- 1Chronic Respiratory Diseases Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Anesthesiology and Critical Care Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Department of Nursing, Sabzevar Branch, Islamic Azad University, Sabzevar, Iran
- 5Department of Nursing, Tabas Branch, Islamic Azad University, Tabas, Iran
- 6Research Center for Health Management in Mass Gathering, Red Crescent Society of the Islamic Republic of Iran, Tehran, Iran
- 7Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, MD, United States
- 8Mycobacteriology Research Center (MRC), NRITLD, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Department of Naturopathic Medicine, Tehran, Iran
- 10Priority Research Centre for Asthma and Respiratory Disease, Hunter Medical Research Institute, University of Newcastle, Newcastle, NSW, Australia
Introduction: COVID-19 (coronavirus disease-2019) still causes a high rate of death globally with no definite curative treatment described. The traditional plant Borage (Borago officinalis L.) is a good source of gamma-linolenic (GLA). We hypothesized that Borage plus syrup (BPS) would be beneficial in severe COVID-19 patients within an intensive care unit (ICU) setting.
Materials and methods: A pilot single center, randomized trial with no placebo was undertaken. A total of 60 PCR-positive severe COVID-19 participants admitted to ICU from June 2020–December 2020 at Masih Daneshvari Hospital Tehran-Iran gave informed consent. The participants were randomly assigned to either Borage Plus Syrup (BPS, 5 ml for 5 days) (n = 30) or standard care (IFN-β and favipiravir) as a control group (n = 30). Pao2/Fio2, serum ferritin, CRP, bilirubin, IL-6, TNF-α, ALT, AST, PCT and serum IL-8 was measured upon admission and on release.
Results: All the measured parameters decreased significantly with BPS treatment. In the control group, most parameters significantly improved apart from AST and PCT. In addition, the suppression of serum TNF levels in the BPS group was greater than that seen in the control group (P ≤ 0.05). Moreover, the length of ICU stay was significantly lower in the BPS group compared with the control group (P ≤ 0.05).
Conclusion: Our study shows that addition of BPS to the standard treatment regime of COVID-19 patients in ICU improved outcomes and reduced the length of ICU treatment. Natural products could be considered as new approaches for reducting the harmful consequences of COVID-19.
Introduction
Coronavirus 2019 (COVID-19) is a pandemic that has increased mortality worldwide. According to the WHO statistics, as of 29 May 2022, over 526 million confirmed cases and over six million deaths have been reported globally leading to nearly 4,680,008 deaths (1). Iran was among the countries most affected by the pandemic with daily reports from the Health and Medical Education Ministry showing high numbers of infected subjects. By September 2021, nearly 5,378,408 Iranians were infected with COVID-19 leading to approximately 116,072 deaths (1).
The disease typically starts with mild symptoms resembling the common cold with clinical manifestations such as fever (in 98–88% of the cases), fatigue, dry cough, upper respiratory tract obstruction, dyspnea, sputum production, muscle pain, and gastrointestinal, blood lymphopenia, increased the prothrombin (PT) prolongation, elevated C reactive protein (CRP), and lactate dehydrogenase (LDH). Lung opacity, significantly reduced lymphocyte count, as well as the increased neutrophil count is observed in patients with severe disease. Serum levels of inflammatory cytokines including IL-6, G-CSF, IP-10, MCP1, MIP1A, and TNF are elevated in COVID-19 patients within intensive care units (ICU), which indicates the occurrence of cytokine storm in these patients (2–4).
The cytokine storm severely damages several organs, causes a deterioration in the patient’s health status, increases the ICU stay time, increases the patient’s dependence on mechanical ventilation and is associated with an increased mortality rate (3, 5). Considering the COVID-19 pandemic and the then absence of any specific treatments for the disease, many attempts were made to re-purpose different therapies in an effort to reduce the mortality rate and improve the clinical symptoms of the patients (3). Among the proposed treatments for controlling the cytokine storm in patients with COVID-19 is treatment with a high-dose corticosteroid (6). Corticosteroids induce severe immune suppression, which increases the risk of nosocomial infections, such as ventilator-associated pneumonia (VAP) which results in a raised mortality rate (6).
The use of complementary medicine and herbal medicine as safe and low-cost treatment approaches have, therefore, been considered (7). For example, AM3 supplement is a glycophosphopeptide derived from Ricinus communis protein and phosphorylated glucomannan polysaccharide from Candida utilis yeast. AM3 supplementation improves COVID-19 outcomes by decreasing the systemic levels of IL-6, TNF-α, CRP and ferritin and the enzymes ALT, AST, LDH, CK, and MB (8). Using medicinal plants for alleviating physical problems, e.g., inflammation is a custom in Iranian traditional medicine (9). Among the herbal candidates is an extract from borage–the Borage plus syrup (BPS) which was recently shown to alleviate inflammation in various in vivo and in vitro studies (10–13). For example, borage can alleviate of Alzheimer’s disease (AD)-induced cognitive dysfunction by halting the decline in hippocampal antioxidant status (10). Borage officinalis treatment also reduced the clinical symptoms of asthma including coughing, dyspnea, wheezing, nocturnal symptoms and airway hyperresponsiveness, but it was unable to reduce the inflammation that is a feature of asthma (11). The effects of borage and it’s metabolites extensively proposed by Das and co-workers (14–16). The Iranian Borage flower has also been used to treat infectious disorders and have antifebrile properties. Borage syrup was considered to be a treatment for jaundice, itch, and ringworm in addition to treating fever (12). Thus, in the current study we aimed to assess the effect of the BPS in severe COVID-19 patients in the ICU.
