- 1Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, China
- 3Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 4Department of Hematology, Shengjing Hospital of China Medical University, Shenyang, China
Epidemiological studies have suggested that dietary acid load (DAL) might be related to the risk and prognosis of cancer, whereas the evidence is contentious. Several high-quality observational studies have been published following a prior systematic review with only one study included. Consequently, we conducted an updated systematic review and meta-analysis to comprehensively investigate the relationship between DAL and cancer risk and prognosis. A systematic literature search was conducted in the PubMed, Embase, and Web of Science databases from inception to 26 October 2021. Summary relative risks (RRs) with 95% CIs were calculated using a random-effects model. Publication bias, subgroup, meta-regression, and sensitivity analyses were also conducted. Ten observational studies (six cohorts and four case–control studies) with 227,253 participants were included in this systematic review and meta-analysis. The summary RRs revealed a statistically significant associations between DAL and cancer risk (RR = 1.58, 95% CI = 1.23–2.05, I2 = 71.9%, n = 7) and prognosis (RR = 1.53, 95% CI = 1.10–2.13, I2 = 77.1%, n = 3). No evidence of publication bias was observed in the current analysis. Positive associations were observed in most subgroup analyses stratified by predefined factors, including region, study design, study quality, study population, participants’ gender, age of participants, cancer type, DAL assessment indicator, and adjustment of potential confounding parameters. No evidence of heterogeneity between subgroups was indicated by meta-regression analyses. The high DAL might be associated with an increased risk of cancer, as well as a poor prognosis of cancer. More high-quality prospective studies are warranted to further determine the associations between DAL and risk and prognosis for specific cancers.
Introduction
Cancer is a leading cause of death and an important barrier to prolonging life (1). Globally, more than 19 million new cases of cancers were diagnosed, and nearly 10 million deaths from cancer occurred in 2020 (2). Most cancers were caused by a complex etiology such as environment, genetics, and lifestyle factors (3), and evidence had suggested that over 40% of cancer deaths could be prevented through changes in lifestyles, including diet (4). Due to the potential interaction between food and nutrients, the studies of dietary patterns or overall diet quality may better measure the impact of diet on health outcomes (5).
Dietary acid load (DAL) is one of the indexes to evaluate the quality of the whole diet, which provides more comprehensive information about the dietary intakes of subjects (6). It has been recently proposed that higher DAL, representing the consumption of diets characterized by a higher intake of meat and eggs and a lower intake of vegetables and fruits, could lead to changes or imbalances in blood pH and acid-base balance (7). DAL could be calculated through the potential renal acid load (PRAL), the net endogenous acid production (NEAP), the protein to potassium (Pro:K) ratio, and the net acid excretion (NAE), which are validated methods to assess DAL from dietary composition data (8, 9). Negative values of PRAL and lower values of NEAP, Pro:K, and NAE reflect alkaline-forming potential, whereas positive values of PRAL and higher values of NEAP, Pro: K, and NAE indicate acid-forming potential.
Experimental evidence has indicated that an acidic environment had a benign effect on the survival of cancer cells and promoted the invasion and metastasis of tumors (10, 11). The alkaline environment had the opposite effect on cancer cell survival compared with acidic environments (12). Several observational studies have also suggested that DAL is positively associated with some chronic diseases, such as metabolic syndrome (13) and type 2 diabetes (14). In 2016, Fenton and Huang (15) conducted a systematic review and found only one study focused on the association between DAL and cancer risk, which suggested null results. Interestingly, several epidemiological studies have published their results in recent years, but the findings have been controversial (16–25). For example, a large cohort study with 43,570 participants showed that consumption of high DAL food increased the risk of breast cancer (19). In contrast, a cohort study of 27,096 male smokers suggested a significant relationship between high DAL and an increased risk of bladder cancer (23).
To the best of our knowledge, there has been no updated systematic review and meta-analysis comprehensively verifying whether DAL plays a vital role in cancer risk and prognosis after the study of Fenton and Huang (15). Therefore, given the controversial findings as well as the current lack of high-level evidence of this issue, we conducted the present study to further understand and investigate the aforementioned topic.
Methods
Search strategy
This systematic review and meta-analysis were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (26) and the Meta-analysis of Observational Studies in Epidemiology group (27). PubMed, Embase, and the Web of Science databases were searched systematically to obtain studies published up to 26 October 2021 by two independent investigators (RW and ZYW). The following search keywords were utilized: (diet or dietary or diet dependent) and (acid or acid-base or NEAP or potential renal net acid load or DAL) and (cancer or neoplasms or oncology). Our search was completed by an additional manual search of reference lists of all the retrieved articles.
Dietary acid load definitions
There were four ways to estimate DAL: (i) PRAL, which considered the absorption rates for dietary proteins and minerals, ionic dissociation, and sulfur metabolism (28); (ii) NEAP, which took into account the acidification of proteins and the alkalization of potassium (8); (iii) Pro:K that also involved animal proteins and potassium (8); and (iv) NAE that similar to PRAL, which further included estimated excretion of organic acids (12).
Study selection and exclusion
To be included in this review, the following criteria were used for inclusion: (i) studies had an observational design, including cross-sectional, case–control, and cohort studies; (ii) studies assessing the relationship between DAL and cancer risk and prognosis; and (iii) studies recommending relative risk (RR), hazard ratio (HR), odds ratio (OR), or required data for an estimate. The studies were excluded for the following reasons: (i) studies that were not original research, including editors, case reports, and reviews; (ii) studies with randomized controlled or ecological design; and (iii) studies published in other languages instead of English.
Data extraction and quality assessment
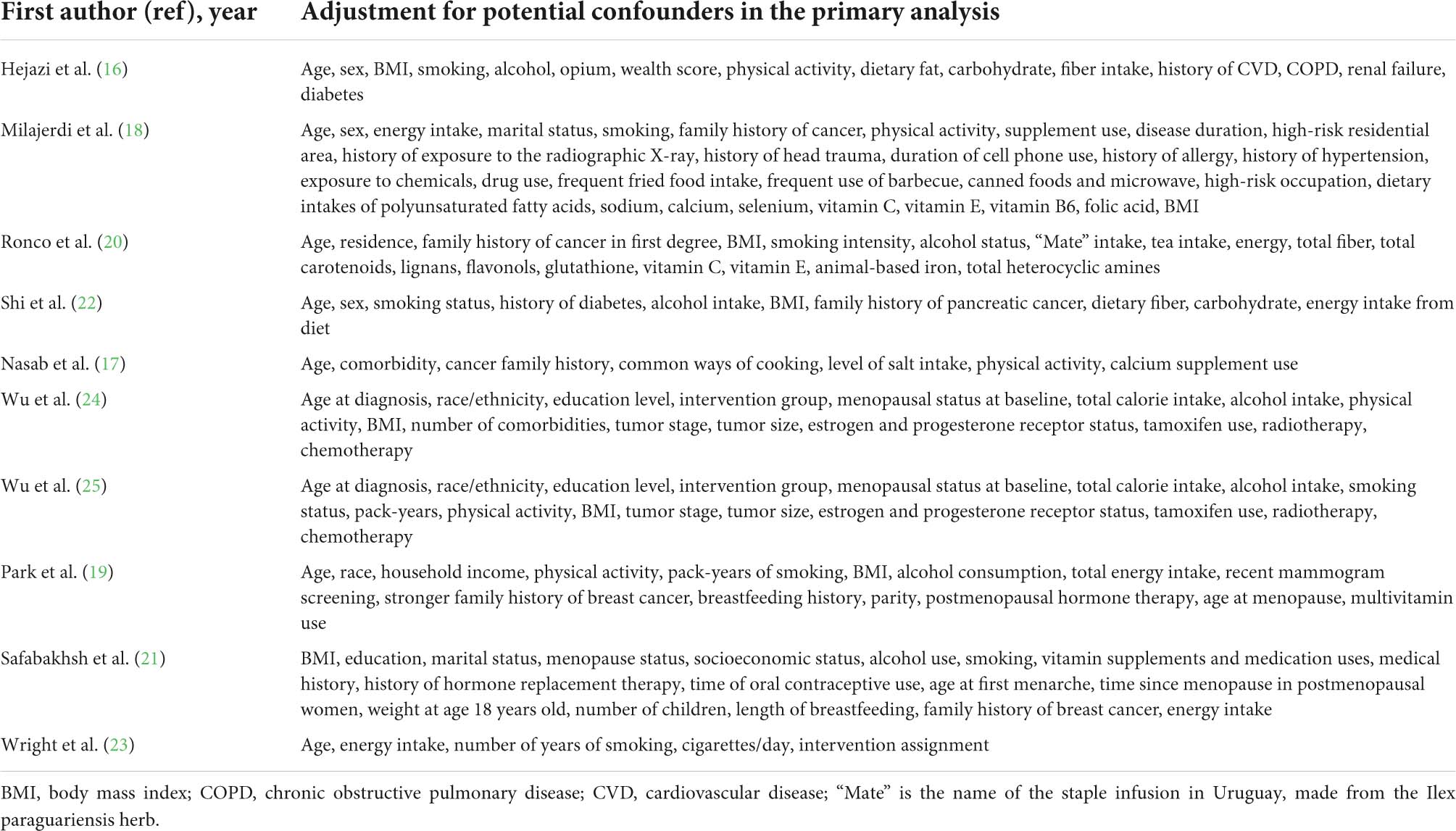
The studies that fulfilled all the inclusion criteria were qualitatively evaluated by two investigators (RW and ZYW), and any disagreements were settled by a discussion with a third investigator (QJW). The extracted data included the first author, the year of publication, country, design of studies included, number of cases, dietary assessment index, exposure categories, risk estimates, and adjusted variables. We assessed the quality of the articles according to the Newcastle–Ottawa Scale (NOS; 29). The NOS consisted of three fields: selection, comparability, and outcome. These studies received full marks in at least two categories of selection, comparability, or outcome assessment and were classified as low-risk bias (30, 31).
