- 1Department of Breast Surgery, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Key Laboratory of Molecular Biology, Hainan Medical University, Haikou, China
- 3Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, China
- 4Biotechnology and Biochemistry Laboratory, Hainan Medical University, Haikou, China
A whole-grain (WG) diet affects human health in multiple ways. However, the effect of WG on the gut microbiota of the elderly individuals is still largely unknown. In this study, WG did not affect the microbial α-diversity but had a profound impact on the microbes' abundance in the elderly individuals. WG increased the abundance of Verrucomicrobia and decreased the abundance of Firmicutes. The prediction of microbial function showed that glucose metabolism and lipid metabolism were inhibited. In addition, the effects of WG on the gut microbiota of normal-weight (NW) and overweight (OW) individuals were different. WG increased Verrucomicrobia in the NW group and decreased Firmicutes in the OW group. Meanwhile, the effect of WG on gut microbiota showed gender characteristics, Firmicutes/Bacteroidetes ratio was decreased in women, while Verrucomicrobia abundance was increased in men. The use of WG could improve the microbial composition and promote the growth of beneficial microbes, which may be beneficial to the health of the elderly individuals.
Introduction
Healthy aging is the key to alleviating the pressure of population aging (1). With the increase of age, the physiological functions of the various organs of the elderly individuals would significantly decrease. The metabolic function of the elderly individuals would be disordered, and their immunity would be significantly decreased (2). The elderly individuals are prone to various kinds of chronic diseases, such as hypertension, diabetes, coronary heart disease, and cancer (3, 4). A healthy lifestyle is important for the health of the elderly individuals (5). The most direct way of health management is to intervene in the diet of the elderly individuals.
Studies have shown that gut microbiota is related to the occurrence and development of various diseases (6, 7). Recently, large-scale observational epidemiological studies and animal studies have suggested that microbial disorder and hypertension might be causally related (8, 9). There may be some connection between Firmicutes/Bacteroidetes ratio and blood pressure (10). Studies suggest that changes in the gut microbiota have been involved in the pathogenesis of diabetes (11). Increasing evidence suggests that the microbial ecosystem is associated with the progression of multiple cancers, such as prostate cancer, pancreatic cancer, and liver cancer (12). Gut microbiota imbalance can affect the production of short-chain fatty acids (SCFAs), bile acid metabolism, and intestinal mucosa, leading to the occurrence of diseases (13). Therefore, it is important to maintain the intestinal microenvironment for human health.
Whole grain is characterized by the retention of the endosperm, germ, and bran found in whole grain (WG). WG is a good source of dietary fiber, protein, B vitamins, vitamin E, minerals (such as selenium, copper, and magnesium), flavonoids, and polyphenols (14–16). Numerous studies have shown that they can help to reduce the risk of many diseases, such as overweight (OW)/obesity, cardiovascular disease, type 2 diabetes, intestinal disease, and certain cancers, as compared to refined grains. Several studies have shown that WG can significantly alter the diversity, structure, and function of the gut microbiota. A recent study shows that WG can increase the abundances of Ruminococcaceae_UCG-014, Ruminococcaceae_UCG-014, Ruminocostridium_9, and Ruminococcaceae_NK4A214_group (17). This study explored the possible effects of WG on the health of the elderly individuals from the perspective of gut microbiota.
Materials and Methods
Data Sources
The American Gut Program (AGP) collected 25,376 samples through voluntary and self-reported methods that included basic information (age, gender, height, and weight) and lifestyle-related information. The collection, sequencing, and quality control of samples were carried out according to the standards of the Earth Microbiome Project.
American Gut Program sequencing data had been stored in the Sequence Read Archive (SRA) database in SRA file format (https://www.ncbi.nlm.nih.gov/sra/), and the registration number was PRJEB11419. AGP questionnaire results could also be downloaded from the SRA database.
In the study, samples were strictly screened and matched. Those who did not fill in the basic information in the questionnaire, who showed serious diseases, who received antibiotic treatment within 6 months, and who had been traveling recently were excluded. Non-fecal samples were also excluded. In addition, according to the sequencing quality, samples with a sequencing depth of <8,000 were excluded. Finally, we found 70 elderly individuals who daily fed on/ate WG (DWG) and matched 70 elderly individuals who never fed on/ate WG (NWG) according to gender, body mass index (BMI), and age (Table 1 and Supplementary Table S1).
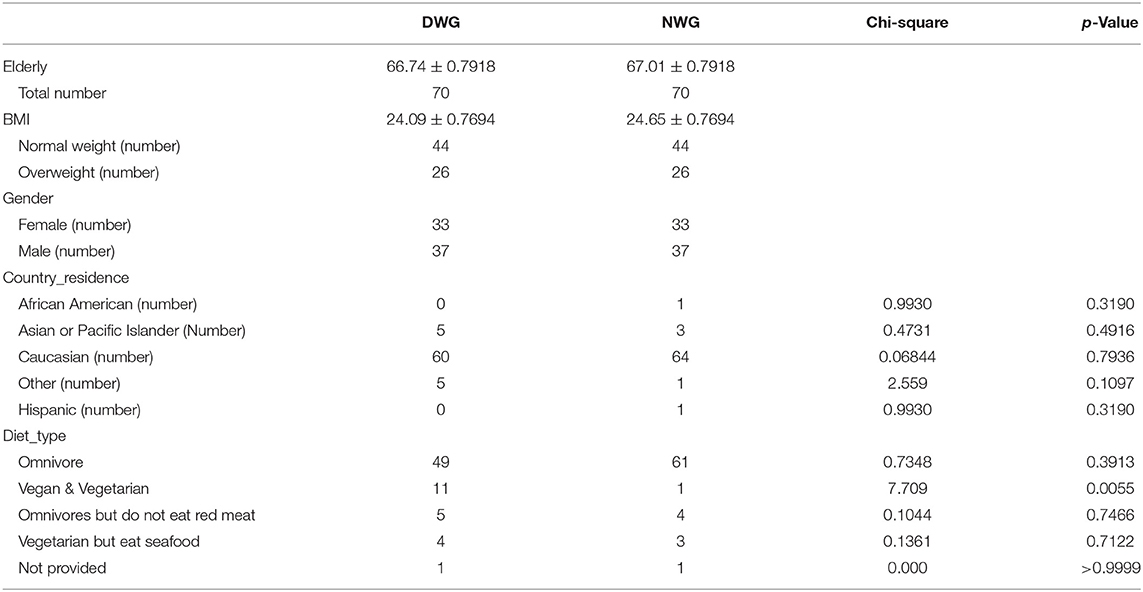
Table 1. Demographic and anthropometric characteristics in the elderly individuals of the daily whole-grain (WG) diet and the never whole grain diet.
