
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 19 July 2022
Sec. Nutrition and Microbes
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.939571
This article is part of the Research TopicFood, Nutrition and Microecological HealthView all 27 articles
Gut microbiota, a group of microorganisms that live in the gastrointestinal tract, plays important roles in health and disease. One mechanism that gut microbiota in modulation of the functions of hosts is achieved through synthesizing and releasing a series of metabolites such as short-chain fatty acids. In recent years, increasing evidence has indicated that dietary compounds can interact with gut microbiota. On one hand, dietary compounds can modulate the composition and function of gut microbiota; on the other hand, gut microbiota can metabolize the dietary compounds. Although there are several reviews on gut microbiota and diets, there is no focused review on the effects of dietary compounds on gut microbiota-derived metabolites. In this review, we first briefly discussed the types of gut microbiota metabolites, their origins, and the reasons that dietary compounds can interact with gut microbiota. Then, focusing on gut microbiota-derived compounds, we discussed the effects of dietary compounds on gut microbiota-derived compounds and the following effects on health. Furthermore, we give our perspectives on the research direction of the related research fields. Understanding the roles of dietary compounds on gut microbiota-derived metabolites will expand our knowledge of how diets affect the host health and disease, thus eventually enable the personalized diets and nutrients.
Gut microbiota is a group of microorganisms including bacteria, archaea, eukarya, etc., that dwell in the human gastrointestinal tract (1). The number of microorganisms in human gut microbiota is estimated to be as many as 1014 bacterial cells, which is 10 times larger than the number of human cells (2). In recent decades, numerous studies have demonstrated that gut microbiota plays important role in maintenance of health by acting as a barrier to defend against pathogens, maintaining the integrity of the epithelial barrier, modulating the metabolism and immune functions of hosts, communicating with central nervous system (3). On the contrary, gut microbiota dysbiosis can lead to a large number of diseases, such as non-alcoholic fatty liver disease, obesity, inflammatory bowel disease, cancer, allergy, and depression (2, 4). Because the importance of gut microbiota in health and disease, gut microbiota has become an important research frontier in health-related research fields.
Normally, the gut microbiota is confined within the gastrointestinal lumen by gut barrier and translocation of gut bacteria can lead to local and systematic inflammation (5). To overcome the spatial limitation, gut microbiota develops a tactic of releasing a large number of gut microbiota metabolites from different chemical classes to exert extensive effects on host organs that are near or far away from gastrointestinal lumen (6). Gut microbiota contains 5 × 106 unique microbial genes collectively, which outnumber the genes of humans (7). With these genes, gut microbiota is capable of synthesizing a large group of metabolites. These gut microbiota-derived metabolites include, but not limited to, short chain fatty acids (SCFAs), bile acids, vitamins, tryptophan and indole derivatives (8). These metabolites can exert the functions of regulating of gut microbiota composition, host metabolism, nutrition absorption, gut motility, local and systemic immune response, circadian rhythm, etc., to maintain the health or promote the development of diseases (6).
Diet is one of the most important regulators of gut microbiota. Many dietary compounds exhibit low bioavailability or cannot not be absorbed directly, such as polysaccharides and polyphenol (9). When orally taken, these compounds can pass the small intestine and enter the colon, a comfortable place that most gut bacteria live. In the colon, dietary compounds can interact with gut microbiota. On one side, gut microbiota can transform the food compounds and produce new compounds derived from dietary compounds. For example, rutin can be transformed to quercetin and further into protocatechuic acid, 3,4-dihydroxyphenyl-acetic acid, etc., by gut microbiota (10). On the other side, food compounds can induce the functional and compositional change of gut microbiota. For example, coffee and its major components caffeine and chlorogenic acid can alter gut microbial community and SCFAs levels (11). The gut microbiota transformation of dietary compounds and dietary compounds modulation of gut microbiota composition have been extensively reviewed (12–14), however, there is no focused review on the effects of dietary compounds on gut microbiota metabolites. In this review, we focus on the effects of dietary compounds on gut microbiota-derived compounds and the following effects on health.
Gut microbiota not only contain a large number of bacteria that belong to more than 1,000 species, it also contain millions of microbial genes that is 150-fold larger than the number of human gene complements (7). With the aid of these genes, gut microbiota is capable of producing a large number of enzymes. These enzymes can ferment diverse food compounds that are not digested by human enzymes such as fiber or compounds that are released by human body such as primary bile acids. Consequently, gut microbiota can synthesis and release a large number of metabolites with great diverse of chemical structures. These gut microbiota-derived metabolites can be broadly grouped into 3 categories: (I) metabolites that are produced directly from dietary compounds by gut microbiota, such as SCFAs, tryptophan, and indole derivatives of tryptophan; (II) metabolites that are originally synthesized by hosts and chemically transformed by gut microbiota, such as secondary bile acids; (III) metabolites that are produced by gut microbiota de novo (15). A lot of metabolites show similar structure and bioactivities, and they are therefore grouped together. Currently, the extensively studied gut microbiota-derived metabolites include SCFAs, bile acids, branched chain amino acids (BCAAs), tryptophan and indole derivatives, etc. In Table 1, we have listed the typical groups of gut microbiota metabolites and their functions.
After the production and releasing, gut microbiota metabolites can be absorbed and transferred into circulating system. It is estimated that gut microbiota metabolites can account for 10% of the total metabolites in blood of mammalian (37). When absorbed, gut microbiota metabolites can be transported to target organs and tissues that are remote from gastrointestinal tract, and therefore exert a wide range of activities on hosts. These activities include, but not limited to, regulating the composition and function of gut microbiota, acting as nutrition, influencing nutrition absorption, modulating host metabolism, influencing intestinal barrier and gut motility, impacting the local and systemic immune response, modulating circadian rhythm and nervous system (6). With these bioactivities, gut microbiota metabolites play important roles in the development and progress of a variety of diseases such as cancer, non-alcoholic fatty liver disease, hypertension, Parkinson's disease, and ulcerative diseases (30, 38–40).
In gastrointestinal tract, most of the dietary compounds can be digested and absorbed by human body. Even so, significant amounts of digestible dietary compounds can reach the colon. For instance, the majority of proteins can be digested and absorbed in the small intestine, a relatively large amounts of proteins and amino acids (about 6–18 g per day) can reach the colon (41). In addition, the bioavailability of some types of dietary compounds is very low and thus these compounds can reach the colon as well. For example, the bioavailability of polyphenols is very poor and is often <10% (42). Thus, the unabsorbed dietary compounds such as polyphenols and amino acids can enter the colon. The colon is an ideal place for the interactions between gut microbiota and dietary compounds because colon alone contains over 70% of all the microbes in the human body including the surface of body (2). Other reasons that render colon an ideal place also include the suitable pH and the long time that enable the direct contact between gut microbiota and dietary compounds. The direct interactions between dietary compounds and gut microbiota include (I) gut microbiota can transform the dietary compounds directly; (II) dietary compounds can modulate the composition of gut microbiota; (III) dietary compounds can modulate the metabolites of gut microbiota. In addition to direct reactions, dietary compounds can also interact indirectly with gut microbiota via modulation of gastrointestinal pH, gastrointestinal transit time, the synthesis and release of immune materials such as antimicrobial peptides and secretory immunoglobulin A (43). For example, dietary compounds such as polyphenols and peptides can modulate the gastrointestinal pH, which can further modulate the composition of gut microbiota and the catalytic activity of enzymes (42, 44, 45). Whatever the indirection, the final reactions of indirect reactions can be only attributed to the types of direct reactions we discussed.
The bioavailability of many dietary compounds and herbal compounds is very low, yet they possess strong bioactivities. This discrepancy has perplexed researchers for a long time (46). Studies in recent decade have revealed that the interaction between gut microbiota and these compounds is one of the keys to decipher this conundrum. Gut microbiota can transform the chemical structure of dietary compounds and thus improve the bioavailability or potency. For example, curcumin, a polyphenolic compound with high hydrophobicity and poor solubility, exhibits wide spectrum of pharmacologic effects on diseases such as inflammatory diseases, cancers, cardiovascular diseases, and metabolic diseases (47). Animal studies and clinical trials indicated that the bioavailability of curcumin is very poor (about 1%) (48). Recent studies showed that gut microbiota can transform the curcumin into ferulic acid, dihydrocurcumin, tetrahydrocurcumin, curcumin-L-cysteine, bisdemethylcurcumin, etc., via gut bacteria such as Blautia sp. MRG-PMF1, Bacillus megaterium DCMB002 (49, 50). Some of these products, such as dimethoxycurcumin, have superior bioactivities compared with their parent compound curcumin (51). Thus, the biotransformation of curcumin by gut microbiota can explain the contradiction between low bioavailability and strong bioactivities of curcumin. In addition to biotransformation, the gut microbiota metabolites may also explain this contradiction as well. Hydroxysafflor yellow A (HSYA) is a water-soluble compound isolated from Carthamus tinctorius L. with only 1.2% oral bioavailability (52). Yet it shows a group of bioactivities such as anti-tumor, neuroprotective, hepatoprotective, and pulmonary protective effects (53). In a high-fat diet-induced obese mice, oral administration of HSYA increased the abundances of Akkermansia and Romboutsia, as well as SCFAs-producing bacteria Butyricimonas and Alloprevotella (54). In addition, HSYA significantly increased acetic acid, propionic acid, and butyric acid, a group of SCFAs that is directly associated with obesity and intestinal integrity when their levels are decreased (54). The study suggested that increase of SCFAs may contribute to the pharmacological effects of HSYA. Taken together, transformation of dietary compounds by gut microbiota and modulation of gut microbiota metabolites by dietary compounds can explain the contradiction between low bioavailability and strong bioactivities of some dietary compounds.
In general, dietary compounds in modulation of gut microbiota metabolites can be achieved by the following ways: (1) dietary compounds directly be metabolized into gut microbiota metabolites by gut microbiota; (2) dietary compounds modulate the gut microbiota composition and thus influence the metabolites-producing activity; (3) dietary compounds directly inhibiting enzymes responsible for production of gut microbiota metabolites; (4) regulating the expression or activity of hepatic enzymes responsible for host metabolism of gut microbiota metabolites; (5) a combination of these effects. The typical metabolites modulated by dietary compounds are SCFAs, bile acids, trimethylamine, branched-chain amino acids, tryptophan and indole metabolites (Figure 1).
