- 1School of Nuclear Science and Technology, University of Science and Technology of China, Hefei, China
- 2Joint Laboratory of Plasma Application Technology, Institute of Advanced Technology, University of Science and Technology of China, Hefei, China
- 3Anhui Academy of Medical Sciences, Hefei, China
- 4Institute of Advanced Technology, University of Science and Technology of China, Hefei, China
- 5CAS Key Laboratory of Geospace Environment, University of Science and Technology of China, Hefei, China
Winter jujube (Ziziphus jujuba Mill. cv. Dongzao) is a very popular horticultural fruit worldwide, which contains a high number of bioactive compounds. Nevertheless, jujube is perishable by microbial contamination and has a short shelf life under non-controlled conditions. Cold atmospheric plasma (CAP) presents a great potential for food sterilization, maintain postharvest quality, and prolonged storage time. Herein, this study investigated the potential effect of CAP with different exposure times (0, 5, 10, and 20 min) on the physicochemical and biochemical changes in jujube during 15-day storage at 4°C and 90% relative humidity (RH). The results showed that CAP treatment could obviously delay ripening, but displayed no effects on the speed of weight loss and moisture content. Meanwhile, the total native aerobic bacterial count in each jujube group was restrained during whole storage. However, CAP treatment showed a time-dependent manner to improve gene expression (PAL, 4CL, DFR, ANS, LAR, and ANR) related to phenolic biosynthesis. As compared to other groups, 20-min CAP treatment can keep or increase total phenolic content (TPC), maintain antioxidant activity, and reduce oxidative damage. Furthermore, the hydrogen peroxide (H2O2) and malondialdehyde (MDA) content in jujube during middle storage were visibly reduced by 20-min CAP treatment. All in all, our findings concluded that appropriate CAP exposure time can be a promising candidate for the postharvest preservation of jujube.
Introduction
Winter jujube (Ziziphus jujuba Mill. cv. Dongzao) is a traditional plant of China, which has been cultivated for more than 4,000 years. It is regarded as one of the most popular fruits in the world due to its incomparable nutritive value, susceptive aroma, and delicious taste. Winter jujube contains many bioactive compounds such as polysaccharides, ascorbic acid, phenolic acids, amino acid, flavonoids, and mineral constituents, so that displays antioxidant, anti-inflammatory, antimicrobial, and anticancer abilities (1–3). Compared with dried jujube, fresh jujube fruit has more antioxidants and tastes better. However, after harvesting, jujube is highly susceptible to get contaminated by pathogenic including Alternaria alternata, Botrytis cinerea, and Penicillium expansum (4, 5). On the other hand, numerous uncontrollable elements that include respiration, transpiration, temperature, and relative humidity during postharvest storage and transportation have resulted in many quality losses of jujube and critically reduce farmer’s income. Thus, it is of importance to apply an effective strategy to preserve the postharvest quality of jujube during transportation and storage.
So far, various approaches that include food additives, fungicides, and special gas have been developed to retain nutrition, reduce decay, and prolonged shelf life of jujube as far as possible. Hydroxychloroquine (HCQ) (6), melatonin (MET) (7), natamycin (NATA) (8), 1-methylcyclopropene (1-MCP) (9), β-aminobutyric acid (BABA) (10), oligochitosan (OCH) (11), essential mineral (Fe, Zn, Cu, Mn, and Se) mixture (12), carbon monoxide (CO) (13), nitric oxide (NO) (14), and even their combinations were used to enhance antioxidant nutrients, delay senescence, and suppress pathogenic fungi of jujube during postharvest storage (15–18). Moreover, it also has been reported that low temperature (0°C) could be used to delay ripening rate, improve antioxidant levels, and maintain storage quality of jujube (19). Nevertheless, long-term usage of fungicides and pesticides will lead to a negative effect to produce more drug-resistant pathogens. To some extent, residual chemical fungicides located on the surface or inside of fruit will endanger human health. Therefore, the discovery of alternative, easy controlled, lower energy consumption, fruit safe, and environment friendly approaches to preserve the postharvest quality and delay senescence during transportation and storage of jujube are required.
Plasma, known as a chaos state, contains various excited species including O, O2, O3, OH, NO, NO2, and NOx and even electric fields, ultraviolet radiation (UV), free radicals, and high-energy particles (20). Cold atmospheric plasma (CAP) is another type of plasma produced under standard pressure and simple gas environment besides thermal plasma and characterized with lower temperature around 300–1,000 K. Over the recent years, cold plasma has shown unique merits and applications in textiles, automotive, aerospace, packaging materials, water purification, clinical medicine, and food industry among others (21, 22). With the increasing demand for safe, convenient, and tasty food of consumer, numerous alternative approaches to preserve the storage quality of fruit and vegetable are needed. According to the previous literature, many studies have well demonstrated that plasma can effectively exterminate pathogens (23), degrade pesticide residue and mycotoxins (24, 25), inactivate metabolism enzymes (26–28), increase bioactive compound contents, and improve antioxidant activity (29). Liu et al. pointed out that CAP treatment can reduce rot rate and inhibit B. cinerea increment in mulberry fruit (30). Besides, increasing phenolic compounds and antioxidant ability and longer shelf-life of strawberry treated by surface dielectric barrier discharge (SDBD, 6 kVpp and 42 kHz) for 10 min and kumquat (Citrus japonica) fruits treated by intermittent corona discharge plasma jet (ICDPJ, 8 kV and 4.0 A) for 120 s were acquired (31, 32). As it well known, the preservation effects of cold plasma on postharvest quality of fruits and vegetables are closely related to the carrier gases (air, oxygen, nitrogen, argon, helium, or a combination thereof), generating sources (dielectric barrier discharges (DBDs), corona discharge (CD), microwave discharges (MWs), atmospheric cold plasma jet (APPJ), etc.), treating ways (direct and indirect), and time (33). Nevertheless, to the best of our knowledge, there is rare information about the effects of cold plasma on jujube, but Bao et al. elucidated the cold plasma treatment for 15 s on each side of jujube slice could effectively enhance drying rate and effective diffusivity at 60 and 70°C. Consequently, dried jujube slices with higher polyphenol content and stronger antioxidant capacity are prepared (34). Therefore, it is of importance to unravel the physicochemical and biochemical effects of CAP pretreatment on fresh jujube during cold storage.
Accordingly, the objective of this study was to apply CAP pretreatment to delay ripening and senescence of jujube. Finally, our work will provide a theoretical support for proposing CAP as an effective storage and preservation strategy for jujube fruit.
Materials and Methods
Jujube Processing
Winter jujube was harvested from an orchard located Dali, Shaanxi, China and immediately transported to our laboratory within 3 days. A total of 700 jujubes (weight: 12–16 g) of uniform size, color, and without visual defects were collected and randomly allocated into four groups of 175 fruit each.
These jujubes were first disinfected with 200 μl L–1 sodium hypochlorite for 2 min and then washed with sterilized distilled water and air-dried prior to use. Afterward, the jujube was treated with the following conditions: (1) for CAP treatment, the jujubes were irradiated with a DBD plasma device (10 mm × 15 mm) at 12 cm distance for 5, 10, and 20 min (labeled as CAP5, CAP10, and CAP 20, respectively) (Supplementary Figure 1). (2) for the control group, jujubes without any treatment were coded as control. After treatment, all jujubes were stored at 4°C and 90 % RH for 15 days. Each sampling experiment was carried out at 5-day intervals. For each group, 10 fruits were used for the measurement of weight loss, and 5 fruits for appearance changes during whole storage. Besides, every 5 fruits were used for evaluating the moisture content and 5 fruits for detecting the total native aerobic bacterial counts. Furthermore, 30 fruits in each group were randomly collected every 5 days, cut into slice, frozen by liquid nitrogen, pulverized, and then stored at –80°C for the assessment of quality parameters including TPC, antioxidant activities, lipid oxidation degree, and phenylpropane pathway-related gene expression.