Converting SRA to FASTQ Format
The files in the SRA database were in SRA format and needed to be converted to FASTQ format for further analysis. The SRA Toolkit tool was employed for format conversion.
Data Processing
The FASTQ format file was used for flora analysis. This process used the QIIME2 software. The FASTQ format file was first packaged into a demux.qza file. Then the Deblur plug-in was used to perform denoising and Operational Taxonomic Unit (OTU) clustering and generate result files with representative sequences, feature tables, and sample statistics. Unrooted trees are obtained through the “qiime-phylogeny-align-to-tree-mafft-fastree” plug-in. Alpha diversity is obtained through the “qiime diversity alpha sparse” plugin and converted to qzv files. QZV file can view OTU number and Shannon index through the browser.
Then, we compared it with the Greengenes database (version 13.8), and the comparison method was UCLUST, identity 0.9, to complete the classification of bacteria. In addition, we deleted the bacteria with low distribution (which only existed in <1% of the samples) for subsequent analysis.
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) was used to predict functional abundance based on marker gene sequences.
Statistical Analysis
Statistical Analysis of Metagenomic Profiles (STAMP 2.1.3) was used to analyze microbial data, such as microbial community composition and function. Adjusted p-values were calculated by Benjamin Hochberg's false discovery rate (FDR) method and used to analyze significant differences (padj < 0.05).
Result
The present study detected the effects of WG on the gut microbiota of the elderly individuals. As shown in Table 1, there is no significant difference in age, BMI, and gender between the DWG and NWG dietary groups.
Effects of WG on Gut Microbiota in the Elderly Individuals
To detect the effect of WG on the gut microbiota of the elderly individuals, α-diversity was analyzed (Figures 1A,B). The OTU numbers of DWG and NWG were 183.2 and 177.9, respectively (p > 0.05), and the Shannon index values were 5.192 and 5.239, respectively (p > 0.05). The results showed that the microbial diversity in the elderly individuals was not affected by WG.
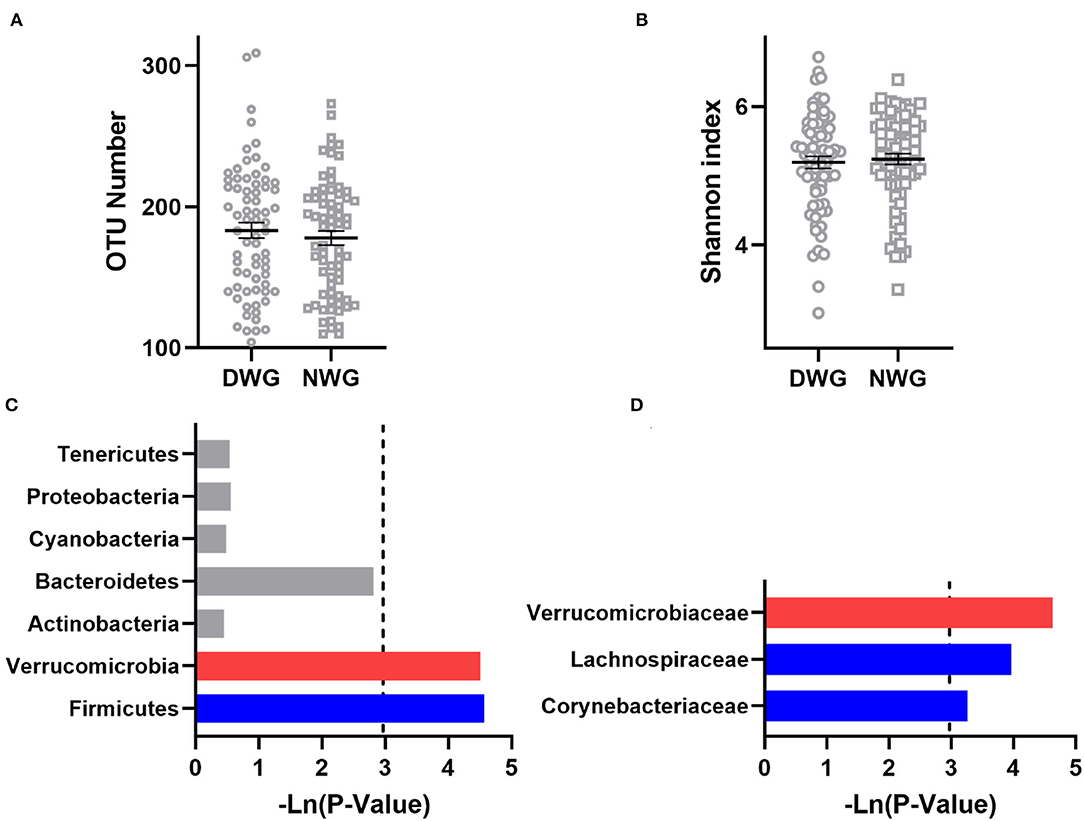
Figure 1. The effect of whole-grain (WG) diet on the gut microbiota composition of elderly individuals. (A) Number of OTUs, (B) Shannon index, (C) phylum level, and (D) family level. The red bar represents a significant increase, while the blue bar represents a significant decrease.
The effect of WG on the gut microbiota composition was further analyzed (Figures 1C,D). At the phylum level, when compared with NWG, the relative abundance of Verrucomicrobia was increased in the DWG, while the relative abundance of bacteria Firmicutes was decreased. At the family level, the relative abundance of Verrucomicrobiaceae was increased, while that of Lachnospiraceae and Actinomycetaceae was decreased.
In addition, the microbial function predicted that 58 metabolic pathways had significant changes, i.e., 16 amino acid metabolism-related pathways, five nucleotide metabolism-related pathways, one biological oxidation pathway, 11 carbohydrate metabolism-related pathways, two terpenoid synthesis pathways, three vitamins related pathways, seven cell wall-related metabolic pathways, and six lipid metabolism pathways. Generally, the metabolism of amino acids, nucleotides, biological oxidation, sugar, vitamins, and lipids was inhibited in the DWG group (Figure 2).
