
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 28 July 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.964484
This article is part of the Research Topic Nutrition, Metabolism and Infection View all 17 articles
Objectives: Vitamin C deficiency is common among patients with sepsis and has been associated with poor clinical outcomes. Nevertheless, the effect of intravenous (IV) vitamin C for the treatment of sepsis remains controversial. The purpose of this meta-analysis was to evaluate the effect of IV vitamin C in patients with sepsis or septic shock.
Methods: Electronic databases (PubMed, Embase, Scopus, and Cochrane Library) were searched from inception through May 25, 2022 for randomized controlled trials evaluating the effect of IV vitamin C treatment in patients with sepsis. The primary outcome was short-term mortality, and secondary outcomes including the duration of vasopressor, length of intensive care unit (ICU) stay, and Sequential Organ Failure Assessment (SOFA) score after vitamin C treatment. Subgroup analyses were performed based on the type of disease, dose and duration of IV vitamin C.
Results: A total of 10 studies were included, with a total sample of 755 septic patients. The IV vitamin C was associated with a significant reduction in the short-term mortality (OR 0.51, 95% CI 0.37–0.69, I2 = 0%) and duration of vasopressor (MD −27.88, 95% CI −49.84 to −5.92, I2 = 95%). The length of ICU stay (MD −0.68, 95% CI −2.13 to 0.78, I2 = 74%) and SOFA score (MD −0.05, 95% CI −1.69 to 1.58, I2 = 86%) were not significantly different between two groups.
Conclusion: In patients with sepsis or septic shock, the IV vitamin C reduced the short-term mortality rate and duration of vasopressor, with no effect on the length of ICU stay and SOFA score. Further trials are required to explore the optimal dosage and duration of IV vitamin C.
Systematic Review Registration: https://inplasy.com/inplasy-2022-6-0013/, identifier INPLASY202260013.
Sepsis is a life-threatening syndrome associated with physiological, pathological, and biological abnormalities due to a dysregulated immune response to infections (1). Despite the advances in sepsis research improving the diagnosis and treatment (2), sepsis continues to be the most major cause of intensive care unit (ICU) admission and death (3, 4). Based on the Global Burden of Diseases 2017 estimates, there were 48.9 million incident sepsis cases worldwide, with nearly 11.0 million sepsis-related deaths, accounting for 19.7% of all global deaths (5). Even survivors are at high risk of developing functional limitations, cognitive impairment, and mental health problems, which significantly impair the quality of life (6).
It is well-established that patients with sepsis have decreased levels of vitamin C (also known as ascorbic acid), and this depletion has a dose-dependent association with increased organ dysfunction and mortality (7). The beneficial effects and associated mechanisms of vitamin C in sepsis including its anti-inflammatory and anti-oxidant properties (8, 9), acting as an enzymatic cofactor in the synthesis of vasopressin, cortisol, and catecholamine (10, 11), inhibiting the nitric oxide synthase and regulating the clearance of alveolar fluid (12, 13).
In recent years, multiple randomized controlled trials (RCTs) evaluating the effect of intravenous (IV) vitamin C with or without hydrocortisone and thiamine have been completed. Several recently published meta-analyses (14–20) assessed the combination of hydrocortisone, ascorbic acid, and thiamine (HAT) treatment in septic patients. The results indicated that the HAT treatment improved the Sequential Organ Failure Assessment (SOFA) score and reduced the duration of vasopressor, but was not associated with lower short-term mortality. However, the evidence-based medical evidence for using IV vitamin C as monotherapy in septic population are scarce. Therefore, this current study aimed to evaluate the effect of IV vitamin C alone on clinical outcomes among patients with sepsis or septic shock. Furthermore, we performed subgroup analyses to better understand the effectiveness of IV vitamin C in different populations and examined whether a dose-effect modified the treatment effect of vitamin C.
We conducted our study on the basis of the updated PRISRMA statement (21) (checklist in Supplementary Material), and the study protocol was registered in International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY 202260013). We systematically searched the PubMed, Embase, Scopus, and Cochrane Library for relevant studies in English from inception through May 25, 2022. The search used broad search terms containing “sepsis,” “septic shock,” “vitamin C,” “ascorbic acid,” and “randomized” (the comprehensive search strategies are listed in Supplementary Data Sheet 2).
The inclusion criteria were as follows: 1. Population: adult patients (≥18 years of age) with sepsis or septic shock. Sepsis was defined as reported by the original authors, septic shock was defined as sepsis with the need for vasopressor support; 2. Intervention: IV vitamin C as monotherapy; 3. Comparison: placebo, or no intervention; 4. Outcomes: the primary outcome was short-term mortality, including hospital mortality, and 28/30-day mortality. Secondary outcomes including the duration of vasopressor, length of ICU stay, and SOFA score after vitamin C treatment; 5. Design: RCT.
