
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 01 July 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.929891
Background: The role of leucine in sarcopenia prevention remains unclear. We aimed to summarize the published data from randomized controlled trials (RCTs) to estimate the effect of leucine supplementation on sarcopenia-related measures in older adults.
Methods: A systematic literature search was performed using the electronic databases PubMed, Embase, and Web of Science with restriction to randomized controlled trials design from January 1, 2009 to March 19, 2022. Sarcopenia-related measures included handgrip strength, total lean mass, gait speed, leg press, 6-min walk test, short-physical performance battery, timed up-and-go test and 30-s chair-stand test. Fixed- and random-effects meta-analysis models were used to generate pooled weighted mean differences (WMDs) and 95% CIs. Heterogeneity was examined in subgroup and sensitivity analyses. Publication bias assessments were performed.
Results: A total of 17 RCTs enrolling 1418 subjects were identified. Leucine-isolated supplementation showed no effect on total lean mass (WMD = 0.03 kg, 95% CI: –0.51, 0.57, P = 0.917), handgrip strength (WMD = 1.23 kg, 95% CI: –0.58, 3.03, P = 0.183) and leg press (WMD = –1.35 kg, 95% CI: –7.46, 4.77, P = 0.666). However, leucine-combined supplementation including vitamin D showed a significant improvement in handgrip strength (WMD = 2.17 kg, 95% CI: 0.24, 4.10, P = 0.027) and gait speed (WMD = 0.03 m/s, 95% CI: 0.01, 0.05, P = 0.008).
Conclusion: Leucine-isolated supplementation did not improve muscle mass and strength in elderly. However, leucine-combined supplementation including vitamin D exhibited a significant benefit for muscle strength and performance including handgrip strength and gait speed in older adults. A combination of nutritional supplements would be a viable option for improving sarcopenia.
Sarcopenia is a progressive loss of muscle mass, strength, and function (1), which usually develops with advanced age (2). The estimated prevalence of sarcopenia in people aged over 60 years was 10% according to a meta-analysis including 58404 individuals around the world (3). After the age of 60, the estimated muscle mass decreased at a rate of 3% per year, while the grip strength and gait speed decreased at a rate of 1.9–5.0% and 2.0–2.3% per year, respectively (4, 5). Sarcopenia was reported as one of the leading health issues in the older adults which could reduce the quality of life in the long term (6), and cause adverse health consequences including malnutrition (7), falls (8), disability, and even death (9). Although accumulating studies have focused on sarcopenia (10), there are still challenges in prevention and treatment of the disease. The homeostasis of amino acids has been increasingly suggested to be critical to maintaining muscle health (11).
L-leucine is an essential non-polar aliphatic, branched-chain amino acid (12), which activates the transducer of regulated cAMP response element-binding protein activity 1 (TORC1) in human skeletal muscle. The activation of TORC1 contributes to the initial stimulus of muscle protein synthesis, increasing the availability of amino acids through translation (13, 14). Leucine has a strong effect on energy and lipid metabolism (15). Increased energy expenditure and toxic lipids removal by increasing the prevalence and activity of leucine may be a promising therapeutic strategy to treat obesity and its consequent conditions (16, 17). Furthermore, some observational studies and randomized controlled trials (RCTs) have reported associations between leucine and muscle mass, muscle properties, and muscle functions (18–20).
Studies suggested that L-leucine supplementation was able to enhance muscle protein synthesis in the elderly (21–23). Some RCTs indicated that leucine could improve clinical indicators of sarcopenia in the elderly, including functional performance, and improve bone mineral-free lean tissue mass (12, 24). In contrast, several trials reported that prolonged leucine supplementation could not modulate body composition, muscle mass, and strength in elderly individuals (25–27). Inconsistent findings motivated a comprehensive systematic review and meta-analysis that evaluates the relationship between leucine and sarcopenia measures. Therefore, we summarized the latest evidence for the effect of leucine supplementation on sarcopenia measures in older adults based on published data from RCTs.
We searched the literature in the electronic bibliographic databases of PubMed, Embase, and Web of Science from January 1, 2009 to March 19, 2022 by following keywords or phrases: (“amino acid” OR “L-Leucine” OR “L-isomer Leucine” OR “leucine” OR “Leu”) AND (“RCT” OR “controlled trial” OR “randomized trial”) AND (“sarcopenia”). The detailed search strategies were listed in Supplementary Table 1. The search was restricted to human studies published in the English language with full text available.
Screening of the literature and extraction of the data were done by XF and YG. First, we exported the literature from the database, and then we browsed every reference according to the title and abstract after removing the duplicate references. After deleting the irrelevant literature, we further browsed the full text of each article. Studies incorporated into the final analysis need to meet the following criteria: (1) were original investigations; (2) were randomized controlled trials; (3) the subjects were over 60 years old; (4) reported at least one diagnostic criterion for sarcopenia, including muscle mass (total lean mass), muscle strength (handgrip strength and leg press), physical performance (gait speed, short-physical performance battery, 6-min walk test, 30-s chair-stand test, and timed up-and-go test); (5) reported the doses of leucine.
The contents to be extracted included authors, year of publication, study design, sample size, mean age, gender, population, duration of follow-up, duration of intervention, leucine-isolated (yes/no), with/without vitamin D, physical exercise, type of leucine and dosage (g/day), muscle mass, muscle strength, and muscle performance outcomes. Study quality was assessed by the Modified Jadad Scale (scores ranged from 0 to 7).
