
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 04 August 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.960674
This article is part of the Research Topic Recent Advances in Nanofabricated Delivery Systems of Bioactive Components for Food Applications View all 5 articles
 Rutika R. Jagtap1
Rutika R. Jagtap1 Aniket Garud2
Aniket Garud2 Shubhangi S. Puranik1
Shubhangi S. Puranik1 Mithun Rudrapal2*
Mithun Rudrapal2* Mohammad Azam Ansari3
Mohammad Azam Ansari3 Mohammad N. Alomary4
Mohammad N. Alomary4 Meshal Alshamrani5
Meshal Alshamrani5 Ahmad Salawi5
Ahmad Salawi5 Yosif Almoshari5
Yosif Almoshari5 Johra Khan6,7
Johra Khan6,7 Bhagyashri Warude2
Bhagyashri Warude2Nanobiotechnology is a burgeoning field of research with applications in cancer treatment, targeted chemotherapy, and molecular diagnosis. This study aims at the fabrication of silver nanoparticles using embelin derived from Embelia ribes to evaluate its anticancer property. Silver nanoparticles (AgNPs) have emerged as a novel nano-carrier for therapeutic agents with a wide range of medical capabilities due to their unique structural, physicochemical, and optical features. In our study, the particle size of fabricated AgNPs was measured as 25 nm, and the zeta potential was recorded as −5.42 mV, which indicates the good stability of embelin-derived AgNPs. The crystalline surface morphology was observed by SEM analysis. The FT-IR spectrum confirmed the reduction in silver ions (Ag+) by embelin, and the TEM analysis exhibited polydispersed Ag+ of 20–30 nm. The anticancer potential of embelin-fabricated AgNPs was investigated using in vitro studies on lung cancer cells by the MTT assay. The results revealed significant dose-dependent inhibition of cell proliferation against A549 cell lines. Embelin AgNP-induced apoptosis was measured by the annexin-V PI apoptosis assay, which exhibited significantly low necrotic cells as compared to apoptotic cells. Finally, the findings of our study suggest the anticancer potential of biofabricated embelin AgNPs, particularly against lung cancer cells.
The development of nanotechnology, which offers remarkable solutions to cope with life-threatening disorders, has boosted advancement in the field of medical science (1). Nanotechnology is a significant milestone that has numerous applications in a variety of fields, including electronics (2), textiles (3), cosmetics, and, most crucially, healthcare as targeted drug delivery, diagnosis, treatment, and biosensing for the benefit of humanity (4). Nanoparticles are an appealing platform for a wide range of biological applications. They are more precise therapeutic strategies for difficult-to-control disorders such as cancer.
There are a variety of metal-based nanoparticles, including gold, silver, zinc, iron, titanium, and magnesium (5). Among them, silver nanoparticles are fascinating due to their biomedical applications; silver can upregulate or downregulate cellular mechanisms as well as act as a medium to detect and diagnose body problems (6), and silver has been used since ancient times in ayurvedic medicines. Silver nanoparticles are used in wound dressing due to antimicrobial nature as well as an anticancer agent (7). The synthesis of nanoparticles is a major aspect as various methods are available, viz., chemical, physical, and biological. The alternative to the harmful chemical and physical method for the synthesis of the nanoparticle is “green synthesis”; an eco-friendly and cost-effective approach. Biological systems, such as plants and microorganisms, operate as reducing and capping agents in green synthesis, converting metal ions into metal nanoparticles without the use of complex instruments or chemicals (8). Plants, being a significant reservoir of phytoconstituents, act as a reducing, stabilizing, and capping agent in the creation of nanoparticles.