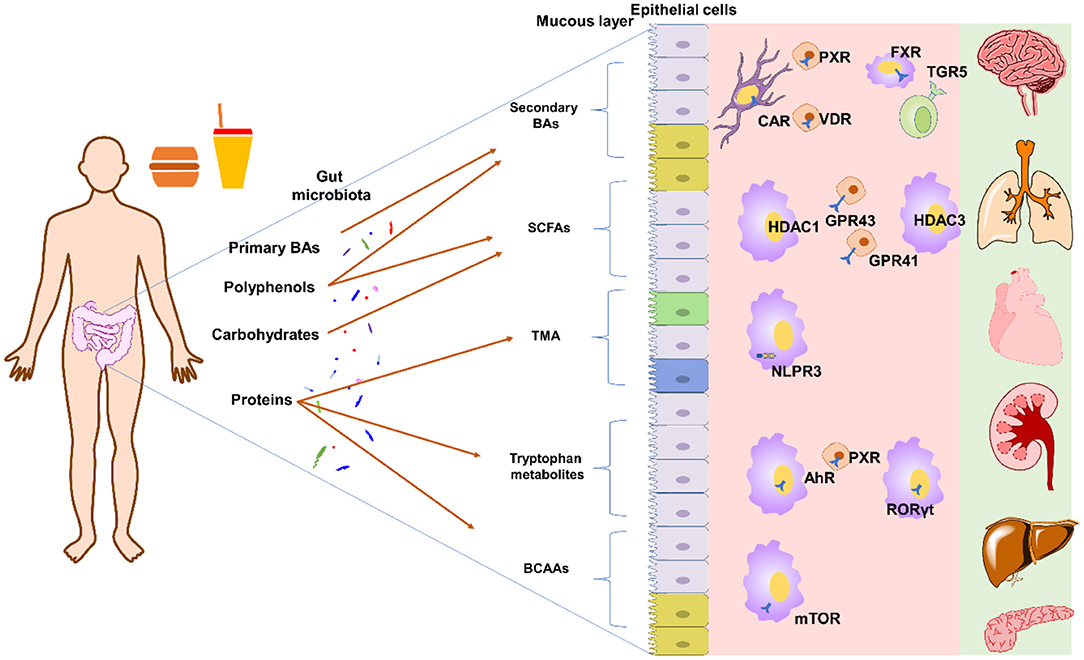
Figure 1. Typical gut microbiota derived metabolites and their targets. Primary BAs can by transformed into secondary BAs by bile salt hydrolase and 7α-dehydroxylase[[Inline Image]]. Polyphenols can modulate the composition of gut microbiota to affect SCFAs production or be directed degraded into SCFAs. Polyphenols can also affect 7α-dehydroxylase activity to influence BA pool. Carbohydrates can be directly fermented into SCFAs. Proteins can be degraded into peptides and amino acids, which can further be transformed into TMA, tryptophan metabolites, and others. Please refer to main text for detailed metabolites and targets. AhR, aryl hydrocarbon receptor; BAs, bile acids; BCAAs, branched-chain amino acids; CAR, constitutive androstane receptor; FXR, farnesoid X receptor; GPR41, G protein-coupled receptor 41; GPR43, G protein-coupled receptor 43; HDAC1, histone deacetylase 1; HDAC3, histone deacetylase 3; NLPR3, inflammasome NOD-like receptor protein 3; PXR, pregnane X receptor; RORγt, retinoid-related orphan receptor gamma-t; SCFAs, short-chain fatty acids; TMA, trimethylamine; TGR5, Takeda G-protein receptor 5; VDR, vitamin D3 receptor.
SCFAs are a group of small organic monocarboxylic acids with one to six carbon atoms (55), and they are the most widely studied gut microbiota metabolites in recent years. SCFAs are comprised mostly of acetate (C2), propionate (C3), and butyrate (C4) with a molar rate of 60:20:20 in gastrointestinal tract (95%) (56, 57). After the production, SCFAs can be readily absorbed by colonocytes via hydrogen-coupled monocarboxylate transporters and sodium-coupled monocarboxylate transporters, with only about 5–10 % of the SCFAs can be detected in feces (58, 59). The concentration gradient of SCFAs falls from the gut lumen to the periphery tissues and organs because of the consumption of butyrate at the epithelium, propionate at the liver and acetate in the periphery (60). After absorption, part of SCFAs will be directly utilized as energy resource for gluconeogenesis and lipid synthesis, while the remaining SCFAs play important roles in regulating various host biological responses such as inflammation and oxidative stress. SCFAs can activate several G protein-coupled receptors (GPCRs) directly, such as GPR43 (free fatty acid receptor 2, FFAR2) and GPR41 (FFAR3), GPR109a/HCAR2 (hydrocarboxylic acid receptor) and GPR164, and thus regulate energy metabolism, inflammation, oxidative stress, and other reactions (16, 61). SCFAs can also inhibit nuclear class I histone deacetylases (HDACs) including HDAC1 and HDAC3, and thus end up with regulation of inflammatory signaling pathways (16, 62). Under physiological condition, SCFAs can regulate gut microbiota composition, gut barrier, gut hormone, appetite, energy homeostasis, and immune function, and circadian clocks (18). On the contrary, disturbance of SCFAs is associated with a group of diseases such as diabetes, obesity, non-alcoholic fatty liver disease, hypertension, ulcerative colitis, Parkinson's disease, and colorectal cancer (18, 63).
Dietary compounds can directly modulate the production of SCFAs by serving as the resource for SCFAs synthesis. Non-digestible dietary carbohydrates such as fibers that escape the digestion and absorption of small intestine can be partially or completely fermented by gut microbiota and produce SCFAs. During fermentation, acetate can be produced by acetyl-CoA pathway or via the Wood-Ljungdahl pathway under the action of bacteria such as Bifidobacterium spp., Lactobacillus spp., and Akkermansia muciniphila (64). Propionate can be produced by succinate pathway, propanediol pathway, or acrylate pathway under the action of bacteria such as Megasphaera elsdenii, Veillonella spp. and Roseburia inulinivorans (65). Butyrate can be produced by butyryl-CoA:acetate CoA-transferase routes or phosphotransbutyrylase/butyrate kinase routes under the action of bacteria such as Eubacterium rectale, and Faecalibacterium prausnitzii (64, 65). In addition to carbohydrates, proteins or peptides that contain branched-chain amino acids and escape the digestion and absorption of small intestine can be fermented to SCFAs as well. However, the SCFAs produced from proteins and peptides are typically branched-chain fatty acids, such as 2-methylbutyrate, isobutyrate, and iso-valerate (66). Polyphenols can affect the SCFAs production as well. For example, the sugar moiety of quercetin-3-glucoside can be fermented to formate and acetate by Enterococcus casseliflavus, and the resulting quercetin can be further degraded into 3,4-dihydroxyphenylacetic acid, phloroglucinol, butyrate and acetate by Eubacterium ramulus (67).
The type of food and the intake amount can affect the types and amounts of SCFAs significantly. Dietary fiber, according to the definition by CODEX Alimentarius Commission, are carbohydrate polymers with ten or more monomeric units that are not hydrolyzed by the endogenous enzymes in the small intestine of humans (68). In animal models, the addition of fiber can lead to increase of cecal SCFAs in comparison with control diets. However, no significant linear correlation between fiber intake amount and SCFA concentrations can be observed (69). The study of SCFA concentrations in human is mainly carried by measuring the fecal SCFAs because of the convenience of the sampling method. However, concentrations of fecal SCFAs reflect little information on actual intestinal SCFA concentrations since SCFAs are readily absorbed by host after production. Therefore, in vitro fermentation is widely used to study the relationship between diet and SCFAs, and it was found out that different fiber showed significant difference in production of SCFAs (56). Specifically, the physical form and chemical properties of fibers including monosaccharide compositions, linkage of monosaccharide, molecular size and arrangements of the sugars are important factors influencing the production of SCFAs (70). For example, in vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. showed that acetic and butyric acids are produced mainly due to the fermentation of aldehydes and xylose, whereas propionic acid is mainly generated by fermentation of arabinose and xylose (71). Flammulina velutipes polysaccharide with the monosaccharide composition at the ratio of glucose: galactose: mannose = 60.66:19.96:19.38 can increase the levels of isobutyric and butyric acid (72). On the contrary, the Flammulina velutipes polysaccharide with the monosaccharide composition at the ratio of mannose: glucose: xylose: arabinose: fucose = 6.6:27.8:18:1.5:5.2 increased the levels of acetic, propanoic and butyric acids (73). Because SCFAs play important roles in health and the type of food can affect the production of SCFAs, modulation of SCFAs by diet has been suggested to be a good approach to maintain health and treat diseases. In Table 2, we have listed the common dietary polysaccharides on SCFAs.
In recent years, a large number of animal and clinical studies have carried to investigate the supplement of dietary compounds on diseases, especially fiber. In mice, supplement of dietary fermentable fiber changed the composition of gut and lung microbiota, especially the ratio of Firmicutes to Bacteroidetes. In addition, mice received a high-fiber diet showed increased circulating SCFAs and were protected from allergic lung inflammation (105). Compared with the animal studies, the clinical studies on supplement of dietary compounds have received more attentions. In a randomized clinical study, supplement of specifically designed isoenergetic diets showed that a high-fiber diet can change the composition of the gut microbiota and improve glucose homeostasis in patients with type 2 diabetes mellitus (T2DM). In addition, the high-fiber diet can selectively elevate bacteria producing SCFAs and the levels of bacteria producing SCFAs correlated positively with hemoglobin A1c levels in participants with T2DM (106). The results suggested that targeted promotion of bacteria producing SCFAs can be effective to treat T2DM in clinic. Considering that carbohydrates such as fibers can be fermented and transformed into SCFAs, it is no wonder that dietary supplement of these compounds can increase circulating SCFAs and can be conducive to amelioration of diseases. In fact, in addition to fermentable carbohydrates, many other dietary compounds especially polyphenol can increase SCFAs as well by changing the levels of bacteria producing SCFAs (107). For example, a major bioactive compound from green tea epigallocatechin-3-gallate protected the mice from colitis by increasing abundance of SCFAs-producing bacteria such as Akkermansia and levels of SCFAs (108). The mounting evidence suggested that manipulating gut bacterial SCFAs by dietary compounds can be useful to treat diseases.
