- 1Department of Food and Nutrition, Inha University, Incheon, South Korea
- 2Department of Home Economics Education, Korea National University of Education, Cheongju-si, South Korea
Epidemiological studies have demonstrated the inverse association between the intake of fruits and vegetables and inflammation. However, the mechanisms by which inflammation-related genes interact with fruit and vegetable intake and the role of these combinations in inflammation remain unclear. Therefore, we assessed the effect of interactions between fruit and vegetable intake and the hepatic nuclear factor 1 alpha (HNF1A) genetic variants on the C-reactive protein (CRP) levels. Baseline data from the Ansan and Ansung Cohort Study of the Korean Genome and Epidemiology Study (KoGES) were used. A total of 7,634 participants (3,700 men and 3,934 women) were included in the analyses. Fruit and vegetable intake was assessed using semi-quantitative food frequency questionnaire data. Genotyping information for HNF1A was extracted from the Affymetrix Genome-Wide Human SNP array 5.0. Inflammation was determined after overnight fasting by measuring CRP levels using automated analyzers. Multivariable logistic regression was used to estimate the adjusted odds ratio (AOR) with a 95% confidence interval (CI). In the fully adjusted model, men and women with the GG genotype of HNF1A rs2393791 and high fruit intake had lower odds of elevated CRP levels compared to those with the AA genotype and low fruit intake (AOR 0.50, 95% CI 0.38–0.67; AOR 0.73, 95% CI 0.55–0.97, respectively). Men and women with the rs2393791 GG genotype and high vegetable intake had lower odds of having elevated CRP levels compared to those with the AA genotype and low fruit intake (AOR 0.57, 95% CI 0.43–0.75; AOR 0.65, 95% CI 0.49–0.86, respectively). Men and women with the GG genotype and high total fruit and vegetable intake had lower odds of having elevated CRP levels. These findings indicate that fruit and vegetable intake interacts with HNF1A genetic polymorphisms, consequently influencing the inflammation levels.
Introduction
A growing body of evidence indicates that inflammation plays a crucial role in the pathogenesis of several diseases, such as cardiovascular diseases (1), metabolic diseases (2), type 2 diabetes (3), and cancer (4). Inflammation is closely associated with dietary factors (5–7). Specifically, antioxidants, such as β-carotene, vitamin C, fiber, and other phytochemicals abundant in fruits and vegetables, have been inversely associated with inflammation markers, such as C-reactive protein (CRP) (8–10). In Korea, the current recommended intake of fruits and vegetables is more than 500 grams per day (11), underscoring the beneficial effects of consuming fruits and vegetables to optimize nutritional intake and offer various health benefits, such as reducing disease risk and maximizing positive health outcomes (12). Fruit and vegetable intake reportedly reduced inflammation in healthy, well-nourished, non-smoking German men in a randomized controlled trial, which indicated that those who consumed eight servings of fruits and vegetables a day had significantly lower plasma CRP levels within 4 weeks compared to those who only consumed two servings a day (13).
Strong evidence has indicated that genetic factors play a vital role in the onset of inflammation. Hepatic nuclear factor 1 alpha (HNF1A) encodes the transcription factor HNF-1 α, an important transcriptional regulator of various hepatic genes (14). HNF-1α binding sites exist in the CRP promoter region (15), and this illustrates the role of HNF1A expression in regulating CRP expression (16). Genetic polymorphisms in the HNF1A gene are closely associated with CRP. Carriers of rs2259816 A allele in HNF1A have been associated with approximately 15% decreased CRP levels in the German population (17). In healthy Filipino women, carriers of the rs7305618 T allele in HNF1A were positively associated with plasma CRP levels (β = 0.288, P = 1.0 × 10−8) (18).
Although the relationship between fruit and vegetable intake and inflammation has been investigated (13), the mechanisms by which inflammation-related genes interact with fruit and vegetable intake and the role of these combinations in inflammation remain unclear. Therefore, gene–diet interactions must be investigated to understand the underlying pathophysiology of the disease, which could potentially be useful in inflammation-based assessment for the risk stratification. Therefore, we assessed the effects of fruit and vegetable intake and HNF1A genetic variants on inflammation.
Methods
Data Set
Study Design and Participants
The data set from the Ansan-Ansung Cohort Study of the Korean Genome and Epidemiology Study (KoGES), an ongoing large-scale prospective study conducted by the Korea National Institute of Health, was used for this study. The Ansan-Ansung study began in 2001–2002 (baseline) to investigate genetic and environmental risk factors, including dietary factors, that affect chronic diseases in the Korean population (19). Initially, 10,030 adults (40–69 years old) residing in Ansan (urban) and Ansung (rural) cities were included in the study. The participants were followed up biannually, and their follow-up data collected up until 2012 was included.
From the 10,030 participants at baseline examination, we excluded those without genotyping data (n = 1,633), who had a diagnosis of myocardial infarction, coronary artery or congestive heart disease, stroke, or cancer (n = 350), no dietary information or energy intake <500 kcal/day or >5,000 kcal/day (n = 342), and no information on BMI or lifestyle variables (n = 71). The final analytical dataset comprised 7,634 individuals (3,700 men and 3,934 women). The protocol was reviewed and approved by the Institutional Review Board (IRB) of Inha University, Korea, on February 18, 2022 (IRB no. 220215-1A).