Fruit Quality Assessment
Visual Appearance
The visual appearance images of jujubes at days 0, 5, 10, and 15 were obtained by a mobile phone in a darkroom with a single light source. After pictured, the jujubes were put back into the incubator and keep stored for the next record till the end of the experiment.
Weight Loss
The weight loss of jujubes during 15-day storage was carried out by weight method, using an electronic balance (0.0001 g). The results were calculated by the following equation:
where M0 represents the initial weight of jujubes at day 0, and Mt means the weight value of jujubes at days 5, 10, and 15, respectively (t = 5, 10, 15).
Moisture Content
The moisture content of jujubes in each group at every sampling day was determined according to the Chinese standard named GB 5009.3-2016, with some adjustments. A total of 5 jujubes were cut into small slices and put into five glass petri dishes, respectively, followed hot air-dried at 105 ± 5°C for 2 h. Subsequently, the first-dried sample was cooled to ambient temperature and dried again till the final weight was 50 mg lower than the last. The moisture content of jujubes was calculated by the equation below:
where M0 and Mf symbolize the weight of jujubes before and after hot air-drying treatment, respectively.
Total Aerobic Bacterial (TAB) Count Determination
The total native aerobic bacterial count was determined by the National Food Safety Standards of China (GB 4789.2-2016), with slight modifications. A total of 5 jujubes were separately cut into slices, diluted with desterilized normal saline (0.9 % NaCl) at a ratio of 1:10, and tapped for 2 min to prepare the original TAB solution. About 100 μl of above solution was spread on the plate count agar (PCA) medium, cultured at 37 ± 1°C for 48 h. The plates with the number of colonies between 30 and 300 CFU were recorded. Each value was obtained two times by technical replications, and the result was averaged by three values. The results were expressed as log10 CFU g–1.
Antioxidant Activity Determination
The antioxidant activity of jujubes was investigated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) methods proposed by the previous study, with some simplified changes (35). About 0.1 g freeze-dried jujube powder was added to a 2-ml tube and immersed with 1 ml of 60% ethanol reagent. Then, the mixture was put into a 60°C water bath for 1 h and around 700 ul extract was obtained. Subsequently, 400 μl extract was mixed with 5.6 ml of 200 μm DPPH solution for 30 min in a darkroom. The absorbance was measured at 515 nm, and the final DPPH scavenging rate was calculated by the equation below:
where A0 and As represent the absorbance values of the mixture prepared by 400 μl ethanol or 400 μl jujube extract mixed with 5.6 ml DPPH solution, respectively.
Besides, 10 μl jujube extract was reacted with 90 μl ABTS solution for 20 min. Then, the absorbance was read at 732 nm, and the ABTS scavenging rate was calculated followed by the equation below:
where A0 and At represent the absorbance values of the mixture prepared by 10 μl ethanol or 10 μl jujube extract mixed with 90 μl ABTS solution, respectively.
Total Phenolic Content (TPC)
The TPC of jujubes was determined using the Folin–Ciocalteu colorimetric method according to Dzimitrowicz et al., with some modifications (36). About 0.1 g of freeze-dried jujube powder was blended with 1.6 ml of 60% ethanol solution, ultrasonic extracted at 40°C for 2 h, and centrifuged at 5,600 × g for 15 min to obtain the TPC extract. Subsequently, 200 μl of extract was brought into a 10-ml tube, and 2 ml of 0.2 M Folin–Ciocalteu reagent was added and vortexed thoroughly. After 5 min, 2 ml of 7.5% (w/v) sodium carbonate solution and 2.8 ml of deionized water were further added and mixed vigorously and put in a darkroom for 30 min. Finally, the absorbance was measured at 765 nm in a microplate reader (CLARIO star, Munich, Germany) with gallic acid used as the standard for calibration curve. The TPC was expressed as g gallic acid equivalence per kg of dry weight (g GAE kg–1 DW).
Real-Time Quantitative PCR (RT-qPCR)
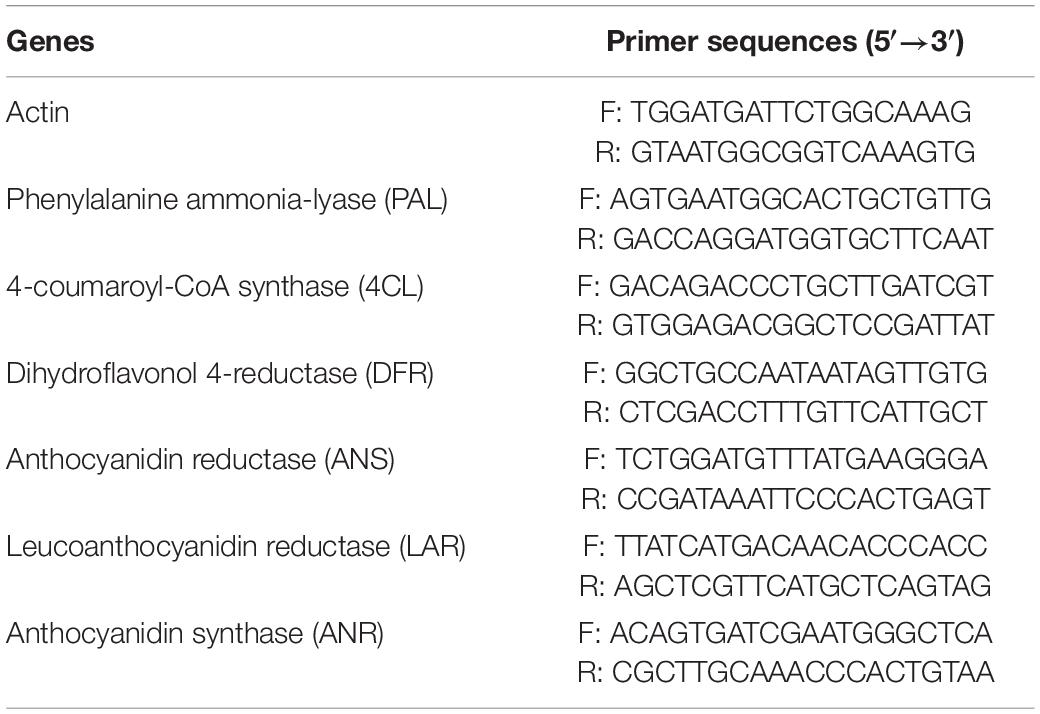
In this experiment, the major genes involved in phenylpropanoid pathway including PAL, 4CL, DFR, ANS, LAR, and ANR were selected according to Wang et al. (37). Total RNAs from jujubes on days 0, 5, 10, and 15 were extracted with the cetyltrimethylammonium bromide (CTAB) method. RT-qPCR experiment was conducted followed by the manufacturer’s instruction. The results were calculated using 2–ΔΔCt against the original values of the sample at day 0 as relative expression levels. Finally, the genes and primer sequences designed in the present studies are listed in Table 1, and the actin gene was used as the reference gene. Each sample was carried out for three technical replications, and the results were averaged by three samples.
Determination of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content
The H2O2 content was carried out based on titanium sulfate colorimetric method, with slight modifications (38). First, 0.1 g of freeze-dried jujube powder was decolored using 2 ml pre-cooled acetone, accompanied by grinding for 1 min. The mixture (∼2 ml) was collected, the residual jujube powder in mortar was washed by another 3 ml acetone, and 5 ml of jujube-acetone mixture was obtained. After centrifugation at 5,600 × g for 15 min, 1 ml of supernatant was added to a 2-ml tube, and 100 μl of 5 % titanous sulfate regnant and 200 μl of ammonium hydroxide were successively added. After reaction for 5 min and centrifuged at 5,600 × g for 10 min, the precipitate was preserved and used for next procedure. Finally, the precipitate was washed by acetone for two times and 1.5 ml of 2 M vitriol solution was added. The absorbance value of 150 μl reaction solution was obtained at 415 nm. The standard curve was built using different concentrations of H2O2 solution by the same processing. The results were expressed as mmol kg–1 dry weight (mmol kg–1 DW).
