- 1Department of Endocrinology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Provincial Clinical Research Center for Diabetes and Metabolic Disorders, Wuhan, China
- 3Department of Emergency Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: The preoperative nutritional status of cancer patients is closely related to prognosis. The prognostic nutritional index (PNI) has been shown to predict the prognosis of a variety of tumors, but its study in pancreatic neuroendocrine neoplasms (pNENs) is lacking. The aim of the present study is to investigate the predictive value of the preoperative PNI for postoperative progression in patients with pNENs.
Methods: The medical records of 181 patients with pNENs, who underwent surgery, were retrospectively analyzed. A time-dependent receiver operating characteristic (ROC) curve was plotted to determine the optimal cut-off value of the preoperative PNI. Correlations between the preoperative PNI and clinicopathological parameters were analyzed using multiple linear regression. A Kaplan-Meier curve was applied to assess the progression-free survival (PFS) rate, which was tested using a log rank. Univariate and multivariate Cox proportional risk regression models were used to analyze the predictive value of the preoperative PNI on prognosis.
Results: The optimal cut-off value of the preoperative PNI was 48.275. The patients were divided into a high PNI group (PNI > 48.275, n = 92) and a low PNI group (PNI ≤ 48.275, n = 89). The proportion of patients with tumor progression after surgery was significantly higher in the low PNI group compared with that in the high PNI group (P = 0.004). The Kaplan-Meier curve showed that the PFS rate after surgery was significantly lower in the low PNI group compared with that in the high PNI group (P = 0.026). The preoperative PNI was an independent predictor of PFS (HR: 2.727, 95% CI: 1.174∼6.333, P = 0.020).
Conclusion: The preoperative PNI has a predictive value for postoperative progression in patients with pNENs.
Introduction
Pancreatic neuroendocrine neoplasms (pNENs) are a group of rare heterogeneous tumors originating from pancreatic neuroendocrine cells, accounting for approximately 2% of pancreatic tumors (1, 2). The slow growth rate of the tumor and the lack of a specific clinical presentation make the diagnosis of this disease more difficult. With the widespread use of high-quality imaging techniques, the incidence of this disease is increasing every year (3, 4). Currently, surgery occupies an important place in the management of this disease (5–8). However, tumor recurrence and metastasis can still be observed during patients’ follow-up after surgery, which reflects a poor prognosis. Like other tumors, the grading and staging of pNENs are crucial for the prognostic assessment of patients. In addition, clinicopathological characteristics such as age, gender, race, tumor size, and location, have been shown to be closely related to overall survival (OS) (9). The study by Landoni et al. found that the functional status of the tumor, lymph node status, tumor grade, and vascular infiltration are independent predictors of disease progression in pNENs (10). However, due to individual differences and tumor heterogeneity, these prognostic factors have limitations in practical clinical application (11), and therefore, the predictors of postoperative progression of pNENs still need to be investigated in depth.
It has been found that the nutritional status is closely related to disease prognosis and is particularly significant for oncology patients (12, 13). For surgery patients, there is a significant statistical correlation between preoperative malnutrition and poor postoperative wound healing, the increased incidence of complications (14, 15). The prognostic nutritional index (PNI) is an index used to assess the nutritional and immune statuses of surgical patients that can be calculated from serum albumin levels and total peripheral blood lymphocyte count. Compared with other nutritional assessment tools, this index has the advantage of being non-invasive and simple to calculate. Therefore, it has been widely used in the prognostic assessment of various diseases such as cancer, adverse cardiovascular events, cerebrovascular accidents, and in the treatment and management of diseases (16–19). Several existing meta-analyses have shown that cancer patients with low PNI have lower postoperative OS, recurrence-free survival, and PFS compared with those in patients with high PNI (20–22). This suggests that low PNI is a risk factor affecting the postoperative prognosis of cancer patients, and PNI can be used to assess the postoperative prognosis of cancer patients.
However, although the use of PNI has become more popular in assessing the prognosis of tumors in various organs, no study has yet analyzed the association between preoperative PNI and postoperative progression of pNENs. This indicates that there is still a gap in this research field as to whether preoperative PNI can be used as a predictor of postoperative progression of pNENs. Therefore, to clarify this question, we conducted the present study.
Materials and methods
Study population and data collection
Patients who underwent surgery and had pathologically confirmed pNENs from April 2009 to September 2021 at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, were retrospectively included. The clinicopathological characteristics of patients were collected from the hospital’s electronic medical record system, including age, gender, residence, height, weight, and personal history (whether smoking or drinking). The date of surgery was recorded, and routine blood test and blood biochemical indices such as total and percentage of peripheral blood lymphocytes, hemoglobin, total serum protein, serum albumin, triglycerides, and cholesterol within a week before surgery, were summarized. Following these procedures, the patients were followed up. Patients with exocrine pancreatic malignancy and hereditary diseases such as multiple endocrine neoplasia type 1 (MEN1), neurofibromatosis type 1 (NF1), and too many missing items in the medical records, and missed visits, were excluded.
The location of the tumor was divided into the head or neck and the body or tail of pancreas. Tumors were classified as functional and non-functional, based on whether they could secrete substances, such as peptide hormones or biogenic amines, and produce the corresponding clinical symptoms. The tumor grading and staging were performed using the grading criteria in the 2019 World Health Organization (WHO) classification of endocrine organ tumors and the 8th American Joint Committee on Cancer (AJCC) TNM staging criteria for pancreatic neuroendocrine tumors, respectively (23, 24).
Places where patients lived continuously for more than 10 years were defined as residence and were divided into urban and rural groups. Patients were classified into a smoking or drinking group if they had smoked (≥10 cigarettes/day) or consumed alcohol (≥100 ml/day) for more than 3 years prior to admission. The body mass index (BMI) was defined as weight (kg)/height squared (m2). For the Chinese population, the reference range of normal BMI was 18.5∼23.9 kg/m2. Preoperative PNI was calculated from preoperative serological indicators (serum albumin level and total peripheral blood lymphocyte count) as PNI = serum albumin (g/L) + 5 × total peripheral blood lymphocyte count (*109/L).