Materials and methods
The present study is a randomized clinical trial that was conducted without a placebo control arm. All participants completed an informed consent form before enrolling in the study. The trial was approved by the Masih Daneshvari Ethical committee (IR.SBMU.NRITLD.REC.1399.059). The study population consisted of patients admitted to the COVID-19 ICU wards June 2020–December 2020 at Masih Daneshvari Hospital Tehran-Iran. A total of 60 patients were included in this study.
The inclusion criteria of the study included a confirmed COVID-19 diagnosis based on the clinical criteria agreed by a specialist, confirmed positive PCR, CT images of lungs, willingness for participation in the study and written informed consent. The exclusion criteria of this study was patient death or unwillingness to continue the study at any point for any reason. The participants were randomly divided into a control (n = 30) and an intervention group (n = 30). Serum levels of ferritin, CRP, bilirubin, IL-6, TNF-α, ALT, AST, PCT, IL-8 were measured in both control and it intervention groups at admission time and 5 days after treatment. All participants were treated according to the standard WHO protocol at the time with IFN-β and Favipiravir (600 mg three times a day) with patients in the intervention group receiving an additional 5 ml of BPS orally for 5 days. The dose of 5 ml for 5 days was selected based on the previous described effect on the cytokine storm (11, 17).
The sample size has been calculated by one of the key variables in this study, length of stay in ICU. Standard deviation of length of stay was estimated based on a pilot sample included 15 patients, (S = 2.5).
Borage plus syrup preparation
Borage plus syrup is produced by the Iranian Maad Lotus Company (Tehran, Iran) and licensed by Iranian Health Ministry (number 11849). The fatty acid composition of the borage oil was linoleic acid (35–38%), oleic acid (16–20%), palmitic acid (10–11%), stearic acid (3.5–4.5%), eicosenoic acid (3.5–5.5%), and erucic acid (1.5–3.5%) (18). BPS syrup (5 ml) was given to patients at a dose of 100 mg/ml for 5 days which gave a GLA dose of 20 mg/ml (19).
Blood sampling
Before the start of the trial and 5 days after the first treatment with Borage, venous blood samples were collected. The blood samples were centrifuged for 15 min at 380 × g to obtain serum. Serum samples were isolated and then stored at −20°C until analysis.
Serum cytokine determination
A total of 3 ml whole blood without anticoagulant was harvested and after isolation of serum used to measure analytes using ELISA. The serum levels of TNF, IL-6, and IL-8 were measured using commercially available ELISA kits (DIASource, Belgium and eBioscience, USA) exactly according to the manufacturer’s instructions. Assays were read at 450 nM wavelength in an ELISA plate reader.
Statistical analysis
The data was analyzed using SPSS version 22 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered significant. Quantitative data were presented as mean ± SD and qualitative data were shown as a number (percentage). The normality of the quantitative variables was tested using the Kolmogorov–Smirnov test. To compare parametric variables between the two groups, a t-test and, if necessary, a non-parametric Mann–Whitney U test was used. Comparisons of before and after treatment was analyzed using a paired t-test for normal data and Wilcoxon’s test for non-parametric data. Relationships between categorical variables were performed using Cross Tabulation (Pearson Chi-Square Test).
