
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 16 May 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.888960
This article is part of the Research Topic Metabolic Consequences of Malnutrition: How to Balance Nutrients and Genes View all 15 articles
 Hui Chen1,2,3
Hui Chen1,2,3 Huizhen Zheng1,2
Huizhen Zheng1,2 Tiejun Li4
Tiejun Li4 Qihong Jiang1,2
Qihong Jiang1,2 Shulai Liu1,2
Shulai Liu1,2 Xuxia Zhou1,2
Xuxia Zhou1,2 Yuting Ding1,2
Yuting Ding1,2 Xingwei Xiang1,2*
Xingwei Xiang1,2*Oyster peptide (OP) has exhibited useful biological activities and can be used in multi-functional foods. OP has been reported to play a significant role in intestinal protection, but its specific mechanism is still not completely understood. The aim of this study was to analyze the potential effect of OP on oxidative damage of mice intestine induced by cyclophosphamide (Cy). The experimental results revealed that intragastric administration of OP significantly increased average bodyweight, improved ileum tissue morphology and villus structure, as well as increased the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) in oxidized mice serum and liver. The content of malondialdehyde (MDA) in the mice serum and liver homogenate was found to be markedly decreased. Moreover, OP significantly increased the relative mRNA expression levels of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), quinone oxidoreductase (NQO1) and heme oxidase-1 (HO-1) in ileum. Western-blot results indicated that prior administration of OP significantly up-regulated the Nrf2 production in ileum, and substantially decreased then Keap1 gene expression. In conclusion, intake of OP was found to markedly improve intestinal oxidative stress in vivo, and this effect was primarily mediated through the simulation of antioxidant Nrf2-Keap1 signaling pathway. This study is beneficial to the application of peptide nutrients in the prevention or mitigation of intestinal oxidative damage.
Oxidative stress related injury refers to the imbalance between oxidative and anti-oxidative reactions caused by excessive production of reactive oxygen species (ROS) in the body (1). The various ROS species are primarily generated and exhibit their functions in mitochondria. These include superoxide anion radical, hydrogen peroxide (H2O2), alkane peroxide radical, lipid peroxide radical, nitric oxide (NO·) and hydroxyl radical (HO·) (2). At low concentrations, ROS generally plays a beneficial regulatory role through inducing apoptosis of injured or senescent cells and acting as the different mediators of cell signaling pathway regulation (3). However, excessive production of ROS can be harmful to the body, leading to significant oxidative stress and can substantially weaken intestinal mucosal immune barrier function (4). The intestine is a vital organ of the human body and the major site of digestion and absorption. As a biological barrier (5), it is frequently exposed to oxidative stress and other stress conditions, resulting in increased infiltration of the toxins. Excessive accumulation of oxygen free radicals can effectively destroy the gut mucosal barrier and disrupt intestinal flora, thus affecting the body's homeostasis system (6). Furthermore, oxidative stress can function as an important factor in intestinal inflammation. Accumulating evidences suggest that the use of antioxidants could be useful for the treatment of inflammatory bowel disease, intestinal mucosal infections, ulcerative colitis and colon cancer (7–9).
Cyclophosphamide (Cy) is an alkylating compound, which is often used as an antitumor agent and chemotherapeutic agent (10, 11). It mainly acts through causing cross-linking in DNA, blocking DNA replication as well as synthesis, and thus preventing cell proliferation. The effects of Cy have been found to be non-specific and it can not only eliminate the malignant cells, but at the same time can also damage the various normal cells (such as lymphocytes, gastrointestinal mucosa cells, etc.) (12). It can also disrupt the normal structure of gastrointestinal mucosa and adversely affect intestinal mucosa thereby causing immune disorders and severe side-effects. Extensive use of Cy can cause nausea and vomiting, diarrhea, intestinal barrier destruction, increase intestinal permeability, gastrointestinal mucosal damage, enhance exposure to immune deficiency and secondary infection, oxidative stress, intestinal microflora structural disorders and other side effects (13). Thus, in this study, Cy was used as an inducer to establish a model of intestinal oxidative damage in mice.