Follow-up
The patients who were included in the study were followed up by telephone or through outpatient visits, with an endpoint event defined as any form of tumor progression such as recurrence and metastasis, or patient death from any cause, with a follow-up deadline of March 2022. Progression-free survival (PFS) was defined as the time between the day of the patient’s surgery and the occurrence of the endpoint event.
Statistical analysis
For continuous variable data, the Kolmogorov-Smirnov test was used to analyze whether the data conformed to a normal distribution, and if so, the mean ± standard deviation (SD) was used, and vice versa, the median M (P25, P75) was used. Categorical information was expressed using numbers and percentages (n, %). The time-dependent receiver operating characteristic (ROC) curve was plotted and the Youden index was calculated to find the best cut-off value for preoperative PNI. The independent-samples t-test, the non-parametric rank sum test, and the chi-square test were used for comparison between groups, respectively, when applicable. Multiple linear regression was used to analyze the correlation between preoperative PNI and clinicopathological parameters. The Kaplan-Meier curve and the log rank test were applied to evaluate PFS rate. Cox proportional risk models were used for univariate and multivariate analyses to identify indicators affecting prognosis. The above statistical analyses were performed using the SPSS 26.0 software, and a two-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics of all patients
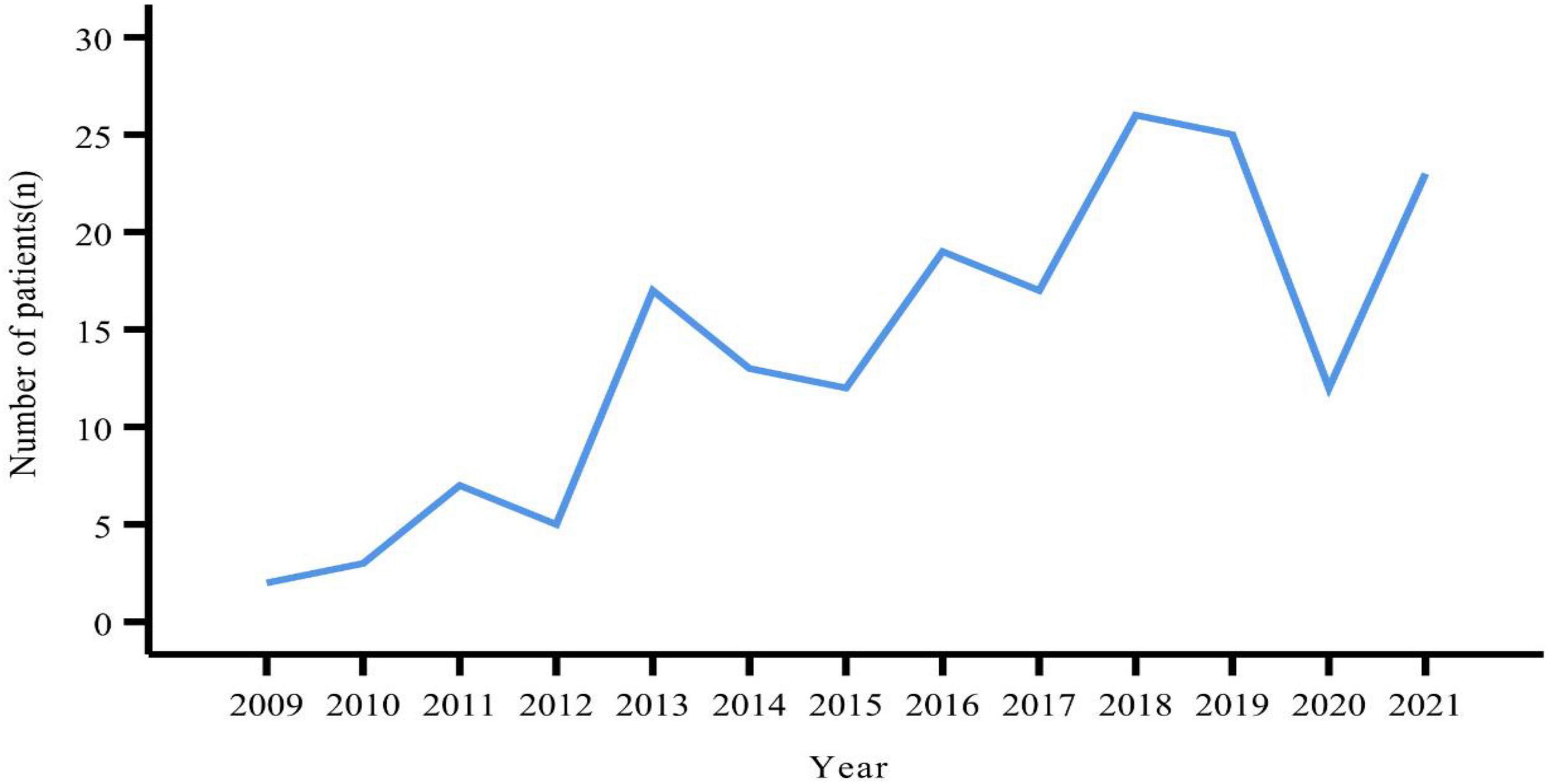
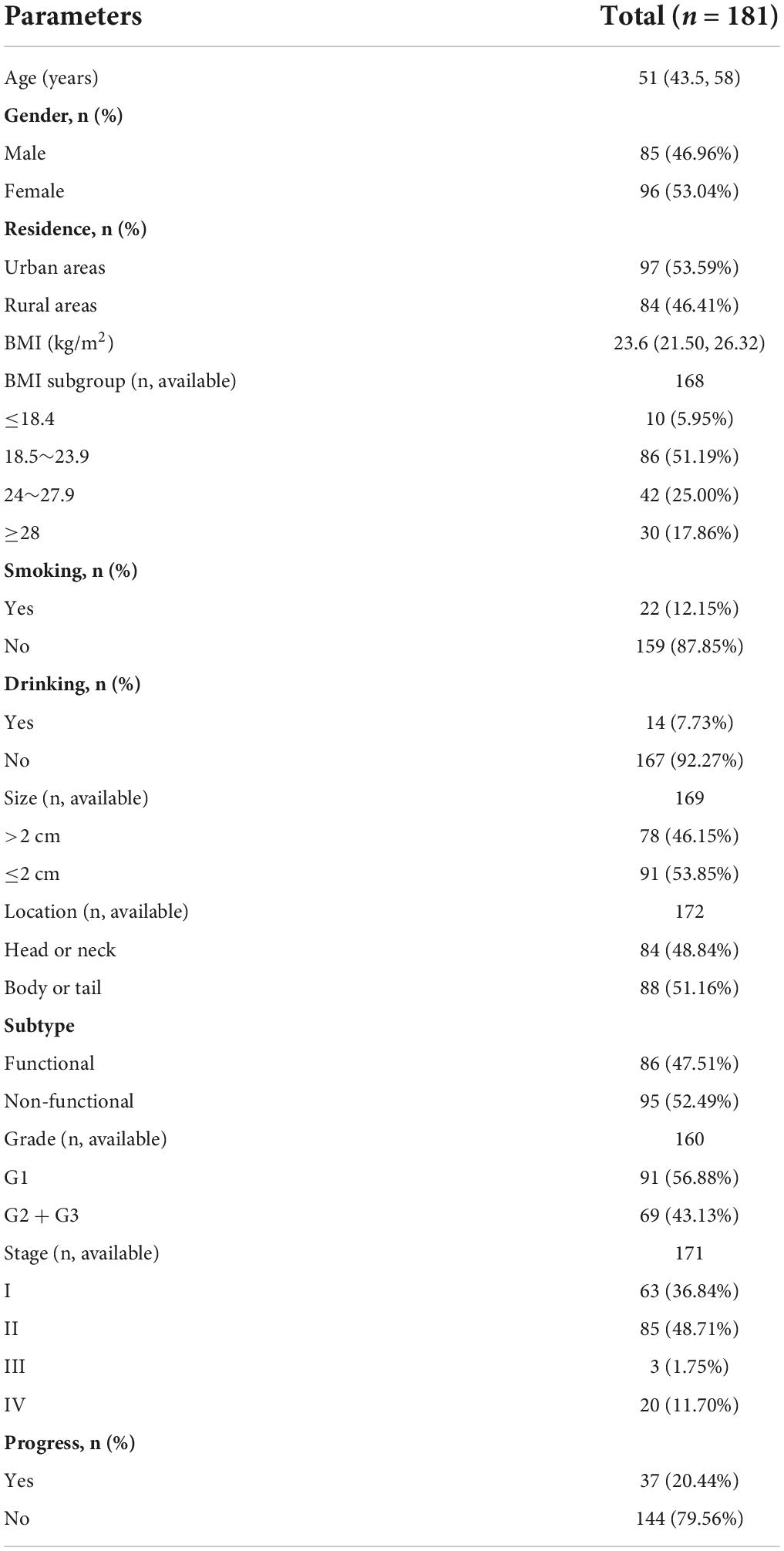
A total of 181 patients were eventually included in this study. The number of patients peaked in 2018 (n = 26), followed by 2019 (n = 25) (Figure 1). There were slightly more females (n = 96, 53.04%) than males (n = 85, 46.96%). The age of the patients ranged from 12 to 83 years, with a median age of 51 years. Slightly more patients were from urban than rural areas (53.59% vs. 46.41%). BMI values were obtained for a total of 168 patients, with a median value of 23.60 kg/m2. More than half of the patients (n = 86, 51.19%) had a BMI within the normal reference range. Smokers and alcohol drinkers accounted for 12.15 and 7.73% of all patients, respectively (Table 1).
The median tumor size was 2 cm, and 46.15% of the tumors were > 2 cm in size. Non-functional tumors were more frequent than functional tumors (52.49% vs. 47.51%). In addition, highly differentiated G1 tumors were the most frequent, with 91 cases, followed by medium and low differentiated G2 and G3 tumors. According to TNM staging criteria, we counted the tumor stages of 171 patients, among which, 63 (36.84%) were stage I, 85 (48.71%) were stage II, 3 (1.75%) were stage III, and 20 (11.70%) were stage IV (Table 1).
Preoperative prognostic nutritional index and clinicopathological parameters
The ROC curve showed an area under the curve (AUC) of 0.642 (95% CI: 0.540∼0.743, P = 0.008) (Figure 2). The optimal cut-off value for PNI was 48.275, which was used to divide all patients into a high PNI group (PNI > 48.275) and a low PNI group (PNI ≤ 48.275).
