- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 3Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Background: Phytosterol is a bioactive compound existing in all plant foods, which might have anticancer properties. The aim of this study was to first assess the impact of the pre-diagnosis phytosterol intake on overall survival (OS) of patients with ovarian cancer (OC).
Materials and methods: This ambispective cohort study recruited 703 newly diagnosed OC patients to investigate the aforementioned associations. Dietary intake was assessed using a validated 111-item food frequency questionnaire. Deaths were ascertained until March 31, 2021, through active follow-up and medical records. Cox proportional hazards regression models were applied to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: During the median follow-up of 37.17 months, 130 deaths occurred. The median age at diagnosis of 703 OC patients was 53.00 (interquartile: 48.00–60.00) years. Of these, almost half patients (48.08%) were diagnosed in advanced International Federation of Gynecology and Obstetrics (FIGO) stage (III-IV). Additionally, more than half patients were serous carcinoma (68.14%), poorly differentiated (85.21%), and no residual lesions (78.66%). Patients consumed the highest tertile of dietary campesterol (HR = 0.54, 95% CI = 0.31–0.94, P trend < 0.05), stigmasterol (HR = 0.60, 95% CI = 0.37–0.98), and β-sitosterol (HR = 0.63, 95% CI = 0.40–0.99) were significantly associated with better OS compared with those with the lowest tertile of intake. The curvilinear associations were observed between total phytosterols and β-sitosterol intake and OC survival (P non-linear < 0.05). Significant associations were generally consistent across different subgroups stratified by demographical, clinical, and immunohistochemical characteristics. Moreover, there were significant interactions between phytosterol intake and age at diagnosis, body mass index, as well as expressions of Wilms’ tumor-1 and Progestogen Receptor (all P interaction < 0.05).
Conclusion: Pre-diagnosis higher campesterol, stigmasterol, and β-sitosterol intake were associated with better survival among OC patients.
Introduction
Ovarian cancer (OC) is one of the most common malignant tumors in women, due to insidious onset and lack of typical symptoms, most patients were diagnosed in the advanced stages (1), and the mortality rate ranks first among gynecological malignancies (2). In 2020, there were 313,959 new cases of OC and 207,252 deaths worldwide (3). Despite surgical treatment and systematic treatment of OC have made great progress, the 5-year survival rate of developed regions still below 50% (4). Accumulated evidence indicated that several factors, including clinical stage (5), histological type (6), menopausal hormone therapy, and breastfeeding (7), were associated with the prognosis of OC. However, these factors are difficult to modify. Thus, it is particularly crucial to find modifiable prognostic factors that can improve the prognosis of OC. Of note, diet is a modifiable factor that could contribute to the improvement of OC survival, which has been verified by our previous research (8–10).
Emerging evidence has revealed that plant food consumption such as vegetables and fruits were inversely associated with OC survival (11). Phytosterol is a kind of bioactive compound found in all foods of plant origin (12), which is abundant in vegetables, fruits, legumes, cereals, and oils (13). Although more than 250 phytosterols have been reported so far, campesterol, β-sitosterol, stigmasterol, β-sitostanol, and campestanol are the main phytosterols found in food (14). Previous studies have suggested that phytosterol exhibited anticancer effect on multiple cancers (15–20). For example, McCann et al. found higher intake of stigmasterol was associated with the decreased risk of OC (20). Several epidemiological studies similarly indicated that phytosterol was related to the lower risk of stomach (15), colorectal (16), esophagus (17), breast (18), and lung (19) cancer. Potential biological mechanisms might account for the aforementioned associations, including promoting apoptosis, arresting the cell cycle (21), and reducing the production of reactive oxygen species (ROS) (22), all of which have been experimentally verified.
Notwithstanding, to our knowledge, no study has investigated the correlations between phytosterol intake and the survival among OC patients. Given the lack of prospective evidence, we performed the present study to evaluate the associations of pre-diagnosis phytosterol consumption with OC survival based on the Ovarian Cancer Follow-Up Study (OOPS).
Materials and methods
Study population
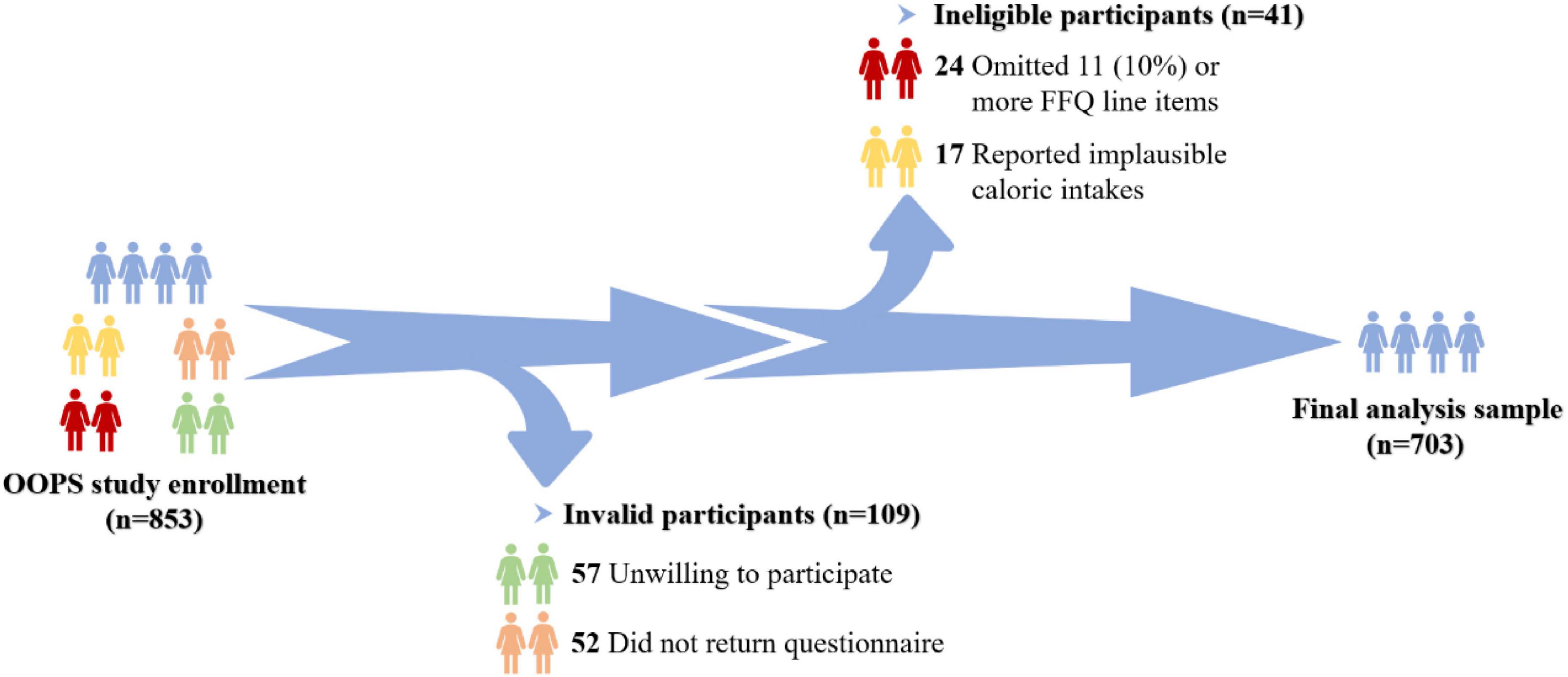
The OOPS is an ongoing ambispective cohort study of newly diagnosed OC patients, designed to assess the associations of demographic and clinical characteristics as well as lifestyle factors with the OC prognosis (8, 23, 24). During the time period between January 2015 and December 2020, 853 newly diagnosed OC patients aged 18–75 years were recruited. Among them, 796 women (93%) agreed to participate, and 744 (87%) women returned the completed study questionnaire. Furthermore, participants who omitted 11 (10%) or more food items (n = 24) and those reported implausible caloric intakes (< 500 or > 3,500 calories per day, n = 17) were excluded. Ultimately, aggregated to 703 women were eligible for the final analysis (Figure 1). The OOPS was approved by the Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China, and all women signed informed consent prior to participation.