Antioxidants present in the food can significantly improve the oxidative state of the body and help to maintain the oxidative/antioxidant balance in the gut (14, 15). The use of food proteins and their constituent peptides which exhibit relatively fewer side effects as antioxidants can serve as an effective approach. Oyster is one of the largest shellfish varieties found in the world. It is extremely rich in different nutrients, including the trace elements, proteins, fatty acids, glycogen, vitamins and taurine. Oyster, as the largest shellfish cultured in the world, is rich in various marine based active peptides, which has become a research hotspot at home and abroad (16, 17). The Marine oyster peptides (OP) possess specific amino acid compositions and sequences, which are quite different from those of the terrestrial proteins. There may be some peptides with specific biological activities. In recent years, a number of studies have reported that oyster active peptide can display multifarious biological activities, such as those related to anti-oxidation (18), lowering of blood pressure (19), inhibition of tumor cancer (20), improving learning and memory (21), and enhancing immunity (22). In addition, a few studies have found that the peptides obtained from the various substances can also exhibit potent antioxidant properties. For example, the antioxidant peptide (ATVY) obtained by Yang et al. from the hydrolyzed protein of black shark skin has displayed good free radical scavenging activity and can be used for the production of antioxidant food additives (23). In addition, the purified peptides obtained from the extract of Marphysa sanguinea have demonstrated significant antioxidant activity and reduced the contents of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and malondialdehyde (MDA) (24). It also inhibited the abnormal secretion of proinflammatory cytokines such as nitric oxide (NO), inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2). In another study, Mirzapour-kouhdasht et al. prepared gelatin peptides from fish by-products which displayed potent free radical scavenging effects and pronounced antioxidant effects after gastrointestinal digestion in vitro (25). In addition, the small molecule peptides obtained from ALI have exhibited strong DPPH (26), hydroxyl radical and superoxide radical scavenging activities, iron reducing antioxidant and metal chelating properties, and inhibited enzymatic activities of ACE, α-amylase and glucosidase. However, there are only few studies which have examined the effect of OP on oxidative damage of intestinal mucosa induced by Cy.
Here, the potential protective effects of OP on intestinal mucosa were explored by establishing a oxidative injury mouse model of intestinal mucosa, and the underlying mechanism was elucidated. As the advance and the novelty, this study analyzed the relationship between antioxidative peptide and Nrf2-Keap1 pathway in vivo. The findings of this study might provide evidence for further understanding the protective mechanism of OP on intestinal mucosal injury, and thus provide a better natural alternative health food both for the prevention and treatment of oxidative stress injury.
Male SPF BALB/C mice aged 5–7 weeks and weighing 15–18 g were used in the present study. The mice were purchased from Slack Laboratory Animal Co., LTD. (Shanghai, China), and the license number was: SCXK (Shanghai) 2017-0005. Before the formal beginning of the experiments, the mice were placed in the animal room for adaptive feeding for 7 days, during which they were free to ingest food and water. Environmental conditions were set as: temperature 25 ± 1°C, humidity 50 ± 3%, light and dark cycle for 12 h. This study was carried out by the guidelines of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, which is approved by the Animal Ethics Committee of Zhejiang University of Technology (20210308038).
Cyclophosphamide was obtained from Aladdin Chemical Co., LTD (Shanghai, China). Catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and malondialdehyde (MDA) detection kits were purchased from Nanjing Jianguo Institute of Biological Engineering (Nanjing, China). Antibodies against Nrf2 and Keap1 were obtained from Abcam (Cambridge, UK). In addition, monoclonal antibody against β-actin was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All other reagents obtained were commercially available and of analytical grade.
The acclimated mice were randomly divided into four different groups according to body weight (n = 8): control group (C), Cy model group (Y), OP high-dose group (HP) and low-dose group (LP). Mice were intragastrically administered OP for 21 successive days, and intraperitoneal Cy was injected for 3 days (days 18–21). The dose of Cy was determined according to our previous research, as shown in Table 1. The body weight and the vital physical signs of the mice were examined and recorded daily. At the end of the experiment, all the mice were sacrificed by cervical dislocation. The cardiac blood samples were collected at 2,000 r/min and centrifuged for 10 min, −80 °C. The samples of thymus, spleen, liver, ileum, serum and feces were obtained and stored at −80°C for further experiment, respectively.