Results
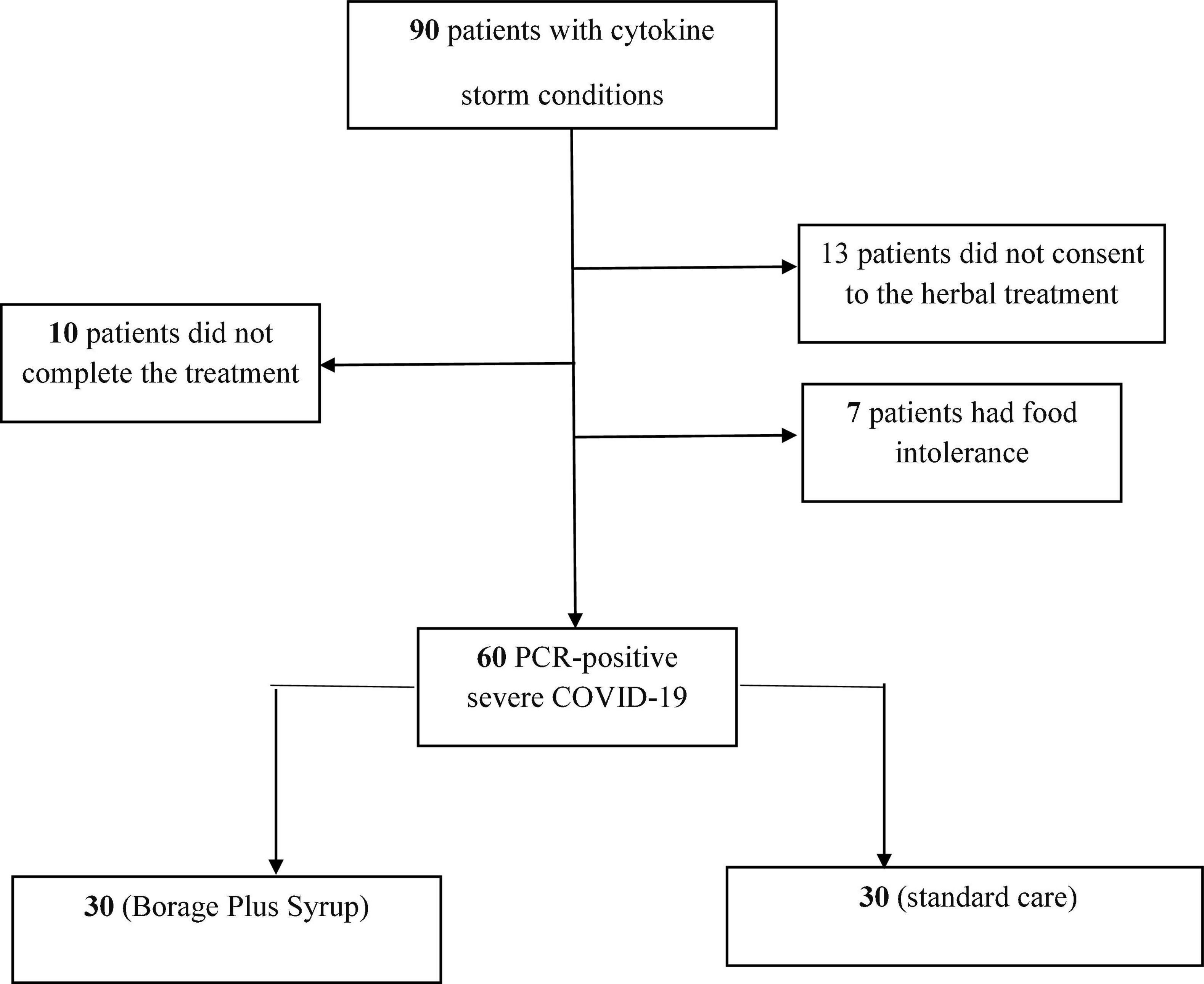
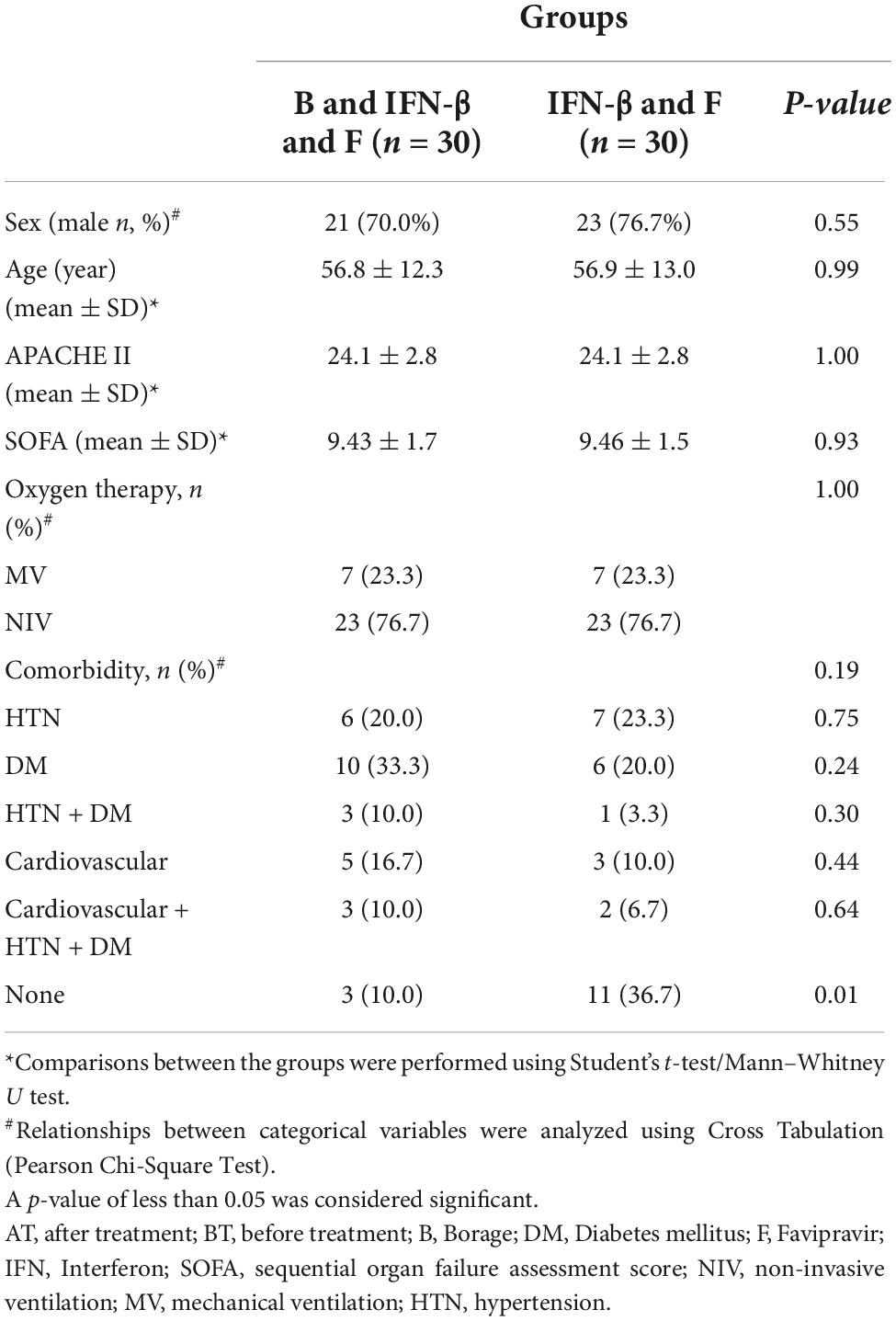
The flowchart for patient recruitment shows the number of patients excluded from the study (30) and the number of subjects (60) included (Figure 1). The average age of the patients was 56.9 ± 13 years in the control group and 56.8 ± 12.3 years in the intervention (BPS) group. The mean APACHE score was 24.1 ± 2.8 in both groups. The average SOFA score in the intervention (9.43 ± 1.7) and control (9.46 ± 1.5) groups were also similar. A total of 14 patients (23.33%) of the 60 participants in this study received mechanical ventilation (MV), while 46 patients (76.67%) received non-invasive ventilation (NIV). Regarding underlying diseases; 13 patients (21.66%) suffered hypertension and 16 patients (26.66%) had diabetes mellitus whilst 4 patients (6.66%) suffered both diabetes mellitus and hypertension. A total of 8 patients (13.34%) suffered cardiovascular diseases with 5 patients (8.34%) suffering from diabetes mellitus, cardiovascular diseases and hypertension. Finally, 14 patients (23.34%) did not have any known underlying diseases (Table 1). None of these variables differed between the BPS and usual care study groups.
Cytokines and serum analytes
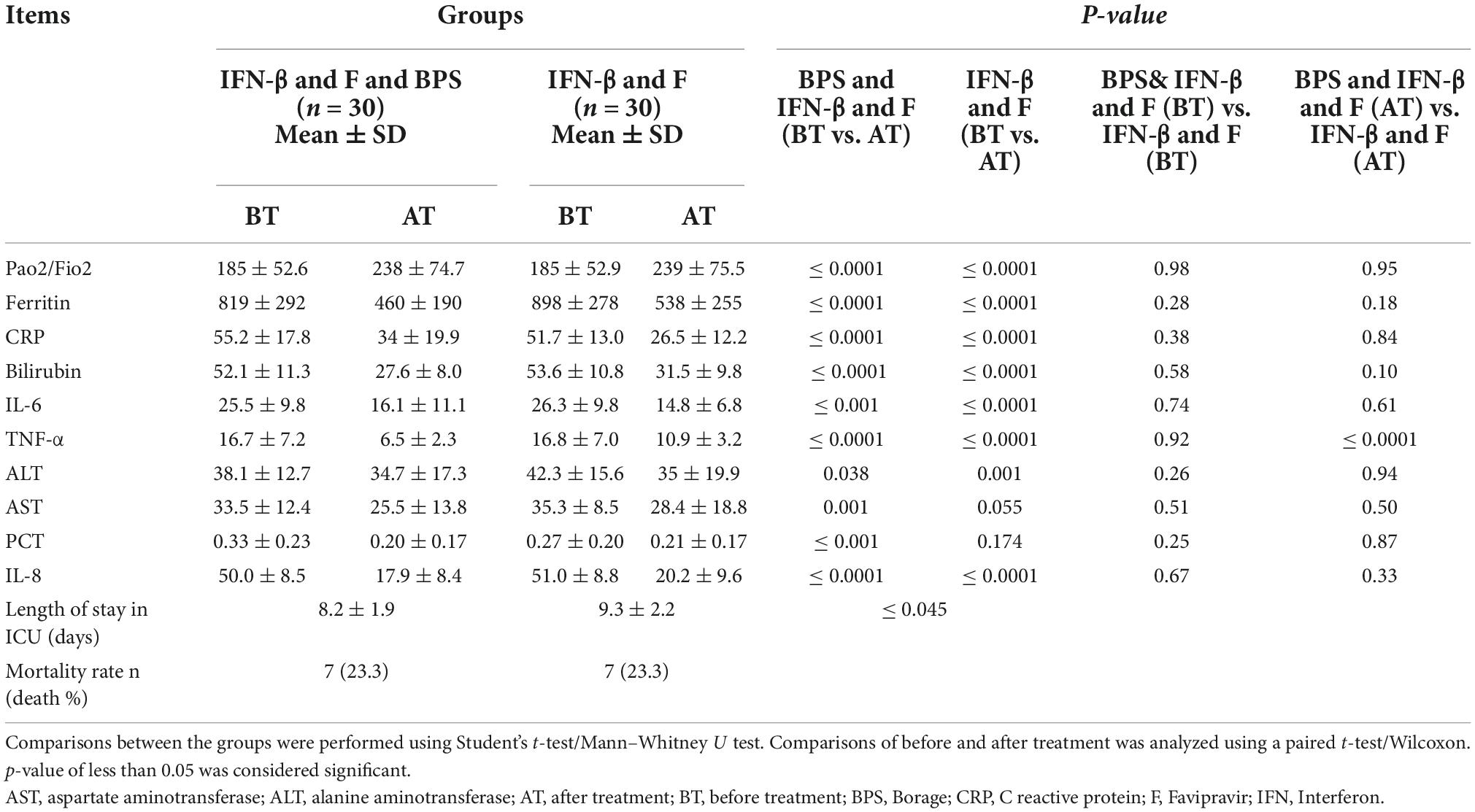
Table 2 shows the comparison of the serum level of cytokines between the BPS intervention and control groups before and after treatment. There were no significant differences in the levels of the analytes measures at baseline between the control and BPS treatment groups. The levels of