
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 12 May 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.917349
This article is part of the Research Topic Selenium and Human Health View all 11 articles
This study aimed to compare the protective effects of different selenium supplements against heat stress and exercise fatigue-induced liver injury and to investigate the potential mechanisms of action. Selenium-enriched soybean protein (SePro), selenium-enriched soybean peptides (SePPs), and selenomethionine (SeMet) are organic selenium supplements in which selenium replaces the sulfur in their sulfur-containing amino acids. Common peptides (PPs) are obtained by enzymatic hydrolysis of soybean protein which was extracted from common soybean. The SePPs with higher hydrolysis degree and selenium retention were isolated via alkaline solubilization and acid precipitation and the enzymatic hydrolysis of alkaline protease, neutral protease, and papain. The results showed that SePPs could significantly increase the antioxidant levels in rats, inhibit lipid peroxidation, and reduce liver enzyme levels in rat serum, while the histological findings indicated that the inflammatory cell infiltration in the liver tissue was reduced, and new cells appeared after treatment with SePPs. Moreover, SePPs could increase glutathione (GSH) and GSH peroxidase (GSH-Px) in the liver, as well as protect the liver by regulating the NF-κB/IκB pathway, prevent interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α) release in the liver. The SePPs displayed higher antioxidant and anti-inflammatory activity in vivo than SePro, SeMet, Sodium selenite (Na2SeO3), and PPs. Therefore, SePPs could be used as a priority selenium resource to develop heatstroke prevention products or nutritional supplements.
In recent years, with the general increase in the concept of health, more and more people are running, hiking, and doing other sports to promote their health. Meanwhile, some occupational groups (such as athletes, workers, etc.) require higher exercise intensity, which increases the incidence of exercise-related diseases. Therefore, sports health has drawn increasing attention during the past decades. Heatstroke is one of the common sports injury-related diseases, mainly caused by heat stress and exercise fatigue. Hyperthermic exercise is associated with severe fluid loss, which leads to the formation of reactive oxygen species (ROS) and affects the body’s redox balance (1). ROS can disrupt cell membranes and allow endotoxins and pathogens to leak into the human circulation, resulting in the destruction of the liver detoxification function and accompanying inflammatory response, eventually causing hepatocyte necrosis or liver tissue damage (2). Electrolyte or water supplementation alone has limited recovery from heatstroke induced inflammatory response and tissue damage. The most important treatment for patients with heatstroke is rapid and effective body cooling, while the endpoint of the cooling is controversial. The controversial lies in whether to stop cooling by targeting the patient’s core body temperature or behavioral performance (3). Consequently, developing functional foods or heatstroke preventive drinks has become a crucial and practical strategy to avoid heatstroke.
Selenium is an essential trace element for the body and a key component of the GSH-Px. GSH-Px is expressed in the cytoplasm and mitochondria, which can enhance the antioxidant capacity of the body and protect the structure and function of cells (4). The functions of selenium have been demonstrated previously, including detoxification, antioxidant and immune enhancement. It was reported that low selenium consumption was linked to various human disorders. Zhang et al. found that selenium deficiency disrupted the normal membrane structure of spleen cells, downregulated the expression levels of immune response-related genes, and activated T lymphocyte differentiation via the DUSP1/NF-κB pathway, affecting the immune response in the spleen and causing tissue damage (5). Selenium is an element that cannot be synthesized by the human body and needs to be consumed from food. Selenium supplements are classified as inorganic selenium (selenates and selenites) and organic selenium (selenocysteine [SeCys], selenomethionine [SeMet], selenoproteins and their hydrolysates) (6). Nevertheless, selenium is considered both beneficial and toxic depending on its form and amount ingested (7). The organic form of selenium is superior to the inorganic form in terms of bioavailability and toxicity for meeting human dietary requirements (8). Consequently, it is necessary to explore new selenium carriers to address the potential problems associated with selenium deficiency and selenium toxicity.
Soybean protein has a high nutritional value and contains a complete range of amino acids and eight essential amino acids (9). More importantly, soybean exhibits a high selenium enrichment capacity, with more than 75% of selenium in soybean bound to protein. Up to 82% of selenium is present in high molecular weight form, primarily in SeCys and SeMet (10). The bioavailability of selenium from soy protein can be as high as 86-96% (11). Previous results have shown that selenium-enriched soy protein hydrolysates are healthy nutrients. According to Liu et al., selenium biofortified soy peptides could reduce CCl4-induced liver fibrosis by increasing GSH-Px activity and GSH content, improving hepatocyte survival, inhibiting hepatic stellate cell activation, and improving antioxidant capacity (12). Zhang et al. observed that SePPs could regulate immunoglobulin (Ig)M, IgG, and IgA secretion, significantly increase splenic interleukin 2 (IL-2), interferon-gamma (IFN-γ), nitric oxide (NO), and cyclic guanosine monophosphate (cGMP) production, and IL-2, IFN-γ, and inducible NO synthase (iNOs) mRNA expression, as well as enhance host-specific and non-specific immunity via a cyclophosphamide-induced immunosuppression mice model (13). The beneficial effects of selenium-enriched peptides on health were confirmed previously, whereas, the protective mechanism of selenium-enriched peptides against heatstroke-induced liver injury is still unclear. Meanwhile few studies attempted to compare the effects of different selenium supplements.
This study, isolated SePro from naturally grown selenium-enriched soybeans, which were enzymatically separated to produce SePPs. A modeling approach of rats swimming in hot water under heat stress and exercise fatigue-induced heatstroke was used to investigate the protective effect of SePPs against sports injury and the possible mechanisms of action. The impact of different forms of selenium supplements was compared comprehensively. It was predicted that SePPs would prevent heatstroke and increase exercise tolerance by reducing oxidative stress and regulating inflammatory responses. This study provides a basis for preventing sports diseases and also provides new ideas for selenium nutrition supplementation.
Selenium-enriched soybeans were purchased from Enshi Se-Run Health Tech Development Co., Ltd. (Enshi city, China), while L-SeMet was obtained from Shanghai Macklin Biochemical Co., Ltd. Na2SeO3 was purchased from Sigma Chemical Co. (St. Louis, MO, United States). Xi’an Ruidi Biotechnology Co., Ltd., supplied the honeysuckle extract. The alanine aminotransferase (ALT), aspartate aminotransferase (AST), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), GSH, and GSH-Px kits were obtained from Nanjing Jiancheng Bioengineering Institute. The IL-1β, IL-6, TNF-α, GSH, and GSH-Px ELISA kits were acquired from Jiangsu Meibiao Biotechnology Co., Ltd., while the BCA protein concentration assay kit was purchased from Beijing Solarbio Science & Technology Co., Ltd. The skim milk powder was supplied by Becton, Dickinson, and Company. Furthermore, the PVDF membrane, TBST, NF-κB p65 rabbit monoclonal antibody, phospho-IκBα (Ser32/36) rabbit polyclonal antibody, α-Tubulin rabbit polyclonal antibody, horseradish peroxidase-labeled goat anti-rabbit IgG (H + L), Western secondary antibody dilution, and BeyoECL Star (extra super-sensitive enhanced chemiluminescence kit) were purchased from Beyotime Biotechnology.
The SePPs was prepared as described in a previous study (14). The selenium-enriched soybeans were crushed, defatted, and dried to obtain a powder, after which the SePro was extracted via alkaline solubilization and acid deposition. The preparation process is shown in Figure 1. Alkaline solubilization was performed at 40°C for 2 h at a 1:20 mater-liquid ratio and pH = 8. The SePro was obtained via centrifugation at 2,100 g for 20 min, after which the supernatant was adjusted to pH 4.0 and centrifuged at 2,100 g for 20 min. Subsequently, the SePro was dissolved in water to obtain a SePro solution with a mass fraction of 8%, using alkaline protease, neutral protease, and papain. Enzymatic digestion occurred at 50°C for 4 h a ratio of 2:1:1. Finally, the enzymatic digest was heated at 95°C for 15 min to inactivate the protease, to ensure that the degree of hydrolysis reached 68%. After centrifugation, the supernatant was collected and filtered through a 0.45 μm microporous membrane. SePPs were obtained after freeze-drying.
Protein content, selenium content and amino acid composition were determined as described in previous reports (14). The protein content in the SePro, SePPs, and common soy peptides (PPs) was assessed using the Kjeldahl method (Kjeltec 8000, FOSS analysis A/S) at a conversion factor setting of 6.25. The total selenium content in the solid SePro, SePPs and PPs powders was measured using hydride generation-atomic fluorescence spectrometry (LC-AFS6500, Beijing Haiguang Instruments Co., Ltd.). The amino acid composition of SePro, SePPs, and PPs was assessed using an amino acid analyzer (Biochrom 30 amino acid analyzer; Biochrom Ltd.) and a Na cation exchange column (8 mm, 4.6 × 200 mm), which was purchased from the Waters Corporation. The amino acids were derivatized with ninhydrin reagent after they passed through the exchange column. The absorbance of the subsequent compounds was measured at 440 nm (proline [Pro]) and 570 nm (all other amino acids). The molecular weight distribution of SePPs was determined following a previously reported method (13). Standard molecular weight samples of aprotinin (6500 Da), bacitracin (1422 Da), Gly-Gly-Tyr-Arg (451 Da), and reduced GSH (307 Da) were passed through a 0.22 μm filter and deposited in a Superdex 200 10/300 GL column. The chromatographic analysis was performed using an ÄKTA pure system (AKTA pure 25, Cytiva). The phosphate buffer (PBS, concentration 0.05 M, pH = 7) mobile phase was eluted at a 0.5 ml/min flow rate and detected at 220 nm.
