
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 22 August 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.926429
This article is part of the Research Topic Breast Milk Composition and Infant Metabolism View all 14 articles
Fatty acid (FA) in breast milk is beneficial to the growth and neurodevelopment of infants. However, the structure profiles of breast milk FAs and the influencing factors which are crucial for normal function have not been fully elucidated. This study aimed to characterize the profiles of total and sn-2 FAs in human mature milk based on two representative urban areas in China and explore potential sociodemographic determinants. Mothers (n = 70) at 40–100 d postpartum from Beijing and Danyang were recruited according to unified inclusion and exclusion criteria. Total and sn-2 FA compositions were examined by gas chromatography and quantified. Using the Spearman correlation and multiple regression model, we found that the location and maternal education level were the most conspicuous correlated factor. The milk of mothers from Beijing had higher levels of the n-6 series of long-chain polyunsaturated fatty acids (LCPUFA) (C20:2, C20:3n-6, C20:4n-6, n-6PUFA/n-3PUFA, LA/ALA, and ARA/DHA) than that of Danyang, while the opposite was observed in the n-3 series of LCPUFA (C18:3n-3 and Total n-3PUFA). Compared to the milk of mothers with a high school degree or below, those with a bachelor's degree or above had lower SFAs (C10:0, C12:0, C14:0, and Total SFA), n-3 series of LCPUFA (C18:3n-3 and Total n-3PUFA), C18:1n-9t, and higher n-6 series of LCPUFA (C18:2n-6c, C20:2, C20:4n-6, Total n-6PUFA, and n-6PUFA/n-3PUFA). Maternal age, infant gender, pre-conception body mass index (BMI), parity, delivery mode, and gestational weight gain were also associated with total FAs. However, fewer associations were found between the above factors and sn-2 FAs. This study will promote an understanding of human breast milk's lipid profile and help develop a formula more suitable for infants.
Human milk is the optimal food for infants during the first 6 months of life. It provides adequate nutrients and numerous bioactive ingredients such as water, carbohydrate, fat, protein, minerals, vitamins, immunoglobulin, and lactoferrin (1–3). However, the composition of human milk differs significantly between and within mothers. Human milk fatty acids (FAs) are the most variable macronutrient (4). The lipid content of human milk varies between 3 and 5% mostly (wider variation also has been reported) (5); it is the major energy source in breast milk and provides 40–50% of an infant's daily energy requirement (6–8). Triacylglycerol (TAG) is the major compound of lipid in breast milk. The property of TAGs mainly depends on the composition and specific position distribution of FAs. Studies have found that saturated fatty acid (SFA), especially esterified palmitic acid (PA; C16:0), preferentially occupies the sn-2 position in human milk (9, 10), while in formulas, PA is mainly located at the sn-1,3 positions (11, 12). A high level of PA at the sn-2 position is reported to promote the absorption of fat and calcium in infants (13), reduce insoluble calcium soap in feces (14), and aid in intestinal microbiota development of infants (15). However, the FA composition in human milk is easily influenced by maternal characteristics (dietary habits (3), lactation, and gestational age (16), duration of pregnancy (12), the stage of lactation (9, 17), and body mass index (BMI) (18, 19) and also external factors such as maternal geographic location (3, 17) and socioeconomic status (2)).
A number of studies have investigated the impact of maternal dietary habits and lactation stage on FA composition in mother's milk (3, 8, 20, 21). Nevertheless, the investigation of profiles of sn-2 FAs in human mature milk is limited, with only a few reported data for the Chinese population (10, 22, 23). Meanwhile, the exploration of influencing sociodemographic factors of sn-2 FAs is still rarely reported.
In this study, we assessed the total and sn-2 FA profiles of mature milk from mothers living in Beijing and Danyang, China. The associations between FA composition and sociodemographic factors (maternal age, BMI before pregnancy, gestational weight gain, maternal education level, parity, infant gender, and region) were explored to elucidate the main characteristics of total and sn-2 FA profiles in Chinese mature milk and to explore the potential factors influencing their composition.
From June to October 2018, women with singleton pregnancies and no diabetes, hypertension, and other chronic diseases were recruited during their obstetrician visits in Beijing (Beijing Maternity Hospital affiliated to the Capital Medical University) and Danyang (Danyang People's Hospital), China (Characteristics between the two study sites were shown in Table A1). These subjects were considered eligible if their infants were delivered at full term and breastfed. For this study, we excluded mothers with the following criteria: birth weight of infants <2,500 g or > 4,000 g; with mental health disorders; unable to answer questions and poor postpartum mood; participated in any nutrition or drug intervention research; took hormones and antibiotics recently; and tobacco use.
A sample size of 64 subjects was estimated by the G*Power 3.1.9 software with a significance level of 5%, power of 80%, and expected effect size of 0.3, considering the correlation between total and sn-2 fatty acid of human mature milk and their correlated factors by correlation test. Evaluated by the G*Power 3.1.9 software, the statistical power was 83% with a sample size of 70, a significance level of 5%, and an expected effect size of 0.3.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. All the procedures involving human subjects were approved by the Chinese Clinical Trial Registry with the registration number ChiCTR1800018766 (http://www.chictr.org.cn/listbycreater.aspx). Written informed consent was obtained from all subjects.
Data on height, weight (pre-conception weight and gestational weight gain), age, parity, delivery mode, and infant gender were obtained via a questionnaire during sample collection. Information such as the mothers' physical condition and lifestyle aspects were also included in the questionnaire.
On the day when mothers were scheduled for an obstetrical examination, 40–100 days post-delivery, they were asked to breastfeed at 6–8 am and then use an electric breast pump to empty the milk from one of their breasts at home. When they went to the hospital later (9–11 am), the milk from the breast that was previously emptied was collected by an electric breast pump with the help of sampling persons. The milk was mixed and poured into centrifuge tubes which were later wrapped with tin foil and stored at −80°C for further analysis.