Two authors (Huiyan Zhu, Qiaoping Ye) independently retrieved relevant studies, extracted characteristics of studies (first author, years of publication, population, intervention and control methods, vitamin C level) and predefined outcomes from included studies.
The Cochrane risk of bias tool (22) was utilized for assessing the methodological quality of including studies by two authors (Huiyan Zhu, Qiaoping Ye), any differences in opinion were resolved by a third adjudicator (Xiaoya Xu).
We computed the pooled odds ratio (OR) with 95% confidence interval (CI) for dichotomous outcomes, and mean difference (MD) with 95% CI for continuous outcomes. The heterogeneity was assessed by the Higgins inconsistency (I2) statistics (23). Substantial heterogeneity was identified when I2 value > 30% and a random-effects model was employed to perform the analysis, otherwise a fixed-effects model would be used. Publication bias was assessed by using the funnel plot and Egger’s regression test (24).
A prespecified subgroup analysis stratified by the types of disease (sepsis vs. septic shock), dose [high dose was set to a daily dose of ≥100 mg/kg or 10000 mg/day, according to the review of Patel et al. (25)], and duration [<5 days vs. ≥5 days, according to the study by Jung et al. (26)] of IV vitamin C treatment. Finally, a sensitivity analysis was conducted to explore the effect of individual study by consecutive exclusion of each study at one time.
During the primary search, we identified 506 articles. Among them, 319 were duplicated articles, and 142 studies were excluded by screening the abstracts. During the evaluation of the full text, 35 studies were further removed with various reasons. Eventually, a total of ten RCTs (27–36) were included in our study (follow chart in Figure 1).
Table 1 presents the characteristics of included studies. A total of 755 patients were included in the analysis, whereof 384 patients received IV vitamin C and 371 patients received placebos or no intervention during the study period. The number of patients in each study ranged from a minimum of 20 up to 167. Seven trials (29–33, 35, 36) enrolled patients with sepsis or septic shock based on the Sepsis-3 criteria, three trials (27, 28, 34) diagnosed sepsis by the original investigators. According to whether the patients required vasopressor support, patients were categorized into sepsis (27–29, 35) and septic shock (30–34, 36) cohort. The dose and duration of IV vitamin C varied amongst included trials. Five trials administered low-dose vitamin C, four administered high-dose vitamin C, and one trial included one low-dose vitamin C cohort and one high-dose vitamin C cohort. In six trials (28, 29, 31–34), patients received IV vitamin C for 3–4 days, two trials (27, 35) administered vitamin C for 6–7 days, and the rest two trials (30, 36) administered vitamin C until ICU discharge. Four trials (28, 29, 31, 32) reported the pre-trial and post-trial plasma vitamin C level, the IV vitamin C treatment could increase the plasma vitamin C concentration. The serum level of vitamin C was significantly higher in intervention group compared with control group.
In addition, the duration of vasopressor, length of ICU stay, and SOFA score were expressed in the form of median and interquartile range in several trials. Thus, we used the methodology of Wan et al. (37) to convert these data into mean and standard deviation.
The results of risk of bias assessment (Figure 2) showed that three studies were rated as high risk of bias: Habib et al. (36) used an open-label design, which carried the risk of bias; in the trial by Mahmoodpoor et al. (31), the SOFA score in intervention group was significantly higher than that in control group; Zhang et al. (35) enrolled critically ill patients with severe SARS-CoV-2-related pneumonia and SOFA score ≥ 2, which were different from other trials. Four trials (27, 30, 31, 36) did not provide the methods of random sequence generation or allocation concealment, four trials (27, 30, 32, 36) did not report the blinding method, which would either underestimate or overestimate the size of the effect. Moreover, six trials (27, 30, 32–34, 36) were rated as having an unclear risk of other bias since because the serum level of vitamin C of intervention and control groups were not provided.
In terms of publication bias, the funnel plot and Egger’s test showed that there was no significant risk of publication bias (Egger’s test, P > 0.05; Supplementary Data Sheet 1).
All included trials reported the short-term mortality with different definitions. Three trials reported in-hospital mortality, five trials reported 28-day mortality, two trials reported multiple results and we chose in-hospital mortality in the analysis. The incidence of short-term mortality in vitamin C group was lower than that in control group, 26.6% (102/384) vs. 41.2% (153/371), respectively. The pooled result indicated that IV vitamin C treatment was associated with a significant lower short-term mortality (OR 0.51, 95% CI 0.37 to 0.69, I2 = 0%, Figure 3).
