- 1Graduate School, Ning Xia Medical University, Yinchuan, China
- 2General Surgery Clinical Medical Center, Gansu Provincial Hospital, Lanzhou, China
- 3Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province, Gansu Provincial Hospital, Lanzhou, China
- 4First Clinical Medical College, Gansu University of Chinese Medicine, Lanzhou, China
- 5Medical College of Jiangsu University, Zhenjiang, China
- 6First Clinical College of Medicine, Lanzhou University, Lanzhou, China
- 7NHC Key Laboratory of Diagnosis and Therapy of Gastrointestinal Tumor, Gansu Provincial Hospital, Lanzhou, China
Background: Colorectal cancer (CRC) risk is linked to serum and dietary retinol and carotenoids, according to clinical and epidemiological research. However, the findings are not consistent. As a result, we did this meta-analysis to determine the link between them.
Methods: From 2000 through 2022, the PubMed, Web of Science, and Embase databases, as well as pertinent article references, were searched and filtered based on inclusion and exclusion criteria and literature quality ratings. High and low intake were used as controls, and OR (odds ratio) or RR (relative risk) and 95% confidence interval were extracted. The extracted data were plotted and analyzed using Stata12.0 software.
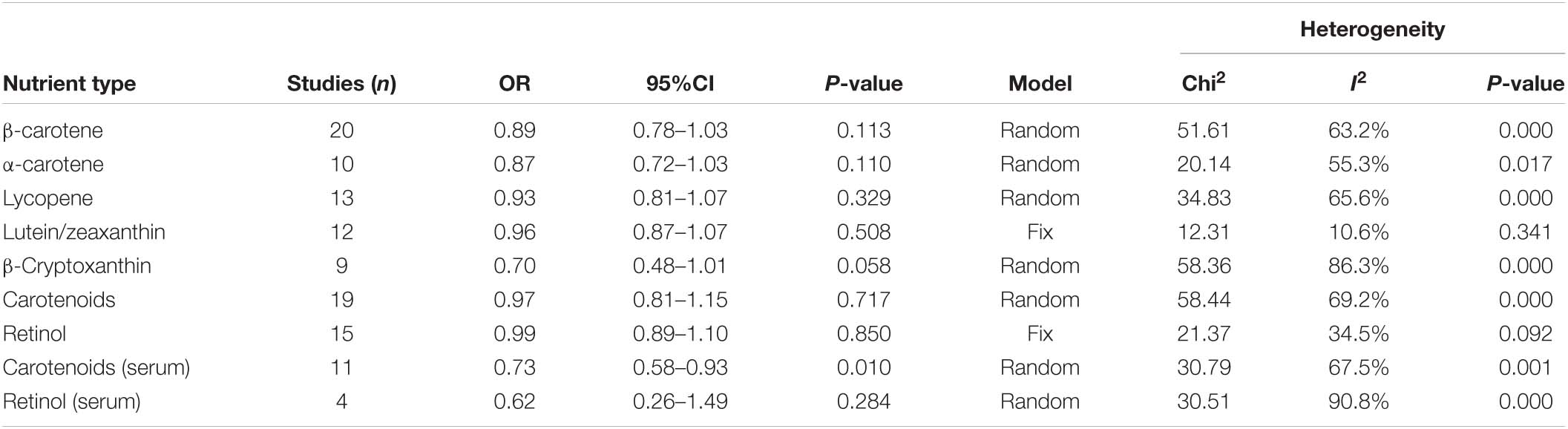
Results: A total of 22 relevant studies were included, including 18 studies related to diet and 4 studies related to serum. For high and low intake or concentration controls, the pooled OR was as follows: β-carotene (OR = 0.89, 95% CI: 0.78–1.03), α-carotene (OR = 0.87, 95% CI: 0.72–1.03), lycopene (OR = 0.93, 95% CI: 0.81–1.07), lutein/zeaxanthin (OR = 0.96, 95% CI: 0.87–1.07), β-cryptoxanthin (OR = 0.70, 95% CI: 0.48–1.01), total carotenoids (OR = 0.97, 95% CI: 0.81–1.15), retinol (OR = 0.99, 95% CI: 0.89–1.10), serum carotenoids (OR = 0.73, 95% CI: 0.58–0.93), serum retinol (OR = 0.62, 95% CI: 0.26–1.49). Subgroup analysis was performed according to tumor type, study type and sex.
Conclusion: Total carotenoid intake and Lutein/Zeaxanthin intake were not associated with CRC risk. High β-carotene, α-carotene, lycopene, and β-cryptoxanthin all tended to reduce CRC risk. Serum carotenoid concentrations were significantly inversely associated with CRC risk.
Introduction
In recent years, the incidence and mortality of malignant tumors have been increasing year by year, even exceeding other chronic diseases, becoming a veritable human health killer (1). Although the efficacy of cancer treatment has been improved due to comprehensive therapies such as surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy, the prognosis and early diagnosis remain poor and the mortality rate remains high (2). Colorectal cancer (CRC) is the world’s third most frequent cancer and the second largest cause of cancer mortality, with a significant number of new cases and deaths every year (3). CRC has caused great burden and harm to the economy and society of the country (4). Economic development and changes in lifestyle and dietary choices have increased the prevalence and mortality of CRC in China in recent years, putting a strain on the health-care system (3, 5). The etiology of CRC is heavily influenced by environmental and genetic factors. Diet, history of benign adenomatous polyps and inflammatory bowel disease, age, diabetes, obesity, lack of physical activity, and a family history of CRC are all risk factors for CRC (6). Therefore, the prevention of CRC by changing dietary habits and lifestyle is an area that we should focus on.
Fruits and vegetables are among the daily foods required for good health since they include high levels of minerals, vitamins, carbs, proteins, dietary fiber, and different substances with nutritional medicinal value that can help prevent a variety of ailments (7). Many studies have indicated that eating fruits and vegetables helps prevent cancer, with vegetable-related protection being more substantial (8, 9). Vitamin A is an unsaturated hydrocarbon group that includes retinol and its derivatives such as retinaldehyde, retinoic acid, and retinyl ester (10). Cell development and differentiation, embryogenesis, reproduction, epithelial cell integrity, and immunological function are all regulated by vitamin A (11, 12). It also has antioxidant properties (13) and helps to reduce oxidative stress damage and inflammation (11, 14). Carotenoids are a good source of vitamin A and may be turned into it by the body (15). Carotenoids are natural pigments found in a wide range of fruits and vegetables, including lycopene, β-carotene, lutein, zeaxanthin, and β-cryptoxanthin (16). Carotenoids and retinoids share many biological actions, including antioxidant capabilities, suppression of malignant tumor development, and activation of apoptosis (17). In addition, carotenoids can influence cell development, as well as gene expression and immunological responses (18, 19). Thus, retinol and carotenoids are indispensable in the human body. But retinol cannot be synthesized in the human body, and it must be obtained from the diet (20). As a result, research into the relationship between their consumption and human illnesses, including cancer, is required.
Over the last two decades, researchers have conducted substantial research on the link between nutrition and cancer. Epidemiological studies have found a link between food and cancer incidence and aggressiveness (21). A high intake of dietary carotenoids or vitamin A (retinol) has been linked to a decreased risk of CRC in several studies (22–25). However, other studies have shown no substantial link between their use and the risk of cancer onset (25–27). In addition to diet, there has been interest in the research of serum retinol and carotenoids, and some studies have shown that their levels in the blood are related to the risk of colon cancer. As a result, we completed our meta-analysis in time to incorporate the most recent relevant data, providing more credible scientific support for CRC prevention.
Materials and Methods
Search Strategy for Literature
Two writers (Xiaoyong Han and Rangyin Zhao) separately conducted a literature search for the association between retinol, carotenoids, and related derivatives and the risk of CRC in humans using the PubMed, Web of Science and Embase databases. The following keywords were used in the search: “retinol” or “carotenoids” or “carotene” or α-carotene or “β-carotene” or “cryptoxanthin” or “lycopene” or “lutein” or “zeaxanthin” combined with “colorectal cancer” or “colon cancer” or “rectal cancer.” All relevant literature was searched from 2000 to April 2022. In addition, we performed a manual search of the reference lists of reviews, meta-analyses, and other relevant publications to prevent potentially missed articles. The language of included articles was limited to English.
Inclusion and Exclusion Criteria
Studies were included according to the following criteria: (1) patients were diagnosed with colon or rectal cancer; (2) observational studies, including cohort or case-control studies; (3) The associations of interest are about the association of serum or dietary retinol or carotenoids with CRC risk, and there are comparisons of high and low content.; (4) Included studies contained relative risks (RR) or odds ratios (OR) with 95% confidence intervals for CRC. The following exclusion criteria were used: (1) reviews or conferences or abstracts or letters to the editor; (2) duplicate study populations; (3) animal studies; (4) other cancer studies; (5) lack of RR or OR data; (6) other vitamin supplement studies.
Data Extraction and Quality Evaluation
All included papers were examined and relevant data were retrieved independently by two researchers. Inclusion basic information included: name of first author, date of publication, country, type of study, vitamin type, cancer type, sample size of cases and controls, RR or OR and 95% CI for cancer, covariate correction. The disagreement between these two researchers was decided jointly by a third author. The quality of the included studies was assessed using the NOS scoring criteria (0–9 points), and those with a score > 6 were included in the meta-analysis.
Statistical Analysis
RR or OR with 95% confidence intervals were extracted from each study to assess the association of high retinol or carotenoids intake with cancer risk. The results generally combined in cohort studies are RR values, and the results generally combined in case-control studies are OR values. In order to better calculate and combine the results of studies, the difference between the two is negligible, and all the results are expressed as OR values. In addition, heterogeneity among studies was assessed by Q-test and I2 statistic. Q-test (PQ) p-value < 0.1 and I2 > 50% indicated that there was significant statistical heterogeneity between studies, and the results were analyzed using a random-effects model. Otherwise, a fixed effects model was used. We used forest plots to present the meta-analysis results and used Begg’s test as well as Begg’s funnel plots to assess publication bias. In addition, by eliminating each study one by one, a sensitivity analysis was performed to check the stability of the results. Analyses were performed using Stata12.0 for Windows (Stata, College Station, TX, United States) and p < 0.05 was considered statistically significant.