Cancer is a diverse category of diseases that are fatal and characterized by uncontrolled cellular development. In a multistage linear process, cancer cells progress from a precancerous lesion to a heterogeneous malignant tumor capable of spreading to other organs (9). According to the World Health Organization, cancer is the world's top cause of death, accounting for ~10 million deaths in 2020 (10). Lung cancer is one of the leading causes of death among all cancers; it accounted for ~1.8 million deaths in 2020 (10). Based on clinicopathological stages and cytology, lung cancer can be divided into small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC) (11, 12). The rise of lung cancer cases is due to tobacco/cigarette smoking, and it is the single most factor accounting for up to 90% of all lung cancer cases. Other factors responsible for causing lung cancer are radon, a radioactive gas released during the decay of uranium, thorium, and radium; asbestos, the heat and corrosion resistive fibrous material; environmental tobacco smoke and air pollutants, such as arsenic, nickel, and benzo[a]pyrene (13). A hereditary predisposition to lung cancer has been linked to a 1.7-fold increased chance of developing lung cancer. Infecting agents such as human papillomavirus (HPV) and Epstein-Barr virus are also linked with lung cancer (13, 14).
Despite substantial advances in disease biology and many therapeutic tools such as surgery, radiation therapy, chemotherapy, and targeted therapy, successful cancer care remains elusive (15). Conventional therapies are unsuitable because of a large number of side effects, nonspecificity, high treatment costs, recurrence, and cancer spread (16). As a result, there is a strong need to find a therapeutic treatment that is distinctive, target-oriented, safe, and low-cost. The advancement of medical science has been accelerated by the development of nanotechnology, which offers extraordinary methods for dealing with life-threatening illnesses.
Medicinal plants have been used to treat diseases since antiquity, and they constitute an essential component of traditional healthcare systems throughout the world. The creation of medicinal products and medications has remained a major source of hope for treating a number of human degenerative conditions, such as cancer (17). A large range of medicinal plants has been tested for their broad-spectrum anticancer properties. Eventually, anticancer medicines such as paclitaxel, vincristine, vinblastine, vinorelbine, and camptothecin were isolated as a result of this effort (18). The major research goal in the field of medicinal plants is to address the nature of medicinal plants in cancer therapy in a comprehensive and integrative approach.
Embelia ribes is a traditional medicinal plant that belongs to the Myrsinaceae family and is frequently used in Ayurvedic medicines. It is most recognized for its antihelmentic activity, which is called Krmighna. It has anti-inflammatory, antioxidant, cytotoxic, antimicrobial, antifungal, and wound-healing properties (19). Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone, molecular weight: 294.4) (Figure 1) is a strong quinine-derivative phytoconstituent of E. ribes (especially in fruits) that has been extensively explored for its antihelmintic, antitumor (20), anti-inflammatory, antidiabetic, anticancer, and anticonvulsant properties (21). Embelin has anticancer benefits in the pancreas (22), colon (23), and liver tumors in animals, according to in vivo research (24–26). Thus, embelin is a potent molecule to consider for cancer treatment.
Silver metal nanoparticles, which exhibit amazing broad-spectrum activities, are the most extensively studied. Plants can synthesize silver nanoparticles; embelin is a phytoconstituent that can reduce the number of silver ions (27–29). In view of the preceding, we aimed to conduct the current work on embelin-aided production of silver nanoparticles, their characterization, and anticancer potential against A549 lung cancer cells. An annexin PI FITC assay was also carried out to investigate the effect of the embelin-derived silver nanoparticles in inducing apoptosis.
Embelia ribes was collected from the Koyna region, Maharashtra, and embelin, a major phytoconstituent, was isolated from the fruits. Briefly, the fruits of E. ribes were dried, coarsely pulverized, and extracted in chloroform using Soxhlet apparatus. By eluting the extract with benzene, embelin was isolated using silica column chromatography (30). The isolated embelin was characterized by a chemical test, FT-IR, and 1H NMR spectral analyses.
A chemical test was performed for confirmation of embelin. Embelin was dissolved in pet ether, and diluted ammonia was added to the solution. The presence of embelin was indicated by a bluish-violet precipitate (31).
The FT-IR spectrum was obtained using an FT-IR spectrophotometer (Bruker, Germany). The IR spectrum was recorded by scanning over a wavelength of 400–4,000 cm−1 using the Origin software. The characteristic IR peaks were observed and compared with the reference spectrum of embelin (31). 1H NMR was done by a sophisticated NMR spectrophotometer (Bruker), and the 1H frequency was 500 MHz.