The MDA content of jujubes was determined based on the thiobarbituric acid (TBA) method, with some adjustments (39). The absorbance of the supernatant was read at 450, 532, and 600 nm. The MDA concentration was calculated by the following equations:
where c displays the MDA content of reaction solution, μmol L–1; A450, A532, and A600 represent the absorbance values of reaction solution at 450, 532, and 600 nm, respectively; Ve, Vr, and Vd mean the total volume of sample extract, the volume of sample extract taken for chromogenic reaction, and the volume used for absorbance detection, respectively, ml; m symbols the dry weight of jujube used for extraction, g.
Statistical Analysis
All data were collected, recorded, and calculated by Excel 2016. Every figure was plotted using Origin 8.5, 2019b version. Moreover, one-way analysis of variance (ANOVA) tools including least-significant difference (LSD) and Waller-Duncan were used to compare and label statistically differences between groups. The results were recorded as mean ± SD (standard deviation) and p < 0.05 represented the significant differences.
Results
Surface Morphology
Figure 1 shows the visual images of jujube during 15-day postharvest storage, which can directly reflect the ripening, senescence, surface morphology, and color change on jujube. At day 10, the jujubes in control, CAP5, and CAP10 are gradually turning red and epidermal tissue is getting dehydrated and shrunk. However, the jujube in CAP20 still displayed a yellow and glossy surface. At the last sampling day, all fruits in control, CAP5, and CAP10 were red, and numerous trenches formed on the surface. Nevertheless, there still were some immature jujubes with half yellow area and highly acceptable appearance in CAP20.
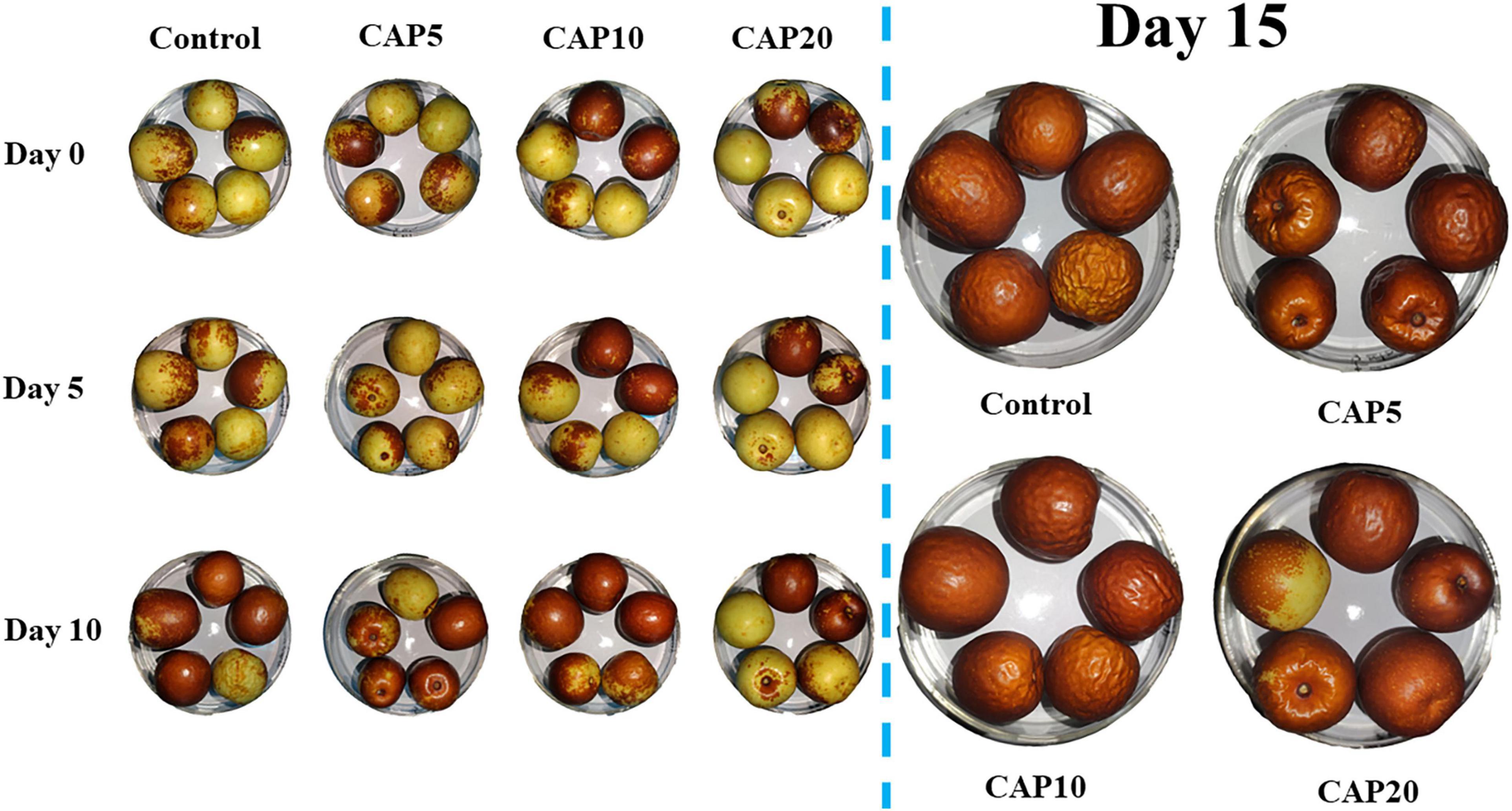
Figure 1. Visual appearance of jujubes in CAP5, CAP10, CAP20, and control during 15-day storage at 4°C and 90% relative humidity (RH).
Weight Loss and Moisture Content
Figure 2 shows the weight loss and moisture content of fresh jujube pretreated or untreated by CAP during 15-day cold storage. As shown in Figure 2A, the weight loss of jujube in every group was gradually increased from days 5 to 15. The weight loss of jujube in CAP5 and CAP20 was slightly decreased as compared to that in control. However, an obviously diminution on weight loss was observed in CAP10 during the whole storage (p < 0.05).
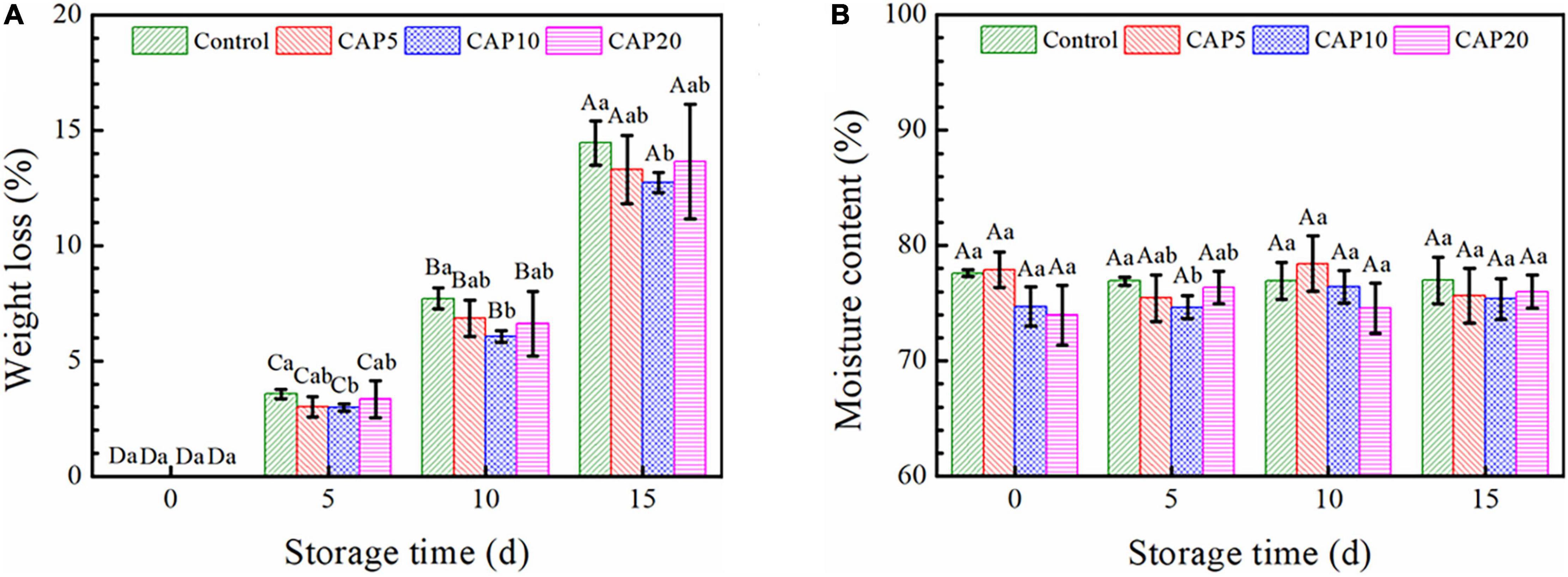
Figure 2. The weight loss (A) and moisture content (B) of jujubes in CAP5, CAP10, CAP20, and control during 15-day storage at 4°C and 90 % relative humidity (RH). Different small letters indicate significant differences among different groups at the same storage time (p < 0.05), whereas different capital letters represent significant differences among different storage times within the same group (p < 0.05).