Statistical analysis
In the meta-analysis, effect sizes for DAL were extracted from original studies, including standardized incidence ratio, HR, and RRs. The OR estimate and HR estimate were considered an approximation of the RR estimate (31). We calculated RR and 95% CI with a random-effects model (32) as a measure of the effect size for all the studies. A random-effects model accounted for variation between studies, as this can provide more conservative results than a fixed-effects model (33).
Heterogeneity in the relationship between DAL and cancer risk and prognosis across studies was quantified using I2 statistics. Cutoff points of ≤25, ≤50, ≤75, and >75% were used to indicate no, small, moderate, and substantial levels of heterogeneity, respectively, (34). To explore the sources of heterogeneity among studies, we conducted subgroup analyses and sensitivity analyses. Subgroup analyses were conducted based on region, study design, study quality, study population, gender, age, cancer type, DAL assessment indicator, and adjustments made for potential confounders, including body mass index, cigarette smoking, alcohol consumption, and physical activity. We also made a meta-regression model to identify potential sources of heterogeneity between subgroups. Sensitivity analysis was performed in which each study was eliminated from the study to evaluate the influence of that study (35). Publication bias was assessed by Begg’s test (36), Egger’s test (37), and visual inspection of funnel plots. A probability (P) value of <0.05 was considered statistically significant. All the analyses were conducted using Stata version 11.2 software (StataCorp, College Station, TX, United States).
Results
Search results, study characteristics, and quality assessment
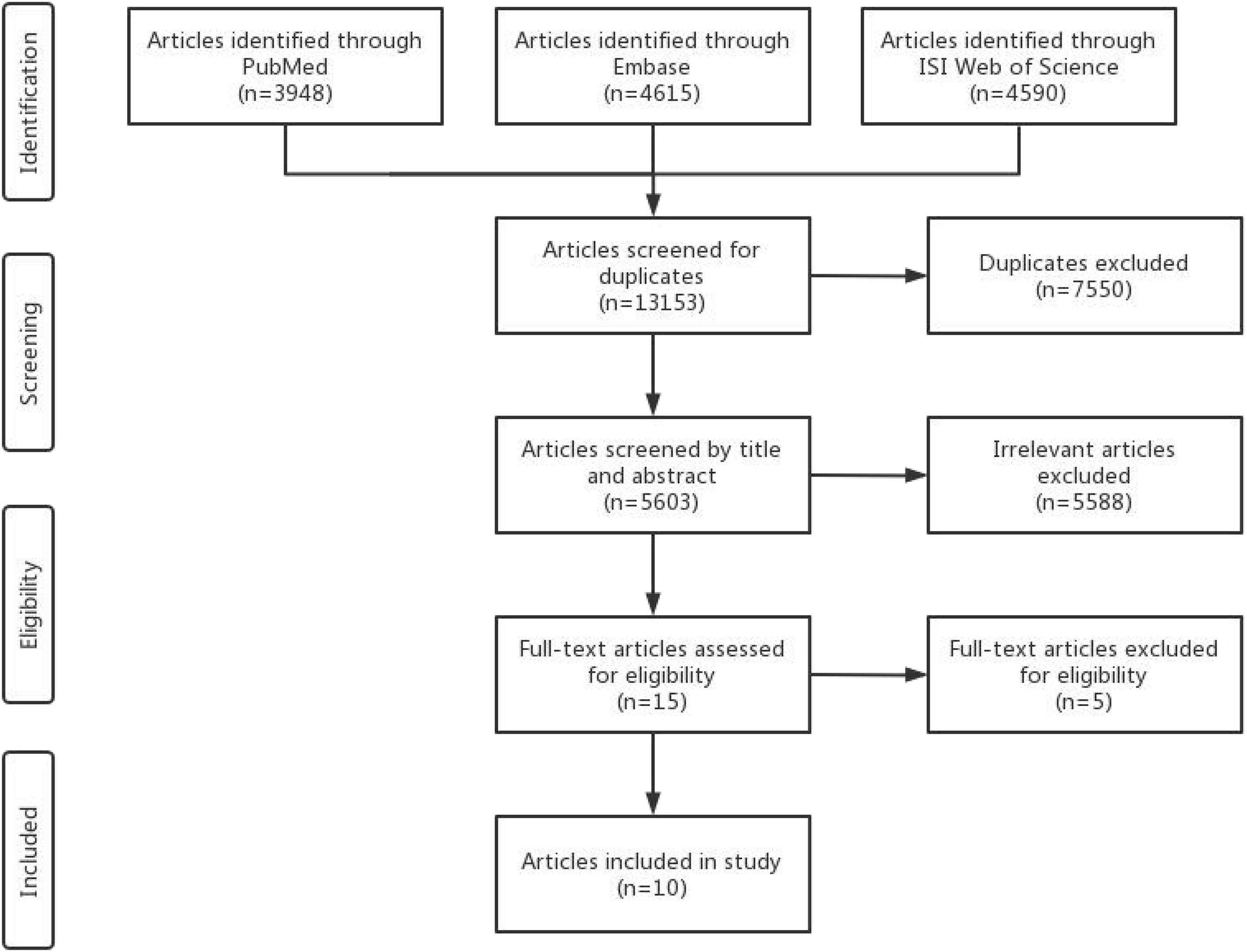
The search strategy retrieved 13,153 articles from databases, of which 5,605 articles remained after removing the 7,550 duplicate articles. After the initial screening based on titles or abstracts, 5,588 studies were excluded, leaving 17 studies included. Of these, 5 articles (38–42) were further eliminated because of the duplicated study population and incomplete results. The final selection yielded 10 articles (16–25; 7 studies for cancer risk and 3 studies for cancer prognosis; Figure 1) included in the meta-analysis.
