- 1Non-communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 2Chronic Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute Tehran University of Medical Sciences, Tehran, Iran
- 3Dietary Supplements and Probiotic Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 4Research Development Center, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 5Cardiac Primary Prevention Research Center, Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 6Social Determinants of Health Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 7Department of Internal Medicine, Faculty of Medicine, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 8Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease Isfahan University of Medical Sciences, Isfahan, Iran
Introduction: Metabolic syndrome comprises a set of metabolic risk factors associated with cardiovascular disease and type 2 diabetes. Zinc plays an essential role in numerous enzyme functions that may be associated with metabolic dysfunctions. The relationship between serum zinc levels and metabolic syndrome in adolescents has not been specifically studied. Therefore, this study was performed to determine the relationship between serum zinc levels and metabolic syndrome in Iranian children and adolescents.
Materials and methods: This cross-sectional study was performed using data collected in the CASPIAN-V study. In this project, data were collected using interviews, examinations, biochemical assessments, anthropometric studies, and the nutritional status of participants. The variables considered in this study included serum zinc levels, triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), fasting blood sugar, height, weight, abdominal circumference, and systolic and diastolic blood pressure.
Results: A total of 1371 participants were included in this study, with a mean age of 12.24 ± 3.23 years. In total, 12.40% (n = 170) of the study population had metabolic syndrome, of which 55.7% were boys and 44.3% were girls. Mean zinc levels (μg/dL) in patients with and without metabolic syndrome were 107.03 and 110.6, respectively (p-value = 0.211) and 111.8 for boys and 109.10 for girls (p-value = 0.677).
Conclusion: This cross-sectional study showed no association between serum zinc levels and metabolic syndrome in children. Further similar studies and cohort studies with large sample sizes are needed to reveal the exact relationship between serum zinc levels and metabolic syndrome.
Introduction
Metabolic syndrome is a set of metabolic risk factors associated with cardiovascular diseases and type 2 diabetes (1). Numerous definitions of components and their diagnostic boundaries have been proposed for this syndrome, all of which are associated with metabolic disorders such as elevated blood pressure, impaired glucose and insulin metabolism, dyslipidemia, and central obesity (Measured parameters include: abdominal obesity or waist circumference equal to or more than 90th percentile of the age and sex; Systolic or diastolic blood pressure (SBP or DBP) equal to or greater than 90th percentile by height, age, and sex; Serum triglyceride (TG) level greater than 110 mg/dL, High-density lipoprotein (HDL) equal to or less than 40 mg/dL, fasting glucose level equal to or more than 100 mg/dL) (1–3). Metabolic syndrome is one of the main health hazards worldwide, resulting in a significant number of years lost (DALY) due to its associated morbidity and mortality (3, 4). Although many studies have been conducted on the prevalence of this syndrome in adults based on different definitions and its relationship with cardiovascular diseases (2, 3), there is no precise definition for it in childhood and adolescence. As nutrition plays a major role in growth and development in children, and deficiencies in various nutrients could result in metabolic defects (5), it seems that deficiencies in trace elements such as zinc which plays an essential role in numerous enzyme functions could result in metabolic dysfunction as well (6).
Zinc is a rare and essential element in the body involved in the metabolism of nucleic acids and their stability in protein synthesis, cell division, and gene expression. Furthermore, zinc plays an essential role in the activity of more than 300 enzymes in the body. Its deficiency can result in many skin diseases, mental disorders, pregnancy, lactation, growth disturbances, and susceptibility to infections (7). Hence, in theory, zinc deficiency can result in metabolism defects and metabolic disorders. Since the body does not have a proper zinc reserve, nutrition plays a significant role in providing this micronutrient (8).
Although severe zinc deficiency is rare, studies have shown that mild to moderate zinc deficiency occurs on a large scale in unbalanced diets (9). In Iran, due to the calcareous nature of agricultural soils, bicarbonate water, and the lack of zinc-containing fertilizers, the amount of zinc absorbed by plants is minimal (10–12).