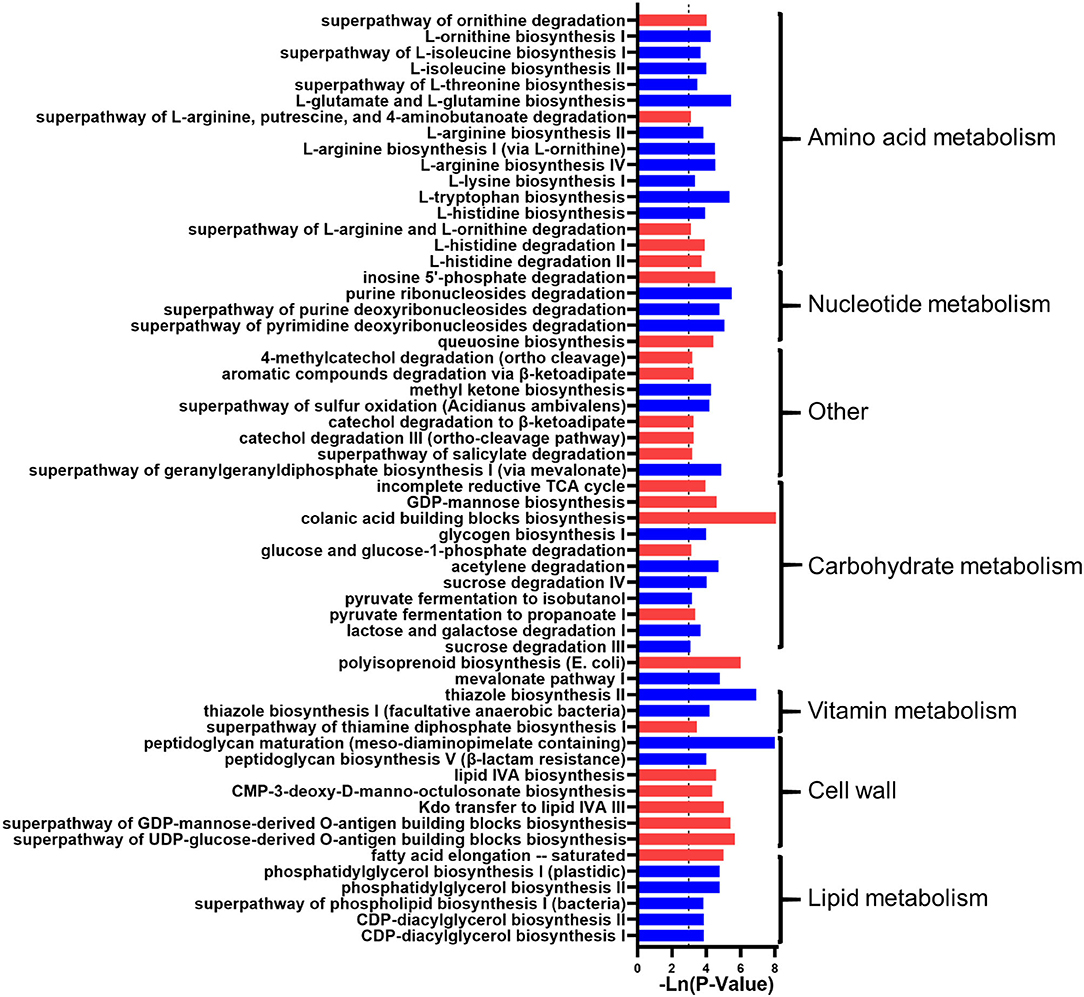
Figure 2. The effect of whole-grain (WG) diet on the gut microbiota function of elderly individuals. The significant effect of the WG diet on microbial metabolism pathways. The red bar represents a significant increase, while the blue bar represents a significant decrease.
Effects of WG on the Gut Microbiota of Normal Weight (NW) Group and OW Group in the Elderly Individuals
To further explore the effect of WG on the gut microbiota of different BMI elderly individuals, the samples were divided into NW and OW (BMI > 25) groups. As shown in Figure 3, the OTU values of NW_DWG, NW_NWG, OW_DWG, and OW_NWG are 179.5, 173.7, 189.5, and 185.1, respectively (p > 0.05), and the Shannon index values are 5.113, 5.117, 5.326, and 5.446, respectively (p > 0.05). In addition, when compared with the NW group, both OTU and Shannon index of the OW group showed an upward trend, which indicated that the intestinal microbial diversity of the elderly individuals changed with the increase in BMI.
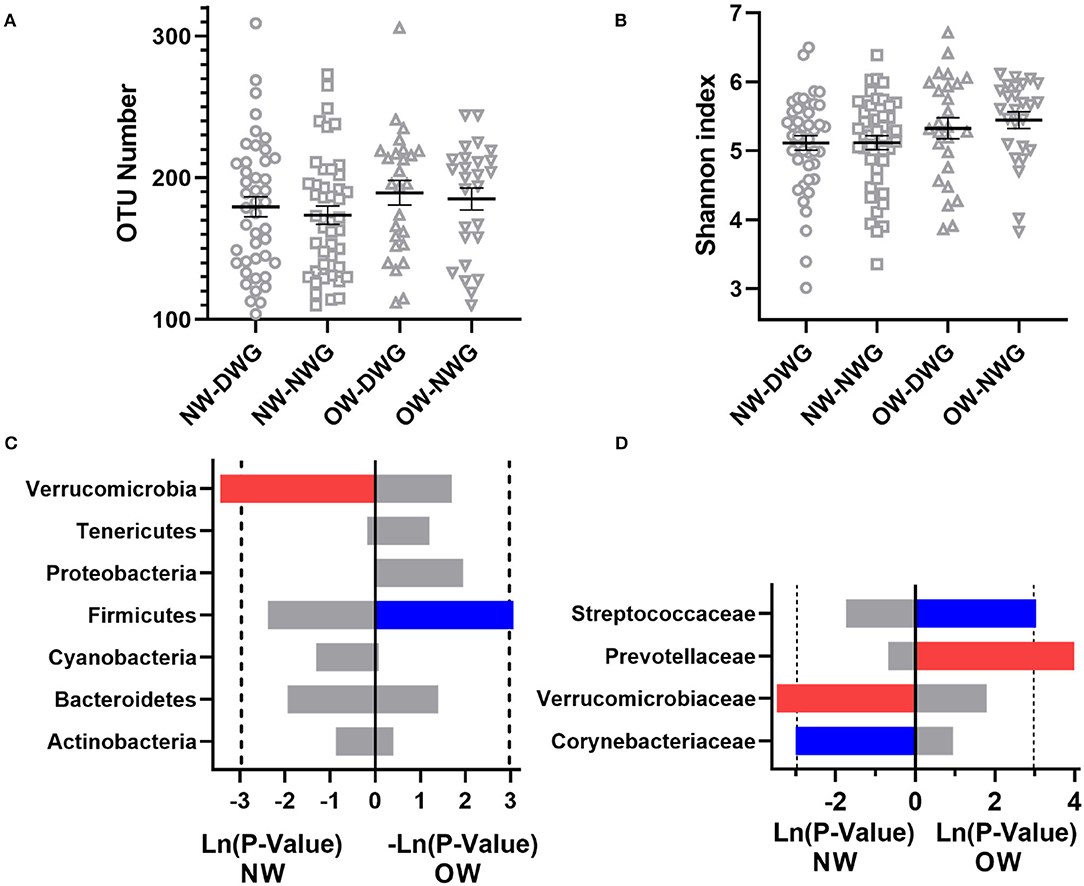
Figure 3. The effect of whole-grain (WG) diet on the gut microbiota composition of female and male elderly individuals. (A) Number of OTUs, (B) Shannon index, (C) phylum level, and (D) family level. The red bar represents a significant increase, while the blue bar represents a significant decrease.