As can be seen in Figure 2B, from days 5 to 15, the moisture content of jujube with or without CAP treatment presented no changes as compared to day 0. On the other hand, CAP treatment for 5, 10, and 20 min displayed no effects on the moisture content of jujube at days 0, 10, and 15, whereas the value was decreased in CAP10 group as compared to that in control at day 5 (p < 0.05).
Total Native Aerobic Bacterial (TAB) Count
As shown in Figure 3, the native TAB count of jujube in CAP5 and CAP20 decreased to 2.83 and 3.07 log10 CFU g–1, respectively, whereas the number of that in CAP10 was increased to 3.28 log10 CFU g–1 as compared to control (3.15 log10 CFU g–1). At the end of 15-day storage, the TAB count of CAP-treated jujube all presented no differences as compared to control. Moreover, with the prolonged storage time, the TAB count of jujube in control and CAP treatment was not changed.
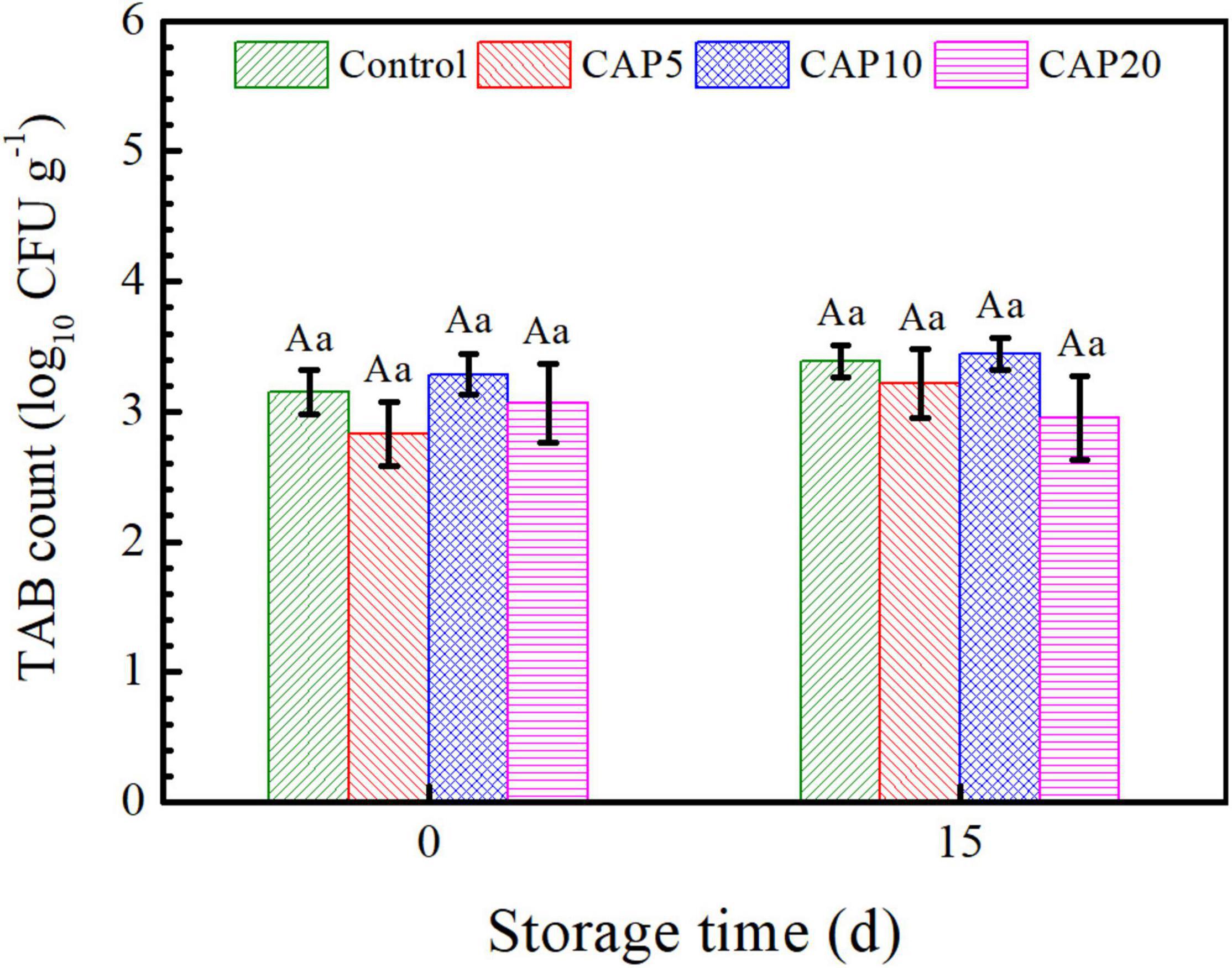
Figure 3. Total native aerobic bacterial (TAB) count of jujubes in CAP5, CAP10, CAP20, and control at days 0 and 15. Small and capital letters show the similar meanings as Figure 2.
Antioxidant Ability and Total Phenolic Content (TPC)
The antioxidant ability of fruit and vegetable is strongly associated with antioxidant compounds and usually characterized by the free radical scavenging capacity including DPPH and ABTS. As shown in Table 2, the TPC was gradually decreased with the prolonged storage time. At day 0, the TPC of freeze-dried jujube was reduced by the CAP treatment for 10 and 20 min (p < 0.05), whereas there was no difference of that in CAP5 as compared to control. From days 5 to 10, a slight increment of TPC was observed in CAP5, CAP10, and CAP20 as compared to control. However, at the end of 15-day storage, the TPC of jujube in each group was further decreased and still presented no differences with each other.