Results
Screening Process for Eligible Literatures
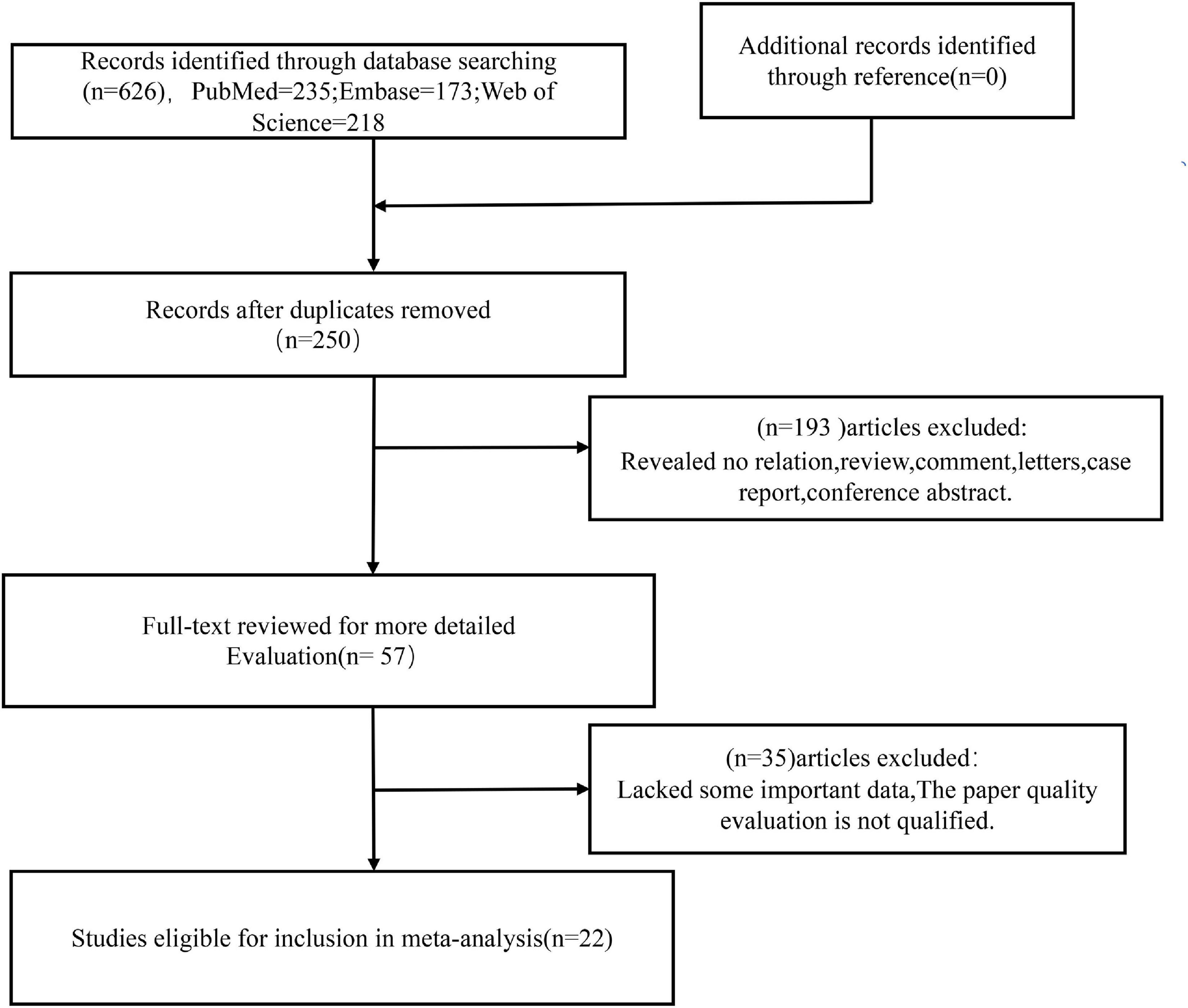
The relevant literatures were searched in three main English databases according to the search strategy: PubMed (n = 235), Web of Science (n = 218), Embase (n = 173). After de-duplication (n = 376), the titles and abstracts of the remaining articles (n = 250) were examined and evaluated. A total of 193 articles were rejected for purpose, article type (review, case study, or conference abstract), or irrelevant findings. Fifty-seven full-text articles were downloaded, of which 35 studies were rejected after initial analysis due to lack of important data or unsatisfactory quality of NOS scores. Finally, the meta-analysis comprised 22 papers that fully fulfilled the inclusion criteria and quality evaluation. Figure 1 depicts the search flow chart.
Characteristics of Included Research Projects
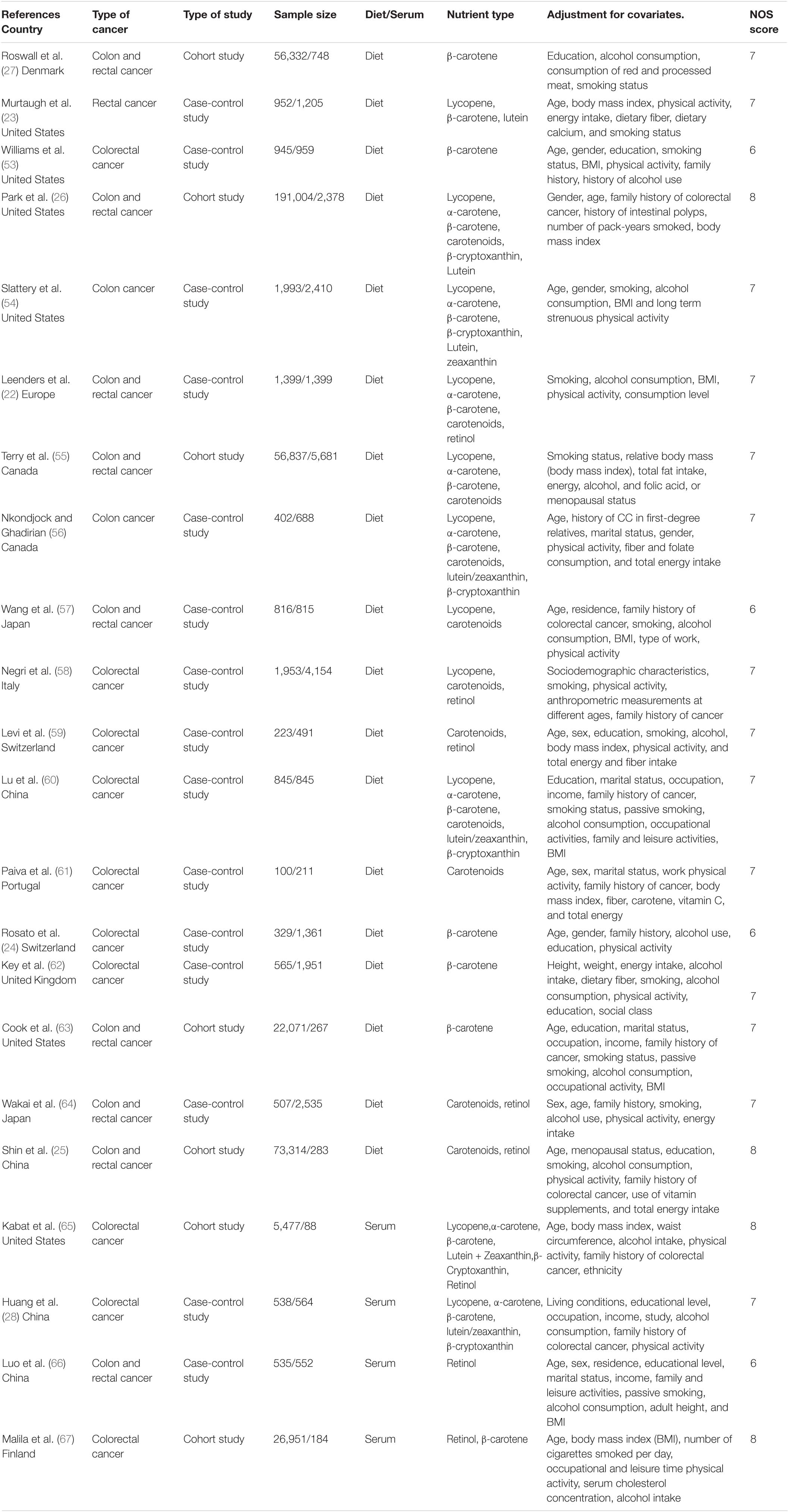
Table 1 shows the main characteristics of the 22 included studies. Regarding dietary aspects, a total of five cohort studies were included, and 399,558 individuals were followed up for 5–15 years, eventually resulting in 6,919 CRC patients. A total of 13 case-control studies involving 11,029 cases and 19,024 controls were included. With respect to serum, two cohort studies were included, with 32,428 participants and, ultimately, 272 patients with CRC. Two case-control studies involving 1,073 cases and 1,116 controls were included. Studies were published between 2000 and 2019. Eight studies were from European countries, eight from North American countries and six from Asian countries. The major nutrient species studied were carotenoids, lycopene, α-carotene, β-carotene, lutein/zeaxanthin, β-cryptoxanthin, and retinol. The included studies were adjusted for covariates, mainly including: gender, age, smoking, alcohol consumption, family history of CRC, and physical activity. The NOS was scored from 6 to 8. The original data of the included studies are found in Supplementary Table S1.
Association Between Dietary Retinol and Various Carotenoids and Colorectal Cancer Risk
β-Carotene
We combined a total of 20 sets of data from 11 studies. Comparing low intakes, dietary high intake of β-carotene reduced the risk of CRC by 11% (OR = 0.89, 95% CI: 0.78–1.03, Figure 2A), but the association between the two was not significant (pt = 0.113), and due to significant heterogeneity (I2 = 63.2%, p < 0.001), we used a random-effects model for pooled analysis. According to subgroup analysis by tumor type, it can be seen that there is no significant correlation between dietary intake and the risk of colon cancer (OR = 0.96, 95% CI: 0.86–1.06, Figure 3A) and rectal cancer (OR = 1.06, 95% CI: 0.89–1.25, Figure 3A). In the subgroup analysis by study type, both the cohort study (OR = 0.95, 95% CI: 0.85–1.07, Figure 4A) and the case-control study (OR = 0.81, 95% CI: 0.63–1.05, Figure 4A) showed a trend of β-carotene to reduce the risk of CRC, but none of them were significantly associated. Finally, according to gender subgroup analysis, β-carotene intake was not significantly associated with the risk of CRC in female (OR = 0.97, 95% CI: 0.79–1.19, Figure 5A), but β-carotene intake was negatively associated with the risk of CRC in male (OR = 0.74, 95% CI: 0.55–0.99, Figure 5A).