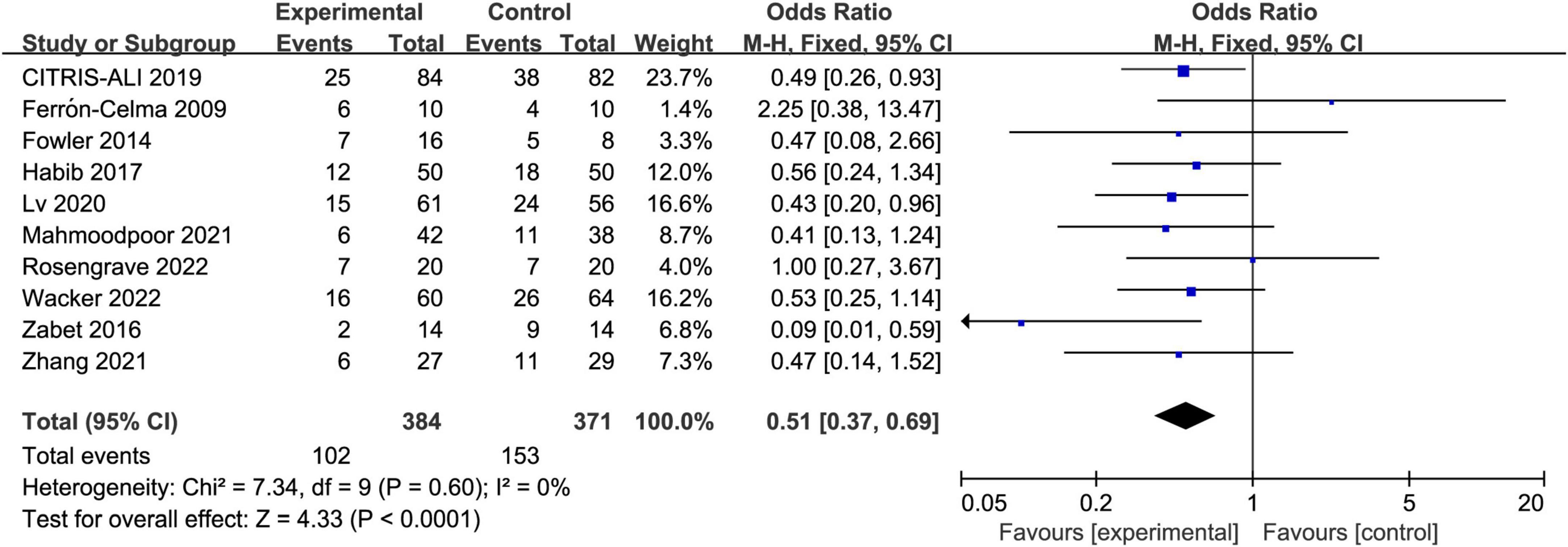
Figure 3. Forest plot showing the association between IV vitamin C and the risk of short-term mortality.
Six trials reported the duration of vasopressor, seven reported the length of ICU stay, and five reported the SOFA score. The IV vitamin C treatment was associated with a reduction in the duration of vasopressor (MD −27.88, 95% CI −49.84 to −5.92, I2 = 95%, Figure 4A) among patients with septic shock. However, there was no significant difference in length of ICU stay (MD −0.68, 95% CI −2.13 to 0.78, I2 = 74%, Figure 4B) and SOFA score (MD −0.05, 95% CI −1.69 to 1.58, I2 = 86%, Figure 4C) between two groups. Notably, the results were greatly weakened by significant heterogeneity.