Total lipids were extracted from human milk by a revised Mojonnier method (24). The extracted lipids were saponified and the FA methyl esters were obtained by FA methylation, and then analyzed by gas chromatography (GC). Sn-2 monoglyceride (MAG) was hydrolyzed from triglyceride (TAG) and then analyzed by GC following the method by Sahin et al. (25) (see Supplementary material 1, which demonstrates detailed experimental steps).
The contents of each FA and sn-2 FA were expressed as mean ± SD and range (minimum~maximum). All the data were tested for normal distribution using SPSS (Version 20) before analysis. The Spearman correlations between differences in the contents of the FA/sn-2 FA vs. differences in characteristics of mothers and infants (age, infant gender, pre-conception BMI, gestational weight gain, delivery mode, parity, maternal education level, and sampling site) were calculated. The multiple regression model was adopted to estimate the importance of the differences in characteristics of mothers and infants in explaining the dissimilarities in FA/sn-2 FA. All the statistical analyses were performed in the R (Version 4.1.2), using “psych” (26), “reshape2” (27), “relaimpo” (28), and “packfor” (29) packages. Statistical significance was set at a P < 0.05. Plots were generated by the “ggplot2” (30) package in R.
Among the 100 screened healthy volunteers, 25 were excluded for not providing complete questionnaire information and five subjects withdrew from the study. A total of 70 mature milk samples were obtained from 70 mothers. The main characteristics of the participants are described in Table 1.
As displayed in Table 2, the total lipid content varied amongst subjects, ranging from 1.33 to 7.25 g/100 g (mean: 3.47±1.52 g/100 g). In total, 34 FAs were detected and only 23 FAs whose levels of more than 0.1% (total fatty acids) were shown in Table 2. C4:0, C6:0, C11:0, C13:0, C21:0, C22:0, C24:0, C14:1, C24:1, C22:2, and C20:5n-3 were also detected, but the levels were less than 0.1%. In general, monounsaturated fatty acid (MUFA) was the predominant FA (37.57 ± 3.82%), in which C18:ln-9c was found to make up the largest proportion (34.50 ± 3.44%). SFA was the second most abundant FA (34.50 ±3.44%), the largest component of which was C16:0 (20.06 ± 2.20%). The proportion of polyunsaturated fatty acids (PUFA) was the least (27.94±4.36%), among which C18:2n-6c accounted for more than 85% (24.08 ± 4.42%).
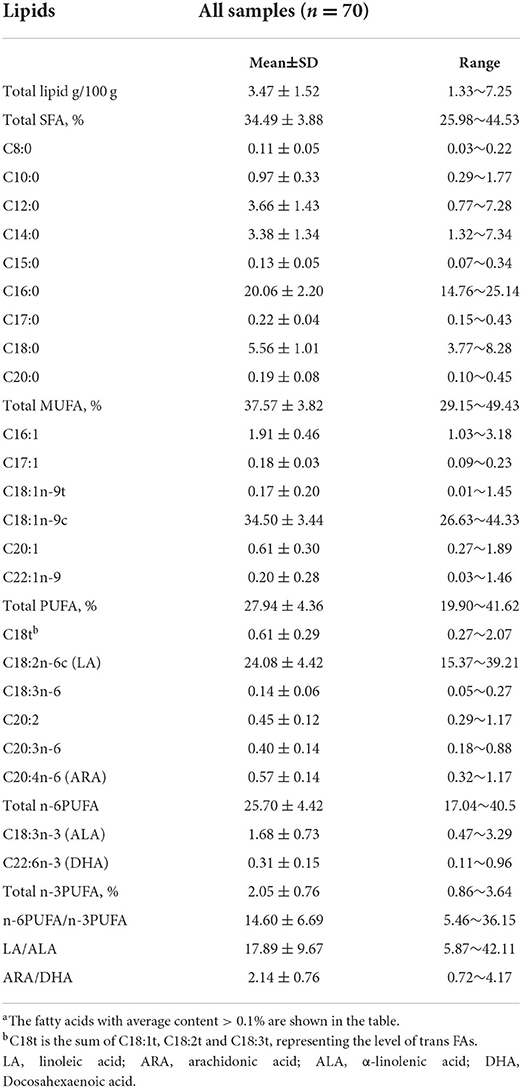
Table 2. Total lipid content (g/100 g milk) and fatty acid (% total fatty acids) levels in mature milka.
Some potential correlated factors of human milk FAs, namely, pre-conception BMI, parity, maternal education level, location, infant gender, gestational weight gain, delivery mode, and maternal age were individually explored using the Spearman correlation analysis and later tested using the multiple regression model (Figure 1).
