
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 17 June 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.938829
This article is part of the Research Topic Advances in Anthocyanins: Sources, Preparation, Analysis Methods, Bioavailability, Physiochemical Properties, and Structural Features View all 5 articles
Although evidence shows that anthocyanins present promising health benefits, their poor stability still limits their applications in the food industry. Increasing the stability of anthocyanins is necessary to promote their absorption and metabolism and improve their health benefits. Numerous encapsulation approaches have been developed for the targeted release of anthocyanins to retain their bioactivities and ameliorate their unsatisfactory stability. Generally, choosing suitable edible encapsulation materials based on biopolymers is important in achieving the expected goals. This paper presented an ambitious task of summarizing the current understanding and challenges of biopolymer-based anthocyanin encapsulation in detail. The food-grade edible microencapsulation materials, especially for proteins and polysaccharides, should be employed to improve the stability of anthocyanins for effective application in the food industry. The influence factors involved in anthocyanin stability were systematically reviewed and highlighted. Food-grade proteins, especially whey protein, caseinate, gelatin, and soy protein, are attractive in the food industry for encapsulation owing to the improvement of stability and their health benefits. Polysaccharides, such as starch, pectin, chitosan, cellulose, mucilages, and their derivatives, are used as encapsulation materials because of their satisfactory biocompatibility and biodegradability. Moreover, the challenges and perspectives for the application of anthocyanins in food products were presented based on current knowledge. The proposed perspective can provide new insights into the amelioration of anthocyanin bioavailability by edible biopolymer encapsulation.
- The interactions between food matrix and anthocyanins were discussed in detail.
- The influence factors involved in the stability of anthocyanins were introduced.
- Performance of proteins or/and polysaccharides-based encapsulation was concluded.
- Advantages of protein-polysaccharide systems for encapsulation were summarized.
Recently, numerous studies have updated the current understanding of the health-promoting effects of dietary polyphenols and related food products (1–3). As an important and well-considered type of polyphenol, non-toxic water-soluble anthocyanins contribute to food color and present a wide range of biological activities, including antibacterial, anti-inflammatory, anti-diabetic, anti-obesity, and anticancer effects (4–6). However, the low stability and non-targeted release of anthocyanins have become the main obstacles in realizing their biological benefits in food systems (7, 8).
The main challenges for the application of anthocyanins in the food industry are how to decrease anthocyanin loss and control anthocyanin reaction to obtain more products with high stability (9, 10). Encapsulation systems can introduce physical protection for anthocyanins to achieve the stimulus-responsive controlled release and site-specific delivery of anthocyanins (11, 12). Many encapsulation methods have been performed for the controlled release of anthocyanins to overcome the poor stability, oral bioavailability, and intestinal absorption of anthocyanins.
In addition to delivery techniques or carriers, various cross-linked biopolymers have also been studied for anthocyanin encapsulation (13, 14). Suitable encapsulation materials are important for achieving the expected performance of anthocyanins. Undoubtedly, only edible materials can be developed for the delivery of anthocyanins in food applications (10, 15). Edible biopolymer-based systems, including proteins and carbohydrates, are preferred for anthocyanin encapsulation (16, 17).
Encapsulated systems based on protein or/and polysaccharide particles can protect anthocyanins in food products during storage and retain the bioavailability of anthocyanins within the gastrointestinal tract (18, 19). In this perspective, the current understanding of biopolymer-based anthocyanin encapsulation is presented in this paper in detail. The influence factors involved in anthocyanin stability are introduced, and the properties and performances of anthocyanins encapsulated by proteins or/and polysaccharide-based systems are summarized in detail. Moreover, the challenges and future perspectives of the application of anthocyanins in food products are highlighted. Retaining the bioavailability of anthocyanins by means of edible biopolymers encapsulation can provide much information for the promising application of anthocyanins in food products.
Anthocyanins have a carbon skeleton made up of C6–C3–C6 unit (xanthine cation) and are composed of anthocyanidin (aglycone units) linked to sugar, which is usually located at the 3-position on the C-ring and methoxyl and hydroxyl groups (20), as shown in Figure 1. However, the stability of anthocyanins is strongly related to the substitution pattern in the B-ring; the stability can be improved with the increase in methoxyl group or deteriorate with the increase in hydroxyl group. Glycosylation and acylation can improve the stability of anthocyanins (21, 22).
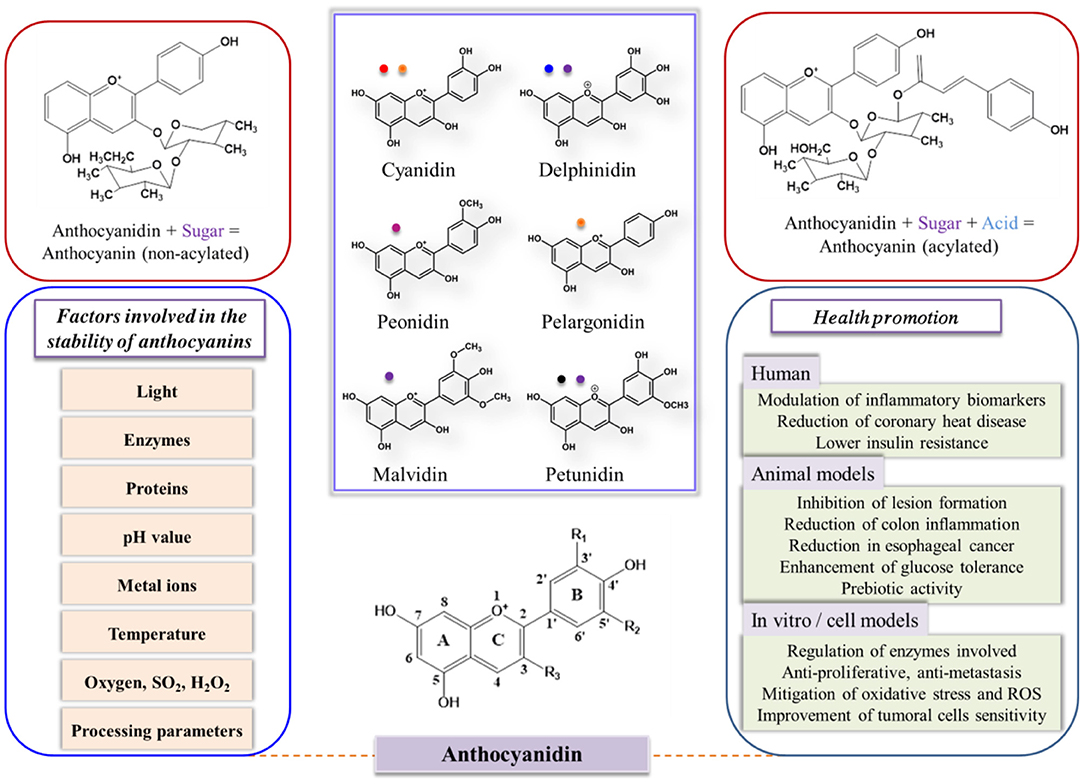
Figure 1. Structures, main colors involved, and bioactivity of six important food anthocyanidins, as well as factors affecting the stability of anthocyanins. R1, R2 = H or OH; R3 = H or glucose. The parameters were adapted from (12, 21, 23–27) with permission.