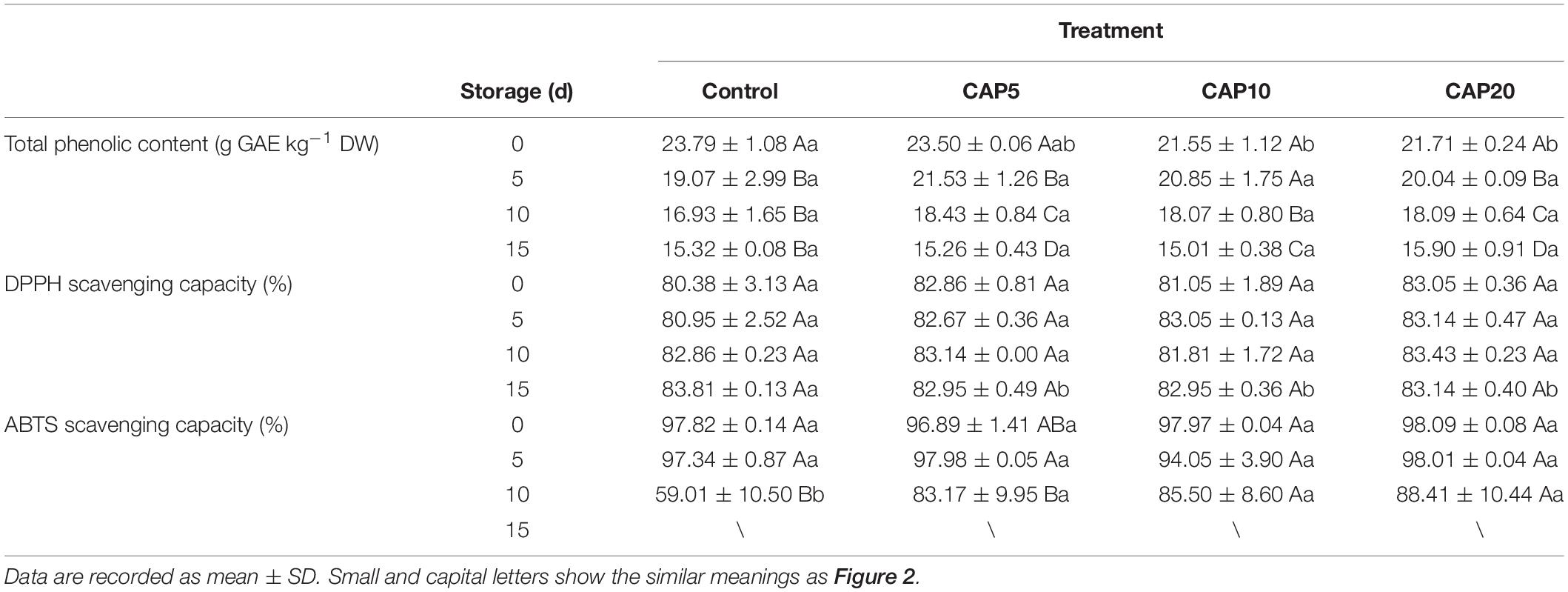
Table 2. The antioxidant ability characterized by diphenyl-1-picrylhydrazyl (DPPH)and ABTS scavenging capacity, and total phenolic content (TPC) of jujubes in cold atmospheric plasma 5 (CAP5), CAP10, CAP20, and control during 15-day storage at 4°C and 90% relative humidity (RH).
For antioxidant activity, the results showed that the DPPH scavenging capacity in CAP-treated groups showed no differences as compared to control from days 0 to 10. However, at day 15, the DPPH scavenging capacity of CAP-treated jujubes was decreased as compared to control (p < 0.05). Moreover, the DPPH scavenging capacity of jujube with or without CAP treatment displays no obvious changes during the 15-day storage. Likewise, the same trend was observed in the ABTS scavenging capacity of jujube at day 0 and 5, which displayed no differences of that in CAP-treated groups as compared to control. Differently, at day 10, the ABTS scavenging capacity of jujube in control was sharply decreased to 59.01%, while of that in CAP5, CAP10, and CAP20 still maintained a high level around 85.00% (p < 0.05).
Phenolic Acid Synthesis-Related Gene Expression
To unravel the potential mechanism of CAP treatment on the total phenolic accumulation of jujube during cold storage, the transcriptional expression degrees of 6 genes involved in phenolic biosynthesis were studied. As depicted in Figure 4, CAP treatment for 5 min has no impacts on all 6 genes as compared to control from days 0 to 15. However, 10- and 20-min CAP treatment could improve the PAL, LAR, and ANR expression at days 5 and 10 (p < 0.05). Then, at day 15, these genes’ expression declined but still higher than that in control. On the other hand, the expression of 4CL, DFR, and ANS of jujube in control and CAP-treated groups was notably decreased at day 5 as compared to day 0. But from days 10 to 15, 10- and 20-min CAP treatment suppressed the decline rate as compared to control. Furthermore, the expression of DFR and ANS at day 15 was higher than that in control at day 0 (p < 0.05).
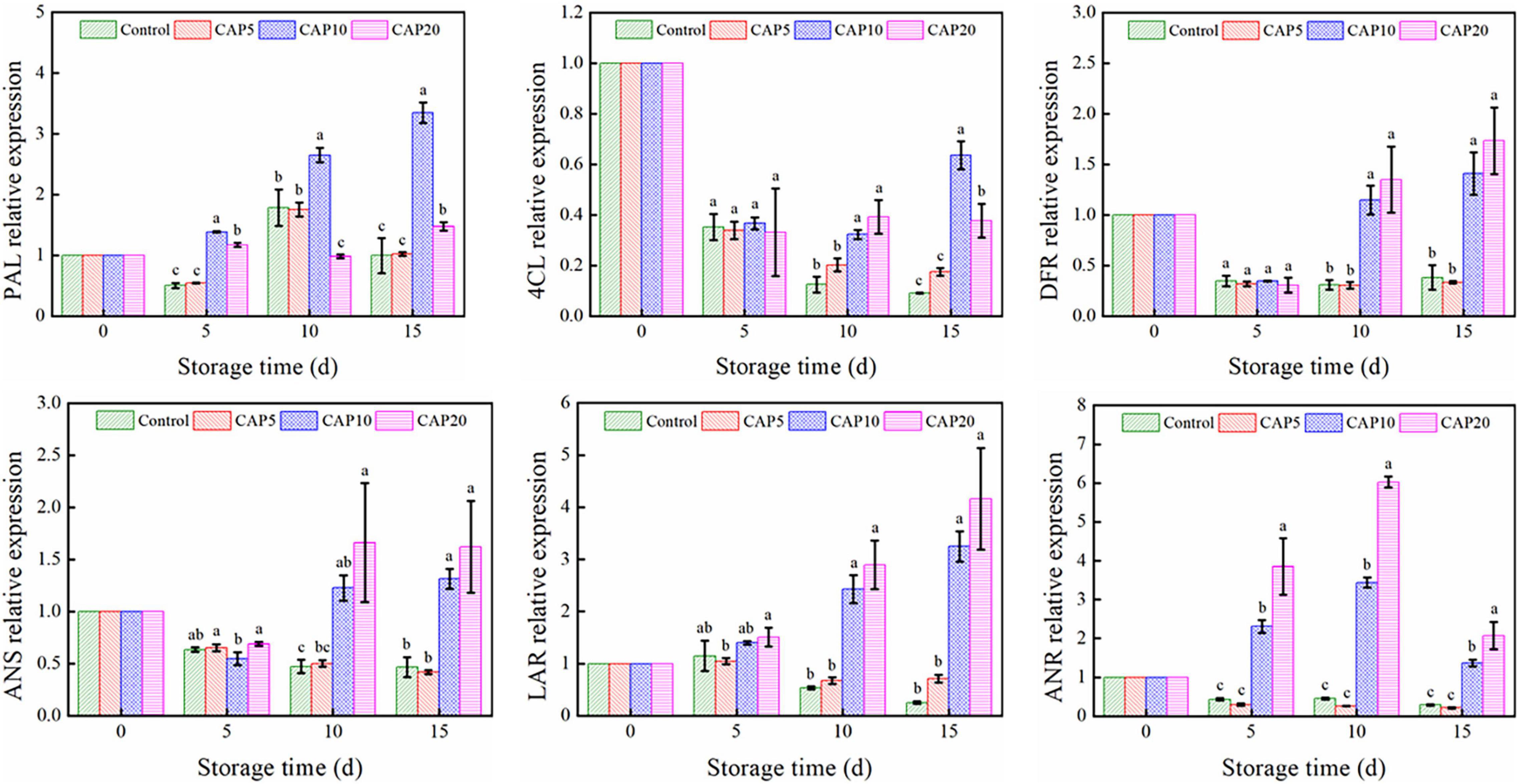
Figure 4. Gene expression of jujubes in CAP5, CAP10, CAP20, and control during 15-day storage at 4°C and 90% relative humidity (RH). Different small letters indicate significant differences among different groups at the same storage time (p < 0.05).