A solution of 1 mM aqueous silver nitrate (AgNO3) was added to the embelin solution and vigorously stirred, and stirring was continued for 30 min to get colloids. The tubes were kept in the dark for the reaction to proceed further. The color of the AgNO3 solution changes from colorless to light brown after 30 min when embelin is added to it. After 24 h of incubation at room temperature, the hue deepens and turns to a dark reddish-brown tone (32). The AgNPs were synthesized by a process of reduction aided by embelin.
Different factors influence the features of synthesized nanoparticles, and there are a variety of characterization techniques for studying the traits and properties of nanoparticles.
As nanoparticles have unique optical properties, UV-visible spectroscopy is used to characterize AgNPs. The UV-visible spectrum was analyzed in the range of 200–800 nm in the UV-visible spectrophotometer (Shimadzu) 30 min after the addition of embelin solution with vigorous stirring (32).
The particle size of synthesized nanoparticles was analyzed by using the NANOPHOX-SympaTec (Germany) apparatus. The required analysis volume ranges between 50 μl and 4 ml, and the size range of scattering is 0.5–10,000 nm. The zeta potential of synthesized AgNPs is analyzed using Delsa Nano C by Beckmen Counter Inc. in order to analyze the stability of NPs. The liquid sample was diluted 10 times with distilled water and centrifuged. The zeta potential of the generated AgNPs was assessed in the presence of water as dispersion medium (33).
The existence of numerous reducing and stabilizing functional groups of embelin was confirmed using an infrared spectrum, as well as their likely role in the manufacture of AgNPs. FT-IR (Bruker) was used to investigate the functional group responsible for the AgNPs in the wavelength range from 4,000 to 400 cm−1 (32).
The morphology, size, and shape of embelin biofabricated AgNPs were determined by TEM analysis. The dispersed solution of AgNPs was sonicated for 20 min, and the TEM grid was prepared by placing a drop of diluted solution on a carbon-coated grid and later drying overnight. TEM measurements were done by a JEOL JEM-2100 (JEOL, Peabody, MA, USA) high-resolution transmission electron microscope (33).
A FESEM is used to visualize extremely fine topographic characteristics on the surface of whole or fractioned objects. The morphology and shape of the AgNPs were examined using field emission electron microscopy (Icon Analytical, Quanta 250, FEI, United States). The AgNPs suspension was air-dried, loaded to the sample holder, and coated, and images were obtained at 20 kV with a different magnification (32).
Human lung cancer cell line A549 was procured from the National Centre for Cell Science (NCCS), Pune (India). The cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Gibco1X) and antibiotic Antimycotic (Gibco). The cell line was maintained at 37 C in a humidified atmosphere of 5% CO2.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell viability. Adhered cells were trypsanized and seeded in 96-well plates after detaching from the surface. After allowing the cells to adhere for 24 h, they were treated in triplicate with the relevant drug concentrations for another 24 h. Each well was filled with 0.5 mg/ml MTT and covered with aluminum foil. For 4 h, plates were incubated at 37°C. Following the incubation period, the culture medium was withdrawn from each well and dimethyl sulfoxide (DMSO) was applied to dissolve the blue-purple formazan crystals. At 570 nm, the absorbance of the blank, control, and treatment wells was measured on a microplate reader (26).
The FITC annexin V/dead cell apoptosis kit (catalog no. V13241, Invitrogen) containing FITC annexin V and propidium iodide (PI) was used to assess embelin AgNPs-mediated apoptotic induction, as directed by the manufacturer (34). Briefly, cells were cultured and treated with drugs and incubated. Phosphate-buffered saline (PBS) was used to wash the cells after they were triggered to apoptosis. The cells were then resuspended in an annexin-binding buffer. The cells were stained with FITC annexin V and PI and incubated at room temperature for 15 min and then washed. After the incubation period, cells were resuspended in annexin-binding buffer and kept on ice until analyzed by a flow cytometer (Attune NxT acoustic flow cytometer Invitrogen, using the Attune nxt software v. 2.1) (35). Flow cytometry was used to examine both untreated (negative control) and positive control cells (doxorubicin with 6 g/ml-treated cells).