Data collection
Baseline information about socio-demographic and lifestyle characteristics, including education, income, smoking status (the smoking status before OC diagnosis), alcohol drinking (the alcohol drinking status before OC diagnosis), dietary change (defined as participants who had deliberately changed their eating habits recently with three response: 3 years ago, 1 to 2 years ago, and this year), menopausal status, parity, and physical activity, were recorded via self-administered questionnaires at the baseline interview. Anthropometrics were measured by trained personnel following a standard protocol, and body mass index (BMI) was calculated as weight in kilograms divided by height in squared meters. Physical activity was evaluated in OC patients according to previous study (25). In brief, all participants were required to report usual type and duration of activities related to work, commuting, household chores, and leisure-time exercise during the past 12 months (25). We calculated total physical activity through metabolic equivalent tasks (METs) from the 2011 update of a major compendium of physical activities (26). Clinical characteristics were extracted from the electronic medical records of the Shengjing hospital information system, including age at diagnosis, histological type, histopathologic grade, International Federation of Gynecology and Obstetrics (FIGO) stage, residual lesions, and comorbidities. On the basis of the routine protocol, specimens of OC tissues and adjacent tissues acquired after surgery were applied to immunohistochemistry (IHC) analysis. All indicators, including Wilms’ tumour-1 (WT-1), Estrogen Receptor (ER), Progesterone Receptor (PR), Vimentin, and p53, were divided into positive and negative. IHC expression was manually confirmed by two independent experienced pathologists.
Dietary exposure and assessment
All participants were recruited at Shengjing Hospital of China Medical University, Shenyang, China and required to complete a 111-item semi-quantitative food frequency questionnaire (FFQ). In the present study, the FFQ was a modified version FFQ used in the Tianjin Chronic Low-grade Systemic Inflammation and Health cohort study (27). The FFQ was performed by well-trained and skilled researchers through face-to-face interviews. Pre-diagnosis dietary information was measured at baseline through the FFQ, its validity and reliability have been verified by previous studies (8, 9). The reproducibility coefficients (Spearman correlation coefficients and intraclass correlation coefficients) were above 0.5 for most food groups, and the correlation coefficients (Spearman correlation coefficients) were between 0.3 and 0.7 for most food groups between the FFQ and weighed dietary records (8–10). All newly diagnosed OC patients were required to report their usual intake frequency of each food item during the year prior to OC diagnosis, with seven response options: almost never, 2–3 times per month, 1 time per week, 2–3 times per week, 4–6 times per week, 1–2 times per day, and more than two times per day. The consumption of each food item was calculated by multiplying fitted portion sizes (gram/time) by the frequency at which each food item was consumed per day (28). Additionally, the Chinese food composition tables were applied as the nutrient database to obtain the nutrient content of each food item (29). By linking the information from the FFQ to the Chinese food composition table, the nutrient intake was calculated by first multiplying the amount of consumption for each food item by its nutrient content and subsequently summing nutrient contributions across all food items (30). The consumption of the following phytosterols from plant food were available for analysis: campestanol, β-sitostanol, campesterol, stigmasterol, and β-sitosterol. Besides, we calculated total phytosterols by summing the above variates. All nutrients were adjusted for total energy intake based on the residual method (31).
Follow-up and outcome
At the present study, the outcome was overall survival (OS). All participants were followed up until the occurrence of mortality from any cause or the last follow-up (March 31, 2021). Information about the vital status of OC patients was ascertained from medical records every 6 months and by active follow-up. Survival time was calculated from the date of histologic OC diagnosis to the date of all-cause death or the date of the last follow-up for women who were still alive.
Statistical analysis
Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile (IQR). And categorical variables were expressed as number with percentage. Distribution of OC patients in terms of demographic and clinical characteristics across total phytosterols intake was examined using one-way ANOVA or Kruskal–Wallis test for continuous variables and Chi-square tests for categorical variables. We estimated crude OS probabilities and plotted crude survival curves by the Kaplan-Meier technique. The proportional hazards assumption was examined through adding an interaction term between each activity variable and log survival time, and variables in this analysis satisfied the conditions (all P > 0.05). Associations between pre-diagnosis phytosterol intake and OS of OC patients were examined using the Cox proportional hazards regression models. Phytosterol intake was categorized into tertiles, the hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the lowest tertile served as the reference group. Continuous intakes of phytosterol were calculated by per SD increment. The linear trend test was assessed by assigning the median value of consumption for each tertile of phytosterol and treating it as a continuous variable in the respective regression model.
The final multivariate models were adjusted for age at diagnosis (< 50 or ≥ 50 years), education (junior secondary or below, senior high school/technical secondary school, and junior college/university or above), cigarette smoking (yes or no), alcohol drinking (yes or no), dietary change (yes or no), menopausal status (yes or no), parity (≤ 1 or ≥ 2), BMI (continuous, kg/m2), physical activity (continuous, MET/hours/day), FIGO stage (I–II, III–IV), histological type (serous or non-serous), histopathologic grade (well, moderately, and poorly differentiated), residual lesions (none, < 1, and ≥ 1 cm), comorbidities (yes or no), and total energy (continuous, kcal/day), isoflavone (continuous, mg/day), and monounsaturated fatty acid (continuous, g/day) intake. Selected covariates for the final models were based on correlation with phytosterol, clinical significance, and previous studies. Carbohydrate, total fiber, and polyunsaturated fatty acids intake excluded from the final analysis, due to the multicollinearity between covariates. We also conducted restricted cubic spline model with three knots (i.e., 5, 50, and 95th percentiles) to test the non-linear relationships between phytosterol intake and OC survival (32).
Stratified analyses were performed according to age at diagnosis (< 50 compared to ≥ 50 years), menopausal status (“no” compared to “yes”), BMI (< 25 compared to ≥ 25 kg/m2), histological type (serous compared to non-serous), FIGO stage (I-II compared to III-IV), and residual lesions (“no” compared to “yes”). Moreover, we also performed stratified analyses by IHC biomarkers, included WT-1 (“positive” compared to “negative”), ER (“positive” compared to “negative”), PR (“positive” compared to “negative”), Vimentin (“positive” compared to “negative”), and p53 (“positive” compared to “negative”). Potential interactions of phytosterol intake with these stratified variables were analyzed by adding cross-product terms in the multivariable Cox regression models. We implemented several sensitivity analyses to test the robustness of the primary findings. First, we adjusted the mean energy intake per day using the nutrient density method. Moreover, we excluded the patients having less than 1-year follow-up period to evaluate whether the associations were independent of follow-up periods. All statistical analyses were conducted by SAS version 9.4 (SAS Institute, Cary, NC, United States), and two-sided P values < 0.05 were considered statistically significant.