Dietary Assessment
Dietary data were collected using a 103-item semi-quantitative food frequency questionnaire (FFQ) by well-trained interviewers during the baseline examination. To assess typical dietary intake, the validated FFQ was developed and utilized for Korean adults in the KoGES. Fruit intake included the consumption of the 12 fruit items: persimmon/dried persimmon, tangerine, melon/oriental melon, banana, pear, apple/apple juice, orange/orange juice, watermelon, peach/prune, strawberry, grape/grape juice, and tomato/tomato juice. Vegetable intake comprised 23 items: Korean cabbage, radish/salted radish, watery kimchi made of sliced radishes/radish water kimchi, other kimchi (such as green onion, leaf mustard, and Korean lettuce kimchi), green pepper, hot pepper leaves, spinach, lettuce, perilla leaves, chives/water parsley, other green vegetables (such as horseradish, crown daisy, mallow, aster, and turnip green), radish (soup or braised), pickled radish, bellflower/deodeok, onion, cabbage/cabbage soup, cucumber, bean sprouts or mungbean sprouts, carrot, carrot juice, pumpkin (soup or juice), zucchini, vegetable juice, bracken/sweet potato leaves, and pickled vegetables (20). Nine possible responses regarding the intake were provided, ranging from never or seldom to ≥ 3 times per day. For the portion size, three options for each food (0.5 serving, 1 serving (standard), and ≥2 servings) were provided. One serving of fruit or vegetable corresponded to 100 g or 70 g, respectively. To calculate the usual intake of fruits and vegetables, the consumption frequency for each fruit and vegetable food item was multiplied by the nutrient content of its corresponding food item based on a nutrient database (CAN-Pro 2.0) developed by the Korean Nutrition Society (21). The separate intake of fruits and vegetables and the total intake of fruits and vegetables were considered for this study.
Genotyping
Genomic DNA samples were isolated from whole blood and genotyped using an Affymetrix Genome-Wide Human single nucleotide polymorphism (SNP) array 5.0 (Affymetrix, Inc., Santa Clara, CA, USA). A total of 10,004 KARE study samples were genotyped using this platform. Single nucleotide polymorphisms (SNPs) are the most common type of genetic variations between alleles (22). The quality control procedures were as follows: samples were excluded if they had low genotyping calls (<96%), high heterozygosity, sex inconsistencies, cryptic relatedness, or serious concomitant illness. Markers with high missing gene call rates (>5%), low minor allele frequency (< 0.01), or significant deviation from the Hardy–Weinberg equilibrium (P <1 × 10−6) were excluded (23). Imputation analysis was performed using IMPUTE program for the Asian HapMap (JPT + CHB) population (release 22/NCBI, build 36, and dbSNP build 126) as a reference panel (23). Three SNPs (rs2393791, rs11065386, and rs2259816) in HNF1A were identified, and rs2393791 was chosen for the final analysis based on the results of the tag SNP section (Figure 1).
Measurement of Inflammation
After overnight fasting, CRP levels were measured using an automated analyzer (CRP: Hitachi Automatic Analyzer 7600, Hitachi, Nittobo, Japan). CRP levels >0.2 mg/dL were considered as elevated (24).
Statistical Analyses
Descriptive statistics were used to calculate the mean, standard deviations, and frequencies based on CRP status. To assess the relationship between CRP status and demographics, alcohol intake, metabolic equivalent of task (MET) (hours/week), BMI, and fruit and vegetable intake were measured. MET was defined as the amount of oxygen consumed in the resting state and was equal to 3.5 mL O2 per kg body weight × min (25).
Multivariable linear regression analyses were performed to determine the association between rs237391 genotype, fruit intake, and vegetable intake with log-transformed CRP after controlling for covariates. Multivariable logistic regression analyses were performed to assess the association of intake of fruits and vegetables and rs2393791 genotypes (AA, GA, GG) with elevated CRP (>0.2 mg/dL) after controlling for covariates. Men and women were divided into groups based on their median intake of fruit (131.3 g and 175.6 g, respectively), vegetable (78 g and 78.4 g, respectively), or total fruit and vegetable (224.1 g and 271.5 g, respectively).