Embelin was isolated from E. ribes berries/fruits by the hot percolation method. Chloroform was a solvent required to isolate the embelin through Soxhlation. Embelin is a benzoquinone, and the presence of a long alkyl chain makes it a nonpolar compound. Hexane as an extraction solvent has been reported earlier, but it shows impurities of other phytochemicals during purification. The purification of embelin was done through column chromatography using a silica column, and on elution with benzene, embelin flakes were recovered. Silica is more polar than the mobile phase benzene and hence embelin being nonpolar is carried by benzene more readily during elution (30).
The purity of embelin was confirmed by physical appearance such as color, consistency, and chemical tests. The color of embelin was observed as golden orange, and flakes were crystalline. The chemical test was performed to indicate the presence of embelin; the formation of a bluish-violet precipitate confirmed the presence of embelin. Further characterization was done by FT-IR and 1H NMR analyses.
The FT-IR analysis revealed major functional groups of embelin and was analyzed using the Origin Pro software and is shown in Supplementary Figure 1. The observed peaks in the IR spectrum of isolated embelin were in accordance with the functional groups of standard embelin (31).
Proton nuclear magnetic resonance is used for the structural determination of molecules. The proton NMR (1 H NMR−500 MHz, CDCl3) spectrum revealed chemical shifts at δ 7.65 (2H, broad spectra, 2× OH), 6.0 (1H, singlet), 2.43–2.46 (2H, triplet), 1.46–1.47 (2H, triplet), 1.26–1.30 (4H, broad spectra), 1.26 (12H, broad spectra), and 0.86–0.89 (3H, triplet). 1H NMR of isolated embelin is shown in Supplementary Figure 2.
The colorless AgNO3 solution turned pinkish brown and colloids developed after the addition of embelin to AgNO3. The color intensified to brown after 24 h of incubation at room temperature (32). The reduction of silver nitrate to elemental silver (Ag+ to Ag0) was due to the interaction of AgNO3 with embelin.
The UV/vis spectrum of AgNPs synthesized in the range of 200–800 nm gave the maximum absorption peak at 384.5 nm. The conjugated system of embelin affects its UV/vis spectral profile. The presence of free electrons in metal nanoparticles produces a surface plasmon resonance (SPR) absorption band due to collective oscillation in resonance with the light wave (32). The synthesized AgNPs were analyzed using a UV-visible spectrophotometer (Shimadzu). The absorption spectrum is shown in Supplementary Figure 3.
For the determination of the surface charge and stability of the formulation, a zeta potential analysis is carried out (33). Measuring the velocity of the nano-sized particles also assesses the colloidal stability of AgNPs. A distinct peak between 25 and 30 nm clearly indicates the formation of AgNPs with uniformity. The higher peak value indicates the monodispersity of nanoparticles. The zeta potential of −5.42 mV was recorded, which indicates the good stability of AgNPs (Supplementary Figures 4A,B).
The FT-IR analysis of AgNPs is used to identify the molecules that act as coating and stabilizing agents, as well as to detect silver ion reduction (31, 32). The FT-IR spectrum of AgNPs (Supplementary Figure 5) synthesized using embelin showed sharp absorption peaks at 952.84, 1,029, and 1,043 cm−1 corresponding to secondary alcohol ring stretching. A broad peak in between 3,340 and 3,588 cm−1 is characteristic of the presence of the hydroxyl group (OH) of biomolecules present in the synthesized AgNPs. The various peaks represent biomolecules attached to AgNPs and reduce Ag+ to AgNPs by acting as capping and stabilizing agents.
The image of a nanoparticle's surface at high resolution provides useful information such as size, shape, topography, composition, electrical conductivity, and other properties. The FESEM images of the embelin-derived silver nanoparticles are shown in Figures 2A,B. The surface morphology of AgNPs showed an even shape and spherical nature. The particle size ranges from 20 to 30 nm.