Results
Socio-demographic and lifestyle characteristics of 703 OC patients were presented in Table 1, according to tertiles of total phytosterols intake. During the median follow-up 37.17 (IQR: 24.73–50.17) months, a total of 130 confirmed deaths were documented. The median age at diagnosis of 703 OC patients was 53.00 (IQR: 48.00–60.00) years. Of these, 48.65% were diagnosed in early FIGO stage (I-II), 48.08% were diagnosed in advanced FIGO stage (III-IV). In addition, among these OC patients, 68.14% were serous carcinoma, 13.94% were clear cell carcinoma, 12.52% were endometrioid carcinoma, and 2.28% were mucinous carcinoma. Moreover, most OC patients included in our study were poorly differentiated (85.21%) and no residual lesions (78.66%). OC patients with higher total phytosterols intake had longer follow-up time (P < 0.05). Moreover, participants with higher consumption of total phytosterols tended to consume more carbohydrate, total fiber, isoflavones, monounsaturated fatty acids, polyunsaturated fatty acids, and total energy (all P < 0.05). Advanced FIGO stage, larger residual lesions, and non-serous histological subtypes were statistically significant correlated to worse OC survival (Supplementary Table 1). Besides, we found the negative expressions of WT-1, ER, and PR were related to poorer survival of OC (Supplementary Table 2).
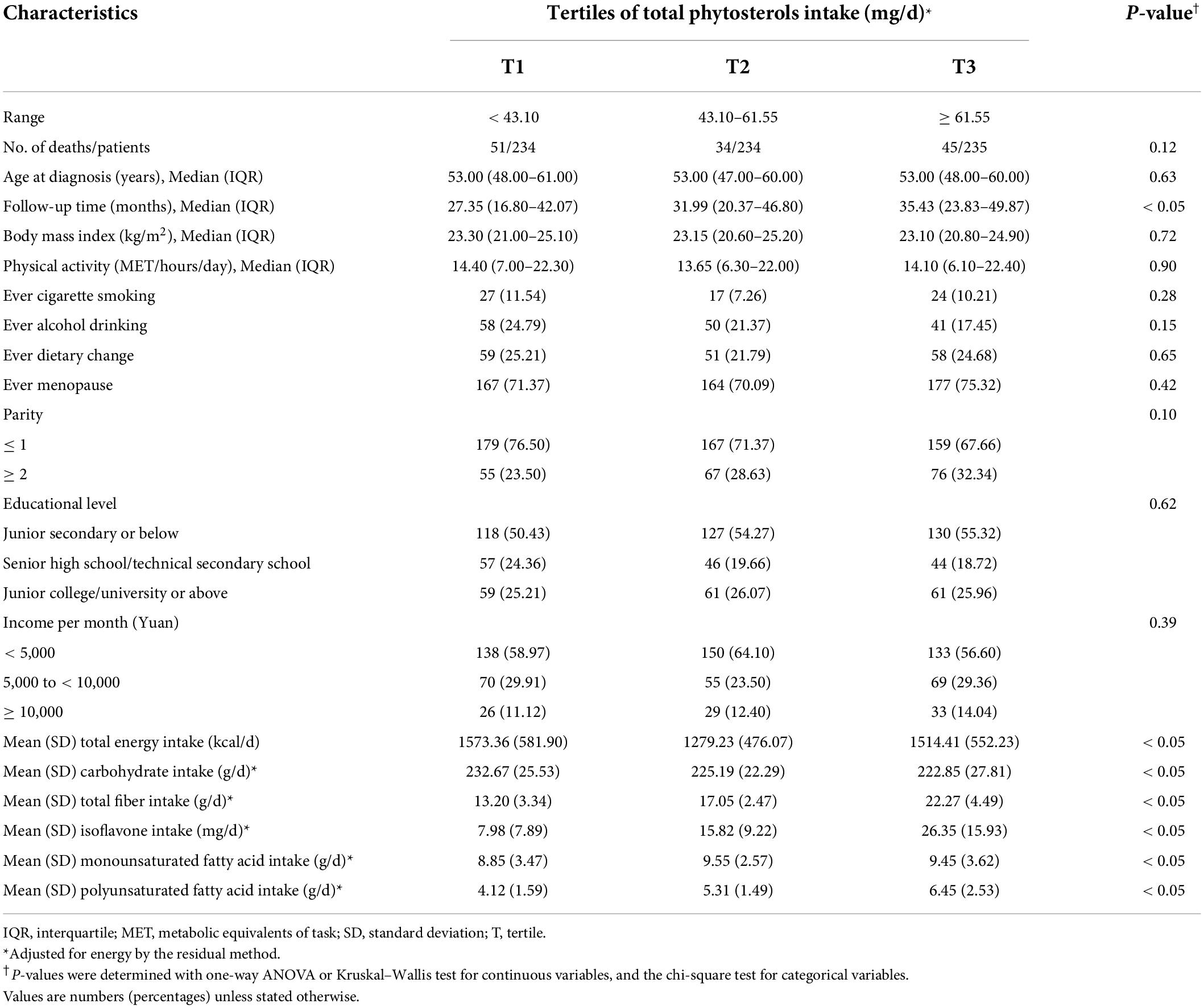
Table 1. Baseline characteristics of ovarian cancer patients by tertile of total phytosterols intake (N = 703).
Table 2 revealed the associations of dietary phytosterol intake with OS of OC patients. After controlling for potential confounders, although we failed to observe significant association between total phytosterols and OC survival (HRT3 vs. T1 = 0.64, 95% CI = 0.40–1.03), we found that the highest tertile of campesterol intake was associated with better OS than the lowest tertile of intake (HR = 0.54, 95% CI = 0.31–0.94) with an evident linear trend (P trend < 0.05) (Figure 2). Moreover, dietary β-sitosterol intake was related to the favorable OC survival (HRT3 vs. T1 = 0.63, 95% CI = 0.40–0.99), similar pattern was also noticed in stigmasterol (HRT3 vs. T1 = 0.60, 95% CI = 0.37–0.98) (Figure 2). However, we failed to observe significant linear trend in these aforementioned variables. Furthermore, our results suggested that there probably existed curvilinear relationships between total phytosterols and β-sitosterol intake and OC survival (P non-linear < 0.05) (Figure 3).