The relationship between serum zinc levels and the incidence of metabolic syndrome in adolescents has not been specifically studied, and there has been controversy regarding the effects of zinc on metabolic syndrome (13). Therefore, this study was performed to determine the relationship between serum zinc levels and metabolic syndrome in children and adolescents.
Materials and methods
Study design
This cross-sectional study was performed using data collected in the Caspian-V study. (Caspian-V study, was a care system for health-related behaviors and risk factors for diseases in students in Iran).
Data collection
Sampling was done by multi-stage using cluster and stratified sampling method. Class sampling was performed in each province of the country according to the student’s residence (city or village) and educational level (primary and secondary) in a manner commensurate with the size with an equal sex ratio. This means that the number of boys and girls in each province was equal, and their ratio in urban and rural areas was also proportional to the number of urban and rural students. Similarly, the number of samples was divided between the educational levels in the city and the village in proportion to the number of students studying at each level. In this study, 480 students were selected from each province (i.e., 48 clusters of 10 people in each province). In total, according to the study in 31 provinces, 14,880 people were surveyed using the standard questionnaire “WHO-GSHS,” which has been translated into Persian, and its validity has been evaluated and approved in previous studies (14). Trained professionals collected information about health-related behaviors and risk factors for diseases; and assessed anthropometric measurements, including height, weight, waist circumference, hip, neck, wrist circumferences, and blood pressure, for all participating students. One-third of the number of clusters from each province were randomly selected for blood sampling, and a skilled blood sampler took six ccs of their venous blood with consideration of all health issues to measure blood glucose indices, lipid profile, liver enzymes, and serum zinc level. It should be noted that the waist circumference was measured as a waist from the tangent and above the iliac crest to the ground with a metal meter. Metabolic syndrome was defined based on NHANES III (Third National Health And Nutrition Examination Survey)(15) as the presence of 3 out of 5 criteria for the diagnosis of this syndrome, including abdominal obesity or waist equal to or more than 90th percentile in terms of age and sex; SBP or DBP equal to or greater than 90th percentile by height, age, and sex; serum TG level greater than 110 mg/dL, HDL serum equal to or less than 40 mg/dL, fasting serum glucose levels equal to or greater than 100 mg/dL.
Inclusion and exclusion criteria
The study population was children and adolescents studying in primary or secondary school whose information was recorded in the Caspian Cohort. Participants with incomplete data, according to the studied variables, as well as individuals with reduced renal function (glomerular filtration rate; GFR < 30), Chronic liver diseases, and glucocorticoids were excluded from the study.
Data analysis
The SPSS software version 21 was used for data analyzes. Descriptive analysis results on quantitative variables are presented as mean and standard deviation, and qualitative variables as frequency and relative frequency. Furthermore, the correlation of coefficients was calculated. A p-value less than 0.05 was considered statistically significant.
Ethical considerations
No information about the identities of individuals entered the study process; hence, the data were anonymous, and at no stage of the study, the individuals’ information was recorded or mentioned. Furthermore, this study was approved by the ethics committee of the Alborz University of Medical Sciences.
Results
A total of 1,371 participants were included in this study, with a mean age of 12.24 ± 3.22 years. In total, 6.41% (n = 88) of the study population had metabolic syndrome, of which 55.68% were boys, and 44.32% were girls. The means of height, weight, waist circumference, SBP, DBP, TG and HDL were 147.24 ± 18.13 cm, 42.64 ± 16.5 kg, 67.7 ± 11.72 cm, 101.33 ± 13.46 mmHg, 65.45 ± 10.76 mmHg, 88.08 ± 44.58 mg/dL, and 46.88 ± 9.24 mg/dL, respectively. Serum zinc level mean was 107.23 ± 25.81 μg/dL. The presence or absence of metabolic syndrome based on demographic parameters has been categorized in Table 1. As shown in this table, no significant difference was observed between zinc levels in children and adolescents with or without metabolic syndrome (p-value = 0.211). Among the different variables among children with metabolic syndrome and others, only a significant difference in their TG levels can be seen. Participants with metabolic syndrome had greater TG and FBS compared to those without metabolic syndrome (109.29 ± 68.25 vs. 86.62 ± 41.18; p-value < 0.0001 and 99.26 ± 8.65 vs. 92.95 ± 8.32; p-value < 0.0001, respectively).