all analytes were significantly suppressed in the BPS intervention group after treatment. The control group also saw significant reductions in most analytes over time except for AST and PCT. In addition, Pao2/Fio2 in both groups was significantly increased with treatment.
Importantly, the reduction in TNF expression was significantly higher in the BPS intervention group than in the control group (P ≤ 0.0001). Moreover, the length of ICU stay was significantly lower in the intervention group compared with the control group (Table 2).
Discussion
In current study we applied the herbal extract BPS to severe COVID-19 patients within ICU as an add-on therapy to standard treatment. We show that the Pao2/Fio2 in intervention and control groups is increased however serum ferritin, CRP, bilirubin, IL-6, IL-8, TNF-α, ALT, AST, and PCT were decreased in the BPS intervention group. Similar effects on cytokine levels were seen with standard care although no significant attenuation of AST or PCT levels was observed. Interestingly, the reduction in TNF levels was significantly greater in the BPS intervention group. There was a small but significant reduction in the length of ICU stay in the BPS treatment group compared to the standard intervention group.
So far, no effective antiviral treatment is available to treatment of COVID-19 disease and many treatment approaches are geared toward reducing the inflammatory response and to preventing severe lung damage. BPS contains a mixture of medicinal plants such as Borage, lemongrass, yarrow, chamomile, and chicory, all of