On the 21st day of the experiment, the organ index of the mice after the death was determined by using the following formula:
Organ Index = organ weight (mg)/body weight (g) × 10
The fresh ileum of mice was rinsed several times with the normal saline and placed in a clean eppendorf tube. The ileum was fixed in 10 mL 4% paraformaldehyde at room temperature for 24 h. Thereafter, ethanol dehydration, xylene transparent and paraffin embedding were carried out, respectively. Finally, the paraffin blocks were continuously cut by a slicer to obtain intestinal sections with a thickness of approximately 6 μm. Three sections of each tissue block were randomly selected for hematoxylin-eosin (HE) staining (27), and the histopathological changes of the intestine were observed under the microscope (Olympus CX23, Japan).
The ileum was removed after mouse the dissection, washed with PBS solution, and dried with filter article. A section of the empty intestine was obtained and fixed in a centrifuge tube containing glutaraldehyde. Transmission electron microscope (TEM) samples were treated as follows: The ileum of mice was washed several times with PBS solution and fixed in 1% osmium solution for 1 h. After rinsing with the distilled water for three times, the ileum was dehydrated with gradient ethanol and pure acetone successively. The ileum tissue was placed overnight in Spurr resin and polymerized at the temperature of 70°C. Thereafter, the intestine was cut into slices and then stained with uranium acetate and lead citrate. The ileum tissue was observed by TEM and SEM photographed (28). The sample was dehydrated with the gradient ethanol and embedded in the sample section. The samples were dried with liquid carbon dioxide critical point. The ileum surface was coated with a layer of metal composite material with a thickness of about 30 nm by ion sputtering.
The mice were anesthetized and sacrificed by neck amputation, followed by blood collection. The blood was subjected to the centrifugation at 4000 r/min and incubated at 4°C for 15 min. SOD, GSH-Px, CAT and MDA antioxidant kits were used to detect the contents of the various antioxidant factors in the serum of different groups of mice, according to the manufacturer's instructions.
The mouse livers were weighed to about 100 mg and thoroughly ground in a tissue homogenizer. The supernatant was obtained after centrifugation at 3000 r/min for 10 min and stored at the temperature of 4°C. The contents of antioxidant enzymes SOD, GSH-Px, CAT and MDA were detected on the basis of the manufacturer's instructions of the commercial antioxidant kits.
The relative mRNA expression levels of GSH-PX, SOD, CAT, NQO1 and HO-1 were detected through RT-PCR. The total RNA of the sample was extracted from the ileum by using 1 mL ice-cold TRIzol reagent (Ambion, Carlsbad, USA). The mass and concentrations of extracted RNA were determined via spectrophotometry at 260 and 280 nm, respectively. The first strand of cDNA was synthesized by using a Prime-Script 1st Strand cDNA Synthesis Kit (Takara, Dalian, Japan). The different primer sequences were designed by Primer 5 software and synthesized by Shanghai Shenggong Biotechnology Co., Ltd. (Shanghai, China), the details of which have been shown in Table 2. The qRT-PCR was performed by using the Applied Biosystems ViiA™7 Real-Time PCR system. Thereafter, PCR reaction was carried out according to the standard operation process of SYBR Green qPCR test kit. The RT-PCR reaction was carried out based on the following procedure: pre-denaturation at the temperature of 95°C for 30 s, followed by 40 cycles of the conditions: 95°C for 5 s and 65°C for 34 s. The cyclic threshold (CT) values of each gene were normalized using β-actin. With β-actin as internal reference gene, the relative mRNA expression level of target gene was calculated according to the 2−ΔΔCt method (29). The experiment was repeated in triplicate.