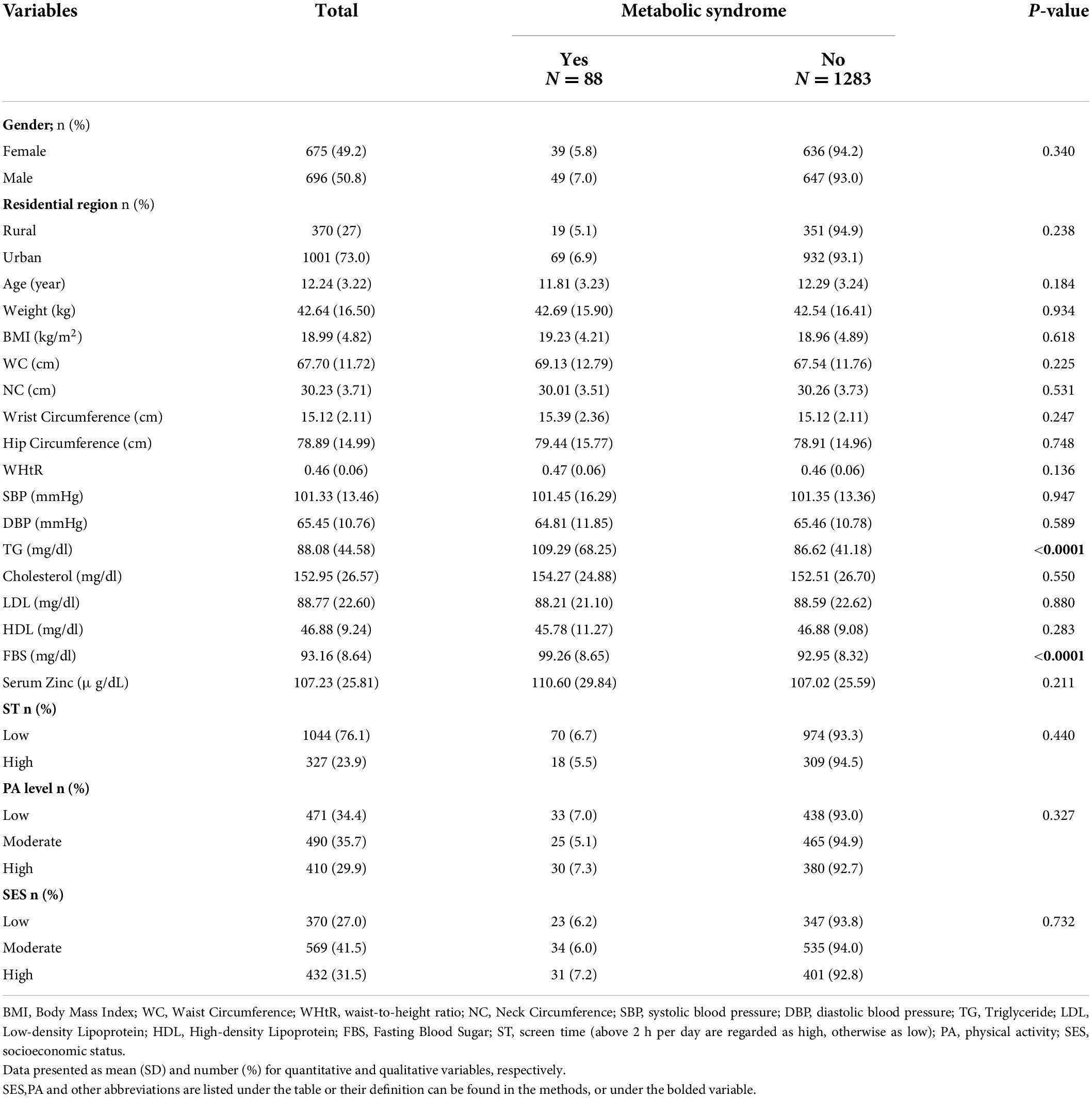
Table 1. Demographic status of the study population according to the presence or absence of metabolic syndrome (n = 1371).