Comparisons were made between the leucine-isolated/combined supplementation and control groups with reference to the difference in mean and standard deviation (SD) from baseline to final. We converted variances, standard errors, or confidence intervals to SD according to Cochrane Handbook when the data description form was not SD. Furthermore, we calculated the changes in mean and SD if only baseline and final data were available. Mean change values were calculated as the final mean minus the baseline mean. SD change values were estimated from the baseline and final SD using the following equation, derived from the Cochrane Handbook for Systematic Review of Interventions (28):
In this equation, we used 0.8 as the assumed correlation (Use correlation coefficients obtained from studies according to the Cochrane Handbook).
The heterogeneity of the results was assessed using Cochran Q (Chi-square test) and I2 statistics. Statistical significance was set at P < 0.10 for Cochran Q test. When I2 > 50%, it was calculated by random effect. When I2 < 50%, the fixed effect was adopted. We performed subgroup analyses to explore the heterogeneity of the effect estimates based on modified Jadad score, vitamin D supplementation, physical activity, region of study and leucine-isolated/combined supplementation, dosage of leucine supplementation. All results were submitted for sensitivity analysis using the “remove-1” strategy. Publication bias was assessed by the Funnel plots and Egger’s regression model. Statistical significance was considered if the 95% CI did not contain 0. All the statistical analyses were performed using Stata version 15.1 (StataCorp, College Station, TX, United States).
Figure 1 shows a flowchart of the study screening and selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We retrieved 2,346 records after searching the three databases, 177 from PubMed, 1,019 from Web of Science, 1,148 from Embase, and 2 through manual search. After removing duplicates (n = 576) and browsing the remaining articles (n = 1,770) by title and abstract, with three additional articles identified through manual searches, 31 records were screened out for further evaluation by carefully checking the full-length articles. Finally, we obtained 17 RCTs meeting the inclusion criteria. The quality of these studies is shown in Supplementary Table 2.
Demographic data of the subjects and study characteristics of the included RCTs are summarized in Table 1. The total number of subjects from all the included studies was 1,418 (range of study sample size: 19,380). The mean age of the subjects ranged from 67.7 to 82.3. There were 11 studies that included men and women (12, 24, 25, 29–36), and six studies (26, 27, 37–40) included only male or female subjects. The duration of the intervention varied from 4 to 48 weeks. Subjects of nine studies (25, 26, 30, 33–36, 38, 39) took part in a controlled physical activity program. Total lean mass (TLM) was measured in nine studies (24, 26, 27, 30, 32, 34, 35, 37, 40). Handgrip strength was assessed in 11 studies (12, 26, 29–36, 40). Leg press was assessed in three studies (26, 27, 39). Gait speed was assessed in seven studies with eight treatment arms (29–33, 38, 40). Short-physical performance battery (SPPB) was assessed in six studies (25, 29, 31, 35, 36, 40). 6-min walk test (6-WT) was assessed in five studies with six treatment arms (24, 25, 33, 35, 39). 30-s chair-stand test (30-CST) was assessed in five studies with six treatment arms (24, 26, 29, 36, 40). Timed up-and-go test (TUG) was assessed in three studies (26, 29, 33).