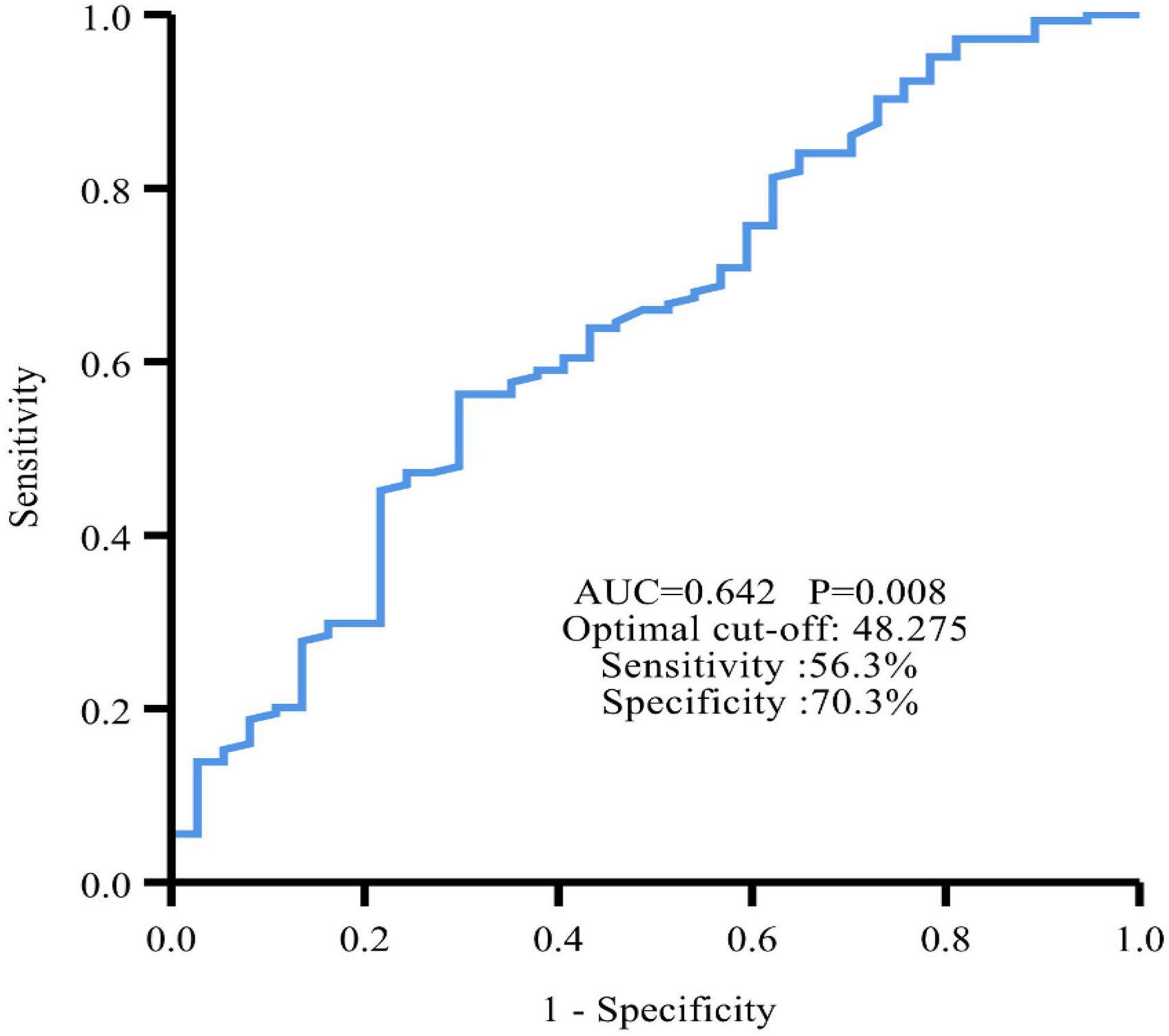
Figure 2. Time-dependent ROC curve revealed that the best cut-off value of PNI to predict PFS was 48.275 with 56.3% sensitivity and 70.3% specificity.
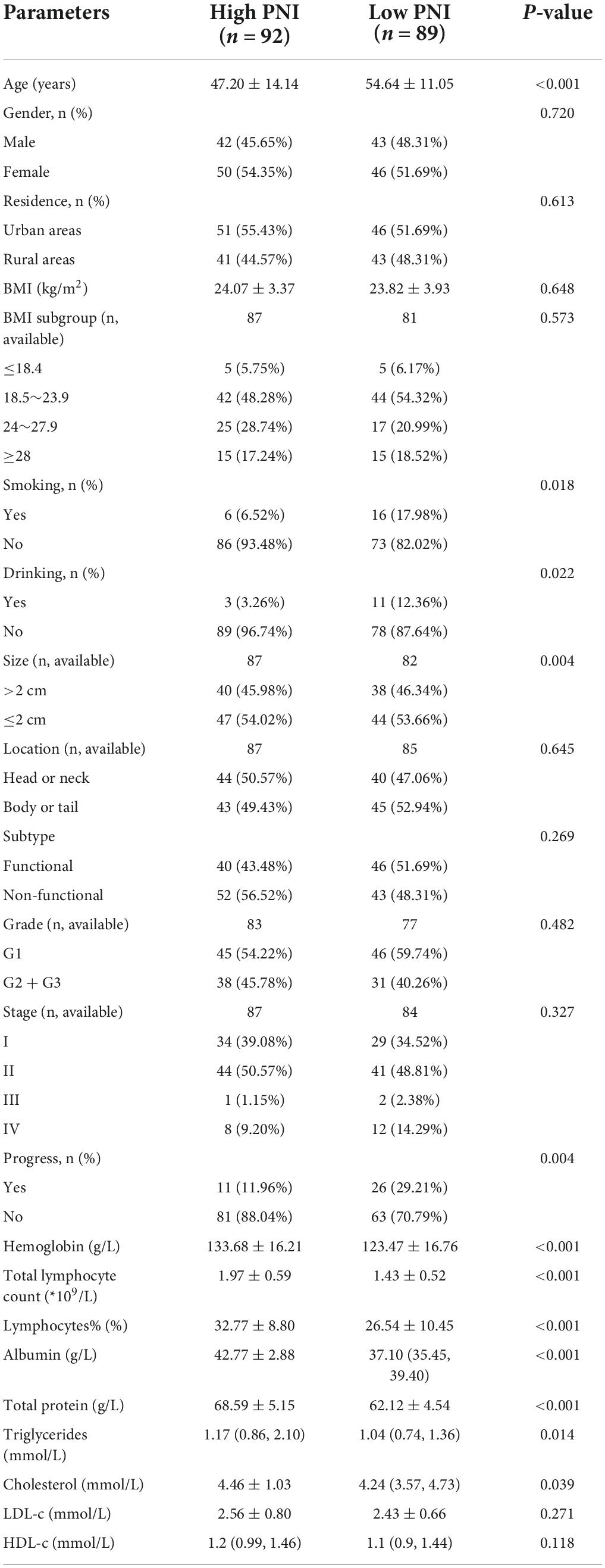
There were slightly more people in the high PNI group than in the low PNI group (n = 92 vs. n = 89). In both groups, there were more women than men. The gender difference between the two groups was statistically insignificant (P = 0.720). Patients in the low PNI group were significantly older than those in the high PNI group (54.64 ± 11.05 years vs. 47.20 ± 14.14 years, P < 0.001), and the BMI was similar in both groups (P = 0.648). The proportion of smokers (P = 0.018) and alcohol drinkers (P = 0.022) was significantly higher in the low PNI group compared with that in the high PNI group. Although there were mostly non-functional tumors in the high PNI group (56.52%) and functional tumors in the low PNI group (51.69%), the difference between the two groups was statistically insignificant (P = 0.269). The proportion of tumors size > 2 cm was higher in the low PNI group than in the high PNI group (P = 0.004). No significant differences were observed between the two groups based on location, grading and staging of tumors (P > 0.05).