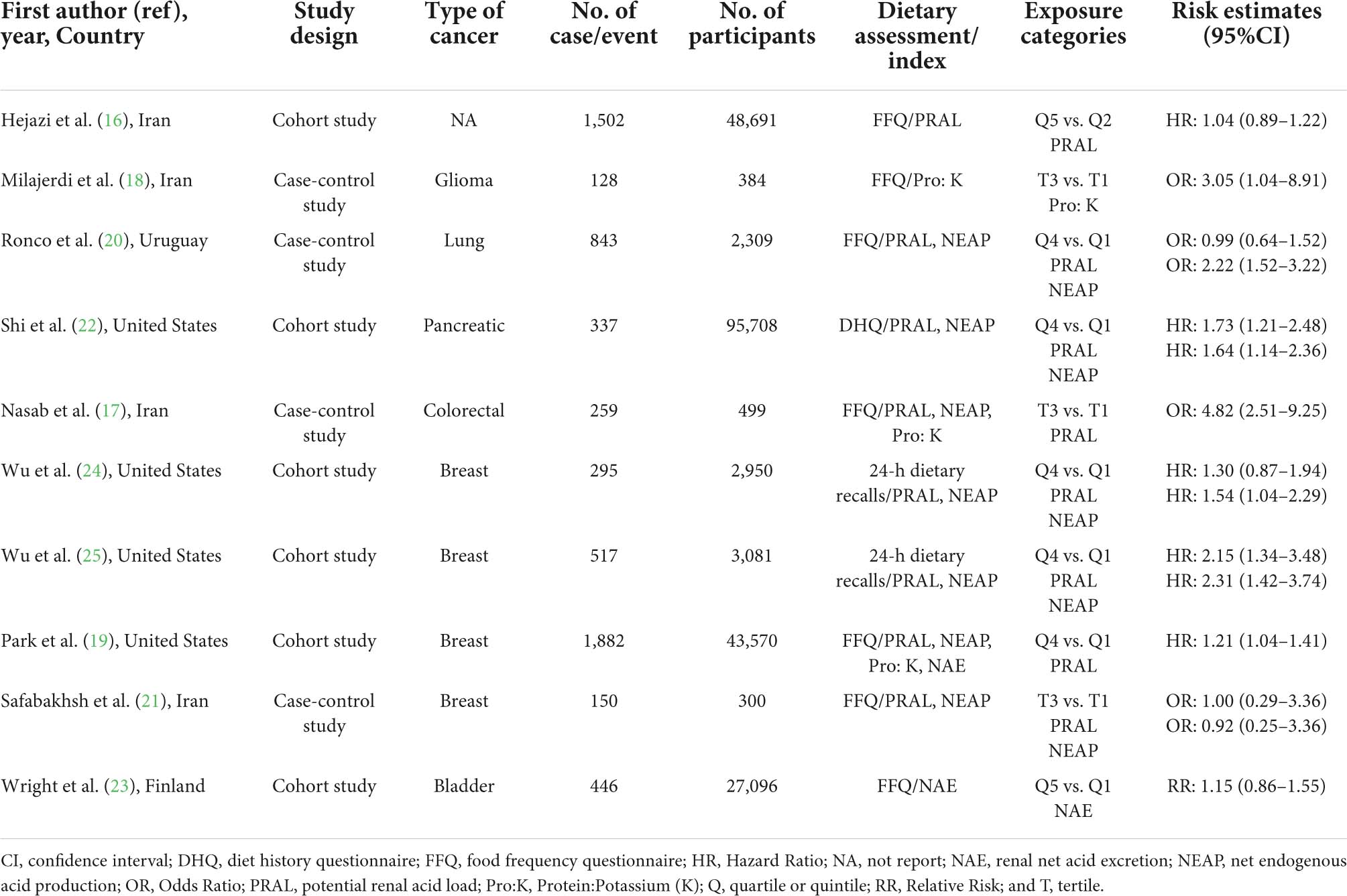
Seven studies focused on cancer risk were published between 2005 and 2021 (Table 1). Among them, four were case–control studies (17, 18, 20, 21), and three were cohort studies (19, 22, 23). Three studies were performed in Asia (17, 18, 21), two in North America (19, 22), one in South America (20), and one in Europe (23), respectively. DAL had been assessed using the PRAL and NEAP methods in five studies (17, 19–22), NAE in two studies (22, 23), and Pro:K in three studies (17, 20, 22). The included articles were assessed by dietary intake through the Food Frequency Questionnaire (FFQ) and the Diet History Questionnaire (DHQ). Potential confounders were adapted for age (n = 6), energy intake (n = 6), family history of cancer (n = 6), smoking status (n = 6), and body mass index (n = 5; Table 2). Five studies (lung, glioma, colorectal, breast, and pancreatic cancers) indicated a relationship between higher DAL intake and an increased risk of cancer (17–20, 22), whereas two studies (breast and bladder cancers) demonstrated a null association (21, 23).
Table 1 demonstrates the characteristics of the cancer prognosis studies (16, 24, 25), which were referred to as cohort studies. Of them, two studies were undertaken in North America (24, 25) and one study was undertaken in Asia (16). PRAL was assessed in all the studies, whereas NEAP was applied in two studies (24, 25). Dietary intake was evaluated through FFQ and 24-h dietary recall in all the included studies. Risk estimates were adjusted for body mass index (n = 3), smoking status (n = 3), physical activity (n = 3), and age at diagnosis (n = 2; Table 2). Two cohort studies indicated a significant relationship between higher DAL (represented by NEAP) intake and poor survival among patients with breast cancer (24, 25), whereas one cohort study demonstrated a null association (16).
The information on quality assessment is given in Tables 3, 4. Five cohort studies (16, 19, 22, 24, 25) were graded as low risk, whereas only one cohort study (23) was graded as high risk (Table 3). For the item of “control for important factor or additional factor,” four studies (16, 23–25) were not awarded two stars since these studies adjusted for less than two important confounder factors. For the classification of “outcome,” two studies (22, 23) were not assigned full stars because of the inadequacy of the follow-up rate of cohorts. Most included case–control studies (75%) were at high risk (Table 4). For the “selection” classification, three studies (17, 18, 20) were not assigned full stars. For the item of “control for important factor or additional factor,” one study (17) was not awarded two stars since these studies had adjusted for less than two important confounder factors in their analysis. For the classification of “exposure,” two studies (18, 20) were not assigned full stars because there was a significant difference in the response rate between cases and controls.
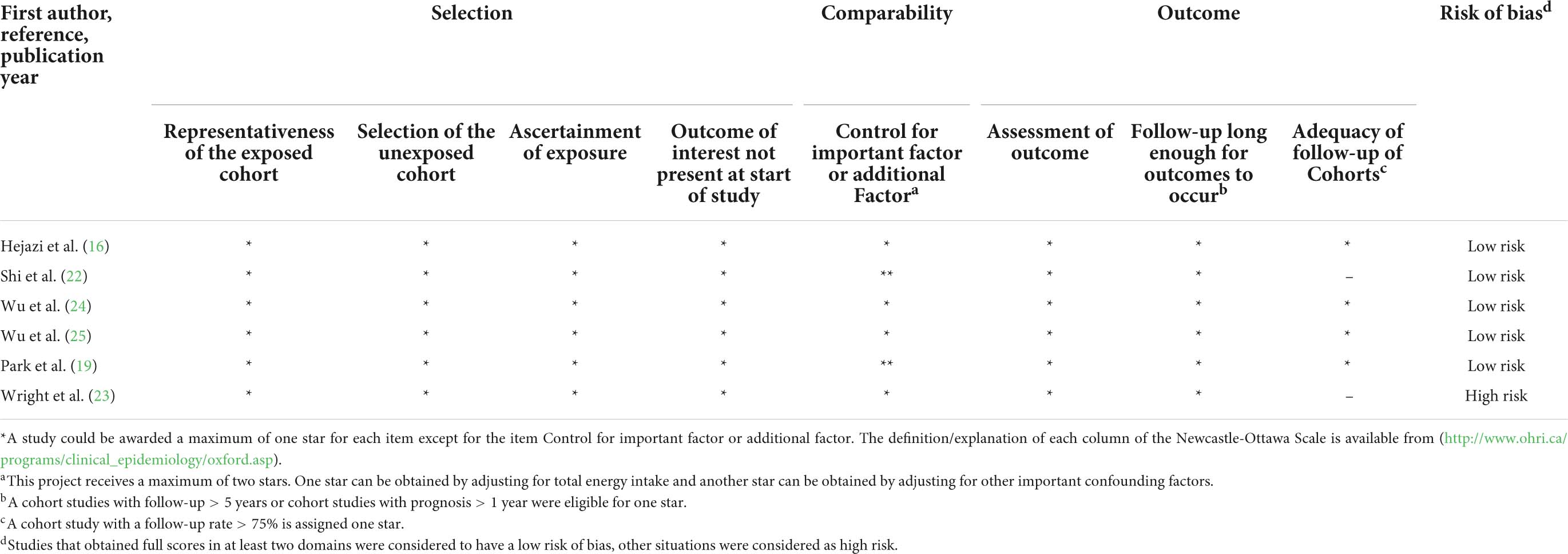
Table 3. Methodological quality of cohort studies included in the systematic review and meta-analysis.