Eighty male Sprague-Dawley (SD) rats (6 weeks old, weight 170 ± 10 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., license No. SCXK (Beijing) 2016-0006. The rats received a standard diet and were provided free access to water. They were kept at a temperature of 22 ± 2°C, a humidity of 50 ± 10%, and a 12 h light/12 h dark cycle. The rats were acclimatized for one week before the experiment. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the Pony Testing International Group Co., Ltd. (PONY-2021-FL-15).
The 80 male SD rats were randomly divided into eight groups (n = 10): control group, model group, positive control (PC) honeysuckle group (15), SePro group, SePPs group, PPs group, SeMet group, and Na2SeO3 group. All rats were administered via gavage, while the control and model groups were given equal volumes of deionized water, in which the selenium content 18 μg/kg in selenium-containing groups was equal, equivalent to 3.57 μg/kg in adults (Reference Chinese Nutrition Association: The recommended adult daily intake of selenium is 50-250 μg, 200 μg was selected for conversion). The protein composition of the PPs group was the same as that of SePPs group, and the rats were gavaged once daily for 7 d. The last gavage was administered after a 12-h fasting period. After 10 min of gavage, the rats in the control group were kept at room temperature (22 ± 2°C), while the rats in the model group were placed in hot water to swim at 40 ± 2°C to observe their status. When the activity of the rats decreased, the hot water near them was agitated with a wooden stick to keep them moving continuously. The rats were removed immediately when they started to sink, and the time was recorded. The remaining groups of rats swam in hot water for the same duration as the model group. After completing the swimming process, the rats were anesthetized with 3% sodium pentobarbital and dissected to collect the material. Blood was taken from the abdominal aorta, serum was separated for blood indicator analysis, and liver tissue was collected for histomorphometric analysis, while the rest was flash-frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
The SOD, CAT, GSH-Px, MDA, and GSH levels in serum were measured using commercial kits according to the instructions of the manufacturer.
The ALT and AST viability in serum were measured using commercial kits according to the instructions of the manufacturer. The optical density (OD) values of the solutions were measured at 510 nm using an automated microplate reader (Infinite 200 Pro Nanoquant, Tecan).
Tissue samples were collected and rinsed with saline. The filter paper was blotted to dry the surrounding water, after which the tissues were cut, weighed, and placed on ice with a delay of 5 s after every 5 s of grinding. The grinding process was repeated five times for tissue homogenization, and the tissue homogenate used PBS (pH = 7.2-7.4, concentration of 0.01 mol/L) with a homogenization ratio of 10%. The supernatant was collected for analysis after centrifugation at 2296 g for 15 min. The GSH and GSH-Px levels in liver tissue were measured using a commercial rat ELISA quantification kit according to the instructions of the manufacturer. The OD values of the solutions were measured at 450 nm using an automated microplate reader (Infinite 200 Pro Nanoquant, Tecan).
Some of the liver tissue was immediately fixed in formalin for 48 h, embedded in paraffin, stained with hematoxylin and eosin, and connected to a microscope for observation and morphological analysis, which was used to assess the degree of liver tissue damage.
The liver tissue was pre-treated as described in Section 2.7. The IL-1β, IL-6, and TNF-α concentrations in the liver tissue were measured using commercial rat ELISA quantification kits according to the instructions of the manufacturer. The OD values of the solutions were measured at 450 nm using an automated microplate reader (Infinite 200 Pro Nanoquant, Tecan).
The liver samples were added to the lysate (RIPA) with phosphatase inhibitors, placed on ice and ground, and delayed for 5 s after every 5 s of grinding. The grinding process was repeated five times, after which the extracts were centrifuged for 4 min at 12000 g. The protein concentration was determined by adding diluent to the BCA supernatant to unify the protein concentrations of all the samples. Then 5 × loading buffer was added and placed in a 100°C water bath. The mixture was heated for 5 min for denaturing, cooled, and centrifuged at 9184 g for 2 min, after which the supernatant was collected for determination. The protein was separated in 10% SDS-PAGE and transferred to a PVDF membrane. After blocking with 5% skim milk, the primary antibody (NF-κB p65 rabbit monoclonal antibody, phospho-IκBα (Ser32/36) rabbit polyclonal antibody, 1:1000) was incubated overnight at 4°C, after which the primary antibody was washed off. The secondary antibody [anti-rabbit IgG(H + L) 1:1000] was incubated for 1 h at room temperature, and protein expression was normalized with α-Tubulin via ECL chemiluminescence. Band intensities were measured using Image J software.
The data were expressed as mean ± standard deviation (SD). The results were analyzed via one-way analysis of variance (ANOVA) and Tukey’s method using SPSS 23 software. A P value less than 0.05 indicated statistical significance.
The protein contents of the SePro, SePPs, and PPs were 89.36 ± 0.24%, 84.46 ± 0.03%, and 88.98 ± 0.15%, respectively, while the total selenium contents in the solid SePro, SePPs, and PPs powders were 44.00 ± 0.95 mg/kg, 26.00 ± 0.7 mg/kg and 0.06 ± 0.001 mg/kg, respectively. During alkaline solubilization and acid deposition, the proteins were separated via acid-base washing and dialyzed to remove the small molecules (including inorganic selenium not attached to the proteins), leaving only organic selenium in SePro and SePPs. Table 1 showed the amino acid compositions of the SePro, SePPs, and PPs. SePro, SePPs, and PPs contained a comprehensive variety of amino acids, including eight essential amino acids, as well as aliphatic, aromatic, and basic amino acids.
As shown in Table 2, more than 90% of the components of SePPs and PPs have molecular weights less than 3000 Da. The molecular weight of peptides represents a vital influencing factor of their functional activity, while bioactive peptides are typically a mixture of protein hydrolysis products and low molecular weight peptides.
When the rats were removed from the hot water, their eyes were dull and bulging, while most were not moving, indicating the occurrence of heatstroke. The CAT, SOD, GSH-Px, MDA, and GSH levels in the serum were measured to evaluate the ameliorative effect of SePPs on oxidative damage in vivo in the heat stress and exercise fatigue-induced heatstroke rats. As shown in Figure 2. Heatstroke significantly decreased (p < 0.05) the CAT, SOD, GSH-Px, and GSH content in the serum of the rats in the model group by 61, 46, 13, and 73%, respectively, while the MDA content was 170% higher than that in the control group, suggesting stronger oxidative stress in the rats after heatstroke. Compared with the model group, significant differences were found in the CAT, SOD, MDA, GSH, and GSH-Px levels in serum between the SePro and SePPs groups (p < 0.05), while no statistical difference was observed in the MDA content levels in the serum between the PPs and model groups (p > 0.05). Additionally, there were no statistical differences between the SOD activity and MDA content in the serum of the rats in the SeMet and model groups (p > 0.05), while the serum SOD, GSH-Px activities, and MDA content of the rats in the Na2SeO3 group were not statistically different from those in the model group (p > 0.05). Our results indicated that SePPs could improve oxidative damage due to heatstroke by increasing the antioxidant enzyme activity and inhibiting lipid peroxidation. Furthermore, its ability to increase antioxidant enzyme activity in the body was higher than that of SePro, PPs, SeMet, and Na2SeO3, while the degree of lipid peroxidation inhibition was not statistically different from that of SePro (p > 0.05).