The TEM provides structural and chemical behavior of nanoparticles under high electron beam conditions with great resolution (1). The shape and size of the resultant AgNPs were analyzed with the help of TEM (Figures 3A,B). The AgNP solution was placed on a carbon-coated copper grid and allowed to dry, and TEM images were recorded. The sizes of AgNPs were ~20–30 nm as per the TEM micrographs and were found to be spherical.
The cytotoxic potential of embelin-derived AgNPs against the human lung cancer cell line A549 was assessed by using the MTT assay, which is widely used in cytotoxicity and cell viability assays. For the cytotoxicity study, A549 lung cancer cells were incubated with different concentrations of embelin-aided AgNPs (10, 25, 50, 100, 150, and 200 μg/ml). After 24 h of incubation, cell viability was assessed by the MTT assay. It was observed that the effects of embelin-derived AgNPs were in a dose-dependent manner. Embelin-based AgNPs could exhibit cytotoxicity at low doses. At 10 μg/ml concentration, % inhibition was 80.131 ± 0.068, whereas in doxorubicin 10 μg/ml, it was 89.364 ± 0.080. Doxorubicin, a standard anticancer drug, exerts antimitotic activity by intercalating between DNA base pairs. When compared, embelin-AgNPs showed slightly lower activity than doxorubicin. At 200 μg/ml, embelin AgNPs showed a % inhibition of 96.864 ± 0.112, suggesting an increase in % inhibition in a dose-dependent manner (Figure 4).
However, the exact molecular mechanism of cytotoxicity needs to be explored. As per Maeda 2003, the possible mechanism of nanoparticles in drug targeting is enhanced permeability and retention [EPR]. The EPR results in the passive accumulation of medications and drug carriers due to extravasation through leaky vasculature. Nano-carriers or nanoparticles supposedly use the same EPR mechanism to deliver the anticancer drugs at the targeted site (35).
Embelin is a phytoconstituent and is therefore considered safer for normal cells, and at the same time, it exhibits cytotoxicity against cancer cells. Silver has been used since ancient times in Ayurveda, which possesses anticancer properties (1, 36). The combination of Ag+ and embelin acted upon A549 cells to show cytotoxicity. Mahendran et al. (38) synthesized AgNPs from embelin and studied their cytotoxicity against human MG-63 (osteosarcoma cells). In their study, they found that the AgNPs derived from embelin were efficient to deliver embelin to cancer cells and showed cytotoxicity (37, 38). The study also suggested that embelin AgNPs were more efficient than pure embelin due to the water solubility of embelin AgNPs and the presence of Ag+ ions.
For embelin-based AgNPs, the IC50 value was used in triplicate to treat human lung cancer A549 cells, and it exhibited cytotoxic action. Cells were stained with FITC-labeled annexin V and PI dyes after apoptosis induction, and the cell suspension was analyzed by flow cytometry. The annexin V assay revealed that embelin-derived AgNPs induced early and late stages of apoptosis in A549 cells. The annexin V-FITC graphs in Figure 5 show the distribution of A549 cells in four quadrants (Q1, Q2, Q3, and Q4), and it is one of three independent studies performed.