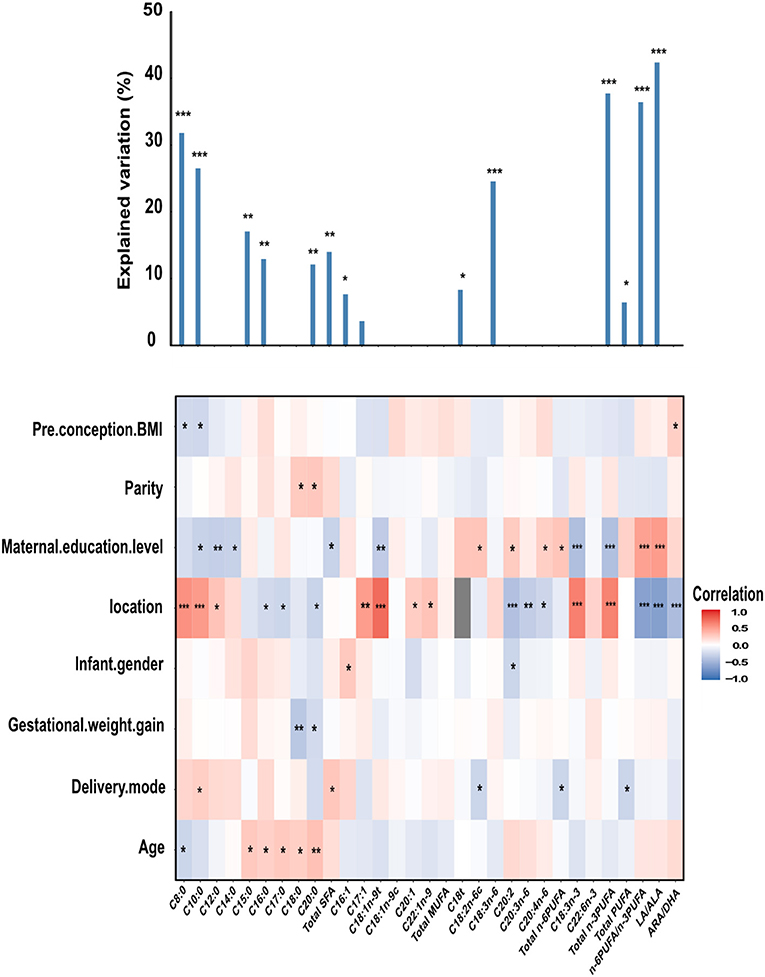
Figure 1. The column chart shows the total contribution of indicators to the interpretation of FAs variation (obtained by multiple linear regressions). The heat map shows the Spearman correlations between total FAs and correlated factors. Coloring reflects the direction and magnitude of the correlation coefficients. For pre-conception BMI, parity, maternal education level, gestational weight gain, and age, the higher the value/level of the correlated factors, the higher the fatty acid content. For the location, red indicates FAs in Danyang are higher than that in Beijing. For infant gender, red indicates FAs in mothers with baby girls are higher than that with baby boys. For delivery mode, red indicates FAs of mothers with natural childbirth are lower than with other modes of delivery. The major predictors were identified based on the correlation and best multiple regression model. C18t is the sum of C18:1t, C18:2t, and C18:3t, representing the level of trans FAs. LA, linoleic acid (C18:2n-6c); ARA, arachidonic acid (C20:4n-6); ALA, α-linolenic acid (C18:3n-3); DHA, Docosahexaenoic acid (C22:6n-3). *P < 0.05; **P < 0.01; ***P < 0.001.
A significant correlation was found between maternal education level and FAs (C10:0, C12:0, C14:0, Total SFA, C18:1n-9t, C18:2n-6c, C20:2, C20:4n-6, Total n-6PUFA, C18:3n-3, Total n-3PUFA, n-6PUFA/n-3PUFA, and LA/ALA), and location and FAs (C8:0, C10:0, C12:0, C16:0, C17:0, C20:0, Total SFA, C17:1, C18:1n-9t, C20:1, C22:1n-9, C20:2, C20:3n-6, C20:4n-6, C18:3n-3, Total n-3PUFA, n-6PUFA/n-3PUFA, and LA/ALA). The milk of mothers from Danyang had higher levels of C8:0, C10:0, C12:0, C17:1, C18:1n-9t, C20:1, C22:1n-9, C18:3n-3, and total n-3PUFA, and lower levels of C16:0, C17:0, C20:0, C20:2, C20:3n-6, C20:4n-6 and lower n-6PUFA/n-3PUFA, LA/ALA, and ARA/DHA ratios. Compared to the milk of mothers with a high school degree or below, those with a bachelor's degree or above had lower C10:0, C12:0, C14:0, Total SFA, C18:1n-9t, C18:3n-3, Total n-3PUFA, and higher C18:2n-6c, C20:2, C20:4n-6, Total n-6PUFA, and n-6PUFA/n-3PUFA, and LA/ALA ratios.
Several associations were observed between age and some SFAs (C8:0, C15:0, C16:0, C17:0, C18:0, and C20:0), delivery mode, and C10:0, Total SFA, C18:2n-6C, Total n-6PUFA, and Total PUFA. For pre-conception BMI, parity, infant gender, and gestational weight gain, there are few correlations shown between these characteristics and FAs.
For C8:0, LA/ALA, n-6PUFA/ n-3PUFA, and total n-3PUFA, these factors can explain more than 30% of the variance in mature milk FAs.
In this study, 26 sn-2 FAs were examined. In total, 8 major sn-2 FAs were detected in mature breast milk (Table 3), namely, C16:0, C18:2n-6, C18:1, C14:0, C12:0, C18:0, C16:1, and C18:3n-3. These FAs collectively accounted for 92.43% (mean) of sn-2 FAs. Different from the total FAs composition, total SFA (mean: 67.74 ± 5.21%) was predominant in the sn-2 position, followed by MUFA (16.26 ± 2.54%) and PUFA (16.00 ± 3.87%). Notably, C16:0 accounted for more than half of the total sn-2 FAs (50.18 ± 4.74%). Furthermore, approximately 80% of the total C16:0 was in the sn-2 position (79.72 ± 4.29%). C14:0 and C15:0 were also mainly found in the sn-2 position; the average relative molar percentages at the sn-2 position were 66.80 and 75.31%, respectively. However, most MUFAs and PUFAs were located at sn-1, 3 positions.