Results
General Characteristics of Study Participants
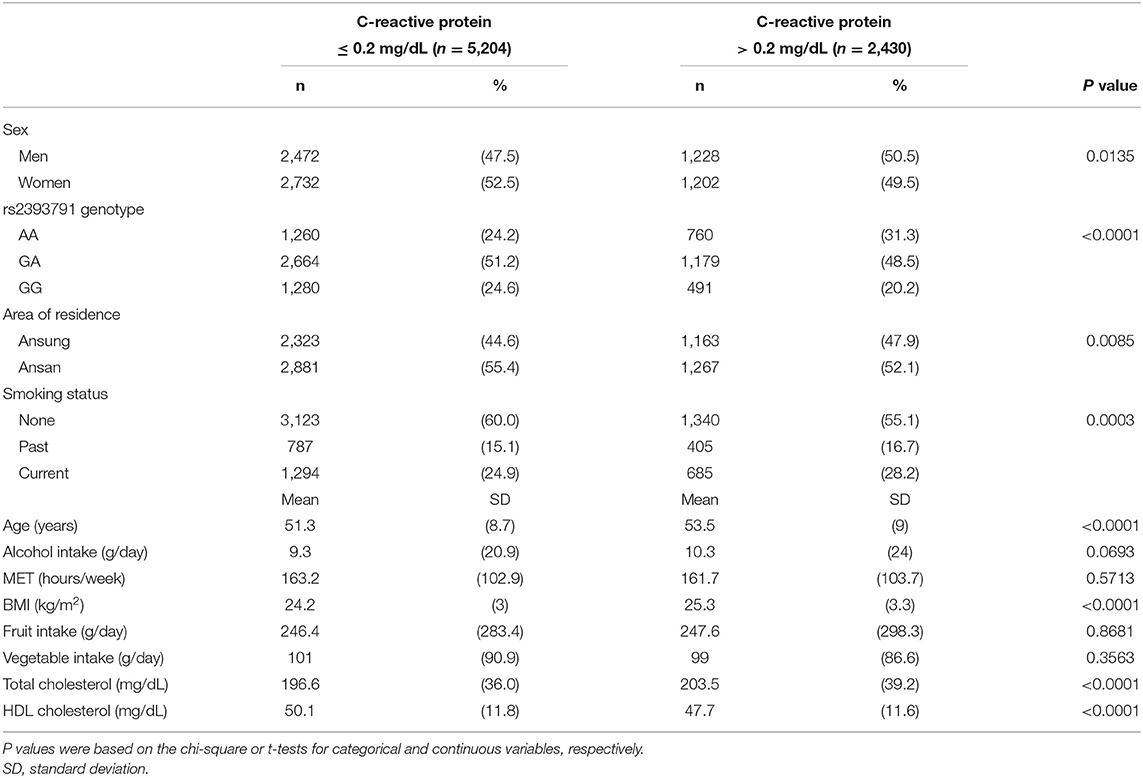
Table 1 presents the descriptive statistics for demographic characteristics, lifestyle factors, rs2393791 genotype, alcohol intake, MET (hours/week), BMI (kg/m2), and fruit and vegetable intake. Sex, HNF1A rs2393791 genotype, region, and smoking status contributed to significant differences in the CRP status (P <0.05). Participants with CRP >0.2 mg/dL were significantly older, had significantly higher BMI, and had higher total and HDL cholesterol levels than those with CRP ≤ 0.2 mg/dL.
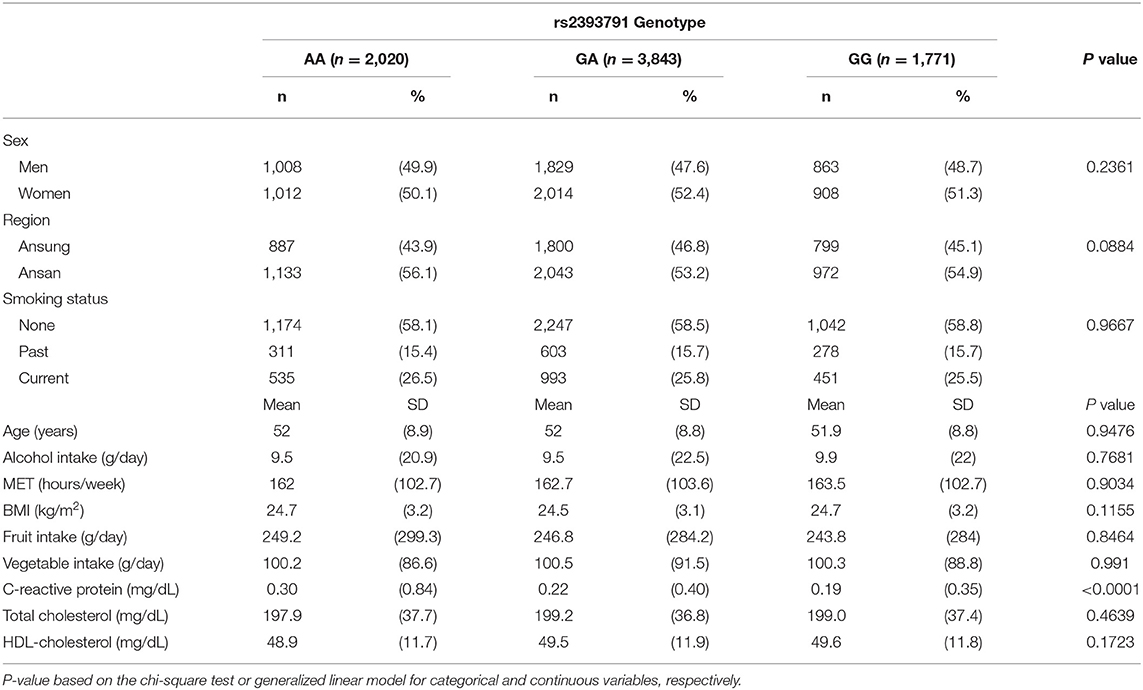
Demographic characteristics, lifestyle factors, fruit and vegetable intake, and CRP levels by rs2393791 genotype are presented in Table 2. CRP levels significantly differed according to the rs2393791 genotype (P <0.0001). CRP was highest in rs2393791 AA genotype (0.30 ± 0.84 mg/dL) compared to that in GA or GG genotypes (vs. 0.22 ± 0.40 vs. 0.19 ± 0.35 mg/dL). Sex, region, smoking status, age, alcohol intake, MET, BMI, and fruit and vegetable intake did not significantly differ according to the rs2393791 genotype.