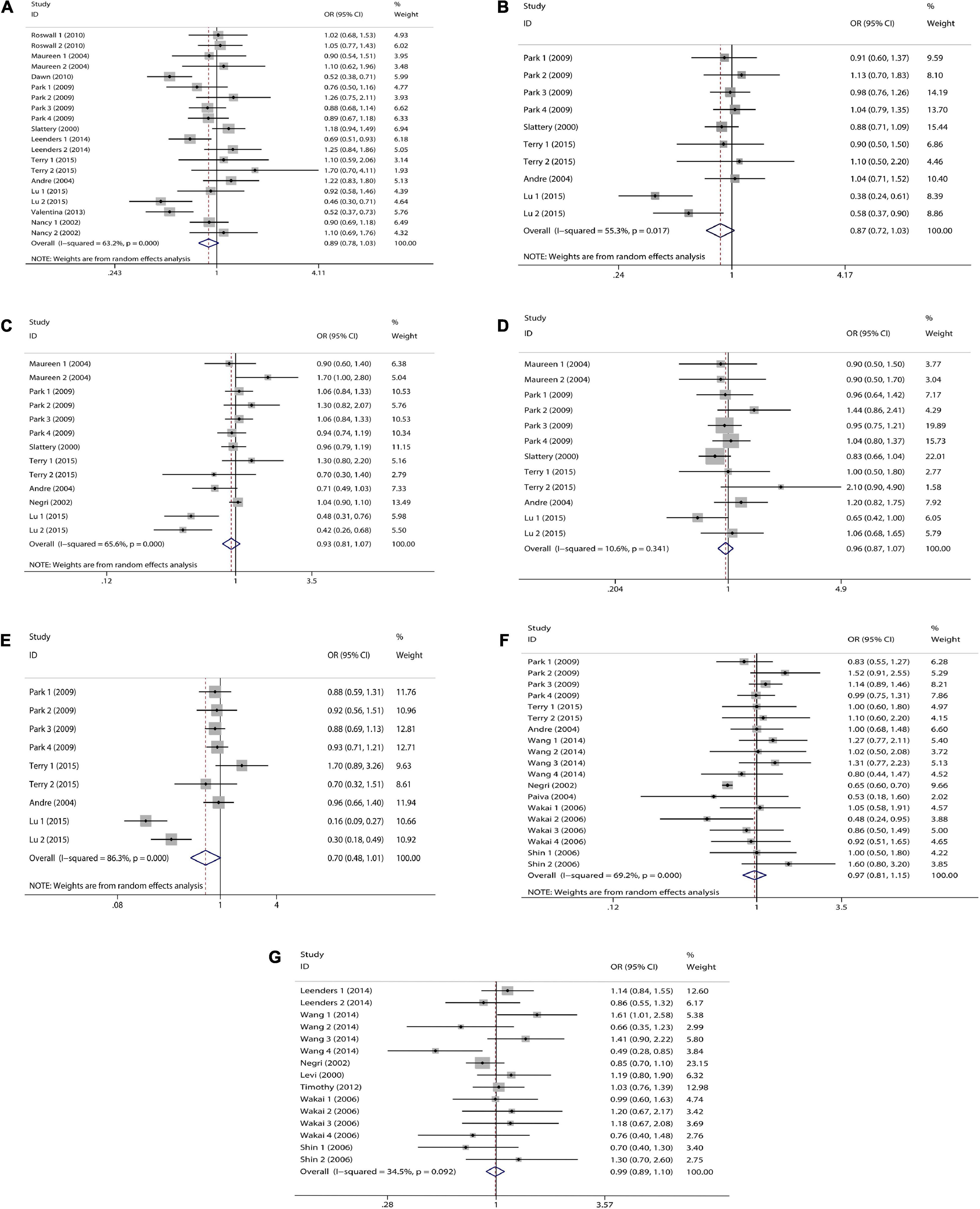
Figure 2. Forest plot on dietary intake of carotenoids and retinol and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-Cryptoxanthin; (F) carotenoids; (G) retinol.
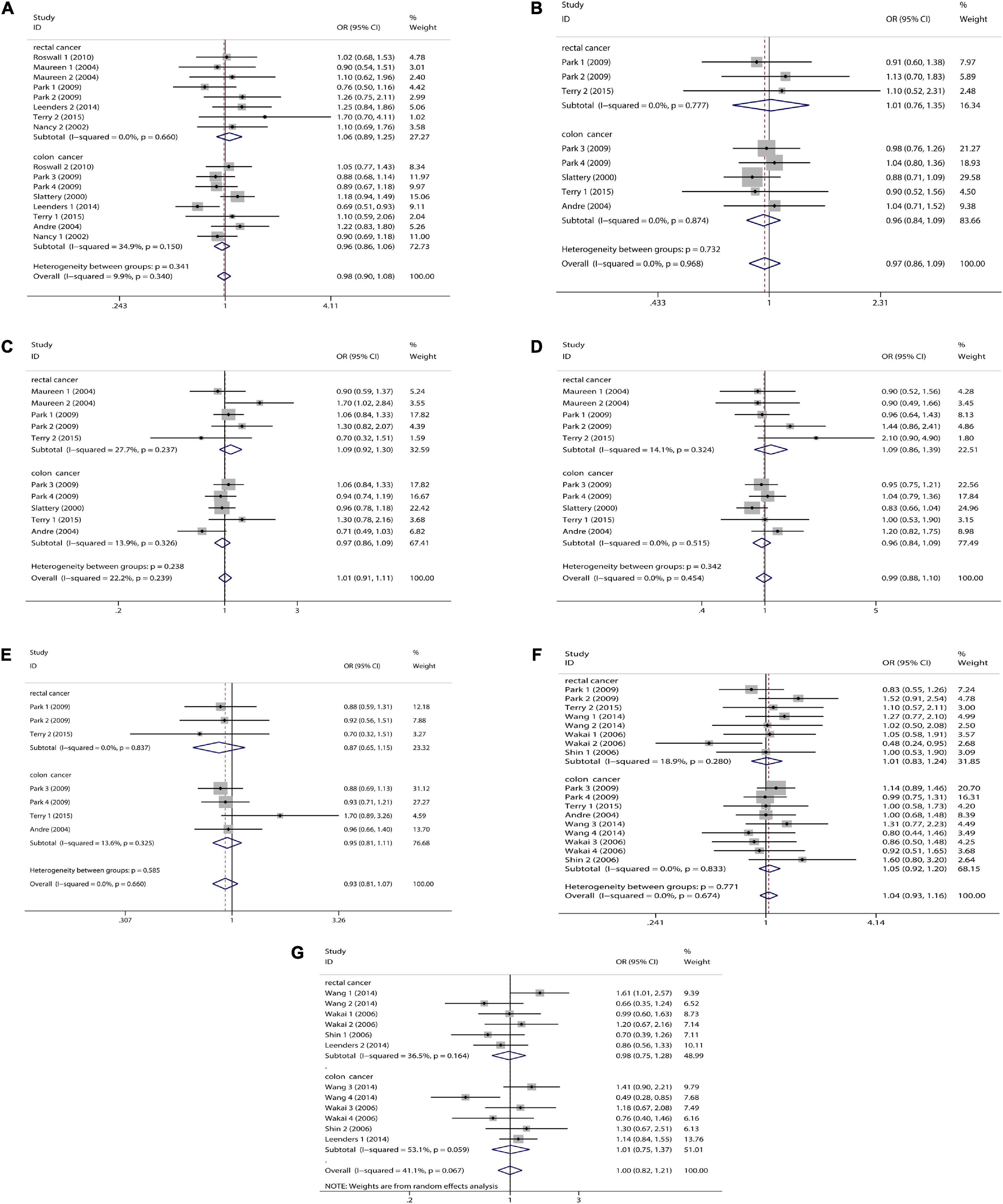
Figure 3. Tumor subgroup analysis of dietary carotenoid and retinol intake and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-Cryptoxanthin; (F) carotenoids; (G) retinol.
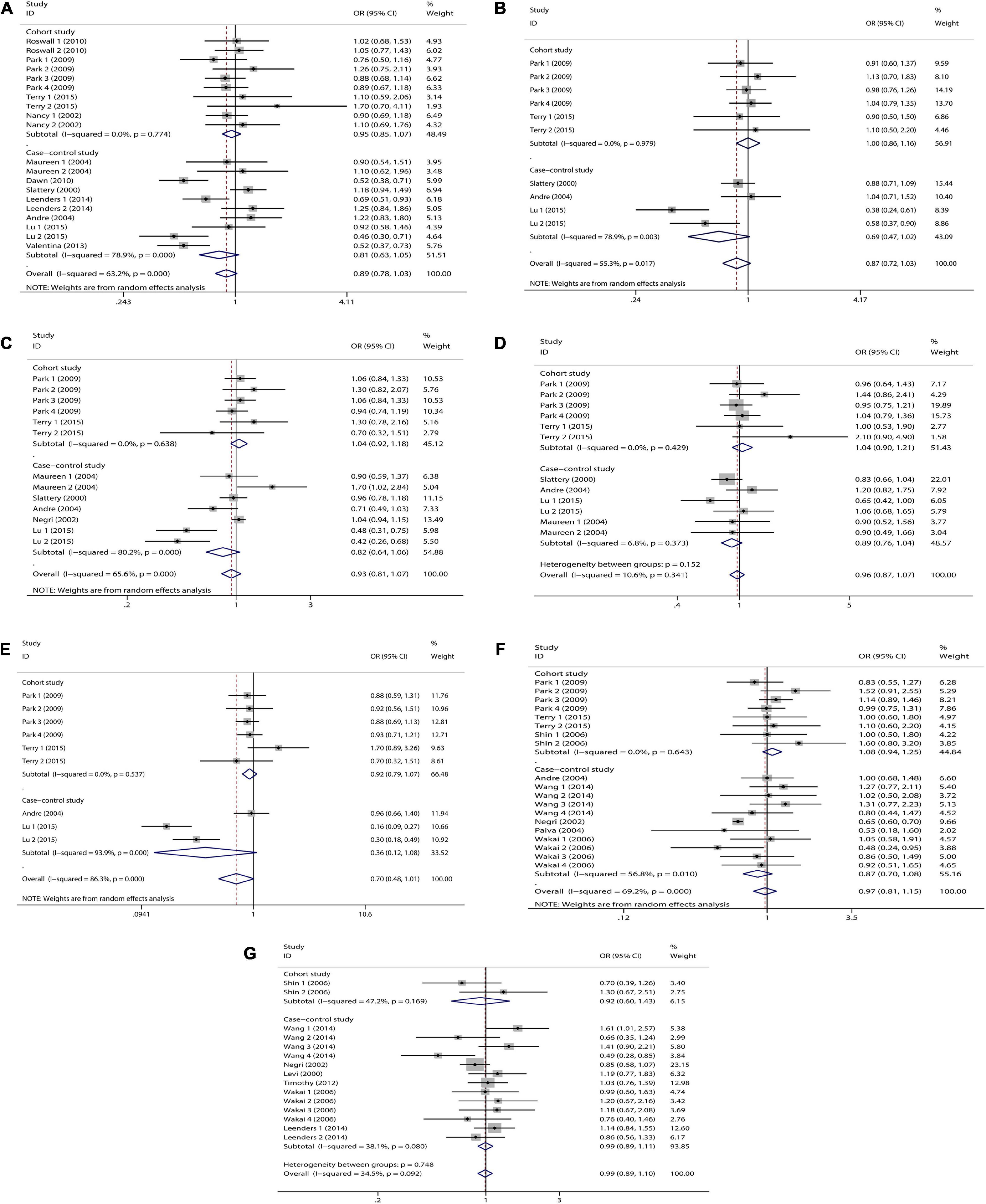
Figure 4. Study type subgroup analysis of dietary carotenoid and retinol intake and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-Cryptoxanthin; (F) carotenoids; (G) retinol.