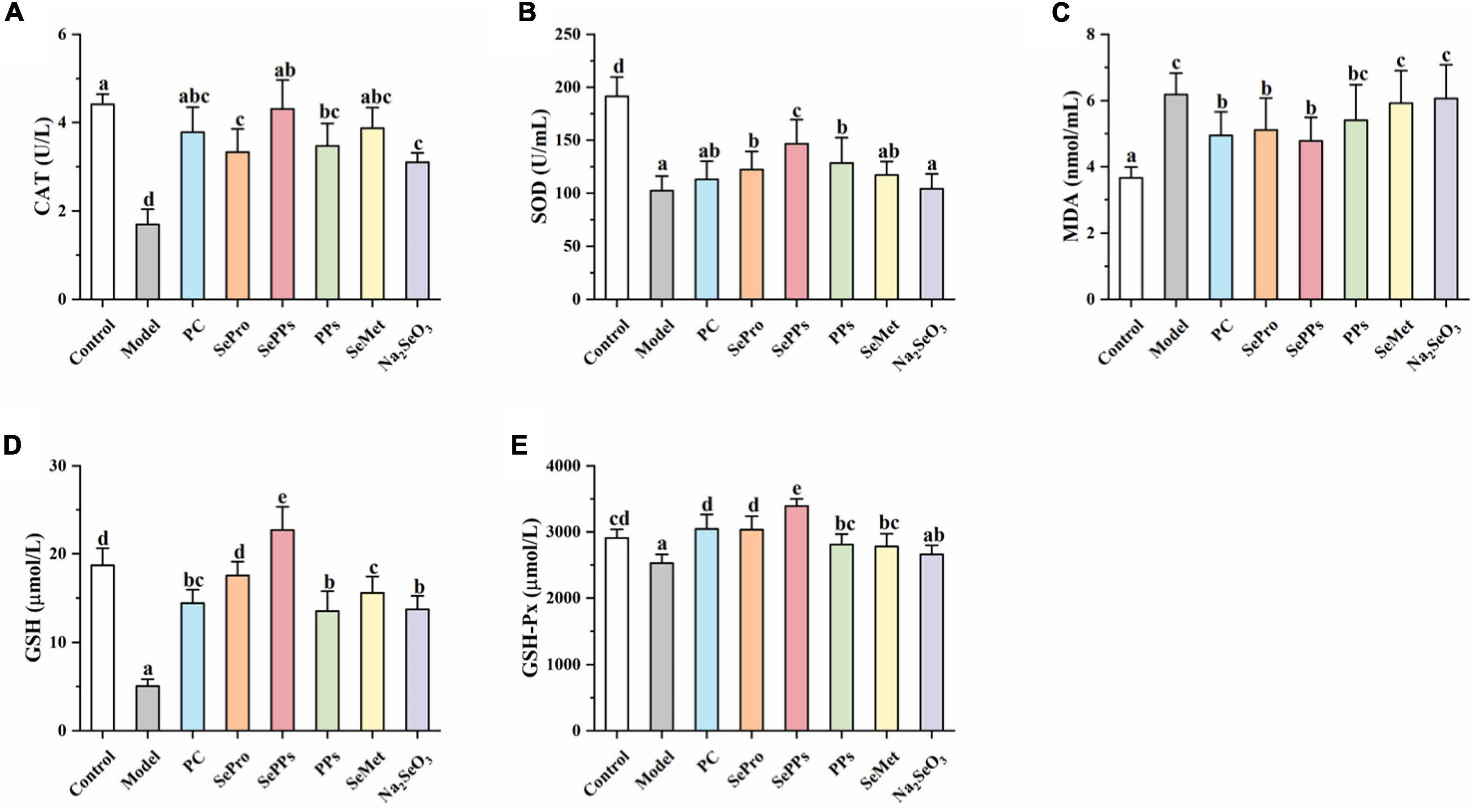
Figure 2. Effects of different selenium supplements on CAT (A), SOD (B), MDA (C), GSH (D), and GSH-Px (E) in rat serum. Values are expressed as the mean ± SD, results marked with the same letters were not significantly different (p > 0.05).
The ALT and AST levels in the serum of the rats were determined to assess the impact of heat stress and exercise fatigue on the liver. It has been shown in Figure 3 that the liver enzyme levels in the serum of the rats in the model group were significantly higher (p < 0.05), which were 2.0 and 2.2 times higher than those in the control group. The data indicate the impaired liver function after heatstroke. The SePro, SePPs, PPs, and SeMet groups displayed significantly lower (p < 0.05) liver enzyme blood levels than the model group, while the serum ALT in the Na2SeO3 group did not differ considerably from the model group (p > 0.05). SePro, SePPs, and PPs were more effective in reducing the serum ALT levels than SeMet and Na2SeO3, while SePPs were the most effective in lowering serum AST among the other treatment groups. Overall, SePPs were superior to other supplements in preventing liver damage.
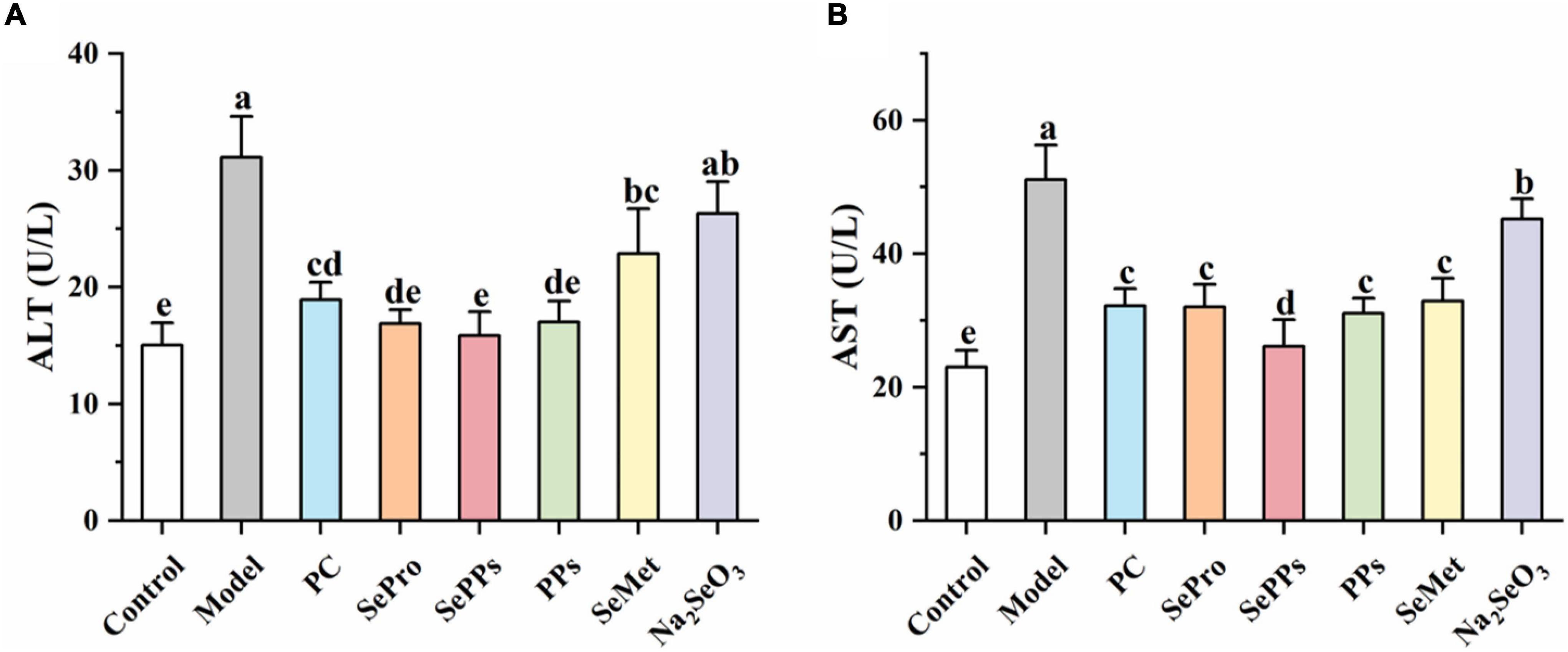
Figure 3. Effects of different selenium supplements on ALT (A) and AST (B) in rat serum. Values are expressed as the mean ± SD, results marked with the same letters were not significantly different (p > 0.05).
Selenium is a vital component of GSH-Px and is closely related to the GSH system. Therefore, the GSH and GSH-Px content in the liver tissues were determined further. The results are shown in Figure 4. The GSH and GSH-Px content in rat livers was considerably reduced (p < 0.05) after heatstroke, which was 64 and 59% of the control, respectively. Supplementation with SePro, SePPs, PPs, and SeMet before heat stress and exercise improved the decrease in GSH and GSH-Px content (p < 0.05). However, Na2SeO3 supplementation did not significantly augment the GSH and GSH-Px levels in the liver (p > 0.05). The GSH contents in the SePPs group were higher than in the control group.