Bile acids (BAs) are a group of acidic, amphipathic, and water-soluble metabolites with a steroid structure that are originally produced from cholesterol in the hepatocytes. The primary BAs are synthesized mainly by classic pathway, in which 7α-hydroxylase acts as the rate-limiting enzyme, and to a lesser extent by alternative pathway (109). The main BAs synthesized in human are chenodeoxycholic acid and cholic acid whereas rodents can also produce muricholic acids, and thus BAs show evident species-specific differences (109). After the production, BAs can conjugate with taurine or glycine to form bile salts in the liver (110). When triggered by food intake, BAs are released into duodenum to exert their detergent-like activity to facilitate absorption of intestinal lipids. Meanwhile, most of the BAs can be reabsorbed and delivered back to liver, a process that is also known as enterohepatic circulation, to maintain the BA pool (111). For the unreabsorbed BAs, they can be deconjugated by gut microbiota under the catalysis of bile salt hydrolase, and further transformed into secondary BAs by 7α-dehydroxylase (20). A part of secondary BAs can also be absorbed from gut and get enriched through enterohepatic circulation, and thus may acting on hosts as important molecules to regulate the health and disease (20). The BA receptors include Takeda G-protein receptor 5 (TGR5, also known as G-protein-coupled BA receptor, GPBAR1), farnesoid X receptor (FXR), vitamin D3 receptor, pregnane X receptor/steroid and xenobiotic-sensing receptor, constitutive androstane receptor and others (19). By acting on these targets, BAs play important roles in regulation of lipids, amino acids, glucose metabolism and are implicated in a number of diseases such as obesity, insulin resistance, liver cirrhosis, non-alcoholic steatohepatitis and colitis (19). Worth notice is that BA receptors exhibit different affinity for different BAs. For example, TGR5 can be activated by BAs with the order of potency lithocholic acid > deoxycholic acid > chenodeoxycholic acid > cholic acid (including in both conjugated and unconjugated states) (112). This phenomenon indicates that variations of BA pool can result in perturbation of BA signaling in host, and this is confirmed by the fact that BA metabolism is disturbed in a number of diseases (113).
Given that dietary compounds can affect the composition of gut microbiota, dietary supplement may alter the gut microbial BA metabolism and thus further bring beneficial effects on hosts. In fact, a group of recent studies have continuously supported this idea. Curcumin is a compound that is extracted from turmeric, and is widely used as a dietary agent and traditional medicine for many years (114). In high-fat diet-induced obese wild type mice, curcumin treatment ameliorated obesity by reconstruction of gut microbiota composition and increasing of microbial derived secondary BAs including deoxycholic acid and lithocholic acid, two potent ligands for TGR5 (115). Moreover, the enhanced effects of curcumin on thermogenesis were eliminated inTGR5 knockout mice, which further confirmed that the effects of curcumin on obesity were achieved through modulation of microbial BA metabolism. Capsaicin, a naturally occurring alkaloid that derived from chillies, are widely used as food additives due to its hot pungent taste. In type 2 diabetic db/db mice, capsaicin remarkably improved glucose tolerance and insulin sensitivity, and this process was associated with decreasing the abundance of Lactobacillus and microbial bile salt hydrolase activity (116). The modulation of microbial abundance and enzyme activity further leaded to accumulation of tauro-β-muricholic acid, an antagonist of the FXR, and thus improved glucose metabolism and insulin sensitivity in diabetic mice (116). In another study by same group, capsaicin ameliorated high fat diet-induced obesity and adipose tissue accumulation, and this process was associated with the increase of Bacteroides abundance and 7α-dehydroxylase activity and the decrease of bile salt hydrolase activity. The modulation of microbial abundance and enzyme activity further leaded to the increase of lithocholic acid, an agonist of TGR5, and thus improved glucose metabolism and insulin sensitivity (117). These two studies showed similar pharmacological effects of capsaicin, yet the mechanisms were different. The reason may be that the animal models are different as one was leptin receptor-deficient diabetic mice whereas the other was high-fat diet-induced obese mice. Resveratrol, a natural polyphenol that is present largely in grapes and berries, improved glucose homeostasis by modulation of microbial BA metabolism and TGR5 in db/db mice (118). These studies suggested that the healthy effects of dietary compounds can be partly attribute to the modulation of gut microbial BA metabolism.
Under the action of gut microbial trimethylamine (TMA) lyases, dietary quaternary amines that are not absorbed such as choline, betaine, phosphatidylcholine, and L-carnitine can be transformed into TMA (119). After absorption, TMA can be oxidized to trimethylamine N-oxide TMAO under the action of hepatic flavin-containing monooxygenases (FMOs) such as FMO1 and FMO3 (120). Increased TMAO then promotes oxidative stress and inflammation of endothelial cells, activates the inflammasome NOD-like receptor protein 3 and nuclear factor kappa B signaling in vascular smooth muscle cells, promotes the transformation of macrophages into foam cells, promotes platelet hyperreactivity, and alters cholesterol transport and bile acid synthesis (121). Correspondingly, high level of blood TMAO might contribute to heart disease, diabetes, atherosclerosis, and even cancer (122, 123).
Worth notice is that while a large number of epidemiologic research have showed strong association between the increased plasma TMAO concentrations and the risk of cardiovascular diseases, the roles of dietary compounds on final concentrations of TMAO in blood and the risk of diseases are still worth investigating as controversy remains over this topic. For example, a study on a longitudinal cohort of US men showed that intake of higher levels of red meat and choline was significantly associated with higher TMAO levels in participants with rich TMAO-producing bacteria especially Alistipes shahii but not in other participants (124). On the contrary, a study comparing the TMAO levels in patients with carotid artery atherosclerosis and healthy controls showed that there was no association between the level of TMAO and carotid atherosclerosis or cardiovascular death (125). In addition, fish-rich diets can lead to obvious increase of plasma TMAO; however, meta-analysis, cross-sectional and prospective studies showed that fish consumption is associated with lower risk of cardiovascular diseases (126). Furthermore, some studies even reported the beneficial effects of TMAO or TMA in cardiovascular diseases (119).
Nevertheless, in addition to the compounds such as choline and L-carnitine that can be metabolized to TMA directly, modulation of TMA and TMAO metabolism is still regarded as one important mechanism of other types of dietary compounds in regulation of cardiovascular diseases. For example, administration of Lonicera caerulea berry extract, which is rich in polyphenols, to high cholesterol-induced hypercholesterolemic male SD rats for 12 weeks attenuated serum dyslipidemia (127), and this process was associated with decreasing of serum TMAO levels. In mice supplemented with 1.3% carnitine, flavonoids from oolong tea and citrus peels remarkedly reduced plasma TMAO and aortic inflammation, and this process was associated with modulation of Lactobacillus and Akkermansia (128). In addition to crude extracts, pure compounds in diet can also modulate TMA and TMAO to modulate cardiovascular diseases. For example, oral resveratrol can attenuate TMAO-induced atherosclerosis in ApoE−/− mice, and this process was associated with inhibiting of gut microbial TMA production (129). Besides, treatment with antibiotics abolished the decreasing effects of resveratrol on TMAO levels and the inhibiting effects on atherosclerosis.
Branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, are essential amino acids with branched aliphatic side chains. BCAAs play critical roles in nutrients metabolism such as muscle protein synthesis, glucose and lipid metabolism via phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway (130). In contrast to the beneficial effects, evidence has indicated that BCAAs can contribute to a number of diseases such as insulin resistance, type 2 diabetes mellitus, cancer, and cardiovascular diseases (26, 130). Therefore, BCAAs has been suggested to be developed as biomarkers to predict the outcomes of diseases such as obesity, insulin resistance, and cardiovascular diseases (131). Although mammalians lack the enzymes needed for the de novo synthesis of BCAAs, gut microbiota contain rich enzymes responsible for synthesis of BCAAs (132). Many gut bacteria are capable of de novo synthesis of BCAAs, such as Prevotella copri, Bacteroides vulgatus, and Clostridium clusters (133). Since BCAAs can be degraded from food and synthesized by gut microbiota, both the daily supplementation of BCAAs-containing food and modulating the composition of gut microbiota can affect the circulating BCAAs and further affect the health and disease associated with BCAAs. For example, diet with specifically reduced BCAAs can reverse the diet-induced obesity and restore the glucose tolerance and insulin sensitivity (134).
In addition to the dietary proteins, peptides, and amino acids that can be directly metabolized to BCAAs by gut microbiota, other dietary compounds can also influence the levels of circulating BCAAs and thus attenuate diseases. For example, virgin olive oil and high-oleic acid peanut oil are dietary vegetable oil with a high monounsaturated fatty acid and can attenuate metabolic syndrome (135). When the two types of oils were fed to rats with a high-fat diet, the β-diversity and abundance of Bifidobacterium were increased (oleic acid peanut oil also decreased Lachnospiraceae and Blautia) and the levels of BCAAs were revised, suggesting of modulation of gut microbial BCAA metabolism as the mechanism of the two oils in attenuation of metabolic syndrome (135). As a favorite vegetable, Luffa cylindrica (L.) Roem (luffa) is a very common in daily diet, and it is rich in polyphenols, saponin, triterpenoids, flavonoids, and oleanolic acid (136). Dietary oral administration of luffa to diet-induced obese mice can reduce circulating BCAA levels and selectively decrease the relative abundances of bacteria such as Enterortabdus and Butyricicoccus positively correlated with BCAA levels (136). In addition, this effect on BCAA catabolism was not observed in antibiotic-treated obese mice. The results suggested that luffa ameliorated obesity by modulation of gut microbial BCAA metabolism. In another study, citrus polymethoxyflavones ameliorated high-fat diet-induced metabolic syndrome via regulation of gut microbial BCAA metabolism (137). Antibiotic treatment, fecal microbiome transplantation, and single bacterium gavage showed that Bacteroides ovatus was responsible for the reduction of BCAA levels and alleviation of metabolic syndrome (137).
Tryptophan, an essential amino acid for human body, is an important metabolite that can significantly influence both the physiology and pathology of mammalians. In addition to the main role in protein synthesis, tryptophan in host cells can act as an important precursor for production of crucial metabolites following the kynurenine and serotonin pathways (28). In contrast, unabsorbed tryptophan in the colon can interact with gut microbiota and can be metabolized into indole and indole derivatives such as skatole and indole acrylic acid under the action of the tryptophanases, which are expressed in many Gram-negative and Gram-positive gut bacteria such as Clostridium spp., Bacteroides spp. and Escherichia coli (28). Many of the indole derivatives such as indole acrylic acid, indole-3-aldehyde, indole-3-propionic acid, indole-3-acetic acid, and indole-3-acetaldehyde can act as ligands for aryl hydrocarbon receptor (AhR), pregnane X receptor and retinoid-related orphan receptor gamma-t (29, 138). Among these targets, aryl hydrocarbon receptor plays important roles in intestinal homeostasis by acting on a group of cells, such as Th17 cells, macrophages, dendritic cells, neutrophils, and other cells (139). Correspondingly, microbial tryptophan metabolites can regulate the intestinal barrier integrity, epithelial proliferation, and intestinal resistance to pathogens (27, 28). Since intestinal barrier are directly associated with a number of diseases, tryptophan and tryptophan microbial metabolites have been demonstrated to be linked to a number of diseases such as ulcerative colitis, Crohn's disease, obesity, autism spectrum disorder, Alzheimer's disease, Parkinson's disease, and irritable bowel syndrome (28, 140, 141).