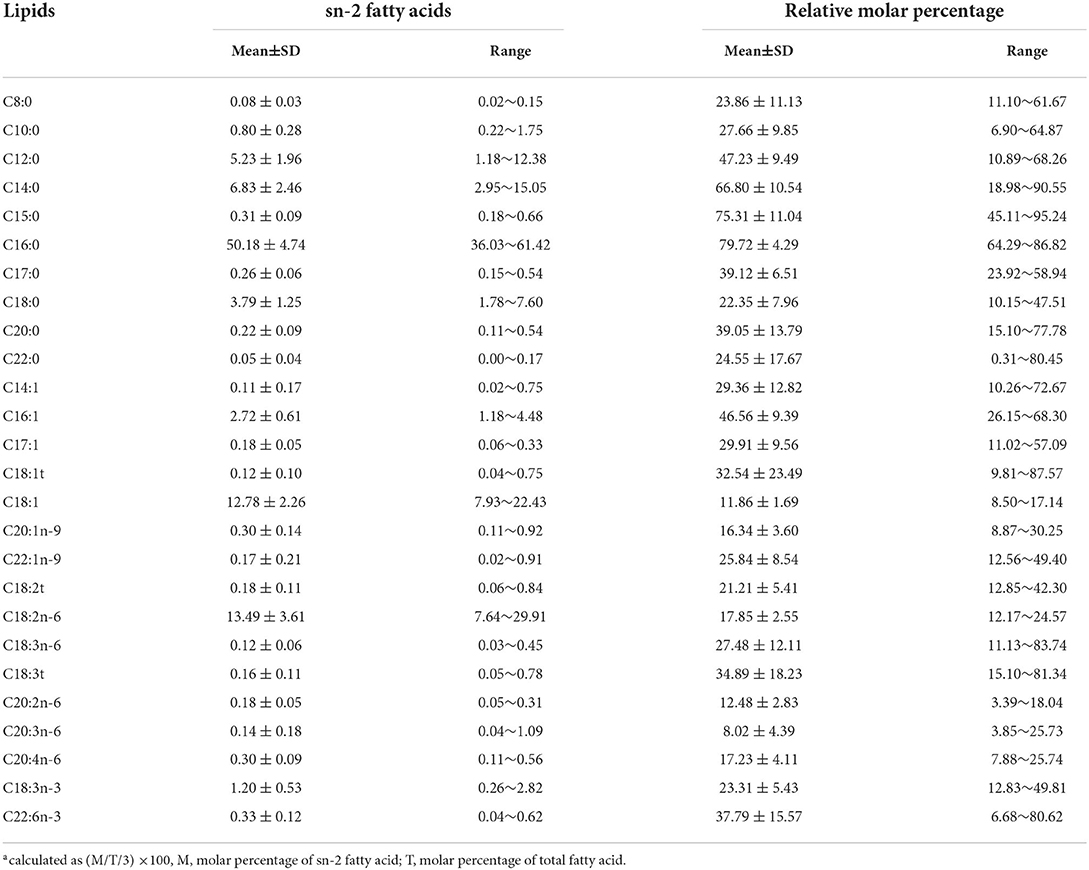
Table 3. Composition of sn-2 fatty acids and relative molar percentagea of each fatty acid in mature milk at the sn-2 position (%).
As shown in Figure 2, no associations with statistical significance were observed between sn-2 FAs and parity. Similar to FAs, maternal education level and location were strong predictors for differences in the constitution of mature milk sn-2 FAs. Mothers from Beijing had lower C20:1n-9, C18:3n-3, and C18:3n-6 and higher percentages of C10:0, C14:1, C16:1, C17:1, C18:2t, and C20:2n-6. Mothers with a bachelor's degree or above had higher C14:1, C16:1, C17:1, C18:2t, C18:2n-6, C20:2n-6, and lower C18:3n-3 compared to the mothers with a high school degree or below. Several correlations were also found between age and C18:0, C20:0, and C20:2n-6; delivery mode and C16:0, C17:1 and C18:2n-6; gestational weight gain and C18:0, C17:1; infant gender and C14:1, C18:1, C20:1n-9; pre-conception BMI and C16:1, C18:3n-6.
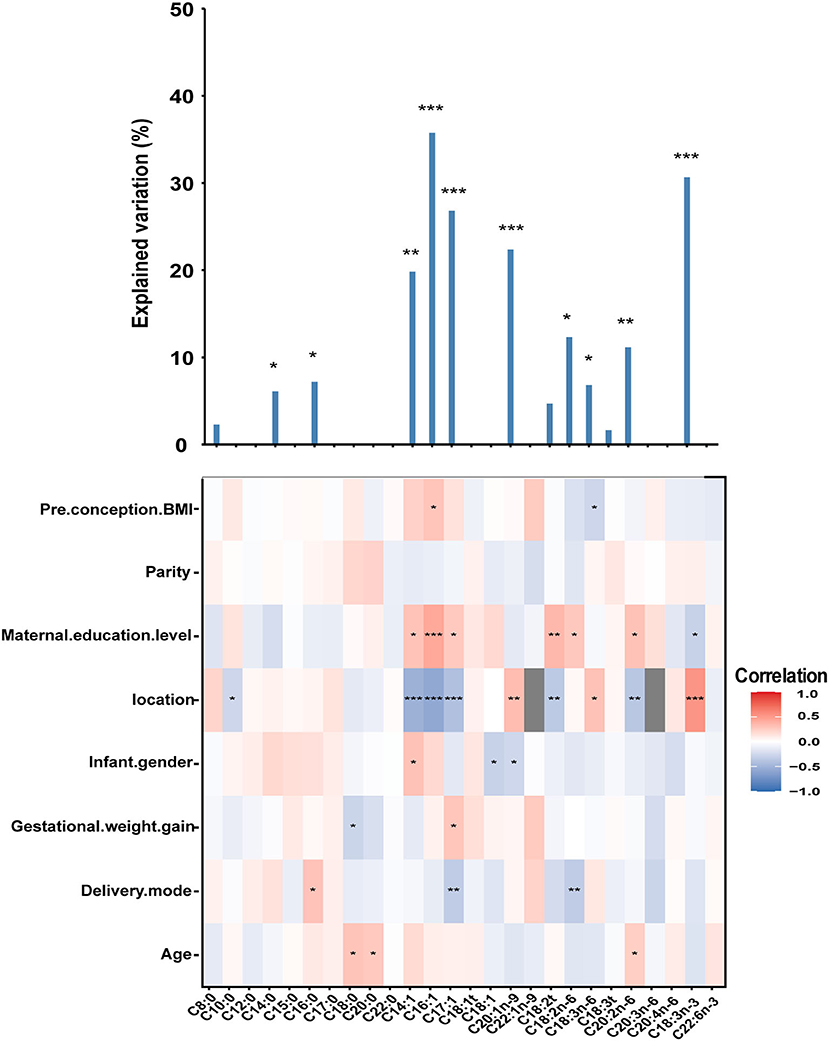
Figure 2. The column chart shows the total contribution of indicators to the interpretation of sn-2 FAs variation (obtained by multiple linear regressions). The heat map shows the Spearman correlations between sn-2 FAs and correlated factors. Coloring reflects the direction and magnitude of the correlation coefficients. For pre-conception BMI, parity, maternal education level, gestational weight gain, and age, the higher the value/level of the correlated factors, the higher the fatty acid content. For the location, red indicates FAs in Danyang are higher than that in Beijing. For infant gender, red indicates FAs in mothers with baby girls are higher than that with baby boys. For delivery mode, red indicates FAs of mothers with natural childbirth are lower than with other modes of delivery. The major predictors were identified based on correlation and the best multiple regression model. DHA, Docosahexaenoic acid (C22:6n-3). *P < 0.05; **P < 0.01; ***, P < 0.001.