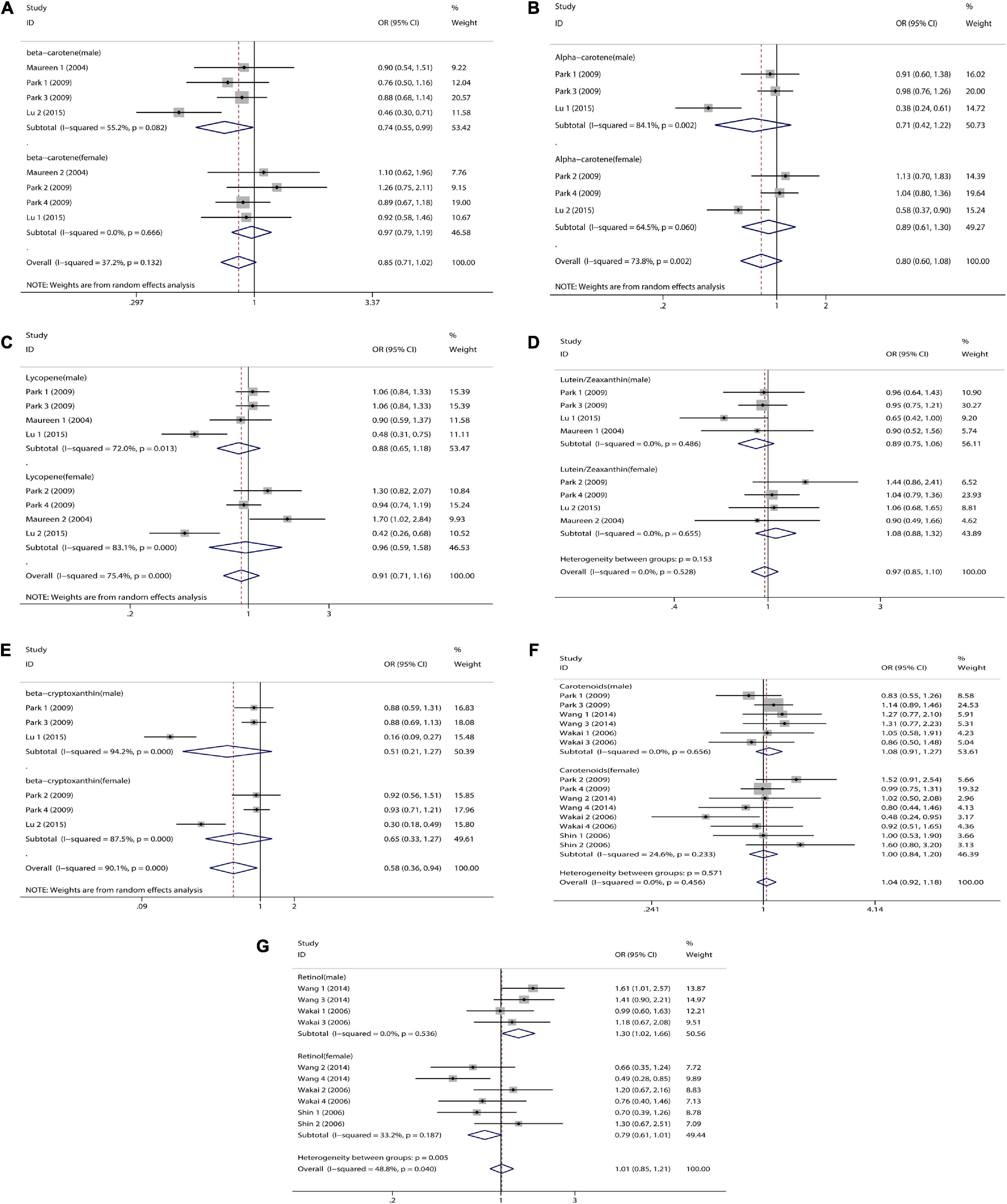
Figure 5. Sex subgroup analysis of dietary carotenoid and retinol intake and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-Cryptoxanthin; (F) carotenoids; (G) retinol.
α-Carotene
We combined a total of 10 sets of data from 5 studies. Comparing low intakes, dietary high intake of α-carotene reduced the risk of CRC by 13% (OR = 0.87, 95% CI: 0.72–1.03, Figure 2B), but the association between the two was not significant (pt = 0.110), and due to significant heterogeneity (I2 = 55.3%, p = 0.017), we used a random-effects model for pooled analysis. Subgroup analysis by tumor type showed that dietary intake was not significantly associated with the risk of colon cancer (OR = 0.96, 95% CI: 0.84–1.09, Figure 3B) and rectal cancer (OR = 1.01, 95% CI: 0.76–1.35, Figure 3B). In the subgroup analysis by study type, the cohort study (OR = 1.00, 95% CI: 0.86–1.16, Figure 4B) showed no significant association between their intake and CRC, and the case-control studies (OR = 0.69, 95% CI: 0.47–1.02, Figure 4B) showed that their high intake tended to reduce CRC risk, but there was no significant association. Finally, high intake of α-carotene tended to reduce CRC in male (OR = 0.71, 95% CI: 0.42–1.22, Figure 5B) and female (OR = 0.89, 95% CI: 0.61–1.30, Figure 5B) according to gender subgroup analysis, but there was no significant association.
Lycopene
Seven studies were included to combine a total of 13 sets of data. High lycopene (OR = 0.93, 95% CI: 0.81–1.07, Figure 2C) intake slightly, but not significantly (pt = 0.329), reduced CRC risk. Due to significant heterogeneity (I2 = 65.6%, p = 0.000), pooling was performed with a random-effects model. Subgroup analysis was performed according to tumor type, study type and gender. Colon cancer (OR = 0.97, 95% CI: 0.86–1.09, Figure 3C), rectal cancer (OR = 1.09, 95% CI: 0.92–1.30, Figure 3C), cohort study (OR = 1.04, 95% CI: 0.92–1.18, Figure 4C), case-control study (OR = 0.82, 95% CI: 0.64–1.06, Figure 4C), male (OR = 0.88, 95% CI: 0.65–1.18, Figure 5C), female (OR = 0.96, 95% CI: 0.59–1.58, Figure 5C). In subgroup analyses, case-control studies showed a non-significant inverse association between lycopene intake and CRC risk. There was also a risk reduction effect in male, although it was not significant.
Lutein/Zeaxanthin
Six studies were included and a total of 12 sets of data were combined. There was no significant (pt = 0.508) association between high lutein/zeaxanthin (OR = 0.96, 95% CI: 0.87–1.07, Figure 2D) intake and CRC risk. No significant heterogeneity was found (I2 = 10.6%, p = 0.341), which was summarized using a fixed-effect model. Subgroup analysis was performed according to tumor type, study type and gender. Colon cancer (OR = 0.96, 95% CI: 0.84–1.09, Figure 3D), rectal cancer (OR = 1.09, 95% CI: 0.86–1.39, Figure 3D), cohort study (OR = 1.04, 95% CI: 0.90–1.21, Figure 4D), case-control study (OR = 0.89, 95% CI: 0.76–1.04, Figure 4D), male (OR = 0.89, 95% CI: 0.75–1.06, Figure 5D), female (OR = 1.08, 95% CI: 0.88–1.32, Figure 5D). In subgroup analysis, case-control studies showed a non-significant inverse association between the intake of lutein/zeaxanthin and CRC risk. The risk reduction effect was also present in male, but was not significant.
β-Cryptoxanthin
Four studies were included and a total of 9 sets of data were combined. High β-cryptoxanthin (OR = 0.70, 95% CI: 0.48–1.01, Figure 2E) intake was able to reduce CRC risk by 30%, but not statistically significant (pt = 0.058). High heterogeneity was found (I2 = 86.3%, p = 0.000), which was combined using the random-effects model. Subgroup analysis was performed according to tumor type, study type, and gender. Colon cancer (OR = 0.95, 95% CI: 0.81–1.11, Figure 3E), rectal cancer (OR = 0.87, 95% CI: 0.65–1.15, Figure 3E), cohort study (OR = 0.92, 95% CI: 0.79–1.07, Figure 4E), case-control study (OR = 0.36, 95% CI: 0.12–1.08, Figure 4E), male (OR = 0.51, 95% CI: 0.21–1.27, Figure 5E), female (OR = 0.65, 95% CI: 0.33–1.27, Figure 5E). In subgroup analysis, high β-cryptoxanthin intake tended to decrease risk of CRC, but this was not significant.
Total Carotenoids
Eight studies were included and a total of 19 sets of data were combined. There was no significant (pt = 0.717) association between high carotenoids (OR = 0.97, 95% CI: 0.81–1.15, Figure 2F) intake and CRC risk. There was significant heterogeneity (I2 = 69.2%, p = 0.000), which was combined using the random-effects model. Subgroup analysis was performed according to tumor type, study type and gender. Colon cancer (OR = 1.05, 95% CI: 0.92–1.20, Figure 3F), rectal cancer (OR = 1.01, 95% CI: 0.83–1.24, Figure 3F), cohort study (OR = 1.08, 95% CI: 0.94–1.25, Figure 4F), case-control study (OR = 0.87, 95% CI: 0.70–1.08, Figure 4F), male (OR = 1.08, 95% CI: 0.91–1.27, Figure 5F), female (OR = 1.00, 95% CI: 0.84–1.20, Figure 5F). No association was found between high carotenoids intake and the risk of CRC in any Subgroup group.