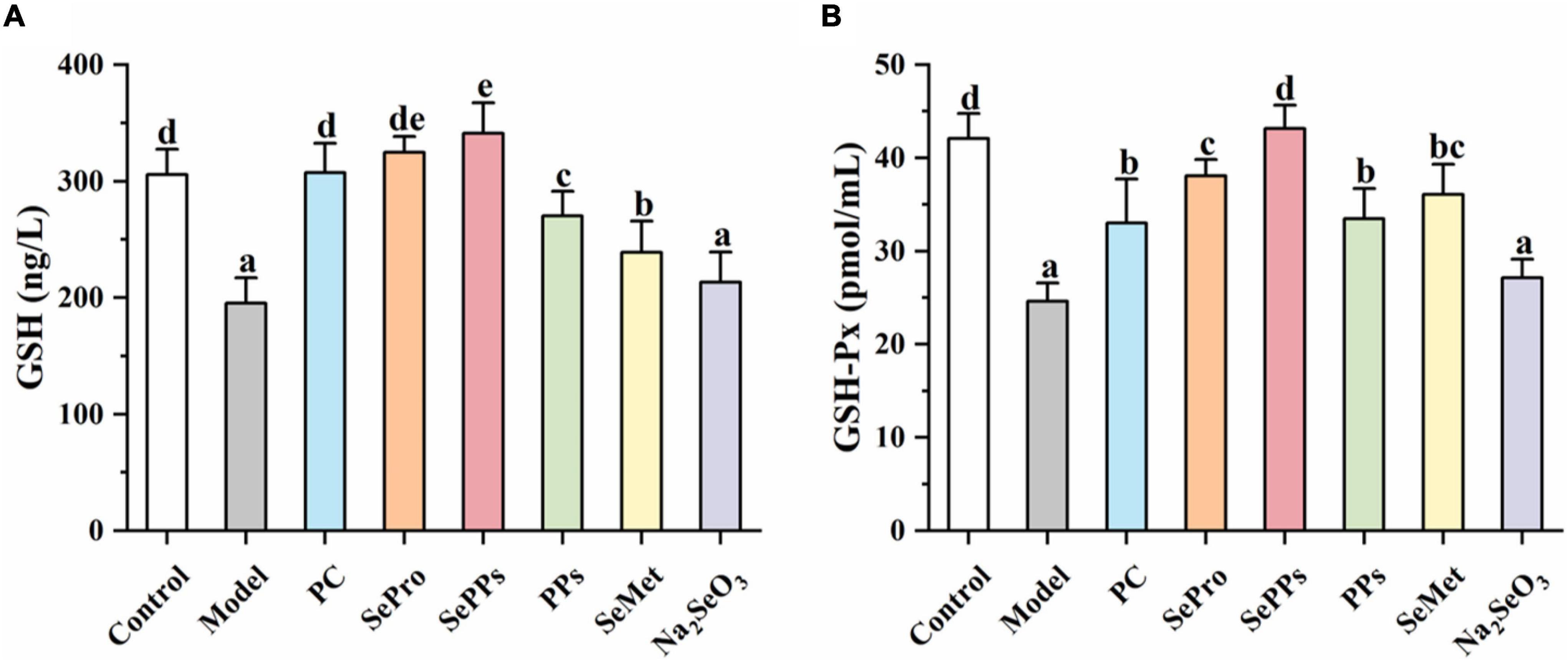
Figure 4. Effects of different selenium supplements on GSH (A) and GSH-Px (B) in rat liver. Values are expressed as the mean ± SD, results marked with the same letters were not significantly different (p > 0.05).
The results of histopathological analysis were presented in Figure 5. The overall structure of the liver tissue in the control group was normal, with a complete hepatocyte structure, and a clearly visible central vein, while the hepatic sinusoids were arranged radially along the central vein, as shown by the yellow arrows. The white arrows indicated the hepatic sinusoidal macrophages, and no obvious inflammatory cell infiltration was evident in the tissue. In contrast, the overall structure of the liver tissue in the model group was abnormal, with a loose hepatocyte structure, hepatocyte necrosis, and the disappearance of nucleus fixation and lysis, as shown by the blue arrows, while the black arrows indicated the presence of inflammatory cell infiltration in the tissue. The addition of SePro, SePPs, PPs, and SeMet improved liver tissue damage to varying degrees, while Na2SeO3 supplementation did not facilitate significant improvement. The overall structure of the liver tissue was mildly abnormal in the SePro, PPs, and SeMet groups, with visible inflammatory cell infiltration, as shown by the black arrows. Moreover, significantly dilated hepatic sinusoids were evident in the SePro and SeMet groups, as shown by the yellow arrows. Notably, the SePPs group displayed a complete hepatocyte structure, and a clearly visible central vein. The tissue did not show obvious sparing edema necrosis of the hepatocytes, and no noticeable inflammatory cell infiltration was apparent. Additionally, several new hepatocytes were visible, as shown by the green arrows in Figure 5E.
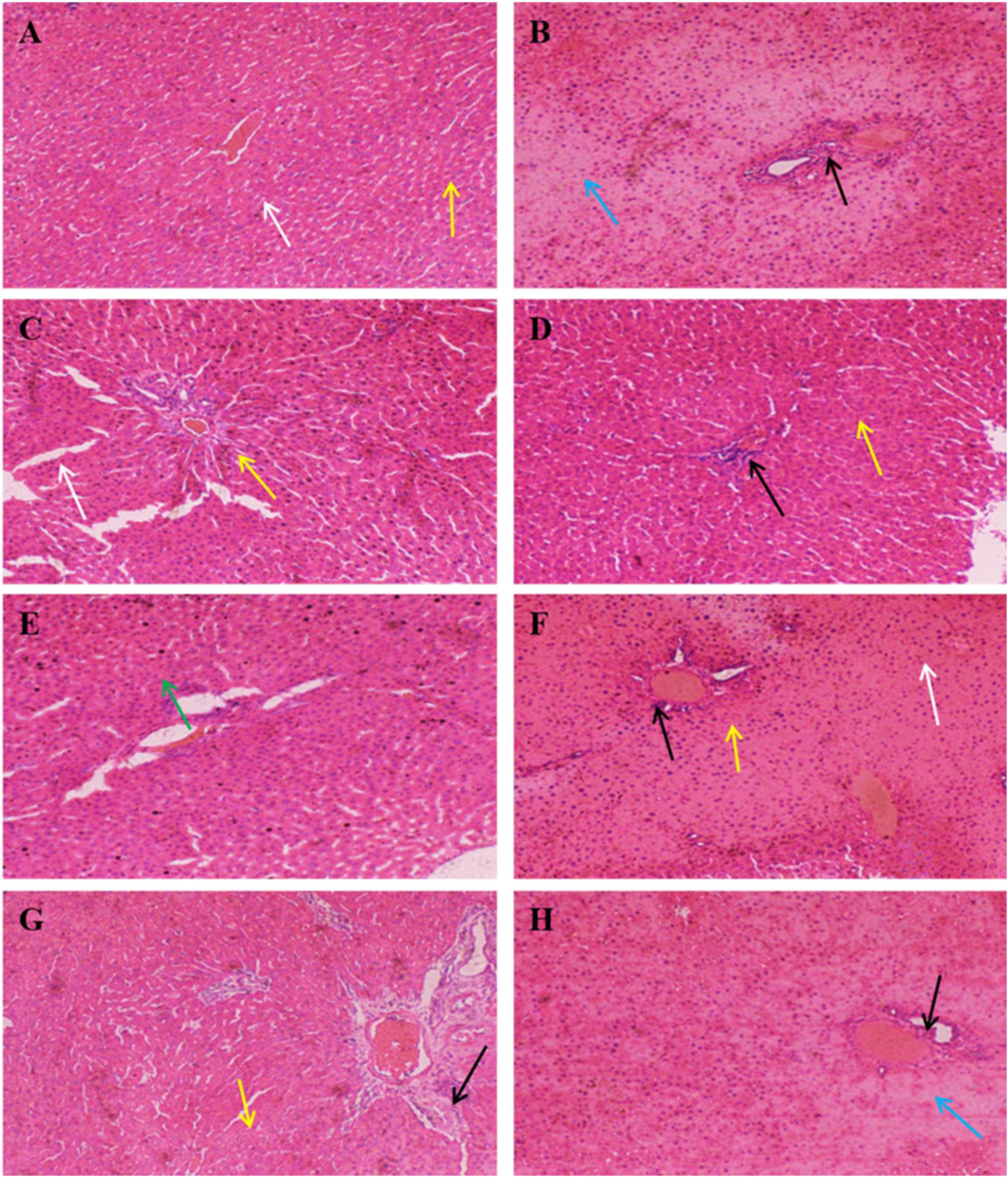
Figure 5. Histopathology of liver (H&E, × 100). Yellow arrows indicate hepatic sinusoids; blue arrows indicate necrosis of hepatocytes and disappearance of nuclei consolidation and lysis; white arrows indicate hepatic sinusoidal macrophages; black arrows indicate inflammatory cells; green arrows indicate neoplastic cells. (A) Control group, (B) Model group, (C) PC group, (D) SePro group, (E) SePPs group, (F) PPs group, (G) SeMet group, (H) Na2SeO3 group.
Various physiological responses, such as the inflammatory response, occur in vivo in heat stress conditions. IL-1β, IL-6, and TNF-α are regarded as important indicators during heatstroke studies. As shown in Figures 6A-C, the IL-1β, IL-6, and TNF-α levels in the livers of the rats in the model group were 1.5, 1.5, and 1.4 times of in the control group (p < 0.05) after exposure to heat stress and exercise, indicating a stronger inflammatory response in the liver tissue. Compared to the model group, IL-1β, IL-6, and TNF-α were substantially lower in the SePro, SePPs, PPs, SeMet, and Na2SeO3 groups (p < 0.05). It seemed that selenium and nutritional protein supplementation suppressed these substances after heat stress and exercise, while SePro, SePPs, and PPs were more effective than SeMet and Na2SeO3.