In terms of routine blood tests and blood biochemical indices, the high PNI group had significantly higher levels of hemoglobin (P < 0.001), total and percentage of peripheral blood lymphocytes (P < 0.001), and a significantly higher level of serum total protein (P < 0.001), serum albumin (P < 0.001), triglycerides (P = 0.014), and cholesterol (P = 0.039) (Table 2).
Correlations
The preoperative PNI negatively correlated with age (β = −0.226, P = 0.004) and cholesterol (β = −0.653, P = 0.002) and positively correlated with hemoglobin (β = 0.314, P < 0.001), triglycerides (β = 0.351, P < 0.001), low-density lipoprotein cholesterol (LDL-c) (β = 0.474, P = 0.007), and high-density lipoprotein cholesterol (HDL-c) (β = 0.290, P = 0.007) (Table 3).
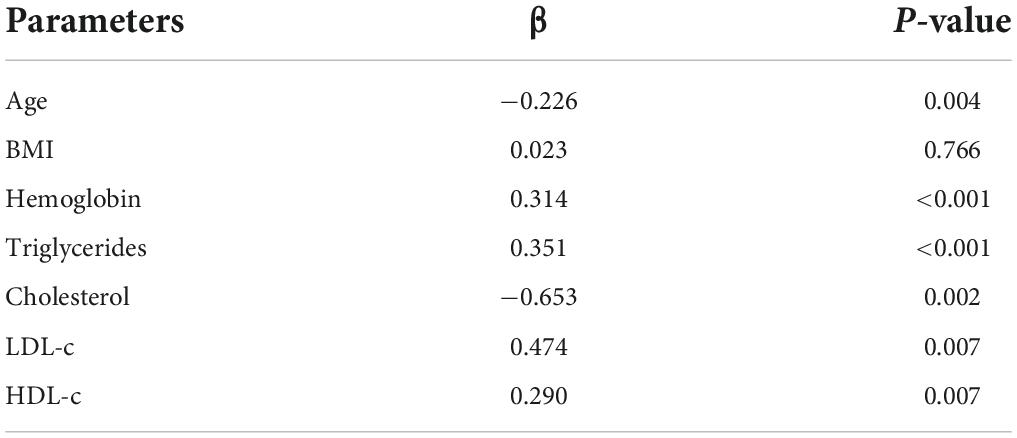
Table 3. Multiple linear regression analysis of the correlation between PNI and clinicopathological parameters.
Preoperative prognostic nutritional index and prognosis
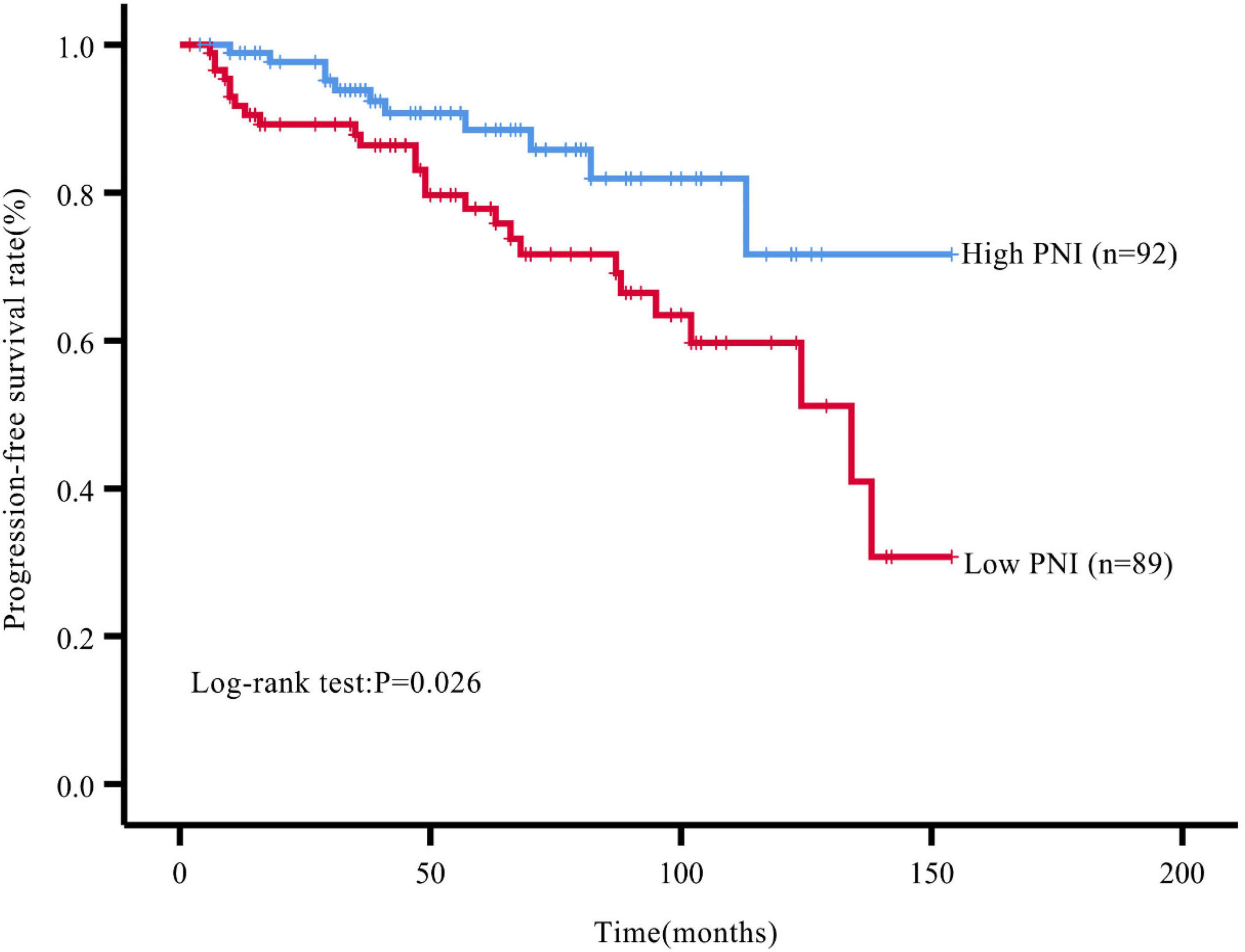
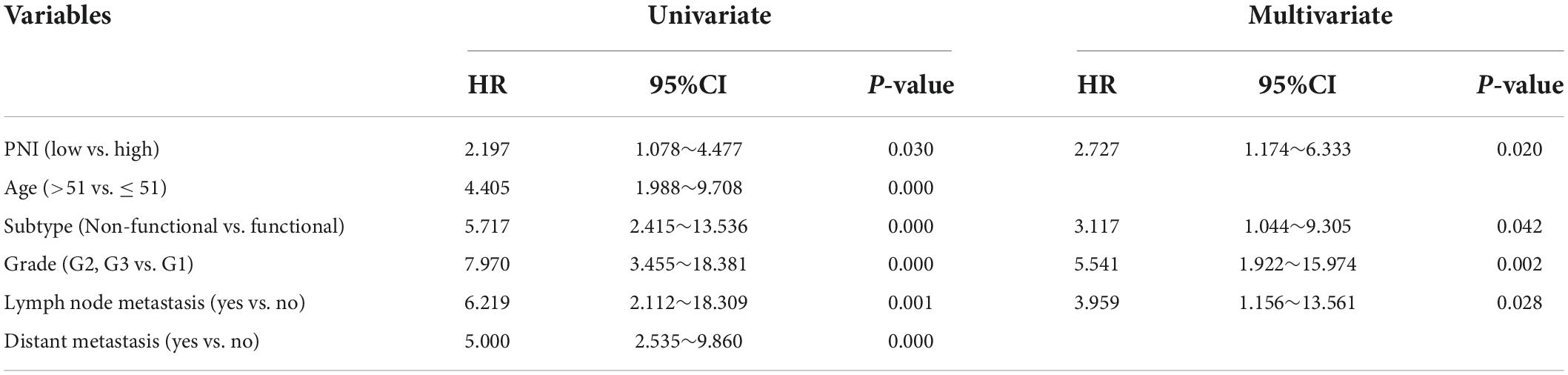
During the follow-up, we found a total of 37 patients (20.44%) with postoperative tumor progression (Table 1). The post-surgery percentage of tumor progression was significantly higher in the low PNI group than in the high PNI group (29.21% vs. 11.96%, P = 0.004) (Table 2), and the result of the Kaplan-Meier survival analysis confirmed this result (P = 0.026) (Figure 3). The univariate Cox regression analysis showed a significant association between PFS and preoperative PNI, age, tumor functionality, pathological grade, lymph node status, and distant metastasis. Among these, the patients’ post-surgery hazard ratio (HR) for tumor progression in the low PNI group to those in the high PNI group was 2.197 (95% CI: 1.078∼4.477, P = 0.030). Next, by incorporating the above statistically different indicators into a multivariate Cox regression analysis, we found that the preoperative PNI (HR: 2.727, 95% CI: 1.174–6.333), tumor functionality (HR: 3.117, 95% CI: 1.0446∼9.305), pathological grade (HR: 5.541, 95% CI: 1.922∼15.974), and lymph node status (HR: 3.959, 95% CI: 1.156∼13.561) were independent predictors of PFS (Table 4). Thus, even after adjusting for confounding factors, the post-surgery risk of tumor progression was significantly higher in the low PNI group compared with that in the high PNI group.
Discussion
pNENs are the second most common epithelial malignancies of the pancreas (25). Although they are still rare diseases, their incidence is gradually increasing worldwide. Gender differences in the incidence of pNENs are influenced by race, region, and other factors. In the American population, more men than women were reported to have these diseases (3), while the opposite finding was reported in the Italian population (26). The tumors’ high heterogeneity allows for significant differences in individual prognosis, which is also the case for patients with pNENs undergoing surgery. Therefore, it is essential to find appropriate preoperative prognostic predictors.