Furthermore, there was no significant difference between zinc levels and sex in participants with and without metabolic syndrome. (p-value = 0.34). Similarly, there was no significant relationship between serum zinc levels and the age of children in children without metabolic syndrome (p-value = 0.184).
In Table 2, the association between metabolic syndrome components and dyslipidemia with serum zinc levels based on linear logistic regression analysis can be seen. No significant association was found between serum zinc levels and any metabolic syndrome components. Table 3 illustrates the same data but is based on serum zinc tertiles. As can be seen in Tables 2, 3, no significant relationship between serum zinc levels and the aforementioned variables was observed.
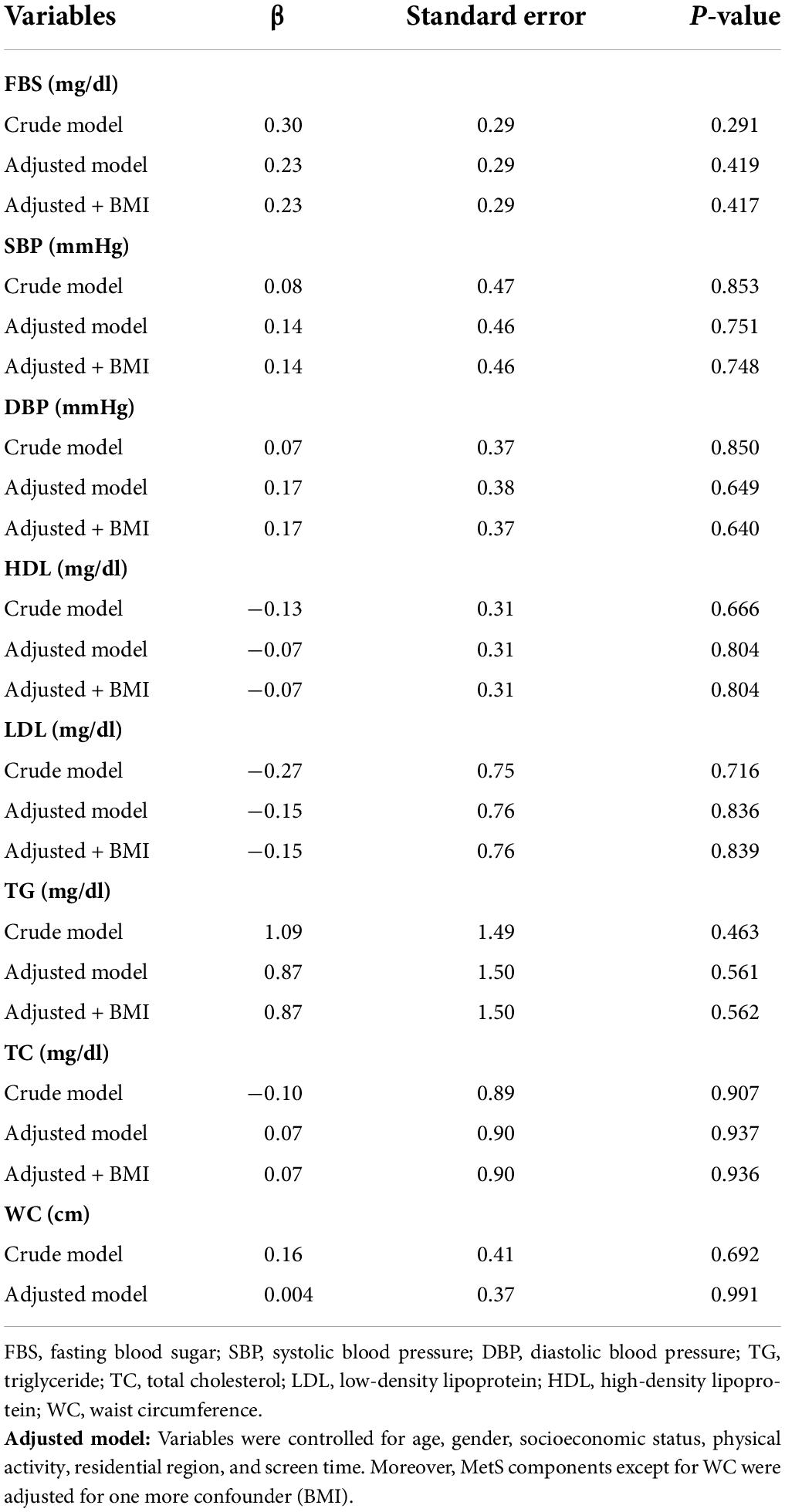
Table 2. Association between metabolic syndrome components and dyslipidemia with serum zinc level in children: linear regression analysis.