Next, the relative abundance of microbial components was detected at the phylum and family level (Figures 3C,D). At the phylum level, the relative abundance of Verrucomicrobia in the NW_DWG group was significantly higher than that in the NW_NWG group. Compared with the OW_NWG group, the relative abundance of Firmicutes was significantly lower in the OW_DWG group. At the family level, the relative abundance of Verrucomicrobiaceae was increased, while the relative abundance of Actinomycetaceae was decreased in the NW_DWG group, when compared with the NW_NWG group. The relative abundance of Streptococcaceae was decreased significantly, while the relative abundance of Prevotellaceae was increased significantly in OW_DWG, when compared with OW_NWG.
In addition, the prediction of microbial function showed that 20 and 66 metabolic pathways were changed significantly (p < 0.05; Figure 4). In NW_DWG, the metabolic pathways related to sugars, cell walls, and lipids were significantly increased, while the metabolism of nucleotides, biological oxidation, and vitamins was significantly decreased. In OW_DWG, the metabolic pathways of amino acids, vitamins, and lipids were significantly decreased, while those of SCFAs, biological oxidation, sugars, and other substances were significantly increased.
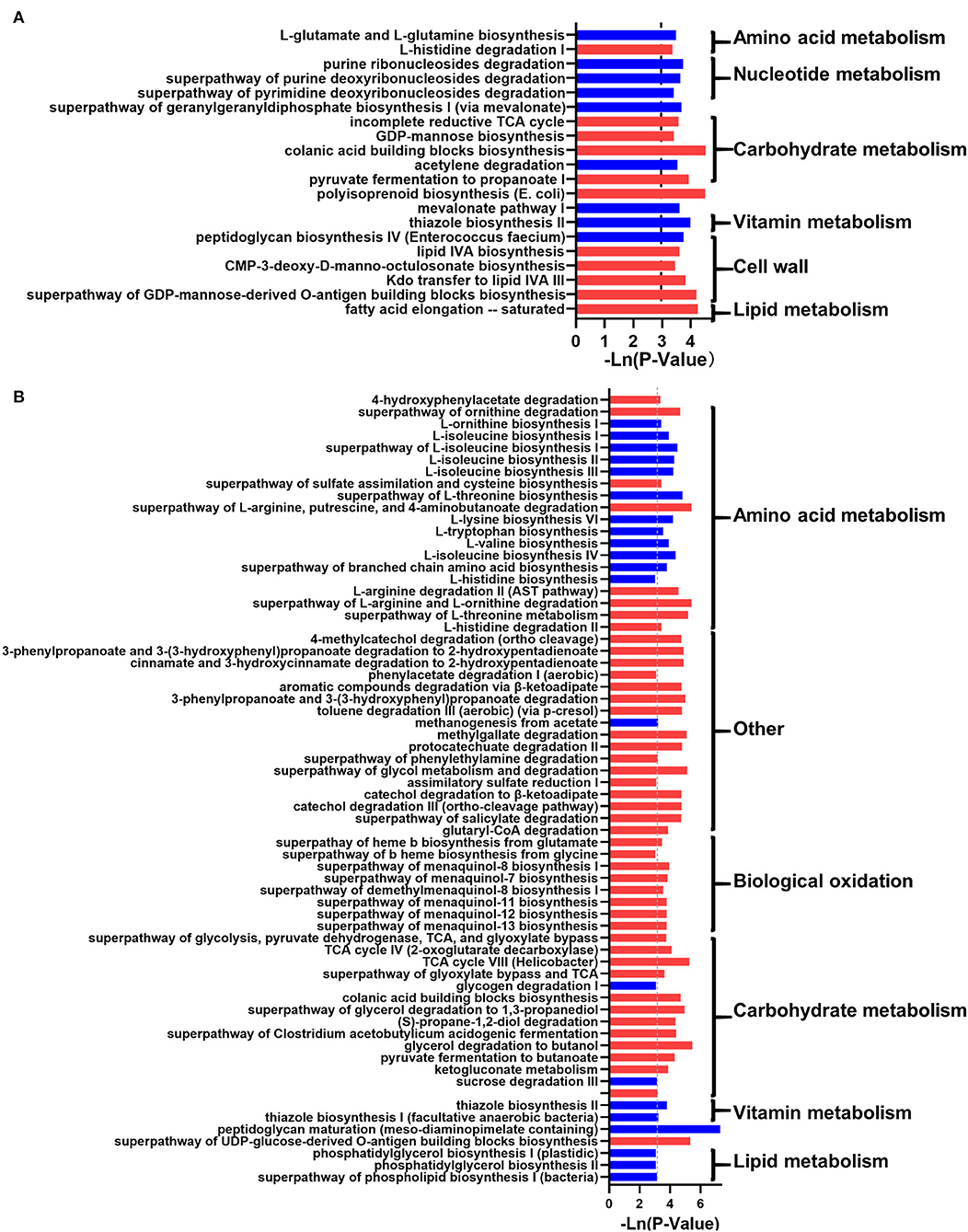
Figure 4. The effect of the whole-grain (WG) diet on the gut microbiota function of female and male individuals. The significant effect of the WG diet on microbial metabolism pathways in (A) female and (B) male individuals. The red bar represents a significant increase, while the blue bar represents a significant decrease.
Effects of WG on the Gut Microbiota of Female and Male Elderly Individuals
Furthermore, the effects of WG on the gut microbiota of female and male elderly individuals were analyzed. The results showed that the DWG did not affect microbial α-diversity of male and female elderly individuals (Figures 5A,B). At the phylum level, the relative abundance of Firmicutes was significantly reduced, while that of Bacteroidetes was significantly increased in female DWG, when compared with that of female NWG. The relative abundance of Verrucomicrobia and Proteobacteria was significantly increased in Male_DWG, when compared with Male_NWG. At the family level, the relative abundance of Prevotellaceae was significantly increased in female DWG, when compared with that of the female NWG group. The relative abundance of Streptococcaceae was significantly decreased, while that of Verrucomicrobiaceae and Enterobacteriaceae was significantly increased in Male_DWG, when compared with that of Male_NWG (Figures 5C,D).