H2O2 and MDA Content
As shown in Figure 5A, the H2O2 content of freeze-dried jujube in CAP10 and CAP20 both decreased at day 0 (p < 0.05), while of that in CAP5 was not changed as compared to control. It is noteworthy that there were no concentration changes occurred in control and CAP5 during the whole storage. However, in CAP10 group, the H2O2 content sharply increased at day 5 and then slowly decreased from days 10 to 15. Furthermore, the H2O2 content of jujube in CAP20 was lower than that in control and presented a gradually decreasing trend during 15-day storage (p < 0.05).
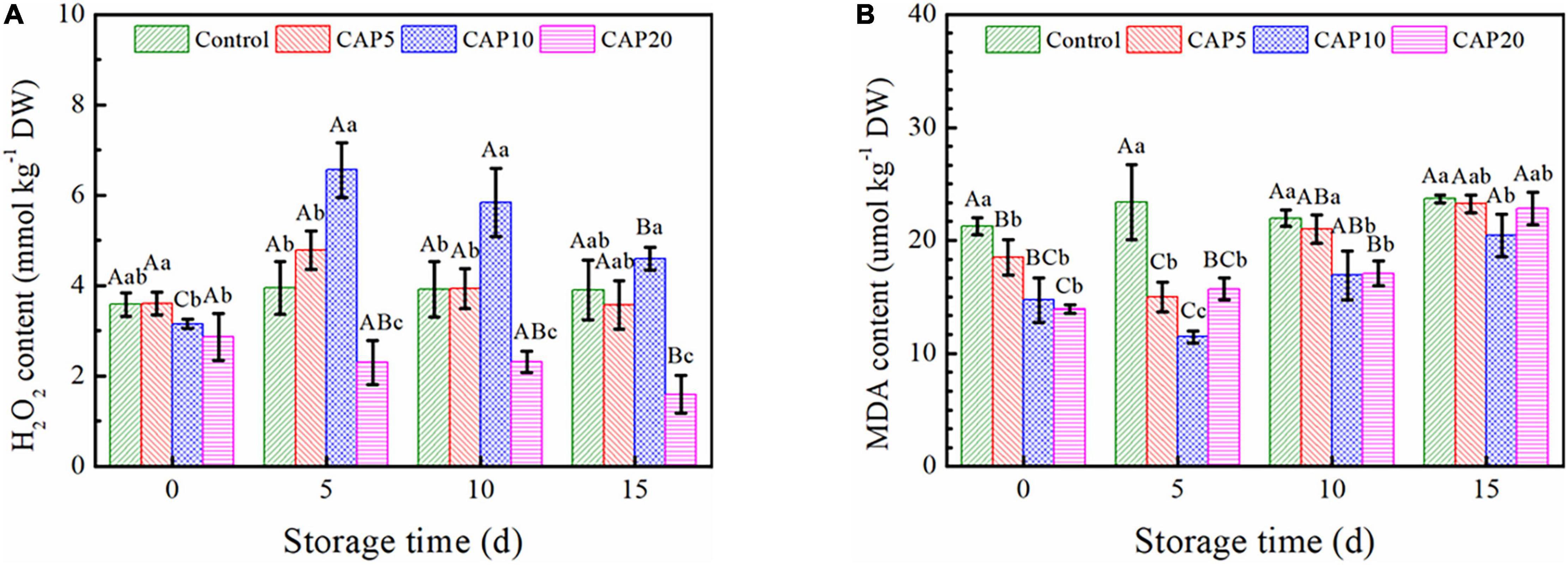
Figure 5. Oxidative stress assessment on H2O2 (A) and MDA (B) content of jujubes in CAP5, CAP10, CAP20, and control during 15-day storage at 4°C and 90% relative humidity (RH). Small and capital letters show the same meanings as Figure 2.
Figure 5B elucidated the MDA accumulation in jujube during 15-day cold storage. At day 0, the MDA content of jujube in CAP-treated groups was reduced and displayed a time-dependent manner. Then, at day 5, the MDA concentration of jujube in control was not changed, while of that in CAP5 and CAP10 was further decreased, and of that in CAP20 start increasing as compared to day 0. As compared to CAP5 and CAP20, the MDA content of jujube in CAP10 was continually increased but still lower than that of control from days 10 to 15 (p < 0.05).
Discussion
In this study, three exposure times of CAP treatment have been used to delay the ripening and senescence of jujube during 15-day storage at 4°C and 90% RH. The results were evident that CAP treatment can reduce the postharvest quality losses and improve oxidative stress response of jujube. As shown in Figure 1, CAP treatment presented a time-dependent manner to postpone turning red and shrinking progress of jujube during 15-day storage. The result was in line with previous study which stated that cherry tomatoes pretreated by CAP activated water still displayed higher acceptable after storage for 14 days (40).
As investigated in Figure 2, CAP treatment can effectively reduce the weight loss of jujube, but this has no concern with moisture content changes. The reason might be due to there were higher content bound water than free water in jujube, and the bound water combined with cell wall polysaccharides and macromolecules was hard to dislodge (41, 42). Besides, the high relative humidity can restrain the water diffusion from peel to air under a stable pressure difference, which already verified by (43–45).
As seen in Figure 3, the result demonstrated that 5-, 10-, and 20-min CAP treatment all have no impacts on TAB count as compared to control. This may be due to 200 μl L–1 sodium hypochlorite displayed a brilliant disinfect effect on Jujube and then, the TAB count stood in a safe range (35). Our result was in good agreement with the finding of Won et al., who formulated that P. italicum were reduced by microwave-powered cold plasms from 96.6 to 85.0 and 16.0% with an independent input power manner (46). Furthermore, low/ice temperature environment is strictly unfavorable for bacteria growth and proliferation (47).
It is worthwhile to mention that CAP treatment not only can delay decay, reduce weight loss, but also play an important role in antioxidant system. As seen in Table 2, the jujube treated by CAP presented stronger ABTS free radical scavenging capacity and higher TPC than control during middle stage of storage. These differences in antioxidant activity of jujube treated or untreated by CAP were closely depended on the accumulation of antioxidants, which further regulated by the genes related to phenylpropanoid biosynthetic pathway. According to Figure 4, it can be concluded that CAP treatment effectively accelerated the flavonoid and phenylpropanoid biosynthesis in fresh jujube during postharvest storage. In other words, CAP treatment could upregulate key genes’ expression to promote larger phenolic compounds that transform into smaller ones, so that it maintain high-level TPC in jujube. As it is previously reported, CAP treatment could trigger excited stress response to abominable environment through increasing antioxidant compound content in fruits and vegetables, so that defense the oxidative damage (48, 49).
According to the previous studies, H2O2 is a kind of reactive oxygen species (ROS) produced by stress response in the cells of fruit and vegetable when meet injure, whereas MDA is the main by-product of cell membrane lipid peroxidation in fruits (50). Thus, the H2O2 and MDA content can reflect the cell membrane permeability, degree of oxidative damage, and fruit aging. Our results showed that short-time CAP treatment could induce seriously the oxidative stress response and increase H2O2 content, whereas CAP treatment for a longer time would decrease H2O2 content (Figure 5A). This may be due to longer time exposure can produce more ROS and reactive nitrogen species (RNS), which could effectively lead to serious stress response, so that improve the antioxidant enzyme activities including superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX). Rather, short-time treatment could only increase the H2O2 content and further damage the cell membrane and other tissues of fruits. Besides, the MDA content in our study was first decreased and then increased with a dynamically change trend (Figure 5B). This may be due to CAP treatment not only improved the antioxidant enzymes activity, but also inhibited the lipid peroxidase enzyme expression in jujube during storage. Our results were identical to previous studies, which pointed out that dielectric barrier discharge cold plasma treatment for 10 min can inactivate the litchi peroxidase (POD) to 47.16% (51).