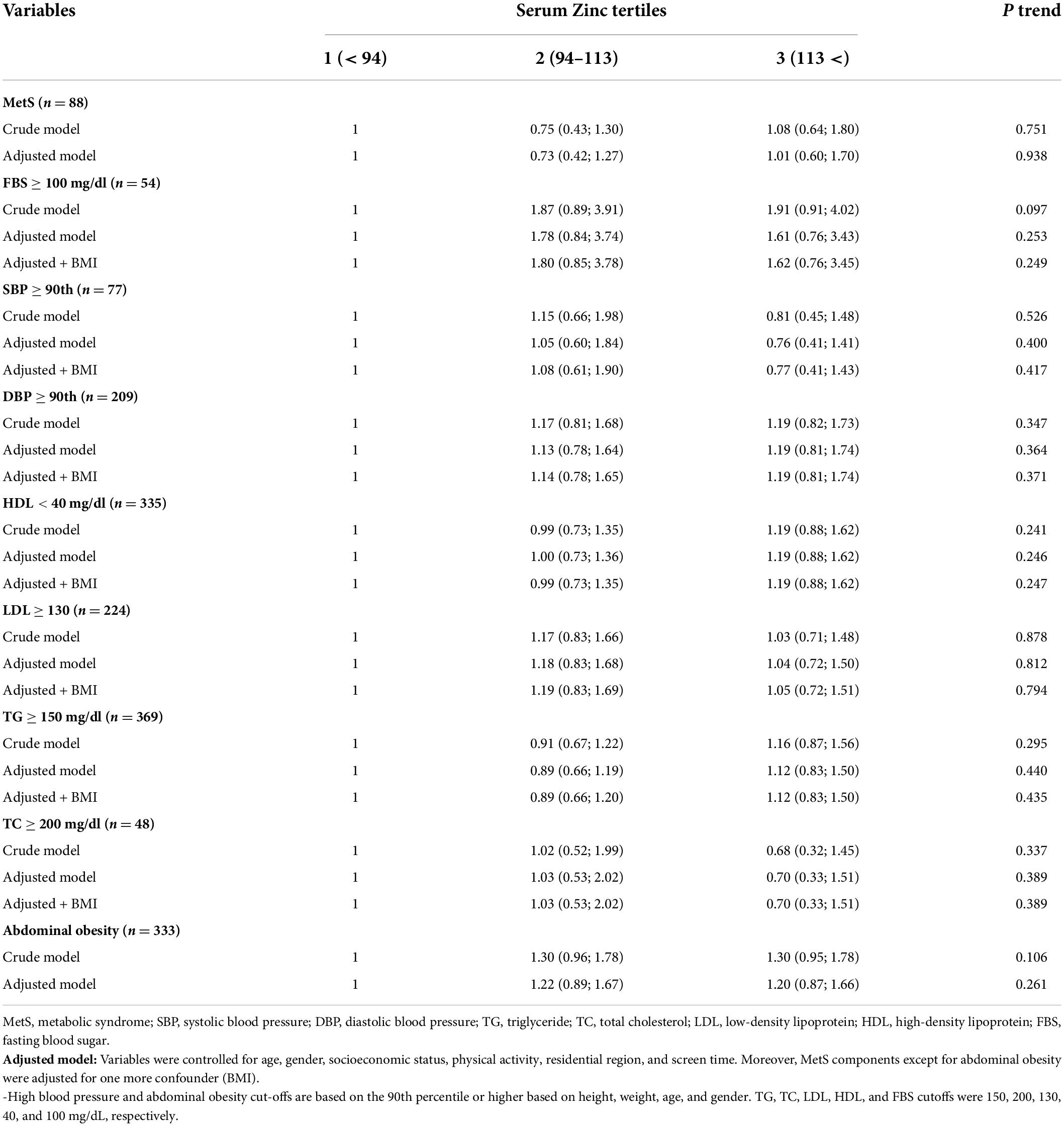
Table 3. Association between metabolic syndrome components and dyslipidemia with tertiles of serum zinc in children: logistic regression analysis.
Discussion
This study investigated the serum level of zinc in children and adolescents and the factors affecting metabolic syndrome. Metabolic syndrome is one of the most significant risk factors associated with cardiovascular disease and type 2 diabetes (3). Studies estimate that more than 100 million children worldwide are obese (16), highlighting the importance of paying attention to metabolic syndrome and its factors in this age group. Moreover, studies also suggest that serum zinc and manganese levels may be essential factors in controlling and synthesizing insulin and controlling fat profile in individuals (17).
This cross-sectional study, based on the population of the Caspian cohort, showed no significant difference in the overall picture between children and adolescents with metabolic syndrome in terms of serum zinc levels. Also, in subgroup analysis, there was no significant correlation between serum zinc levels and age, abdominal obesity, or different fat profiles, and in all evaluations, the numbers did not differ significantly in children with metabolic syndrome or others. However, based on the findings of this study, it seems that healthy girls with metabolic syndrome have lower serum zinc levels than boys. This difference, In addition to biological factors, can also be attributed to the different eating habits of boys and girls. In addition, the studies showed a slight difference between serum zinc levels and blood pressure status in children with metabolic syndrome. Among children with metabolic syndrome, serum zinc levels were significantly lower in children with elevated blood pressure than in others. Compared to this study, few studies have reported a more significant zinc effect than what was seen in this