Association of Fruit and Vegetable Intake and rs2393791 With CRP
The results on the association between fruit and vegetable intake and rs2393791 genotypes with log-transformed CRP levels are listed in Table 3. Dietary intake of fruits and vegetables was negatively and significantly associated with log-transformed CRP (β = −0.0001 for fruits, β = −0.0005 for vegetables, and β = −0.126 for rs2393791 genotypes).
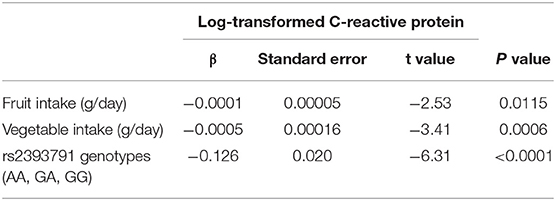
Table 3. General linear model examining the association between fruit and vegetable intake and HNF1A rs2393791 with log-transformed C-reactive protein.
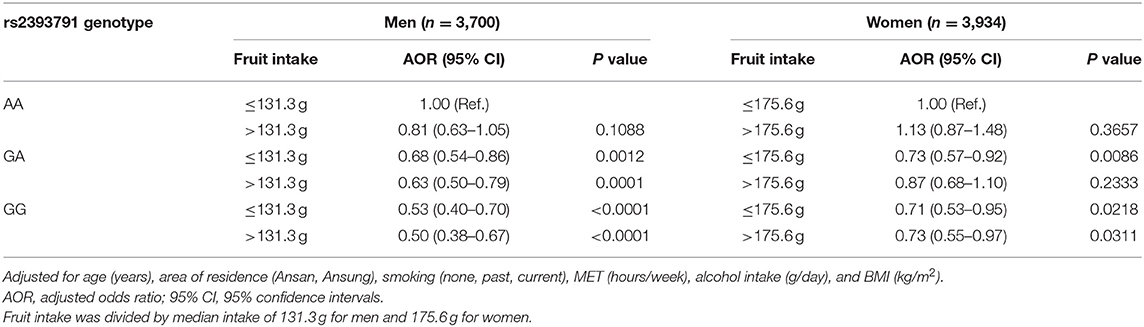
Association of Fruit Intake and rs2393791 Genotype With CRP
Associations of rs2393791 genotype and fruit intake divided by the median intake with elevated CRP (>0.2 mg/dL) by sex are presented in Table 4. Compared to men with the AA genotype of rs2393731 who consumed low fruit, those with the GA or GG genotype had significantly lower odds of elevated inflammation regardless of the fruit intake levels after controlling for covariates such as age, region, smoking, MET, alcohol intake, and BMI. Notably, men with the GG genotype and high fruit intake had the lowest odds of having elevated CRP levels after controlling for covariates (adjusted odds ratios (AOR) 0.50, 95% confidence interval [CI] 0.38–0.67) compared to those with the AA genotype and low fruit intake. In women, compared to those with the rs2393731 AA genotype and low fruit intake, those with the GG genotype and high fruit intake had lower odds of having elevated CRP levels after controlling for covariates (AOR 0.73, 95% CI 0.55–0.97).
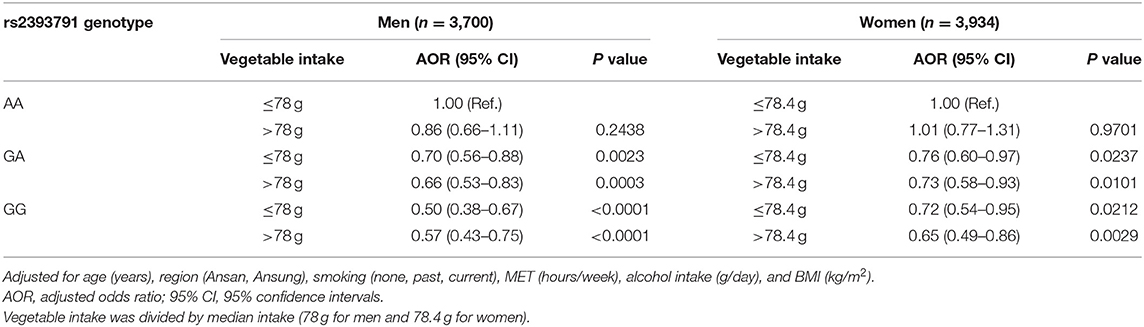
Association of Vegetable Intake and rs2393791 Genotype With CRP
Associations of rs2393791 genotype and vegetable intake divided by to the median intake with elevated CRP (>0.2 mg/dL) by sex are presented in Table 5. Compared to men with the rs2393731 AA genotype who consumed low amounts of vegetables, those with the GA or GG genotype had significantly lower odds of elevated inflammation regardless of vegetable intake levels after controlling for covariates. Specifically, men with the rs2393791 GG genotype and high vegetable intake had lower odds of having elevated CRP (AOR 0.57, 95% CI 0.43–0.75) after controlling for covariates. In addition, compared to women with the rs2393791 AA genotype and low vegetable intake, those with the GA or GG genotype had lower odds of having elevated CRP, regardless of vegetable intake in the fully adjusted model. Notably, women with the GG genotype and high vegetable intake had the lowest odds of having elevated CRP (AOR 0.65, 95% CI 0.49–0.86) in the fully adjusted model.