In general, the application of anthocyanins as food additives is seriously limited by their instability. The absorption of anthocyanins is small in comparison with the dietary consumption of anthocyanins, indicating the low bioavailability of anthocyanins (28, 29). Anthocyanins may easily be degraded in vivo before reaching the target locations because of the harsh environment. As shown in Figure 1, anthocyanin stability can be easily impacted by pH, structure, enzymes, light, temperature, oxygen, solvents, concentrations, and other compounds that can interact with anthocyanins (12, 24). All of these factors restrict the wide applications of anthocyanins, because anthocyanins are extremely unstable and can easily degrade. Hence, the industrialized applications of anthocyanins in food products are challenging.
To date, although evidence shows that anthocyanins present promising health benefits, their poor stability still limits their applications in food industry. Foods containing anthocyanins can only enter the bloodstream for further absorption and metabolism after reaching the gut lumen (30). Therefore, increasing the stability of anthocyanins is necessary to promote their absorption and metabolism and improve their health benefits. Encapsulated delivery systems have been reported to protect anthocyanins from adverse environmental conditions (31–34).
Although several wall materials can be employed for encapsulation, some properties, such as affinity, film-forming ability, degradability, intestinal resistance, and viscosity, should be optimized before the selection of wall materials (23, 35). Edible wall materials can be made from gum, protein, polysaccharides (natural or modified), and synthetic polymers (36, 37). The food-grade proteins and polysaccharides that are generally recognized as suitable materials for food products are shown in Table 1. Therefore, edible microencapsulation materials, especially for proteins and polysaccharides, should be clarified to improve the stability of anthocyanins for effective application in the food industry (Figure 2).
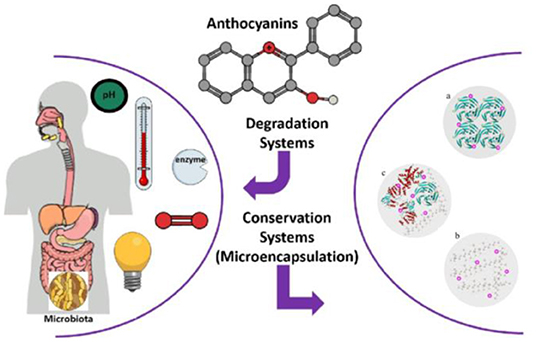
Figure 2. Schematics of anthocyanin degradation and biopolymer-based microencapsulation systems (a-protein particles, b-polysaccharide particles, and c-protein plus polysaccharide particles). Reprinted or adapted from references (12, 32–34) with permission.
Food-grade proteins, especially whey protein, caseinate, gelatin, and soy protein, are attractive in the food industry owing to their health benefits. Their functional properties, including gelation, emulsification, and binding capacity, support their use as alternatives in the development of anthocyanin delivery systems (46, 47). In addition, proteins' hydrophobic region can interact with the benzene ring of anthocyanins. The carbonyl and amine groups of proteins form hydrogen bonds with the hydrophilic region of anthocyanins (48).
Whey proteins could be developed as wall materials to deliver anthocyanins with enhanced bioavailability (21, 35). Whey protein microgels as an anthocyanin encapsulation material can dissolve rapidly in the gastrointestinal tract and form liquid particles that impede anthocyanin release and degradation (49). The interactions between whey proteins and anthocyanins affect the color and heat/light stability of anthocyanins. The encapsulation of anthocyanins from blackcurrant using whey protein via spray drying or freeze drying has been suggested to develop nutritional food products (50). The encapsulation of anthocyanins from sour cherry skins using whey proteins with suitable encapsulation efficiency (over 70%) decreases gastric digestion and thus presents a potential as a functional matrix for food products (51).
Whey protein, casein, and soy protein isolates are efficient for improving anthocyanin bioavailability (Table 1). Casein and whey protein have been used as wall materials to encapsulate blueberry anthocyanins using spray drying technique. Anthocyanin encapsulation is helpful in decreasing the rapid release and degradation of anthocyanin, especially during digestion in simulated gastric fluid. However, casein and whey protein showed different protection mechanisms as shown in Figure 3. The formation of casein–anthocyanin microparticles with poor solubility effectively inhibited the release and degradation of anthocyanins. The highly soluble whey protein–anthocyanin microparticles had decreased anthocyanin release. Casein and whey protein isolate could be employed to hinder the release of encapsulated anthocyanins, indicating that the proteins' physicochemical properties and structural changes caused by digestion contributed to anthocyanin delivery. Obviously, the individual digestion behaviors of different proteins or composites as wall materials for anthocyanin encapsulation should be investigated in future research. The conformational change of the amphiphilic peptides of 18 amino acids (C6M1) from an α-helical structure to a β-sheet structure was caused by co-assembly when used for anthocyanin encapsulation (Figure 4). The C6M1 peptide improved the resistance of anthocyanin to pH, high temperature, and metallic ions and improved the bioactivity for scavenging free radicals (52).
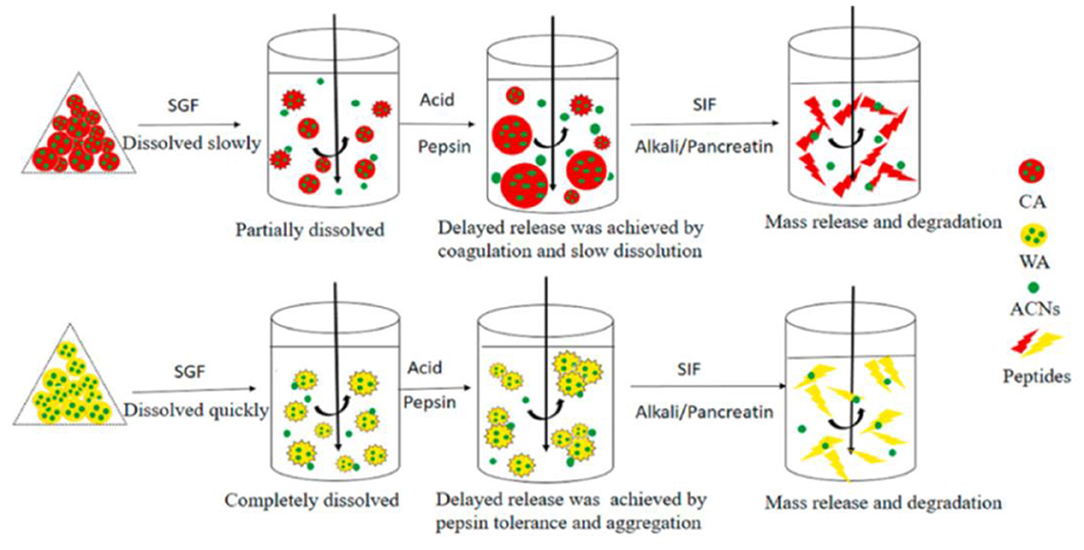
Figure 3. Release mechanisms of CA-ACN and WA-ACN microparticles during in vitro digestion. Reproduced from reference (36) with permission. CA, casein; WA, Whey protein isolate; ACN, anthocyanin; SGF, simulated gastric fluid; SIF, simulated intestinal fluid.