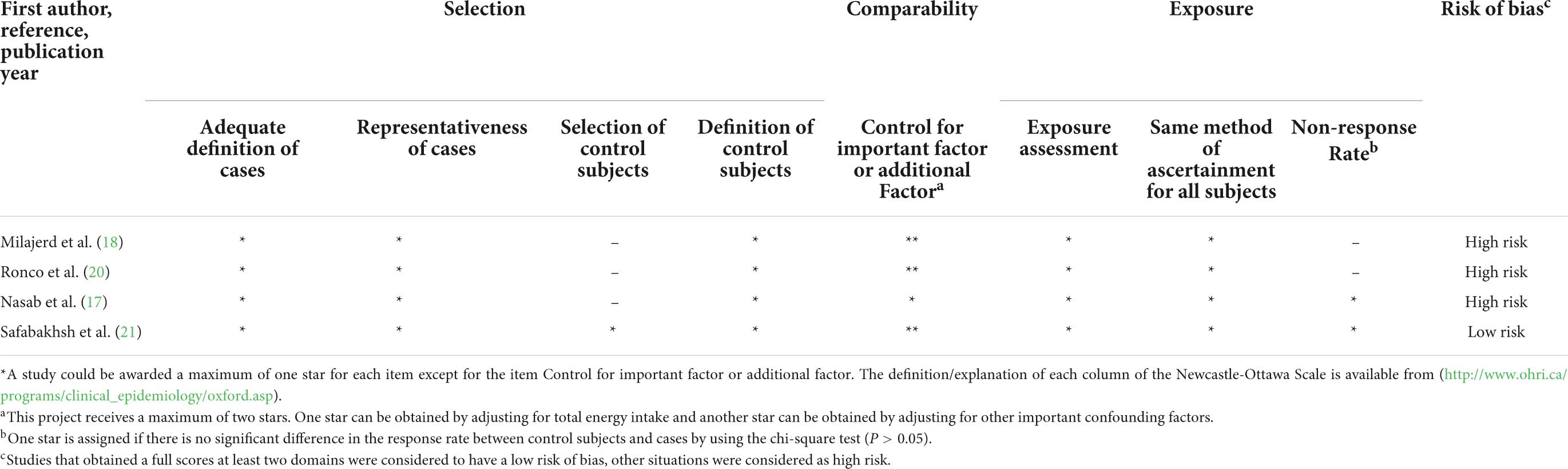
Table 4. Methodological quality of case–control studies included in the systematic review and meta-analysis.
Association of dietary acid load with cancer risk
Higher DAL was associated with a 58% increased risk of cancer (RR = 1.58, 95% CI = 1.23–2.05, I2 = 71.9%; Figure 2). No publication bias was discovered (Supplementary Figure 1; Egger’s P = 0.21 and Begg’s P = 0.47).
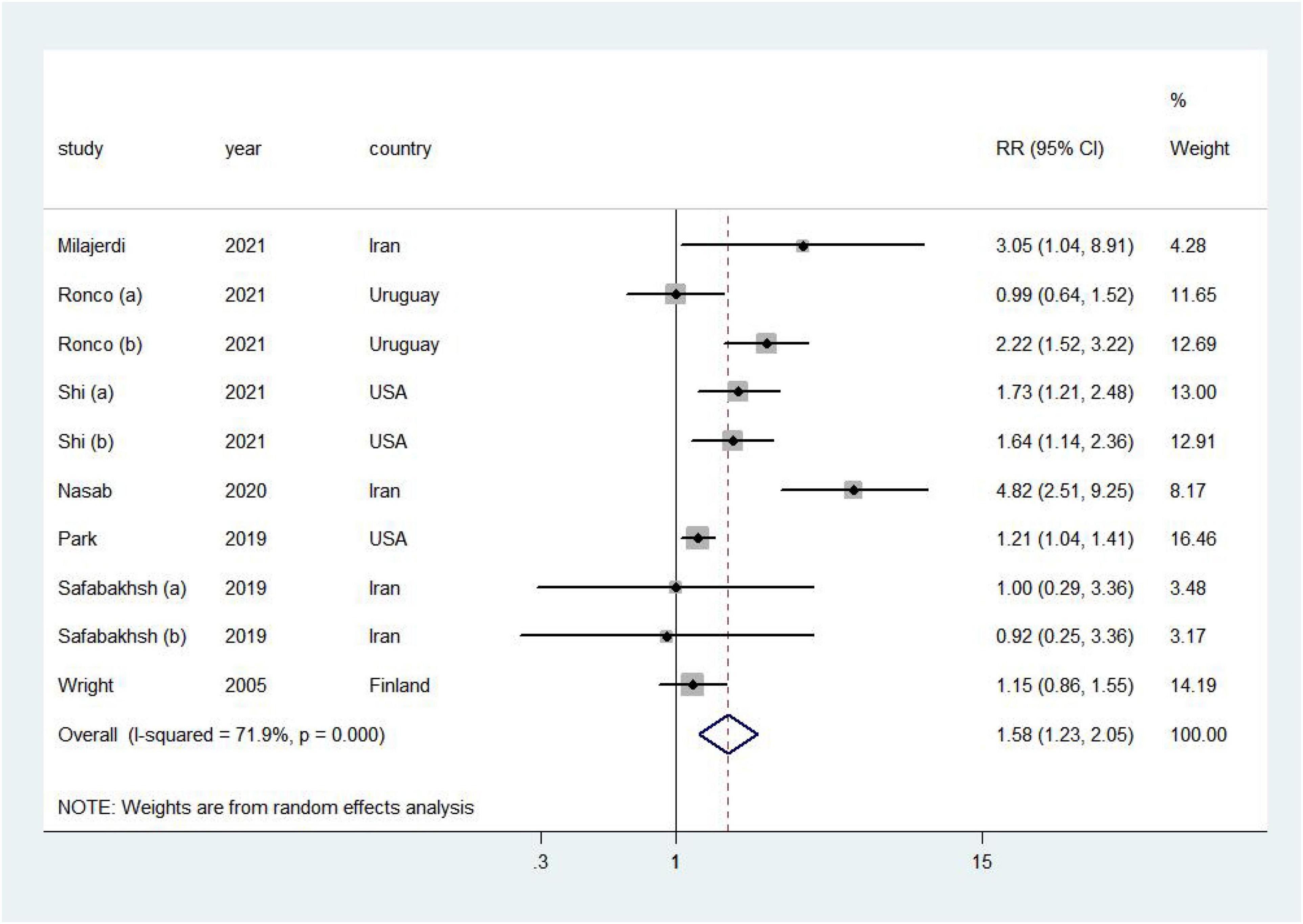
Figure 2. Forest plot (a random-effects model) of the association between DAL and cancer risk (highest vs. lowest). Squares indicate study-specific relative risk (RR), where the size of the square reflects the study-specific statistical weight; horizontal lines indicate the 95% CI; and diamonds denote the summary RR with 95% CI.
Positive associations were found in most subgroup analyses (Table 5). Notably, in the stratified analysis, we observed significant positive associations in studies in non-Asia (RR = 1.41, 95% CI = 1.14–1.76), age of participants ≥50 years (RR = 1.60, 95% CI = 1.21–2.11), breast cancer (RR: 1.20, 95% CI = 1.03–1.40), and pancreatic cancer (RR = 1.69, 95% CI = 1.31–2.18). Furthermore, the risk of cancer incidence increased by 57% (RR = 1.57, 95% CI = 1.03–2.41) and 83% (RR = 1.83, 95% CI = 1.36–2.47) by high PRAL and NEAP, respectively. Additionally, meta-regression analysis revealed that there was no evidence of heterogeneity between these subgroup analyses.