Conclusion
In this study, a native Chinese winter jujube was used to investigate the potential effects of CAP treatment on the postharvest quality and genes expression. Our results proved that 5-, 10-, and 20-min CAP pretreatment showed no differences on moisture content and total native aerobic bacterial count during 15-day cold storage. However, some meaningful findings were obtained that suitable CAP treatment (10 and 20 min) could reduce oxidative damage and maintain high-level TPC and antioxidant activity through improving key genes’ expression. Taken together, our work demonstrated that CAP treatment can act as a prospective tool to preserve the postharvest qualities of fruits and vegetables. In the later researches, comprehensive effects of CAP on postharvest quality of fruit and vegetable should be further studied including sensory evaluation, color, aroma, texture, polyphenol oxidases, antioxidant enzymes, and cuticle, etc.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
TJ: investigation, methodology, formal analysis, and writing – original draft. CD: methodology and writing – original draft. YX: methodology, validation, and writing – review and editing. YC: data curation, validation, and formal analysis. QX: resources, project administration, and funding acquisition. ZW: conceptualization, resources, writing – review and editing, supervision, and funding acquisition. All authors contribution to the article and approved the submitted version.
Funding
The Key R&D plan of Anhui Province, under Grant No. 201904a07020013; and the joint Laboratory of Plasma Application Technology Funding, under Grant No. JL06120001H.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.934841/full#supplementary-material
References
1. Rashwan AK, Karim N, Shishir MRI, Bao T, Lu Y, Chen W. Jujube fruit: a potential nutritious fruit for the development of functional food products. J Funct Foods. (2020) 75:104205. doi: 10.1016/j.jff.2020.104205
2. Ji X, Guo J, Ding D, Gao J, Hao L, Guo X, et al. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao. J Food Meas Charact. (2022) 16:2191–200. doi: 10.1007/s11694-022-01288-3
3. Yuan L, Qiu Z, Yang Y, Liu C, Zhang R. Preparation, structural characterization and antioxidant activity of water-soluble polysaccharides and purified fractions from blackened jujube by an activity-oriented approach. Food Chem. (2022) 385:132637. doi: 10.1016/j.foodchem.2022.132637
4. Wang Y, Tang F, Xia J, Yu T, Wang J, Azhati R, et al. A combination of marine yeast and food additive enhances preventive effects on postharvest decay of jujubes (Zizyphus jujuba). Food Chem. (2011) 125:835–40. doi: 10.1016/j.foodchem.2010.09.032
5. Cao J, Yan J, Zhao Y, Jiang W. Effects of postharvest salicylic acid dipping on Alternaria rot and disease resistance of jujube fruit during storage. J Sci Food Agric. (2013) 93:3252–8. doi: 10.1002/jsfa.6167
6. Deng B, Guo M, Liu H, Tian S, Zhao X. Inhibition of autophagy by hydroxychloroquine enhances antioxidant nutrients and delays postharvest fruit senescence of Ziziphus jujuba. Food Chem. (2019) 296:56–62. doi: 10.1016/j.foodchem.2019.05.189
7. Deng B, Xia C, Tian S, Shi H. Melatonin reduces pesticide residue, delays senescence, and improves antioxidant nutrient accumulation in postharvest jujube fruit. Postharvest Biol Technol. (2021) 173:111419. doi: 10.1016/j.postharvbio.2020.111419
8. He C, Zhang Z, Li B, Xu Y, Tian S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—postharvest pathogens of grape berries and jujube fruit. Postharvest Biol Technol. (2019) 151:134–41. doi: 10.1016/j.postharvbio.2019.02.009
9. Ozturk B, Yildiz M, Yildiz K, Gun S. Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv. ‘Li’) fruit by applying 1-methylcyclopropene. Sci Hortic. (2021) 275:109671. doi: 10.1016/j.scienta.2020.109671
10. Yan J, Yuan S, Wang C, Ding X, Cao J, Jiang W. Enhanced resistance of jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit against postharvest Alternaria rot by β-aminobutyric acid dipping. Sci Hortic. (2015) 186:108–14. doi: 10.1016/j.scienta.2015.02.018
11. Yan J, Cao J, Jiang W, Zhao Y. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci Hortic. (2012) 142:196–204. doi: 10.1016/j.scienta.2012.05.025
12. Deng B, Shi H, Liu H, Li S, Tian S, Zhao X. Soaking with an essential mineral (Fe, Zn, Cu, Mn and Se) mixture delays senescence and improves nutrient accumulation in postharvest fruit of Ziziphus jujuba. Postharvest Biol Technol. (2020) 166:111186. doi: 10.1016/j.postharvbio.2020.111186
13. Zhang S, Wang Q, Guo Y, Kang L, Yu Y. Carbon monoxide enhances the resistance of jujube fruit against postharvest Alternaria rot. Postharvest Biol Technol. (2020) 168:111268. doi: 10.1016/j.postharvbio.2020.111268
14. Zhao Y, Zhu X, Hou Y, Wang X, Li X. Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Sci Hortic. (2020) 261:109009. doi: 10.1016/j.scienta.2019.109009
15. Ban Z, Zhang J, Li L, Luo Z, Wang Y, Yuan Q, et al. Ginger essential oil-based microencapsulation as an efficient delivery system for the improvement of Jujube (Ziziphus jujuba Mill.) fruit quality. Food Chem. (2020) 306:125628. doi: 10.1016/j.foodchem.2019.125628
16. Cheng S, Yu Y, Guo J, Chen G, Guo M. Effect of 1-methylcyclopropene and chitosan treatment on the storage quality of jujube fruit and its related enzyme activities. Sci Hortic. (2020) 265:109281. doi: 10.1016/j.scienta.2020.109281
17. Gong L, Zhao Z, Yin C, Gupta VK, Zhang X, Jiang Y. Synergistic interaction of natamycin with carboxymethyl chitosan for controlling Alternata alternara, a cause of black spot rot in postharvest jujube fruit. Postharvest Biol Technol. (2019) 156:110919. doi: 10.1016/j.postharvbio.2019.05.020
18. Nikkhah M, Hashemi M. Boosting antifungal effect of essential oils using combination approach as an efficient strategy to control postharvest spoilage and preserving the jujube fruit quality. Postharvest Biol Technol. (2020) 164:111159. doi: 10.1016/j.postharvbio.2020.111159
19. Sang Y, Yang W, Liu Y, Zhang W, Guo T, Shen P, et al. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao). LWT. (2022) 154:112876. doi: 10.1016/j.lwt.2021.112876
20. Laroque DA, Seó ST, Valencia GA, Laurindo JB, Carciofi BAM. Cold plasma in food processing: design, mechanisms, and application. J Food Eng. (2022) 312:110748. doi: 10.1016/j.jfoodeng.2021.110748
21. Sruthi NU, Josna K, Pandiselvam R, Kothakota A, Gavahian M, Mousavi Khaneghah A. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: a comprehensive review. Food Chem. (2022) 368:130809. doi: 10.1016/j.foodchem.2021.130809
22. Asl PJ, Rajulapati V, Gavahian M, Kapusta I, Putnik P, Mousavi Khaneghah A, et al. Non-thermal plasma technique for preservation of fresh foods: a review. Food Control. (2022) 134:108560. doi: 10.1016/j.foodcont.2021.108560