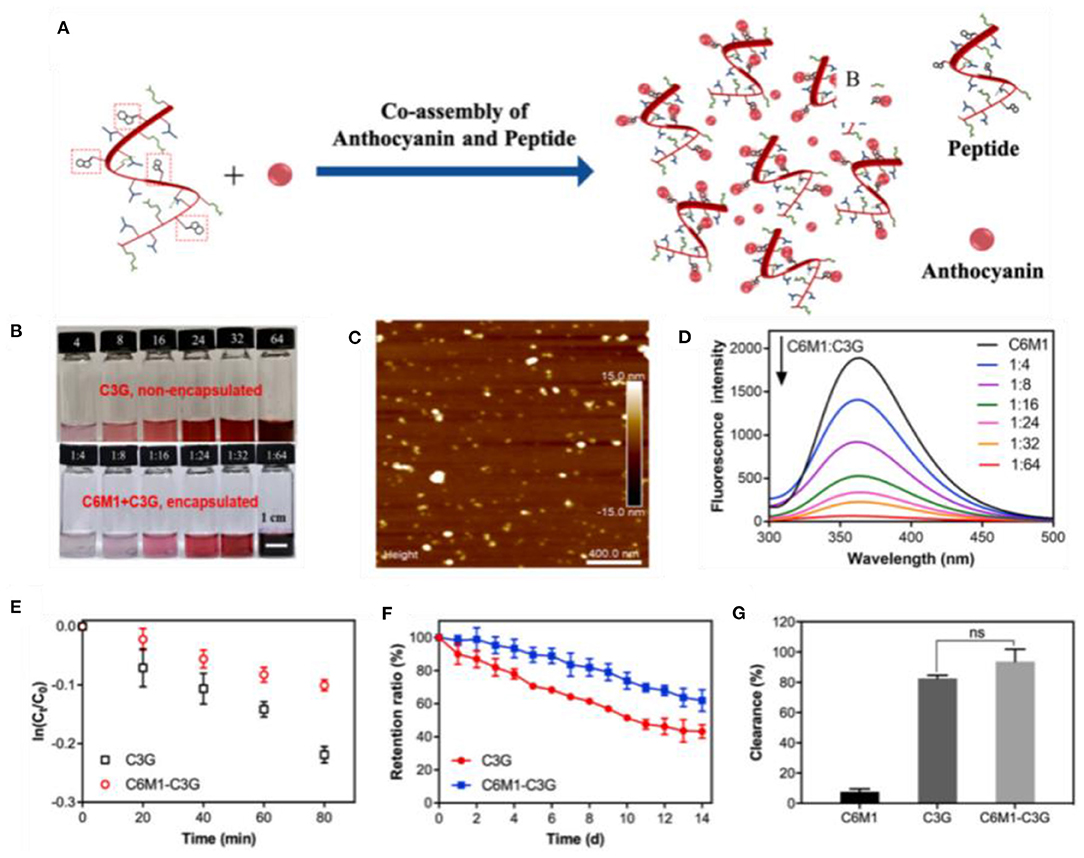
Figure 4. (A) Schematic of the co-assembly of the peptide (C6M1) and anthocyanin (C3G) into a nanocomposite. (B) Encapsulated and non-encapsulated anthocyanins. (C) Atomic force microscopy image and (D) fluorescence spectra of C6M1–C3G nanocomposites. (E) Thermal stability (80 °C), (F) retention ratio during storage (25 °C), and (G) activity tests of C3G and C6M1–C3G. Reproduced from reference (52) with permission.
Although anthocyanin–protein interactions have been extensively studied, many parameters still need to be evaluated (53). The chemical structures of anthocyanins contribute to binding affinity. Moreover, different anthocyanins may produce different binding forces with proteins; hence, binding affinity to specific anthocyanins should be explored (54). The protein concentrations used in combination with anthocyanins still need to be optimized because they influence the rheological and sensory properties of anthocyanin–protein complexes, which are crucial parameters for food and beverage products (21).
Polysaccharides, such as starch, pectin, chitosan, cellulose, mucilages, and their derivatives, are used as encapsulation materials because of their satisfactory biocompatibility and biodegradability (55). The performances of starch and its derivatives have been evaluated for anthocyanin encapsulation. Non-toxic and biodegradable chitosan has been widely utilized for anthocyanin encapsulation. Anthocyanin–chitosan nanoparticles are formed via non-covalent bonds (e.g., weak ionic binding and hydrogen binding) (56). As reported, dual coating with chitosan and polyanionic polysaccharide to stabilize anthocyanins had high encapsulation efficacy and achieved resistance against auto-oxidation, heat, ascorbic acid, and neutral environment (57).
In addition, as the most widely reported cyclic oligosaccharide material, cyclodextrin can form complexes with anthocyanins through hydrogen bonding and hydrophobic interactions (58). Maltodextrin is also commonly introduced in the food industry as a wall material. The dextrose equivalent of maltodextrin is of paramount importance for retaining the stability and other properties of anthocyanins (59). Short-chain maltodextrin with high dextrose equivalent resulted in browning, hygroscopicity, and solubility. However, maltodextrin with s a higher dextrose equivalent showed better performance in retarding anthocyanin degradation (60, 61).
The combination of xanthan gum and carboxymethyl starch produced a high encapsulation efficiency (over 96%) and contributed to the stability of blueberry anthocyanins (62). The co-encapsulation of blackberry juice and Lactobacillus acidophilus by gum arabic–maltodextrin could be effective to protect anthocyanins and probiotic bacteria (63). In addition, alginate–pectin hydrogel particles have been reported to encapsulate blueberry anthocyanins with high encapsulation efficiency (116%) (64).
Covalent interaction is the main pathway that contributes to the interactions between proteins and polysaccharides. Several factors affect covalent interactions, such as intrinsic factors, including free amino groups, carbonyl groups, molecular structure, hydrophilicity, and hydrophobicity. Similarly, extrinsic factors, such as pressure, temperature, processing methods (i.e., microwave, ultrasonic, and pulsed electric field), crosslinkers, and the molar ratio between biopolymers, affect the interactions between proteins and polysaccharides.
The covalent bonds formed by proteins and polysaccharides are involved in the enhancement of the stability and impediment of anthocyanin release in harsh environments (65). During this process, polysaccharides and proteins or peptides form electrostatic complexes by opposite charges under particular pH conditions. The covalent bonds can be achieved via chemical cross-linking or Maillard reactions. Anthocyanins interact with proteins via hydrophobic interactions and hydrogen bonds because of the high affinity between anthocyanins and proteins (13, 66). Afterward, the loaded proteins can be cross-linked by electrostatic interaction with oppositely charged polysaccharides to form double polymers (67, 68).
Electrostatic interactions between differently charged acrosome molecules lead to the formation of protein–polysaccharide complexes. This technique consists of two parts: the phase separation of biopolymer mixtures and the subsequent deposition of a cohesive phase near the active ingredients (69, 70). Three main steps, namely, the solubilization of biopolymers, mixing the biopolymers with appropriate proportions, and the acidification of the medium, are required to form complexes. Moreover, the acidification phase is critical because it strongly affects the complex dimensions of formation (32).
The biopolymers formed by proteins or peptides and polysaccharides are promising for anthocyanin encapsulation because they could achieve high loading capacity and encapsulation efficiency and controlled release (71). Whey protein, gum arabic, and maltodextrin have been employed for anthocyanin extract encapsulation using freeze drying with encapsulation efficiency over 82%; they could reduce anthocyanin degradation during heat processing (72). Moreover, the biopolymer particles fabricated with beet pectin and whey protein have been used to encapsulate anthocyanins to improve their heat stability (31). Anthocyanins from elderberry were encapsulated through whey proteins and pectin with high encapsulating efficiency (98%), and the remarkable anti-oxidation of the system highlighted the potential utilization of the microcapsules in food products (41).
As shown, the biopolymers of proteins and polysaccharides for anthocyanin encapsulation can be formed by covalent interactions and non-covalent complexations, and the possible factors that might be involved in the formation are summarized in previous studies (73). In comparison with the anthocyanin encapsulation based on proteins or polysaccharides, the protein-polysaccharide systems for anthocyanin encapsulation are comparable or more excellent for the improvement of stability in harsh environments and may overcome the limitation of single utilization (9, 74).