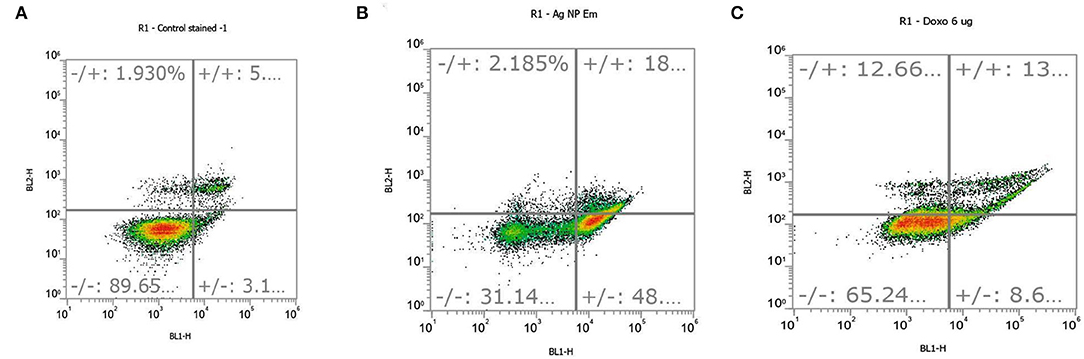
Figure 5. Apoptosis occurred in (A) control cells, (B) embelin AgNP-treated cells, and (C) doxorubicin (standard)-treated cells at a concentration of 6 μg/ml. Quadrants are as follows: Q1, necrotic cells; Q2, late apoptotic cells; Q3, viable cells; and Q4, early apoptotic cells.
In comparison to control cells, all treated cells showed a lower percentage of viable cells. There was very less cell dispersion in Q1, Q2, and Q4 in the control cells, indicating a very low amount of necrotic, late, and early apoptotic cells, respectively. After treatment with embelin-based AgNPs, the distribution of cells in these quadrants (Q1, Q2, and Q4) increased. As per the triplicate study, 48% of the embelin-derived AgNPs-treated cells were in the early stages of apoptosis (Q4), whereas 18% were in the late stages of apoptosis (Q2) (Figure 5B). Q1 denoted necrotic cells, which had a minor increase in cell dispersion.
In the control cells, only 1.93% of necrotic cells were observed along with 5% of late apoptotic cells (Figure 5A), while, in embelin AgNP-treated cells, necrotic cells were found to be 2.18%. In the positive control, cells were treated with doxorubicin, a well-known anticancer drug, at a concentration of 6 μg/ml, which resulted in increased apoptosis. Doxorubicin showed 13% of cells in early apoptosis, while 8.6% of cells in late apoptosis, but it also showed 12.66% of necrotic cells (Figure 5C). The graphical representation of the annexin PI apoptosis assay is shown in Figure 6. Embelin is a phytoconstituent; hence, embelin AgNPs resulted in less necrotic cells.
Apoptotic cell death is a closely regulated process characterized by morphological alterations in the cellular membrane structure. Cell shrinkage, blebbing of the plasma membrane, cell separation, phosphatidylserine translocation, nuclear condensation, and DNA fragmentation are all signs of apoptosis. Embelin AgNPs caused apoptosis in A549 cells, according to the current apoptotic investigation. Various underlying mechanisms can cause apoptosis to occur. The exact signaling pathway and the apoptotic process can be determined through a further investigation of the molecular mechanisms.
The therapeutic potential of embelin of E. ribes-derived AgNPs in the treatment of lung cancer is the outcome of this investigation. The embelin fabricated AgNPs possess a substantial anticancer effect against A549 lung cancer cells. Further apoptosis assay reveals considerable induction of apoptosis in A549 cells by embelin AgNPs. The use of embelin in the formulation of AgNPs has opened new doors to design safer nanomedicine for the treatment of cancer with the application of nanotechnology. It is necessary to explore the possible cellular and molecular mechanisms along with toxicity issues of embelin AgNPs in future work. However, the amalgamation of the silver ions with embelin at the molecular nano level can work marvels and could be a viable therapeutic strategy in the management of cancer.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
RJ, AG, SP, MR, and BW: conceptualization, methodology, software, investigation, writing-original draft, review and editing, resources, and supervision. MAn, MAlo, and MAls: validation and formal analysis. MAn, MAlo, MAls, AS, YA, and JK: funding acquisition. AS, YA, and JK: visualization and software. MR: critical analysis and final draft-review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.960674/full#supplementary-material
Supplementary Figure 1. FT-IR spectrum of embelin.
Supplementary Figure 2. 1H NMR spectrum of isolated embelin.
Supplementary Figure 3. UV-visible spectrum of embelin-derived AgNPs.
Supplementary Figure 4. (A) Cross-correlation for particle size analysis [NANOPHOX (NX0088)]; (B) zeta potential of embelin AgNPs.