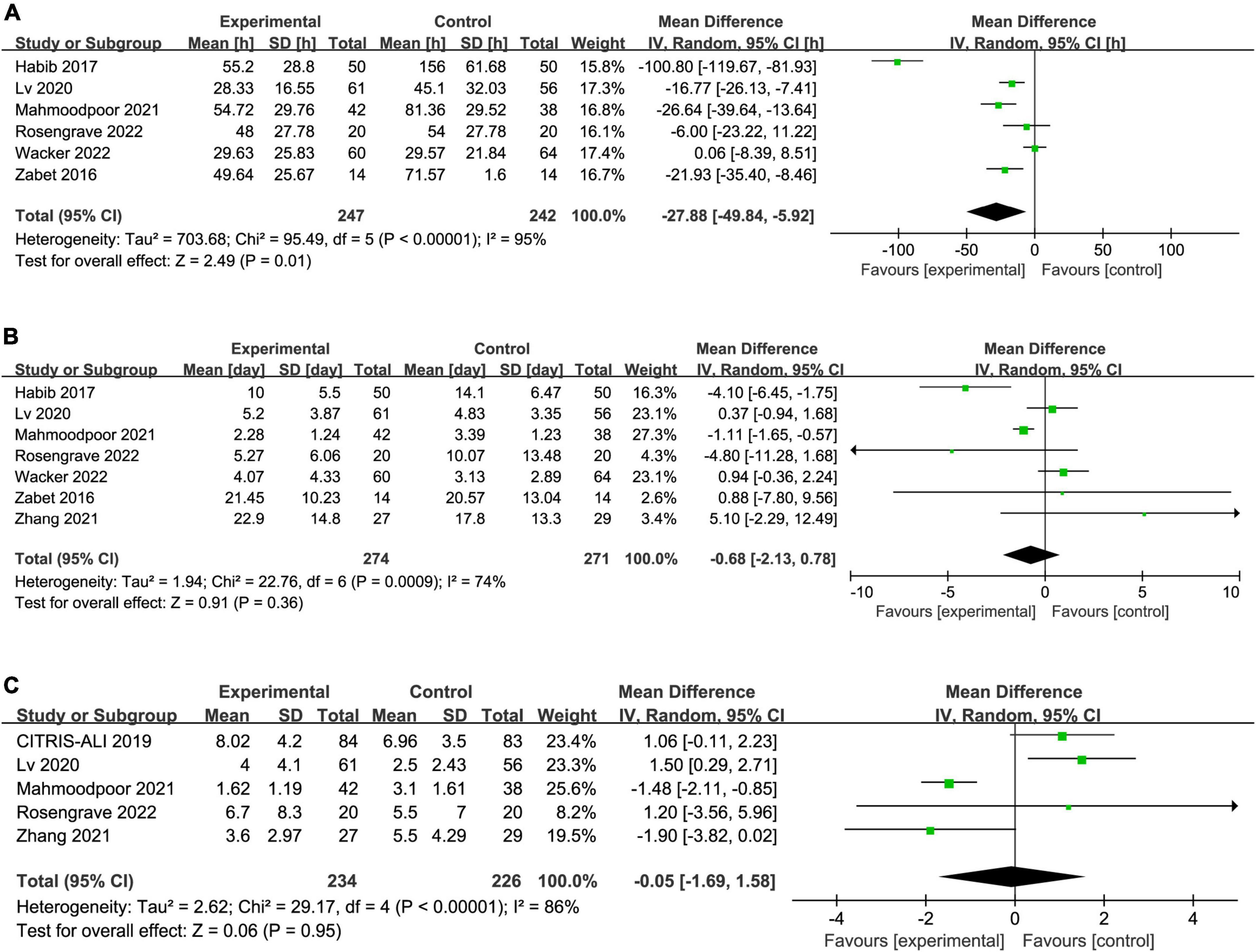
Figure 4. Forest plot showing the association between IV vitamin C and (A) duration of vasopressor, (B) length of ICU stay, (C) SOFA score.
We assessed the effect of every single trial on the pooled result by omitting each study. Furthermore, the sensitivity analysis showed similar results to the overall analysis, indicating the good robustness (Supplementary Data Sheet 1).
We performed subgroup analyses to assess whether the types of disease, dose and duration of IV vitamin C treatment would affect the clinical outcomes. Four trials enrolled patients with sepsis (27–29, 35) and six enrolled patients with septic shock (30–34, 36). Five trials (27, 30, 31, 33, 36) administered low-dose vitamin C, four (29, 32, 34, 35) administered high-dose vitamin C, and one trial (28) included one low-dose vitamin C cohort and one high-dose vitamin C cohort. In six trials (28, 29, 31–34), patients received IV vitamin C for 3–4 days, two trials (27, 35) administered vitamin C for 6–7 days, and the rest two trials (30, 36) administered vitamin C until ICU discharge.
The IV vitamin C treatment was associated with a reduced mortality rate in both the patients with sepsis (OR 0.55, 95% CI 0.33–0.92, I2 = 0%, Figure 5) and septic shock (OR 0.48, 95% CI 0.32–0.71, I2 = 0%, Figure 5). Furthermore, the survival benefit was not associated with the dose or duration of IV vitamin C.