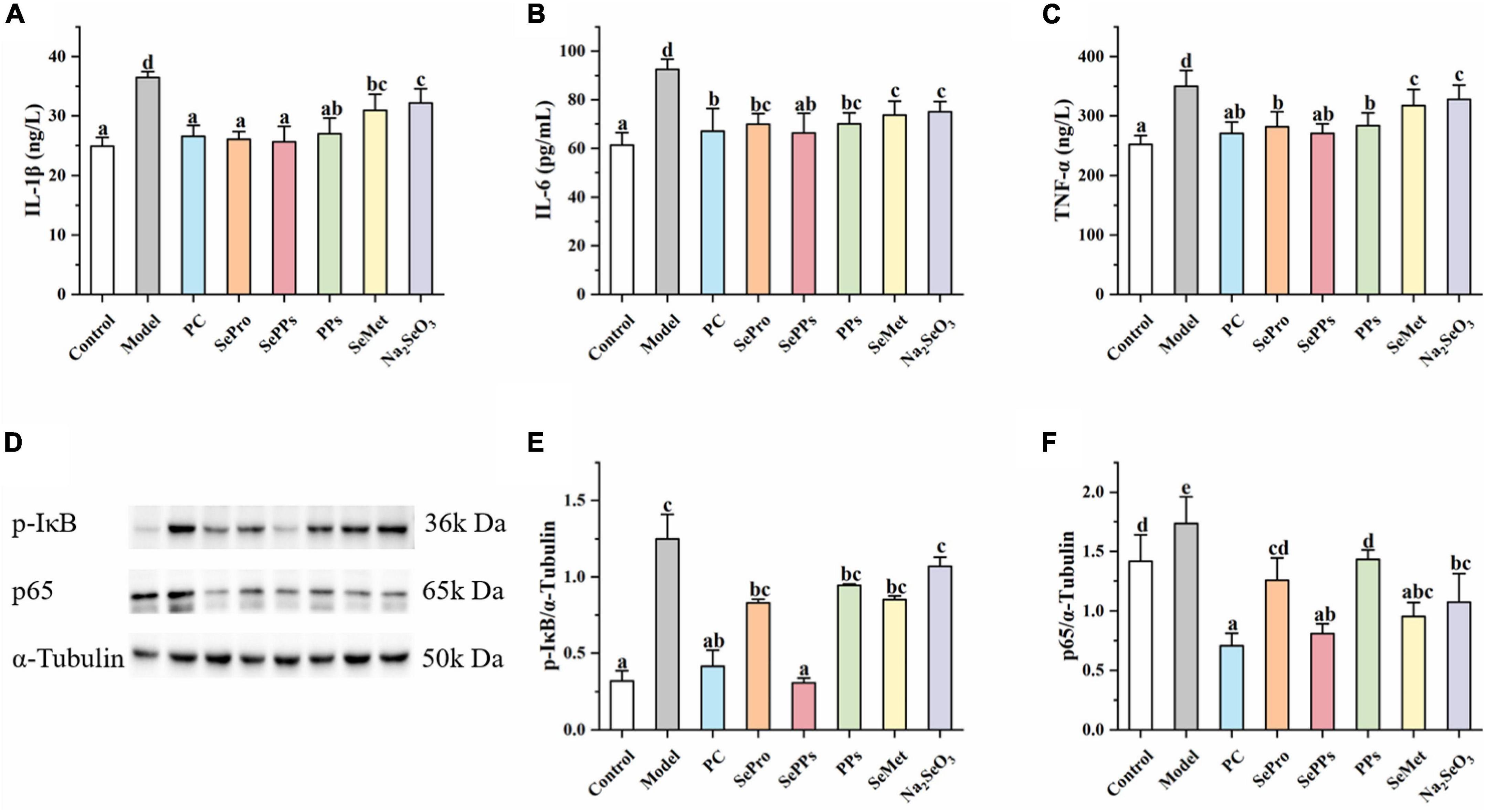
Figure 6. Effects of different selenium supplements on IL-1β (A), IL-6 (B), TNF-α (C), Western blot analysis (D), p-IκB (E), and p65 (F) in rat liver. Values are expressed as the mean ± SD, results marked with the same letters were not significantly different (p > 0.05).
The NF-κB p65 and p-IκB protein levels in the different treatment groups were measured to investigate the role of the NF-κB/IκB signaling pathway in heatstroke. As shown in Figures 6D-F, the protein expressions of p-IκB and NF-κB p65 were upregulated in the livers of rats after heat stress and exercise. The SePPs treatments significantly reduced p-IκB and NF-κB p65 protein expressions compared with the model group (p < 0.05). The protein expression of p-IκB in SePro, PPs, SeMet, and Na2SeO3 treatment groups were not significantly different from that of the model group (p > 0.05). The results indicated that supplementation with selenium and nutritional proteins could improve the inflammatory response by inhibiting the expressions of p-IκB and NF-κB p65 protein in vivo. SePPs were the most effective of the selenium supplements, variation was evident in the regulation of different transcription factors, while the regulation of p65 protein expression was superior to that of p-IκB.
The morbidity and mortality of sports diseases have attracted increasing attention in recent years. However, in the context of dramatic global warming, there is no specific and effective treatment strategy for heat stress and exercise fatigue-induced heatstroke. Therefore, it is essential to study on the prevention of heatstroke. The primary aim of this study was to investigate the protective effect and potential action mechanism of SePPs in heat stress and exercise fatigue-induced heatstroke rats. This study focuses on the liver since it is more sensitive to heat stress, while liver injury and dysfunction represent the most common and often fatal pathological changes in almost all cases of heat stress (16). Additionally, the liver is the central organ for selenium metabolism. Selenium ingested via food is absorbed in the intestine and transported to the liver for metabolism, from where it is transported and distributed to other tissues in the body (17). This study selected honeysuckle as a PC due to its antioxidant and anti-inflammatory effect. Using Freund’s adjuvant-induced arthritis rat model, Wu et al. found that honeysuckle extract inhibited the production of pro-inflammatory factors (TNF-α, IL-1β, IL-6, NO/iNOS, and cyclooxygenase-2 [COX-2]) and increased the activity of antioxidant enzymes (SOD, GSH-Px, and heme oxygenase-1 [HO-1]), attenuating arthritis symptoms (18). Honeysuckle also exhibits hepatoprotective effects (19).
Heatstroke causes oxidative damage, which is related to the pathophysiology of various disorders (20), including respiratory muscle dysfunction in heart failure (21) and skeletal muscle exhaustion in vitro (22). CAT, SOD, and GSH-Px are key components of the antioxidant enzyme system in living organisms and play a significant role in maintaining oxidative and antioxidant equilibrium in the body. GSH helps maintain normal immune system function, displays antioxidant effects, and integrates detoxification. MDA is the end product of lipid peroxidation and induces the cross-linking polymerization of proteins, nucleic acids, and other biological macromolecules. This study showed a decrease in antioxidant enzyme activity and an increase in lipid peroxidation after heat stress and exercise, consequently causing oxidative stress. Zhang et al. found that selenium-containing soybean antioxidant peptides inhibited D-galactose-induced liver injury and oxidative brain damage via the MAPK/NF-κB pathway, and played a key role in reducing organic oxidative damage during inflammation and aging. They compared the antioxidant activity of inorganic selenium, organic selenium, and selenium-free peptides and found that the effect of the selenium-enriched peptide treatment group was more significant (23). Consistent with these findings, this study revealed that PPs exhibited an ameliorative effect on the oxidative damage caused by heat stress and exercise fatigue. However, the effect of the SePPs treatment group was superior to PPs and other forms of selenium supplementation, indicating that both selenium and peptides play a vital role in antioxidant activity. Many studies have reported the antioxidant effect of selenium (24), while its bioavailability is essential for the strength of functional expression. We improved selenium bioavailability via selenium biofortification with soy protein, soy peptides, and methionine as carriers, respectively. Protein stability and transport pathways in vivo determine the true efficacy of functional applications. The oral bioavailability of such macromolecules is generally low due to poor membrane permeability. Proteins are typically denatured by pepsin and produce a mixture of peptides with molecular weights > 10 kDa. These gastric digestion products are further hydrolyzed by pancreatic enzymes, indicating that they exhibit low stability in the gastrointestinal tract and are susceptible to degradation by multiple digestive enzymes. Therefore, they are difficult to be absorbed by the human body completely (25). SeMet is absorbed through the intestinal methionine transporter after ingestion, following the active amino acid absorption pathway. SeMet and Met can share the same Na+-dependent, carrier-mediated transport mechanism (26), which is also an important way for multiple amino acids to be absorbed by the intestine. This may decrease SeMet absorption efficiency due to the structural similarity of individual amino acids and mutual competition. The higher bioavailability exhibited by SePPs may be due to their higher stability and membrane permeability, as well as their rapid in vivo transport and less saturable carriers. Furthermore, the results indicated that different forms of selenium exhibit varying bioavailability, while that of organic selenium was significantly higher than inorganic selenium. The body can absorb inorganic and organic selenium via the digestive tract, particularly the small intestine. However, a large amount of inorganic selenium is lost via urine as selenite and selenate before further metabolism, resulting in the bioavailability of selenium as selenite and selenate at levels lower than organic selenium (27). In line with previous findings, acute heat exposure increased lipid peroxidation and protein oxidation in rat plasma, ultimately resulting in oxidative damage to the body (28). As a metabolic crossroads, the liver is vulnerable to heat stress and exercise state, disturbing the metabolic equilibrium of the liver (29). Moreover, the liver is high in selenium, accounting for 29% of the total selenium in rats (27). It is the first organ encountered after selenium absorption in the small intestine and plays a crucial role in selenium metabolism (30). Consequently, more research regarding heat stress and exercise fatigue-induced liver injury was conducted.