study. For example, in the study of the relationship between serum zinc levels and metabolic syndrome in Korea in 2014, 1,926 people were studied and analyzed. Serum zinc levels were negatively correlated with elevated fasting blood sugar and positively correlated with elevated TG levels in a statistically significant manner. It was also found that in women, serum zinc levels are associated with an increase in the incidence of metabolic syndrome (18). Although, some studies report an association between zinc levels and blood pressure (19), and in theory, zinc could potentially affect blood pressure, due to the effects of zinc on the endothelial cells and vasodilation/constriction through its interactions with various enzymes and proteins (further explained by Tubek) (20); in this study, serum zinc levels were not significantly correlated with blood pressure.
However, it should be noted that the findings on this subject are not entirely consistent. For example, a study by Ghasemi et al. in 2014 in Tehran that was performed on 2401 participants, indicated that there is a significant difference between the genders of participants regarding the relationship between serum zinc levels and metabolic syndrome;(21).
The difference in the populations being studied. (children, adults, specific groups of patients), different adjustments, different settings, confounding variables, and most importantly serum zinc level differences across studies could be the cause of the observed controversial findings. As stated zinc plays a part in many metabolic functions, some of these functions are opposite in nature and outcome (20, 22); Thus many metabolic factors, could potentially impact the results of studies (e.g., mean levels of serum zinc, mean age, sex, ethnicity and other related factors of the population). Hence, more detailed studies are recommended to determine the role of serum zinc levels in the progression of metabolic disease.