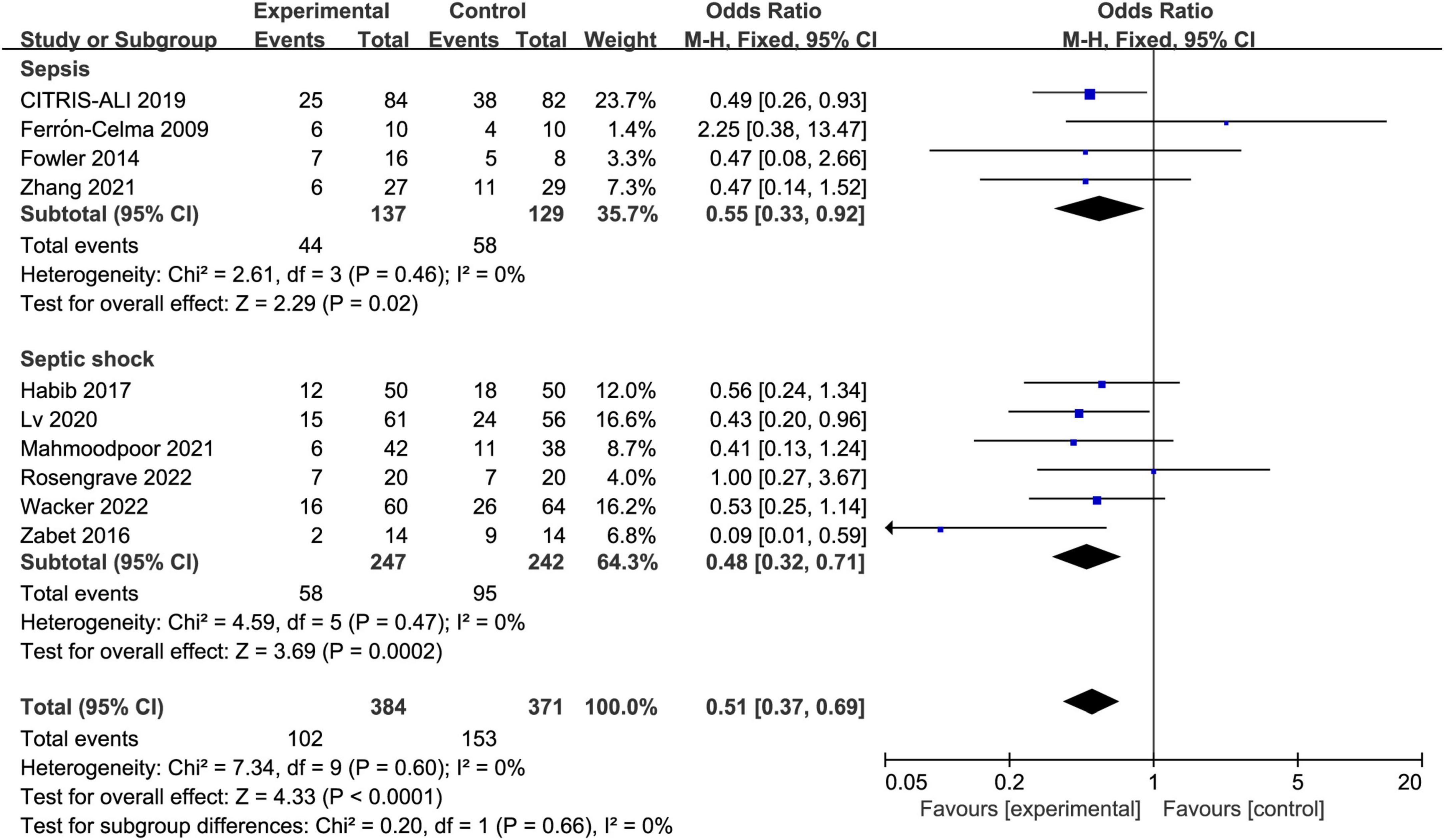
Figure 5. Forest plot showing the subgroup analysis of short-term mortality, patients with sepsis vs. patients with septic shock.
In terms of the duration of vasopressor, the high-does IV vitamin C was associated with reduction in the duration of vasopressor (MD −24.42, 95% CI −47.19 to −1.66, I2 = 95%), whereas the low-does subgroup found no difference (MD −14.10, 95% CI −29.32 to 1.13, I2 = 91%). Moreover, we did not observe significant difference in the duration of vasopressor between patients received IV vitamin C < 5 or ≥5 days.
In addition, the types of disease, dose and duration of IV vitamin C treatment did not have significant effects on the length of ICU and SOFA score (Supplementary Data Sheet 1). The results of subgroup analyses are shown in Table 2.
In this meta-analysis, we included 10 RCTs with 755 patients to analyze the effect of IV vitamin C as monotherapy in patients with sepsis or septic shock. The preliminary analysis showed that the IV vitamin C treatment for patients with sepsis or septic shock was associated with a significant reduction in short-term mortality, but with no effect on the length of ICU stay and SOFA score. Meanwhile, the use of IV vitamin C treatment might reduce the duration of vasopressor for patients with septic shock. Furthermore, the dose and duration of vitamin C showed no significant effect on the clinical outcomes.