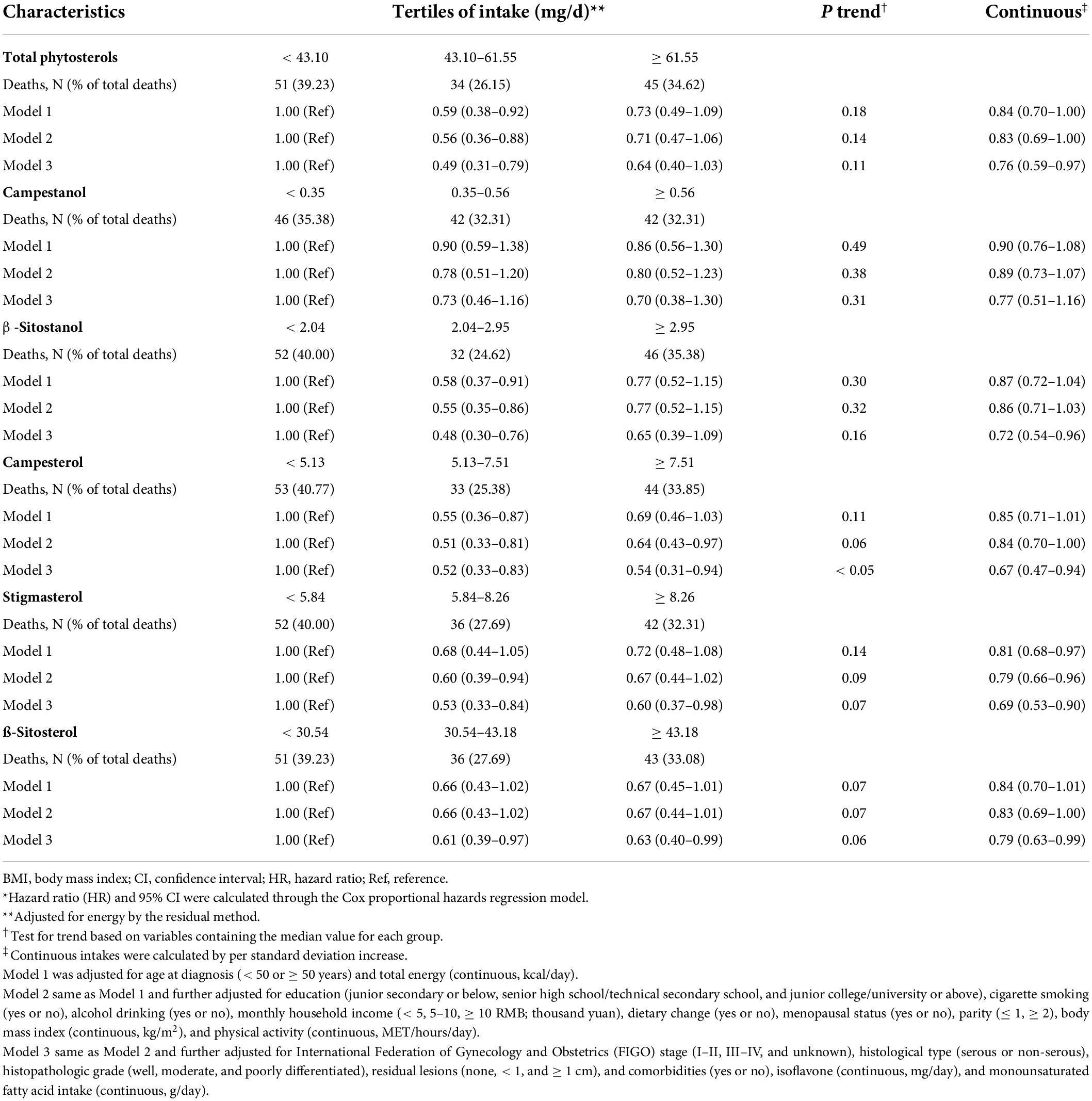
Table 2. Adjusted hazard ratio (HR) and 95% confidence interval (CI) for the associations of phytosterol intake with overall survival among 703 ovarian cancer patients*.
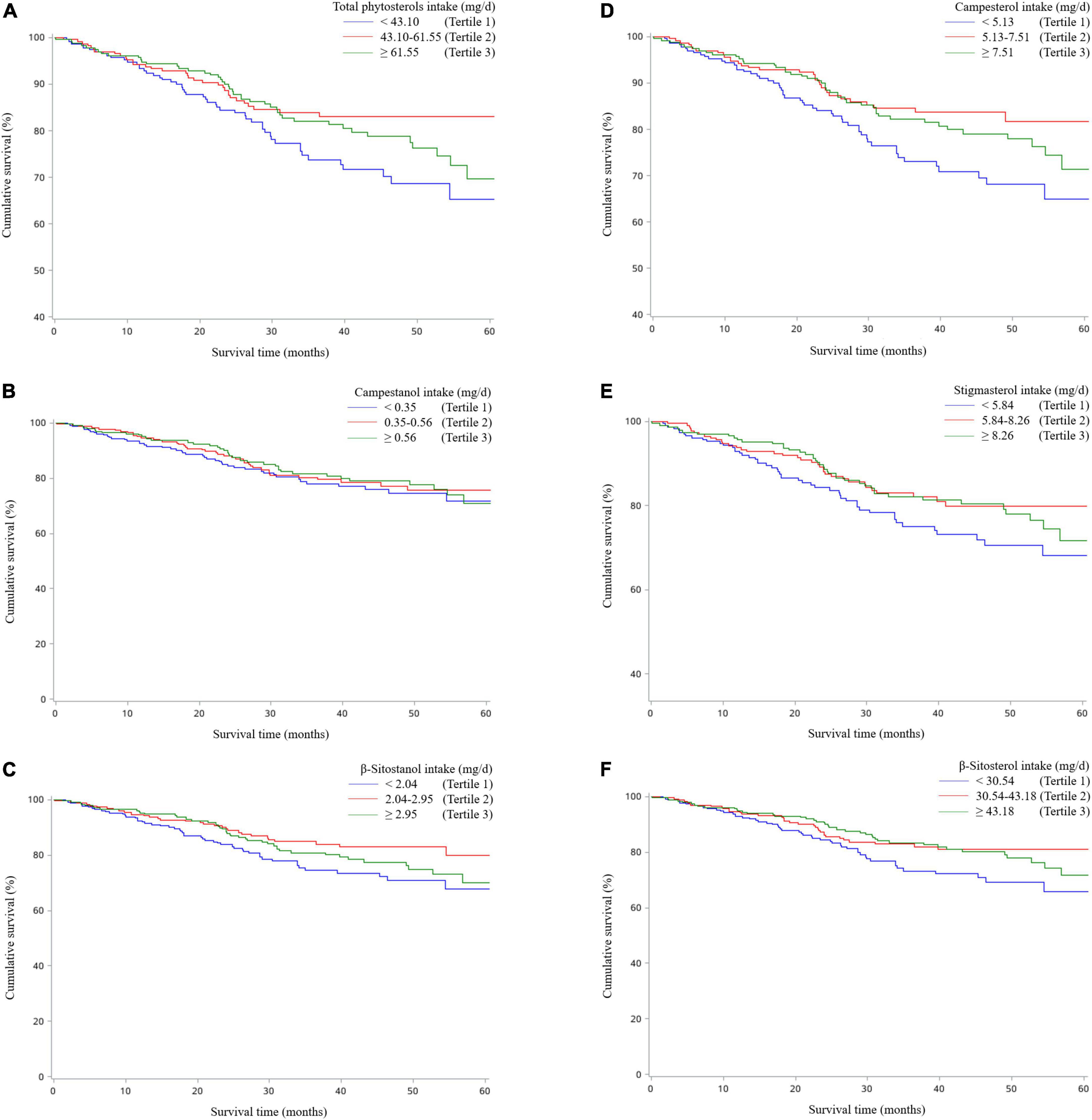
Figure 2. Kaplan–Meier survival curves for total phytosterols (A), campestanol (B), β-sitostanol (C), campesterol (D), stigmasterol (E), and β-sitosterol (F).