Association of Total Fruit and Vegetable Intake and rs2393791 Genotype With CRP
Associations of rs2393791 genotype and total fruit and vegetable intake divided by the median intake with elevated CRP (>0.2 mg/dL) by sex are presented in Table 6. Compared to men with the rs2393791 AA genotype who had low total fruit and vegetable intake, those with the GA or GG genotype had significantly lower inflammation levels after covariates adjustment regardless of the fruit and vegetable intake levels. The lowest Odds of having elevated CRP was shown among men with the GG genotype of rs2393791 with high fruit and vegetable intake (AOR 0.56, 95% CI 0.42–0.75). Compared to women with the rs2393791 AA genotype and low total fruit and vegetable intake, those with the GG genotype with high fruit and vegetable intake had significantly lower odds of elevated CRP (AOR 0.75, 95% CI 0.56–0.99).
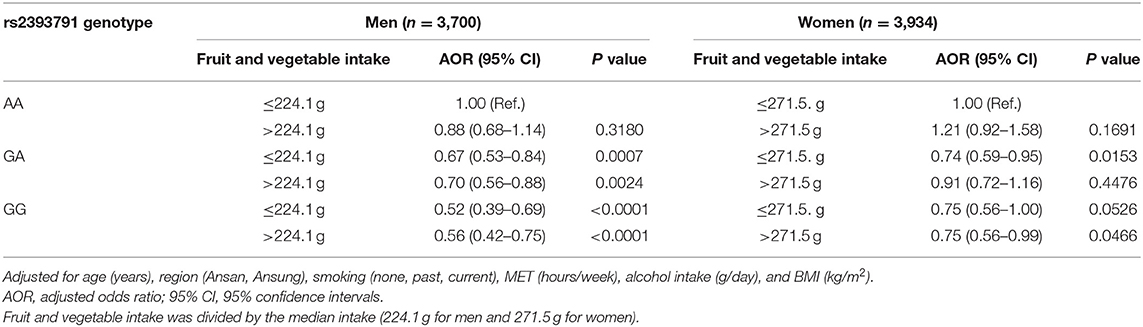
Table 6. Association of HNF1A rs2393791 genotype and total fruit and vegetable intake with inflammation.
Discussion
We observed that dietary fruit and vegetable intake modified the association between the rs2393791 variants and inflammation. rs2393791 is located on chromosome 12 and intron 1 of HNF1A. HNF1A can reportedly regulate the expression of multiple target genes, including albumin secretion, anti-trypsin synthesis, and fibrinogen synthesis genes (26). Specifically, men and women with the rs2393791 GG genotype who consumed more fruit and vegetables had decreased odds of elevated CRP. When stratified by rs2393791 genotype, men harboring the G allele with high consumption of fruits and vegetables were associated with lower odds of elevated CRP. A similar finding was found in women carrying the G allele with higher vegetable intake but not with fruit intake. We also observed that an overall higher fruit and vegetable intake was associated with lower CRP levels.
We reported interactions between rs2393791 genotypes and fruit intake in relation to CRP. Higher fruit intake was inversely associated with elevated CRP, and the lowest odds of elevated CRP were found in men with rs2393791 GG genotype and high fruit intake. High fruit intake in women with the GG genotype was associated with decreased odds of elevated CRP (AOR 0.73, 95% CI 0.55–0.97), which was only slightly higher than that in those with the GG genotype and low fruit intake (AOR 0.71, 95% CI 0.53–0.95). This may be partially due to the small sample size of women who are GG carriers with elevated CRP levels and consumed fewer fruits (n = 119 out of 439; 27.1%). We observed interactions between rs2393791 genotypes and vegetable intake in relation to CRP levels. Higher vegetable intake was associated with lower CRP levels. The decrease in odds was caused by the presence of the G allele, which was stronger among those with higher vegetable intake. The lower odds of elevated CRP levels may be mediated by vegetable intake, which might be a protective factor against inflammation.