These factors explained 1.67–35.76% of the variance in sn-2 FAs, with the lowest proportion (1.67%) for C18:3t and the highest proportion (35.76%) for C16:1.
In this present study, we analyzed the total and sn-2 FA profiles in mature milk samples from healthy lactating women in Beijing and Danyang, China. We found specific associations of pre-conception BMI, maternal education level, location, infant gender, gestational weight gain, delivery mode, and maternal age with total and sn-2 FAs in mature milk. Parity may affect total FA composition but not sn-2 FA. Location and maternal education level were strong predictors for differences in the constitution of mature milk total and sn-2 FAs.
Total lipid content in mature breast milk found in our study was 3.47 ± 1.52 g/100 g, which was close to the level (3.39 ± 1.24 g/dl) found in a recent systematic review in Chinese women (31). Our study showed that the total contents of SFA, MUFA, and PUFA in mature milk were 34.49 ± 3.88%, 37.57 ± 3.82%, and 27.94 ± 4.36%, respectively, which was similar to the pooled results of the Asian populations in a meta-analysis study (37.87~39.91%, 34.64~36.72%, 23.18~29.79% for SFA, MUFA, PUFA, respectively) (32). It is worth noting that the infant milk formula standards of China (GB10765-2010) specify that the ratio of LA/ALA should range from 5:1 to 15:1, which is in line with the standards of international organizations and other countries, e.g., the Food and Agriculture Organization of the United Nations, WHO, Australia and New Zealand (33). However, the mean value for LA:ALA in our study was 17.89:1; other studies conducted among the Chinese population also showed a mean value for LA/ALA in mature milk above 10:1 [Shanghai:18.56:1 (34); Guangzhou:19.70:1 (35); Beijing:15.69:1 (35), Jiangsu:11.9:1 (35)]. It is suggested that we should focus on the characteristics of domestic breast milk to guide the formulation and production of infant formula, rather than just referring to standards that are based on data from the breast milk of mothers in western countries. In our study, the sampling site had a significant effect on PUFAs. The levels of n-6 series of long-chain polyunsaturated fatty acids (LCPUFA) (C20:2, C20:3n-6, C20:4n-6, n-6PUFA/n-3PUFA, LA/ALA, and ARA/DHA) in mature milk from mothers in Beijing were higher than that of Danyang, while the opposite was observed in the n-3 series of LCPUFA (C18:3n-3 and Total n-3PUFA). The discrepancy in eating habits between the two regions might lead to the difference in the proportions of PUFA in the mature milk. Beijing is an inland city, while Danyang is a coastal city abundant in various fishes rich in n-3 PUFA. In addition to PUFA, regional differences were also observed in several types of SFA (C8:0, C10:0, C12:0, C16:0, C17:0, and C20:0) and MUFA (C17:1, C18:1n-9t, C20:1, and C22:1n-9), which might be caused by other site-specific correlated factors, such as gene, climate, or lifestyle.
Maternal education level was also found to be associated with some human milk FAs. Mothers with higher degrees had lower SFAs (C10:0, C12:0, C14:0, and Total SFA), n-3 series of LCPUFA (C18:3n-3 and Total n-3PUFA), C18:1n-9t, and higher n-6 series of LCPUFA (C18:2n-6c, C20:2, C20:4n-6, Total n-6PUFA, and n-6PUFA/n-3PUFA, LA/ALA ratios). In a similar manner, a study on low-income Indian women showed that a higher maternal education resulted in lower concentrations of SFAs and PUFAs (36). However, Al-Tamer and Mahmood (37) found the proportions of the n-3 series of LCPUFA decreased with decreasing socioeconomic status (mother's education and occupation). The effect of maternal education on the FA in human milk is equivocal. A lower level of education usually implies higher unemployment or lower wages and a lower income level (38). Besides, as a reflection of traditional gender roles in society, women are more likely to take on the responsibility of food selection and acquisition. Taking all into consideration, maternal education level may influence the nutritional knowledge and income levels, which may lead to the difference in food consumption and dietary habits, and therefore, affect the profiles of FAs.
The influence of maternal age on FAs is ambiguous. In this study, maternal age is positively associated with some SFAs (C15:0, C16:0, C17:0, C18:0, and C20:0). Antonakou et al. (39) also reported that maternal age was an independent factor of MUFAs. However, two studies (40, 41) reported that maternal age was not related to milk lipids. Moreover, the association between infant gender and FAs was observed in our study. Infant gender was reported to influence hormonal secretions in the placenta during pregnancy, which is related to breast development (42). This may help explain the associations found in our study.