Due to inappropriate secretion of hormones, patients with pNENs can suffer from malabsorption, abdominal pain, diarrhea, and other gastrointestinal symptoms that lead to a state of malnutrition (27). For tumor patients undergoing surgery, the preoperative nutritional status has almost become an accepted factor affecting the patient’s postoperative prognosis (28). However, in recent years, scholars have mostly focused their prognosis for patients on the impact of tumor cells or the tumor microenvironment, such as CD47 expression and CD163+ macrophages (29), cytokeratin-19 (CK-19) (30), and tumor-infiltrating platelets (31). Few studies have assessed whether the preoperative nutritional status has a predictive value in the postoperative progression of patients with pNENs.
PNI was originally proposed by Onodera et al. and used in the prognostic assessment of patients undergoing gastrointestinal surgery (32). In recent years, the PNI has been widely used in clinical practice, with current research focusing on investigating the relationship between the PNI and tumor prognosis. Cadwell et al. found that in elderly cancer patients, PNI was associated with 6-month postoperative mortality (33). In addition, the PNI can be combined with the neutrophil/lymphocyte ratio (NLR), and lactate dehydrogenase (LDH) to synergistically assess PFS and OS in patients with advanced cancer, receiving immunotherapy (34, 35). It has also been reported that the PNI has a significant association with prognosis in patients undergoing surgery and who have digestive system tumors, such as liver and gastric cancer (36, 37). The PNI levels are determined by serum albumin and blood lymphocyte count. Previous studies have shown that serum albumin levels can be used alone to assess the development of several malignancies and to predict the prognosis of patients (38, 39) as it can directly reflect the nutritional status of an organism. Lymphocytes play an important role in tumor immune surveillance and are closely related to the occurrence and development of tumors (40). The total peripheral blood lymphocyte count can reflect the immune function of the body. This retrospective study is the first to use the PNI to reflect the nutritional status of patients with pNENs and fills a gap in the field of research on the predictive value of preoperative PNI for the postoperative progression of pNENs.