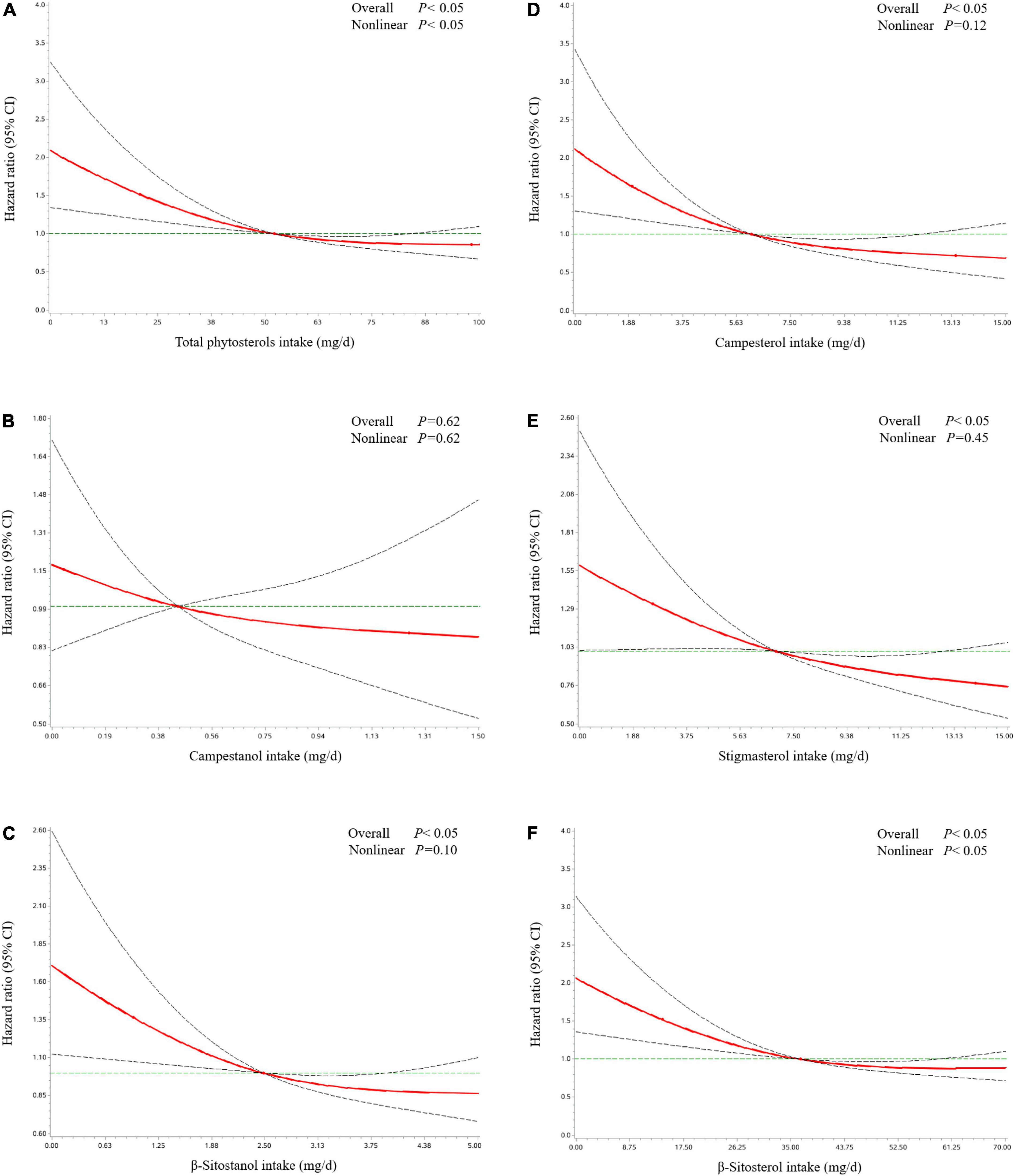
Figure 3. Hazard ratio and 95% confidence interval of overall survival among ovarian cancer patients by total phytosterols (A), campestanol (B), β-sitostanol (C), campesterol (D), stigmasterol (E), and β-sitosterol (F). The associations were adjusted for age at diagnosis, education, cigarette smoking, alcohol drinking, monthly household income, dietary change, menopausal status, parity, body mass index, physical activity, federation of gynecology and obstetrics stage, histological type, histopathologic grade, residual lesions, comorbidities, and total energy, isoflavone, and monounsaturated fatty acid intake. The red line and dashed line represent the estimated HRs and their 95% CIs, respectively. CI, confidence interval; HR, hazard ratio; OC, ovarian cancer.
Consistent findings from subgroup analyses were still observed that higher phytosterol intake was related to better OS of OC patients with I-II FIGO stage, BMI ≥ 25, residual lesions, age at diagnosis > 50, or postmenopausal patients. Additionally, the results of subgroup analyses stratified by IHC biomarkers showed that inversely significant associations were presented in patients with the negative expressions of WT-1, ER, and PR as well as the positive expressions of Vimentin and p53 (Tables 3, 4). Notably, we found significant interactions of age at diagnosis, BMI, and the expressions of WT-1 and PR on the correlations between phytosterol intake and OS of OC patients (all P interaction < 0.05).
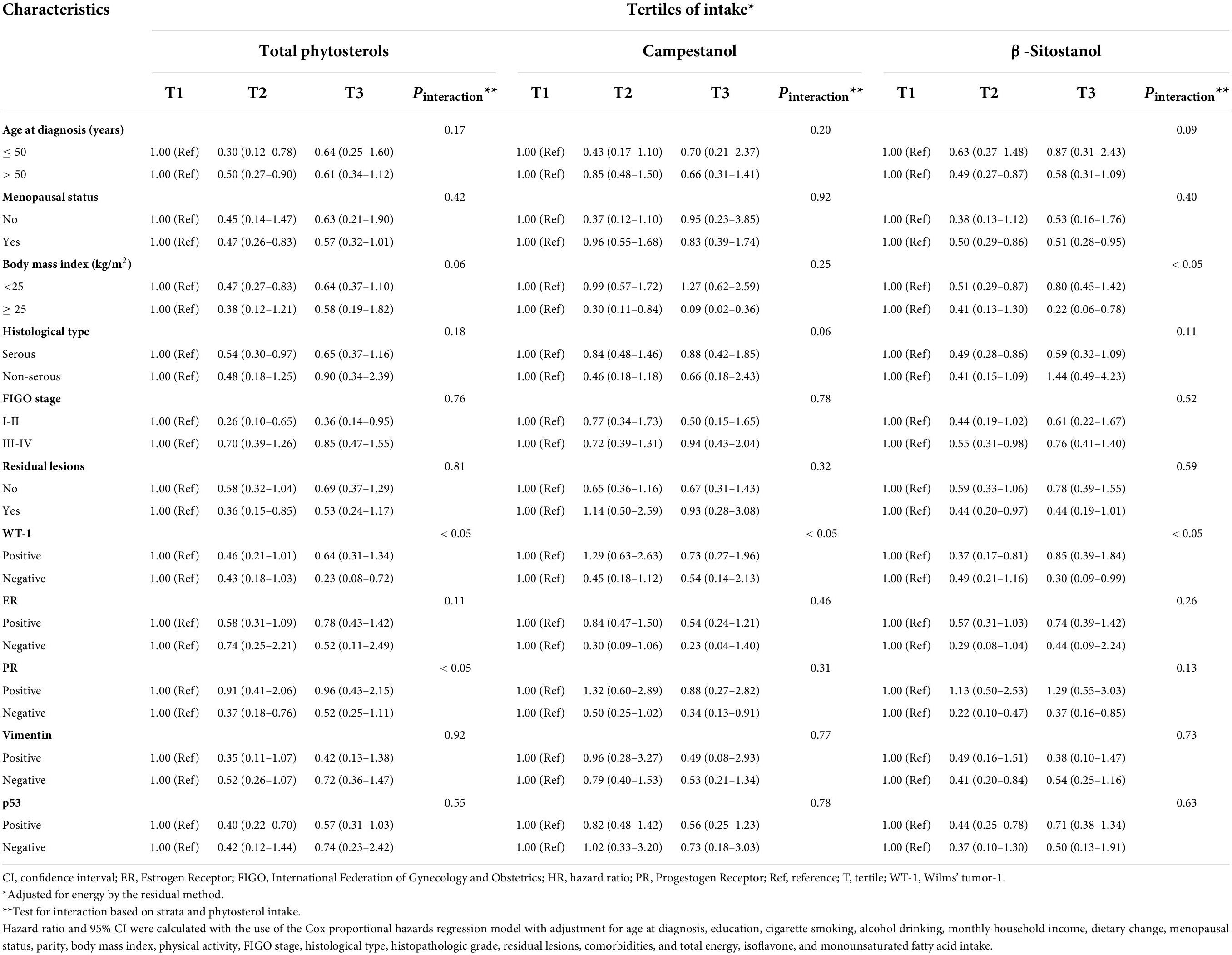
Table 3. Subgroup analyses of demographical, clinical, and immunohistochemical characteristics for adjusted hazard ratio (HR) and 95% confidence interval (CI) for the associations of total phytosterols, campestanol, and β-sitostanol intake with overall survival among ovarian cancer patients.