23. Qian J, Ma L, Yan W, Zhuang H, Huang M, Zhang J, et al. Inactivation kinetics and cell envelope damages of foodborne pathogens Listeria monocytogenes and Salmonella Enteritidis treated with cold plasma. Food Microbiol. (2022) 101:103891. doi: 10.1016/j.fm.2021.103891
24. Gavahian M, Sarangapani C, Misra NN. Cold plasma for mitigating agrochemical and pesticide residue in food and water: similarities with ozone and ultraviolet technologies. Food Res Int. (2021) 141:110138. doi: 10.1016/j.foodres.2021.110138
25. Wang X, Wang S, Yan Y, Wang W, Zhang L, Zong W. The degradation of Alternaria mycotoxins by dielectric barrier discharge cold plasma. Food Control. (2020) 117:107333. doi: 10.1016/j.foodcont.2020.107333
26. Tappi S, Berardinelli A, Ragni L, Dalla Rosa M, Guarnieri A, Rocculi P. Atmospheric gas plasma treatment of fresh-cut apples. Innov Food Sci Emerg Technol. (2014) 21:114–22. doi: 10.1016/j.ifset.2013.09.012
27. Pankaj SK, Misra NN, Cullen PJ. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov Food Sci Emerg Technol. (2013) 19:153–7. doi: 10.1016/j.ifset.2013.03.001
28. Takai E, Kitano K, Kuwabara J, Shiraki K. Protein inactivation by low-temperature atmospheric pressure plasma in aqueous solution. Plasma Process Polym. (2012) 9:77–82. doi: 10.1002/ppap.201100063
29. Hemmati V, Garavand F, Khorshidian N, Cacciotti I, Goudarzi M, Chaichi M, et al. Impact of cold atmospheric plasma on microbial safety, total phenolic and flavonoid contents, antioxidant activity, volatile compounds, surface morphology, and sensory quality of green tea powder. Food Biosci. (2021) 44:101348. doi: 10.1016/j.fbio.2021.101348
30. Yinxin L, Can Z, Menglu H, Cui S, Jinping C, Jingyu W, et al. Effect of cold atmospheric plasma on the gray mold rot of postharvest mulberry fruit. Food Control. (2022) 137:108906. doi: 10.1016/j.foodcont.2022.108906
31. Giannoglou M, Xanthou Z-M, Chanioti S, Stergiou P, Christopoulos M, Dimitrakellis P, et al. Effect of cold atmospheric plasma and pulsed electromagnetic fields on strawberry quality and shelf-life. Innov Food Sci Emerg Technol. (2021) 68:102631. doi: 10.1016/j.ifset.2021.102631
32. Puligundla P, Lee T, Mok C. Effect of intermittent corona discharge plasma treatment for improving microbial quality and shelf life of kumquat (Citrus japonica) fruits. LWT. (2018) 91:8–13. doi: 10.1016/j.lwt.2018.01.019
33. Mao L, Mhaske P, Zing X, Kasapis S, Majzoobi M, Farahnaky A. Cold plasma: microbial inactivation and effects on quality attributes of fresh and minimally processed fruits and ready-to-eat vegetables. Trends Food Sci Technol. (2021) 116:146–75. doi: 10.1016/j.tifs.2021.07.002
34. Bao T, Hao X, Shishir MRI, Karim N, Chen W. Cold plasma: an emerging pretreatment technology for the drying of jujube slices. Food Chem. (2021) 337:127783. doi: 10.1016/j.foodchem.2020.127783
35. Zheng H, Liu W, Liu S, Liu C, Zheng L. Effects of melatonin treatment on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. (2019) 299:125116. doi: 10.1016/j.foodchem.2019.125116
36. Dzimitrowicz A, Jamroz P, Cyganowski P, Bielawska-Pohl A, Klimczak A, Pohl P. Application of cold atmospheric pressure plasmas for high-throughput production of safe-to-consume beetroot juice with improved nutritional quality. Food Chem. (2021) 336:127635. doi: 10.1016/j.foodchem.2020.127635
37. Wang L, Luo Z, Ban Z, Jiang N, Yang M, Li L. Role of exogenous melatonin involved in phenolic metabolism of Zizyphus jujuba fruit. Food Chem. (2021) 341:128268. doi: 10.1016/j.foodchem.2020.128268
38. Li X, Li M, Ji N, Jin P, Zhang J, Zheng Y, et al. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT. (2019) 115:108447. doi: 10.1016/j.lwt.2019.108447
39. Yu Y, Guo W, Liu Y, Sang Y, Yang W, Guo M, et al. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao). Sci Hortic. (2021) 288:110372.
40. Bremenkamp I, Ramos AV, Lu P, Patange A, Bourke P, Sousa-Gallagher MJ. Combined effect of plasma treatment and equilibrium modified atmosphere packaging on safety and quality of cherry tomatoes. Future Foods (2021) 3:100011. doi: 10.1016/j.fufo.2021.100011
41. Li L, Zhang M, Bhandari B, Zhou L. LF-NMR online detection of water dynamics in apple cubes during microwave vacuum drying. Dry Technol. (2018) 36:2006–15. doi: 10.1080/07373937.2018.1432643
42. Sun Q, Zhang M, Yang P. Combination of LF-NMR and BP-ANN to monitor water states of typical fruits and vegetables during microwave vacuum drying. LWT. (2019) 116:108548. doi: 10.1016/j.lwt.2019.108548
43. Romero P, Lafuente MT. Relative humidity regimes modify epicuticular wax metabolism and fruit properties during Navelate orange conservation in an ABA-dependent manner. Food Chem. (2022) 369:130946. doi: 10.1016/j.foodchem.2021.130946
44. Zuo X, Cao S, Zhang M, Cheng Z, Cao T, Jin P, et al. High relative humidity (HRH) storage alleviates chilling injury of zucchini fruit by promoting the accumulation of proline and ABA. Postharvest Biol Technol. (2021) 171:111344.
45. Lufu R, Ambaw A, Opara UL. Water loss of fresh fruit: influencing pre-harvest, harvest and postharvest factors. Sci Hortic. (2020) 272:109519. doi: 10.1016/j.scienta.2020.109519
46. Won MY, Lee SJ, Min SC. Mandarin preservation by microwave-powered cold plasma treatment. Innov Food Sci Emerg Technol. (2017) 39:25–32. doi: 10.1016/j.ifset.2016.10.021
47. Nian Y, Wang N, Li R, Shao Y, Li W. Cold shock treatment alleviates chilling injury in papaya fruit during storage by improving antioxidant capacity and related gene expression. Sci Hortic. (2022) 294:110784. doi: 10.1016/j.scienta.2021.110784
48. Sarangapani C, Thirumdas R, Devi Y, Trimukhe A, Deshmukh RR, Annapure US. Effect of low-pressure plasma on physico–chemical and functional properties of parboiled rice flour. LWT Food Sci Technol. (2016) 69:482–9. doi: 10.1016/j.lwt.2016.02.003
49. Sarangapani C, O’Toole G, Cullen PJ, Bourke P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov Food Sci Emerg Technol. (2017) 44:235–41. doi: 10.1016/j.ifset.2017.02.012
50. Hu X, Sun H, Yang X, Cui D, Wang Y, Zhuang J, et al. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol Technol. (2021) 179:111564. doi: 10.1016/j.postharvbio.2021.111564
Keywords: cold atmospheric plasma, winter jujube, antioxidant activity, gene expression, postharvest qualities
Citation: Jin T, Dai C, Xu Y, Chen Y, Xu Q and Wu Z (2022) Applying Cold Atmospheric Plasma to Preserve the Postharvest Qualities of Winter Jujube (Ziziphus jujuba Mill. cv. Dongzao) During Cold Storage. Front. Nutr. 9:934841. doi: 10.3389/fnut.2022.934841
Received: 03 May 2022; Accepted: 13 June 2022;
Published: 06 July 2022.
Edited by:
Fabián Guillén, Miguel Hernández University of Elche, SpainReviewed by:
Xiaolong Ji, Zhengzhou University of Light Industry, ChinaJiaqi Yan, China Agricultural University, China
Copyright © 2022 Jin, Dai, Xu, Chen, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Xu, eHFoMTEyNkBzaW5hLmNvbQ==; Zhengwei Wu, d3V6d0B1c3RjLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Tao Jin
Tao Jin Chenwei Dai3†
Chenwei Dai3† Zhengwei Wu
Zhengwei Wu