In the present study, HNF1A SNP rs2393791 was significantly associated with CRP levels. Specifically, the G allele was associated with lower CRP levels. The present findings indicate that five HNF1A SNPs (rs1920792, rs1169288, rs7310409, rs2464196, and rs1169310) are significantly associated with CRP levels in Taiwanese patients (27). CRP levels are influenced by CRP gene expression, which, in turn, is affected by HNF1A SNPs. The promoter region of the human CRP gene includes two different regions or acute-phase response elements. Each region includes a binding site with a low affinity for the liver-specific transcription factor HNF-1. Two HNF-1 molecules can activate CRP gene expression by binding simultaneously to its promoter (15). Furthermore, HNF1A (rs1169288) variant is associated with an increased risk of type 2 diabetes and dysglycemic status in normal-weight Japanese individuals (28). HNF1A genotypes for rs1169288 and rs2464196 were positively associated with total and LDL cholesterol levels in older European and American adults (29). These data suggested that HNF1A polymorphisms influence glucose metabolism and its related traits.
The interplay between diet and genes influences phenotypic traits. Under a hypocaloric and high-fat diet, carriers of the HNF1A rs7957197 T allele demonstrated a higher drop in weight loss and improved insulin resistance levels than non-carriers under the same diet (30). These findings are similar to our findings that HNF1A affects CRP levels by choosing different fruit and vegetable intake levels. The underlying mechanisms governing the association between HNF1A, dietary fruit and vegetable intake, and inflammation remain to be elucidated. However, those with a high intake of fruits and vegetables may alter the gene-association changes in inflammation.
In this cross-sectional study of Korean adults using data set from the Ansan and Ansung cohort study, “fruit” and “vegetable” dietary patterns were identified and examined in relation to CRP (31). In this study, the “vegetable” dietary pattern was only inversely associated with CRP. In contrast, no such association was observed with the “fruit” dietary pattern, indicating the difficulty in observing the isolated effect of fruits (31). Increased fruit and vegetable intake reduces inflammation (13, 32, 33). In a four-week trial, a high intake of fruits and vegetables reduced plasma CRP levels in healthy and non-smoking men. Furthermore, dietary patterns characterized by a high intake of fruits and vegetables are negatively associated with CRP levels (34–37). The inverse association between fruits and vegetables intake and CRP may be due to several nutritional components of fruits and vegetables, such as numerous phytochemicals, including flavonoids (38), which demonstrate the anti-inflammatory activity by reducing pro-inflammatory cytokines such as interleukin (IL-6), tumor necrosis factor-alpha (TNF-α), IL-1β, and cyclooxygenase-2 (COX-2), and eventually downregulating inflammatory markers such as CRP (39).
In the present study, the association between HNF1A genotypes and CRP differed by the intake of fruits, vegetables, and their combination. The underlying reason may be due to different amounts of flavonoids and antioxidants present in fruits and vegetables mostly consumed by Koreans in terms of lowering inflammation. Certain types of fruits, such as grapes and berries, contain high levels of antioxidants and flavonoids (40). Specifically, grapes and berry fruits have been shown to possess anti-inflammatory and antioxidative effects (40, 41). However, Koreans do not consume grapes and berry fruits as frequently. Vegetables such as garlic (42), mushroom, and white cabbage (43), which are widely consumed by Koreans, possess antioxidant properties. Fruit and vegetable items mostly consumed by Koreans contain different antioxidant properties, and these yielded the associations between CRP and HNF1A differ with the intake of fruits, vegetables, and their combination. Our findings indicate that men and women showed associations between CRP and HNF1A irrespective of fruits, vegetables, or their combination. Men and women differ substantially with respect to inflammatory regulation (44). Women demonstrate more pronounced inflammatory and innate immune responses during bacterial and viral infections than men (45). In addition, sex hormones, including estrogens, progesterone, and androgens, contribute to the differential regulation of immune responses between the sexes, and dietary factors may alter the development and functioning of the immune system differently in men and women (46).
Our study had several limitations that were considered when analyzing our findings. First, the nature of cross-sectional analyses of inflammation hinders causal inference. Second, our findings cannot be extrapolated to the entire South Korean population. The study participants resided in Ansan and Ansung in South Korea; therefore, generalizing our study findings to middle-aged Korean adults is difficult. Despite these limitations, our study had several strengths. A relatively large sample size and long follow-up period are evident in this study. Furthermore, representation of both rural and urban populations is advantageous.
In conclusion, we observed that the presence of the G allele was associated with lower CRP levels in men and women with high fruit and vegetable intake. These significant interactions may indicate that the decrease in the odds of elevated CRP levels by the rs2393791 genotype may be dependent on fruit and vegetable intake. Our findings elucidated the mechanism by which fruit and vegetable intake interact with genetic background. These novel findings indicate that the susceptibility of individuals to inflammation is affected by their dietary fruit and vegetable intake.
Data Availability Statement
The dataset used in this study (Ansan-Ansung Cohort Study of the KoGES) was obtained after reviewing and evaluating the research plan of the Korea National Institute of Health, Korea Disease Control and Prevention Agency (http://nih.go.kr/contents.es?mid=a50401010400).