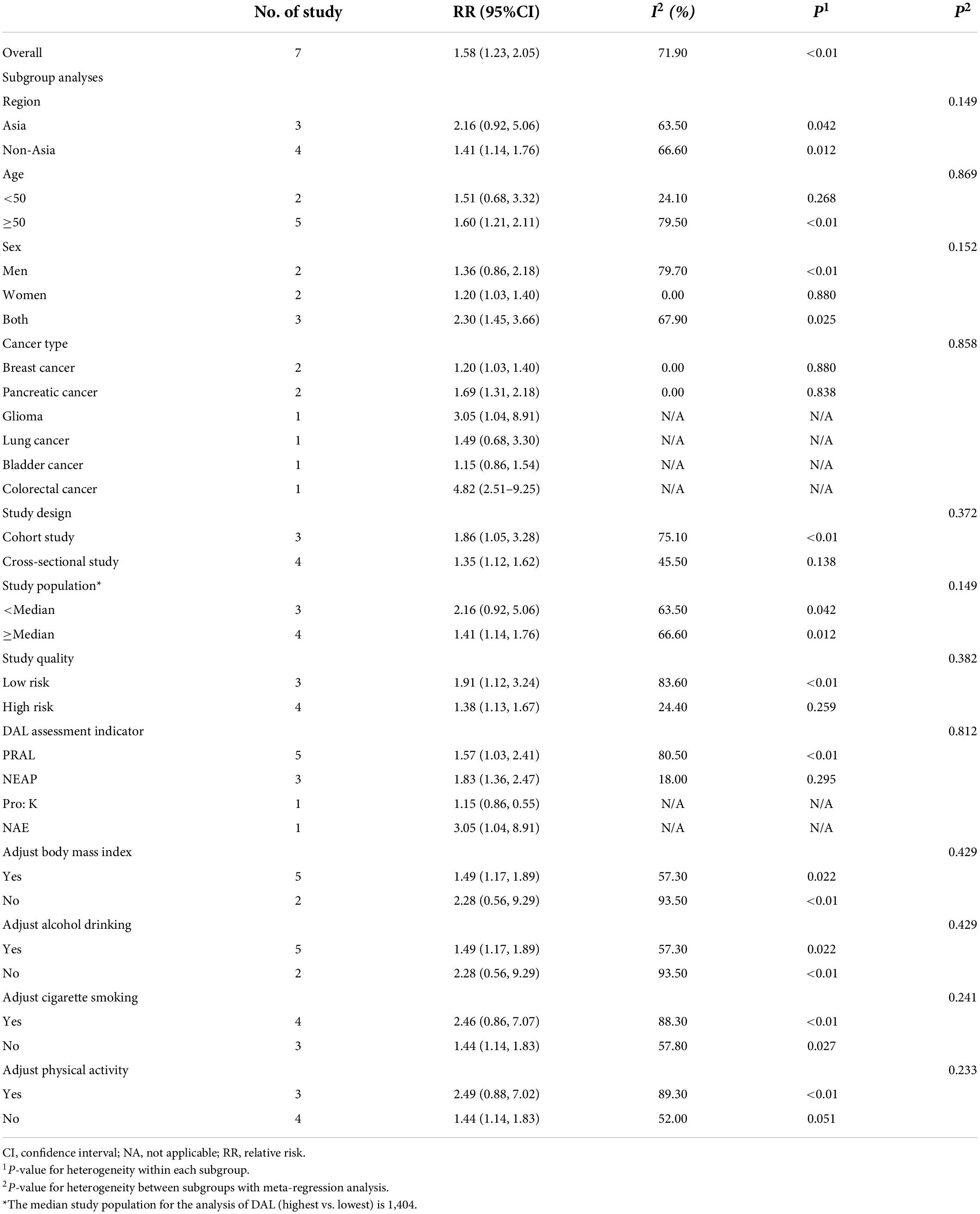
Table 5. Summary risk estimates of the association between dietary acid load and risk of cancer (highest vs. lowest).
In sensitivity analyses, we sequentially removed one study; in turn, the pooled RR did not change substantially. Our sensitivity analysis showed that the RR for cancer ranged from a low of 1.50 (95% CI = 1.16–1.95, I2 = 68.4%) after removing the study by Ronco et al. (20) to a high of 1.68 (95% CI = 1.23–2.29, I2 = 69.5%) after removing the study by Park et al. (19; Supplementary Figure 2).
Association of dietary acid load with cancer prognosis
Higher DAL was associated with a poor prognosis of cancer (RR = 1.53, 95% CI = 1.10–2.13, I2 = 77.1%; Figure 3). No publication bias was discovered (Supplementary Figure 3; Egger’s P = 0.02 and Begg’s P = 0.09). In sensitivity analyses, we sequentially removed one study; in turn, the pooled RR did not change substantially. Our sensitivity analysis showed that the RR for cancer ranged from a low of 1.41 (95% CI = 1.01–1.98, I2 = 74.6%) after removing the study by Wu et al. (25) to a high of 1.62 (95% CI = 1.05–2.48, I2 = 82.7%) after removing the study by Wu et al. (24; Supplementary Figure 4).
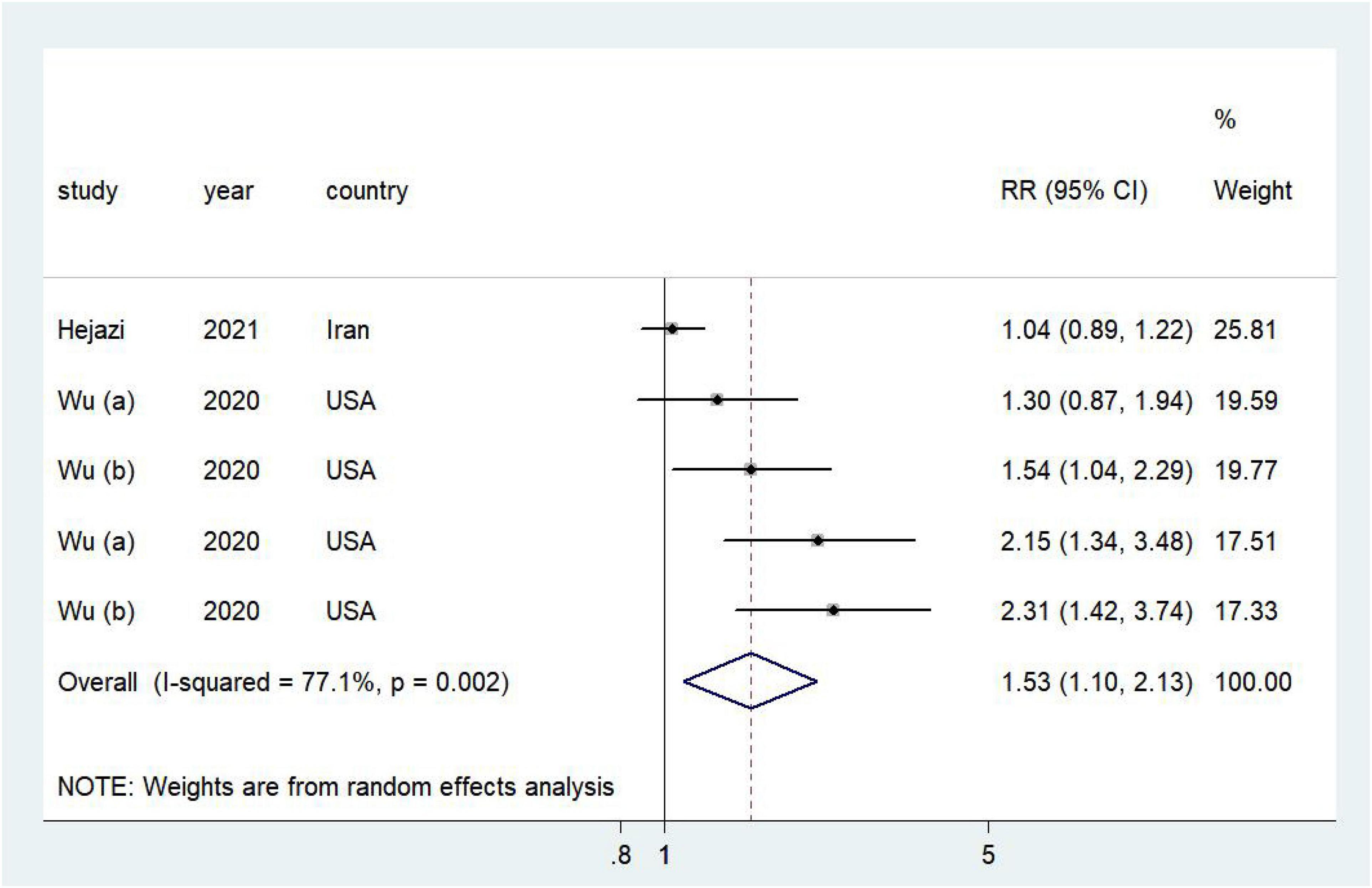
Figure 3. Forest plot (a random-effects model) of the association between DAL and cancer prognosis (highest vs. lowest). Squares indicate study-specific relative risk (RR), where the size of the square reflects the study-specific statistical weight; horizontal lines indicate the 95% CI; and diamonds denote the summary RR with 95% CI.