Retinol
Seven studies were included and a total of 15 sets of data were combined. There was no significant (pt = 0.850) association between high retinol (OR = 0.99, 95% CI: 0.89–1.10, Figure 2G) intake and CRC risk. There was no significant heterogeneity (I2 = 34.5%, p = 0.092), and fixed effect model was used for combination. Subgroup analysis was performed according to tumor type, study type and gender. Colon cancer (OR = 1.01, 95% CI: 0.75–1.37, Figure 3G), rectal cancer (OR = 0.98, 95% CI: 0.75–1.28, Figure 3G), cohort study (OR = 0.92, 95% CI: 0.60–1.43, Figure 4G), case-control study (OR = 0.99, 95% CI: 0.89–1.11, Figure 4G), male (OR = 1.30, 95% CI: 1.02–1.66, Figure 5G), female (OR = 0.79, 95% CI: 0.61–1.01, Figure 5G). Retinol appeared to play a protective role in women, reducing CRC risk by 21%, although there was no significant association. For men, retinol intake was significantly positively associated with the risk of CRC.
Association of Serum Retinol and Carotenoid Levels With Colorectal Cancer Risk
With regard to serum carotenoids, three studies were included and a total of 11 sets of data were combined. Serum total carotenoids (OR = 0.73, 95% CI: 0.58–0.93, Figure 6A) were significantly (pt = 0.01) negatively associated with CRC risk. The results showed significant heterogeneity (I2 = 67.5%, p = 0.001), which was combined using the random-effects model. The subgroup analysis was performed according to the type of nutrients. Serum α-carotene (OR = 0.61, 95% CI: 0.37–0.99, Figure 6B) was significantly inversely associated with CRC risk. However, the serum content of β-carotene (OR = 0.83, 95% CI: 0.64–1.08, Figure 6B), Lycopene (OR = 0.58, 95% CI: 0.22–1.54, Figure 6B), and β-Cryptoxanthin (OR = 0.69, 95% CI: 0.28–1.69, Figure 6B), although negatively correlated with CRC risk, was not significant. There was no correlation between serum Lutein/Zeaxanthin (OR = 0.99, 95% CI: 0.63–1.56, Figure 6B) content and CRC risk.
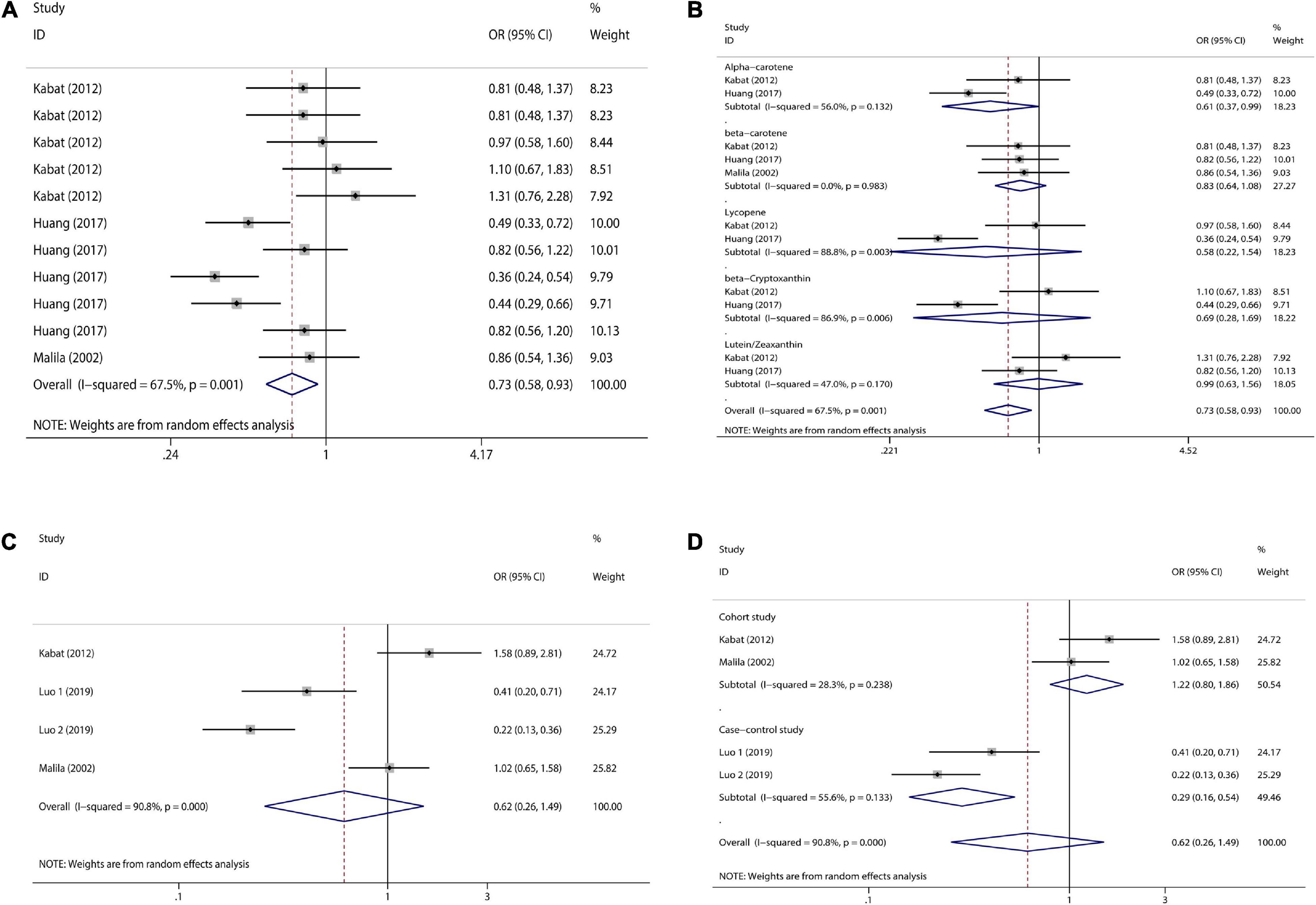
Figure 6. Forest plot of serum carotenoid and retinol concentrations and colorectal cancer risk. (A) serum carotenoid; (B) subgroup analysis of serum carotenoids according to their types; (C) serum retinol; (D) subgroup analysis of serum retinol by study type.
With regard to serum retinol, three studies were included and a total of four sets of data were combined. High serum retinol (OR = 0.62, 95% CI: 0.26–1.49, Figure 6C) was inversely associated with CRC risk, but the association was not significant (pt = 0.284). The results showed significant heterogeneity (I2 = 90.8%, p = 0.000), and random effects model was used for combination. Subgroup analysis were also performed according to study type. Cohort studies (OR = 1.22, 95% CI: 0.80–1.86, Figure 6D) showed no association between serum retinol and CRC risk, but case-control studies (OR = 0.29, 95% CI: 0.16–0.54, Figure 6D) showed a significant inverse association between serum retinol and CRC risk. Meta-analysis results of the above various nutrients are shown in Table 2.
Publication Bias and Sensitivity Analysis
Due to the less in serological studies included, bias testing and sensitivity analysis were not necessary. Therefore, we performed bias test and sensitivity analysis on the combined results of dietary retinol and carotenoids. We used Begg’s test as well as Begg’s funnel plot to assess publication bias. Begg’s test results (Figure 7): β-carotene (Pr > | z | = 0.417), α-carotene (Pr > | z | = 0.721), lycopene (Pr > | z | = 0.464), β-Cryptoxanthin (Pr > | z | = 0.075), Lutein/Zeaxanthin (Pr > | z | = 0.304), Carotenoids (Pr > | z | = 0.234), retinol (Pr > | z | = 0.692). The results of bias test showed that all funnel plots were symmetrical and (Pr > | z | > 0.05), indicating that no significant publication bias was found in the combined results. Sensitivity analysis (Figure 8) of the results was performed and the pooled OR varied in a limited range without significant change after removing each study, indicating that our results were stable. From this, it can be seen that the relevant conclusions we draw are stable and reliable.
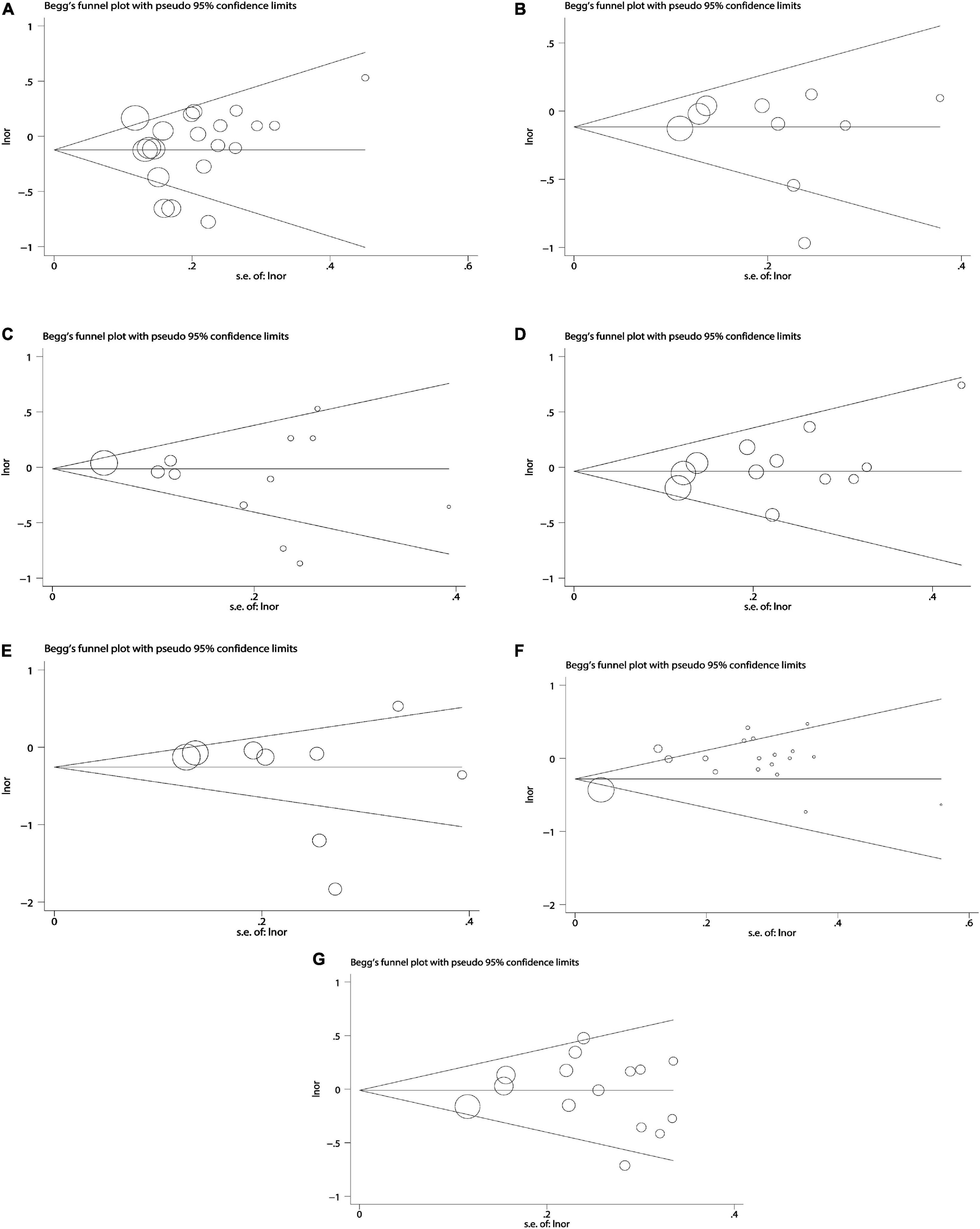
Figure 7. Begg’ s publication bias plots on dietary carotenoids and retinol and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-cryptoxanthin; (F) carotenoids; (G) retinol.