As microbial tryptophan metabolism is associated with host functions and diseases, it is no wonder that dietary tryptophan is associated with health. In the aging mice, a diet containing 0.4% tryptophan significantly attenuated the inflammation and oxidative stress via increasing the colon indoles and the relative abundance of Akkermansia (142). Similarly, in non-obese diabetic mice expressing DQ8, a tryptophan-rich diet decreased gluten immunopathology via modulation of gut microbiota composition and enhancing the production of AhR ligands such as indole-3-aldehyde and indole3-lactic acid (143). On the contrary, in an aged mouse model, tryptophan-deficient diets (0.1% tryptophan) showed an elevated levels of pro-inflammatory cytokines in comparison with those fed a normal (0.2% tryptophan) and a tryptophan-rich (1.25% tryptophan) diet (144). And the effect was associated with modulation of gut bacteria such as Clostridium spp., which is involved in microbial tryptophan metabolism (144).
In addition to the diet containing tryptophan, other types of dietary compounds can modulate the gut microbiota tryptophan metabolism as well. For example, turmeric polysaccharides can improve the pathological phenotype of colitis mice via increasing the relative abundance of gut bacteria associated with tryptophan metabolism such as Lactobacillus and increasing the levels of cecal indole-3-acetic acid (145). Similarly, Fuzhuan brick tea polysaccharide ameliorated the ulcerative colitis of mice via promoting the proliferation of beneficial bacteria such as Akkermansia and increasing of fecal indole-3-acetaldehyde and indole-3-acetic acid (146). 1-Deoxynojirimycin, a major bioactive compound from functional food mulberry leaves, can ameliorate hyperlipidemia via increasing of Akkermansia and Clostridium and enhancing the microbial production of indole-3-propionic acid (147). Besides the animal-based studies, clinical studies have also suggested the beneficial effects of the other types of dietary compounds on microbial tryptophan metabolism. For instance, in a randomized, controlled, crossover trial, a polyphenol-rich diet significantly increased the microbial tryptophan metabolite indole 3-propionic acid in older people with normal renal function, and this effect was associated with shift of bacteria Bacteroidales and Clostridiales (148).
Gut microbiota can produce gases (H2S, H2, CO2, CH4, NO) (6, 35). CH4 has significant effect on gastrointestinal tract as it is associated with constipation and can slow the colon motility (149). NO plays important roles in neuronal communication, modulation of blood vessels and immune response, and is possibly participated in diseases such as stroke and Parkinson's disease (150). While high concentration of H2S is generally believed to be toxic to hosts by inducing mucus disruption and inflammation, low levels H2S can be beneficial to hosts by stabilizing mucus layer, inhibiting adherence of bacteria to the epithelium, preventing the invasive bacteria, and reducing inflammation and tissue injury (151).
CO2 is mainly produced in the stomach whereas H2, CH4, CO2 and H2S are mainly generated in the small intestine and colon (31). The gases H2 and CO2 are produced by fermentation of undigested carbohydrates and, to a much lesser extent, by dietary or hosts-released proteins, whereas the gas CH4 is produced with the help of archaea in the colon by metabolism of CO2 and H2 (152). H2S is generated by fermentation of sulfur-containing proteins with the help of bacteria that are capable of reducing sulfates and sulfites (153). In mammalian cells, H2S can also be generated from L-cysteine, thio sulfate and homocysteine with the help of enzymes such as cystathionine β-and γ-lyase, aspartate aminotransferase and 3-mercaptopyruvate sulfurtransferase (154, 155). Since chemical constituents and fermentable substrates are affected by dietary intake, the diet can change the production of gases. For instance, cellulose and corn bran can improve the production of H2, whereas the production of CH4 was not affected (156, 157). In healthy volunteers, a diet with high levels of short-chain carbohydrates that are poorly absorbed and non-digestible in the small intestine leaded to an increase of H2 levels and a decrease of CH4 (158). In addition, consuming of two purified fibers xylans and pectin increased the levels of CH4, whereas lactulose did not show this effect (156, 159). Taken together, dietary compounds can be metabolized into gases and the types of diet can influence the gas production.
In Table 3, we have listed the non-polysaccharide dietary compounds and extracts on aforementioned gut microbiota-derived metabolites. In addition to these metabolites, gut microbiota can also produce other metabolites such as lipids (lipopolysaccharides, conjugated fatty acids, etc.), vitamins, ethanol, triphosadenine, organic acids (such as benzoate and hippurate), polysaccharide A, imidazole propionate, dipeptide aldehydes (35, 176–179). Among these metabolites, of special note are the metabolites produced from amino acids. In addition to tryptophan, other amino acids can also interact with gut microbiota and be transformed. Dietary tyrosine can be transformed into phenol, a molecule with possible roles in large bowel cancer and leukemia, under the action of gut bacterial-specific tyrosine phenol-lyase (180). Another study reported that tyrosine can be transformed into phenol and p-cresol under the action of tyrosine phenol-lyase and (or) hydroxyarylic acid decarboxylase, and p-hydroxyphenylacetate decarboxylase and (or) tyrosine lyase, respectively (181). p-cresol exhibits deleterious metabolic and genotoxic effects on colonic epithelial cells, and its level is associated with chronic kidney disease, cardiovascular disease, and autistic-like behaviors (182, 183). Phenylalanine can be transformed into phenylacetate under a group of enzymes such as phenylacetate-CoA ligase in bacteria such as Escherichia coli and Pseudomonas putida (184). Methionine can be metabolized into methanethiol, NH3, and 2-oxobutanoate under the action of methionine γ-lyase, an enzyme that exists in Pseudomonas putida and other bacteria (185). Gut microbiota can transform amino acid into polyamines such as agmatine, putrescine, spermidine, cadaverine. For example, lysine can be transformed into cadaverine through diaminopimelic acid route and the α-aminoadipic acid pathway (186). High concentration of cadaverine can be toxic and potentiate histamine toxicity and is associated with ulcerative colitis (179). Gut microbiota can also produce neuroactive compounds and neurotransmitters, such as γ-aminobutyrate (GABA), norepinephrine, dopamine, histamine, and serotonin, by catabolism of amino acids (187, 188). For example, glutamate can be transformed into γ-aminobutyrate via the enzyme glutamate decarboxylase and bacteria such as Lactobacillus spp. and Bifidobacterium spp. (189). These gut microbiota-derived neuroactive compounds and neurotransmitters play important roles in gut motility disorders, behavioral disorders, neurodegenerative diseases, cerebrovascular diseases, and neuroimmune-mediated disorders (190). Taken together, amino acids can be transformed into neuroactive compounds, sulfide-containing metabolites (H2S, methanethiol), aromatic compounds (phenol, p-cresol), and polyamines, under the action of gut microbiota.
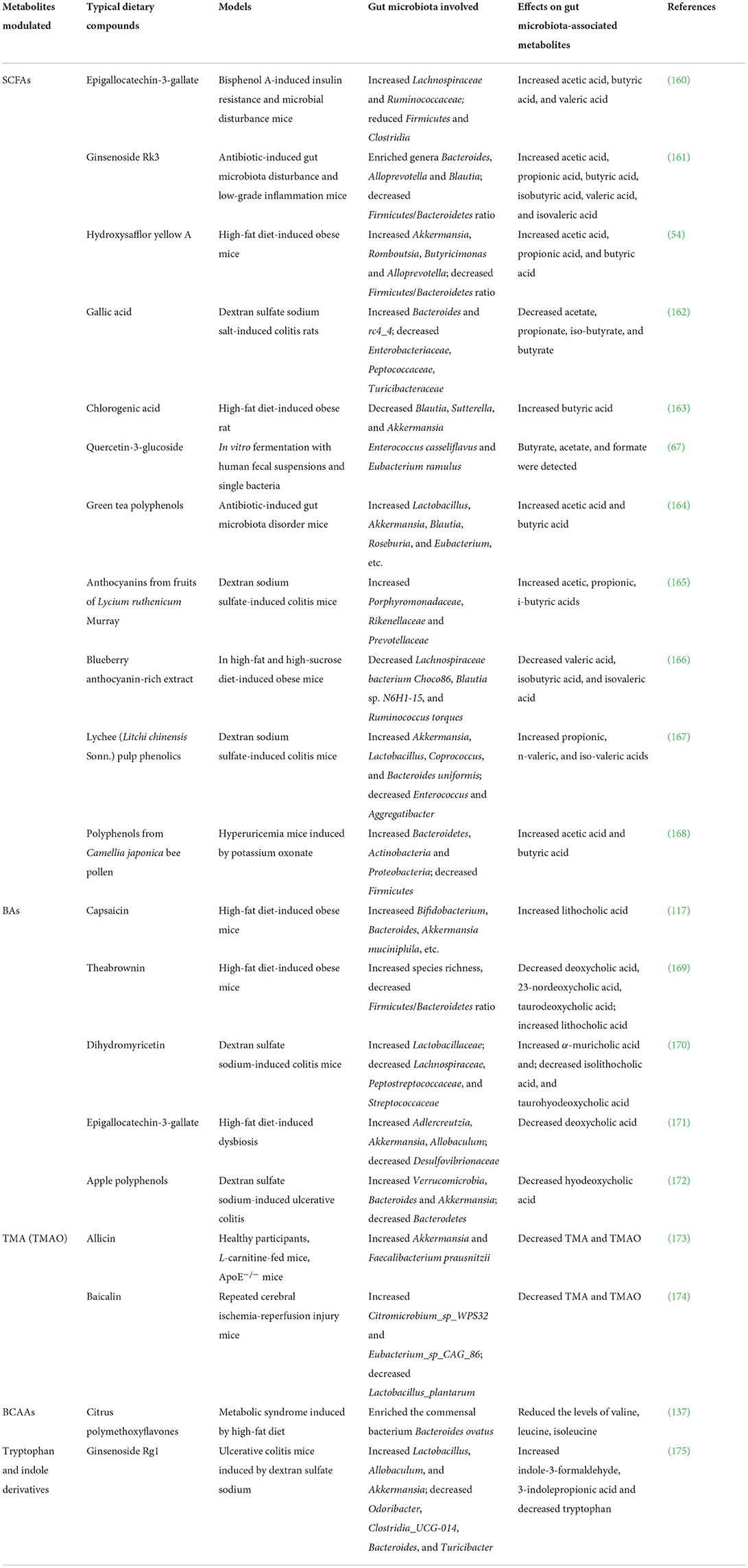
Table 3. Non-polysaccharide dietary compounds and extracts in modulation of typical gut microbiota-derived metabolites.