In this study, we first determined the optimal cut-off value for the preoperative PNI at 48.275. By analyzing the differences in clinicopathological characteristics between patients in the low PNI and high PNI groups, we found that patients in the low PNI group have a relatively poor baseline condition, which is particularly evident with old age. This result was consistent with most studies (17, 19, 41). Not only were albumin levels and the total peripheral blood lymphocyte count lower in the low PNI group, but also hemoglobin levels, lymphocyte percentage, total protein, triglycerides, and cholesterol, which can also indirectly reflect the nutritional and immune status of the body. BMI is calculated based on weight and height, which has been used as a measure of human nutritional status since the 1990s. Since then, in order to harmonize international terminology, the European Society of Clinical Nutrition and Metabolism (ESPEN) issued a statement in 2015 stating that malnutrition should be diagnosed based on BMI (42). The existing studies found that the relationship between BMI and PNI still seems to be contradictory. For example, the study by Park et al. found a positive correlation between preoperative BMI and PNI (43), while the present study, in agreement with the study by He et al. did not observe a correlation between the two (44), which may be related to the different diseases studied and the differences in the populations included, but the exact reasons for this still need further study with large sample data. In addition, our study also found that patients in the low PNI group had a relatively higher likelihood of tumor progression after surgery compared to the high PNI group. The Kaplan-Meier curve also showed a significantly higher PFS after surgery in the high PNI group compared with that in the low PNI group. The preoperative PNI negatively correlated with age, however, after adjusting for many confounding factors, the preoperative PNI was indeed a significant predictor of postoperative tumor progression. This has been confirmed in other tumors of the pancreas, such as pancreatic ductal adenocarcinoma (41) and pancreatic solid pseudopapillary tumor (45). However, to our knowledge, this is the first time it has been confirmed in pancreatic neuroendocrine tumors.
There is no doubt that our study is a retrospective single-center study, and although it can relatively illustrate our point, it still has limitations. Therefore, our existing findings require further validation by prospective multicenter studies.
Conclusion
In conclusion, this study shows for the first time that preoperative PNI has a predictive value for postoperative progression in patients with pNENs, and that a low PNI is an independent risk factor of PFS. For patients who undergo surgery, attention should be paid to their nutritional and immune statuses before surgery, and an extra attention should be paid to the close follow-up of patients with a low PNI after surgery.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HS conceived the study. MF and LYu contributed to the concept and design. LYa, YC, XC, and QH contributed to the data collect, analysis, and interpretation. MF drafted the manuscript. All authors were involved in the revision of the manuscript and approved the submitted version.
Acknowledgments
We would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. (2018) 68:471–87. doi: 10.3322/caac.21493
2. Perri G, Prakash LR, Katz MHG. Pancreatic neuroendocrine tumors. Curr Opin Gastroenterol. (2019) 35:468–77. doi: 10.1097/MOG.0000000000000571
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
4. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. (2016) 103:153–71. doi: 10.1159/000443171
5. Tierney JF, Chivukula SV, Wang X, Pappas SG, Schadde E, Hertl M, et al. Resection of primary tumor may prolong survival in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. (2019) 165:644–51. doi: 10.1016/j.surg.2018.09.006
6. Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis. (2021) 53:171–82. doi: 10.1016/j.dld.2020.08.031
7. Albers MB, Almquist M, Bergenfelz A, Nordenstrom E. Complications of surgery for gastro-entero-pancreatic neuroendocrine neoplasias. Langenbecks Arch Surg. (2020) 405:137–43. doi: 10.1007/s00423-020-01869-0
8. Andreasi V, Muffatti F, Guarneri G, Falconi M, Partelli S. Surgical principles in the management of pancreatic neuroendocrine neoplasms. Curr Treat Options Oncol. (2020) 21:48. doi: 10.1007/s11864-020-00736-w
9. Xu Z, Wang L, Dai S, Chen M, Li F, Sun J, et al. Epidemiologic trends of and factors associated with overall survival for patients with gastroenteropancreatic neuroendocrine tumors in the United States. JAMA Netw Open. (2021) 4:e2124750. doi: 10.1001/jamanetworkopen.2021.24750
10. Landoni L, Marchegiani G, Pollini T, Cingarlini S, D’Onofrio M, Capelli P, et al. The evolution of surgical strategies for pancreatic neuroendocrine tumors (Pan-NENs): time-trend and outcome analysis from 587 consecutive resections at a high-volume institution. Ann Surg. (2019) 269:725–32. doi: 10.1016/j.pan.2018.05.377
11. Lee L, Ito T, Jensen RT. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: recent advances and controversies. Expert Rev Anticancer Ther. (2019) 19:1029–50. doi: 10.1080/14737140.2019.1693893
12. Deftereos I, Kiss N, Isenring E, Carter VM, Yeung JM. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol. (2020) 46:1423–34. doi: 10.1016/j.ejso.2020.04.008
13. Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment—focusing on metabolism and supportive care. Ann Oncol. (2018) 29:ii27–34. doi: 10.1093/annonc/mdy093
14. Wleklik M, Uchmanowicz I, Jankowska-Polanska B, Andreae C, Regulska-Ilow B. The role of nutritional status in elderly patients with heart failure. J Nutr Health Aging. (2018) 22:581–8. doi: 10.1007/s12603-017-0985-1
15. Lakananurak N, Gramlich L. The role of preoperative parenteral nutrition. Nutrients. (2020) 12:1320. doi: 10.3390/nu12051320
16. Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. (2017) 6:e004876. doi: 10.1161/JAHA.116.004876
17. Mirili C, Yilmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. (2019) 24:1301–10. doi: 10.1007/s10147-019-01461-7
18. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/SLA.0000000000002985
19. Wang Z, Zhao L, He S. Prognostic nutritional index and the risk of mortality in patients with hypertrophic cardiomyopathy. Int J Cardiol. (2021) 331:152–7. doi: 10.1016/j.ijcard.2021.01.023
20. Kim SI, Kim SJ, Kim SJ, Cho DS. Prognostic nutritional index and prognosis in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol. (2021) 39:623–30. doi: 10.1016/j.urolonc.2021.05.028
21. Hao J, Chen C, Wan F, Zhu Y, Jin H, Zhou J, et al. Prognostic value of pre-treatment prognostic nutritional index in esophageal cancer: a systematic review and meta-analysis. Front Oncol. (2020) 10:797. doi: 10.3389/fonc.2020.00797
22. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: review and meta-analysis. Clin Chim Acta. (2018) 486:303–10. doi: 10.1016/j.cca.2018.08.030
23. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76:182–8. doi: 10.1111/his.13975
24. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
25. Mpilla GB, Philip PA, El-Rayes B, Azmi AS. Pancreatic neuroendocrine tumors: therapeutic challenges and research limitations. World J Gastroenterol. (2020) 26:4036–54. doi: 10.3748/wjg.v26.i28.4036
26. Muscogiuri G, Altieri B, Albertelli M, Dotto A, Modica R, Barrea L, et al. Epidemiology of pancreatic neuroendocrine neoplasms: a gender perspective. Endocrine. (2020) 69:441–50. doi: 10.1007/s12020-020-02331-3
27. Gallo M, Muscogiuri G, Pizza G, Ruggeri RM, Barrea L, Faggiano A, et al. The management of neuroendocrine tumours: a nutritional viewpoint. Crit Rev Food Sci Nutr. (2019) 59:1046–57. doi: 10.1080/10408398.2017.1390729
28. Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. (2020) 39:3211–27. doi: 10.1016/j.clnu.2020.03.038
29. Imam R, Chang Q, Black M, Yu C, Cao W. CD47 expression and CD163+ macrophages correlated with prognosis of pancreatic neuroendocrine tumor. BMC Cancer. (2021) 21:320. s doi: 10.1186/s12885-021-08045-7
30. Cen D, Chen J, Li Z, Zhao J, Cai X. Prognostic significance of cytokeratin 19 expression in pancreatic neuroendocrine tumor: a meta-analysis. PLoS One. (2017) 12:e0187588. doi: 10.1371/journal.pone.0187588
31. Xu SS, Xu HX, Wang WQ, Li S, Li H, Li TJ, et al. Tumor-infiltrating platelets predict postoperative recurrence and survival in resectable pancreatic neuroendocrine tumor. World J Gastroenterol. (2019) 25:6248–57. doi: 10.3748/wjg.v25.i41.6248
32. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5. doi: <doi>
33. Cadwell JB, Afonso AM, Shahrokni A. Prognostic nutritional index (PNI), independent of frailty is associated with six-month postoperative mortality. J Geriatr Oncol. (2020) 11:880–4. doi: 10.1016/j.jgo.2020.03.013
34. Guller M, Herberg M, Amin N, Alkhatib H, Maroun C, Wu E, et al. Nutritional status as a predictive biomarker for immunotherapy outcomes in advanced head and neck cancer. Cancers (2021) 13:5772. doi: 10.3390/cancers13225772
35. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. (2020) 69:1813–22. doi: 10.1007/s00262-020-02585-w
36. Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, Fukumoto Y, et al. Influence of prognostic nutritional index and tumor markers on survival in gastric cancer surgery patients. Langenbecks Arch Surg. (2017) 402:501–7. doi: 10.1007/s00423-017-1572-y
37. Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC. J Gastrointest Surg. (2021) 25:421–7. doi: 10.1007/s11605-019-04492-7
38. Yang Z, Zheng Y, Wu Z, Wen Y, Wang G, Chen S, et al. Association between pre-diagnostic serum albumin and cancer risk: results from a prospective population-based study. Cancer Med. (2021) 10:4054–65. doi: 10.1002/cam4.3937
39. Takeda K, Umezawa R, Takahashi N, Matsushita H, Kozumi M, Ishikawa Y, et al. Impact of change in serum albumin level during and after chemoradiotherapy in patients with locally advanced esophageal cancer. Esophagus. (2018) 15:190–7. doi: 10.1007/s10388-018-0612-1
40. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. (2018) 32:1267–84. doi: 10.1101/gad.314617.118
41. Itoh S, Tsujita E, Fukuzawa K, Sugimachi K, Iguchi T, Ninomiya M, et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology. (2021) 21:1356–63. doi: 10.1016/j.pan.2021.08.003
42. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition – an ESPEN consensus statement. Clin Nutr. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
43. Park SH, Lee S, Song JH, Choi S, Cho M, Kwon IG, et al. Prognostic significance of body mass index and prognostic nutritional index in stage II/III gastric cancer. Eur J Surg Oncol. (2020) 46:620–5. doi: 10.1016/j.ejso.2019.10.024
44. He ZQ, Ke C, Al-Nahari F, Duan H, Guo CC, Wang Y, et al. Low preoperative prognostic nutritional index predicts poor survival in patients with newly diagnosed high-grade gliomas. J Neurooncol. (2017) 132:239–47. doi: 10.1007/s11060-016-2361-0
Keywords: prognostic nutritional index, pancreatic neuroendocrine neoplasms, nutritional status, progression-free survival, prognosis
Citation: Fu M, Yu L, Yang L, Chen Y, Chen X, Hu Q and Sun H (2022) Predictive value of the preoperative prognostic nutritional index for postoperative progression in patients with pancreatic neuroendocrine neoplasms. Front. Nutr. 9:945833. doi: 10.3389/fnut.2022.945833
Received: 17 May 2022; Accepted: 23 August 2022;
Published: 08 September 2022.
Edited by:
Mohammad Alizadeh, Tabriz University of Medical Sciences, IranReviewed by:
Xiangyi Kong, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaZhongqiu Wang, Affiliated Hospital of Nanjing University of Chinese Medicine, China
Copyright © 2022 Fu, Yu, Yang, Chen, Chen, Hu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Sun, c3Vubnk2OEBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Mengfei Fu
Mengfei Fu Li Yu3†
Li Yu3† Hui Sun
Hui Sun