The strategy of anthocyanin encapsulation has presented functionalities in improving stability, increasing gastric residence time, and targeting release to enhance anthocyanin uptake and absorption by the formation of nanogels, microgels, microparticles, or emulsion systems (17, 75). The protein- and polysaccharide-based biopolymers for anthocyanin encapsulation (Figure 5) provide new insights for further research on how to protect anthocyanins against the external harsh environment by the utilization of environmentally friendly biopolymers.
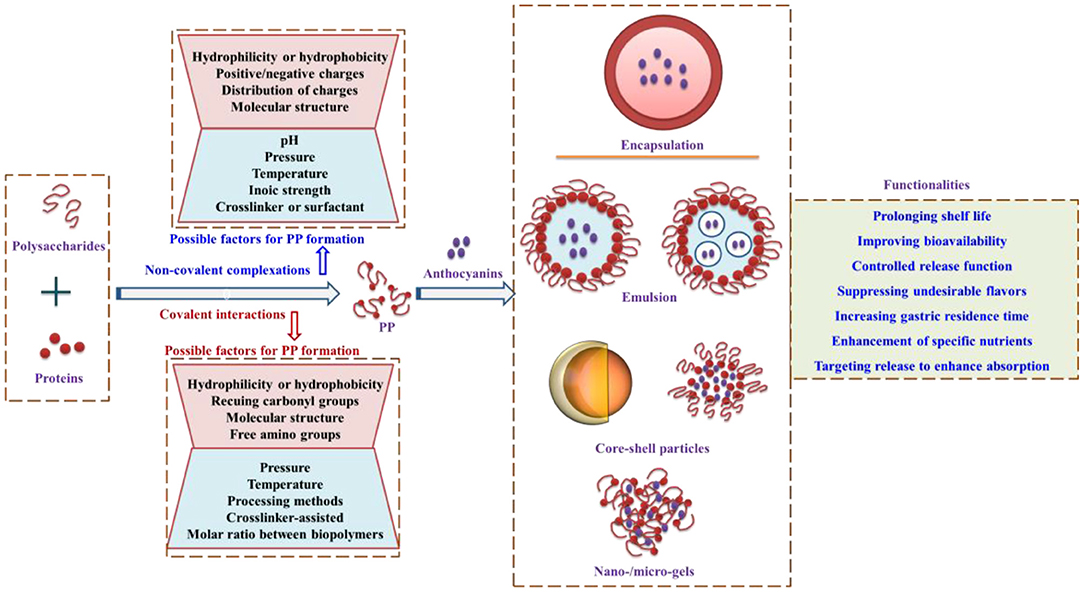
Figure 5. Biopolymers formed by proteins and polysaccharides for anthocyanin encapsulation, the possible factors that affect biopolymer formation, and the functionality of encapsulation. PP, Protein–polysaccharides. Adapted from reference (57, 73, 76–78) with permission.
The absorption and excretion of anthocyanins are associated with many factors; among which, the food matrix's effects are important to maintain the bioactivities of dietary anthocyanins (79, 80). As important parameters, the non-covalent interactions of anthocyanins with proteins, and/or carbohydrates have attracted intensive research attention (81). These interactions with macronutrients, which are driven by van der Waals interactions, hydrogen bond, and hydrophobic interaction, could affect anthocyanins' properties, including bioavailability and radical scavenging (82).
Anthocyanin–protein complexes can be formed by crosslinking or aggregation via non-covalent binding. The hydroxyl and terminal galloyl groups of anthocyanins may contribute to the modulation of crosslinking owing to their molecular flexibility (82, 83). Moreover, anthocyanin–protein (non-enzyme) interactions may also be involved in subtle conformational changes (84). Non-covalent interactions may also occur between anthocyanins and carbohydrates (Figure 2). Generally, the consumption of plant anthocyanins involves the ingestion of starch and fibers, which may help improve their stability by counteracting the pH variations in different in vivo digestion phases (85). The physical entrapping induced by these molecules restricts the mixing process between digestive fluids and anthocyanins to avoid their degradation to some extent and facilitate the biomolecules to reach the gut wall, which can improve their bioavailability and health-promotion benefits (12, 34).
Proteins, polysaccharides, and other components in the food matrix are commonly worked together to affect anthocyanin or macronutrient digestion. All ingredients work together to produce a final result, which highlights that the effects of the food matrix should be evaluated by taking into account all the ingredients or at least the main contributors. The observed effects and interactions of the matrix with anthocyanins remain elusive and require further investigation (77, 78, 86).
The non-targeted release and low stability are the major obstacles of anthocyanins to present health benefits in food systems (87–89). Recently, encapsulation approaches have been developed to address the low stability, low oral bioavailability, and poor intestinal absorption of anthocyanins. Several emerging micro/nanoencapsulation approaches are effective to some extent for improving anthocyanins' stability against the harsh environment of the gastrointestinal tract with bio-efficacy enhancement (90, 91). In encapsulation, particle aggregation and particle size control, the sensitivity to pH and ionic strength of the prepared particles, as well as other related factors, should be optimized for the practice applications with satisfactory stability and bioavailability (92, 93). As above, the application of emerging micro/nanoencapsulation techniques in the food industry is still challenging.
Only food-grade biomaterials can be employed and accepted for delivering anthocyanins in the food industry. Regardless of nano/microcapsulation technique, food-grade materials, such as proteins, and polysaccharides, are utilized as wall materials for anthocyanin encapsulation with the promising performance of high encapsulated efficacy, enhanced stability, and excellent biocompatibility. The interactions between anthocyanins (e.g., proteins/peptides and polysaccharides) and biomaterials are important in designing delivery systems (Figure 6). The biomaterials properties, satisfactory stability, and the interactions between the biopolymers and anthocyanins should be considered when the edible biopolymers were selected for anthocyanin encapsulation. Although each method has advantages for specific applications, evaluating the requirements according to the advantages and disadvantages of encapsulation approaches is neccessary before selection.
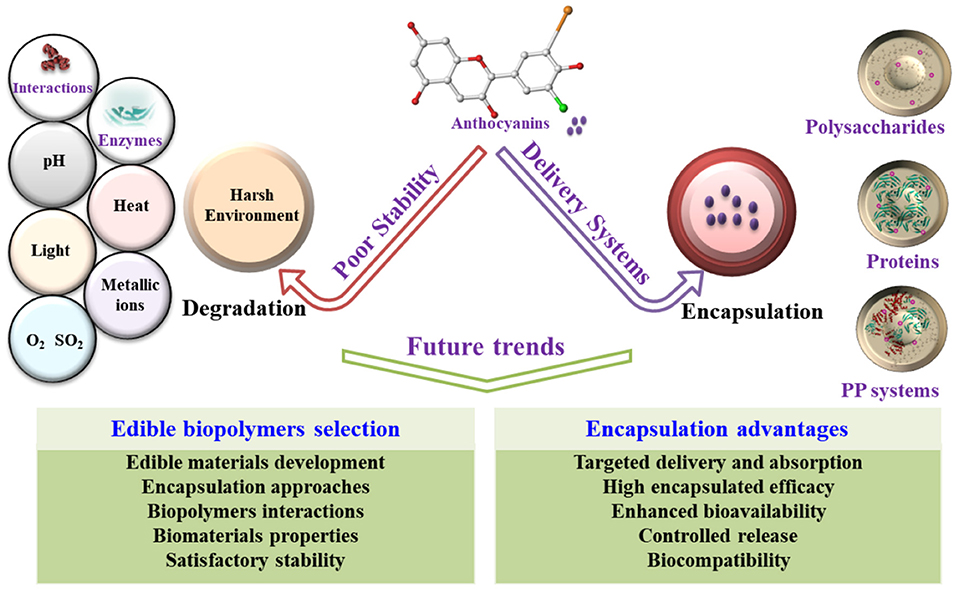
Figure 6. Framework of the future trends or advantages of the micro-/nanoencapsulation of anthocyanins using edible biopolymers, including proteins or/and polysaccharides. PP, protein-polysaccharide.