The liver secretes several endogenous and exogenous compounds during detoxification. ALT and AST are hepatic enzymes, the levels of which can be used as key indicators of liver function. Our results showed that heatstroke compromised the integrity of hepatocyte membranes, allowing aminotransferases to escape into the serum from the cytoplasm. Generally, the increase in the degree of ALT and AST in serum reflects the degree of hepatocyte injury. ALT is mainly present in the cytoplasm of hepatocytes, while AST is primarily distributed in the cytoplasm and mitochondria of hepatocytes. Elevated ALT or AST level in the blood suggested hepatocyte membrane injury and high membrane permeability or organelle damage, respectively (31). Guo et al. found that selenium biofortified maize peptide exerted a substantial ameliorative impact on the high ALT and AST caused by concanavalin A (32). Similarly, the present study revealed that advanced supplementation with different forms of selenium reduced liver damages induced by heat stress and exercise fatigue and PPs displayed hepatoprotective benefits.
As protein degradation products, peptides play an essential role in the development of various liver disorders. In the investigation of Lin et al., marine collagen peptides inhibited early alcoholic liver injury in female rats by improving oxidative stress and lipid metabolism (33). Lv et al. observed that maize peptides significantly reduced MDA, NO, hydroxyproline (HYP), transforming growth factor-β1 (TGF-β1) levels and lactate dehydrogenase activity in the liver and substantially increased the SOD level. The action mechanism of maize peptides might be related to their antioxidant activity in hepatocytes and TGF-β1 secretion inhibition (34). Recently, SePPs were found to exhibit better immunomodulatory effects than PPs in a mouse model involving cyclophosphamide-induced immunosuppression (13). In our study, SePPs had a more significant impact on the GSH system than PPs. The serum indicator results demonstrated that SePPs were most effective in regulating GSH and GSH-Px. Consequently, the GSH and GSH-Px levels in the liver were investigated further, showing that the GSH system was altered after heat stress and exercise fatigue. Using a broiler model, Yang et al. found that acute heat exposure could inhibit the mitochondrial respiratory chain activity, resulting in excessive ROS production and eventually oxidative stress (35). Excessive ROS production overwhelms cellular antioxidant defenses, causing damage to various cellular components (36). The liver is responsible for neutralizing excess ROS by disrupting the mitochondrial membrane proton gradient, causing oxidative damage by breaking the loops of enzymes, cellular lipids, and mitochondrial membranes in the liver. Furthermore, ROS can also act as signaling molecules, affecting the expression of related genes, requiring regulation by the antioxidant system (37–39), which includes GSH and GSH-Px. Similar to the results obtained by Liu et al. (12), the GSH content and GSH-Px activity in the serum and liver of the rats in the SePPs group were higher than those in both PPs group and control group, which may be attributed to the unique relationship between selenium and the GSH system. The increased SePPs on GSH-Px activity may be related to the presence of SeCys peptides in SePPs. Since SeCys represents the active GSH-Px center (40), SePPs can be used as a synthetic GSH-Px and increase its activity, converting more GSH to oxidized GSH (GSSG). Then, catalyzed by GSH reductase, GSSG can be reduced to GSH again by nicotinamide adenine dinucleotide phosphate (NADPH) (41). Throughout the redox cycle, higher GSH-Px activity stimulated the involvement of GSH in the reaction, resulting in higher GSH levels in the SePPs-treated group. This indicated that SePPs more successfully regulated the GSH system in the liver than PPs, reducing oxidative damage. In addition, the GSH content affected the TNF-α sensitivity to hepatocyte destruction. The cell death process includes the mitochondrial release of cytochrome C, while a decrease in the cytoplasmic GSH content is necessary for cytochrome C to promote apoptosis (42). According to Matsumaru et al., GSH depletion in the body enhanced TNF-α sensitivity to induce apoptosis in primary murine hepatocytes (43). The histological results of the current research showed higher hepatocyte death after heatstroke, which was alleviated to varying degrees by SePro, SePPs, PPs, and SeMet supplementation. This may be related to GSH content changes in the liver.
The inflammatory response is another significant mechanism of heat stress and exercise fatigue-induced heatstroke (44). Selenium has been demonstrated to be able to attenuate the inflammatory response (45). In this study, SePPs inhibited the release of inflammatory factors (IL-1β, IL-6, and TNF-α) in the liver. The p-IκB and p65 protein expressions in the NF-κB/IκB signaling pathway were measured in the liver tissue to investigate the action mechanism of SePPs in suppressing the inflammatory response (see Figure 7). NF-κB is a nuclear transcription factor that also functions as an inflammatory mediator. The p50/p65 heterodimer is the common form of NF-κB present in hepatocytes and is inactivated by the binding of IκB to subunit p50/p65 in the cytoplasm (46). Xie et al. found that the NF-κB/IκB pathway plays a vital role in heat stress-induced ROS generation and cytotoxicity in the microvascular endothelial cells in rat lungs (47). In accordance to their results, heatstroke affected the NF-κB/IκB pathway and produced phosphorylated IκB, which was released from subunit p50/p65. It was ubiquitinated, converting subunit p50/p65 from an inhibited to an activated state, while activating the transcription of the gene-encoding proteins involved in the cellular response to stress factors (48). NF-κB controls pro-inflammatory cytokines and chemokines by increasing cell proliferation and stimulating angiogenesis. Consequently, limiting NF-κB activation potentially blocks cytokine expression, preventing the inflammatory response. Ge et al. reported that selenium supplementation attenuated Cd accumulation in the heart and reduced cardiac injury by affecting the NF-κB/IκB signaling pathway (49). In this investigation, supplementation with different forms of selenium and PPs before heat stress and exercise inhibited the p-IκB and p65 protein expression. Comparative analysis of the protective effects showed that SePPs inhibited the inflammatory response more effectively than other selenium supplements, indicating that selenium exerts an anti-inflammatory impact, while selenium and peptide binding is more easily absorbed and utilized by the body. SePPs could reduce inflammation by inhibiting the expressions of p-IκB and p65 proteins in the NF-κB signaling pathway. The DNA-binding activity of NF-κB can be regulated by redox, while the oxidation of its cysteine residues is necessary for the initiation of downstream effector proteins (50). The selenium and GSH Sytems display a unique relationship, rendering the liver GSH and GSH-Px significantly higher in the SePPs group than in the other groups. GSH can be engaged in various cellular reactions and can directly or indirectly modify the redox state of the liver via enzymatic reactions to scavenge free radicals and other reactive oxygen species (51). Furthermore, GSH also functions as a modifier of redox-sensitive proteins, especially since the protein contains cysteine and has a low pKa value. GSH can form mixed disulfides with cysteine in target proteins, a process known as glutathionylation (52). It is considered as a redox-dependent modification with potential relevance to signal transduction, metabolism, inflammation, and apoptosis, suggesting that GSH is an essential antioxidant and a crucial signaling molecule. Zhou et al. showed that GSH supplementation inhibited FKB-induced hepatocyte death via NF-κB and MAPK signaling (53). Proteomic studies have identified many proteins regulated by glutathionylation, including actin, Protein Kinase (PK) A, PKC, and NF-κB (54). NF-κB includes various subunits, of which p50 and p65 are the most abundant. The cysteine residue in the DNA-binding domain of the NF-κB p50 subunit can be glutathione, resulting in activity loss (55). The p65 subunit can also be modified by glutathionylation, causing a loss in activity (56). Since only p65 NF-κB is responsible for transcriptional activation (57), the effect of glutathionylation of the p65 subunit should have a stronger impact on NF-κB activation than that of the p50 subunit. These findings suggest that internal cysteine glutathionylation may be an important mechanism for protein expression regulation. Therefore, the reduced p65 protein level may also be due to the elevated GSH level leading to p65 glutathionylation. This protects sensitive cysteine residues from oxidation, restores protein function in oxidative stress conditions, and inhibits NF-κB activation, consequently reducing the degree of inflammation.