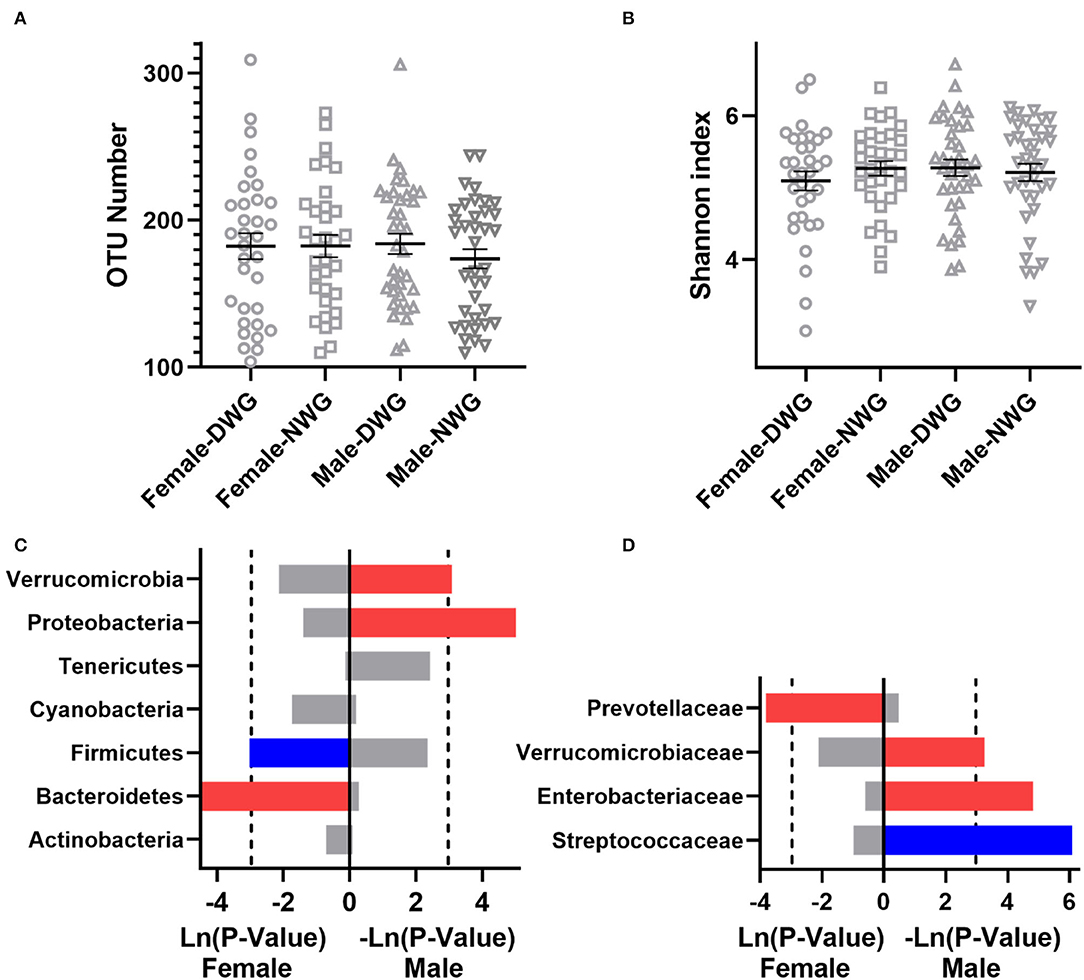
Figure 5. The effect of whole-grain (WG) diet on the gut microbiota composition of normal-weight and overweight elderly individuals. (A) Number of OTUs, (B) Shannon index, (C) phylum level, and (D) family level. The red bar represents a significant increase, while the blue bar represents a significant decrease.
In addition, functional predictions indicated that 13 and 105 related metabolic pathways were significantly changed in Female_DWG and Male_DWG (p < 0.05; Figure 6). In Female_DWG, the related metabolic pathways of terpenoids, cell walls, and lipids were significantly increased, and the metabolism of vitamins is significantly reduced. In Male_DWG, the metabolic pathways related to SCFA, nucleic acid bio-oxidation, and lipo-sugar were significantly increased, while the related metabolic pathways of amino acids, nucleotides, terpenoids, vitamins, and lipids were significantly reduced.
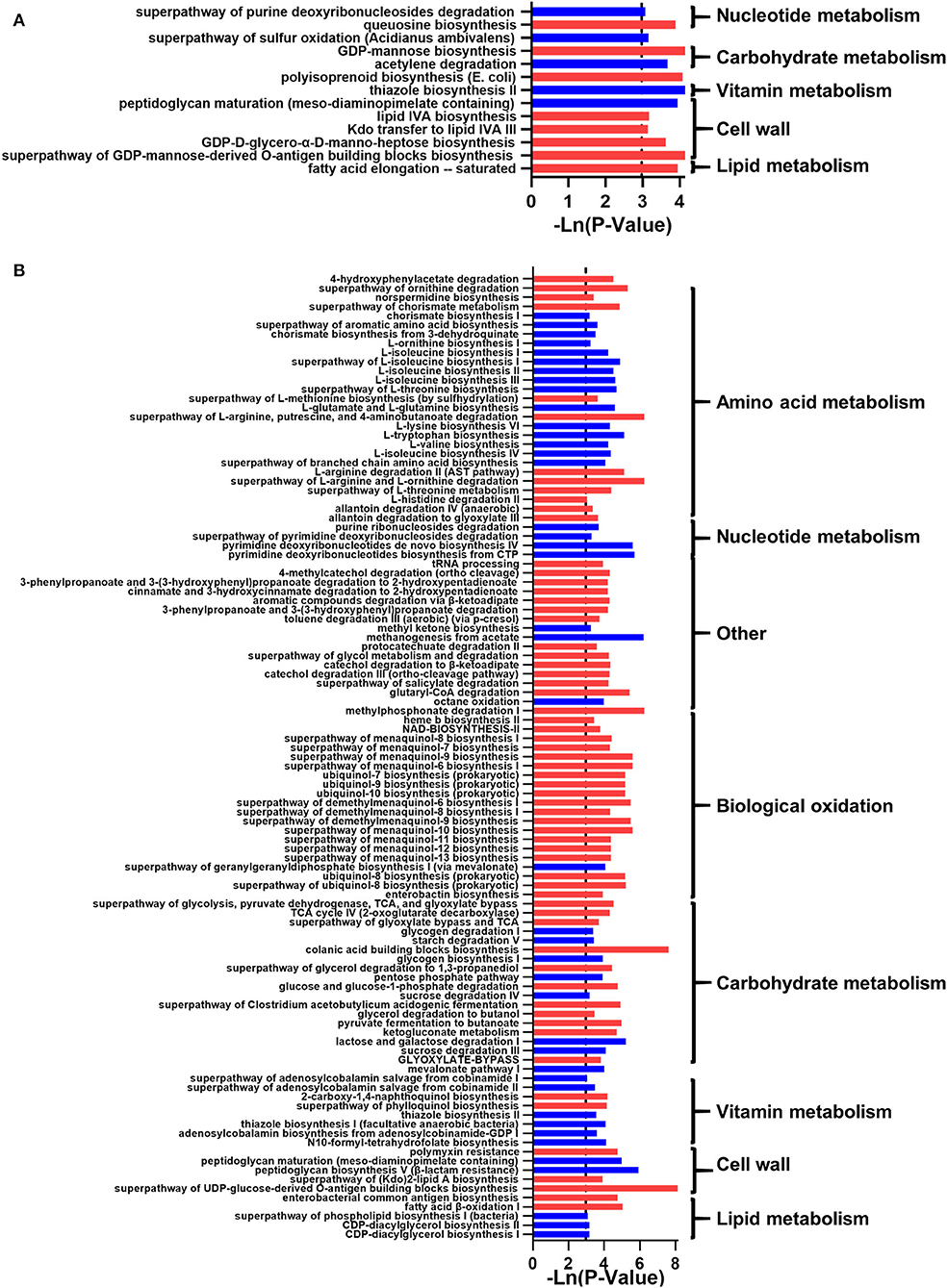
Figure 6. The effect of the whole-grain (WG) diet on the gut microbiota function of normal weight and overweight individuals. The significant effect of the WG diet on microbial metabolism pathways, (A) normal-weight and (B) overweight individuals. The red bar represents a significant increase, while the blue bar represents a significant decrease.