which have confirmed anti-inflammatory effects in traditional medicine (20). Hashemian et al. showed that BPS can effectively reduce the level of inflammatory cytokines in ARDS patients (17). This resulted in diminishing the duration of mechanical ventilation (MV) as well as disease morbidity and mortality in patients with ARDS (20).
Borage contains several anti-inflammatory and anti-oxidant components (21, 22). This is proposed to account for the beneficial effects seen including anxiolytic, sedative, anti-inflammatory and analgesic actions against the common cold (19). In addition, to antioxidant effects, BPS can stimulate lymphocyte proliferation and antibacterial actions against staphylococcus aureus for example (23). Borage, containing mainly borage extract with the addition of mild hyssop and mint extract, GLA and eicosapentaenoic acid (EPA), GLA and EPA reduce the levels of inflammatory cytokines and chemotactic factors in bronchial alveolar fluid (BAL) of ARDS patients (24). GLA administered in the form of borage oil gets metabolized to dihomo-γ-linolenic acid (DHLA), arachidonic acid (AA) and prostaglandine E2 (PGE1) and other eicosanoids (25). Its GLA content has the capacity to inhibit TNF-α (11). There are no published direct effects of GLA/LA on the SARS-CoV-2 demonstrated using in vitro studies. However, polyunsaturated ω-3 fatty acids can inhibit ACE2-SARS-CoV-2 binding and cellular entry (26).
Furthermore, Borage reduces the mortality rate, duration of mechanical ventilation and the insufficiency of vital organs in patients with ARDS (27).