To our knowledge, this is the first comprehensive meta-analysis of RCTs to evaluate the effect of IV vitamin C as monotherapy in patients with sepsis or septic shock. Since recent meta-analyses (14–20) failed to find the association between HAT treatment and improved mortality among patients with sepsis, further research evaluating the HAT treatment in sepsis appears to be less necessary. In contrast, Patel et al. (25) performed the first meta-analysis to evaluate the role of IV vitamin C monotherapy in critically ill patients and revealed a significant treatment effect. On that basis, we hypothesized that IV vitamin C monotherapy still remain protective effect against sepsis, and further evaluated the effects of IV vitamin C in patients with sepsis or septic shock. The results of our meta-analysis are approximately consistent with the research by Patel et al. (25) that IV vitamin C treatment was associated with a significant lower short-term mortality, whereas with no difference in length of ICU stay and SOFA score.
The possible mechanisms of the benefit of IV vitamin C treatment in patients with sepsis or septic shock can be explained in several ways. First of all, serum levels of vitamin C decline rapidly among septic patients, confirming their critical involvement in a worsening prognosis (38, 39). The IV vitamin C treatment could restore the plasma vitamin C concentration. All the trials analyzed in our meta-analysis reported an increased serum level of vitamin C in intervention group.
Secondly, some of the physiological effects of vitamin C are of great significance to improve the prognosis of septic patients. Vitamin C is an important antioxidant of the body (40), supports the synthesis of vasopressin, cortisol, and catecholamine (10, 11), increases lymphocytic and neutrophilic activity while attenuating neutrophil necrosis (41, 42). Furthermore, vitamin C also regulates gene expression of pro-inflammatory and coagulation (39, 43), nuclear cellular responses to stress and hypoxia (44), and orchestrates the immune system and circulating cytokine homeostasis in pleotropic ways (42). Therefore, the vital role of vitamin C and its depletion in septic states justifies the use of IV vitamin C in patients with sepsis or septic shock.
However, as suggested by the negative results of some secondary outcomes in our meta-analysis, the beneficial effect of reducing SOFA score or length of ICU stay does not happen. Previous research has demonstrated that some patients might experience hypovitaminosis C as early as 48 h after discontinuation of vitamin C infusion, regardless of the dosing regimen (45). Given that most included trials limited IV vitamin C use to a maximum of 4 days, sustained therapy may be needed to obtain the favorable effects of vitamin C over time. In the CITRIS-ALI trial (29), patients in the 4-day IV vitamin C treatment group had lower 28-day mortality rate, but the survival curve parallel to that of placebo after cessation of vitamin C infusion. The subgroup analysis showed that septic patients received high-dose vitamin C had lower mortality rate and shorter duration of vasopressor, indicating the improvements in clinical outcomes might be dose dependent. Considering the higher dose or longer medication time of IV vitamin C may have produced different results (46), the most effective dose of IV vitamin C and duration of treatment as well as its effects on clinical outcomes also remains to be seen.
However, our study has several limitations. First of all, this meta-analysis was limited by the small sample size of included RCTs, the sample size was relatively small (number of participants < 100 per arm), which may introduce small-study effects and get larger beneficial treatment effects conclusion (47).
Secondly, since sepsis is a clinically common syndrome with high heterogeneity, the studied population represents a heterogeneous population. For example, some were surgical patients after major operation, some had severe pneumonia or respiratory failure. Similarly, the clinical characteristics of included studies were heterogeneous. The baseline of vitamin C level, dose and duration of IV vitamin C, as well as the disease severity of enrolled patients are varied across all the studies. Thus, the pooled estimates should be interpreted with caution since the significant heterogeneity.
Moreover, renal impairment is one of the important adverse effects when people receive high-dose IV vitamin C (48). Considering only a few articles reported the incidence of acute kidney injury as vitamin C related adverse event, there was not enough data to evaluate the incidence of acute kidney injury between vitamin C and control group.
Finally, the data for continuous variables were reported using the median, interquartile range, or range in several trials, which were calculated into mean and standard deviation. But there was a certain deviation from the real value that leading to bias into our results.