Bioderived colloidal particles, including protein–polysaccharide conjugates, micro/nanogels, and microfibers, provide new insights into the development of biopolymer interfaces to replace emulsifier layers (94). The potential of stabilized interface for particles has attracted great attention for food colloidal structure research (95). Complex coacervation, which has received a growing interest, presents excellent loading capacity, mild operating conditions, and controlled release (96). These controlled parameters for polysaccharide–protein complexes can enhance functional properties without enzymatic and chemical modifications and support the excellent encapsulation of anthocyanins.
Future recommendations include the utilization of microencapsulated anthocyanins with satisfactory bioavailability and stability as food fortification components (97). Developing more biopolymers with health benefits as wall materials is also crucial. New edible biomaterials or the new combinations of known biomaterials for the effective microencapsulation or nanoencapsulation of anthocyanins are important for the satisfactory design of micro/nanomaterials with novel characteristics (98). In particular, research interest on the microcapsules of anthocyanins and other polyphenols for biologically triggering their release in living cells is increasing (99, 100). Additionally, further research is still suggested to combine the feasibility of different anthocyanin encapsulation techniques. However, seeking and strengthening the optimal techniques combined with environmental protection, high yield, and low cost are still needed.
The booming food industry will no longer be regarded as a low-profit commodity and will be a source of well-being and a revenue potential. The utilization of functional biopolymers via edible materials for food structure design provides new insights into the development of future foods with excellent sensory properties and health benefits, avoiding synthetic additives and negative nutrients. Importantly, investigating new edible biomaterials or creating new colloidal structures with underutilized edible biopolymers for future food design is an exciting and promising research direction.
JS, YY, ZL, and ZM designed the topic. JS and YY prepared the manuscript. JS, ZR, and ZL prepared the figures. YY, ZM, MC, ZR, LC, and CF reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31902204), Natural Science Foundation of Fujian Province (2021J01835), National Key Research and Development Program of China (2021YFD2100200/2021YFD2100204), and Science and Technology project of Fujian health and Family Planning Commission (2021GGA054).
1. Yu X, Chu M, Chu C, Du Y, Shi J, Liu X, et al. Wild rice (Zizania spp): a review of its nutritional constituents, phytochemicals, antioxidant activities, and health-promoting effects. Food Chem. (2020) 331:127293. doi: 10.1016/j.foodchem.2020.127293
2. Rabelo ACS, Borghesi J, Noratto GD. The role of dietary polyphenols in osteosarcoma: a possible clue about the molecular mechanisms involved in a process that is just in its infancy. J Food Biochem. (2022) 46:e14026. doi: 10.1111/jfbc.14026
3. Koch W. Dietary polyphenols—important non-nutrients in the prevention of chronic noncommunicable diseases: a systematic review. Nutrients. (2019) 11:1039. doi: 10.3390/nu11051039
4. Braga MB, Veggi PC, Codolo MC, Giaconia MA, Rodrigues CL, Braga ARC. Evaluation of freeze-dried milk-blackberry pulp mixture: influence of adjuvants over the physical properties of the powder, anthocyanin content and antioxidant activity. Int Food Res J . (2019) 125:108557. doi: 10.1016/j.foodres.2019.108557
5. Jia Y, Cai S, Muhoza B, Qi B, Li Y. Advance in dietary polyphenols as dipeptidyl peptidase-IV inhibitors to alleviate type 2 diabetes mellitus: aspects from structure-activity relationship and characterization methods. Crit Rev Food Sci Nutr. (2021) 1–16. doi: 10.1080/10408398.2021.1989659
6. Yu Y, Li Z, Cao G, Huang S, Yang H. Bamboo leaf flavonoids extracts alleviate oxidative stress in HepG2 cells via naturally modulating reactive oxygen species production and Nrf2-mediated antioxidant defense responses. J Food Sci. (2019) 84:1609–20. doi: 10.1111/1750-3841.14609
7. Bendokas V, Skemiene K, Trumbeckaite S, Stanys V, Passamonti S, Borutaite V, et al. Anthocyanins: from plant pigments to health benefits at mitochondrial level. Crit Rev Food Sci Nutr. (2020) 60:3352–65. doi: 10.1080/10408398.2019.1687421
8. Muche BM, Speers RA, Rupasinghe HP. Storage temperature impacts on anthocyanins degradation, color changes and haze development in juice of “Merlot” and “Ruby” grapes (Vitis vinifera). Front Nutr. (2018) 5:100. doi: 10.3389/fnut.2018.00100
9. Tan C, Dadmohammadi Y, Lee MC, Abbaspourrad A. Combination of copigmentation and encapsulation strategies for the synergistic stabilization of anthocyanins. Comp Rev Food Sci F. (2021) 20:3164–91. doi: 10.1111/1541-4337.12772
10. de Oliveira Filho JG, Braga ARC, de Oliveira BR, Gomes FP, Moreira VL, Pereira VAC, et al. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: a review. Food Res Int. (2021) 142:110202. doi: 10.1016/j.foodres.2021.110202
11. Sun Y, Chi J, Ye X, Wang S, Liang J, Yue P, et al. Nanoliposomes as delivery system for anthocyanins: physicochemical characterization, cellular uptake, and antioxidant properties. LWT. (2021) 139:110554. doi: 10.1016/j.lwt.2020.110554
12. Rashwan AK, Karim N, Xu Y, Xie J, Cui H, Mozafari M Chen W. Potential micro-/nano-encapsulation systems for improving stability and bioavailability of anthocyanins: an updated review. Crit Rev Food Sci Nutr. (2021) 1–24. doi: 10.1080/10408398.2021.1987858
13. Zhang X, Zeng Q, Liu Y, Cai Z. Enhancing the resistance of anthocyanins to environmental stress by constructing ovalbumin-propylene glycol alginate nanocarriers with novel configurations. Food Hydrocoll. (2021) 118:106668. doi: 10.1016/j.foodhyd.2021.106668
14. Tan C, Arshadi M, Lee MC, Godec M, Azizi M, Yan B, et al. A robust aqueous core–shell–shell coconut-like nanostructure for stimuli-responsive delivery of hydrophilic cargo. ACS Nano. (2019) 13:9016–27. doi: 10.1021/acsnano.9b03049
15. Liu Y, Peng B. A novel hyaluronic acid-black rice anthocyanins nanocomposite: preparation, characterization, and its xanthine oxidase (XO)-inhibiting properties. Front Nutr. (2022) 9:879354. doi: 10.3389/fnut.2022.879354
16. Ribeiro JS, Veloso CM. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. (2021) 112:106374. doi: 10.1016/j.foodhyd.2020.106374