It should be noted that in these studies, adults were studied, while in this study, the target population was focused on children and adolescents. Therefore, differences in the final findings are to be expected. In this regard, a study was performed on 60 obese Iranian children by giving zinc and placebo supplements. This intervention showed that zinc supplementation reduces LDL, Total Cholesterol, C-reactive protein (CRP), markers of insulin resistance, fasting blood sugar, insulin, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) in children. It should be noted that this study was interventional and prospective, not a cross-sectional one (23).
Our study showed no significant association between serum zinc levels, Mets, and its components. In a similar study conducted on Chinese children and adolescents regarding the association of serum zinc level and metabolic syndrome alongside its components, similarly, no association between serum zinc levels and MetS was found, However serum zinc levels were inversely associated with low-HDL levels and elevated fasting blood glucose with a significant trend (24).
Recommendation for future research
Further research is needed on the need, deficiency, and biological effects of these supplements to further determine the factors affecting the usefulness of zinc supplementation in children with metabolic syndrome. Furthermore, this study shows that there is no significant difference between children with metabolic syndrome and others in terms of weight; however, the fat profile significantly affected the incidence of metabolic syndrome. Thus, studies need to be conducted, not focusing on obesity but on the global standards of metabolic syndrome in children and adolescents.
Limitations and strength
To the best of our knowledge, it is the first study that assessed the relationship between serum zinc levels and MetS components, especially with consideration of confounder variables and impressive sample size. However, there are probably some other unknown confounders that are effective in the exact association. Moreover, this study was cross-sectional and due to its natural cause and effect relationship, could not distinguish.
Conclusion
This cross-sectional study showed no association between serum zinc levels and metabolic syndrome in children. Hence it seems that zinc supplementation in children without zinc deficiency who suffer from Mets or any of its components is redundant. Nonetheless, clinicians must keep racial and age aspects in mind since conflicting findings across different races, sexes, and ages were seen.
Further studies similar studies and cohort studies are needed to reveal the exact relationship between serum zinc levels and metabolic syndrome and warrant our findings.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Alborz University of Medical Sciences. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
MQ and NM conceived the study. NMK and ED substantially contributed to the conception and design, data analysis and interpretation, drafted the manuscript, and revised it critically. RK, HA, and LS participated in preparing the manuscript. AM-G, ME, and RH participated in the study design and data acquisition. MQ carried out the analysis of the data. All authors read and approved the final version of the manuscript.
Acknowledgments
This project was conducted as part of a national school-based surveillance program and was funded by Alborz University of Medical Sciences. The authors are thankful for the large team working on this national project and all participants who cooperated with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin. (2014) 43:1–23. doi: 10.1016/j.ecl.2013.09.009
2. Huang PL. A comprehensive definition for metabolic syndrome. Dis Models Mech. (2009) 2:231–7. doi: 10.1242/dmm.001180
3. Wild SH, Byrne CD. The global burden of the metabolic syndrome and its consequences for diabetes and cardiovascular disease. Metab Syndr. (2005) 1–41. doi: 10.1002/0470025131.ch1
4. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
5. Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, et al. Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal. (2012) 17:282–301. doi: 10.1089/ars.2011.4381
7. Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. (2013) 18:144.
8. Tuerk MJ, Fazel N. Zinc deficiency. Curr Opin Gastroenterol. (2009) 25:136–43. doi: 10.1097/MOG.0b013e328321b395
10. Iyengar B, Raja ME. Zinc adsorption as related to its availability in some soils of Karnataka. J Indian Soc Soil Sci. (1983) 31:432–8.