Among patients with sepsis or septic shock, the IV vitamin C treatment was associated with significant reduction in short-term mortality and duration of vasopressor. Although the statistical heterogeneity considerably weakens the conclusions, the observed favorable effect of IV vitamin C on reducing short-term mortality and duration of vasopressor should be considered. However, we cannot draw a definitive conclusion from this current meta-analysis regarding the optimal dosage or duration of IV vitamin C treatment. Further studies evaluating the effect of different dose or duration of IV vitamin C in septic population are warranted.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HZ and QY conceived the idea, performed the analysis, and drafted the initial writing of this manuscript. XX contributed to the collection and interpretation of data. KZ helped to frame the idea of the study and provided technical support. QY contributed to the revision of this manuscript and to the final approval of the version to be published. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.964484/full#supplementary-material
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/ccm.0000000000005337
3. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC
4. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority - a who resolution. N Engl J Med. (2017) 377:414–7. doi: 10.1056/NEJMp1707170
5. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/s0140-6736(19)32989-7
6. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. (2018) 319:62–75. doi: 10.1001/jama.2017.17687
7. Wald EL, Badke CM, Hintz LK, Spewak M, Sanchez-Pinto LN. Vitamin therapy in sepsis. Pediatr Res. (2022) 91:328–36. doi: 10.1038/s41390-021-01673-6
8. Carr AC, Maggini S. Vitamin c and immune function. Nutrients. (2017) 9:1211. doi: 10.3390/nu9111211
9. Spoelstra-de Man AME, Elbers PWG, Oudemans-van Straaten HM. Making sense of early high-dose intravenous vitamin C in ischemia/reperfusion injury. Crit Care. (2018) 22:70. doi: 10.1186/s13054-018-1996-y
10. Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. (2015) 19:418. doi: 10.1186/s13054-015-1131-2
11. Wilson JX. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid Redox Signal. (2013) 19:2129–40. doi: 10.1089/ars.2013.5401
12. Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, et al. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. (2012) 303:L20–32. doi: 10.1152/ajplung.00300.2011
13. Galley HF, Walker BE, Howdle PD, Webster NR. Regulation of nitric oxide synthase activity in cultured human endothelial cells: effect of antioxidants. Free Radic Biol Med. (1996) 21:97–101. doi: 10.1016/0891-5849(95)02216-3
14. Fujii T, Salanti G, Belletti A, Bellomo R, Carr A, Furukawa TA, et al. Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: a systematic review and a component network meta-analysis. Intensive Care Med. (2022) 48:16–24. doi: 10.1007/s00134-021-06558-0
15. Assouline B, Faivre A, Verissimo T, Sangla F, Berchtold L, Giraud R, et al. Thiamine, ascorbic acid, and hydrocortisone as a metabolic resuscitation cocktail in sepsis: a meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care Med. (2021) 49:2112–20. doi: 10.1097/ccm.0000000000005262
16. Yao R, Zhu Y, Yu Y, Li Z, Wang L, Zheng L, et al. Combination therapy of thiamine, vitamin C and hydrocortisone in treating patients with sepsis and septic shock: a meta-analysis and trial sequential analysis. Burns Trauma. (2021) 9:tkab040. doi: 10.1093/burnst/tkab040
17. Zayed Y, Alzghoul BN, Banifadel M, Venigandla H, Hyde R, Sutchu S, et al. Vitamin C, thiamine, and hydrocortisone in the treatment of sepsis: a meta-analysis and trial sequential analysis of randomized controlled trials. J Intensive Care Med. (2022) 37:327–36. doi: 10.1177/0885066620987809
18. Na W, Shen H, Li Y, Qu D. Hydrocortisone, ascorbic acid, and thiamine (HAT) for sepsis and septic shock: a meta-analysis with sequential trial analysis. J Intensive Care. (2021) 9:75. doi: 10.1186/s40560-021-00589-x
19. Cai B, Lv X, Lin M, Feng C, Chen C. Clinical efficacy and safety of vitamin C in the treatment of septic shock patients: systematic review and meta-analysis. Ann Palliat Med. (2022) 11:1369–80. doi: 10.21037/apm-22-225
20. Li T, Zeng J, Li DH, Yang GY, Wang K, Deng HF, et al. Efficacy of intravenous vitamin C intervention for septic patients: a systematic review and meta-analysis based on randomized controlled trials. Am J Emerg Med. (2021) 50:242–50. doi: 10.1016/j.ajem.2021.08.012
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ.(2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