The effects of leucine-isolated/combined supplements on muscle mass were assessed by TLM. A total of nine RCTs (n = 438) reported TLM as an outcome indicator. No significant difference in TLM was observed in leucine-isolated/combined supplementation group compared with the placebo group (WMD = 0.29 kg, 95% CI: –0.06, 0.63, P = 0.102; Supplementary Figure 1).
The effect of leucine-isolated/combined supplement on muscle strength was assessed by handgrip strength and leg press. Handgrip strength was assessed in 11 RCTs (n = 983), and it was significantly improved in the leucine-isolated/combined supplementation group compared with the control group (WMD = 1.50 kg, 95% CI: 0.24, 2.76, P = 0.019; Figure 2A). Leg press was assessed in three RCTs (n = 120), and no significant effect was observed for leucine-isolated supplement (WMD = –1.35 kg, 95% CI: –7.46, 4.77, P = 0.666; Figure 2B).
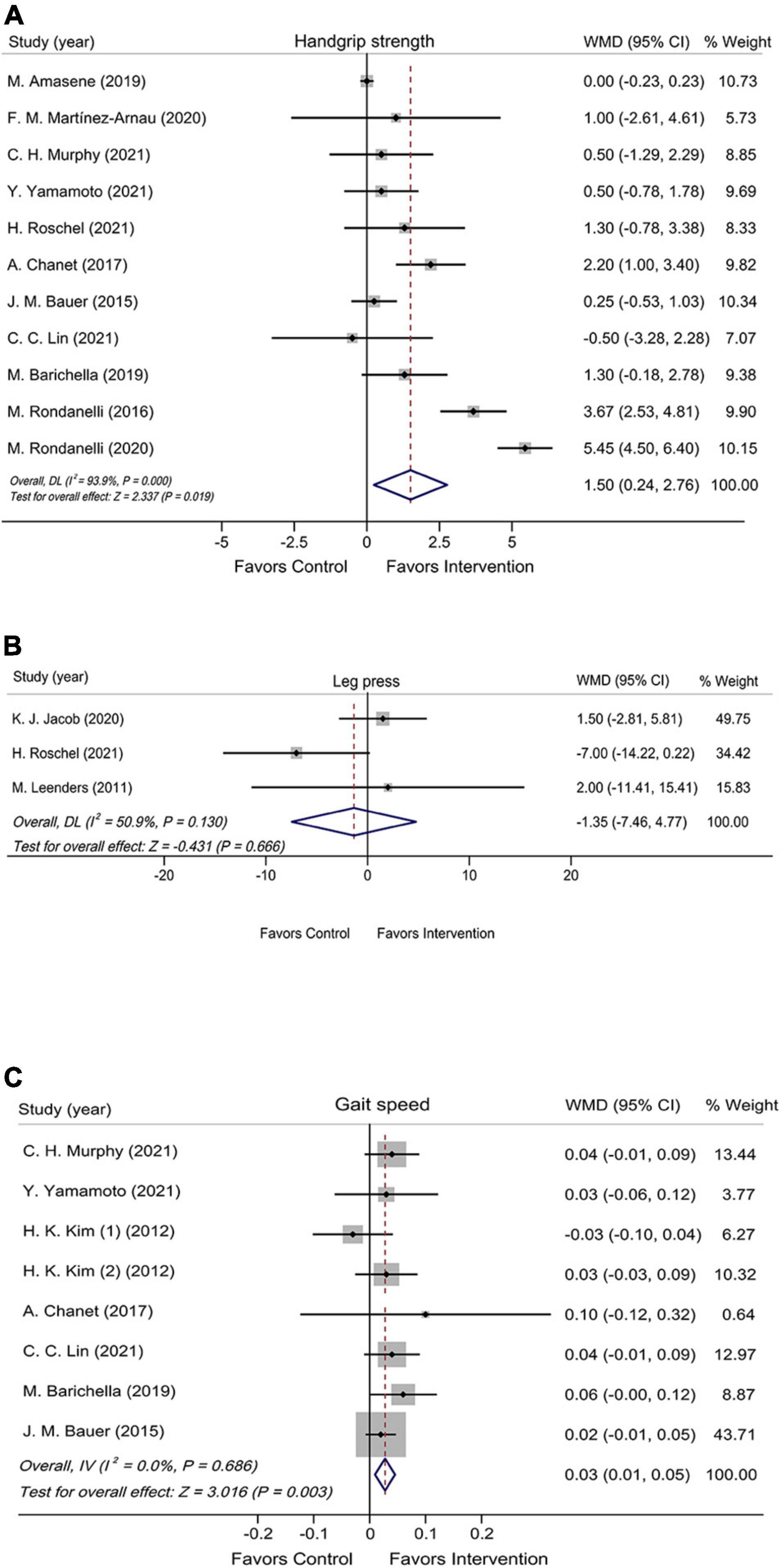
Figure 2. Forest plots assessing the effect of leucine-isolated/combined supplementation on handgrip strength (A), leg press (B), and gait speed (C).
Muscle performance was assessed by gait speed, SPPB, 6-WT, 30sec-CST, and TUG. The pooled results of seven RCTs (n = 772) showed that leucine-isolated/combined supplements significantly accelerated gait speed (WMD = 0.03 m/s, 95% CI: 0.01, 0.05, P = 0.003; Figure 2C), whereas the intervention had no effect on SPPB (WMD = 0.34 scores, 95% CI: –0.55, 1.24, P = 0.453; Supplementary Figure 2A) and 6-WT (WMD = –3.58 m, 95% CI: –13.31, 6.15, P = 0.470; Supplementary Figure 2B). Among the five RCTs (n = 280) and three RCTs (n = 246) assessed 30sec-CST and TUG, neither of which showed significant improvement after supplementation (30sec-CST: WMD = 2.50 times, 95% CI: –0.26, 5.25, P = 0.076; Supplementary Figure 3A); (TUG: WMD = –0.05 s, 95% CI: –0.56, 0.46, P = 0.847; Supplementary Figure 3B).
Subgroup analyses were performed by modified Jadad score, presence/absence of vitamin D supplementation, physical activity, country/region of study, leucine-isolated/combined supplementation and dosage of leucine supplementation. Improvement in handgrip strength was observed in the studies where modified Jadad scores >3 (WMD = 1.79 kg, 95% CI: 0.32, 3.25, P = 0.017), but not in those studies with modified Jadad scores ≤ 3 (WMD = 0.32 kg, 95% CI: –0.84, 1.49, P = 0.584; Supplementary Figure 4). A significantly beneficial effect on handgrip strength was found in the group supplemented with vitamin D (WMD = 2.17 kg, 95% CI: 0.24, 4.10, P = 0.027) compared with the group without vitamin D (WMD = 0.04 kg, 95% CI: –0.18, 0.26, P = 0.715; Figure 3A). Besides, there was an improvement in handgrip strength in the leucine-combined group (WMD = 1.55 kg, 95% CI: 0.16, 2.95, P = 0.029), but not in the leucine-isolated group (WMD = 1.23 kg, 95% CI: –0.58, 3.03, P = 0.183; Figure 4A). In addition, gait speed was significantly improved among the studies from Europe and America (WMD = 0.03 m/s, 95% CI: 0.01, 0.05, P = 0.008; Supplementary Figure 5A), those with leucine-combined supplements containing vitamin D (WMD = 0.03 m/s, 95% CI: 0.01, 0.05, P = 0.008; Figure 3B) and those with supplementation doses ≥ 5 g/day (WMD = 0.03 m/s, 95% CI: 0.01, 0.05, P = 0.009; Supplementary Figure 5B). Additionally, there was no difference in TLM between the leucine-combined group (WMD = 0.62kg, 95% CI: –0.35, 1.59, P = 0.209) and the leucine-isolated group (WMD = 0.03 kg, 95% CI: –0.51, 0.57, P = 0.917; Figure 4B). Besides, no difference in handgrip strength and gait speed was observed when the subjects were stratified by physical activity. Also, when the subgroup analysis was based on the modified Jadad score, vitamin D supplementation, physical activity, country/region of study, and dosage of leucine supplementation, there was no difference in the other indicators of muscle mass, muscle strength and performance.