Discussion
To the best of our knowledge, the present review is the most comprehensive study reporting the relationship between DAL and cancer risk and prognosis. Findings from this systematic review and meta-analysis indicated that higher DAL might be an unfavorable factor for cancer risk and prognosis. These findings were consistently detected in numerous subgroups and sensitivity analyses.
Our findings are inconsistent with the previous systematic review, which included articles published before April 2015, and concluded that DAL was overall not significantly associated with an increased risk of cancer (15). However, this systematic review included only one study comprising 27,542 participants and 446 bladder cancers (23). Our systematic review and meta-analysis further included nine studies involving 227,253 participants published during the last 3 years (16–22, 24, 25). Of note, six low-risk studies were included in the present systematic review and meta-analysis (16, 19, 21, 22, 24, 25). Furthermore, numerous subgroup analyses and meta-regression analyses were conducted based on study characteristics and confounding factors.
In the subgroup analysis stratified by region, we only observed positive associations in studies carried out in the non-Asia region. This phenomenon could partly be attributed to the different DAL scores in patients with cancer from diverse regions. For example, when investigating 1,882 patients with breast cancer in the United States, it was found that the mean value was 2.25 for the PRAL score (19), whereas Safabakhsh et al. (21) reported that the mean value was –26.1 for the PRAL score based on 150 patients with breast cancer in Iran. Furthermore, a Western dietary pattern characterized by a high score of PRAL was associated with an increased risk of patients with cancer (43, 44).
The subgroup analyses suggested that DAL was positively associated with the risk of cancer in participants of age ≥50 years. Indeed, Frassetto et al. found that increasing age was associated with indicative of a progressively worsening low-level metabolic acidosis, and the changes seemed to be the most striking starting at about age 50 years (45). In addition, potential long-term effects of acidogenic diets are further compounded by the reduction of renal function typically from aging (45, 46). However, for the risk of cancer in participants at the age ≥50 years, more studies are warranted, mainly due to the presence of the high heterogeneity of these results.
Compared to the results of PRAL, the risk of cancer was considered to be substantially higher in NEAP. Both the PRAL and NEAP are approximate to DAL and highly correlated (r = 0.9; 8). However, the assessment of PRAL may be imprecise due to the error in the measurement of minerals or the protein intakes with low or high ranges (9, 47). In fact, Ronco et al. proposed that NEAP was found to be a better predictor of breast cancer risk than PRAL (20). One explanation is that PRAL relies on more information from the dietary database, which means that it may be more susceptible to confounding factors. Therefore, future studies should focus more on the accuracy of PRAL calculations.
Results of our subgroup analyses demonstrated that DAL increased the risk of breast and pancreatic cancers. PRAL is inversely correlated with the consumption of vegetables, while phytochemicals contained in vegetables may contribute to decreasing the level of the epidermal growth factor receptor (48, 49), which is known to be a major growth-stimulating factor exclusively in breast cancer (50). Furthermore, metabolic acidosis is found to reduce circulating adiponectin levels by inhibiting the transcription of the adiponectin gene (51); both the experimental and epidemiological studies have suggested a high level of adiponectin against the risk of pancreatic cancer (13, 52). In addition, we have previously found that a higher intake of red meat and dairy was statistically related to an increased risk of breast and pancreatic cancers (53–55). However, due to the limited number of studies, we yielded a null association between DAL and other cancers. Therefore, more prospective cohort studies of a specific cancer are needed to clarify these issues.
Several studies have indicated that consumption of high DAL dietary might be linked with a worse prognosis among patients with cancer (24, 25). Wu et al. (24) indicated that higher DAL was related to breast cancer-specific mortality and total mortality. Furthermore, Wu et al. (25) also found the same trend among 3,081 United States patients with breast cancer. Hejazi et al. (16), however, suggested that DAL was unrelated to the overall survival of cancer. They might miss an association between DAL and cancer survival because of unmeasured confounding and dietary changes. Of note, since dietary information was not updated during follow-up, they could not account for any changes in dietary consumption over time.
Although there was no evident mechanism to explain the relationship between DAL and cancer risk, several consensuses have been proposed. First of all, metabolic acidosis caused by DAL could promote cancer. Acid-base imbalance had been shown to regulate molecular activities, including insulin growth factor-1 (IGF-1; 56, 57) and osteoclast activation (58, 59), which may serve as intermediaries for cancer occurrence and promotion (60–62). In addition, acid-producing diets were often high in animal and processed proteins and low in fruits and vegetables, which were associated with a higher carcinogenic effect (63–65). The evidence also showed that DAL reduces circulating adiponectin (66), and both the experimental and epidemiological studies (13, 51, 52, 67) had shown that it played a role in the occurrence of cancer.
Regarding cancer prognosis, several studies existed to interpret this phenomenon. Metabolic acidosis had been shown to stimulate cancer metastasis in cell and animal models (68–70). In addition, metabolic acidosis depleted endogenous bicarbonate levels, which neutralize acids. A cross-sectional study showed that lower bicarbonate levels were associated with loss of muscle mass and reduced body function (32). As the precursors of bases, potassium (71), magnesium (72), and calcium (73) could inhibit the metastasis and the growth of cancer cells.
The principal strengths were that the present study was the most comprehensive systematic review to estimate the relationship between DAL and the risk and prognosis of cancer. We conducted a rigorous literature search to include all the pertinent studies. In consideration of study features and main adjustments for confounding variables, subgroup, sensitivity, and meta-regression analyses were conducted to probe into possible sources of heterogeneity. In addition, most of the selected articles had a low risk after using the NOS to evaluate the quality of all the included literature. Nevertheless, some limitations of this study should be recognized. First of all, measurement and recall bias in the assessment of dietary intake were inevitable. The calculation of PRAL, NEAP, NAE, and Pro:K based on self-reported data was collected by FFQ, DHQ, and 24-h dietary recalls. However, the majority of the included studies used valid and reliable FFQ, and it had been proved that FFQ could be more precise in assessing the association between diet and diseases (74). Second, the estimation methods of DAL had not been unified (8, 9). PRAL and NEAP were widely recognized and used, while Pro:K and NAE were seldom used, suggesting that one of the estimation methods could be used as the main calculation and the other three methods could be used as a sensitivity analysis in future studies. Third, we only located observational studies that fitted inclusion criteria, which means a large space for future research on DAL and cancer incidence and prognosis, especially in terms of prognosis. The studies on cancer prognosis were mainly concentrated on breast cancer, while other types of cancer had not been covered. Fourth, even though several confounding factors were considered, the included studies cannot rule out the possibility that unmeasured factors might have contributed to these associations.