17. Sharif N, Khoshnoudi-Nia S, Jafari SM. Nano/microencapsulation of anthocyanins: a systematic review and meta-analysis. Int Food Res J. (2020) 132:109077. doi: 10.1016/j.foodres.2020.109077
18. Nishimoto-Sauceda D, Romero-Robles LE, Antunes-Ricardo M. Biopolymer nanoparticles: a strategy to enhance stability, bioavailability, and biological effects of phenolic compounds as functional ingredients. J Sci Food Agric. (2022) 102:41–52. doi: 10.1002/jsfa.11512
19. Koop BL, da Silva MN, da Silva FD, dos Santos Lima KT, Soares LS, de Andrade CJ, Valencia GA, and Monteiro AR, Flavonoids, anthocyanins, betalains, curcumin, and carotenoids: sources classification and enhanced stabilization by encapsulation and adsorption. Int Food Res J. (2022) 110929. doi: 10.1016/j.foodres.2021.110929
20. Ghosh S, Sarkar T, Das A, Chakraborty R. Micro and nanoencapsulation of natural colors: A holistic view. Appl Biochem Biotechnol. (2021) 193:3787–811. doi: 10.1007/s12010-021-03631-8
21. Ren S, Jiménez-Flores R, Giusti MM. The interactions between anthocyanin and whey protein: A review. Comp Rev Food Sci F. (2021) 20:5992–6011. doi: 10.1111/1541-4337.12854
22. Vidana Gamage GC, Lim YY, Choo WS. Sources and relative stabilities of acylated and nonacylated anthocyanins in beverage systems. J Food Sci Technol. (2022) 59:831–45. doi: 10.1007/s13197-021-05054-z
23. Mohammadalinejhad S, Kurek MA. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl Sci. (2021) 11:3936. doi: 10.3390/app11093936
24. Cai D, Li X, Chen J, Jiang X, Ma X, Sun J, et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. (2022) 366:130611. doi: 10.1016/j.foodchem.2021.130611
25. Kalt W. Anthocyanins and their C6-C3-C6 metabolites in humans and animals. Molecules. (2019) 24:4024. doi: 10.3390/molecules24224024
26. Dini C, Zaro MJ, Viña SZ. Bioactivity and functionality of anthocyanins: a review. Curr Bioact Compd. (2019) 15:507–23. doi: 10.2174/1573407214666180821115312
27. Chen B-H, Stephen Inbaraj B. Nanoemulsion and nanoliposome based strategies for improving anthocyanin stability and bioavailability. Nutrients. (2019) 11:1052. doi: 10.3390/nu11051052
28. Braga ARC, Murador DC, de Souza Mesquita LM, de Rosso VV. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J Food Compos Anal. (2018) 68:31–40. doi: 10.1016/j.jfca.2017.07.031
29. Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: The role of bioavailability. Nutrients. (2021) 13:273. doi: 10.3390/nu13010273
30. Gui H, Sun L, Liu R, Si X, Li D, Wang Y, Shu C, Sun X, Jiang Q, and Qiao Y, Current Current knowledge of anthocyanin metabolism in the digestive tract: absorption, distribution, degradation, and interconversion. Critic Rev Food Scie Nutr. (2022) 1–14. doi: 10.1080/10408398.2022.2026291
31. Arroyo-Maya IJ, McClements DJ. Biopolymer nanoparticles as potential delivery systems for anthocyanins: fabrication and properties. Int Food Res J. (2015) 69:1–8. doi: 10.1016/j.foodres.2014.12.005
32. Tarone AG, Cazarin CBB, Marostica Junior MR. Anthocyanins: new techniques and challenges in microencapsulation. Int Food Res J. (2020) 133:109092. doi: 10.1016/j.foodres.2020.109092
33. Yin Z, Zheng T, Ho C-T, Huang Q, Wu Q, Zhang M. Improving the stability and bioavailability of tea polyphenols by encapsulations: a review. Food Sci Hum Wellness. (2022) 11:537–56. doi: 10.1016/j.fshw.2021.12.011
34. Guldiken B, Gibis M, Boyacioglu D, Capanoglu E, Weiss J. Physical and chemical stability of anthocyanin-rich black carrot extract-loaded liposomes during storage. Int Food Res J. (2018) 108:491–7. doi: 10.1016/j.foodres.2018.03.071
35. Norkaew O, Thitisut P, Mahatheeranont S, Pawin B, Sookwong P, Yodpitak S, et al. Effect of wall materials on some physicochemical properties and release characteristics of encapsulated black rice anthocyanin microcapsules. Food Chem. (2019) 294:493–502. doi: 10.1016/j.foodchem.2019.05.086
36. Liao M, Ma L, Miao S, Hu X, Liao X, Chen F, et al. The in-vitro digestion behaviors of milk proteins acting as wall materials in spray-dried microparticles: effects on the release of loaded blueberry anthocyanins. Food Hydrocoll. (2021) 115:106620. doi: 10.1016/j.foodhyd.2021.106620
37. Garrido-Bañuelos G, Buica A, and du Toit W, Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. a current state-of-art review. Critic Rev Food Scie Nutr. (2021) 1–17. doi: 10.1080/10408398.2021.1918056
38. Souza ACP, Gurak PD, Marczak LDF. Maltodextrin, pectin and soy protein isolate as carrier agents in the encapsulation of anthocyanins-rich extract from jaboticaba pomace. Food Bioprod Process. (2017) 102:186–94. doi: 10.1016/j.fbp.2016.12.012
39. Chen Z, Wang C, Gao X, Chen Y, Santhanam RK, Wang C, et al. Interaction characterization of preheated soy protein isolate with cyanidin-3-O-glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts. Food Chem. (2019) 271:266–73. doi: 10.1016/j.foodchem.2018.07.170
40. Moser P, Telis VRN, de Andrade Neves N, García-Romero E, Gómez-Alonso S, Hermosín-Gutiérrez I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. (2017) 214:308–18. doi: 10.1016/j.foodchem.2016.07.081
41. Stănciuc N, Oancea AM, Aprodu I, Turturică M, Barbu V, Ionită E. et al. Investigations on binding mechanism of bioactives from elderberry (Sambucus nigra L) by whey proteins for efficient microencapsulation J Food Eng. (2018) 223:197–207. doi: 10.1016/j.jfoodeng.2017.10.019
42. Ferron L, Milanese C, Colombo R, Pugliese R, Papetti A. Selection and optimization of an innovative polysaccharide-based carrier to improve anthocyanins stability in purple corn cob extracts. Antioxidants. (2022) 11:916. doi: 10.3390/antiox11050916
43. Enache IM, Vasile AM, Enachi E, Barbu V, Stănciuc N, Vizireanu C. Co-microencapsulation of anthocyanins from black currant extract and lactic acid bacteria in biopolymeric matrices. Molecules. (2020) 25:1700. doi: 10.3390/molecules25071700
44. Milea A?, Vasile AM, Cîrciumaru A, Dumitra?cu L, Barbu V, Râpeanu G, et al. Valorizations of sweet cherries skins phytochemicals by extraction, microencapsulation and development of value-added food products. Foods. (2019) 8:188. doi: 10.3390/foods8060188
45. Zang Z, Chou S, Geng L, Si X, Ding Y, Lang Y, et al. Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT. (2021) 152:112269. doi: 10.1016/j.lwt.2021.112269
46. Ju M, Zhu G, Huang G, Shen X, Zhang Y, Jiang L, et al. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. (2020) 99:105329. doi: 10.1016/j.foodhyd.2019.105329
47. Shen Y, Zhang N, Tian J, Xin G, Liu L, Sun X, et al. Advanced approaches for improving bioavailability and controlled release of anthocyanins. J Control Release. (2022) 341:285–99. doi: 10.1016/j.jconrel.2021.11.031