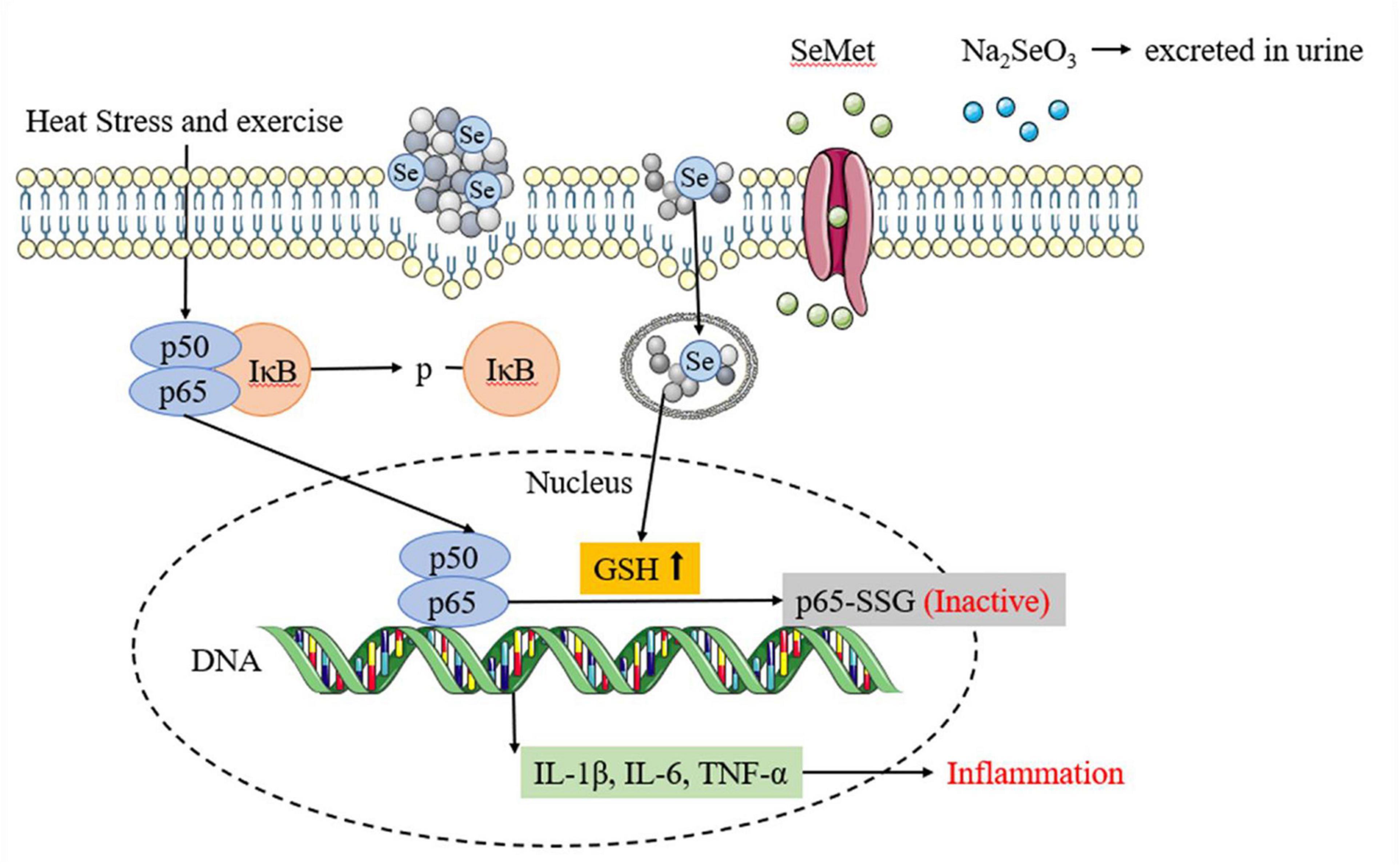
Figure 7. Different selenium supplements inhibits inflammatory response by affecting NF-κB/IκB signaling pathway.
This study provides in vivo evidence that SePPs protect rats from heat stress and exercise fatigue-induced heat stroke. SePPs improve the antioxidant capacity and inflammatory response of the liver in rats caused by heatstroke and reduce liver damage by inhibiting p-IκB and p65 protein expressions in the NF-κB/IκB pathway. It suggests that NF-κB/IκB may serve as a target for heatstroke treatment. In terms of boosting the GSH system, SePPs have a more significant impact than PPs. A comparison between the different selenium supplements revealed that combining selenium and peptides was more successful in vivo, suggesting that SePPs can be used as a preferential selenium resource. Alternatively, our study illustrates the potential of SePPs to prevent heatstroke and mitigate sports injury. Future research can focus on the structural interactions and metabolic pathways of SePPs to thoroughly understand their mode of action.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee at the Pony Testing International Group Co., Ltd. (PONY-2021-FL-15).
XC designed the research. XC and WL carried out experiments. XC and JZ analyzed the data. XC and HL wrote the initial manuscript, with contributions from YX and XL. All authors contributed to the article and approved the submitted version.
The National Key Research and Development Program of China, Grant/Award number: 2021YFD2100400.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Melo-Marins D, Farinha JB, Rodrigues-Krause J, Laitano O, Reischak-Oliveira A. Redox balance during exercise in the heat in healthy adults: a systematic review. J Therm Biol. (2021) 99:102943. doi: 10.1016/j.jtherbio.2021.102943
3. Laitano O, Leon LR, Roberts WO, Sawka MN. Controversies in exertional heat stroke diagnosis, prevention, and treatment. J Appl Physiol. (2019) 127:1338–48. doi: 10.1152/japplphysiol.00452.2019
4. Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. (2000) 28:754–66. doi: 10.1016/S0891-5849(00)00161-1
5. Zhang Y, Liu Q, Yin H, Min Y, Li S. Selenium deficiency causes immune damage by activating the DUSP1/NF-κB pathway and endoplasmic reticulum stress in chicken spleen. Food Funct. (2020) 11:6467–75. doi: 10.1039/D0FO00394H
6. Zhang X, He H, Xiang J, Yin H, Hou T. Selenium-containing proteins/peptides from plants: a review on the structures and functions. J Agric Food Chem. (2020) 68:15061–73. doi: 10.1021/acs.jafc.0c05594
7. Filippini T, Michalke B, Wise LA, Malagoli C, Malavolti M, Vescovi L, et al. Diet composition and serum levels of selenium species: a cross-sectional study. Food Chem Toxicol. (2018) 115:482–90. doi: 10.1016/j.fct.2018.03.048
8. Zhang J, Zhou H, Li H, Ying Z, Liu X. Research progress on separation of selenoproteins/Se-enriched peptides and their physiological activities. Food Funct. (2021) 12:1390–401. doi: 10.1039/d0fo02236e
9. Singh BP, Vij S, Hati S. Functional significance of bioactive peptides derived from soybean. Peptides. (2014) 54:171–9. doi: 10.1016/j.peptides.2014.01.022
10. Chan Q, Afton SE, Caruso JA. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics. (2010) 2:147–53. doi: 10.1039/b916194e
11. Sathe SK, Mason AC, Rodibaugh R, Weaver CM. Chemical form of selenium in soybean (Glycine max L.) lectin. J Agric Food Chem. (1992) 40:2084–91. doi: 10.1021/jf00023a010
12. Liu W, Hou T, Shi W, Guo D, He H. Hepatoprotective effects of selenium-biofortified soybean peptides on liver fibrosis induced by tetrachloromethane. J Funct Food. (2018) 50:183–91. doi: 10.1016/j.jff.2018.09.034
13. Zhang J, Gao S, Li H, Cao M, Li W, Liu X. Immunomodulatory effects of selenium-enriched peptides from soybean in cyclophosphamide-induced immunosuppressed mice. Food Sci Nutr. (2021) 9:6322–34. doi: 10.1002/fsn3.2594
14. Gao S, Zhang J, Zhang Q, Li W, Li H, Yu T, et al. Preparation and in vivo absorption characteristics of selenium-enriched soybean peptides. Food Sci. (2021) 42:165–72. doi: 10.7506/spkx1002-6630-20200102-014
15. Wang M, Srinivasan M, Dasari S, Narvekar P, Samy ALPA, Dontaraju VS, et al. Antioxidant activity of Yichun Blue Honeysuckle (YBHS) berry counteracts CCl4-induced toxicity in liver injury model of mice. Antioxidants. (2017) 6:50. doi: 10.3390/antiox6030050
16. Geng Y, Ma Q, Liu Y, Peng N, Yuan F, Li X, et al. Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. J Hepatol. (2015) 63:622–33. doi: 10.1016/j.jhep.2015.04.010
17. Ha HY, Alfulaij N, Berry MJ, Seale LA. From selenium absorption to selenoprotein degradation. Biol Trace Elem Res. (2019) 192:26–37. doi: 10.1007/s12011-019-01771-x
18. Wu S, He X, Wu X, Qin S, He J, Zhang S, et al. Inhibitory effects of blue honeysuckle (Lonicera caerulea L) on adjuvant-induced arthritis in rats: crosstalk of anti-inflammatory and antioxidant effects. J Funct Food. (2015) 17:514–23. doi: 10.1016/j.jff.2015.06.007
19. Lee Y, Cho IJ, Kim JW, Lee M, Ku SK, Choi J, et al. Hepatoprotective effects of blue honeysuckle on CCl4-induced acute liver damaged mice. Food Sci Nutr. (2019) 7:322–38. doi: 10.1002/fsn3.893
20. Yu J, Liu F, Yin P, Zhao H, Luan W, Hou X, et al. Involvement of oxidative stress and mitogen-activated protein kinase signaling pathways in heat stress-induced injury in the rat small intestine. Stress. (2013) 16:99–113. doi: 10.3109/10253890.2012.680526
21. Souza-Silva AA, Moreira E, Melo-Marins D, Scholer CM, Bittencourt PIHJ, Laitano O. High intensity interval training in the heat enhances exercise-induced lipid peroxidation, but prevents protein oxidation in physically active men. Temperature. (2016) 3:167–75. doi: 10.1080/23328940.2015.1132101
22. Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. (1992) 73:1797–804. doi: 10.1152/jappl.1992.73.5.1797
23. Zhang X, He H, Xiang J, Li B, Zhao M, Hou T. Selenium-containing soybean antioxidant peptides: preparation and comprehensive comparison of different selenium supplements. Food Chem. (2021) 358:129888. doi: 10.1016/j.foodchem.2021.129888
24. Ahmadvand H, Babaeenezhad E, Nayeri H, Nezhad ZZ. Selenium effects on antioxidant and inflammatory indices in renal ischemia-reperfusion injury in rats. J Renal Inj Prev. (2019) 8:71–7. doi: 10.15171/jrip.2019.14
25. Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev. (1989) 4:171–207. doi: 10.1016/0169-409X(89)90018-5
26. Wolffram S, Berger B, Grenacher B, Scharrer E. Transport of selenoamino acids and their sulfur analogues across the intestinal brush border membrane of pigs. J Nutr. (1989) 119:706–12. doi: 10.1093/jn/119.5.706
27. Burk RF, Hill KE. Regulation of selenium metabolism and transport. Annu Rev Nutr. (2015) 35:109–34. doi: 10.1146/annurev-nutr-071714-034250
28. Mladenov M, Gjorgoski I, Stafilov T, Duridanova D. Effect of vitamin C on lipid hydroperoxides and carbonyl groups content of rat plasma depending on age and acute heat exposure. J Therm Biol. (2006) 31:588–93. doi: 10.1016/j.jtherbio.2006.08.001
29. Das A. Heat stress-induced hepatotoxicity and its prevention by resveratrol in rats. Toxicol Mech Methods. (2011) 21:393–9. doi: 10.3109/15376516.2010.550016
30. Kato T, Read R, Rozga J, Burk RF. Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am J Physiol-Gastroint Liver Physiol. (1992) 262:G854–8. doi: 10.1152/ajpgi.1992.262.5.G854
31. Li C, Lan M, Lv J, Zhang Y, Gao X, Gao X, et al. Screening of the hepatotoxic components in Fructus Gardeniae and their effects on rat liver BRL-3A Cells. Molecules. (2019) 24:3920. doi: 10.3390/molecules24213920
32. Guo D, Zhang Y, Zhao J, He H, Hou T. Selenium-biofortified corn peptides: attenuating concanavalin A-induced liver injury and structure characterization. J Trace Elem Med Biol. (2019) 51:57–64. doi: 10.1016/j.jtemb.2018.09.010
33. Lin B, Zhang F, Yu Y, Jiang Q, Zhang Z, Wang J, et al. Marine collagen peptides protect against early alcoholic liver injury in rats. Br J Nutr. (2012) 107:1160–6. doi: 10.1017/S0007114511004211
34. Lv J, Nie Z, Zhang J, Liu F, Wang Z, Ma Z, et al. Corn peptides protect against thioacetamide-induced hepatic fibrosis in rats. J Med Food. (2013) 16:912–9.
35. Yang L, Tan G, Fu Y, Feng J, Zhang M. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C-Toxicol Pharmacol. (2010) 151:204–8.
36. Powers SK, Deruisseau KC, Quindry J, Hamilton KL. Dietary antioxidants and exercise. J Sports Sci. (2004) 22:81–94. doi: 10.1080/0264041031000140563
37. Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. (2019) 8:235. doi: 10.3390/antiox8070235
38. Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. (2018) 80:50–64. doi: 10.1016/j.semcdb.2017.05.023
39. Santoro MM. Fashioning blood vessels by ROS signalling and metabolism. Semin Cell Dev Biol. (2018) 80:35–42. doi: 10.1016/j.semcdb.2017.08.002
40. Cone JE, Del RRM, Davis JN, Stadtman TC. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. (1976) 73:2659–63. doi: 10.1073/pnas.73.8.2659
41. Bhabak KP, Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Accounts Chem Res. (2010) 43:1408–19. doi: 10.1021/ar100059g
42. Brown GC, Borutaite V. Regulation of apoptosis by the redox state of cytochrome c. Biochim Biophys Acta-Bioenerg. (2008) 1777:877–81. doi: 10.1016/j.bbabio.2008.03.024
43. Matsumaru K, Ji C, Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. (2003) 37:1425–34. doi: 10.1053/jhep.2003.50230
44. He S, Hou X, Xu X, Wan C, Yin P, Liu X, et al. Quantitative proteomic analysis reveals heat stress-induced injury in rat small intestine via activation of the MAPK and NF-κB signaling pathways. Mol Biosyst. (2015) 11:826–34. doi: 10.1039/C4MB00495G
45. Owumi SE, Dim UJ. Biochemical alterations in diclofenac-treated rats: effect of selenium on oxidative stress, inflammation, and hematological changes. Toxicol Res Appl. (2019) 3:2397847319874359. doi: 10.1177/2397847319874359
46. Liu T, Zhang L, Joo D, Sun S. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:1–9. doi: 10.1038/sigtrans.2017.23
47. Xie W, Huang W, Cai S, Chen H, Fu W, Chen Z, et al. NF−κB/IκBα signaling pathways are essential for resistance to heat stress-induced ROS production in pulmonary microvascular endothelial cells. Mol Med Rep. (2021) 24:1–8. doi: 10.3892/mmr.2021.12454
48. Oeckinghaus A, Ghosh S. The NF- κB family of transcription factors and its regulation. Cold Spring Harbor Perspect Biol. (2009) 1:a000034. doi: 10.1101/cshperspect.a000034
49. Ge J, Guo K, Zhang C, Talukder M, Lv M, Li J, et al. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci Total Environ. (2021) 773:145442. doi: 10.1016/j.scitotenv.2021.145442
50. Nishi T, Shimizu N, Hiramoto M, Kataoka K, Watanabe H, Handa H, et al. Spatial redox regulation of a critical cysteine residue of NF-kappa B in vivo. J Biol Chem. (2002) 277:44548–56. doi: 10.1074/jbc.M202970200
51. Fang Y, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. (2002) 18:872–9. doi: 10.1016/s0899-9007(02)00916-4
52. Zhang J, Ye Z, Singh S, Townsend DM, Tew KD. An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic Biol Med. (2018) 120:204–16. doi: 10.1016/j.freeradbiomed.2018.03.038
53. Zhou P, Gross S, Liu J, Yu B, Feng L, Nolta J, et al. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF−κB and MAPK signaling pathways. FASEB J. (2010) 24:4722–32. doi: 10.1096/fj.10.163311
54. Fratelli M, Gianazza E, Ghezzi P. Redox proteomics: identification and functional role of glutathionylated proteins. Expert Rev Proteomics. (2004) 1:365–76. doi: 10.1586/14789450.1.3.365
55. Brar SS, Grigg C, Wilson KS, Holder WD Jr., Dreau D, Austin C, et al. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther. (2004) 3:1049–60. doi: 10.1016/j.lungcan.2004.03.001
56. Qanungo S, Starke D, Pai H, Mieyal J, Nieminen A. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. (2007) 282:18427–36. doi: 10.1074/jbc.M610934200
Keywords: selenium-enriched soybean peptides, heat stress, exercise fatigue, heatstroke, oxidative damage, inflammation
Citation: Chen X, Zhang J, Li H, Liu W, Xi Y and Liu X (2022) A Comprehensive Comparison of Different Selenium Supplements: Mitigation of Heat Stress and Exercise Fatigue-Induced Liver Injury. Front. Nutr. 9:917349. doi: 10.3389/fnut.2022.917349
Received: 11 April 2022; Accepted: 28 April 2022;
Published: 12 May 2022.
Edited by:
Tao Hou, Huazhong Agricultural University, ChinaReviewed by:
Dai Cheng, Tianjin University of Science and Technology, ChinaCopyright © 2022 Chen, Zhang, Li, Liu, Xi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Li, bGloZUBidGJ1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.