013) 28:2554–61. doi: 10.1093/humrep/det270
14. Ashrafi M, Gosili R, Hosseini R, Arabipoor A, Ahmadi J, Chehrazi M. Risk of gestational diabetes mellitus in patients undergoing assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol. (2014) 176:149–52. doi: 10.1016/j.ejogrb.2014.02.009
15. Bosdou JK, Anagnostis P, Goulis DG, Lainas GT, Tarlatzis BC, Grimbizis GF, et al. Risk of gestational diabetes mellitus in women achieving singleton pregnancy spontaneously or after ART: a systematic review and meta-analysis. Hum Reprod Update. (2020) 26:514–44. doi: 10.1093/humupd/dmaa011
16. Szymanska M, Horosz E, Szymusik I, Bomba-Opon D, Wielgos M. Gestational diabetes in IVF and spontaneous pregnancies. Neuro Endocrinol Lett. (2011) 32:885–8.
17. Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. (2003) 110(Suppl. 20):30–3. doi: 10.1016/s1470-0328(03)00021-1
18. McGovern PG, Llorens AJ, Skurnick JH, Weiss G, Goldsmith LT. Increased risk of preterm birth in singleton pregnancies resulting from in vitro fertilization-embryo transfer or gamete intrafallopian transfer: a meta-analysis. Fertil Steril. (2004) 82:1514–20. doi: 10.1016/j.fertnstert.2004.06.038
19. Kouhkan A, Khamseh ME, Moini A, Pirjani R, Valojerdi AE, Arabipoor A, et al. Predictive factors of gestational diabetes in pregnancies following assisted reproductive technology: a nested case-control study. Arch Gynecol Obstet. (2018) 298:199–206. doi: 10.1007/s00404-018-4772-y
20. Kouhkan A, Khamseh ME, Pirjani R, Moini A, Arabipoor A, Maroufizadeh S, et al. Obstetric and perinatal outcomes of singleton pregnancies conceived via assisted reproductive technology complicated by gestational diabetes mellitus: a prospective cohort study. BMC Pregnancy Childbirth. (2018) 18:495. doi: 10.1186/s12884-018-2115-4
21. Mohammadi M, Khedmati Morasae E, Maroufizadeh S, Almasi-Hashiani A, Navid B, Amini P, et al. Assisted reproductive technology and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Middle East Fertil Soc J. (2020) 25:1–12. doi: 10.1186/s43043-020-0018-6
22. Lo JC, Feigenbaum SL, Escobar GJ, Yang J, Crites YM, Ferrara A. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care. (2006) 29:1915–7. doi: 10.2337/dc06-0877
23. Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum Reprod. (2020) 35:1666–74. doi: 10.1093/humrep/deaa099
24. Gorgui J, Sheehy O, Trasler J, Fraser W, Bérard A. Medically assisted reproduction and the risk of preterm birth: a case-control study using data from the Quebec pregnancy cohort. CMAJ Open. (2020) 8:E206–13. doi: 10.9778/cmajo.20190082
25. Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. (2016) 39:982–7. doi: 10.2337/dc16-0160
26. Cetin I, de Santis MS, Taricco E, Radaelli T, Teng C, Ronzoni S, et al. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am J Obstet Gynecol. (2005) 192:610–7. doi: 10.1016/j.ajog.2004.08.011
27. Philipps AF, Rosenkrantz TS, Porte PJ, Raye JR. The effects of chronic fetal hyperglycemia on substrate uptake by the ovine fetus and conceptus. Pediatr Res. (1985) 19:659–66. doi: 10.1203/00006450-198507000-00005
28. Kluwgant D, Wainstock T, Sheiner E, Pariente G. Preterm delivery; who is at risk? J Clin Med. (2021) 10:2279. doi: 10.3390/jcm10112279
29. Geurtsen ML, van Soest EEL, Voerman E, Steegers EAP, Jaddoe VWV, Gaillard R. High maternal early-pregnancy blood glucose levels are associated with altered fetal growth and increased risk of adverse birth outcomes. Diabetologia. (2019) 62:1880–90. doi: 10.1007/s00125-019-4957-3
30. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. (2002) 25:1862–8. doi: 10.2337/diacare.25.10.1862
Keywords: gestational diabetes mellitus, assisted reproductive technology, preterm birth, singleton birth, NVSS
Citation: Gao R, Zhao K, Zhou J, Wang X, Liu T, Lian S, Li J, Huang Y, Qiu C, Wu Y, He J and Liu C (2022) Effects of gestational diabetes mellitus and assisted reproductive technology treatment on the risk of preterm singleton birth. Front. Nutr. 9:977195. doi: 10.3389/fnut.2022.977195
Received: 01 July 2022; Accepted: 29 August 2022;
Published: 14 September 2022.
Edited by:
Sheila A. Skeaff, University of Otago, New ZealandReviewed by:
Yuan Lin, Nanjing Medical University, ChinaKathy L. Gatford, University of Adelaide, Australia
Copyright © 2022 Gao, Zhao, Zhou, Wang, Liu, Lian, Li, Huang, Qiu, Wu, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang He, aGVqaWFuZzAxQHNtdS5lZHUuY24=; Chaoqun Liu, Y2hhb3F1bmxpdUBqbnUuZWR1LmNu
†These authors have contributed equally to this work
 Rui Gao1,2†
Rui Gao1,2† Jiaxin Zhou
Jiaxin Zhou Jiang He
Jiang He Chaoqun Liu
Chaoqun Liu