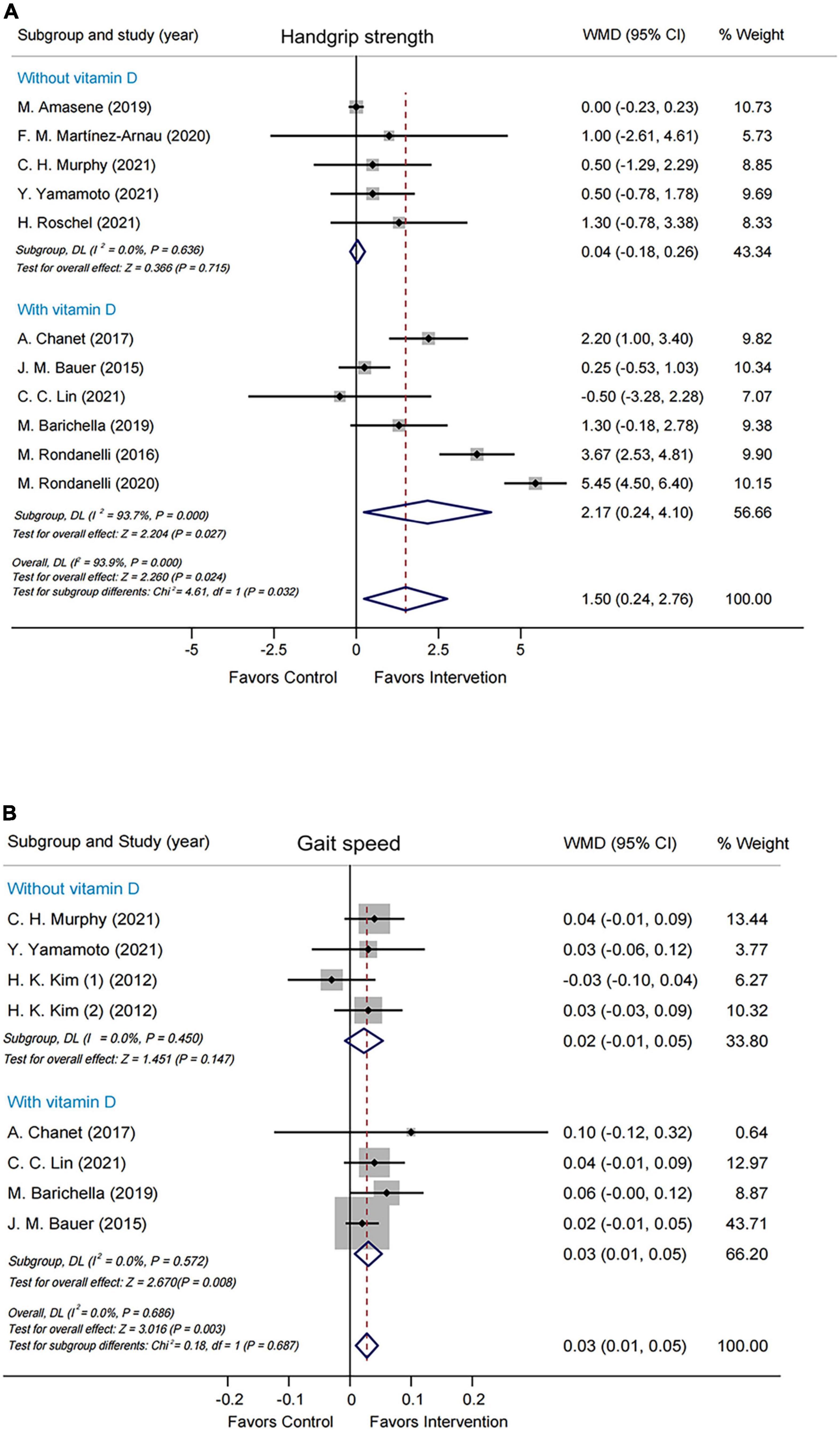
Figure 3. Forest plots assessing the effect of leucine supplementation on handgrip strength (A) and gait speed (B) by groups with or without vitamin D.