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Inha University on February 18, 2022 (IRB No. 220215-1A). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DS conducted statistical analyses and wrote the first draft of the manuscript. KL and DS conceptualized the study design, interpreted the data, and revised the manuscript. KL supervised all aspects of implementation and provided scientific advice. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an Inha University Research Grant.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was conducted with biosources from the National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea (KBN-2020-016).
References
1. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. (2006) 83:456S−60S doi: 10.1093/ajcn/83.2.456S
2. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. doi: 10.1038/nri2921
3. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. (2004) 27:813–23. doi: 10.2337/diacare.27.3.813
5. Galland L. Diet and inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
6. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. J Am Coll Cardiol. (2006) 48:677–85. doi: 10.1016/j.jacc.2006.03.052
7. Bulló M, Casas-Agustench P, Amigó-Correig P, Aranceta J, Salas-Salvadó J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr. (2007) 10:1164–72. doi: 10.1017/S1368980007000663
8. Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. (2006) 83:567–74. doi: 10.1093/ajcn.83.3.567
9. Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. (2009) 109:414–21. doi: 10.1016/j.jada.2008.11.036
10. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. (2006) 84:1489–97. doi: 10.1093/ajcn/84.6.1489
11. Ministry of Health and Welfare. The 3rd National Health Plan (2011-2020). Seoul: Korea Health Promotion Institute (2011).
12. Van Duyn MAS, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Acad Nutr Diet. (2000) 100:1511–21. doi: 10.1016/S0002-8223(00)00420-X
13. Watzl B, Kulling SE, Möseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. (2005) 82:1052–8. doi: 10.1093/ajcn/82.5.1052
14. Reiner AP, Barber MJ, Guan Y, Ridker PM, Lange LA, Chasman Daniel I, et al. Polymorphisms of the HNF1A gene encoding hepatocyte nuclear factor-1α are associated with c-reactive protein. Am J Hum Genet. (2008) 82:1193–201. doi: 10.1016/j.ajhg.2008.03.017
15. Toniatti C, Demartis A, Monaci P, Nicosia A, Ciliberto G. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. (1990) 9:4467–75. doi: 10.1002/j.1460-2075.1990.tb07897.x
16. Owen KR, Thanabalasingham G, James TJ, Karpe F, Farmer AJ, McCarthy MI, et al. Assessment of high-sensitivity C-reactive protein levels as diagnostic discriminator of maturity-onset diabetes of the young due to HNF1A mutations. Diabetes Care. (2010) 33:1919–24. doi: 10.2337/dc10-0288
17. Kleber ME, Grammer TB, Renner W, März W. Effect of the rs2259816 polymorphism in the HNF1A gene on circulating levels of c-reactive protein and coronary artery disease (the ludwigshafen risk and cardiovascular health study). BMC Med Genet. (2010) 11:157. doi: 10.1186/1471-2350-11-157
18. Wu Y, McDade TW, Kuzawa CW, Borja J, Li Y, Adair LS, et al. Genome-wide association with C-reactive protein levels in CLHNS: evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation. (2012) 35:574–83. doi: 10.1007/s10753-011-9348-y
19. Kim Y, Han B-G, Ko GESg. Cohort Profile: The Korean genome and epidemiology study (KoGES) consortium. Int J Epidemiol. (2017) 46:e20. doi: 10.1093/ije/dyv316
20. Korea Disease Control and Prevention Agency Korea Korea National Institute of Health. Manual of Korean Genome and Epidemiology Study. (2014). Available online at: https://nih.go.kr/contents.es?mid=a40504040200 (accessed January 15, 2022).
21. The Korean Nutrition Society. CAN-Pro 4.0: Computer Aided Nutritional Analysis Program. The Korean Nutrition Society, Seoul, Korea. (2006).