48. Zheng Y, Li X, Huang Y, Li H, Chen L, and Liu X Two Two colorimetric films based on chitin whiskers and sodium alginate/gelatin incorporated with anthocyanins for monitoring food freshness. Food Hydrocoll. (2022) 127:107517. doi: 10.1016/j.foodhyd.2022.107517
49. Wang S, Ye X, Sun Y, Liang J, Yue P, Gao X. Nanocomplexes derived from chitosan and whey protein isolate enhance the thermal stability and slow the release of anthocyanins in simulated digestion and prepared instant coffee. Food Chem. (2021) 336:127707. doi: 10.1016/j.foodchem.2020.127707
50. Wu G, Hui X, Mu J, Brennan MA, Brennan CS. Functionalization of whey protein isolate fortified with blackcurrant concentrate by spray-drying and freeze-drying strategies. Int Food Res J. (2021) 141:110025. doi: 10.1016/j.foodres.2020.110025
51. Oancea A-M, Hasan M, Vasile AM, Barbu V, Enachi E, Bahrim G, et al. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT. (2018) 95:129–34. doi: 10.1016/j.lwt.2018.04.083
52. Yao L, Xu J, Zhang L, Liu L, Zhang L. Nanoencapsulation of anthocyanin by an amphiphilic peptide for stability enhancement. Food Hydrocoll. (2021) 118:106741. doi: 10.1016/j.foodhyd.2021.106741
53. Herrera-Balandrano DD, Chai Z, Beta T, Feng J, Huang W. Blueberry anthocyanins: an updated review on approaches to enhancing their bioavailability. Trends Food SciTechnol. (2021) 118:808–21. doi: 10.1016/j.tifs.2021.11.006
54. Ren S, Giusti MM. Monitoring the interaction between thermally induced whey protein and anthocyanin by fluorescence quenching spectroscopy. Foods. (2021) 10:310. doi: 10.3390/foods10020310
55. Mohamed SA, El-Sakhawy M, El-Sakhawy MA-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: a review. Carbohydr Polym. (2020) 238:116178. doi: 10.1016/j.carbpol.2020.116178
56. Sreerekha P, Dara PK, Vijayan DK, Chatterjee NS, Raghavankutty M, Mathew S, et al. Dietary supplementation of encapsulated anthocyanin loaded-chitosan nanoparticles attenuates hyperlipidemic aberrations in male Wistar rats. Carbohydr Polym. (2021) 2:100051. doi: 10.1016/j.carpta.2021.100051
57. Tan C, Wang J, Sun B. Polysaccharide dual coating of yeast capsules for stabilization of anthocyanins. Food Chem. (2021) 357:129652. doi: 10.1016/j.foodchem.2021.129652
58. Fernandes A, Rocha MA, Santos LM, Brás J, Oliveira J, Mateus N, et al. Blackberry anthocyanins: β-cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. (2018) 245:426–31. doi: 10.1016/j.foodchem.2017.10.109
59. Abdin M, Salama MA, Gawad R, Fathi MA, Alnadari F. Two-steps of gelation system enhanced the stability of Syzygium cumini anthocyanins by encapsulation with sodium alginate, maltodextrin, chitosan and gum Arabic. J Polym Environ. (2021) 29:3679–92. doi: 10.1007/s10924-021-02140-3
60. Muhamad II, Jusoh YM, Nawi NM, Aziz AA, Padzil AM, Lian HL. Advanced Natural Food Colorant Encapsulation Methods: Anthocyanin Plant Pigment, Natural and Artificial Flavoring Agents and Food Dyes. Academic Press (2018). p. 495–526. doi: 10.1016/B978-0-12-811518-3.00015-6
61. Rousta LK, Bodbodak S, Nejatian M, Yazdi APG, Rafiee Z, Xiao J, et al. Use of encapsulation technology to enrich and fortify bakery, pasta, and cereal-based products. Trends Food Sci Technol. (2021) 118:688–710. doi: 10.1016/j.tifs.2021.10.029
62. Cai X, Du X, Cui D, Wang X, Yang Z, Zhu G. Improvement of stability of blueberry anthocyanins by carboxymethyl starch/xanthan gum combinations microencapsulation. Food Hydrocoll. (2019) 91:238–45. doi: 10.1016/j.foodhyd.2019.01.034
63. Colín-Cruz M, Pimentel-González D, Carrillo-Navas H, Alvarez-Ramírez J, Guadarrama-Lezama A. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT. (2019) 110:94–101. doi: 10.1016/j.lwt.2019.04.064
64. Guo J, Giusti MM, Kaletunç G. Encapsulation of purple corn and blueberry extracts in alginate-pectin hydrogel particles: impact of processing and storage parameters on encapsulation efficiency. Int Food Res J. (2018) 107:414–22. doi: 10.1016/j.foodres.2018.02.035
65. Gonçalves FJ, Fernandes PA, Wessel DF, Cardoso SM, Rocha SM, Coimbra MA. Interaction of wine mannoproteins and arabinogalactans with anthocyanins. Food Chem. (2018) 243:1–10. doi: 10.1016/j.foodchem.2017.09.097
66. Yang Y, Zhang J, Shen L, Feng L. Zhou Q. Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L) against α-amylase and α-glucosidase. Food Chem. (2021) 359:129934. doi: 10.1016/j.foodchem.2021.129934
67. Yang W, Deng C, Xu L, Jin W, Zeng J, Li B, et al. Protein-neutral polysaccharide nano-and micro-biopolymer complexes fabricated by lactoferrin and oat β-glucan: structural characteristics and molecular interaction mechanisms. Int Food Res J. (2020) 132:109111. doi: 10.1016/j.foodres.2020.109111
68. Dragan ES, Dinu MV. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr Polym. (2019) 225:115210. doi: 10.1016/j.carbpol.2019.115210
69. Albano KM, Cavallieri ÂLF, Nicoletti VR. Electrostatic interaction between proteins and polysaccharides: physicochemical aspects and applications in emulsion stabilization. Int Food Res J. (2019) 35:54–89. doi: 10.1080/87559129.2018.1467442
70. Gentile L. Protein–polysaccharide interactions and aggregates in food formulations. Curr Opin Colloid Interface Sci. (2020) 48:18–27. doi: 10.1016/j.cocis.2020.03.002
71. Tie S, Tan M. Current advances in multifunctional nanocarriers based on marine polysaccharides for colon delivery of food polyphenols. J Agric Food Chem. (2022) 70:903–15. doi: 10.1021/acs.jafc.1c05012
72. Tao Y, Wang P, Wang J, Wu Y, Han Y, Zhou J. Combining various wall materials for encapsulation of blueberry anthocyanin extracts: optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technol. (2017) 311:77–87. doi: 10.1016/j.powtec.2017.01.078
73. Wei Z, Huang Q. Assembly of protein–polysaccharide complexes for delivery of bioactive ingredients: a perspective paper. J Agric Food Chem. (2019) 67:1344–52. doi: 10.1021/acs.jafc.8b06063
74. Cortés-Morales EA, Mendez-Montealvo G, Velazquez G. Interactions of the molecular assembly of polysaccharide-protein systems as encapsulation materials. A review. Advances in Colloid and Interface Science. (2021) 295:102398. doi: 10.1016/j.cis.2021.102398
75. Huang Y, Zhou W. Microencapsulation of anthocyanins through two-step emulsification and release characteristics during in vitro digestion. Food Chem. (2019) 278:357–63. doi: 10.1016/j.foodchem.2018.11.073