Supplementary Figure 5. FT-IR spectrum of embelin-derived AgNPs.
1. Jain N, Priyanshu Jain P, Rajput D, Patil UK. Green synthesized plant-based silver nanoparticles: therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst Lett. (2021) 9:5. doi: 10.1186/s40486-021-00131-6
2. Balantrapu K, Goia DV. Silver nanoparticles for printable electronics and biological applications. J Mater Res. (2009) 24:2828–36. doi: 10.1557/jmr.2009.0336
3. Hasan KMF, Pervez MN, Talukder ME, Sultana MZ, Mahmud S, Meraz MM, et al. Novel Coloration of Polyester Fabric through Green Silver Nanoparticles (G-AgNPs@PET). Nanomaterials. (2019) 9:569. doi: 10.3390/nano9040569
4. Burduşel AC, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel). (2018) 8:681. doi: 10.3390/nano8090681
5. Vardhana J, Kathiravan G. Biosynthesis of silver nanoparticles by endophytic fungi Pestaloptiopsis pauciseta isolated from the leaves of Psidium guajava Linn. Int J Pharm Sci Rev Res. (2015) 31:29–31. doi: 10.7897/2230-8407.0617
6. Mohammadian A, Shojaosadati SA, Rezaee MH. Fusarium oxysporum mediates photogeneration of silver nanoparticles. Sci Iran. (2007) 14:323–6. doi: 10.3390/brainsci10110784
7. Sondi I, Sondi BS. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Col Inter Sci. (2004) 275:177–82. doi: 10.1016/j.jcis.2004.02.012
8. Khan J, Rudrapal M, Bhat EA, Ali A, Alaidarous M, Alshehri B, Banwas S. Perspective iinsights of bio-nanomaterials for the treatment of neurological disorders. Front Bioeng Biotechnol. (2021) 9:1–12. doi: 10.3389/fbioe.2021.724158
9. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
10. Sung H, Ferlay J, Siegel RL, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
11. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. (eds). SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute. Available online at: https://seer.cancer.gov/archive/csr/1975_2010/
12. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol Biomark Prev. (2017) 26:632–41. doi: 10.1158/1055-9965.EPI-16-0520
13. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. (2008) 359:1367–80. doi: 10.1056/NEJMra0802714
14. Aaron J. Cohen, Arden Pope III, lung cancer and air pollution. Environ Health Perspect. (1995) 103:219–24. doi: 10.1289/ehp.95103s8219
15. Charles S. Dela Cruz MD, Lynn TT, Richard A, Matthay. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. (2011) 32:605–544. doi: 10.1016/j.ccm.2011.09.001
16. Chakraborty S, Rahman T. The difficulties in cancer treatment. E Cancer Med Sci. (2012) 6:ed16. doi: 10.3332/ecancer.2012.ed16
17. Karmous I, Pandey A, Ben K, Haj KB, Chaoui A. Efficiency of the green synthesized nanoparticles as new tools in cancer therapy: insights on plant-based bioengineered nanoparticles, biophysical properties, and anticancer roles. Bio Tra Ele Res. (2020) 196:330–42. doi: 10.1007/s12011-019-01895-0
18. Pešic M. Development of natural product drugs in a sustainable manner. Brief for United Nations Global Sustainable Development: Report. (2015). p. 1–4.