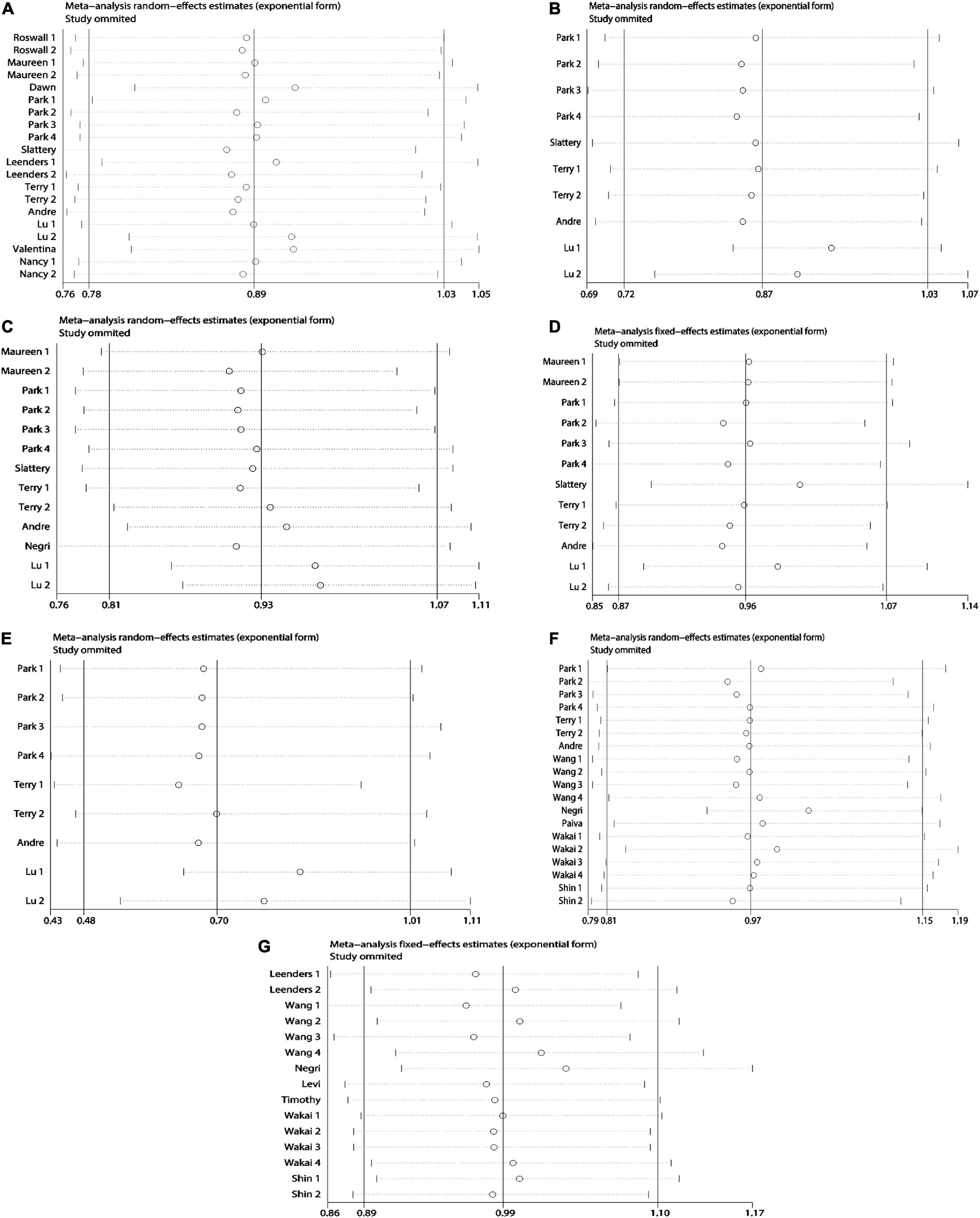
Figure 8. Sensitivity analysis plots on dietary carotenoids and retinol and colorectal cancer risk. (A) β-carotene; (B) α-carotene; (C) lycopene; (D) lutein/zeaxanthin; (E) β-cryptoxanthin; (F) carotenoids; (G) retinol.
Discussion
Although vitamin A (retinol) and carotenoids are widely present in a variety of vegetables and fruits, many people still lack the intake of these nutrients. Therefore, the impact of retinol and carotenoids intake on CRC risk has important public health implications. We included a total of 22 studies that pooled clinical studies on dietary and serum retinol and carotenoids and CRC risk. Subgroup analysis was performed according to tumor type, study category and sex.
The results showed that dietary β-carotene intake was not significantly negatively correlated with CRC risk, but β-carotene could significantly lower CRC risk in the male population, showing a protective and preventive effect. The consumption of α-carotene lowered the risk of CRC, however, the link was not statistically significant. A high lycopene consumption lowered CRC risk marginally but not dramatically. There was no link seen between high lutein/zeaxanthin consumption and CRC risk. High intake of β-cryptoxanthin was able to non-significantly reduce the risk of CRC. There was no link found between high total carotenoids consumption and CRC risk. High retinol consumption had no significant connection with CRC risk, and although it appeared to protect women and reduce CRC risk by 21%, high retinol intake was able to significantly increase the risk of CRC for men, and this difference was very important and could guide dietary matching. In summary, high β-carotene, α-carotene, lycopene, and β-cryptoxanthin all have a tendency to reduce CRC risk, which is more pronounced in the male population, but there is uncertainty that must be explored. Total carotenoids intake and Lutein/Zeaxanthin intake were not associated with CRC risk. Beta-carotene has a preventive effect on CRC in men and retinol seems to have a preventive effect in women and a carcinogenic effect in men, and this difference has led us to new ideas about adjusting the diet by sex.
In addition to focusing on the risk of dietary retinol and carotenoids on CRC, we also focused on serological aspects of the study. A significant inverse association was found between serum carotenoids concentrations and CRC risk. Serum β-carotene was shown to have a substantial negative relationship with CRC risk. Other carotenoids, while adversely associated, were not significant. Case-control studies have found a substantial negative link between serum retinol and CRC risk, while cohort studies have found no significant relationship, hence the relationship between serum retinol and CRC remains unknown and must be confirmed by large prospective investigations. Most previous studies have focused on dietary carotenoids, but in recent years attention has gradually shifted to serum carotenoids, possibly due to the development of serum detection techniques and more stable and accurate quantitative assessment of serum. Serum carotenoids are widely studied, in addition to CRC (28, 29), but also associated with the risk of breast cancer (30), lung cancer (31), prostate cancer (32, 33) and hepatocellular carcinoma (34), which can be used as a key research direction in the future. If the relationship between serum carotenoids and various cancers can be clearly understood, routine admission examination can be performed in high-risk cancer population to preliminarily evaluate and screen related tumors, which has certain application prospects in clinical practice.
We will further explore the mechanisms underlying the prevention and suppression of CRC by carotenoids. β-carotene has been found in animal studies to have anti-colon cancer properties through modulating M2 macrophages and activated fibroblasts (35). By regulating K-ras, PKB, and beta-catenin, dietary lutein can decrease colon carcinogenesis caused by p-dimethylhydrazine in rats (36). Carotenoids isolated from Chlorella ellipsoidea and Chlorella vulgaris have also been shown in cell tests to have antiproliferative and anticancer effects on human colon cancer cells (37). B-carotene has been demonstrated to decrease colon cancer cell development by reducing COX-2 production and down-regulating colon cancer cell homeostasis (38). A growing number of experimental investigations have also proven the mechanism and significance of carotenoids in anti-CRC. Our study surprised us by the finding that a high intake of β-cryptoxanthin (OR = 0.70, 95% CI: 0.48–1.01) was able to reduce CRC risk. β-cryptoxanthin is one of the six primary carotenoids. It is mostly present in citrus fruits, although it is also found in corn, peas, and other yellow animal products (16, 39). β-cryptoxanthin has been demonstrated in animal experiments to have preventative and inhibitory effects on a number of malignancies, including colon cancer (40), gastric cancer (41), lung cancer (42–44), bladder cancer (45), and liver cancer (46) through a variety of molecular mechanisms. It has been demonstrated that β-cryptoxanthin in combination with oxaliplatin dramatically increased the apoptosis of colon cancer cells in vitro and in vivo, indicating anti-tumor and therapeutic actions on CRC (40). From this point of view, although there are few studies on β-cryptoxanthin, it may have a role in preventing and inhibiting tumors in a variety of cancers, especially CRC. The conclusions about β-cryptoxanthin in this meta-analysis should be paid attention to, and strengthening the study of β-cryptoxanthin may bring fruitful results.
There have also been several earlier meta-analyses investigating the relationship between carotenoids and CRC. Männistö et al. performed a meta-analysis of cohort studies on dietary carotenoids and CRC risk in 2006, and discovered no link between any carotenoids and CRC risk (47). Conclusion may be caused by several limitations. On the one hand, we believe that the relevant studies it includes are somewhat old and not suitable for the dietary pattern of modern humans. On the other hand, the studies it included were Caucasian studies in Europe and North America with certain geographical limitations; at the time, communication technology was limited, which easily led to a loss due to follow-up bias. Wang et al. performed a meta-analysis of observational data on lycopene consumption and CRC risk in 2016 (48). The data indicate that lycopene consumption is not related with an increased risk of CRC, which is consistent with our findings. In 2016, Panic et al. performed a meta-analysis of dietary carotenoid consumption and CRC risk, which found no significant link between dietary carotenoid intake and CRC (49). The reason for the inconsistency with our findings is that on the one hand we updated and added several new studies, on the other hand our study was performed in strict accordance with the quality assessment rules and removed several unqualified studies, and his study included these low-quality articles, which may affect the results. Third, we also conducted a gender subgroup analysis that may derive the effect of gender differences, and his study did not consider gender differences. Our findings are not consistent with the above the meta-analyses, but our study is higher credible.