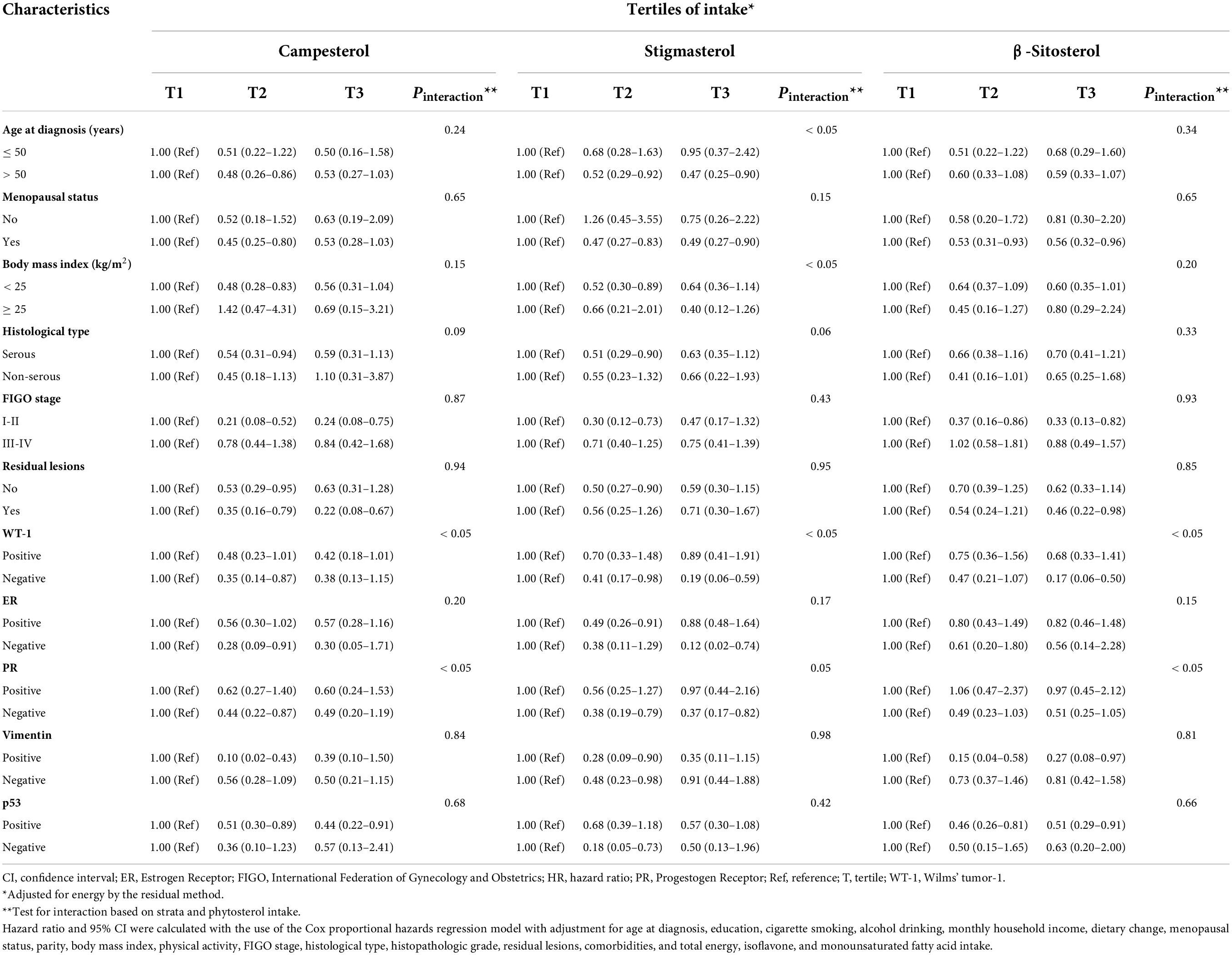
Table 4. Subgroup analyses of demographical, clinical, and immunohistochemical characteristics for adjusted hazard ratio (HR) and 95% confidence interval (CI) for the associations of campesterol, stigmasterol, and β-sitosterol intake with overall survival among ovarian cancer patients.
In sensitivity analyses that adjusted energy with nutrient density method, we found the association between campesterol intake and the OS of OC patients remained significant (Supplementary Table 3). However, we failed to find statistically significant correlations between β-sitosterol and stigmasterol intake with OC survival. Additionally, after excluding the patients less than 1-year follow-up, we found OC patients consumed the highest tertile of dietary total phytosterols, campesterol, and β-sitosterol were significantly associated with better OS compared with those with the lowest tertile of intake (data not shown).
Discussion
In this ambispective cohort study, we firstly examined the correlations between pre-diagnosis phytosterol intake and OS of OC patients. We found that higher campesterol, stigmasterol, and β-sitosterol intake were associated with better OS, total phytosterols and other phytosterols showed null association. Interestingly, we observed significant interactions of age at diagnosis, BMI, and the expressions of WT-1 and PR on the associations of phytosterol intake with OC survival.
Although no research has investigated the relationships between dietary phytosterol consumption and OC survival, one study examined the associations of phytosterol intake with the risk of OC (20). McCann et al. performed a case-control study in western New York involving 124 OC cases and 696 population-based controls, found higher stigmasterol intake was associated with a decreased risk of OC (OR = 0.42, 95% CI = 0.20–0.87) (20). Moreover, several previous studies have provided potential significant evidence for other cancer outcomes (15–19). For example, a hospital-based case-control study conducted in Guangzhou, China, enrolled 1,802 colorectal cases and 1,813 controls, suggested that dietary consumptions of total phytosterols, β-sitosterol, campesterol, and campestanol were inversely associated with colorectal cancer risk, whereas stigmasterol intake was related to an increased risk of colorectal cancer (16). Additionally, several case-control studies implemented in Uruguay with 100–500 newly diagnosed multiple cancer patients manifested that phytosterol were related to the decreased risk of stomach cancer (15), esophageal cancer (17), breast cancer (18), and lung cancer (19). Generally, current studies suggested that phytosterol might have favorable effect on cancers. Given the lack of relevant literature on phytosterol intake and OC survival, further studies are warranted to validate our results.
Despite null association was observed between β-sitostanol intake and OC survival, findings from stratified analysis by BMI showed that β-sitostanol intake was associated with better OS of OC patients among the subgroups of high BMI (BMI ≥ 25 kg/m2). Of note, we also observed a significant interaction between β-sitostanol intake and BMI (P interaction < 0.05). Since one previous meta-analysis reported that high BMI at diagnosis was not correlated to the prognosis of OC (33). Moreover, the distribution of death in present study were mainly concentrated in the normal BMI range. Hence, the inverse association presented in high BMI patients might be attributed to the interaction between BMI and β-sitostanol consumption. Our findings need to be validated by future studies.