19. Greenwell M, Rahman PKSM. Medicinal Plants: Their Use in Anticancer Treatment. Int J Pharm Sci Res. (2015) 6:4103–12. doi: 10.13040/IJPSR.0975-8232.6(10)0.4103-12
21. Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. (1994) 40:109–13. doi: 10.1159/000239181
22. Mahendran S, Thippeswamy BS, Veerapur VP, Badami S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine. (2011) 18:186–8. doi: 10.1016/j.phymed.2010.04.002
23. Peng M, Huang B, Zhang Q, Fu S, Wang D, Cheng X, et al. Embelin inhibits pancreatic cancer progression by directly inducing cancer cell apoptosis and indirectly restricting IL-6 associated inflammatory and immune suppressive cells. Cancer Lett. (2014) 354:407–16. doi: 10.1016/j.canlet.2014.08.011
24. Dai Y, Jiao H, Teng G, Wang W, Zhang R, Wang Y, et al. Embelin reduces colitis-associated tumorigenesis through limiting IL-6/STAT3 signaling. Mol Cancer Ther. (2014) 13:1206–16. doi: 10.1158/1535-7163.MCT-13-0378
25. Sreepriya M, Bali G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia. (2005) 76:549–55. doi: 10.1016/j.fitote.2005.04.014
26. Dipraj G, Shubhangi P. In-Vitro Cytotoxicity Assay of Curry Leaves Silver Nanoparticles against Thp-1 Cell Line. Int J Sci Res Sci Technol. (2020) 7:45–52.
27. Argade P, Puranik S. Effect of Organically Grown Curcuma longa (Turmeric) on Leukemic and MCF-7 Cell Lines. Int J Curr Microbiol Appl Sci. (2015) 2:182–6.
28. Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of Phosphatidylserine on the Surface of Apoptotic Lymphocytes triggers specific recognition and removal by Macrophages. J Immunol. (1992) 148:2207–16.
29. Atsumi T, Murakami Y, Shibuya K, Tonosaki K. Fujisawa S. Induction of Cytotoxicity and Apoptosis and Inhibition of Cyclooxygenase-2 Gene Expression, by Curcumin and its Analog, Diisoeugenol. Anticancer Res. (2005) 25:4029–36.
31. Veerpal K, Supandeep SH, Nidhi AN. Kalia, Neeraj M. Isolation of embelin from and evaluation of its anticancer potential in Embelia ribes breast cancer. Asian J Pharm Pharmacol. (2015) 1:33–9.
32. Geethalakshmi R, Sarada DVLS. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomed. (2012) 7:5375–84. doi: 10.2147/IJN.S36516
33. Zhang XF, Liu ZG, Shen W. Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. (2016) 17:1534. doi: 10.3390/ijms17091534
34. Atsumi T, Murakami Y, Shibuya K, Tonosaki K. Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, ·diisoeugenol. Anticancer Res. (2005) 25:4029–36.
35. Silva LP, Pereira TM, Bonatto CC. Frontiers and perspectives in the green synthesis of silver nanoparticles. Green Synth Characterizat Applicat Nanoparticles. (2019):137–164. doi: 10.1016/B978-0-08-102579-6.00007-1
36. Hussain N, Kakoti BB, Rudrapal M, Junejo JA, Laskar MA, Lal M, et al. Anticancer and antioxidant activities of Cordia dichotoma Forst. Int J Green Pharm. (2020) 14:265–72.
37. Shafaghat A. Synthesis and characterization of silver nanoparticles by photosynthesis method and their biological activity. Inorg Nano-Met Chem. (2015) 45:381–7. doi: 10.1080/15533174.2013.819900
Keywords: biofabrication, silver nanoparticles, embelin, anticancer, lung cancer, MTT assay, apoptosis assay
Citation: Jagtap RR, Garud A, Puranik SS, Rudrapal M, Ansari MA, Alomary MN, Alshamrani M, Salawi A, Almoshari Y, Khan J and Warude B (2022) Biofabrication of Silver Nanoparticles (AgNPs) Using Embelin for Effective Therapeutic Management of Lung Cancer. Front. Nutr. 9:960674. doi: 10.3389/fnut.2022.960674
Received: 03 June 2022; Accepted: 14 June 2022;
Published: 04 August 2022.
Edited by:
Kandi Sridhar, Agrocampus Ouest, FranceReviewed by:
André Mauricio De Oliveira, Federal Center for Technological Education of Minas Gerais, BrazilCopyright © 2022 Jagtap, Garud, Puranik, Rudrapal, Ansari, Alomary, Alshamrani, Salawi, Almoshari, Khan and Warude. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mithun Rudrapal, cnNtcnBhbEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.