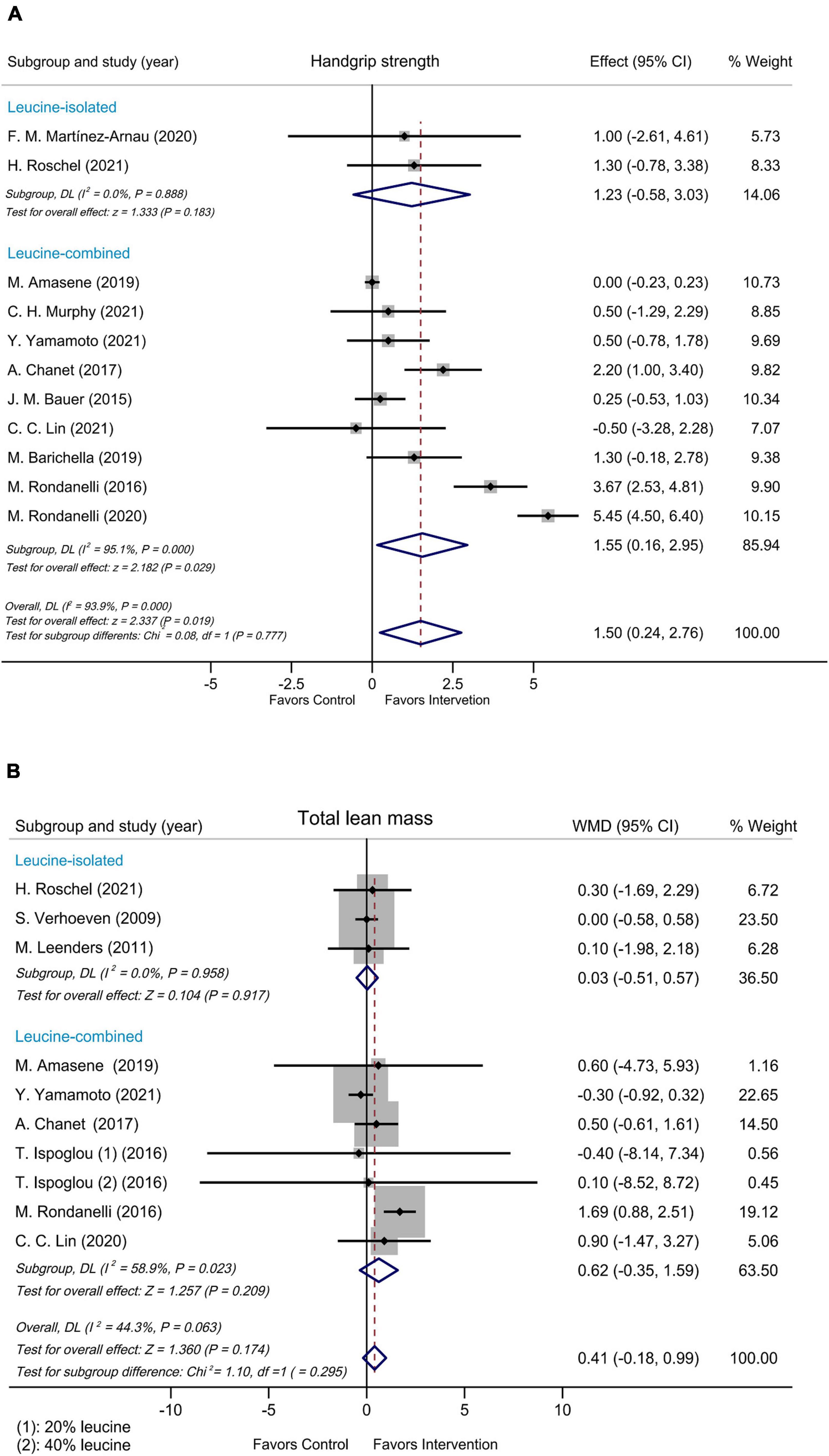
Figure 4. Forest plots assessing the effect on handgrip strength (A) and TLM (B) by leucine-isolated/combined supplementation.
Sensitivity analyses indicated that our findings were robust for TLM, handgrip strength, gait speed, 30sec-CST, TUG, SPPB, 6-WT, and leg press. Moreover, the symmetrical shape of the funnel plot indicated low publication bias (all p-values of Egger’s test > 0.1; data not shown).
Our findings based on 17 RCTs showed that leucine-isolated supplementation had no effect on total lean mass, handgrip strength and leg press, but leucine-combined supplementation including vitamin D could significantly improve handgrip strength and gait speed in older adults. In addition, we observed gait speed was improved for the intake of ≥5 g of leucine supplements and in those studies conducted among non-Asians.
After two previous meta-analyses in 2015 (41, 42), 14 newly published RCTs (12, 24–26, 29–36, 39, 40) were included in the present meta-analysis. Our updated findings indicated no significant difference in changes in lean body mass between the intervention and control group. The meta-analysis by B. Komar et al. included 16 studies testing leucine-combined supplements in a wider variety of participants, who were also frail, sarcopenic, and geriatric hospitalized. In that study, leucine-combined supplementation increased lean body mass (Mean difference = 0.99 kg, 95% CI: 0.43, 1.55), which was inconsistent with our findings. However, null effect of leucine on muscle strength was reported by B. Komar et al. (42), which was similar to our results. In addition, we found a significant improvement in handgrip strength after taking leucine-combined supplementation including vitamin D. Another one from Z. Xu et al included nine RCTs, including healthy participants and participants with cancer and type 2 diabetes, which showed no significant effect of leucine supplementation on lean body mass (41), which was consistent with our results. Studies by Z. Xu et al. have also shown that leucine supplementation increased the rate of muscle protein fraction synthesis (Standardized mean difference = 1.08, 95% CI: 0.5, 1.67), which was not analyzed in our meta-analysis due to the lack of references using the rate of muscle protein fraction synthesis as a primary or secondary measure end-point.
Leucine plays an important role in maintaining skeletal muscle metabolism. Muscle mass is regulated daily by muscle protein synthesis (MPS) and breakdown (MPB). However, aging disrupts the response of MPS to anabolic stimuli and corresponding protein balance. Leucine stimulates mTOR (a major regulator of protein synthesis) and MPS (43). Moreover, leucine can influence proteolysis by inhibiting associated catabolic transcription factors (e.g., FoxO3) (44). A previous study by Chae et al. has observed a positive correlation between daily leucine intake and skeletal muscle mass index in middle-aged individuals, skeletal muscle mass index increased by 0.29%, when each 1g/day increased in leucine (45). Similarly, the study by Lixandrão et al. showed there was a moderate and positive association between total daily leucine intake (g/day) and both quadriceps muscle cross-sectional area (β = 1.7) and maximum dynamic muscle strength (β = 2.4) (When the leucine dose changes by one unit, maximum dynamic muscle strength and muscle cross-sectional area change by β units) (46). However, in our meta-analysis, leucine-isolated supplementation showed no effect on total lean mass, handgrip strength and leg press. There are several possible explanations for this. First, it is possible that leucine-isolated supplements do have a relatively slight effect on muscle mass and muscle strength. Second, the effective dose of leucine may be largely reduced by in vivo metabolism. About 40% of leucine absorbed by muscle accumulates in intracellular free pools, 20% is incorporated into proteins, and 40% is oxidized (47). Third, potential confounding factors may bias the observed results. Moreover, the intervention doses in most of the included RCTs may not be at levels that produce significant effects. There was evidence that the daily protein intake of older adults was insufficient (48). In particular, the elderly tended to consume less animal protein (there was evidence that leucine was more abundant in animal protein than plant protein) (49, 50). Our subgroup analyses showed significant improvement in gait speed with leucine supplementation of 5 g or more. This may indicate a need for a higher dose of leucine intervention, as evidenced by Casperson et al.’s study that long-term leucine supplementation resulted in a higher MPS rate (22). In addition, Park et al. observed a positive correlation between leucine dose and grip strength. when each 1g/day increased in leucine, grip strength increased by 0.796 kg (quartiles 4) (51). This may mean that follow-up studies can set multiple leucine supplementation doses to explore the association between leucine supplementation doses and the risk of sarcopenia. In addition, if the population’s total protein intake is already enough, leucine supplementation may not provide additional benefits (52).