The fatty acids found in Borago officinalis and its Iranian species Echium amoenum include palmitic, linoleic, stearic, and linolenic acids. This plant’s flower has a long history of use in folk medicine as a bronchodilator (11) but borage oil can reduce the production of ROS, eliminates free radicals, and lessens the impact of inflammatory proteins (10). BPS has been prescribed at a dose of 1.4–2.8 g/day for arthritis and other inflammatory diseases in clinical trials (28). For example, borage oil was clinically effective in children with atopic dermatitis in a double-blind, placebo-controlled clinical experiment. In particular, transepidermal water loss (TEWL) on the dorsal skin was significantly reduced in the borage-treatment group. Importantly, patients had no negative effects (29). Furthermore, borage oil containing γ-linoleic acid decreased inflammatory and non-inflammatory acne lesions as well as lesional IL-8 levels with no serious adverse effects being recorded (30).
The effects of boragina on bone mineral density (BMD) may result from greater GLA concentrations. In fact, the cyclooxygenases that transform GLA into PGE1, which has anti-inflammatory properties that may help bone and recover BMD. This theory is supported by the finding that, in the SAMP8 mouse strain (a progeria model), spleen weight and CRP levels were associated with a reduction in IL-6 and IL-6R expression in bone tissues as compared to a wild type control strain (31). Steatohepatitis and cardiovascular illnesses both exhibit high levels of oxidative stress, inflammation, and lipid imbalance and may be good targets for borage treatment supporting data in rat models (32).
In the current study, the reduced level of TNF-α indicates an anti-inflammatory effect of BPS in COVID-19 disease. We speculate, therefore, that this herbal syrup given in the early stages of COVID-19 may improve the condition of patients and reduce the risk of a cytokine storm developing, hospitalization and thereby reduce the mortality rate. Corticosteroids are a good supportive treatment strategy for patients with severe COVID-19 (33) but are associated with several side effects including immune system suppression and delayed healing. The advantages of BPS could be considered as a natural and safe herbal products with very low side effects.
Dynamic abnormalities in liver function parameters are common in COVID-19 patients, and associated with disease severity and mortality of disease (34). Elevated liver enzymes revealed in 15–58% of COVID-19 patients (35). The most common patterns of liver enzyme abnormalities in patients with COVID-19 include elevated AST and ALT (35). In the current study we show that patients who were treated with BPS had reduced serum levels of AST. Previous studies showed that procalcitonin (PCT) levels are increased in severe COVID-19 patients and that this may be considered as a prognostic factor in the severity of COVID-19 disease (36). In the current study we demonstrate that BPS reduced both AST and PCT levels in contrast to the lack of effect in the standard care group. This may contribute to the beneficial effects of BPS in this ICU cohort of COVID-19 patients.
One limitation of current study is small size of the study conducted in a single center. Furthermore, we did not include a placebo group. However, we show that treatment with BPS is able to decrease PCT and TNF-α more than in the standard care control group and reduce the duration of ICU stay. In addition, although APACHE scores were similar between groups there were significantly fewer subjects with no comorbidities in the borage group. The results of the present study show significant effects of combined treatment of herbal and conventional medications on the treatment effectiveness and alleviation of COVID-19 disease symptoms. This suggests the potential role of herbal products such as BPS in the treatment of patients with COVID-19 or ARDS. Considering that there is still no known treatment for the inflammatory phase of COVID-19, the use of safe herbal remedies along with medical treatments will reduce mortality and increase the effectiveness of therapeutic drugs. In addition, it is possible that the efficacy of borage oil is due to GLA alone and future studies should investigate this. Examination of plasma or serum fatty acid components in future studies may help resolve this and indicate correlations with the effects of borage oil on cytokine levels.
Further studies are needed to characterize the effectiveness of BPS in the early stages of COVID-19 particularly in people who remain resistant to being vaccinated. The precise molecular effects of BPS also warrant additional research.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Shahid Beheshti Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SH desinged the proposal. NS, SZ, HJ, MH, MJ, RE, BK, and MF recruited patients and DIS experiments and approved last version of manuscript. ND analyzed statistical methods. MF, IMA, and EM revised last version of manuscript. All authors contributed to the article and approved the submitted version.
Funding
IMA was supported by the EPSRC (EP/T003189/1 and EP/V052462/1), the UK MRC (MR/T010371/1 and MR/M016579/1), the Wellcome Trust (208340/Z/17/Z), and by the Imperial College Jameel Trust for work on COVID-19. MF was supported by the Iranian Maad Lotus Co., Tehran-Iran.
Acknowledgments
We acknowledge all study participants and critical care nursing team of Masih Daneshvari Hospital, especially Mrs. Maryam Yarinejad.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ZS declared a shared affiliation with several of the authors, SH, SZ, HJ, MH, MJ, RE, MV, SA, and MF, to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Worldometer. COVID Live Update: 142,376,091 Cases and 3,037,353 Deaths from the Coronavirus. (2022). Available online at: http://worldometers.info (accessed May 29, 2022).
2. Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. (2020) 50:620–32. doi: 10.3906/sag-2004-168
3. Dezfuli N, Adcock IM, Montazami N, Mortaz E, Velayati A. Update on immunology of COVID-19 disease and potential strategy for controlling. Tanaffos. (2020) 19:274–90.
4. Mortaz E, Tabarsi P, Jamaati H, Dalil Roofchayee N, Dezfuli NK, Hashemian SM, et al. Increased serum levels of soluble TNF-α receptor is associated with mortality of ICU COVID-19 patients. Front Immunol. (2021) 12:592727. doi: 10.3389/fimmu.2021.592727
5. Mortaz E, Bassir A, Dalil Roofchayee N, Dezfuli NK, Jamaati H, Tabarsi P, et al. Serum cytokine levels of COVID-19 patients after 7 days of treatment with favipiravir or kaletra. Int Immunopharmacol. (2021) 93:107407. doi: 10.1016/j.intimp.2021.107407
6. Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. (2020) 19:102567. doi: 10.1016/j.autrev.2020.102567
7. Ang L, Lee HW, Choi JY, Zhang J, Soo Lee M. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. (2020) 9:100407. doi: 10.1016/j.imr.2020.100407
8. Fernandez-Lazaro D, Fernandez-Lazaro CI, Mielgo-Ayuso J, Adams DP, García Hernández JL, González-Bernal J, et al. Glycophosphopeptical AM3 food supplement: a potential adjuvant in the treatment and vaccination of SARS-CoV-2. Front Immunol. (2021) 12:698672. doi: 10.3389/fimmu.2021.698672
9. Sadighara P, Araghi A, Tajdar-Oranj B, Peivasteh-roudsari L, Mohajer A, Behzadi R. The effect of borage (Echium amoenum) on the mouse heart and hematology parameters. Cardiovasc Hematol Disord Drug Targets. (2019) 19:154–9. doi: 10.2174/1871529X18666181105113617
10. Ghahremanitamadon F, Shahidi S, Zargooshnia S, Nikkhah A, Ranjbar A, Soleimani Asl S. Protective effects of Borago officinalis extract on amyloid beta-peptide(25-35)-induced memory impairment in male rats: a behavioral study. Biomed Res Int. (2014) 2014:798535. doi: 10.1155/2014/798535
11. Mirsadraee M, Khashkhashi Moghaddam S, Saeedi P, Ghaffari S. Effect of Borago officinalis extract on moderate persistent asthma: a phase two randomized, double blind, placebocontrolled clinical trial. Tanaffos. (2016) 15:168–74. doi: 10.1183/13993003.congress-2016.PA4116
12. Abolhassani M. Antibacterial effect of borage (Echium amoenum) on Staphylococcus aureus. Braz J Infect Dis. (2004) 8:382–5. doi: 10.1590/S1413-86702004000500008
13. Rezaei K, Delfan B, Javanbakht A, Toulabi T, Gholami M, Ghiasvand A, et al. The therapeutic effect of borage seeds’ oil on Psoriasis vulgaris. Yafte. (2009) 11:23–30.
14. Das UN. Can bioactive lipids inactivate coronavirus (COVID-19)? Arch Med Res. (2020) 51:282–6. doi: 10.1016/j.arcmed.2020.03.004
15. Das UN. Response to: bioactive lipids and coronavirus (COVID-19)-further discussion. Arch Med Res. (2020) 51:445–9. doi: 10.1016/j.arcmed.2020.04.004
16. Das UN. Essential fatty acids and their metabolites in the pathobiology of (coronavirus disease 2019) COVID-19. Nutrition. (2021) 82:111052. doi: 10.1016/j.nut.2020.111052
17. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. (2020) 11:1446. doi: 10.3389/fimmu.2020.01446
18. Asadi-Samani M, Bahmani M, Rafieian-Kopaei M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pac J Trop Med. (2014) 7(Suppl. 1):S22–8. doi: 10.1016/S1995-7645(14)60199-1
19. Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. (1998) 128:1411–4. doi: 10.1093/jn/128.9.1411
20. Hashemian MR, Jamaati HR, Velayati AA. Historical pie: borage, a forgotten iranian heritage now used in ards treatment. Tanaffos. (2010) 9:1–5.
21. Segovia F, Lupo B, Peiró S, Gordon MH, Almajano MP. Extraction of antioxidants from borage (Borago officinalis L.) leaves—optimization by response surface method and application in oil-in-water emulsions. Antioxidants. (2014) 3:339–57. doi: 10.3390/antiox3020339
22. Zemmouri H, Ammar S, Boumendjel A, Messarah M, El Feki A, Bouaziz M. Chemical composition and antioxidant activity of Borago officinalis L. leaf extract growing in Algeria Arabian. J Chem. (2019) 12:1954–63. doi: 10.1016/j.arabjc.2014.11.059
23. Adel Pilerood S, Prakash J. Evaluation of nutritional composition and antioxidant activity of borage (Echium amoenum) and Valerian (Valerian officinalis). J Food Sci Technol. (2014) 51:845–54. doi: 10.1007/s13197-011-0573-z
24. Gillis RC, Daley BJMD, Enderson BL, Karlstad MD. Eicosapentaenoic acid and γ-linolenic acid induce apoptosis in HL-60 cells. J Surg Res. (2002) 107:145–53.