22. Rafalski A. Applications of single nucleotide polymorphisms in crop genetics. Curr Opin Plant Biol. (2002) 5:94–100. doi: 10.1016/S1369-5266(02)00240-6
23. Hwang JY, Sim X, Wu Y, Liang J, Tabara Y, Hu C, et al. Genome-wide association meta-analysis identifies novel variants associated with fasting plasma glucose in East Asians. Diabetes. (2015) 64:291–8. doi: 10.2337/db14-0563
24. Khoo CM, Tan M, Wu Y, Wai CH, Subramaniam T, Lee J, et al. Central obesity and smoking are key modifiable risk factors for elevated C-reactive protein in Asian individuals who are not eligible for statin therapy. Nutr Diabetes. (2011) 1:e8. doi: 10.1038/nutd.2011.4
25. Jetté M, Sidney K, Blümchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. (1990) 13:555–65. doi: 10.1002/clc.4960130809
26. Blumenfeld M, Maury M, Chouard T, Yaniv M, Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. (1991) 113:589–99. doi: 10.1242/dev.113.2.589
27. Hsu L-A, Ko Y-L, Teng M-S, Wu S, Chou H-H, Chang P-Y, et al. Effect of obesity on the association between common variations in the HNF1A gene region and C-reactive protein level in Taiwanese. Clin Chim Acta. (2011) 412:725–9. doi: 10.1016/j.cca.2010.12.027
28. Morita K, Saruwatari J, Tanaka T, Oniki K, Kajiwara A, Otake K, et al. Associations between the common HNF1A gene variant p.I27L (rs1169288) and risk of type 2 diabetes mellitus are influenced by weight. Diabetes Metab. (2015) 41:91–4. doi: 10.1016/j.diabet.2014.04.009
29. Reiner AP, Gross MD, Carlson CS, Bielinski SJ, Lange LA, Fornage M, et al. Common coding variants of the HNF1A gene are associated with multiple cardiovascular risk phenotypes in community-based samples of younger and older european-american adults. Circ Cardiovasc Genet. (2009) 2:244–54. doi: 10.1161/CIRCGENETICS.108.839506
30. Huang T, Wang T, Heianza Y, Sun D, Ivey K, Durst R, et al. HNF1A variant, energy-reduced diets and insulin resistance improvement during weight loss: the POUNDS Lost trial and DIRECT. Diabetes Obes Metab. (2018) 20:1445–52. doi: 10.1111/dom.13250
31. Lee Y, Kang D, Lee SA. Effect of dietary patterns on serum C-reactive protein level. Nutr Metab Cardiovasc Dis. (2014) 24:1004–11. doi: 10.1016/j.numecd.2014.05.001
32. Hosseini B, Berthon BS, Saedisomeolia A, Starkey MR, Collison A, Wark PAB, et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. (2018) 108:136–55. doi: 10.1093/ajcn/nqy082
33. Aggarwal A, Verma S, Ghai R, Nagarajan K. Potential of fruits and vegetables to treat inflammatory conditions. Materials Today: Proceedings. (2021) 47:127–34. doi: 10.1016/j.matpr.2021.04.006
34. Poggio R, Elorriaga N, Gutierrez L, Irazola V, Rubinstein A, Danaei G. Associations between dietary patterns and serum lipids, apo and C-reactive protein in an adult population: evidence from a multi-city cohort in South America. Br J Nutr. (2017) 117:548–55. doi: 10.1017/S0007114517000514
35. Julia C, Meunier N, Touvier M, Ahluwalia N, Sapin V, Papet I, et al. Dietary patterns and risk of elevated C-reactive protein concentrations 12 years later. Br J Nutr. (2013) 110:747–54. doi: 10.1017/S0007114512005636
36. Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. (2004) 80:1029–35. doi: 10.1093/ajcn/80.4.1029
37. Anderson AL, Harris TB, Tylavsky FA, Perry SE, Houston DK, Lee JS, et al. Dietary patterns, insulin sensitivity and inflammation in older adults. Eur J Clin Nutr. (2012) 66:18–24. doi: 10.1038/ejcn.2011.162
39. Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. (2018) 153:105–15. doi: 10.1016/j.ejmech.2017.09.001
40. Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994-2005. Prev Chronic Dis. (2008) 5:A35.
41. Meyer AS, Jepsen SM, Sørensen NS. Enzymatic release of antioxidants for human low-density lipoprotein from grape pomace. J Agric Food Chem. (1998) 46:2439–46. doi: 10.1021/jf971012f
42. Al-Saikhan M, Howard L. Miller Jr J. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum, L). J Food Sci. (1995) 60:341–3. doi: 10.1111/j.1365-2621.1995.tb05668.x
43. Gazzani G, Papetti A, Massolini G, Daglia M. Anti-and prooxidant activity of water soluble components of some common diet vegetables and the effect of thermal treatment. J Agric Food Chem. (1998) 46:4118–22. doi: 10.1021/jf980300o
44. Bédard A, Lamarche B, Corneau L, Dodin S, Lemieux S. Sex differences in the impact of the Mediterranean diet on systemic inflammation. Nutr J. (2015) 14:46. doi: 10.1186/s12937-015-0035-y
45. Wegner A, Benson S, Rebernik L, Spreitzer I, Jäger M, Schedlowski M, et al. Sex differences in the pro-inflammatory cytokine response to endotoxin unfold in vivo but not ex vivo in healthy humans. Innate Immun. (2017) 23:432–9. doi: 10.1177/1753425917707026
Keywords: HNF1A gene variants, fruit intake, vegetable intake, C-reactive protein, inflammation
Citation: Shin D and Lee KW (2022) Fruit and Vegetable Consumption Interacts With HNF1A Variants on the C-Reactive Protein. Front. Nutr. 9:900867. doi: 10.3389/fnut.2022.900867
Received: 21 March 2022; Accepted: 02 June 2022;
Published: 07 July 2022.
Edited by:
Alok Agrawal, East Tennessee State University, United StatesReviewed by:
Johannes Zeller, Baker Heart and Diabetes Institute, AustraliaShang-Rong Ji, Lanzhou University, China
Copyright © 2022 Shin and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung Won Lee, a3dsZWUmI3gwMDA0MDtrbnVlLmFjLmty
 Dayeon Shin
Dayeon Shin Kyung Won Lee
Kyung Won Lee