The results also showed that the mature milk of mothers who had delivered with a natural birth contained fewer SFAs (C10:0 and Total SFA) and more PUFAs (C18:2n-6c, Total n-6PUFA, and Total PUFA) compared with the cesarean section group. Sinanoglou et al. investigated the factors affecting human colostrum FAs and found that the proportions of C12:0, C14:0, C18:3n-3, C20:4n-6, C20:5n-3, and Total n-3PUFA were significantly (P < 0.05) lower in colostrum fat from cesarean than from vaginal deliveries (43). Gestational weight gain, infant gender, parity, and pre-conception BMI were also found to be associated with several human milk FAs, suggesting a possible role between these characteristics in FAs. As few studies have examined the associations between maternal characteristics and FAs, more research is needed to clarify these factors.
The FAs at the sn-2 position of TAG in human milk were reported to significantly affect the absorption of FAs and calcium, infant intestinal flora (17), and stool consistency (44). TAGs with sn-2 FAs have recently become a target in the optimizing of infant formula (45–47).
Results of our study corresponded with Deng et al. (22), in which several SFAs (C14:0, C15:0, and C16:0) were mainly acylated in the sn-2 position, and most MUFAs and PUFAs showed sn-1,3 positional selectivity in TAGs.
Notably, the sn-2 FA profile seemed to be less affected by the factors that influence total FA. Parity was not found to be associated with sn-2 FAs. The association between location and several FAs (C20:1n-9, C18:3n-3, and C20:2n-6) in total FAs was similar to that in sn-2 FAs. The sn-2 FAs as a part of total FAs may account for the similarity regarding these correlations between total and sn-2 FAs. C16:0 and DHA are proved to be beneficial to infants' health under the sn-2 positional selectivity (17, 44, 48). However, DHA was not observed to be associated with the factors discussed earlier, and C16:0 was only found to be associated with the delivery mode. This may suggest that the levels of the two FAs are relatively constant. The average levels of C16:0 and DHA at the sn-2 position among the domestic population may be a reliable reference for their infant milk powder formulation.
Based investigation of the profiles of FAs and sn-2 FAs in human mature milk samples from two representative areas in China, this study also strived to explore the associations between maternal factors and FAs, especially, sn-2 FAs, which are rarely explored in previous studies. Our study has a few limitations. First, a systematic dietary survey was not conducted, so nutrient intakes cannot be estimated, and the effects of maternal diet on FAs cannot be explored. In addition, we restricted our analyses to several sociodemographic factors, but other elements, namely, lactation stage, genes, and gestational age, were also reported to influence the FA profile. Further research is needed to integrate all the correlated factors. Despite these limitations, one of the outstanding advantages of this study is that the sampling sites were restricted to the hospital, and breast pump sampling was used to reduce the confounding factors of sampling, which significantly improved the reliability of the results.
In conclusion, this study elucidated the total and sn-2 FA profiles of mature milk in women from Beijing and Danyang, China. Correlation analysis revealed that the total FAs composition was variable and independently associated with location, maternal age, infant gender, pre-conception BMI, gestational weight gain, delivery mode, parity, and maternal education level. On the contrary, sn-2 FAs composition seemed more constant than total FAs, as parity was not found to be associated with the levels of sn-2 FAs. The conspicuous contribution of location and maternal education level was observed in both total and sn-2 FAs, which implicated the possible role of economic–related or education–related dietary habits; the delivery mode was also a significantly correlated predictor of the variation in FAs and sn-2 FAs. Together, these findings present a pilot study on the correlated factors of FAs in mature milk and may act as a reference for infant formula or human milk fortifier optimization.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Chinese Clinical Trial. The patients/participants provided their written informed consent to participate in this study.
MN conducted the experiments, analyzed the data, and wrote the first draft of the manuscript. YW contributed to the conception and design of the study. ZY wrote a part of the first draft. XX and HZ provided experimental and technical support. JC contributed to manuscript revision, read, and approved the submitted version. YY and LZ supervised. All the authors contributed to the article and approved the submitted version.
This study was funded by the Yihai Kerry Group.
The authors thank the doctors and nurses who assisted in the recruitment of the subjects and collecting samples.