Discussion
Studies have shown that diet can directly affect the diversity and composition of human gut microbiota (18, 19). WG has a significant effect on the gut microbiota of obesity, DM, and hypertension (20–22). The present study used the data from the AGP to analyze the effect of WG on the gut microbiota of the elderly individuals. To simplify the analysis model, we screened out the microbial data of the elderly individuals who never and/or daily feed on WG for analysis. The results showed that the WG did not affect the microbial α-diversity in the elderly individuals, but had a significant effect on the microbial composition and function.
Studies have shown that WG can significantly affect microbial community composition (23, 24). Wheat WG could significantly increase Bifidobacteria, Lactobacilli, and Prevotella and significantly decrease Dialister, Bifidobacterium, Blautia, and Collinsella (25, 26). Quinoa could alleviate dysbiosis remarkably by increased species richness and diversity, decreased phylum Proteobacteria, and genera Escherichia and Peptoclostridium (23). A 6-week randomized controlled trial shows that WG can effectively reduce the weight of OW, and it is related to Prevotella abundance (27). This may be related to the WG-rich diet reducing systematic low-grade inflammation and keeping the gut microbiota stable (28). WG significantly decreased Firmicutes/Bacteroidetes ratio, Blautia, Roseburia, Bifidobacterium, and Dialister (29, 30). The present study showed that DWG could decrease the abundance of Firmicutes, Lachnospiraceae, and Actinomycetaceae and increase the abundance of Verrucomicrobia and Verrucomicrobiaceae. Therefore, the present study and previous research have shown that WG intake can reduce the Firmicutes/Bacteroidetes ratio, and this study provides more gut bacteria associated with WG intake. Meanwhile, the study provides more intestinal microbes related to WG intake.
Studies have shown that the components of WG (dietary fiber, phenols, and B vitamins) can affect the gut microbiota. High dietary fiber can effectively improve the diversity and composition of gut microbiota, such as Clostridiales, which participate in carbohydrate utilization via polysaccharide degradation (31). The type and composition of dietary fiber from WG can affect the production of SCFA, such as arabinoxylan mixed with β-glucan, which can affect butyrate production. WG is high in phenolic acids, mainly covalently bound to fiber (32). The gut microbiota could release covalently bound phenolics from fiber and undergo metabolic conversion. Biotransformation of phenolic compounds may favor specific bacterial species, which in turn affect dietary fiber degradation pathways (32). In addition, B vitamins also play an important role in the regulation of gut microbiota (33). The present study found that WG not only affected the composition of gut microbiota but also predicted that WG could achieve beneficial effects by modulating the effect of gut microbiota on the metabolism of amino acids, carbohydrates, lipids, and vitamins.
Being OW is an important factor threatening the health of the elderly people (34). Studies have shown that the microbial composition of OW individuals is significantly affected (33, 35). Increased Streptococcaceae is generally considered to be associated with diseases, such as diabetes, obesity, and hypertension (36–38). Moreover, gut microbiota rich in Prevotella can improve glucose metabolism, potentiate weight loss, and reduce cholesterol levels (39–41). Researchers believe that the high diversity of Prevotella can enhance the fermentation ability of the microbiota, which is conducive to human health (42). Akkermansia in Verrucomicrobiaceae is inversely associated with obesity, diabetes, and cardiovascular disease (43). Consistent with previous studies, the study found that being OW was an important factor affecting the gut microbiota of the elderly people. The level of Firmicutes and Streptococcaceae was decreased, while the level of Prevotella was increased in the OW_DWG group. In addition, Verrucomicrobiaceae was significantly increased in the NW_DWG group. In general, WG diets can increase the abundance of probiotics and inhibit the growth of disease-related microbes for both NW and OW individuals.
Several studies have shown that there are significant differences in the gut microbiota of men and women (44, 45). This may be due to the male and female receiving different hormonal disturbances or different dietary patterns (46, 47). Studies have shown that the gut microbiota of men and women respond differently to the same diet and pharmacological interventions (48–50). A study showed an increased abundance of Lactobacillus, Alistipes, Lachnospira, and Clostridium in male mice but not in female mice when fed with HFD (48). A recent study showed that a diet of fructooligosaccharides had differential effects on SCFAs in the gut of male and female rats, while an increased abundance of Bacteroides was found only in female rats (49). A tuna oil and algae oil mixture treatment resulted in different gut microbial composition alterations in different sexes (51). The present study showed that WG significantly increased Verrucomicrobia and decreased Streptococcaceae in men, while significantly suppressing the Firmicutes to Bacteroidetes ratio and increasing Prevotellaceae in women. This study highlights the inconsistencies in the effect of WG on the gut microbiota in the male and female elderly individuals.
However, this study has some shortcomings due to AGP's project design. For example, all data are self-reporting, and its authenticity cannot be traced back. Although AGP collected more than 25,000 samples, there is little data available. Specific to the present study, we only matched 70 samples. In addition, the sample data come from the Caucasian population, and there is a lack of data on different races. These were worthy of further exploration in future research.