The ileum lysates were prepared using the Radio Immunoprecipitation Assay (RIPA) lysate buffer. The protein concentrations of the lysates were analyzed through bicinchoninic acid (BCA) protein determination kit (Beyotime, Shanghai, China). Approximately 30 μg of proteins were isolated using SDS-PAGE (10%) solution and then transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane (Merck Millipore, MA, USA). The membrane was then blocked with 5% skim milk and incubated with the primary antibody (Abcam, Cambridge, UK) at the temperature of 4°C overnight. The membranes were washed three times with TBS for 5 min. Thereafter, the membrane was incubated within HRP-conjugated secondary antibody at the room temperature for about 2 h and rinsed three times for 15 min. The chemiluminescence imaging was carried out using the protein using enhanced chemiluminescence (ECL) reagent (Beyotime, Shanghai, China) (30). Finally, Image J software was used to test and analyze the band density, and each sample was analyzed three times.
All experimental data was analyzed using SPSS17.0 software. The experimental data was expressed as mean ± standard error (SEM). One-way variance (ANOVA) and Tukey tests were adopted for the statistical significance analysis. P < 0.05 was considered to be statistically significant.
The mice in Cy treated group showed significant stress reactions such as hair loss and luster, lethargy, slow movement and yellow feces on the second day after intraperitoneal injection of Cy, compared to the normal group (Figure 1). In addition, the average daily gain of mice after Cy injection was found to be decreased (P < 0.05). Compared with Cy group, early intragastric administration of high-dose OP significantly increased the average daily gain of mice (P < 0.05). However, the mice treated with low dose OP also significantly increased the average daily gain, but the effect was not found to be significant (P > 0.05).
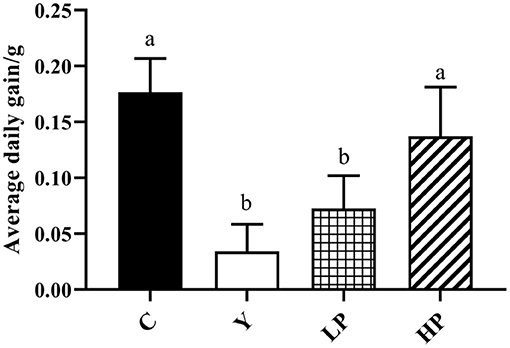
Figure 1. Effects of OP on the average daily gain in oxidative injured mice. Different letters in the figure represent significant differences (P < 0.05). C: the normal group; Y: the Cy-induced group; LP: Cy+200 mg/kg OP; HP: Cy+400 mg/kg OP.
The potential effects of OP on ileum tissue morphology of mice were also analyzed and the results have been shown in Figure 2. It was found that in the normal group of mice ileum, villi was arranged orderly, appeared slender and compact with complete structure as well as thick intestinal wall. In the Cy treated group, the villi of ileum were observed to be loose and short, and some of the villi were broken and detached. After the OP pre-treatment, the length of villi in the LP and HP group was restored, and the structure was observed to be more complete as well as orderly, and the damaged condition was substantially improved. These results suggested that intake of OP can effectively protect ileum tissue of immunosuppressed mice to a certain extent, and reduce intestinal villi damage caused by Cy.