25. Raederstorff D, Moser U. Borage or primrose oil added to standardized diets are equivalent sources for γ-linolenic acid in rats. Lipids. (1992) 27:1018–23. doi: 10.1007/BF02535582
26. Goc A, Niedzwiecki A, Rath M. Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry. Sci Rep. (2021) 11:5207. doi: 10.1038/s41598-021-84850-1
27. Hamilton LA, Trobaugh KA. Acute respiratory distress syndrome: use of specializedn in pediatric patients and infants. Nutr Clin Pract. (2011) 26:26–30. doi: 10.1177/0884533610392922
28. Soeken KL, Miller SA, Ernst E. Herbal medicines for the treatment of rheumatoid arthritis: a systematic review. Rheumatology (Oxford Engl). (2003) 42:652–9. doi: 10.1093/rheumatology/keg183
29. Kanehara S, Ohtani T, Uede K, Furukawa F. Clinical effects of undershirts coated with borage oil on children with atopic dermatitis: a double-blind, placebo-controlled clinical trial. J Dermatol. (2007) 34:811–5. doi: 10.1111/j.1346-8138.2007.00391.x
30. Jung JY, Kwon HH, Hong JS, Yoon JY, Park MS, Jang MY, et al. Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol. (2014) 94:521–5. doi: 10.2340/00015555-1802
31. Wauquier F, Barquissau V, Léotoing L, Davicco MJ, Lebecque P, Mercier S, et al. Borage and fish oils lifelong supplementation decreases inflammation and improves bone health in a murine model of senile osteoporosis. Bone. (2012) 50:553–61. doi: 10.1016/j.bone.2011.05.030
32. Al-Okbi SY, El-Qousy SM, El-Ghlban S, Moawad HF. Role of borage seed oil and fish oil with or without turmeric and alpha- tocopherol in prevention of cardiovascular disease and fatty liver in rats. J Oleo Sci. (2018) 67:1551–62. doi: 10.5650/jos.ess18064
33. De Backer D, Azoulay E, Vincent JL. Corticosteroids in severe COVID-19: a critical view of the evidence. Crit Care. (2020) 24:627. doi: 10.1186/s13054-020-03360-0
34. Xu W, Huang C, Fei L, Li Q, Chen L. Dynamic changes in liver function tests and their correlation with illness severity and mortality in patients with COVID-19: a retrospective cohort study. Clin Interv Aging. (2021) 16:675–85. doi: 10.2147/CIA.S303629
35. Moon AM, Barritt AS. Elevated liver enzymes in patients with COVID-19: look, but not too hard. Dig Dis Sci. (2020) 66:1767–9. doi: 10.1007/s10620-020-06585-9
Keywords: ARDS, COVID-19, Borage, cytokine storm, CRP, TNF-α, IL-6
Citation: Hashemian SM, Mortaz E, Shafigh N, Ziaie S, Jamaati H, Hasheminik M, Jamalinik M, Erfani R, Khoundabi B, Dezfuli NK, Varahram M, Ahmadi S, Fahimi M and Adcock IM (2022) Effectiveness of Borage plus syrup on COVID-19 patients in intensive care units. Front. Nutr. 9:975937. doi: 10.3389/fnut.2022.975937
Received: 22 June 2022; Accepted: 20 October 2022;
Published: 15 November 2022.
Edited by:
Laurent Hiffler, Independent Researcher, Lagny-sur-Marne, FranceReviewed by:
Zahra Vahdat Shariatpanahi, Shahid Beheshti University of Medical Sciences, IranDiego Fernández Lázaro, University of Valladolid, Spain
Prasad Rasane, Lovely Professional University, India
Copyright © 2022 Hashemian, Mortaz, Shafigh, Ziaie, Jamaati, Hasheminik, Jamalinik, Erfani, Khoundabi, Dezfuli, Varahram, Ahmadi, Fahimi and Adcock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esmaeil Mortaz, ZW1vcnRhekBnbWFpbC5jb20=
 Seyed MohammadReza Hashemian
Seyed MohammadReza Hashemian Esmaeil Mortaz
Esmaeil Mortaz Navid Shafigh
Navid Shafigh Shadi Ziaie3
Shadi Ziaie3 Hamidreza Jamaati
Hamidreza Jamaati Neda K. Dezfuli
Neda K. Dezfuli Mohammad Varahram
Mohammad Varahram Shahrzad Ahmadi
Shahrzad Ahmadi Ian M. Adcock
Ian M. Adcock