Because of the importance of gut microbiota metabolites in health and disease, regulation of gut microbiota metabolites by diets has received much attention in recent years. However, gut microbiota itself show great intra- and interindividual variations (1) and thus the extent of the response of gut microbiota metabolites in one person to a diet might be different from the response of another person. In this background, the concept precision nutrition or personalized nutrition has been raised in recent years following the initiative of precision medicine (191). Currently, targeted manipulating of gut microbiota has become an important approach to achieve precision medicine (43). Similar to precision medicine, precision nutrition aims to identify key characteristics of gut microbiota that can predict the response of an individual to specific dietary components, and then design of a diet that is conductive to health. In a proof-of-principle study, analysis of three cohorts of obese adults from Belgium, Finland, and Britain showed that Clostridial species were indicative of the response of gut microbiota to diet, which in turn predicted the hosts' cholesterol response (192). In another study, a machine-learning approach integrating gut microbiota, blood parameters, dietary habits, anthropometrics, and physical activity showed that this approach can accurately predicts individual postprandial glycemic response after real-life meals (193). However, these studies did not correlate the levels of gut microbiota metabolites with diets and clinical outcomes, further studies are needed to identify the metabolites that are capable of predicting clinical outcomes.
After intake of a specific diet, the change of gut bacteria and gut microbiota metabolites may not be limited to a specific bacterium or metabolites. For example, in a mice model of inflammatory bowel disease, ketogenic diet altered gut bacteria such as Proteobacteria, Enterobacteriaceae, Escherichia-Shigella and changed the gut microbiota metabolites such as stearic acid, arachidic acid, and erucic acid (194). In fact, many of the changed bacteria and metabolites only show associative relationship with the change of phenotypes, and the key bacteria and metabolites with causal relationship must be determined. This idea is supported by the recent idea of keystone taxa, which drive the community composition and function of microbiota irrespective of their abundance (195). Therefore, screening of the key bacteria and gut microbiota metabolites responsible for the phenotypes is necessary. To achieve this aim, the combination of in vivo and in vitro experiments, and a variety of advanced technologies can be used. In this case, animal such as germ-free animal, gnotobiotic animal, antibiotic-treated animal and fecal bacteria culturing can be adopted to screening key bacteria. Targeted and untargeted metabolomics combined with oral supplementation or intravenous injection can be adopted to identify the key metabolites.
Gut microbiota-derived metabolites such as SCFAs and BAs play important roles in maintenance of host homeostasis and development of diseases. In the colon, the dietary compounds that pass small intestine can interact with gut microbiota and thus modulate the gut microbiota metabolites. In general, these metabolites can be grouped into 3 types: (I) metabolites that are produced directly from dietary compounds by gut microbiota; (II) metabolites that are originally synthesis by hosts and chemically transformed by gut microbiota; (III) metabolites that are produced by gut microbiota de novo. Depending on the types of dietary compounds, the gut microbiota metabolites affected include, but not limited to, SCFAs, BAs, TMA, BCAAs, and tryptophan metabolites. Because some dietary compounds can be metabolized to a specific group of metabolites under the action of particular bacteria, personalized diets can be applied for targeted manipulation of gut microbiota metabolites. In addition, a specific diet can influence multiple gut bacteria and metabolites, thus, identification of key bacteria and metabolites responsible to the healthy effects of diets are necessary.
Conceptualization and funding acquisition: WF and CP. Writing—original draft preparation: WF. Writing—review and editing: JL, HC, DZ, and YT. Supervision and project administration: CP and YT. All authors have read and agreed to the published version of the manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 82104409, 81891012, 81891010, and U19A2010), Science and Technology Ministry of China (2108ZX09721001–008), China Postdoctoral Science Foundation (No. 2021M690490, China), Sichuan Science and Technology Program (Nos. 2021YJ0466 and 2022C001, China), National Interdisciplinary Innovation Team of Traditional Chinese Medicine (ZYYCXTD-D-202209), and Xinglin Scholar Plan of Chengdu University of Traditional Chinese Medicine (BSH2020017).
The authors thank the support from Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. (2017) 474:1823–36. doi: 10.1042/BCJ20160510
2. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. (2010) 90:859–904. doi: 10.1152/physrev.00045.2009
3. Feng WW, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. (2019) 142:176–91. doi: 10.1016/j.phrs.2019.02.024
4. Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. (2021) 19:241–55. doi: 10.1038/s41579-020-00460-0
5. Fine RL, Manfredo Vieira S, Gilmore MS, Kriegel MA. Mechanisms and consequences of gut commensal translocation in chronic diseases. Gut Microbes. (2020) 11:217–30. doi: 10.1080/19490976.2019.1629236
6. Liu J, Tan YZ, Cheng H, Zhang DD, Feng WW, Peng C, et al. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. (2022) 13.
7. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
8. Cheng H, Liu J, Tan YZ, Feng WW, Peng C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J Pharm Anal. (2022). doi: 10.1016/j.jpha.2021.10.003
9. Zhang F, He F, Li L, Guo L, Zhang B, Yu S, et al. Bioavailability based on the gut microbiota: a new perspective. Microbiol Mol Biol Rev. (2020) 84:e00072. doi: 10.1128/MMBR.00072-19
10. Rechner AR, Smith MA, Kuhnle G, Gibson GR, Debnam ES, Srai SKS, et al. Colonic metabolism of dietary polyphenols: influence of structure on microbial fermentation products. Free Radic Biol Med. (2004) 36:212–25. doi: 10.1016/j.freeradbiomed.2003.09.022
11. Nishitsuji K, Watanabe S, Xiao J, Nagatomo R, Ogawa H, Tsunematsu T, et al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci Rep. (2018) 8:16173. doi: 10.1038/s41598-018-34571-9
12. Danneskiold-Samsøe NB, Barros HDDQ, Santos R, Bicas JL, Cazarin CBB, Madsen L, et al. Interplay between food and gut microbiota in health and disease. Food Res Int. (2019) 115:23–31. doi: 10.1016/j.foodres.2018.07.043
13. Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. (2019) 11:2393. doi: 10.3390/nu11102393
14. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. (2018) 57:1–24. doi: 10.1007/s00394-017-1445-8
15. Postler TS, Ghosh S. Understanding the Holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. (2017) 26:110–30. doi: 10.1016/j.cmet.2017.05.008
16. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. (2014) 121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
17. Canfora EE, Meex RC, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. (2019) 15:261–73. doi: 10.1038/s41574-019-0156-z
18. Feng WW, Ao H, Peng C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharmacol. (2018) 9:1354. doi: 10.3389/fphar.2018.01354
19. Perino A, Demagny H, Velazquez-Villegas L, Schoonjans K. Molecular physiology of bile acid signaling in health, disease, and aging. Physiol Rev. (2021) 101:683–731. doi: 10.1152/physrev.00049.2019
20. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. (2016) 24:41–50. doi: 10.1016/j.cmet.2016.05.005
21. Poland JC, Flynn CR. Bile acids, their receptors, and the gut microbiota. Physiology. (2021) 36:235–45. doi: 10.1152/physiol.00028.2020
22. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. (2019) 40:583–94. doi: 10.1093/eurheartj/ehy799
23. Zhang YX, Wang Y, Ke BB, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. (2021) 228:109–25. doi: 10.1016/j.trsl.2020.08.007
24. Yang SJ Li XY, Yang F, Zhao R, Pan XD, Liang JQ, et al. Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Front Pharmacol. (2019) 10:1360. doi: 10.3389/fphar.2019.01360
25. Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. (2019) 81:139–64. doi: 10.1146/annurev-physiol-020518-114455
26. White PJ, Newgard CB. Branched-chain amino acids in disease. Science. (2019) 363:582–3. doi: 10.1126/science.aav0558
27. Modoux M, Rolhion N, Mani S, Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol Sci. (2021) 42:60–73. doi: 10.1016/j.tips.2020.11.006
28. Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. (2018) 9:3294. doi: 10.1038/s41467-018-05470-4
29. Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
30. McCarville JL, Chen GY, Cuevas VD, Troha K, Ayres JS. Microbiota metabolites in health and disease. Annu Rev Immunol. (2020) 38:147–70. doi: 10.1146/annurev-immunol-071219-125715