Our meta-analysis found that leucine-combined supplementation including vitamin D significantly improved handgrip strength and gait speed. Vitamin D plays an important role in maintaining the physiological function of skeletal muscle. Pfeifer et al. reported that vitamin D could improve muscle mass in the elderly (53), but some researchers reported no significant improvement in muscle mass or strength by vitamin D (54, 55). Moreover, a meta-analysis of the effect of vitamin D monotherapy on sarcopenia showed that vitamin D supplementation had no effect on muscle mass (appendicular lean mass) and muscle strength (handgrip strength) and muscle performance parameters except SPPB (56). The leucine-combined supplementation including vitamin D might be more effective than leucine- or vitamin D-isolated supplements. The reason remains unclear, but it was hypothesized that both vitamin D and leucine inhibit atrophy-related transcription factors, and stimulate mTOR to promote protein synthesis (13, 14, 57, 58).
The present study has several limitations. First, heterogeneity existed among the included RCTs, which might be due to different study populations, leucine dosing, and regimens, interventions, variability in study design, residual bias, etc. Therefore, we used a random-effect model for subgroup analyses and meta-regression analysis for items with heterogeneity > 50%. Heterogeneity was not addressed in this section that might arise from differences in inclusion and exclusion criteria, patients’ baseline risk profiles, different brands of leucine, or differences in methodological quality. Second, few studies used leucine-isolated interventions, and heterogeneity in trial design and subjects might have influenced the results. Also, it might lead to poor representativeness of the samples and affect the extrapolation of results. Therefore, more RCTs are needed to address whether leucine supplements are effective to reduce sarcopenia in older adults. Third, no strictly control or measure of the leucine content in daily food intake of the subjects was taken in the RCTs that we included. Fourth, the cut-off values for the diagnosis of sarcopenia in men and women are different. The same effect size may represent different degrees of measures change in men and women. Our meta-analysis could not present results in men and women separately due to the lack of data. Fifth, we might not be able to eliminate the effect of physical exercise on sarcopenia-related measures. However, the subjects in the intervention and control groups in all the included RCTs either had no exercise or had exercise at the same frequency and intensity. Therefore, we assume that the change in outcome measures was not attributed to physical exercise.
Leucine-isolated supplementation had no significant effect on total lean mass, handgrip strength and leg press in older adults. Instead, leucine-combined supplementation including vitamin D could significantly improve muscle strength and muscle performance. More experimental studies are needed to clarify and better understand the effect of leucine supplementation on sarcopenia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YG and XF performed the literature search, extracted and analyzed the data, and drafted the manuscript. QH assisted the literature search and data analyses. HZ conceptualized and supervised the work, and had primary responsibility for the final content of the manuscript. QH, LC, and HZ critically revised the manuscript for important intellectual content. All authors reviewed and approved the final manuscript.
This work was supported by a project funded by the Priority Academic Program Development (PAPD) of Jiangsu higher education institutions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.929891/full#supplementary-material
Supplementary Figure 1 | Forest plots assessing the effect of leucine supplementation on total lean mass.
Supplementary Figure 2 | Forest plots assessing the effect of leucine supplementation on SPPB (A) and 6-WT (B).
Supplementary Figure 3 | Forest plots assessing the effect of leucine supplementation on 30sec-CST (A), TUG (B).
Supplementary Figure 4 | Forest plots assessing the effect of leucine supplementation on handgrip strength by modified Jadad score ≤3 and modified Jadad score >3.
Supplementary Figure 5 | Forest plots assessing the effect of leucine supplementation on gait speed by Asian countries and Europe and America (A) and doses ≥5 g/day and <5 g/day (B).
Supplementary Table 1 | Literature search strategy for meta-analysis.
Supplementary Table 2 | Quality assessment of the included studies according to modified Jadad score.