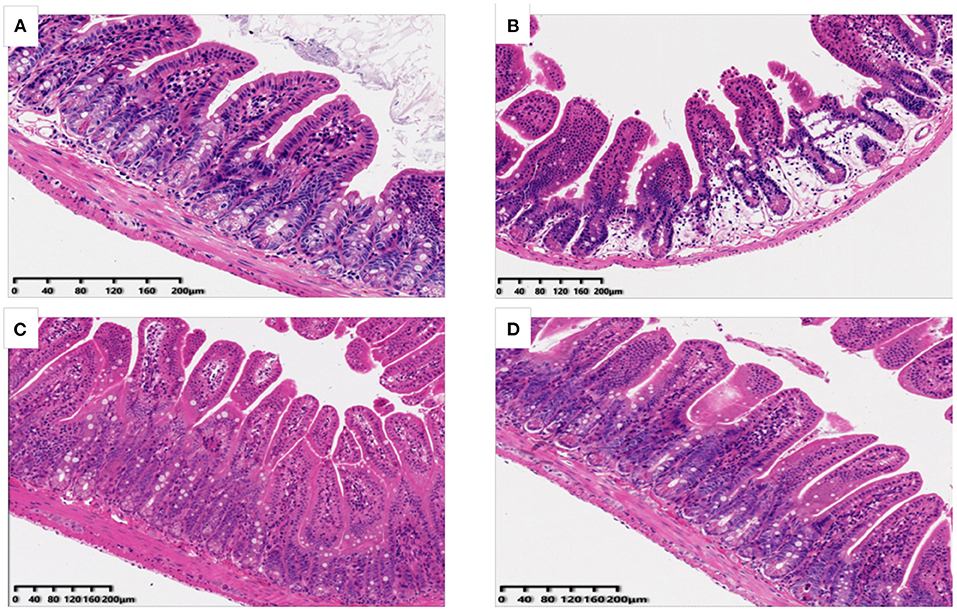
Figure 2. Effects of OP on ileum histomorphology in oxidative injured mice [(A): Normal group; (B): Cy-induced group; (C): LP: Cy+200 mg/kg OP; (D): HP Cy+400 mg/kg OP; 200x].
TEM results of ileum of all mice have been shown in Figures 3A–C. In the normal group, the microvilli were found to be arranged and distributed in a very orderly manner, with long villi and almost no gap, and the close connection was clearly visible in the figure. However, compared with the normal group, Cy induced the intestinal microvilli of mice into rare and disordered state, with irregular tight connections, and the intestinal villi were observed to be severely damaged. After early intragastric administration of OP, the intestinal mucosa structure of the mice was noted to be significantly improved and the structure of microvilli was restored. The SEM results of ileum showed that the ileum structure of mice in the normal group was flat as well as smooth, and the microvilli were closely connected, in Figures 3D–F. The microvilli in the Cy model group were not consistent in height and were arranged irregularly, and some microvilli were found to be broken and fractured. The intestinal microvilli of mice treated with OP appeared to be relatively repaired, with small spaces between them as well as smooth surface, and almost returned to the normal state. These results indicated that intake of OP can markedly repair the damage of Cy induced ileum mucosa in mice.
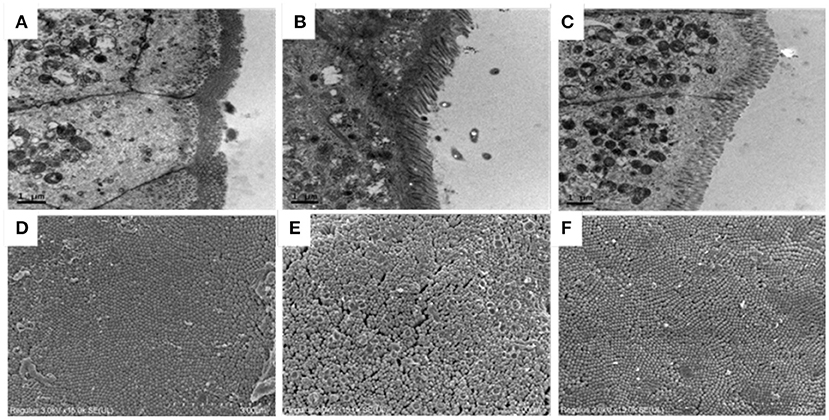
Figure 3. Effects of OP on ileum villi morphology in immunosuppressed mice. (A–C) Transmission electron microscopy, 10K×, (A): Normal group, (B): Cy-induced group, (C): Cy+400 mg/kg OP, (D–F): Scanning electron microscopy, 15K×, (D): Normal group, (E): Cy-induced group, and (F): Cy+400 mg/kg OP.