Authors XX and HZ were the employees of the Wilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd. when this work was done. They participated in the validation of the study and the opinions they expressed were their own and do not necessarily reflect the views or recommendations of their respective affiliations.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.926429/full#supplementary-material
1. Wu K, Zhu J, Zhou L, Shen L, Mao Y, Zhao Y, et al. Lactational changes of fatty acids and fat-soluble antioxidants in human milk from healthy Chinese mothers. Br J Nutr. (2020) 123:841–8. doi: 10.1017/S0007114520000239
2. Bobiński R, Mikulska M, Mojska H, Simon M. Comparison of the fatty acid composition of transitional and mature milk of mothers who delivered healthy full-term babies, preterm babies and full-term small for gestational age infants. Eur J Clin Nutr. (2013) 67:966–71. doi: 10.1038/ejcn.2013.96
3. Liu Y, Liu X, Wang L. The investigation of fatty acid composition of breast milk and its relationship with dietary fatty acid intake in 5 regions of China. Medicine. (2019) 98:e15855. doi: 10.1097/MD.0000000000015855
4. Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T. Physiological aspects of human milk lipids. Early Hum Dev. (2001) 65 (Suppl):S3–S18. doi: 10.1016/S0378-3782(01)00204-3
5. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. doi: 10.1016/j.pcl.2012.10.002
6. Costa AGV, Sabarense CM. Modulation and composition of fatty acids in human milk. Rev Nutr. (2010) 23:445–57. doi: 10.1590/S1415-52732010000300012
7. Silberstein T, Burg A, Blumenfeld J, Sheizaf B, Tzur T, Saphier O. Saturated fatty acid composition of human milk in Israel: a comparison between Jewish and Bedouin women. Isr Med Assoc J. (2013) 15:156–9. Available online at: https://pubmed.ncbi.nlm.nih.gov/23781748/
8. Krešić G, Dujmović M, L Mandić M, Mrduljaš N. Breast milk: fatty acid composition and maternal diet. Mljekarstvo: časopis za unaprjedenje proizvodnje i prerade mlijeka. (2013) 63:158–71. Available online at: https://hrcak.srce.hr/en/file/157006
9. Qi C, Sun J, Xia Y, Yu R, Wei W, Xiang J, et al. Fatty acid profile and the sn-2 position distribution in triacylglycerols of breast milk during different lactation stages. J Agric Food Chem. (2018) 66:3118–26. doi: 10.1021/acs.jafc.8b01085
10. Qin XL, Yang B, Wang YH. Study on composition of fatty acid and distribution of sn-2 fatty acid in colostrum human milk. J Food Sci Tech Mys. (2010) 88:81–4. doi: 10.13386/j.issn1002-0306.2010.05.070
11. Straarup EM, Lauritzen L, Faerk J, Høy Deceased CE, Michaelsen KF. The stereospecific triacylglycerol structures and Fatty Acid profiles of human milk and infant formulas. J Pediatr Gastroenterol Nutr. (2006) 42:293–9. doi: 10.1097/01.mpg.0000214155.51036.4f
12. López-López A, López-Sabater MC, Campoy-Folgoso C, Rivero-Urgell M, Castellote-Bargalló AI. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur J Clin Nutr. (2002) 56:1242–54. doi: 10.1038/sj.ejcn.1601470
13. Bar-Yoseph F, Lifshitz Y, Cohen T. Review of sn-2 palmitate oil implications for infant health. Prostaglandins Leukot Essent Fatty Acids. (2013) 89:139–43. doi: 10.1016/j.plefa.2013.03.002
14. Bar-Yoseph F, Lifshitz Y, Cohen T, Malard P, Xu C. SN2-palmitate reduces fatty acid excretion in chinese formula-fed infants. J Pediatr Gastroenterol Nutr. (2016) 62:341–7. doi: 10.1097/MPG.0000000000000971
15. Yaron S, Shachar D, Abramas L, Riskin A, Bader D, Litmanovitz I, et al. Effect of high β-palmitate content in infant formula on the intestinal microbiota of term infants. J Pediatr Gastroenterol Nutr. (2013) 56:376–81. doi: 10.1097/MPG.0b013e31827e1ee2
16. Jie L, Qi C, Sun J, Yu R, Wang X, Korma SA, et al. The impact of lactation and gestational age on the composition of branched-chain fatty acids in human breast milk. Food Funct. (2018) 9:1747–54. doi: 10.1039/C7FO01979C
17. Jiang T, Liu B, Li J, Dong X, Lin M, Zhang M, et al. Association between sn-2 fatty acid profiles of breast milk and development of the infant intestinal microbiome. Food Funct. (2018) 9:1028–37. doi: 10.1039/C7FO00088J
18. Thakkar SK, De Castro CA, Beauport L, Tolsa JF, Fischer Fumeaux CJ, Affolter M, Giuffrida F. Temporal Progression of Fatty Acids in Preterm and Term Human Milk of Mothers from Switzerland. Nutrients. (2019) 11:112. doi: 10.3390/nu11010112
19. Garcia-Ravelo S, Diaz-Gomez NM, Martin MV, Dorta-Guerra R, Rodríguez C. Fatty acid composition and eicosanoid levels (LTE4 and PGE) of human milk from normal weight and overweight mothers. Breastfeed Med. (2018) 13:702–10. doi: 10.1089/bfm.2017.0214
20. Jiang J, Wu K, Yu Z, Ren Y, Zhao Y, Jiang Y, et al. Changes in fatty acid composition of human milk over lactation stages and relationship with dietary intake in Chinese women. Food Funct. (2016) 7:3154–62. doi: 10.1039/C6FO00304D
21. Roy S, Ghosh S. Fatty acid composition of Bengali mothers' milk: a survey based population study. J Indian Chem Soc. (2013) 90:663–7. Available online at: https://www.researchgate.net/publication/272089384
22. Deng L, Zou Q, Liu B, Ye W, Zhuo C, Chen L, et al. Fatty acid positional distribution in colostrum and mature milk of women living in Inner Mongolia, North Jiangsu and Guangxi of China. Food Funct. (2018) 9:4234–45. doi: 10.1039/C8FO00787J
23. Yuan X, Xiang J, Cao X, Cao M, Jin Q, Wang X. Fatty acid composition and sn-2 fatty acid distribution of human milk from wuxi prefecture(jiangsu,china). China Oils Fats. (2015) 11:44–7.
24. Barbano DM, Clark JL, Dunham CE. Comparison of babcock and ether extraction methods for determination of fat content of milk: collaborative study. J Assoc Off Anal Chem. (1988) 71:898–914. doi: 10.1093/jaoac/71.5.898
25. Sahin N, Akoh CC, Karaali A. Lipase-catalyzed acidolysis of tripalmitin with hazelnut oil fatty acids and stearic acid to produce human milk fat substitutes. J Agric Food Chem. (2005) 53:5779–83. doi: 10.1021/jf050465e
26. Revelle W,. psych: Procedures for Personality Psychological Research, Northwestern University, Evanston, Illinois, USA. (2015). Available online at: http://CRAN.R-project.org/package=psych Version = 1.5.8 (accessed August 30, 2015).