In summary, the current systematic review and meta-analysis revealed that a higher DAL was associated with an increased risk and poor prognosis for cancers. Further large-scale prospective studies were warranted to explore the role of DAL in different cancers.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RW, H-HW, and Q-JW conceived the study. RW, F-HL, Y-HZ, T-TG, and Q-JW contributed to the design. RW, Z-YW, and Q-JW collected the data, cleaned the data and checked the discrepancy, and analyzed the data. RW, Z-YW, Y-FW, H-LX, M-LS, Y-HZ, T-TG, H-HW, and Q-JW interpreted the data. All the authors have interpreted the data, read the manuscript, and approved the final vision of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (No. 2017YFC0907402 to Y-HZ), the Natural Science Foundation of China (No. 82073647 and No. 81602918 to Q-JW and No. 82103914 to T-TG), the LiaoNing Revitalization Talents Program (No. XLYC1907102 to Q-JW), and the 345 Talent Project of Shengjing Hospital of China Medical University (Q-JW and T-TG).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.891936/full#supplementary-material
References
1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385:117–71. doi: 10.1016/S0140-6736(14)61682-2
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Boyle P. The globalisation of cancer. Lancet. (2006) 368:629–30. doi: 10.1016/S0140-6736(06)69225-8
4. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1223–49. doi: 10.1016/S0140-6736(20)30752-2
5. Kant AK. Dietary patterns and health outcomes. J Am Dietetic Assoc. (2004) 104:615–35. doi: 10.1016/j.jada.2004.01.010
6. Lee MR, Huang HL, Lin SW, Cheng MH, Lin YT, Chang SY, et al. Isoniazid concentration and NAT2 genotype predict risk of systemic drug reactions during 3HP for LTBI. J Clin Med. (2019) 8:812. doi: 10.3390/jcm8060812
7. Williams RS, Heilbronn LK, Chen DL, Coster ACF, Greenfield JR, Samocha-Bonet D. Dietary acid load, metabolic acidosis and insulin resistance – Lessons from cross-sectional and overfeeding studies in humans. Clin Nutr. (2016) 35:1084–90. doi: 10.1016/j.clnu.2015.08.002
8. Frassetto LA, Todd KM, Morris RC Jr. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr. (1998) 68:576–83. doi: 10.1093/ajcn/68.3.576
9. Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. (1994) 59:1356–61. doi: 10.1093/ajcn/59.6.1356
10. Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. (2013) 15:1125–37. doi: 10.1593/neo.13946
11. Wang H, Cai J, Du SX, Wei W, Shen XH. LAMC2 modulates the acidity of microenvironments to promote invasion and migration of pancreatic cancer cells via regulating AKT-dependent NHE1 activity. Exp Cell Res. (2020) 391:111984. doi: 10.1016/j.yexcr.2020.111984
12. Itoyama S, Noda E, Takamatsu S, Kondo J, Kawaguchi R, Shimosaka M, et al. Enterococcusspp. have higher fitness for survival, in a pH-dependent manner, in pancreatic juice among duodenal bacterial flora. JGH Open. (2022) 6:85–90. doi: 10.1002/jgh3.12703
13. Jiang JH, Fan YC, Zhang W, Shen YL, Liu TT, Yao M, et al. Adiponectin suppresses human pancreatic cancer growth through attenuating the β-Catenin signaling pathway. Int J Biol Sci. (2019) 15:253–64. doi: 10.7150/ijbs.27420
14. Jayedi A, Shab-Bidar S. Dietary acid load and risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective observational studies. Clin Nutr ESPEN. (2018) 23:10–8. doi: 10.1016/j.clnesp.2017.12.005
15. Fenton TR, Huang T. Systematic review of the association between dietary acid load, alkaline water and cancer. BMJ Open. (2016) 6:e010438. doi: 10.1136/bmjopen-2015-010438
16. Hejazi E, Emamat H, Sharafkhah M, Saidpour A, Poustchi H, Sepanlou S, et al. Dietary acid load and mortality from all causes, CVD and cancer: results from the Golestan Cohort Study. Br J Nutr. (2021):1–7. [Online ahead of print], doi: 10.1017/S0007114521003135
17. Nasab SJ, Rafiee P, Bahrami A, Rezaeimanesh N, Rashidkhani B, Sohrab G, et al. Diet-dependent acid load and the risk of colorectal cancer and adenoma: a case-control study. Public Health Nutr. (2021) 24:4474–81. doi: 10.1017/S1368980020003420
18. Milajerdi A, Shayanfar M, Benisi-Kohansal S, Mohammad-Shirazi M, Sharifi G, Tabibi H, et al. A case-control study on dietary acid load in relation to glioma. Nutr Cancer. (2021) 74:1644–51. doi: 10.1080/01635581.2021.1957134
19. Park YM, Steck SE, Fung TT, Merchant AT, Hodgson ME, Keller JA, et al. Higher diet-dependent acid load is associated with risk of breast cancer: findings from the sister study. Int J Cancer. (2019) 144:1834–43. doi: 10.1002/ijc.31889
20. Ronco AL, Martínez-López W, Calderón JM, Golomar W. Dietary acid load and lung cancer risk: a case-control study in men. Cancer Treat Res Commun. (2021) 28:100382. doi: 10.1016/j.ctarc.2021.100382
21. Safabakhsh M, Imani H, Yaseri M, Omranipour R, Shab-Bidar S. Higher dietary acid load is not associated with risk of breast cancer in Iranian women. Cancer Rep. (2020) 3:e1212. doi: 10.1002/cnr2.1212
22. Shi LW, Wu YL, Hu JJ, Yang PF, Sun WP, Gao J, et al. Dietary acid load and the risk of pancreatic cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prevent. (2021) 30:1009–19. doi: 10.1158/1055-9965.EPI-20-1293
23. Wright ME, Michaud DS, Pietinen P, Taylor PR, Virtamo J, Albanes D. Estimated Urine pH and bladder cancer risk in a cohort of male smokers (Finland)*. Cancer Causes Control. (2005) 16:1117–23. doi: 10.1007/s10552-005-0348-9
24. Wu TY, Hsu FC, Pierce JP. Increased acid-producing diet and past smoking intensity are associated with worse prognoses among breast cancer survivors: a prospective cohort study. J Clin Med. (2020) 9:1817. doi: 10.3390/jcm9061817
25. Wu TY, Hsu FC, Wang SR, Luong D, Pierce JP. Hemoglobin A1C levels modify associations between dietary acid load and breast cancer recurrence. Nutrients. (2020) 12:578. doi: 10.3390/nu12020578
26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
27. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
28. Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Dietetic Assoc. (1995) 95:791–7. doi: 10.1016/S0002-8223(95)00219-7
29. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2013). Available online at: http://www.ohri.ca/programsclinical_epidemiology/oxford.asp
30. Jiang YT, Gong TT, Zhang JY, Li XY, Gao S, Zhao YH, et al. Infertility and ovarian cancer risk: evidence from nine prospective cohort studies. Int J Cancer. (2020) 147:2121–30. doi: 10.1002/ijc.33012
31. Gong TT, Wu QJ, Lin B, Ruan SK, Kushima M, Takimoto M, et al. Observational studies on the association between post-diagnostic metformin use and survival in ovarian cancer: a systematic review and meta-analysis. Front Oncol. (2019) 9:458. doi: 10.3389/fonc.2019.00458
32. Abramowitz MK, Hostetter TH, Melamed ML. Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. Am J Kidney Dis. (2011) 58:29–38. doi: 10.1053/j.ajkd.2010.12.021
33. Ades AE, Lu G, Higgins JPT. The interpretation of random-effects meta-analysis in decision models. Med Decision Making. (2005) 25:646–54. doi: 10.1177/0272989X05282643
34. Jackson D, White IR, Thompson SG. Extending dersimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. (2010) 29:1282–97. doi: 10.1002/sim.3602
35. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
36. Trikalinos TA, Salanti G, Khoury MJ, Ioannidis JPA. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am J Epidemiol. (2006) 163:300–9. doi: 10.1093/aje/kwj046
37. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
38. Ronco A, Calderón J, Mendoza B. Dietary acid load and breast cancer risk: a case-control study in Uruguay. Eur J Cancer. (2020) 138:S122–122.