Conclusion
This study analyzed the effect of WG on the gut microbiota of the elderly people. It was found that although WG did not change the α-diversity of the microbes, it affected the abundance of Verrucomicrobia and Firmicutes. In addition, for the WG, different weights and gender have different outcomes. WG affected the abundance of Verrucomicrobia, Verrucomicrobiaceae, and Corynebacteriaceae bacteria in NW people and affected the abundance of Firmicutes, Streptococcaceae, and Prevotellaceae bacteria in OW people. WG affected the abundance of Firmicutes, Bacteroidetes, and Prevotellaceae in men and Verrucomicrobia, Proteobacteria, Verrucomicrobiaceae, and Streptococcaceae bacteria in women. In general, WG can increase the abundance of beneficial bacteria and partially inhibit the growth of harmful bacteria.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
GD contributed to funding acquisition, investigation, methodology, project administration, resources, and writing—original draft and editing. YZ and ZC contributed to the software. JL contributed to the conceptualization of formal analysis. PF contributed to writing—original drafts and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (No. 81960672 to GD), Natural Science Foundation of Hainan Province (821MS0830 to PF), and had no role in the study design, data collection analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.919838/full#supplementary-material
References
1. Plaza-Diaz J, Bernal MJ, Schutte S, Chenoll E, Genoves S, Codoner FM, et al. Effects of whole-grain and sugar content in infant cereals on gut microbiota at weaning: a randomized trial. Nutrients. (2021) 13:1496. doi: 10.3390/nu13051496
2. Vetrano DL, Triolo F, Maggi S, Malley R, Jackson TA, Poscia A, et al. Fostering healthy aging: the interdependency of infections, immunity and frailty. Ageing Res Rev. (2021) 69:101351. doi: 10.1016/j.arr.2021.101351
3. Almeida I, Magalhaes S, Nunes A. Lipids: biomarkers of healthy aging. Biogerontology. (2021) 22:273–95. doi: 10.1007/s10522-021-09921-2
4. Foscolou A, Chrysohoou C, Dimitriadis K, Masoura K, Vogiatzi G, Gkotzamanis V, et al. The association of healthy aging with multimorbidity: IKARIA study. Nutrients. (2021) 13:1386. doi: 10.3390/nu13041386
5. Wianto E, Sarvia E, Chen CH. Authoritative parents and dominant children as the center of communication for sustainable healthy aging. Int J Environ Res Public Health. (2021) 18:3290. doi: 10.3390/ijerph18063290
6. Almeida C, Barata P, Fernandes R. The influence of gut microbiota in cardiovascular diseases-a brief review. Porto Biomed J. (2021) 6:e106. doi: 10.1097/j.pbj.0000000000000106
7. Duarte L, Gasaly N, Poblete-Aro C, Uribe D, Echeverria F, Gotteland M, et al. Polyphenols and their anti-obesity role mediated by the gut microbiota: a comprehensive review. Rev Endocr Metab Disord. (2021) 22:367–88. doi: 10.1007/s11154-020-09622-0
8. Yokoyama K, Tsuchiya N, Yamauchi R, Miyayama T, Uchida Y, Shibata K, et al. Exploratory research on the relationship between human gut microbiota and portal hypertension. Intern Med. (2020) 59:2089–94. doi: 10.2169/internalmedicine.4628-20
9. Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. (2015) 47:187–97. doi: 10.1152/physiolgenomics.00136.2014
10. Chen Y, Zhu Y, Wu C, Lu A, Deng M, Yu H, et al. Gut dysbiosis contributes to high fructose-induced salt-sensitive hypertension in Sprague-Dawley rats. Nutrition. (2020) 75–76:110766. doi: 10.1016/j.nut.2020.110766
11. Lappi J, Kolehmainen M, Mykkanen H, Poutanen K. Do large intestinal events explain the protective effects of whole grain foods against type 2 diabetes? Crit Rev Food Sci Nutr. (2013) 53:631–40. doi: 10.1080/10408398.2010.550388
12. McQuade JL, Ologun GO, Arora R, Wargo JA. Gut microbiome modulation via fecal microbiota transplant to augment immunotherapy in patients with melanoma or other cancers. Curr Oncol Rep. (2020) 2:74. doi: 10.1007/s11912-020-00913-y
13. Salamone D, Rivellese AA, Vetrani C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta Diabetol. (2021) 58:1131–8. doi: 10.1007/s00592-021-01727-5
14. Tais L, Schulz H, Bottcher C. Comprehensive profiling of semi-polar phytochemicals in whole wheat grains (Triticum aestivum) using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. Metabolomics. (2021) 17:18. doi: 10.1007/s11306-020-01761-4
15. Bowman SA. A vegetarian-style dietary pattern is associated with lower energy, saturated fat, and sodium intakes; and higher whole grains, legumes, nuts, and soy intakes by adults: National Health and Nutrition Examination Surveys 2013-2016. Nutrients. (2020) 12:2668. doi: 10.3390/nu12092668
16. Rebello CJ, Greenway FL, Finley JW. Whole grains and pulses: a comparison of the nutritional and health benefits. J Agric Food Chem. (2014) 62:7029–49. doi: 10.1021/jf500932z
17. van Trijp MPH, Schutte S, Esser D, Wopereis S, Hoevenaars FPM, Hooiveld G, et al. Minor changes in the composition and function of the gut microbiota during a 12-week whole grain wheat or refined wheat intervention correlate with liver fat in overweight and obese adults. J Nutr. (2021) 151:491–502. doi: 10.1093/jn/nxaa312
18. Cronin P, Joyce SA, O'Toole PW, O'Connor EM. Dietary fibre modulates the gut microbiota. Nutrients. (2021) 13:1655. doi: 10.3390/nu13051655
19. Gibiino G, Siena MD, Sbrancia M, Binda C, Sambri V, Gasbarrini A, et al. Dietary habits and gut microbiota in healthy adults: focusing on the right diet. a systematic review. Int J Mol Sci. (2021) 22:6728. doi: 10.3390/ijms22136728
20. Campbell MS, Fleenor BS. Whole grain consumption is negatively correlated with obesity-associated aortic stiffness: a hypothesis. Nutrition. (2018) 45:32–6. doi: 10.1016/j.nut.2017.06.028
21. Aberg S, Mann J, Neumann S, Ross AB, Reynolds AN. Whole-grain processing and glycemic control in type 2 diabetes: a randomized crossover trial. Diabetes Care. (2020) 43:1717–23. doi: 10.2337/dc20-0263
22. Kashino I, Eguchi M, Miki T, Kochi T, Nanri A, Kabe I, et al. Prospective association between whole grain consumption and hypertension: the Furukawa Nutrition and Health Study. Nutrients. (2020) 12:902. doi: 10.3390/nu12040902