Vitro experiments have shown that several phytosterols, including campesterol, β-sitosterol, and stigmasterol, exerted anticancer effect on OC, which provided potential biological mechanisms for our findings. These phytosterols activated proapoptotic signals and induced the apoptosis of OC cells, simultaneously upregulated the calcium levels of cytosolic and mitochondrial and caused excessive calcium overload (34–36), which further promoted the cell apoptosis (37, 38). Besides, these phytosterols promoted the overexpression of unfolded protein response and endoplasmic reticulum-mitochondria axis signals, which triggered cell apoptosis pathway and autophagic stimulus and caused endoplasmic reticulum stress (34–36, 39), the accumulation of endoplasmic reticulum stress induced the cancer cells death (40). Moreover, these phytosterols promoted the production of ROS (34–36), excessive ROS caused cell death through intrinsic apoptotic signals in the mitochondria or extrinsic apoptotic signals by death receptor pathways (35, 41, 42). Additionally, these phytosterols inhibited cell growth and cell cycle progression through inhibiting the expression of the proliferating cell nuclear antigen and PI3K/MAPK signal pathways, similarly reduced OC cells migration and inhibited the aggregation of OC cells (34–36). Despite present evidence suggested phytosterol could improve OC survival, the vitro trials only conducted in the ES2 and OV90 human OC cells and restricted to several phytosterols. Future research should further explore the effect of other phytosterols and total phytosterols on multiple types of OC cells and conduct randomized controlled trial to illustrate exact and detailed biological mechanisms.
Previous studies suggested that the expression of IHC biomarkers, including ER, PR, p53, WT-1, and Vimentin, might have prognostic implications on the OC survival (43–45). Notably, our study found significant interactions between pre-diagnosis phytosterol intake and PR as well as WT-1 in relation to the survival of OC patients. Nevertheless, restricted to the fewer participants in some categories, we could not fully eliminate the possibility of accidental findings. Future studies are needed to validate these interactions.
A major strength of the present study is that we firstly investigated pre-diagnosis phytosterol intake and OS of OC patients. Second, the OOPS was an ambispective cohort study with high participation rates (93%) and a small proportion of loss to follow-up (6%), which could reduce the likelihood of recall bias and selection bias. Third, the comprehensive collection of detailed lifestyle and clinical characteristics in relation to OC survival allowed us to rigorously control potential confounding factors and provide more credible findings. Moreover, we performed multifaceted subgroup analyses and considered the interactions of phytosterol with several key influential factors to strength the reliability of primary results.
Nonetheless, the present study also has some potential limitations. First, the baseline data, including dietary intake, was collected through a self-administered questionnaire, which might lead to recall bias. However, we used a highly reproducible validated FFQ, which was performed by well-trained and skilled researchers through face-to-face interviews. Second, our study only collected dietary intake prior to diagnosis, some participants might change their dietary habits anterior to the year before diagnosis. However, only a small proportion of participants (23.9%) of our study changed their dietary habits and we controlled for dietary change in the final analysis. Third, we failed to evaluate the associations of dietary phytosterol intake with OC specific mortality, due to the data for the cause of death were not obtainable. However, previous study indicated that the results of overall and OC-specific mortality were highly consistent (46). Fourth, we did not obtain the consumption of cooking oil, the consumption of total phytosterols in current study (mean: 55.1 mg/d) was obviously lower than the consumption of total phytosterols in United States (mean for cases: 557.8 mg/d; mean for controls: 646.8 mg/d); thus, it should be cautious to generalize our findings to western populations. Fifth, although we have collected the information about surgery and chemotherapy, the details of these information are relatively limited, potential residual confounding of different surgical and chemotherapy protocols on the associations between phytosterol intake and OC survival might not have been ruled out completely. Sixth, since the present study is a single-center cohort study, several biases such as selection bias are unavoidable. Last, although many confounders were considered, the impact of unknown or unmeasured factors might not be eliminated in any of the observational studies.
Conclusion
In conclusion, the present study provides evidence suggesting that the pre-diagnosis higher consumption of campesterol, β-sitosterol, and stigmasterol are associated with better OS of OC patients. Further studies with extended follow-up period and larger sample size are warranted to confirm our findings.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Ethics Committee of Shengjing Hospital of China Medical University, Shenyang, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-QZ, Y-YH, T-TG, Q-JW, and Y-HZ conceived the study. T-TG, SG, Q-JW, and Y-HZ contributed to the design. T-TG, SY, and SG collected the data. Y-FW, SY, F-HL, and Q-JW cleaned the data and checked the discrepancy. J-QZ, Y-FW, and F-HL analyzed the data. All authors interpreted the data, read the manuscript, and approved the final vision.
Funding
This work was supported by the Natural Science Foundation of China (No. 82073647 to Q-JW and No. 82103914 to T-TG), LiaoNing Revitalization Talents Program (No. XLYC1907102 to Q-JW), Clinical Research Cultivation Project of Shengjing Hospital (SG), and 345 Talent Project of Shengjing Hospital of China Medical University (Nos. M0268 to Q-JW and M0952 to T-TG).
Acknowledgments
We thank the research team for their daily efforts in material collection and manuscript writing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.974367/full#supplementary-material
Abbreviations
BMI, body mass index; CI, confidence interval; ER, Estrogen Receptor; FFQ, food frequency questionnaire; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; Ref, reference; ROS, reactive oxygen species; IHC, immunohistochemistry; IQR, interquartile; MET, metabolic equivalents of task; OOPS, Ovarian Cancer Follow-Up Study; OS, overall survival; OC, ovarian cancer; PR, Progestogen Receptor; WT-1, Wilms’ tumor-1; SD, standard deviation.
References
1. Doubeni CA, Doubeni AR, Myers AE. Diagnosis and management of ovarian cancer. Am Fam Phys. (2016) 93:937–44.
2. Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstetr Gynecol. (2018) 131:909–27. doi: 10.1097/AOG.0000000000002580
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49.
4. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
5. Chudecka-Głaz A, Cymbaluk-Płoska A, Luterek-Puszyńska K, Menkiszak J. Diagnostic usefulness of the risk of ovarian malignancy algorithm using the electrochemiluminescence immunoassay for he4 and the chemiluminescence microparticle immunoassay for CA125. Oncol Lett. (2016) 12:3101–14. doi: 10.3892/ol.2016.5058
6. Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, Rossing MA, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. (2019) 111:60–8.
7. Bešević J, Gunter MJ, Fortner RT, Tsilidis KK, Weiderpass E, Onland-Moret NC, et al. Reproductive factors and epithelial ovarian cancer survival in the EPIC cohort study. Br J Cancer. (2015) 113:1622–31.
8. Wen ZY, Liu C, Liu FH, Wei YF, Xu HL, Wang R, et al. Association between pre-diagnostic dietary pattern and survival of ovarian cancer: evidence from a prospective cohort study. Clin Nutr. (2022) 41:452–9. doi: 10.1016/j.clnu.2021.12.033
9. Wei YF, Hao YY, Gao S, Li XQ, Liu FH, Wen ZY, et al. Pre-diagnosis cruciferous vegetables and isothiocyanates intake and ovarian cancer survival: a prospective cohort study. Front Nutr. (2021) 8:778031. doi: 10.3389/fnut.2021.778031
10. Wei YF, Sun ML, Wen ZY, Liu FH, Liu YS, Yan S, et al. Pre-diagnosis meat intake and cooking method and ovarian cancer survival: results from the ovarian cancer follow-up study (OOPS). Food Funct. (2022) 13:4653–63. doi: 10.1039/d1fo03825g
11. Hurtado-Barroso S, Trius-Soler M, Lamuela-Raventós RM, Zamora-Ros R. Vegetable and fruit consumption and prognosis among cancer survivors: a systematic review and meta-analysis of cohort studies. Adv Nutr. (2020) 11:1569–82. doi: 10.1093/advances/nmaa082
12. Ras RT, van der Schouw YT, Trautwein EA, Sioen I, Dalmeijer GW, Zock PL, et al. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European Prospective Investigation into Cancer and Nutrition-The Netherlands (EPIC-NL) population. Eur J Prev Cardiol. (2015) 22:1067–75. doi: 10.1177/2047487314554864
13. Racette SB, Lin X, Ma L, Ostlund RE Jr. Natural dietary phytosterols. J AOAC Int. (2015) 98:679–84.
14. Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res. (2007) 51:161–70.