39. Tessou KD, Hector Lemus H, Hsu FC, Pierce J, Hong S, Brown L, et al. Independent and joint impacts of acid-producing diets and depression on physical health among breast cancer survivors. Nutrients. (2021) 13:2422. doi: 10.3390/nu13072422
40. Wu TY, Pierce J, Seaver P. Dietary acid load in relation to inflammation and glycemic status in women with breast cancer. Curr Dev Nutr. (2019) 3:nzz039.P18-095-19. doi: 10.1093/cdn/nzz039.P18-095-19
41. Wu TY, Hsu FC, Pierce JP. Acid-producing diet and depressive symptoms among breast cancer survivors: a longitudinal study. Cancers. (2020) 12:3183. doi: 10.3390/cancers12113183
42. Wu TY, Seaver P, Lemus H, Hollenbach K, Wang E, Pierce JP, et al. Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients. (2019) 11:1913. doi: 10.3390/nu11081913
43. Castelló A, Pollán M, Buijsse B, Ruiz A, Casas AM, Lope V, et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer. (2014) 111:1454–62. doi: 10.1038/bjc.2014.434
44. Dianatinasab M, Wesselius A, Salehi-Abargouei A, Yu EYW, Brinkman M, Fararouei M, et al. Adherence to a Western dietary pattern and risk of bladder cancer: a pooled analysis of 13 cohort studies of the Bladder Cancer Epidemiology and Nutritional Determinants international study. Int J Cancer. (2020) 147:3394–403. doi: 10.1002/ijc.33173
45. Frassetto L, Sebastian A. Age and systemic acid-base equilibrium: analysis of published data. J Gerontol Series A Biol Sci Med Sci. (1996) 51:B91–9. doi: 10.1093/gerona/51a.1.b91
46. Frassetto LA, Morris RC Jr., Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. (1996) 271:F1114–22. doi: 10.1152/ajprenal.1996.271.6.F1114
47. Frassetto LA, Morris RC Jr., Sebastian A. A practical approach to the balance between acid production and renal acid excretion in humans. J Nephrol. (2006) 19:S33–40.
48. Moiseeva EP, Heukers R, Manson MM. EGFR and Src are involved in indole-3-carbinol-induced death and cell cycle arrest of human breast cancer cells. Carcinogenesis. (2007) 28:435–45. doi: 10.1093/carcin/bgl171
49. Bao PP, Shu XO, Zheng Y, Cai H, Ruan ZX, Gu K, et al. Fruit, vegetable, and animal food intake and breast cancer risk by hormone receptor status. Nutr Cancer. (2012) 64:806–19. doi: 10.1080/01635581.2012.707277
50. Biswas DK, Cruz AP, Gansberger E, Pardee AB. Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc Natl Acad Sci U S A. (2000) 97:8542–7. doi: 10.1073/pnas.97.15.8542
51. Disthabanchong S, Niticharoenpong K, Radinahamed P, Stitchantrakul W, Ongphiphadhanakul B, Hongeng S. Metabolic acidosis lowers circulating adiponectin through inhibition of adiponectin gene transcription. Nephrol Dialysis Transplant. (2011) 26:592–8. doi: 10.1093/ndt/gfq410
52. Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, et al. A prospective study of Plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Institute. (2013) 105:95–103. doi: 10.1093/jnci/djs474
53. Tavani A, Vecchia CL, Gallus S, Lagiou P, Trichopoulos D, Levi F, et al. Red meat intake and cancer risk: a study in Italy. Int J Cancer. (2000) 86:425–8. doi: 10.1002/(sici)1097-0215(20000501)86:33.0.co;2-s
54. Rudolph A, Knobelsdorff-Brenkenhoff FV, Wassmuth R, Prothmann M, Utz W, Schulz-Menger J, et al. Assessment of nonischemic fibrosis in hypertrophic cardiomyopathy: comparison of gadopentetate dimeglumine and gadobenate dimeglumine for enhanced cardiovascular magnetic resonance imaging. J Magnetic Resonance Imaging. (2014) 39:1153–60. doi: 10.1002/jmri.24264
55. Mesquita HBB, Maisonneuve P, Runia S, Moerman CJ. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int J Cancer. (1991) 48:540–9. doi: 10.1002/ijc.2910480411
56. Challa A, Chan W, Krieg RJ Jr., Thabet MA, Liu F, Hintz RL, et al. Effect of metabolic acidosis on the expression of insulin-like growth factor and growth hormone receptor. Kidney Int. (1993) 44:1224–7. doi: 10.1038/ki.1993.372
57. Clemmons DR, Klibanski A, Underwood LE, McArthur JW, Ridgway EC, Beitins IZ, et al. Reduction of plasma immunoreactive somatomedin C during fasting in humans. J Clin Endocrinol Metabol. (1981) 53:1247. doi: 10.1210/jcem-53-6-1247
58. Pizzorno J, Frassetto LA, Katzinger J. Diet-induced acidosis: is it real and clinically relevant? Br J Nutr. (2010) 103:1185–94. doi: 10.1017/S0007114509993047
59. Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Mineral Res. (2003) 18:1317–25. doi: 10.1359/jbmr.2003.18.7.1317
60. Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. (2002) 5:298–302. doi: 10.1016/s1470-2045(02)00731-3
61. González-Suárez E. RANKL inhibition: a promising novel strategy for breast cancer treatment. Clin Transl Oncol. (2011) 13:222–8. doi: 10.1007/s12094-011-0646-5
62. Wu J, Zeng R, Huang J, Li XF, Zhang JR, Chung-Man Ho J. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. (2016) 8:730. doi: 10.3390/nu8110730
63. Chow WH, Gridley G, McLaughlin JK, Mandel JS, Wacholder S, Blot WJ, et al. Protein intake and risk of renal cell cancer. J Natl Cancer Institute. (1994) 86:1131–9. doi: 10.1093/jnci/86.15.1131
64. Maso LD, Augustin LSA, Karalis A, Talamini R, Franceschi S, Trichopoulos D, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metabol. (2004) 89:1160–3. doi: 10.1210/jc.2003-031716
65. Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. (2007) 92:255–63. doi: 10.1210/jc.2006-1371
66. Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab. (2012) 9:72. doi: 10.1186/1743-7075-9-72
67. Pérez-Neri I, Diéguez-Campa CE. Taurine and aneurysmal subarachnoid hemorrhage prognosis. Postgraduate Med. (2018) 130:669–72. doi: 10.1080/00325481.2018.1533382
68. Huang S, Tang Y, Peng XS, Cai XD, Wa QD, Ren D, et al. Acidic extracellular pH promotes prostate cancer bone metastasis by enhancing PC-3 stem cell characteristics, cell invasiveness and VEGF-induced vasculogenesis of BM-EPCs. Oncol Rep. (2016) 36:2025–32. doi: 10.3892/or.2016.4997
69. Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. (2006) 66:6699–707. doi: 10.1158/0008-5472.CAN-06-0983
70. Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. (2013) 13:89. doi: 10.1186/1475-2867-13-89
71. Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science. (2019) 363:eaau0135. doi: 10.1126/science.aau0135
72. Mahabir S, Wei QY, Barrera SL, Dong YQ, Etzel CJ, Spitz MR, et al. Dietary magnesium and DNA repair capacity as risk factors for lung cancer. Carcinogenesis. (2008) 29:949–56. doi: 10.1093/carcin/bgn043
73. Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. (2009) 29:3687–9.
Keywords: dietary acid load, prognosis, risk, systematic review, cancer, meta-analysis
Citation: Wang R, Wen Z-Y, Liu F-H, Wei Y-F, Xu H-L, Sun M-L, Zhao Y-H, Gong T-T, Wang H-H and Wu Q-J (2022) Association between dietary acid load and cancer risk and prognosis: An updated systematic review and meta-analysis of observational studies. Front. Nutr. 9:891936. doi: 10.3389/fnut.2022.891936
Received: 09 March 2022; Accepted: 04 July 2022;
Published: 27 July 2022.
Edited by:
Rafaela Rosário, University of Minho, PortugalReviewed by:
Fatemeh Toorang, Tehran University of Medical Sciences, IranAgnieszka Micek, Jagiellonian University, Poland
Copyright © 2022 Wang, Wen, Liu, Wei, Xu, Sun, Zhao, Gong, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Han Wang, MTM5MTg2MDc2NEBxcS5jb20=; Qi-Jun Wu, d3VxakBzai1ob3NwaXRhbC5vcmc=
†These authors have contributed equally to this work
 Ran Wang
Ran Wang Zhao-Yan Wen1,2†
Zhao-Yan Wen1,2† Yi-Fan Wei
Yi-Fan Wei Ting-Ting Gong
Ting-Ting Gong Qi-Jun Wu
Qi-Jun Wu