31. Kalantar-Zadeh K, Berean KJ, Burgell RE, Muir JG, Gibson PR. Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat Rev Gastroenterol Hepatol. (2019) 16:733–47. doi: 10.1038/s41575-019-0193-z
32. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. (2007) 87:315–424. doi: 10.1152/physrev.00029.2006
33. Sen N. Functional and molecular insights of hydrogen sulfide signaling and protein sulfhydration. J Mol Biol. (2017) 429:543–61. doi: 10.1016/j.jmb.2016.12.015
34. Rudzki L, Stone T, Maes M, Misiak B, Samochowiec J, Szulc A, et al. Gut microbiota-derived vitamins-underrated powers of a multipotent ally in psychiatric health and disease. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 107:110240. doi: 10.1016/j.pnpbp.2020.110240
35. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. (2012) 336:1262–7. doi: 10.1126/science.1223813
36. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56:1761–72. doi: 10.2337/db06-1491
37. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. (2009) 106:3698–703. doi: 10.1073/pnas.0812874106
38. Waclawiková B, El Aidy S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals. (2018) 11:63. doi: 10.3390/ph11030063
39. Zhu Y, Shui X, Liang Z, Huang Z, Qi Y, He Y, et al. Gut microbiota metabolites as integral mediators in cardiovascular diseases. Int J Mol Med. (2020) 46:936–48. doi: 10.3892/ijmm.2020.4674
40. Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. (2014) 12:661–72. doi: 10.1038/nrmicro3344
41. Gibson JA, Sladen GE, Dawson AM. Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br J Nutr. (1976) 35:61–5. doi: 10.1079/BJN19760009
42. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health, J. Nutr Biochem. (2013) 24:1415–22. doi: 10.1016/j.jnutbio.2013.05.001
43. Feng WW, Liu J, Ao H, Yue SJ, Peng C. Targeting gut microbiota for precision medicine: focusing on the efficacy and toxicity of drugs. Theranostics. (2020) 10:11278. doi: 10.7150/thno.47289
44. Bazzocco S, Mattila I, Guyot S, Renard CM, Aura AM. Factors affecting the conversion of apple polyphenols to phenolic acids and fruit matrix to short-chain fatty acids by human faecal microbiota in vitro. Eur J Nutr. (2008) 47:442–52. doi: 10.1007/s00394-008-0747-2
45. Walker AW, Duncan SH, Leitch EC, Child MW, Flint HJ. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol. (2005) 71:3692–700. doi: 10.1128/AEM.71.7.3692-3700.2005
46. Chen F, Wen Q, Jiang J, Li HL, Tan YF, Li YH, et al. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. (2016) 179:253–64. doi: 10.1016/j.jep.2015.12.031
47. Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. (2009) 30:85–94. doi: 10.1016/j.tips.2008.11.002
48. Siviero A, Gallo E, Maggini V, Gori L, Mugelli A, Firenzuoli F, et al. Curcumin, a golden spice with a low bioavailability. J Herb Med. (2015) 5:57–70. doi: 10.1016/j.hermed.2015.03.001
49. An CY, Sun ZZ, Shen L, Ji HF. Biotransformation of food spice curcumin by gut bacterium Bacillus megaterium DCMB-002 and its pharmacological implications. Food Nutr Res. (2017) 61:1412814. doi: 10.1080/16546628.2017.1412814
50. Burapan S, Kim M, Han J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J Agric Food Chem. (2017) 65:3305–10. doi: 10.1021/acs.jafc.7b00943
51. Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, et al. Metabolism and anticancer activity of the curcumin analogue, dimethoxycurcumin. Clin Cancer Res. (2007) 13:1269–77. doi: 10.1158/1078-0432.CCR-06-1839
52. Gao H, Zhou HM, Yue SJ, Feng LM, Guo DY, Li JJ, et al. Oral bioavailability-enhancing and anti-obesity effects of hydroxysafflor yellow A in natural deep eutectic solvent. ACS Omega. (2022) 7:19225–34. doi: 10.1021/acsomega.2c00457
53. Ao H, Feng WW, Peng C. Hydroxysafflor yellow A: a promising therapeutic agent for a broad spectrum of diseases. Evid Based Complement Alternat Med. (2018) 2018:8259280. doi: 10.1155/2018/8259280
54. Liu J, Yue S, Yang Z, Feng W, Meng X, Wang A, et al. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol Res. (2018) 134:40–50. doi: 10.1016/j.phrs.2018.05.012
55. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
56. Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
57. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. (1987) 28:1221–7. doi: 10.1136/gut.28.10.1221
58. Wong JM, De Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. (2006) 40:235–43. doi: 10.1097/00004836-200603000-00015
59. Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. (2014) 20:1487–98. doi: 10.2174/13816128113199990462
60. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. doi: 10.1080/19490976.2015.1134082
61. Huang W, Guo HL, Deng X, Zhu TT, Xiong JF, Xu YH, et al. Short-chain fatty acids inhibit oxidative stress and inflammation in mesangial cells induced by high glucose and lipopolysaccharide. Exp Clin Endocrinol Diabetes. (2017) 125:98–105. doi: 10.1055/s-0042-121493
62. Fellows R, Denizot J, Stellato C, Cuomo A, Jain P, Stoyanova E, et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun. (2018) 9:105. doi: 10.1038/s41467-017-02651-5
63. van der Beek CM, Dejong CH, Troost FJ, Masclee AA, Lenaerts K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev. (2017) 75:286–305. doi: 10.1093/nutrit/nuw067
64. Fernández J, Redondo-Blanco S, Gutiérrez-del-Río I, Miguélez EM, Villar CJ, Lombó F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: a review. J Funct Foods. (2016) 25:511–22. doi: 10.1016/j.jff.2016.06.032
65. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
66. Smith EA, Macfarlane GT. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe. (1997) 3:327–37. doi: 10.1006/anae.1997.0121
67. Schneider H, Schwiertz A, Collins MD, Blaut M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch Microbiol. (1999) 171:81–91. doi: 10.1007/s002030050682
68. Jones JM. CODEX-aligned dietary fiber definitions help to bridge the 'fiber gap'. Nutr J. (2014) 13:1–10. doi: 10.1186/1475-2891-13-34
69. Levrat MA, Rémésy C, Demigné C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. J Nutr. (1991) 121:1730–7. doi: 10.1093/jn/121.11.1730
70. Wang M, Wichienchot S, He X, Fu X, Huang Q, Zhang B, et al. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci Tech. (2019) 88:1–9. doi: 10.1016/j.tifs.2019.03.005
71. Hu JL, Nie SP, Li C, Xie MY. In vitro fermentation of polysaccharide from the seeds of Plantago asiatica L. by human fecal microbiota Food Hydrocolloid. (2013) 33:384–92. doi: 10.1016/j.foodhyd.2013.04.006
72. Ye J, Wang X, Wang K, Deng Y, Yang Y, Ali R, et al. A novel polysaccharide isolated from Flammulina velutipes, characterization, macrophage immunomodulatory activities and its impact on gut microbiota in rats. J Anim Physiol Anim Nutr. (2020) 104:735–48. doi: 10.1111/jpn.13290
73. Zhao R, Hu Q, Ma G, Su A, Xie M, Li X, et al. Effects of Flammulina velutipes polysaccharide on immune response and intestinal microbiota in mice. J Funct Foods. (2019) 56:255–64. doi: 10.1016/j.jff.2019.03.031
74. Mou J, Li Q, Shi W, Qi X, Song W, Yang J, et al. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota. Carbohydr Polym. (2020) 228:115359. doi: 10.1016/j.carbpol.2019.115359
75. Di T, Chen G, Sun Y, Ou S, Zeng X, Ye H, et al. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J Funct Foods. (2018) 40:18–27. doi: 10.1016/j.jff.2017.10.040
76. Gu W, Wang Y, Zeng L, Dong J, Bi Q, Yang X, et al. Polysaccharides from Polygonatum kingianum improve glucose and lipid metabolism in rats fed a high fat diet. Biomed Pharmacother. (2020) 125:109910. doi: 10.1016/j.biopha.2020.109910
77. Huo W, Feng Z, Hu S, Cui L, Qiao T, Dai L, et al. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. (2020) 11:4291–303. doi: 10.1039/D0FO00597E
78. Tang C, Sun J, Zhou B, Jin C, Liu J, Kan J, et al. Effects of polysaccharides from purple sweet potatoes on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. (2018) 9:937–50. doi: 10.1039/C7FO01302G
79. Sun SS, Wang K, Ma K, Bao L, Liu HW. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin J Nat Med. (2019) 17:3–14. doi: 10.1016/S1875-5364(19)30003-2
80. Dou Z, Chen C, Fu X. Digestive property and bioactivity of blackberry polysaccharides with different molecular weights. J Agric Food Chem. (2019) 67:12428–40. doi: 10.1021/acs.jafc.9b03505
81. Zhong L, Ma N, Zheng H, Ma G, Zhao L, Hu Q, et al. Tuber indicum polysaccharide relieves fatigue by regulating gut microbiota in mice. J Funct Foods. (2019) 63:103580. doi: 10.1016/j.jff.2019.103580
82. Liu G, Liang L, Yu G, Li Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int J Biol Macromol. (2018) 115:711–7. doi: 10.1016/j.ijbiomac.2018.04.127
83. Li H, Fang Q, Nie Q, Hu J, Yang C, Huang T, et al. Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J Agric Food Chem. (2020) 68:10015–28. doi: 10.1021/acs.jafc.0c01968
84. Chen X, Cai B, Wang J, Sheng Z, Yang H, Wang D, et al. Mulberry leaf-derived polysaccharide modulates the immune response and gut microbiota composition in immunosuppressed mice. J Funct Foods. (2021) 83:104545. doi: 10.1016/j.jff.2021.104545
85. Yang C, Xu Z, Deng Q, Huang Q, Wang X, Huang F, et al. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res Int. (2020) 131:108994. doi: 10.1016/j.foodres.2020.108994