1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
2. Chen Z, Ho M, Chau PH. Prevalence, incidence, and associated factors of possible sarcopenia in community-dwelling Chinese older adults: a population-based longitudinal study. Front Med (Lausanne). (2021) 8:769708. doi: 10.3389/fmed.2021.769708
3. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. (2017) 16:21. doi: 10.1186/s40200-017-0302-x
4. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. (2010) 1:129–33. doi: 10.1007/s13539-010-0014-2
5. Auyeung TW, Lee SW, Leung J, Kwok T, Woo J. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatrics Gerontol Int. (2014) 14(Suppl. 1):76–84. doi: 10.1111/ggi.12213
6. Veronese N, Koyanagi A, Cereda E, Maggi S, Barbagallo M, Dominguez LJ, et al. Sarcopenia reduces quality of life in the long-term: longitudinal analyses from the English longitudinal study of ageing. Eur Geriatr Med. (2022) 13:633–9. doi: 10.1007/s41999-022-00627-3
7. Doan G, Ayhan NY. Relationship between malnutrition and sarcopenia in elderly Turkish community-dwellers. Nutr Clin Métabol. (2022) 36:40–5. doi: 10.1016/j.nupar.2021.08.003
8. Lim SK, Kong S. Prevalence, physical characteristics, and fall risk in older adults with and without possible sarcopenia. Aging Clin Exp Res. (2022) 34:1365–71. doi: 10.1007/s40520-022-02078-z
9. Seino S, Kitamura A, Abe T, Taniguchi Y, Murayama H, Amano H, et al. Dose-response relationships of sarcopenia parameters with incident disability and mortality in older Japanese adults. J Cachexia Sarcopenia Muscle. (2022) 13:932–44. doi: 10.1002/jcsm.12958
10. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/s0140-6736(19)31138-9
11. Dai M, Lin T, Yue J, Dai L. Signatures and clinical significance of amino acid flux in sarcopenia: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2021) 12:725518. doi: 10.3389/fendo.2021.725518
12. Martinez-Arnau FM, Fonfria-Vivas R, Buigues C, Castillo Y, Molina P, Hoogland AJ, et al. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients. (2020) 12:932. doi: 10.3390/nu12040932
13. Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. (2000) 130:139–45. doi: 10.1093/jn/130.2.139
14. Adeva-Andany MM, Fernández-Fernández C, López-Pereiro Y, Castro-Calvo I, Carneiro-Freire N. The effects of glucagon and the target of rapamycin (TOR) on skeletal muscle protein synthesis and age-dependent sarcopenia in humans. Clin Nutr ESPEN. (2021) 44:15–25. doi: 10.1016/j.clnesp.2021.06.025
15. Banerjee J, Bruckbauer A, Zemel MB. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism. (2016) 65:1679–91. doi: 10.1016/j.metabol.2016.06.011
16. Jiao J, Han SF, Zhang W, Xu JY, Tong X, Yin XB, et al. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr Res. (2016) 60:31304. doi: 10.3402/fnrv60.31304
17. Yao K, Duan Y, Li F, Tan B, Hou Y, Wu G, et al. Leucine in obesity: therapeutic prospects. Trends Pharmacol Sci. (2016) 37:714–27. doi: 10.1016/j.tips.2016.05.004
18. Bloom I, Shand C, Cooper C, Robinson S, Baird J. Diet quality and sarcopenia in older adults: a systematic review. Nutrients. (2018) 10:308. doi: 10.3390/nu10030308
19. Tessier AJ, Chevalier S. An update on protein, leucine, omega-3 fatty acids, and vitamin d in the prevention and treatment of sarcopenia and functional decline. Nutrients. (2018) 10:1099. doi: 10.3390/nu10081099
20. Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. (2015) 101:279–86. doi: 10.3945/ajcn.114.090290
21. Wall BT, Hamer HM, de Lange A, Kiskini A, Groen BB, Senden JM, et al. Leucine co-ingestion improves post-prandial muscle protein accretion in elderly men. Clin Nutr (Edinburgh, Scotland). (2013) 32:412–9. doi: 10.1016/j.clnu.2012.09.002
22. Casperson SL, Sheffield-Moore M, Hewlings SJ, Paddon-Jones D. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr (Edinburgh, Scotland). (2012) 31:512–9. doi: 10.1016/j.clnu.2012.01.005
23. Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metabol. (2015) 308:E1056–65. doi: 10.1152/ajpendo.00481.2014
24. Ispoglou T, White H, Preston T, McElhone S, McKenna J, Hind K. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65-75 years. Eur J Clin Nutr. (2016) 70:182–8. doi: 10.1038/ejcn.2015.91
25. Kirk B, Mooney K, Amirabdollahian F, Khaiyat O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: liverpool hope university – sarcopenia aging trial (LHU-SAT). Front Physiol. (2019) 10:445. doi: 10.3389/fphys.2019.00445
26. Roschel H, Hayashi AP, Fernandes AL, Jambassi-Filho JC, Hevia-Larraín V, de Capitani M, et al. Supplement-based nutritional strategies to tackle frailty: a multifactorial, double-blind, randomized placebo-controlled trial. Clin Nutr. (2021) 40:4849–58. doi: 10.1016/j.clnu.2021.06.024
27. Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Hartgens F, Wodzig WK, et al. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J Nutr. (2011) 141:1070–6. doi: 10.3945/jn.111.138495
28. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
29. Murphy CH, Flanagan EM, De Vito G, Susta D, Mitchelson KAJ, de Marco Castro E, et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n-3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2021) 113:1411–27. doi: 10.1093/ajcn/nqaa449
30. Yamamoto Y, Nagai Y, Kawanabe S, Hishida Y, Hiraki K, Sone M, et al. Effects of resistance training using elastic bands on muscle strength with or without a leucine supplement for 48 weeks in elderly patients with type 2 diabetes. Endocr J. (2021) 68:291–8. doi: 10.1507/endocrjEJ20-0550
31. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Directors Assoc. (2015) 16:740–7. doi: 10.1016/j.jamda.2015.05.021
32. Lin CC, Shih MH, Chen CD, Yeh SL. Effects of adequate dietary protein with whey protein, leucine, and vitamin D supplementation on sarcopenia in older adults: an open-label, parallel-group study. Clin Nutr (Edinburgh, Scotland). (2021) 40:1323–9. doi: 10.1016/j.clnu.2020.08.017
33. Barichella M, Cereda E, Pinelli G, Iorio L, Caroli D, Masiero I, et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology. (2019) 93:e485–96. doi: 10.1212/wnl.0000000000007858
34. Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. (2016) 103:830–40. doi: 10.3945/ajcn.115.113357
35. Amasene M, Besga A, Echeverria I, Urquiza M, Ruiz JR, Rodriguez-Larrad A, et al. Effects of leucine-enriched whey protein supplementation on physical function in post-hospitalized older adults participating in 12-weeks of resistance training program: a randomized controlled trial. Nutrients. (2019) 11:2337. doi: 10.3390/nu11102337
36. Rondanelli M, Cereda E, Klersy C, Faliva MA, Peroni G, Nichetti M, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle. (2020) 11:1535–47. doi: 10.1002/jcsm.12532
37. Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. (2009) 89:1468–75. doi: 10.3945/ajcn.2008.26668
38. Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatrics Soc. (2012) 60:16–23.