We found clear heterogeneity in the entire summary results for retinol and carotenoids and CRC risk. Heterogeneity is inevitable in meta-analysis, and determining the source of the heterogeneity is an important step. First, where the heterogeneity of the data was considerable, we utilized a random-effects model to combine effect sizes. Second, we conducted a subgroup analysis by tumor type, research type, and gender. Most studies’ heterogeneity was greatly decreased after subgroup analysis. Third, we conducted a sensitivity analysis to exclude the one that had the biggest influence on the research outcomes, hence lowering heterogeneity. In addition, there may be many factors that can increase heterogeneity, such as differences in race, region, dietary structure, ideology, and degree of economic development. Finally, heterogeneity may ensue as a result of non-uniform methodologies and research details, as well as inconsistency between meals and vitamin A content measurement instruments or scales. Heterogeneity is exacerbated by the inconsistency of particular dosage limits for high and low intakes. As a result, the conclusions drawn should be treated with caution.
Our meta-analysis provides a number of advantages. First, for the first time, we not only evaluated the association between dietary carotenoids and retinol and CRC risk, but also performed serological aspects. Furthermore, each of the six major groups of carotenoids was thoroughly examined. Second, because this study included a large number of cases and participants, more reliable estimations of the connection between retinol and carotenoids consumption and CRC risk may be obtained. Third, there was no evidence of significant publication bias in our meta-analysis. Fourth, we conducted a detailed subgroup analysis according to tumor type, study type, and gender. Fifth, the results of our included studies were all adjusted for covariates. Sixth, we included studies from the last 20 years, avoiding that old dietary patterns influence the accuracy of study conclusions.
Our study has several limitations. First, we only included English articles, which may cause selection bias. Second, there is a large heterogeneity in the findings, although the sources of heterogeneity have been explored. Third, study results were not subgroup analyzed by region and race. Fourth, the specific doses of retinol and carotenoids were not stated, and no dose-response meta-analysis was performed. Fifth, detection and transformation tools for retinol and carotenoids contained in ingested foods are not described. Sixth, although all results were adjusted for covariates, it is possible that there are other factors that affect the accuracy of the results.
While the efficacy of early CRC has improved, the prognosis of advanced CRC remains poor, so we must invest more effort in cancer prevention. Through the transformation of scientific research achievements, develop a set of preventive means suitable for the CRC population, strengthen people’s health publicity and education from the aspects of diet, exercise, and mental psychology, and eliminate tumors in the bud. From the results of our study, it can be seen that retinol, β-cryptoxanthin, β-carotene, α-carotene, and lycopene have some value in preventing CRC. We suggest that middle-aged and older adults with a family history of CRC or other risk factors can prevent CRC by modestly increasing carotenoid intake and even by taking supplements. From our gender subgroup analysis, it can be seen that β-carotene has a preventive effect on CRC in men, while retinol seems to have a preventive effect on CRC in women, so we can develop corresponding dietary recipes according to gender, and agents with different allocation ratios can also be made when designing supplement components to make cancer prevention more accurate.
Internationally, most of the research on vitamin prevention of cancer stays at the level of observational studies, and most of them have not been studied more deeply. In the future, on the one hand, perfect inclusion and exclusion criteria can be developed to conduct multicenter large randomized controlled trials (RCTs) to clarify the preventive effect. On the other hand, rigorous animal experiments and tumor cell experiments can be designed to determine the preventive effect, as well as to clarify the preventive mechanism. If validated by multicenter RCT and cell and animal experiments, recipes for relevant populations can be developed and corresponding supplements can be manufactured for promotion and application. Vitamins are one of the essential nutrients for human beings, which contain a wide variety and have different physiological functions and have an important relationship with many diseases. While exploring retinol and carotenoids, we can also try to explore the preventive effects of vitamin B, vitamin C, vitamin D, vitamin E, and folic acid on different cancers. In addition to exploring the value of vitamins in the prevention of cancer, the value of survival and prognosis was explored. In addition, we found that most of the vitamins belong to antioxidants, and in addition to studying the value of vitamins in cancer, the relationship between other antioxidants and cancer can be explored, such as melatonin, anthocyanins, astaxanthin, and quercetin. Finally, it should be noted that excessive use of vitamins will produce corresponding toxic side effects, such as excessive intake of carotenoids will cause loss of appetite, yellow skin, poor sleep, affecting female ovulation and so on (50–52). Therefore, we recommend an appropriate increase in vitamin A intake within a safe dose range, especially an increase in dietary intake of vegetables, fruits, and animal products.
Conclusion
Total carotenoids intake and Lutein/Zeaxanthin intake were not associated with CRC risk. High β-carotene, α-carotene, lycopene, and β-cryptoxanthin all tended to reduce CRC risk, with a more pronounced effect in the male population. In addition, β-carotene had a significant preventive effect on CRC in men. In the female population, high dietary retinol intake can reduce CRC risk, while it has carcinogenic effects in men. On the other hand, serum carotenoids concentrations were significantly and inversely associated with CRC risk. Finally, due to the limitations, large prospective studies with adequate sample size, well-controlled confounders, and long-term follow-up are needed for further exploration.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XH and RZ conceived the study and wrote the draft. GZ and YJ performed the literature search. DW and YW extracted the required data. XH performed the statistical analyses. HC reviewed the manuscript. All authors viewed and gave permission to publish this manuscript.
Funding
This study was supported by the grants from the Central to Guide Local Scientific and Technological Development (ZYYDDFFZZJ-1), Key talent project of Gansu Province of the Organization Department of Gansu provincial Party committee (2020RCXM076), Key Laboratory of gastrointestinal cancer diagnosis and treatment of National Health Commission (2019PT320005), Gansu Provincial Youth Science and Technology Fund Program (21JR7RA642), Gansu Key Laboratory of molecular diagnosis and precision treatment of surgical tumors (18JR2RA033), Guiding plan for scientific and technological development of Lanzhou (2019-ZD-102), and Natural Science Foundation of Gansu Province (21JR11RA186).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the researchers and study participants for their contributions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.918777/full#supplementary-material
References
1. Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. (2021). 127:3029–30. doi: 10.1002/cncr.33587
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. (2017) 67:7–30. doi: 10.3322/caac.21387
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Doubeni CA, Major JM, Laiyemo AO, Schootman M, Zauber AG, Hollenbeck AR, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. (2012) 104:1353–62. doi: 10.1093/jnci/djs346
5. Zhou J, Zheng R, Zhang S, Zeng H, Wang S, Chen R, et al. Colorectal cancer burden and trends: comparison between China and major burden countries in the world. Chin J Cancer Res. (2021) 33:1–10. doi: 10.21147/j.issn.1000-9604.2021.01.01
6. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/s0140-6736(19)32319-0
7. Sharma S, Katoch V, Kumar S, Chatterjee S. Functional relationship of vegetable colors and bioactive compounds: implications in human health. J Nutr Biochem. (2021) 92:108615. doi: 10.1016/j.jnutbio.2021.108615
8. Negri E, La Vecchia C, Franceschi S, D’Avanzo B, Parazzini F. Vegetable and fruit consumption and cancer risk. Int J Cancer. (1991) 48:350–4. doi: 10.1002/ijc.2910480307
9. Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. (2003) 78(Suppl. 3):559s–69s. doi: 10.1093/ajcn/78.3.559S
10. Li C, Imai M, Matsuura T, Hasegawa S, Yamasaki M, Takahashi N. Inhibitory effects of retinol are greater than retinoic acid on the growth and adhesion of human refractory cancer cells. Biol Pharm Bull. (2016) 39:636–40. doi: 10.1248/bpb.b15-00794
11. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of Vitamin A in the immune system. J Clin Med. (2018) 7:258. doi: 10.3390/jcm7090258
12. Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. (2011) 3:385–428. doi: 10.3390/nu3040385
13. Dao DQ, Ngo TC, Thong NM, Nam PC. Is Vitamin A an antioxidant or a pro-oxidant? J Phys Chem B. (2017) 121:9348–57. doi: 10.1021/acs.jpcb.7b07065
14. Siddikuzzaman, Grace VM. Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol Immunotoxicol. (2013) 35:164–73. doi: 10.3109/08923973.2012.736520
15. Zhang X, Dai B, Zhang B, Wang Z. Vitamin A and risk of cervical cancer: a meta-analysis. Gynecol Oncol. (2012) 124:366–73. doi: 10.1016/j.ygyno.2011.10.012
16. Maiani G, Castón MJ, Catasta G, Toti E, Cambrodón IG, Bysted A, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. (2009) 53(Suppl. 2):S194–218. doi: 10.1002/mnfr.200800053
17. Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. (2014) 6:466–88. doi: 10.3390/nu6020466
18. Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. (2012) 17:3202–42. doi: 10.3390/molecules17033202
19. Saini RK, Keum YS, Daglia M, Rengasamy KR. Dietary carotenoids in cancer chemoprevention and chemotherapy: a review of emerging evidence. Pharmacol Res. (2020) 157:104830. doi: 10.1016/j.phrs.2020.104830
20. Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. (2010) 62:1285–98. doi: 10.1016/j.addr.2010.07.003
21. Rowles JL III, Erdman JW Jr. Carotenoids and their role in cancer prevention. Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865:158613. doi: 10.1016/j.bbalip.2020.158613
22. Leenders M, Leufkens AM, Siersema PD, van Duijnhoven FJ, Vrieling A, Hulshof PJ, et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. (2014) 135:2930–9. doi: 10.1002/ijc.28938
23. Murtaugh MA, Ma KN, Benson J, Curtin K, Caan B, Slattery ML. Antioxidants, carotenoids, and risk of rectal cancer. Am J Epidemiol. (2004) 159:32–41. doi: 10.1093/aje/kwh013
24. Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. (2013) 24:335–41. doi: 10.1007/s10552-012-0119-3
25. Shin A, Li H, Shu XO, Yang G, Gao YT, Zheng W. Dietary intake of calcium, fiber and other micronutrients in relation to colorectal cancer risk: results from the Shanghai Women’s Health Study. Int J Cancer. (2006) 119:2938–42. doi: 10.1002/ijc.22196
26. Park SY, Nomura AM, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Carotenoid intake and colorectal cancer risk: the multiethnic cohort study. J Epidemiol. (2009) 19:63–71. doi: 10.2188/jea.je20080078
27. Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjønneland A. Micronutrient intake and risk of colon and rectal cancer in a danish cohort. Cancer Epidemiol. (2010) 34:40–6. doi: 10.1016/j.canep.2009.12.012
28. Huang J, Lu MS, Fang YJ, Xu M, Huang WQ, Pan ZZ, et al. Serum carotenoids and colorectal cancer risk: a case-control study in Guangdong, China. Mol Nutr Food Res. (2017) 61:1700267. doi: 10.1002/mnfr.201700267
29. Guertin KA, Li XS, Graubard BI, Albanes D, Weinstein SJ, Goedert JJ, et al. Serum trimethylamine N-oxide, carnitine, choline, and betaine in relation to colorectal cancer risk in the alpha tocopherol, beta carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev. (2017) 26:945–52. doi: 10.1158/1055-9965.Epi-16-0948
30. Yan B, Lu MS, Wang L, Mo XF, Luo WP, Du YF, et al. Specific serum carotenoids are inversely associated with breast cancer risk among Chinese women: a case-control study. Br J Nutr. (2016) 115:129–37. doi: 10.1017/s000711451500416x
31. Asbaghi S, Saedisomeolia A, Hosseini M, Honarvar NM, Khosravi A, Azargashb E. Dietary intake and serum level of carotenoids in lung cancer patients: a case-control study. Nutr Cancer. (2015) 67:893–8. doi: 10.1080/01635581.2015.1055365
32. Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, et al. Serum lycopene concentration and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. (2011) 20:638–46. doi: 10.1158/1055-9965.Epi-10-1221
33. Beilby J, Ambrosini GL, Rossi E, de Klerk NH, Musk AW. Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur J Clin Nutr. (2010) 64:1235–8. doi: 10.1038/ejcn.2010.124
34. Lai GY, Weinstein SJ, Albanes D, Taylor PR, Virtamo J, McGlynn KA, et al. Association of serum α-tocopherol, β-carotene, and retinol with liver cancer incidence and chronic liver disease mortality. Br J Cancer. (2014) 111:2163–71. doi: 10.1038/bjc.2014.365
35. Lee NY, Kim Y, Kim YS, Shin JH, Rubin LP, Kim Y. β-Carotene exerts anti-colon cancer effects by regulating M2 macrophages and activated fibroblasts. J Nutr Biochem. (2020) 82:108402. doi: 10.1016/j.jnutbio.2020.108402
36. Reynoso-Camacho R, González-Jasso E, Ferriz-Martínez R, Villalón-Corona B, Loarca-Piña GF, Salgado LM, et al. Dietary supplementation of lutein reduces colon carcinogenesis in DMH-treated rats by modulating K-ras. PKB, and β-catenin proteins. Nutr Cancer. (2011) 63:39–45. doi: 10.1080/01635581.2010.516477
37. Cha KH, Koo SY, Lee DU. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem. (2008) 56:10521–6. doi: 10.1021/jf802111x
38. Palozza P, Serini S, Maggiano N, Tringali G, Navarra P, Ranelletti FO, et al. beta-Carotene downregulates the steady-state and heregulin-alpha-induced COX-2 pathways in colon cancer cells. J Nutr. (2005) 135:129–36. doi: 10.1093/jn/135.1.129
39. Granado F, Olmedilla B, Blanco I, Rojas-Hidalgo E. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. Eur J Clin Nutr. (1996) 50:246–50.