11. Karimian N, Moafpouryan G. Zinc adsorption characteristics of selected calcareous soils of Iran and their relationship with soil properties. Commun Soil Sci Plant Anal. (1999) 30:1721–31. doi: 10.1080/00103629909370325
12. Harter RD. Micronutrient adsorption-desorption reactions in soils. Micronutr Agric. (1991) 4:59–87. doi: 10.2136/sssabookser4.2ed.c3
13. Zhang Y, Zhang D-Z. Relationship between serum zinc level and metabolic syndrome: A meta-analysis of observational studies. J Am Coll Nutr. (2018) 37:708–15. doi: 10.1080/07315724.2018.1463876
14. Ziaei R, Dastgiri S, Soares J, Baybordi E, Zeinalzadeh A, Rahimi VA, et al. Reliability and validity of the Persian version of global school-based student health survey adapted for Iranian school students. J Clin Res Gov. (2014) 3:134–40.
15. Statistics NCFH. Plan and operation of the third national health and nutrition examination survey, 1988–94. Hyattsville, MD: National Centre for Health Statistics (1994).
16. Bussler S, Penke M, Flemming G, Elhassan YS, Kratzsch J, Sergeyev E, et al. Novel insights in the metabolic syndrome in childhood and adolescence. Hormone Res Paediatr. (2017) 88:181–93. doi: 10.1159/000479510
17. Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. (2013) 7:31–52. doi: 10.1007/s11684-013-0251-9
18. Seo J-A, Song S-W, Han K, Lee K-J, Kim H-N. The associations between serum zinc levels and metabolic syndrome in the Korean population: Findings from the 2010 Korean national health and nutrition examination survey. PLoS One. (2014) 9:e105990. doi: 10.1371/journal.pone.0105990
19. Carpenter WE, Lam D, Toney GM, Weintraub NL, Qin Z. Zinc, copper, and blood pressure: Human population studies. Med Sci Monit. (2013) 19:1–8. doi: 10.12659/msm.883708
20. Tubek S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res. (2007) 117:39–51. doi: 10.1007/BF02698082
21. Ghasemi A, Zahediasl S, Hosseini-Esfahani F, Azizi F. Gender differences in the relationship between serum zinc concentration and metabolic syndrome. Ann Hum Biol. (2014) 41:436–42. doi: 10.3109/03014460.2013.870228
23. Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. (2010) 8:505–10. doi: 10.1089/met.2010.0020
Keywords: metabolic syndrome, zinc, adolescents, children, CASPIAN-V
Citation: Qorbani M, Movasaghi N, Mohammadian Khonsari N, Daneshzad E, Shafiee G, Ashraf H, Sokoty L, Mahdavi-Gorabi A, Ebrahimi M, Heshmat R and Kelishadi R (2022) Association of zinc serum level with metabolic syndrome in iranian children and adolescents: The CASPIAN-V study. Front. Nutr. 9:932746. doi: 10.3389/fnut.2022.932746
Received: 30 April 2022; Accepted: 11 July 2022;
Published: 09 August 2022.
Edited by:
Mainul Haque, National Defense University of Malaysia, MalaysiaReviewed by:
Parinaz Poursafa, Tampere University, FinlandRahnuma Ahmad, Medical College for Women and Hospital, Bangladesh
Adekunle Rowaiye, National Biotechnology Development Agency, Nigeria
Copyright © 2022 Qorbani, Movasaghi, Mohammadian Khonsari, Daneshzad, Shafiee, Ashraf, Sokoty, Mahdavi-Gorabi, Ebrahimi, Heshmat and Kelishadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Ebrahimi, bV9lYnJhaGltaTQ5QHlhaG9vLmNvbQ==; Ramin Heshmat, cmhlc2htYXRAdHVtcy5hYy5pcg==
 Mostafa Qorbani
Mostafa Qorbani Negar Movasaghi2
Negar Movasaghi2 Nami Mohammadian Khonsari
Nami Mohammadian Khonsari Elnaz Daneshzad
Elnaz Daneshzad Gita Shafiee
Gita Shafiee Leily Sokoty
Leily Sokoty Mehdi Ebrahimi
Mehdi Ebrahimi Ramin Heshmat
Ramin Heshmat Roya Kelishadi
Roya Kelishadi