39. Jacob KJ, Sonjak V, Spendiff S, Hepple RT, Chevalier S, Perez A, et al. Mitochondrial content, but not function, is altered with a multimodal resistance training protocol and adequate protein intake in leucine-supplemented pre/frail women. Front Nutr. (2020) 7:619216. doi: 10.3389/fnut.2020.619216
40. Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, et al. Supplementing breakfast with a Vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. (2017) 147:2262–71. doi: 10.3945/jn.117.252510
41. Xu ZR, Tan ZJ, Zhang Q, Gui QF, Yang YM. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: a systematic review and meta-analysis. Br J Nutr. (2015) 113:25–34. doi: 10.1017/s0007114514002475
42. Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. (2015) 19:437–46. doi: 10.1007/s12603-014-0559-4
43. Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. (2006) 136:227s–31s. doi: 10.1093/jn/136.1.227S
44. Pereira MG, Baptista IL, Carlassara EO, Moriscot AS, Aoki MS, Miyabara EH. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS One. (2014) 9:e85283. doi: 10.1371/journal.pone.0085283
45. Chae M, Park HS, Park K. Association between dietary branched-chain amino acid intake and skeletal muscle mass index among Korean adults: interaction with obesity. Nutr Res Pract. (2021) 15:203–12. doi: 10.4162/nrp.2021.15.2.203
46. Lixandrão ME, Longobardi I, Leitão AE, Morais JVM, Swinton PA, Aihara AY, et al. Daily leucine intake is positively associated with lower limb skeletal muscle mass and strength in the elderly. Nutrients. (2021) 13:3536. doi: 10.3390/nu13103536
47. Alvestrand A, Hagenfeldt L, Merli M, Oureshi A, Eriksson LS. Influence of leucine infusion on intracellular amino acids in humans. Eur J Clin Invest. (1990) 20:293–8.
48. Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. (1988) 254:579–84. doi: 10.1042/bj2540579
49. Ewy MW, Patel A, Abdelmagid MG, Mohamed Elfadil O, Bonnes SL, Salonen BR, et al. Plant-based diet: is it as good as an animal-based diet when it comes to protein? Curr Nutr Rep. (2022) 11:337–46. doi: 10.1007/s13668-022-00401-8
50. Ishikawa-Takata K, Takimoto H. Current protein and amino acid intakes among Japanese people: analysis of the 2012 National Health and Nutrition Survey. Geriatrics Gerontol Int. (2018) 18:723–31. doi: 10.1111/ggi.13239
51. Park S, Chae M, Park H, Park K. Higher branched-chain amino acid intake is associated with handgrip strength among Korean older adults. Nutrients. (2021) 13:1522. doi: 10.3390/nu13051522
52. Plotkin DL, Delcastillo K, Van Every DW, Tipton KD, Aragon AA, Schoenfeld BJ. Isolated leucine and branched-chain amino acid supplementation for enhancing muscular strength and hypertrophy: a narrative review. Int J Sport Nutr Exerc Metab. (2021) 31:292–301. doi: 10.1123/ijsnem.2020-0356
53. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int J Estab Result Cooperation Between Eur Found Osteoporos Natl Osteoporos Found USA. (2009) 20:315–22. doi: 10.1007/s00198-008-0662-7
54. Shea MK, Fielding RA, Dawson-Hughes B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: a randomized controlled trial. Am J Clin Nutr. (2019) 109:369–79. doi: 10.1093/ajcn/nqy290
55. Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J Bone Mineral Res Off J Am Soc Bone Min Res. (2021) 36:1651–60. doi: 10.1002/jbmr.4412
56. Prokopidis K, Giannos P, Katsikas Triantafyllidis K, Kechagias KS, Mesinovic J, Witard OC, et al. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:1642–52. doi: 10.1002/jcsm.12976
57. Bass JJ, Nakhuda A, Deane CS, Brook MS, Wilkinson DJ, Phillips BE, et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol Metab. (2020) 42:101059. doi: 10.1016/j.molmet.2020.101059
Keywords: leucine, sarcopenia, systematic review, meta-analysis, randomized controlled trials (RCTs), the elderly
Citation: Guo Y, Fu X, Hu Q, Chen L and Zuo H (2022) The Effect of Leucine Supplementation on Sarcopenia-Related Measures in Older Adults: A Systematic Review and Meta-Analysis of 17 Randomized Controlled Trials. Front. Nutr. 9:929891. doi: 10.3389/fnut.2022.929891
Received: 06 May 2022; Accepted: 14 June 2022;
Published: 01 July 2022.
Edited by:
Ryu Watanabe, Osaka Metropolitan University, JapanCopyright © 2022 Guo, Fu, Hu, Chen and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zuo, enVvaHVpQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.