40. San Millán C, Soldevilla B, Martín P, Gil-Calderón B, Compte M, Pérez-Sacristán B, et al. β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through ΔNP73 negative regulation in colon cancer. Clin Cancer Res. (2015) 21:4398–409. doi: 10.1158/1078-0432.Ccr-14-2027
41. Gao M, Dang F, Deng C. β-Cryptoxanthin induced anti-proliferation and apoptosis by G0/G1 arrest and AMPK signal inactivation in gastric cancer. Eur J Pharmacol. (2019) 859:172528. doi: 10.1016/j.ejphar.2019.172528
42. Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM, et al. β-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila). (2013) 6:309–20. doi: 10.1158/1940-6207.Capr-12-0368
43. Liu C, Bronson RT, Russell RM, Wang XD. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res (Phila). (2011) 4:1255–66. doi: 10.1158/1940-6207.Capr-10-0384
44. Iskandar AR, Miao B, Li X, Hu KQ, Liu C, Wang XD. β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic acetylcholine receptor α7 signaling. Cancer Prev Res (Phila). (2016) 9:875–86. doi: 10.1158/1940-6207.Capr-16-0161
45. Miyazawa K, Miyamoto S, Suzuki R, Yasui Y, Ikeda R, Kohno H, et al. Dietary beta-cryptoxanthin inhibits N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Oncol Rep. (2007) 17:297–304.
46. Lim JY, Liu C, Hu KQ, Smith DE, Wu D, Lamon-Fava S, et al. Xanthophyll β-cryptoxanthin inhibits highly refined carbohydrate diet-promoted hepatocellular carcinoma progression in mice. Mol Nutr Food Res. (2020) 64:e1900949. doi: 10.1002/mnfr.201900949
47. Männistö S, Yaun SS, Hunter DJ, Spiegelman D, Adami HO, Albanes D, et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am J Epidemiol. (2007) 165:246–55. doi: 10.1093/aje/kwk009
48. Wang X, Yang HH, Liu Y, Zhou Q, Chen ZH. Lycopene consumption and risk of colorectal cancer: a meta-analysis of observational studies. Nutr Cancer. (2016) 68:1083–96. doi: 10.1080/01635581.2016.1206579
49. Panic N, Nedovic D, Pastorino R, Boccia S, Leoncini E. Carotenoid intake from natural sources and colorectal cancer: a systematic review and meta-analysis of epidemiological studies. Eur J Cancer Prev. (2017) 26:27–37. doi: 10.1097/cej.0000000000000251
50. Saito Y. [Current status of health foods including their interactions with drugs and adverse events]. Yakugaku Zasshi. (2018) 138:1511–6. doi: 10.1248/yakushi.18-00155-1
51. Oliveira MR. The neurotoxic effects of vitamin A and retinoids. An Acad Bras Cienc. (2015) 87(Suppl. 2):1361–73. doi: 10.1590/0001-3765201520140677
52. Siems W, Wiswedel I, Salerno C, Crifò C, Augustin W, Schild L, et al. Beta-carotene breakdown products may impair mitochondrial functions–potential side effects of high-dose beta-carotene supplementation. J Nutr Biochem. (2005) 16:385–97. doi: 10.1016/j.jnutbio.2005.01.009
53. Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, et al. Antioxidant and DNA methylation-related nutrients and risk of distal colorectal cancer. Cancer Causes Control. (2010) 21:1171–81. doi: 10.1007/s10552-010-9544-3
54. Slattery ML, Benson J, Curtin K, Ma KN, Schaeffer D, Potter JD. Carotenoids and colon cancer. Am J Clin Nutr. (2000) 71:575–82. doi: 10.1093/ajcn/71.2.575
55. Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary carotenoid intake and colorectal cancer risk. Nutr Cancer. (2002) 42:167–72. doi: 10.1207/s15327914nc422_3
56. Nkondjock A, Ghadirian P. Dietary carotenoids and risk of colon cancer: case-control study. Int J Cancer. (2004) 110:110–6. doi: 10.1002/ijc.20066
57. Wang Z, Joshi AM, Ohnaka K, Morita M, Toyomura K, Kono S, et al. Dietary intakes of retinol, carotenes, vitamin C, and vitamin E and colorectal cancer risk: the Fukuoka colorectal cancer study. Nutr Cancer. (2012) 64:798–805. doi: 10.1080/01635581.2012.690927
58. Negri E, La Vecchia C, Franceschi S. Relations between vegetable, fruit and micronutrient intake. Implications for odds ratios in a case-control study. Eur J Clin Nutr. (2002) 56:166–70. doi: 10.1038/sj.ejcn.1601317
59. Levi F, Pasche C, Lucchini F, La Vecchia C. Selected micronutrients and colorectal cancer. a case-control study from the canton of Vaud, Switzerland. Eur J Cancer. (2000) 36:2115–9. doi: 10.1016/s0959-8049(00)00195-7
60. Lu MS, Fang YJ, Chen YM, Luo WP, Pan ZZ, Zhong X, et al. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: a case-control study. Eur J Nutr. (2015) 54:619–28. doi: 10.1007/s00394-014-0743-7
61. Paiva I, Amaral T, Barros H. Influence of individually estimated portion size on the assessment of nutritional risk in colorectal cancer in Portugal. J Hum Nutr Diet. (2004) 17:529–36. doi: 10.1111/j.1365-277X.2004.00563.x
62. Key TJ, Appleby PN, Masset G, Brunner EJ, Cade JE, Greenwood DC, et al. Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom dietary cohort consortium. Int J Cancer. (2012) 131:E320–5. doi: 10.1002/ijc.27386
63. Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the physicians’ health study (United States). Cancer Causes Control. (2000) 11:617–26. doi: 10.1023/a:1008995430664
64. Wakai K, Hirose K, Matsuo K, Ito H, Kuriki K, Suzuki T, et al. Dietary risk factors for colon and rectal cancers: a comparative case-control study. J Epidemiol. (2006) 16:125–35. doi: 10.2188/jea.16.125
65. Kabat GC, Kim MY, Sarto GE, Shikany JM, Rohan TE. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s health initiative. Eur J Clin Nutr. (2012) 66:549–54. doi: 10.1038/ejcn.2011.207
66. Luo H, Fang YJ, Lu MS, Pan ZZ, Huang J, Chen YM, et al. Dietary and serum vitamins A and E and colorectal cancer risk in Chinese population: a case-control study. Eur J Cancer Prev. (2019) 28:268–77. doi: 10.1097/cej.0000000000000452
Keywords: retinol, carotenoids, colorectal cancer, risk, meta-analysis
Citation: Han X, Zhao R, Zhang G, Jiao Y, Wang Y, Wang D and Cai H (2022) Association of Retinol and Carotenoids Content in Diet and Serum With Risk for Colorectal Cancer: A Meta-Analysis. Front. Nutr. 9:918777. doi: 10.3389/fnut.2022.918777
Received: 13 April 2022; Accepted: 13 June 2022;
Published: 30 June 2022.
Edited by:
Ren-You Gan, Institute of Urban Agriculture (CAAS), ChinaReviewed by:
Bin Li, Shenyang Agricultural University, ChinaCarla Pereira, Centro de Investigação de Montanha (CIMO), Portugal
Copyright © 2022 Han, Zhao, Zhang, Jiao, Wang, Wang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Cai, Y2FpYWxvbnRlYW1AMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaoyong Han
Xiaoyong Han Rangyin Zhao4†
Rangyin Zhao4† Guangming Zhang
Guangming Zhang Hui Cai
Hui Cai