86. Li X, Guo R, Wu X, Liu X, Ai L, Sheng Y, et al. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr Polym. (2020) 239:116194. doi: 10.1016/j.carbpol.2020.116194
87. Zhu K, Fan H, Zeng S, Nie S, Zhang Y, Tan L, et al. Polysaccharide from Artocarpus heterophyllus Lam.(jackfruit) pulp modulates gut microbiota composition and improves short-chain fatty acids production. Food Chem. (2021) 364:130434. doi: 10.1016/j.foodchem.2021.130434
88. Li TT, Huang ZR, Jia RB, Lv XC, Zhao C, Liu B, et al. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res Int. (2021) 147:110530. doi: 10.1016/j.foodres.2021.110530
89. Su L, Mao C, Wang X, Li L, Tong H, Mao J, et al. The anti-colitis effect of Schisandra chinensis polysaccharide is associated with the regulation of the composition and metabolism of gut microbiota. Front Cell Infect Microbiol. (2020) 10:519479. doi: 10.3389/fcimb.2020.519479
90. Sun Y, Liu Y, Ai C, Song S, Chen X. Caulerpa lentillifera polysaccharides enhance the immunostimulatory activity in immunosuppressed mice in correlation with modulating gut microbiota. Food Funct. (2019) 10:4315–29. doi: 10.1039/C9FO00713J
91. Yang C, Wang X, Deng Q, Huang F. Rapeseed polysaccharides alleviate overweight induced by high-fat diet with regulation of gut microbiota in rats. Oil Crop Sci. (2021) 6:192–200. doi: 10.1016/j.ocsci.2021.09.001
92. Yao H, Wang L, Tang X, Yang Z, Li H, Sun C, et al. Two novel polysaccharides from Solanum nigrum L. exert potential prebiotic effects in an in vitro fermentation model. Int J Biol Macromol. (2020) 159:648–58. doi: 10.1016/j.ijbiomac.2020.05.121
93. Zou Q, Zhang X, Liu X, Li Y, Tan Q, Dan Q, et al. Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct. (2020) 11:6666–79. doi: 10.1039/D0FO01162B
94. Mao G, Li S, Orfila C, Shen X, Zhou S, Linhardt RJ, et al. Depolymerized RG-I-enriched pectin from citrus segment membranes modulates gut microbiota. Increases SCFA production, and promotes the growth of Bifidobacterium spp, Lactobacillus spp and Faecalibaculum spp. Food Funct. (2019) 10:7828–43. doi: 10.1039/C9FO01534E
95. Chen G, Xie M, Wan P, Chen D, Ye H, Chen L, et al. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. (2018) 244:331–9. doi: 10.1016/j.foodchem.2017.10.074
96. Zhou W, Yan Y, Mi J, Zhang H, Lu L, Luo Q, et al. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J Agric Food Chem. (2018) 66:898–907. doi: 10.1021/acs.jafc.7b05546
97. Wang S, Li Q, Zang Y, Zhao Y, Liu N, Wang Y, et al. Apple polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int J Biol Macromol. (2017) 99:282–92. doi: 10.1016/j.ijbiomac.2017.02.074
98. Duan M, Sun X, Ma N, Liu Y, Luo T, Song S, et al. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int J Biol Macromol. (2019) 121:996–1004. doi: 10.1016/j.ijbiomac.2018.10.087
99. Liu Y, Li Y, Ke Y, Li C, Zhang Z, Wu Y, et al. In vitro saliva-gastrointestinal digestion and fecal fermentation of Oudemansiella radicata polysaccharides reveal its digestion profile and effect on the modulation of the gut microbiota. Carbohydr Polym. (2021) 251:117041. doi: 10.1016/j.carbpol.2020.117041
100. Su A, Ma G, Xie M, Ji Y, Li X, Zhao L, et al. Characteristic of polysaccharides from Flammulina velutipes in vitro digestion under salivary, simulated gastric and small intestinal conditions and fermentation by human gut microbiota. Int J Food Sci Tech. (2019) 54:2277–87. doi: 10.1111/ijfs.14142
101. Ma Y, Jiang S, Zeng M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Res Int. (2021) 149:110646. doi: 10.1016/j.foodres.2021.110646
102. Liu Y, Duan X, Duan S, Li C, Hu B, Liu A, et al. Effects of in vitro digestion and fecal fermentation on the stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food Funct. (2020) 11:6899–910. doi: 10.1039/D0FO01430C
103. Shao X, Sun C, Tang X, Zhang X, Han D, Liang S, et al. Anti-inflammatory and intestinal microbiota modulation properties of Jinxiang garlic (Allium sativum L.) polysaccharides toward dextran sodium sulfate-induced colitis. J Agric Food Chem. (2020) 68:12295–309. doi: 10.1021/acs.jafc.0c04773
104. Xie J, Song Q, Yu Q, Chen Y, Hong Y, Shen M, et al. Dietary polysaccharide from Mung bean [Vigna radiate (Linn.) Wilczek] skin modulates gut microbiota and short-chain fatty acids in mice. Int J Food Sci Tech. (2022) 57:2581–9. doi: 10.1111/ijfs.15030
105. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20:159–66. doi: 10.1038/nm.3444
106. Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. (2018) 359:1151–6. doi: 10.1126/science.aao5774
107. Lyu M, Wang YF, Fan GW, Wang XY, Xu SY, Zhu Y, et al. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microb. (2017) 8:2146. doi: 10.3389/fmicb.2017.02146
108. Wu Z, Huang S, Li T, Li N, Han D, Zhang B, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. (2021) 9:1–22. doi: 10.1186/s40168-021-01115-9
109. Ikegami T, Honda A. Reciprocal interactions between bile acids and gut microbiota in human liver diseases. J Hepatol. (2020) 72:558–77. doi: 10.1111/hepr.13001
110. Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. (2017) 56:54–65. doi: 10.1016/j.mam.2017.06.002
111. de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Roles of bile acids in metabolism. Cell Metab. (2013) 17:657–69. doi: 10.1016/j.cmet.2013.03.013
112. Joyce SA, Gahan CG. Bile acid modifications at the microbe-host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu Rev Food Sci Technol. (2016) 7:e313–33. doi: 10.1146/annurev-food-041715-033159
113. Jia W, Wei M, Rajani C, Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. (2021) 12:411–25. doi: 10.1007/s13238-020-00804-9
114. Shishodia S. Molecular mechanisms of curcumin action: gene expression. BioFactors. (2013) 39:37–55. doi: 10.1002/biof.1041
115. Han Z, Yao L, Zhong Y, Xiao Y, Gao J, Zheng Z, et al. Gut microbiota mediates the effects of curcumin on enhancing Ucp1-dependent thermogenesis and improving high-fat diet-induced obesity. Food Funct. (2021) 12:6558–75. doi: 10.1039/D1FO00671A
116. Hui S, Liu Y, Chen M, Wang X, Lang H, Zhou M, et al. Capsaicin improves glucose tolerance and insulin sensitivity through modulation of the gut microbiota-bile acid-FXR axis in type 2 diabetic db/db mice. Mol Nutr Food Res. (2019) 63:e1900608. doi: 10.1002/mnfr.201900608
117. Hui S, Huang L, Wang X, Zhu X, Zhou M, Chen M, et al. Capsaicin improves glucose homeostasis by enhancing glucagon-like peptide-1 secretion through the regulation of bile acid metabolism via the remodeling of the gut microbiota in male mice. FASEB J. (2020) 34:8558–73. doi: 10.1096/fj.201902618RR
118. Hui S, Liu Y, Huang L, Zheng L, Zhou M, Lang H, et al. Resveratrol enhances brown adipose tissue activity and white adipose tissue browning in part by regulating bile acid metabolism via gut microbiota remodeling. Int J Obes. (2020) 44:1678–90. doi: 10.1038/s41366-020-0566-y
119. Papandreou C, Moré M, Bellamine A. Trimethylamine N-oxide in relation to cardiometabolic health-cause or effect? Nutrients. (2020) 12:1330. doi: 10.3390/nu12051330
120. Nam HS. Gut microbiota and ischemic stroke: the role of trimethylamine N-oxide. J Stroke. (2019) 21:151–9. doi: 10.5853/jos.2019.00472
121. Wang B, Qiu J, Lian J, Yang X, Zhou J. Gut metabolite trimethylamine-N-oxide in atherosclerosis: from mechanism to therapy. Front Cardiovasc Med. (2021) 8:723886. doi: 10.3389/fcvm.2021.723886
122. Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. (2018) 38:2225–35. doi: 10.1161/ATVBAHA.118.311023
123. Farhangi MA. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: findings from an updated systematic review and meta-analysis. Nutrition. (2020) 78:110856. doi: 10.1016/j.nut.2020.110856
124. Li J, Li Y, Ivey KL, Wang DD, Wilkinson JE, Franke A, et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut. (2022) 71:724–33. doi: 10.1136/gutjnl-2020-322473
125. Skagen K, Trøseid M, Ueland T, Holm S, Abbas A, Gregersen I, et al. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis. (2016) 247:64–9. doi: 10.1016/j.atherosclerosis.2016.01.033
126. Thomas MS, Fernandez ML. Trimethylamine N-oxide (TMAO), diet and cardiovascular disease. Curr Atheroscler Rep. (2021) 23:12. doi: 10.1007/s11883-021-00910-x
127. Liu S, You L, Zhao Y, Chang X. Wild Lonicera caerulea berry polyphenol extract reduces cholesterol accumulation and enhances antioxidant capacity in vitro and in vivo. Food Res Int. (2018) 107:73–83. doi: 10.1016/j.foodres.2018.02.016
128. Chen PY, Li S, Koh YC, Wu JC, Yang MJ, Ho CT, et al. Oolong tea extract and citrus peel polymethoxyflavones reduce transformation of l-carnitine to trimethylamine-N-oxide and decrease vascular inflammation in l-carnitine feeding mice. J Agric Food Chem. (2019) 67:7869–79. doi: 10.1021/acs.jafc.9b03092
129. Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. (2016) 7:e02210–15. doi: 10.1128/mBio.02210-15
130. Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. (2018) 19:954. doi: 10.3390/ijms19040954
131. Batch BC, Hyland K, Svetkey LP. Branch chain amino acids: Biomarkers of health and disease. Curr Opin Clin Nutr Metab Care. (2014) 17:86–9.