27. Wickham H. Reshaping data with the reshape package. J Stat Software. (2007) 21:1–20. doi: 10.18637/jss.v021.i12
28. Grömping U. Relative importance for linear regression in R: the package relaimpo. J Stat Softw. (2006) 17:1–27. doi: 10.18637/jss.v017.i01
29. Stephane Dray with contributions of Pierre Legendre Guillaume Blanchet. packfor: Forward Selection with permutation (Canoco p.46). R package version. 0.0-8/r136. (2016). Available online at: https://R-Forge.R-project.org/projects/sedar/ (acceseed June 17, 2016).
31. Yang T, Zhang L, Bao W, Rong S. Nutritional composition of breast milk in Chinese women: a systematic review. Asia Pac J Clin Nutr. (2018) 27:491–502. doi: 10.6133/apjcn.042017.13
32. Bahreynian M, Feizi A, Kelishadi R. Is fatty acid composition of breast milk different in various populations? A systematic review and meta-analysis. Int J Food Sci Nutr. (2020) 71:909–20. doi: 10.1080/09637486.2020.1746958
33. Blanchard E, Zhu P, Schuck P. Infant formula powders. Handb Food Powders. (2013) 147:465–83. doi: 10.1533/9780857098672.3.465
34. Wu K, Gao R, Tian F, Mao Y, Wang B, Zhou L, et al. Fatty acid positional distribution (sn-2 fatty acids) and phospholipid composition in Chinese breast milk from colostrum to mature stage. Br J Nutr. (2019) 121:65–73. doi: 10.1017/S0007114518002994
35. Giuffrida F, Cruz-Hernandez C, Bertschy E, Fontannaz P, Masserey Elmelegy I, Tavazzi I, et al. Temporal changes of human breast milk lipids of chinese mothers. Nutrients. (2016) 8:715. doi: 10.3390/nu8110715
36. Nayak U, Kanungo S, Zhang D, Ross Colgate E, Carmolli MP, Dey A, et al. Influence of maternal and socioeconomic factors on breast milk fatty acid composition in urban, low-income families. Matern Child Nutr. (2017) 13:e12423. doi: 10.1111/mcn.12423
37. Al-Tamer YY, Mahmood AA. The influence of Iraqi mothers' socioeconomic status on their milk-lipid content. Eur J Clin Nutr. (2006) 60:1400–5. doi: 10.1038/sj.ejcn.1602470
38. Sila U, Dugain V. Income, Wealth and Earnings Inequality in Australia: Evidence from the HILDA survey. OECD Economics Department Working Papers. Paris: OECD Publishing (2019). No. 1538. doi: 10.1787/cab6789d-en
39. Antonakou A, Skenderi KP, Chiou A, Anastasiou CA, Bakoula C, Matalas AL. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. Eur J Nutr. (2013) 52:963–73. doi: 10.1007/s00394-012-0403-8
40. Bachour P, Yafawi R, Jaber F, Choueiri E, Abdel-Razzak Z. Effects of smoking, mother's age, body mass index, and parity number on lipid, protein, and secretory immunoglobulin A concentrations of human milk. Breastfeed Med. (2012) 7:179–88. doi: 10.1089/bfm.2011.0038
41. Rocquelin G, Tapsoba S, Dop MC, Mbemba F, Traissac P, Martin-Prével Y. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers' nutritional status: impact on infants' EFA supply. Eur J Clin Nutr. (1998) 52:164–71. doi: 10.1038/sj.ejcn.1600529
42. Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. (2010) 22:50–4. doi: 10.1002/ajhb.20941
43. Sinanoglou VJ, Cavouras D, Boutsikou T, Briana DD, Lantzouraki DZ, Paliatsiou S, et al. Factors affecting human colostrum fatty acid profile: a case study. PLoS One. (2017) 12:e0175817. doi: 10.1371/journal.pone.0175817
44. Petit V, Sandoz L, Garcia-Rodenas CL. Importance of the regiospecific distribution of long-chain saturated fatty acids on gut comfort, fat and calcium absorption in infants. Prostag Leukotr Ess. (2017) 121:40–51. doi: 10.1016/j.plefa.2017.05.007
45. Álvarez Carlos A, Akoh CC. Enzymatic synthesis of highsn-2 DHA and ARA modified oils for the formulation of infant formula fat analogues. J Am Oil Chem Soc. (2016) 93:383–95. doi: 10.1007/s11746-015-2774-5
46. Álvarez Carlos A, Akoh CC. Preparation of infant formula fat analog containing capric acid and enriched with DHA and ARA at thesn-2 position. J Am Oil Chem Soc. (2016) 93:531–42. doi: 10.1007/s11746-016-2788-7
47. Moreno-Perez S, Luna P, Señorans J, Rocha-Martin J, Guisan JM, Fernandez-Lorente G. Enzymatic transesterification in a solvent-free system: synthesis of sn-2 docosahexaenoyl monoacylglycerol. Biocatal Biotransformation. (2018) 36:265–70. doi: 10.1080/10242422.2017.1319823
Keywords: breast milk, mature milk, total fatty acid, sn-2 fatty acid, sociodemographic factors
Citation: Ni M, Wang Y, Yang Z, Xu X, Zhang H, Yang Y, Zhang L and Chen J (2022) Profiles of total and sn-2 fatty acid of human mature milk and their correlated factors: A cross-sectional study in China. Front. Nutr. 9:926429. doi: 10.3389/fnut.2022.926429
Received: 22 April 2022; Accepted: 22 July 2022;
Published: 22 August 2022.
Edited by:
Defu Ma, Peking University, ChinaCopyright © 2022 Ni, Wang, Yang, Xu, Zhang, Yang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuexin Yang, eXVleGluX3lhbmdAc2luYS5jb20=; Lishi Zhang, bGlzaGl6aGFuZ181NkAxNjMuY29t; Jinyao Chen, dW1icmVsbGF5eUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.