15. De Stefani E, Boffetta P, Ronco AL, Brennan P, Deneo-Pellegrini H, Carzoglio JC, et al. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer. (2000) 37:140–4. doi: 10.1207/S15327914NC372_4
16. Huang J, Xu M, Fang YJ, Lu MS, Pan ZZ, Huang WQ, et al. Association between phytosterol intake and colorectal cancer risk: a case-control study. Br J Nutr. (2017) 117:839–50. doi: 10.1017/S0007114517000617
17. De Stefani E, Brennan P, Boffetta P, Ronco AL, Mendilaharsu M, Deneo-Pellegrini H. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. (2000) 38:23–9. doi: 10.1207/S15327914NC381_4
18. Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. (1999) 35:111–9.
19. Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio J, Ronco A. Phytosterols and risk of lung cancer: a case-control study in Uruguay. Lung Cancer. (1998) 21:37–45. doi: 10.1016/s0169-5002(98)00044-0
20. McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. (2003) 133:1937–42. doi: 10.1093/jn/133.6.1937
21. Shahzad N, Khan W, Md S, Ali A, Saluja SS, Sharma S, et al. Phytosterols as a natural anticancer agent: current status and future perspective. Biomed Pharmacother. (2017) 88:786–94. doi: 10.1016/j.biopha.2017.01.068
22. Ramprasath VR, Awad AB. Role of phytosterols in cancer prevention and treatment. J AOAC Int. (2015) 98:735–8.
23. Jiang L, Gong TT, Gao S, Li XQ, Liu FH, Wen ZY, et al. Pre-diagnosis dairy product intake and ovarian cancer mortality: results from the ovarian cancer follow-up study (OOPS). Front Nutr. (2021) 8:750801. doi: 10.3389/fnut.2021.750801
24. Gu JH, Gong TT, Wu QJ, Liu FH, Wen ZY, Gao C, et al. Association between pre-diagnostic dietary supplements intake and ovarian cancer survival: findings from a prospective cohort study in Chinese women. Front Nutr. (2021) 8:758178. doi: 10.3389/fnut.2021.758178
25. Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. (2013) 97:487–96. doi: 10.3945/ajcn.112.046854
26. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. (2011) 43:1575–81. doi: 10.1249/MSS.0b013e31821ece12
27. Gu Y, Zhang S, Wang J, Chi VTQ, Zhang Q, Liu L, et al. Relationship between consumption of raw garlic and handgrip strength in a large-scale adult population. Clin Nutr. (2020) 39:1234–41. doi: 10.1016/j.clnu.2019.05.015
28. Hehua Z, Yang X, Qing C, Shanyan G, Yuhong Z. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ Int. (2021) 147:106347. doi: 10.1016/j.envint.2020.106347
29. Yang YWG, He M, Pan C, Wang Z. China Food Composition (Standard Edition). Beijing: Peking University Medical Press (2018).
30. Hu Y, Ding M, Yuan C, Wu K, Smith-Warner SA, Hu FB, et al. Association between coffee intake after diagnosis of colorectal cancer and reduced mortality. Gastroenterology. (2018) 154:916–26.e9. doi: 10.1053/j.gastro.2017.11.010
31. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. (1986) 124:17–27. doi: 10.1093/oxfordjournals.aje.a114366
32. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statist Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
33. Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW, Song JY. Obesity and epithelial ovarian cancer survival: a systematic review and meta-analysis. J Ovarian Res. (2014) 7:41.
34. Bae H, Park S, Yang C, Song G, Lim W. Disruption of endoplasmic reticulum and ROS production in human ovarian cancer by campesterol. Antioxidants. (2021) 10:379. doi: 10.3390/antiox10030379
35. Bae H, Park S, Ham J, Song J, Hong T, Choi JH, et al. ER-Mitochondria calcium flux by β-Sitosterol promotes cell death in ovarian cancer. Antioxidants. (2021) 10:1583. doi: 10.3390/antiox10101583
36. Bae H, Song G, Lim W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics. (2020) 12:488. doi: 10.3390/pharmaceutics12060488
37. Azimi I, Roberts-Thomson SJ, Monteith GR. Calcium influx pathways in breast cancer: opportunities for pharmacological intervention. Br J Pharmacol. (2014) 171:945–60. doi: 10.1111/bph.12486
38. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. (1999) 284:339–43.
39. Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, Miller CCJ. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol. (2017) 27:371–85. doi: 10.1016/j.cub.2016.12.038
40. Yoon MJ, Lee AR, Jeong SA, Kim YS, Kim JY, Kwon YJ, et al. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget. (2014) 5:6816–31. doi: 10.18632/oncotarget.2256
41. Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. (2014) 21:396–413. doi: 10.1089/ars.2014.5851
42. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. (2018) 80:50–64. doi: 10.1016/j.semcdb.2017.05.023
43. Dionísio de Sousa IJ, Marques DS, Príncipe C, Portugal RV, Canberk S, Prazeres H, et al. Predictive biomarkers and patient outcome in platinum-resistant (PLD-Treated) ovarian cancer. Diagnostics. (2020) 10:525. doi: 10.3390/diagnostics10080525
44. Carter JH, Deddens JA, Mueller G, Lewis TG, Dooley MK, Robillard MC, et al. Transcription factors WT1 and p53 combined: a prognostic biomarker in ovarian cancer. Br J Cancer. (2018) 119:462–70. doi: 10.1038/s41416-018-0191-x
45. Sieh W, Köbel M, Longacre TA, Bowtell DD, deFazio A, Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: an ovarian tumor tissue analysis consortium study. Lancet Oncol. (2013) 14:853–62. doi: 10.1016/S1470-2045(13)70253-5
Keywords: cohort, diet, ovarian cancer, phytosterol, survival
Citation: Zhao J-Q, Hao Y-Y, Gong T-T, Wei Y-F, Zheng G, Du Z-D, Zou B-J, Yan S, Liu F-H, Gao S, Wu Q-J and Zhao Y-H (2022) Phytosterol intake and overall survival in newly diagnosed ovarian cancer patients: An ambispective cohort study. Front. Nutr. 9:974367. doi: 10.3389/fnut.2022.974367
Received: 21 June 2022; Accepted: 08 August 2022;
Published: 25 August 2022.
Edited by:
Xi Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Renying Xu, Shanghai Jiao Tong University, ChinaXiaofang Jia, National Institute for Nutrition and Health, China
Copyright © 2022 Zhao, Hao, Gong, Wei, Zheng, Du, Zou, Yan, Liu, Gao, Wu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi-Jun Wu, d3VxakBzai1ob3NwaXRhbC5vcmc=; Yu-Hong Zhao, emhhb3l1aG9uZ0Bzai1ob3NwaXRhbC5vcmc=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Jun-Qi Zhao1,2†
Jun-Qi Zhao1,2† Ting-Ting Gong
Ting-Ting Gong Yi-Fan Wei
Yi-Fan Wei Song Gao
Song Gao Qi-Jun Wu
Qi-Jun Wu Yu-Hong Zhao
Yu-Hong Zhao