23. Liu W, Zhang Y, Qiu B, Fan S, Ding H, Liu Z. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Sci Rep. (2018) 8:14916. doi: 10.1038/s41598-018-33092-9
24. Schutte S, Esser D, Hoevenaars FPM, Hooiveld G, Priebe MG, Vonk RJ, et al. A 12-wk whole-grain wheat intervention protects against hepatic fat: the Graandioos study, a randomized trial in overweight subjects. Am J Clin Nutr. (2018) 108:1264–74. doi: 10.1093/ajcn/nqy204
25. Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. (2015) 101:251–61. doi: 10.3945/ajcn.114.088120
26. Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. (2008) 99:110–20. doi: 10.1017/S0007114507793923
27. Christensen L, Vuholm S, Roager HM, Nielsen DS, Krych L, Kristensen M, et al. Prevotella abundance predicts weight loss success in healthy, overweight adults consuming a whole-grain diet ad libitum: a post hoc analysis of a 6-wk randomized controlled trial. J Nutr. (2019) 149:2174–81. doi: 10.1093/jn/nxz198
28. Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrugger S, Maerkedahl RB, et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. (2019) 68:83–93. doi: 10.1136/gutjnl-2017-314786
29. Martinez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. (2013) 7:269–80. doi: 10.1038/ismej.2012.104
30. Han F, Wang Y, Han Y, Zhao J, Song G, Jiang P, et al. Effects of whole-grain rice and wheat on composition of gut microbiota and short-chain fatty acids in rats. J Agric Food Chem. (2018) 66:6326–35. doi: 10.1021/acs.jafc.8b01891
31. Ma W, Nguyen LH, Song M, Wang DD, Franzosa EA, Cao Y, et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. (2021) 13:102. doi: 10.1186/s13073-021-00921-y
32. Calinoiu LF, Vodnar DC. Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability. Nutrients. (2018) 10:1615. doi: 10.3390/nu10111615
33. Jiang S, Zhu Q, Mai M, Yang W, Du G. Vitamin B and vitamin D as modulators of gut microbiota in overweight individuals. Int J Food Sci Nutr. (2020) 71:1001-9. doi: 10.1080/09637486.2020.1748580
34. Liu C, Li G, Laukkanen JA, Hao L, Zhao Q, Zhang J, et al. Overweight and obesity are associated with cardiac adverse structure remodeling in Chinese elderly with hypertension. Sci Rep. (2019) 9:17896. doi: 10.1038/s41598-019-54359-9
35. Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S, et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. (2020) 10:19417. doi: 10.1038/s41598-020-76474-8
36. Mrozinska S, Kapusta P, Gosiewski T, Sroka-Oleksiak A, Ludwig-Slomczynska AH, Matejko B, et al. The gut microbiota profile according to glycemic control in type 1 diabetes patients treated with personal insulin pumps. Microorganisms. (2021) 9:155. doi: 10.3390/microorganisms9010155
37. Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. (2018) 8:9749. doi: 10.1038/s41598-018-28126-1
38. Daliri EB, Ofosu FK, Chelliah R, Lee BH, An H, Elahi F, et al. Influence of fermented soy protein consumption on hypertension and gut microbial modulation in spontaneous hypertensive rats. Biosci Microbiota Food Health. (2020) 39:199–208. doi: 10.12938/bmfh.2020-001
39. Zheng S, Wang Y, Fang J, Geng R, Li M, Zhao Y, et al. Oleuropein ameliorates advanced stage of type 2 diabetes in db/db mice by regulating gut microbiota. Nutrients. (2021) 13:2131. doi: 10.3390/nu13072131
40. Xu C, Liu J, Gao J, Wu X, Cui C, Wei H, et al. The effect of functional fiber on microbiota composition in different intestinal segments of obese mice. Int J Mol Sci. (2021) 22:6525. doi: 10.3390/ijms22126525
41. Zhao C, Qu Q, Yang F, Li Z, Yang P, Han L, et al. Monascus ruber fermented Panax ginseng ameliorates lipid metabolism disorders and modulate gut microbiota in rats fed a high-fat diet. J Ethnopharmacol. (2021) 278:114300. doi: 10.1016/j.jep.2021.114300
42. Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. (2021) 19:585–99. doi: 10.1038/s41579-021-00559-y
43. Zhou Q, Pang G, Zhang Z, Yuan H, Chen C, Zhang N, et al. Association between gut akkermansia and metabolic syndrome is dose-dependent and affected by microbial interactions: a cross-sectional study. Diabetes Metab Syndr Obes. (2021) 14:2177–88. doi: 10.2147/DMSO.S311388
44. Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, et al. The impact of gut microbiota on gender-specific differences in immunity. Front Immunol. (2017) 8:754. doi: 10.3389/fimmu.2017.00754
45. Bridgewater LC, Zhang C, Wu Y, Hu W, Zhang Q, Wang J, et al. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci Rep. (2017) 7:10776. doi: 10.1038/s41598-017-11069-4
46. Yoon K, Kim N. Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. (2021) 27:314–25. doi: 10.5056/jnm20208
47. Fan W, Huo G, Li X, Yang L, Duan C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the six months of life. J Microbiol Biotechnol. (2014) 24:133–43. doi: 10.4014/jmb.1309.09029
48. Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Org E, Parks B, et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. (2014) 5:4500. doi: 10.1038/ncomms5500
49. Shastri P, McCarville J, Kalmokoff M, Brooks SP, Green-Johnson JM. Sex differences in gut fermentation and immune parameters in rats fed an oligofructose-supplemented diet. Biol Sex Differ. (2015) 6:13. doi: 10.1186/s13293-015-0031-0
50. Mu Q, Zhang H, Liao X, Lin K, Liu H, Edwards MR, et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. (2017) 5:73. doi: 10.1186/s40168-017-0300-8
Keywords: gut microbiota, whole grain, American Gut Program, overweight, female
Citation: Cui Z, Li J, Zhen Y, Fan P and Du G (2022) The Effect of Whole-Grain Diet on the Gut Microbiota of the Elderly Individuals. Front. Nutr. 9:919838. doi: 10.3389/fnut.2022.919838
Received: 14 April 2022; Accepted: 31 May 2022;
Published: 27 June 2022.
Edited by:
Xingbin Yang, Shaanxi Normal University, ChinaReviewed by:
Jun Chu, Anhui University of Chinese Medicine, ChinaXiaopeng Qi, Shandong University, China
Copyright © 2022 Cui, Li, Zhen, Fan and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guankui Du, ZHVndWFua3VpQDE2My5jb20=; Pingming Fan, ZnBtaGFpbmFuQDE2My5jb20=
†These authors have contributed equally to this work
 Zeying Cui1,2†
Zeying Cui1,2† Guankui Du
Guankui Du