132. Amorim Franco TM, Blanchard JS. Bacterial branched-chain amino acid biosynthesis: structures, mechanisms, and drugability. Biochemistry. (2017) 56:5849–65. doi: 10.1021/acs.biochem.7b00849
133. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. (2016) 535:376–81. doi: 10.1038/nature18646
134. Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. (2018) 596:623–45. doi: 10.1113/JP275075
135. Zhao Z, Shi A, Wang Q, Zhou J. High oleic acid peanut oil and extra virgin olive oil supplementation attenuate metabolic syndrome in rats by modulating the gut microbiota. Nutrients. (2019) 11:3005. doi: 10.3390/nu11123005
136. Zhang L, Yue Y, Shi M, Tian M, Ji J, Liao X, et al. Dietary Luffa cylindrica (L.) Roem promotes branched-chain amino acid catabolism in the circulation system via gut microbiota in diet-induced obese mice. Food Chem. (2020) 320:126648. doi: 10.1016/j.foodchem.2020.126648
137. Zeng SL, Li SZ, Xiao PT, Cai YY, Chu C, Chen BZ, et al. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci Adv. (2020) 6, eaax6208. doi: 10.1126/sciadv.aax6208
138. Zhang J, Zhu S, Ma N, Johnston LJ, Wu C, Ma X, et al. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: a therapeutic target to control intestinal inflammation. Med Res Rev. (2021) 41:1061–88. doi: 10.1002/med.21752
139. Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. (2018) 11:1024–38. doi: 10.1038/s41385-018-0019-2
140. Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. (2021) 70:1174–82. doi: 10.1136/gutjnl-2020-323071
141. Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. (2021) 22:2973. doi: 10.3390/ijms22062973
142. Yin J, Zhang B, Yu Z, Hu Y, Lv H, Ji X, et al. Ameliorative effect of dietary tryptophan on neurodegeneration and inflammation in d-galactose-induced aging mice with the potential mechanism relying on AMPK/SIRT1/PGC-1α pathway and gut microbiota. J Agric Food Chem. (2021) 69:4732–44. doi: 10.1021/acs.jafc.1c00706
143. Lamas B, Hernandez-Galan L, Galipeau HJ, Constante M, Clarizio A, Jury J, et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci Transl Med. (2020) 12:eaba0624. doi: 10.1126/scitranslmed.aba0624
144. Yusufu I, Ding K, Smith K, Wankhade UD, Sahay B, Patterson GT, et al. A tryptophan-deficient diet induces gut microbiota dysbiosis and increases systemic inflammation in aged mice. Int J Mol Sci. (2021) 22:5005. doi: 10.3390/ijms22095005
145. Yang C, Du Y, Ren D, Yang X, Zhao Y. Gut microbiota-dependent catabolites of tryptophan play a predominant role in the protective effects of turmeric polysaccharides against DSS-induced ulcerative colitis. Food Funct. (2021) 12:9793–807. doi: 10.1039/D1FO01468D
146. Yang W, Ren D, Zhao Y, Liu L, Yang X. Fuzhuan brick tea polysaccharide improved ulcerative colitis in association with gut microbiota-derived tryptophan metabolism. J Agric Food Chem. (2021) 69:8448–59. doi: 10.1021/acs.jafc.1c02774
147. Li Y, Xu W, Zhang F, Zhong S, Sun Y, Huo J, et al. The gut microbiota-produced indole-3-propionic acid confers the antihyperlipidemic effect of mulberry-derived 1-deoxynojirimycin. Msystems. (2020) 5:e00313–20. doi: 10.1128/mSystems.00313-20
148. Peron G, Meroño T, Gargari G, Hidalgo-Liberona N, Miñarro A, Lozano EV, et al. A polyphenol-rich diet increases the gut microbiota metabolite indole 3-propionic acid in older adults with preserved kidney function. Mol Nutr Food Res. (2022) 2022:e2100349. doi: 10.1002/mnfr.202100349
149. Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. (2007) 102:837–41. doi: 10.1111/j.1572-0241.2007.01072.x
150. Bredt DS. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic Res. (1999) 31:577–96. doi: 10.1080/10715769900301161
151. Buret AG, Allain T, Motta JP, Wallace JL. Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid Redox Signal. (2022) 36:211–9. doi: 10.1089/ars.2021.0004
152. Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. (2012) 9:504–18. doi: 10.1038/nrgastro.2012.85
153. Yao CK, Muir JG, Gibson PR. Insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. (2016) 43:181–96. doi: 10.1111/apt.13456
154. Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. (2009) 284:22457–66. doi: 10.1074/jbc.M109.010868
155. Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. (2009) 11:703–14. doi: 10.1089/ars.2008.2253
156. Marthinsen D, Fleming SE. Excretion of breath and flatus gases by humans consuming high-fiber diets. J Nutr. (1982) 112:1133–43. doi: 10.1093/jn/112.6.1133
157. Skoog SM, Bharucha AE, Zinsmeister AR. Comparison of breath testing with fructose and high fructose corn syrups in health and IBS. J Neurogastroenterol Motil. (2008) 20:505–11. doi: 10.1111/j.1365-2982.2007.01074.x
158. Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. (2010) 25:1366–73. doi: 10.1111/j.1440-1746.2010.06370.x
159. Bond JH, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man-indirect method of studying in-situ metabolism of methane-producing colonic bacteria. J Exp Med. (1971) 133:572–88. doi: 10.1084/jem.133.3.572
160. Li X, Zhang Y, Zhao C, Zhang B, Peng B, Zhang Y, et al. Positive effects of Epigallocatechin-3-gallate (EGCG) intervention on insulin resistance and gut microbial dysbiosis induced by bisphenol A. J Funct Foods. (2022) 93:105083. doi: 10.1016/j.jff.2022.105083
161. Bai X, Fu R, Duan Z, Wang P, Zhu C, Fan D, et al. Ginsenoside Rk3 alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Res Int. (2021) 146:110465. doi: 10.1016/j.foodres.2021.110465
162. Li Y, Xie Z, Gao T, Li L, Chen Y, Xiao D, et al. A holistic view of gallic acid-induced attenuation in colitis based on microbiome-metabolomics analysis. Food Funct. (2019) 10:4046–61. doi: 10.1039/C9FO00213H
163. Xie MG, Fei YQ, Wang Y, Wang WY, Wang Z. Chlorogenic acid alleviates colon mucosal damage induced by a high-fat diet via gut microflora adjustment to increase short-chain fatty acid accumulation in rats. Oxid Med Cell Longev. (2021) 2021:3456542. doi: 10.1155/2021/3456542
164. Li J, Chen C, Yang H, Yang X. Tea polyphenols regulate gut microbiota dysbiosis induced by antibiotic in mice. Food Res Int. (2021) 141:110153. doi: 10.1016/j.foodres.2021.110153
165. Peng Y, Yan Y, Wan P, Chen D, Ding Y, Ran L, et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic Biol Med. (2019) 136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005
166. Morissette A, Kropp C, Songpadith JP, Junges Moreira R, Costa J, Mariné-Casadó R, et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am J Physiol Endocrinol Metab. (2020) 318:E965–80. doi: 10.1152/ajpendo.00560.2019
167. Huang G, Wang Z, Wu G, Zhang R, Dong L, Huang F, et al. Lychee (Litchi chinensis Sonn.) pulp phenolics activate the short-chain fatty acid-free fatty acid receptor anti-inflammatory pathway by regulating microbiota and mitigate intestinal barrier damage in dextran sulfate sodium-induced colitis in mice. J Agric Food Chem. (2021) 69:3326–39. doi: 10.1021/acs.jafc.0c07407
168. Xu Y, Cao X, Zhao H, Yang E, Wang Y, Cheng N, et al. Impact of Camellia japonica bee pollen polyphenols on hyperuricemia and gut microbiota in potassium oxonate-induced mice. Nutrients. (2021) 13:2665. doi: 10.3390/nu13082665
169. Kuang J, Zheng X, Huang F, Wang S, Li M, Zhao M, et al. Anti-adipogenic effect of theabrownin is mediated by bile acid alternative synthesis via gut microbiota remodeling. Metabolites. (2020) 10:475. doi: 10.3390/metabo10110475
170. Dong S, Zhu M, Wang K, Zhao X, Hu L, Jing W, et al. Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism. Pharmacol Res. (2021) 171:105767. doi: 10.1016/j.phrs.2021.105767
171. Ushiroda C, Naito Y, Takagi T, Uchiyama K, Mizushima K, Higashimura Y, et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J Clin Biochem Nutr. (2019) 65:34–46. doi: 10.3164/jcbn.18-116
172. Liu F, Wang X, Li D, Cui Y, Li X. Apple polyphenols extract alleviated dextran sulfate sodium-induced ulcerative colitis in C57BL/6 male mice by restoring bile acid metabolism disorder and gut microbiota dysbiosis. Phytother Res. (2021) 35:1468–85. doi: 10.1002/ptr.6910
173. Panyod S, Wu WK, Chen PC, Chong KV, Yang YT, Chuang HL, et al. Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes. (2022) 8:4. doi: 10.1038/s41522-022-00266-3
174. Liu J, Zhang T, Wang Y, Si C, Wang X, Wang RT, et al. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging (Albany NY). (2020) 12:3791. doi: 10.18632/aging.102846
175. Cheng H, Liu J, Zhang D, Wang J, Tan Y, Feng W, et al. Ginsenoside Rg1 alleviates acute ulcerative colitis by modulating gut microbiota and microbial tryptophan metabolism. Front Immunol. (2022) 13:817600. doi: 10.3389/fimmu.2022.817600
176. Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. (2010) 107:12204–9. doi: 10.1073/pnas.0909122107
177. Koh A, Molinaro A, Ståhlman M, Khan MT, Schmidt C, Mannerås-Holm L, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. (2018) 175:947–61. doi: 10.1016/j.cell.2018.09.055
178. Guo CJ, Chang FY, Wyche TP, Backus KM, Acker TM, Funabashi M, et al. Discovery of reactive microbiota-derived metabolites that inhibit host proteases. Cell. (2017) 168:517–26. doi: 10.1016/j.cell.2016.12.021
179. Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. (2019) 7:1–15. doi: 10.1186/s40168-019-0704-8
180. Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. (2019) 10:1–17. doi: 10.1038/s41467-019-09735-4
181. Saito Y, Sato T, Nomoto K, Tsuji H. Identification of phenol-and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol Ecol. (2018) 94:fiy125. doi: 10.1093/femsec/fiy125
182. Andriamihaja M, Lan A, Beaumont M, Audebert M, Wong X, Yamada K, et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic Biol Med. (2015) 85:219–27. doi: 10.1016/j.freeradbiomed.2015.04.004
183. Bermudez-Martin P, Becker JA, Caramello N, Fernandez SP, Costa-Campos R, Canaguier J, et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. (2021) 9:1–23. doi: 10.1186/s40168-021-01103-z
184. Teufel R, Mascaraque V, Ismail W, Voss M, Perera J, Eisenreich W, et al. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci USA. (2010) 107:14390–5. doi: 10.1073/pnas.1005399107
185. Walker A, Schmitt-Kopplin P. The role of fecal sulfur metabolome in inflammatory bowel diseases. Int J Med Microbiol. (2021) 311:151513. doi: 10.1016/j.ijmm.2021.151513
186. Ma W, Chen K, Li Y, Hao N, Wang X, Ouyang P, et al. Advances in cadaverine bacterial production and its applications. Engineering. (2017) 3:308–17. doi: 10.1016/J.ENG.2017.03.012
187. Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C, et al. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. (2014) 817:221–39. doi: 10.1007/978-1-4939-0897-4_10
188. Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok JA, et al. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol. (1998) 27:299–310. doi: 10.1046/j.1365-2958.1998.00676.x
189. Portune KJ, Beaumont M, Davila AM, Tomé D, Blachier F, Sanz Y, et al. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci Tech. (2016) 57:213–32. doi: 10.1016/j.tifs.2016.08.011
190. Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. (2018) 1693:128–33. doi: 10.1016/j.brainres.2018.03.015
191. San-Cristobal R, Navas-Carretero S, Martínez-González MÁ, Ordovas JM, Martínez JA. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. (2020) 16:305–20. doi: 10.1038/s41574-020-0346-8
192. Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, et al. microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS ONE. (2014) 9:e90702. doi: 10.1371/journal.pone.0090702
193. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, et al. Personalized nutrition by prediction of glycemic responses. Cell. (2015) 163:1079–94. doi: 10.1016/j.cell.2015.11.001
194. Li S, Zhuge A, Wang K, Lv L, Bian X, Yang L, et al. Ketogenic diet aggravates colitis, impairs intestinal barrier and alters gut microbiota and metabolism in DSS-induced mice. Food Func. (2021) 12:10210–25. doi: 10.1039/D1FO02288A
Keywords: dietary compounds, gut microbiota-derived metabolites, short-chain fatty acids, bile acids, trimethylamine, branched-chain amino acids, tryptophan and indole derivatives
Citation: Feng W, Liu J, Cheng H, Zhang D, Tan Y and Peng C (2022) Dietary compounds in modulation of gut microbiota-derived metabolites. Front. Nutr. 9:939571. doi: 10.3389/fnut.2022.939571
Received: 09 May 2022; Accepted: 24 June 2022;
Published: 19 July 2022.
Edited by:
Guifang Tian, Agricultural University of Hebei, ChinaReviewed by:
Gian Carlo Tenore, University of Naples Federico II, ItalyCopyright © 2022 Feng, Liu, Cheng, Zhang, Tan and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhu Tan, dGFueXV6aHVAY2R1dGNtLmVkdS5jbg==; Cheng Peng, cGVuZ2NoZW5nY3h5QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.