76. Rosales TKO, da Silva MP, Lourenço FR, Hassimotto NMA. Fabi JP. Nanoencapsulation of anthocyanins from blackberry (Rubus spp) through pectin and lysozyme self-assembling. Food Hydrocoll. (2021) 114:106563. doi: 10.1016/j.foodhyd.2020.106563
77. Zhang Q, Zhou Y, Yue W, Qin W, Dong H, Vasanthan T. Nanostructures of protein-polysaccharide complexes or conjugates for encapsulation of bioactive compounds. Trends Food Sci Technol. (2021) 109:169–96. doi: 10.1016/j.tifs.2021.01.026
78. Li H, Wang T, Hu Y, Wu J, Van der Meeren P. Designing delivery systems for functional ingredients by protein/polysaccharide interactions. Trends Food Sci Technol. (2021) 119:272–87. doi: 10.1016/j.tifs.2021.12.007
79. Mansour M, Salah M, Xu X. Effect of microencapsulation using soy protein isolate and gum Arabic as wall material on red raspberry anthocyanin stability, characterization, and simulated gastrointestinal conditions. Ultrason Sonochem. (2020) 63:104927. doi: 10.1016/j.ultsonch.2019.104927
80. Victoria-Campos CI, de Jesús Ornelas-Paz J, Rocha-Guzmán NE, Gallegos-Infante JA, Failla ML, Pérez-Martínez JD, et al. Gastrointestinal metabolism and bioaccessibility of selected anthocyanins isolated from commonly consumed fruits. Food Chem. (2022) 132451. doi: 10.1016/j.foodchem.2022.132451
81. Zhang Q, Cheng Z, Chen R, Wang Y, Miao S, Li Z, et al. Covalent and non-covalent interactions of cyanidin-3-O-glucoside with milk proteins revealed modifications in protein conformational structures, digestibility, and allergenic characteristics. Food Funct. (2021) 12:10107–20. doi: 10.1039/D1FO01946E
82. Wang Y, Zhang J, and Zhang L. Study on the mechanism of non-covalent interaction between rose anthocyanin extracts and whey protein isolate under different pH conditions. Food Chem. (2022) 132492. doi: 10.1016/j.foodchem.2022.132492
83. Sui X, Sun H, Qi B, Zhang M, Li Y, Jiang L. Functional and conformational changes to soy proteins accompanying anthocyanins: focus on covalent and non-covalent interactions. Food Chem. (2018) 245:871–8. doi: 10.1016/j.foodchem.2017.11.090
84. Arruda HS, Silva EK, Peixoto Araujo NM, Pereira GA, Pastore GM, Marostica Junior MR. Anthocyanins recovered from agri-food by-products using innovative processes: trends, challenges, and perspectives for their application in food systems. Molecules. (2021) 26:2632. doi: 10.3390/molecules26092632
85. Domínguez-Avila JA, Wall-Medrano A, Velderrain-Rodríguez GR, Chen C-YO, Salazar-López NJ, Robles-Sánchez M, et al. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. (2017) 8:15–38. doi: 10.1039/C6FO01475E
86. Kamiloglu S, Tomas M, Ozdal T, Capanoglu E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci Technol. (2021) 117:15–33. doi: 10.1016/j.tifs.2020.10.030
87. Guo Y, Qiao D, Zhao S, Zhang B, Xie F. Starch-based materials encapsulating food ingredients: recent advances in fabrication methods and applications. Carbohydr Polym. (2021) 270:118358. doi: 10.1016/j.carbpol.2021.118358
88. Guldiken B, Gibis M, Boyacioglu D, Capanoglu E, Weiss J. Ascorbic acid-induced degradation of liposome-encapsulated acylated and non-acylated anthocyanins of black carrot extract. J Sci Food Agric. (2021) 101:5707–14. doi: 10.1002/jsfa.11225
89. Wang Y, Ye A, Hou Y, Jin Y, Xu X, Han J, et al. Microcapsule delivery systems of functional ingredients in infant formulae: research progress, technology, and feasible application of liposomes. Trends Food Sci Technol. (2022) 119:36–44. doi: 10.1016/j.tifs.2021.11.016
90. Zhang R, Zhou L, Li J, Oliveira H, Yang N, Jin W, et al. Microencapsulation of anthocyanins extracted from grape skin by emulsification/internal gelation followed by spray/freeze-drying techniques: characterization, stability and bioaccessibility. LWT. (2020) 123:109097. doi: 10.1016/j.lwt.2020.109097
91. Ramos SDP, Giaconia MA, Assis M, Jimenez PC, Mazzo TM, Longo E, et al. Uniaxial and coaxial electrospinning for tailoring jussara pulp nanofibers. Molecules. (2021) 26:1206. doi: 10.3390/molecules26051206
92. Kanha N, Surawang S, Pitchakarn P, Laokuldilok T. Microencapsulation of copigmented anthocyanins using double emulsion followed by complex coacervation: Preparation, characterization and stability. LWT. (2020) 133:110154. doi: 10.1016/j.lwt.2020.110154
93. Ren Z, Cui Y, Wang Y, Shi L, Yang S. Hao G, Weng W. Effect of ionic strength on the structural properties and emulsion characteristics of myofibrillar proteins from hairtail (Trichiurus haumela). Int Food Res J. (2022) 157:111248. doi: 10.1016/j.foodres.2022.111248
94. Sarkar R, Dutta A, Patra A, Saha S. Bio-inspired biopolymeric coacervation for entrapment and targeted release of anthocyanin. Cellulose. (2021) 28:377–88. doi: 10.1007/s10570-020-03523-w
95. Patel AR. Functional and engineered colloids from edible materials for emerging applications in designing the food of the future. Adv Funct Mater. (2020) 30:1806809. doi: 10.1002/adfm.201806809
96. Dumitraşcu L, Stănciuc N, Borda D, Neagu C, Enachi E, Barbu V, et al. Microencapsulation of bioactive compounds from cornelian cherry fruits using different biopolymers with soy proteins. Food Biosci. (2021) 41:101032. doi: 10.1016/j.fbio.2021.101032
97. Ghosh S, Sarkar T, Das A, Chakraborty R. Natural colorants from plant pigments and their encapsulation: an emerging window for the food industry. LWT. (2022) 153:112527. doi: 10.1016/j.lwt.2021.112527
98. Ren Z, Li Z, Chen Z, Zhang Y, Lin X. Weng W. Li B. Characteristics and application of fish oil-in-water pickering emulsions structured with tea water-insoluble proteins/κ-carrageenan complexes. Food Hydrocoll. (2021) 114:106562. doi: 10.1016/j.foodhyd.2020.106562
99. Neuenfeldt NH, Farias CAA, de Oliveira Mello R, Robalo SS, Barin JS, da Silva LP, et al. Effects of blueberry extract co-microencapsulation on the survival of Lactobacillus rhamnosus. LWT. (2022) 155:112886. doi: 10.1016/j.lwt.2021.112886
Keywords: anthocyanins, biopolymer, polysaccharides, encapsulation, stability, bioavailability
Citation: Song J, Yu Y, Chen M, Ren Z, Chen L, Fu C, Ma Zf and Li Z (2022) Advancement of Protein- and Polysaccharide-Based Biopolymers for Anthocyanin Encapsulation. Front. Nutr. 9:938829. doi: 10.3389/fnut.2022.938829
Received: 08 May 2022; Accepted: 30 May 2022;
Published: 17 June 2022.
Edited by:
Wuyang Huang, Jiangsu Academy of Agricultural Sciences (JAAS), ChinaReviewed by:
Bin Li, Shenyang Agricultural University, ChinaCopyright © 2022 Song, Yu, Chen, Ren, Chen, Fu, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Yu, eXV5dWVvZmZpY2UyMDEyQDEyNi5jb20=; Zheng feei Ma, emhlbmdmZWVpLm1hQHhqdGx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.