25. Patel JJ, Ortiz-Reyes A, Dhaliwal R, Clarke J, Hill A, Stoppe C, et al. IV Vitamin C in critically ill patients: a systematic review and meta-analysis. Crit Care Med. (2022) 50:e304–12. doi: 10.1097/ccm.0000000000005320
26. Jung SY, Lee MT, Baek MS, Kim WY. Vitamin C for ≥ 5 days is associated with decreased hospital mortality in sepsis subgroups: a nationwide cohort study. Crit Care. (2022) 26:3. doi: 10.1186/s13054-021-03872-3
27. Ferrón-Celma I, Mansilla A, Hassan L, Garcia-Navarro A, Comino AM, Bueno P, et al. Effect of vitamin C administration on neutrophil apoptosis in septic patients after abdominal surgery. J Surg Res. (2009) 153:224–30. doi: 10.1016/j.jss.2008.04.024
28. Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. (2014) 12:32. doi: 10.1186/1479-5876-12-32
29. Fowler AA III, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. effect of vitamin c infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the citris-ali randomized clinical trial. JAMA. (2019) 322:1261–70. doi: 10.1001/jama.2019.11825
30. Lv SJ, Zhang GH, Xia JM, Yu H, Zhao F. Early use of high-dose vitamin C is beneficial in treatment of sepsis. Ir J Med Sci. (2021) 190:1183–8. doi: 10.1007/s11845-020-02394-1
31. Mahmoodpoor A, Shadvar K, Sanaie S, Hadipoor MR, Pourmoghaddam MA, Saghaleini SH. Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit: a preliminary study. BMC Infect Dis. (2021) 21:616. doi: 10.1186/s12879-021-06288-0
32. Rosengrave P, Spencer E, Williman J, Mehrtens J, Morgan S, Doyle T, et al. Intravenous vitamin C administration to patients with septic shock: a pilot randomised controlled trial. Crit Care. (2022) 26:26. doi: 10.1186/s13054-022-03900-w
33. Wacker DA, Burton SL, Berger JP, Hegg AJ, Heisdorffer J, Wang Q, et al. Evaluating Vitamin C in Septic Shock: a randomized controlled trial of Vitamin c monotherapy. Crit Care Med. (2022) 50:e458–67. doi: 10.1097/CCM.0000000000005427
34. Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose ascorbic acid on vasopressor’s requirement in septic shock. J Res Pharm Pract. (2016) 5:94–100. doi: 10.4103/2279-042X.179569
35. Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. (2021) 11:5. doi: 10.1186/s13613-020-00792-3
36. Habib NT, Ahmed I. Early Adjuvant Intravenous Vitamin C Treatment in Septic Shock may Resolve the Vasopressor Dependence. Int J Microbiol Adv Immunol. (2017) 5:77–81. doi: 10.19070/2329-9967-1700015
37. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
38. Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. (2017) 21:300. doi: 10.1186/s13054-017-1891-y
39. Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. (1996) 24:392–7. doi: 10.1097/00003246-199603000-00006
40. Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. (2009) 35:5–13. doi: 10.1002/biof.7
41. Manning J, Mitchell B, Appadurai DA, Shakya A, Pierce LJ, Wang H, et al. Vitamin C promotes maturation of T-cells. Antioxid Redox Signal. (2013) 19:2054–67. doi: 10.1089/ars.2012.4988
42. Kashiouris MG, L’Heureux M, Cable CA, Fisher BJ, Leichtle SW, Fowler AA. The emerging role of vitamin c as a treatment for sepsis. Nutrients. (2020) 12:292. doi: 10.3390/nu12020292
43. Mikirova N, Riordan N, Casciari J. Modulation of cytokines in cancer patients by intravenous ascorbate therapy. Med Sci Monit. (2016) 22:14–25. doi: 10.12659/msm.895368
44. Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care. (2014) 18:460. doi: 10.1186/s13054-014-0460-x
45. de Grooth H-J, Manubulu-Choo W-P, Zandvliet AS, Spoelstra - de Man AME, Girbes AR, Swart EL, et al. Vitamin C pharmacokinetics in critically ill patients: a randomized trial of four iv regimens. Chest. (2018) 153:1368–77. doi: 10.1016/j.chest.2018.02.025
46. Fujii T, Deane AM, Nair P. Metabolic support in sepsis: corticosteroids and vitamins: the why, the when, the how. Curr Opin Crit Care. (2020) 26:363–8. doi: 10.1097/mcc.0000000000000736
47. Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. (2013) 17:R2. doi: 10.1186/cc11919
Keywords: vitamin C, ascorbic acid, sepsis, septic shock, meta-analysis
Citation: Zhu H, Xu X, Zhang K and Ye Q (2022) The effect of intravenous vitamin C on clinical outcomes in patients with sepsis or septic shock: A meta-analysis of randomized controlled trials. Front. Nutr. 9:964484. doi: 10.3389/fnut.2022.964484
Received: 08 June 2022; Accepted: 06 July 2022;
Published: 28 July 2022.
Edited by:
Filippo Giorgio Di Girolamo, University of Trieste, ItalyReviewed by:
Guosheng Wu, Second Military Medical University, ChinaCopyright © 2022 Zhu, Xu, Zhang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiaoping Ye, MTExNDcwMzk3MUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.