We also investigated the dose dependent effects of OP on the content or activities of serum antioxidant enzyme in mice, as shown in Figure 4. The activities of SOD, CAT and GSH-Px in serum of Cy treated mice were distinctly decreased, and the activity of MDA was significantly increased (P < 0.05), compared with normal group. The intervention with high dose OP increased the contents of SOD, CAT and GSH-Px in serum of mice (P < 0.05), but significantly decreased the activity of MDA (P < 0.05), compared with the Cy group. Interestingly, low dose of OP intake also increased GSH-Px activity of serum in mice (P < 0.05), and decreased the content of MDA in the serum (P < 0.05), but did not display significant effect on the activities of SOD and CAT in the serum (P > 0.05). These findings suggested that the prior administration of OP boosted the antioxidant capacity in vivo, thereby modulating the oxidative stress response caused by Cy even in a dose-dependent manner.
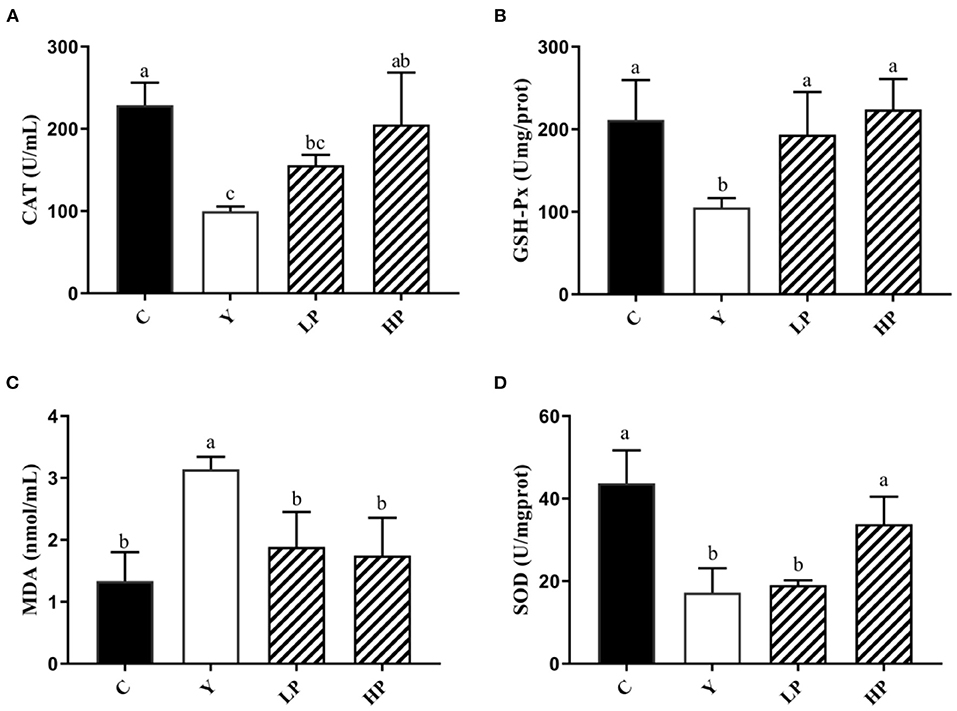
Figure 4. Effects of OP on the contents of CAT (A), GSH-Px (B), MDA (C), and SOD (D) in serum of oxidative injured mice. Different letters in the figure represent significant differences (P < 0.05). C: the normal group; Y: the Cy-induced group; LP: Cy+200 mg/kg OP; HP: Cy+400 mg/kg OP.
In addition, the dose dependent effects of OP on the activities of the various antioxidant factors in the mouse liver tissue was also analyzed (Figure 5). It was found that compared with Cy treated mice, the activities of CAT, GSH-Px and SOD in liver decreased significantly (P < 0.05), while the content of MDA was observed to be increased more than 3 times (P < 0.05), thereby suggesting that serious oxidative stress occurred in liver after Cy was injected into the mice. Moreover, Compared with Cy group, CAT, GSH-Px and SOD in the liver of mice increased substantially (P < 0.05), and MDA production was decreased approximately by 50% in LP mice and 70% in HP mice (P < 0.05) under OP prevention. The above findings indicated that OP also exhibited a specific recovery and regulation effect on oxidative damage caused in the mouse liver tissues, and can display a dose-dependent protective effect.
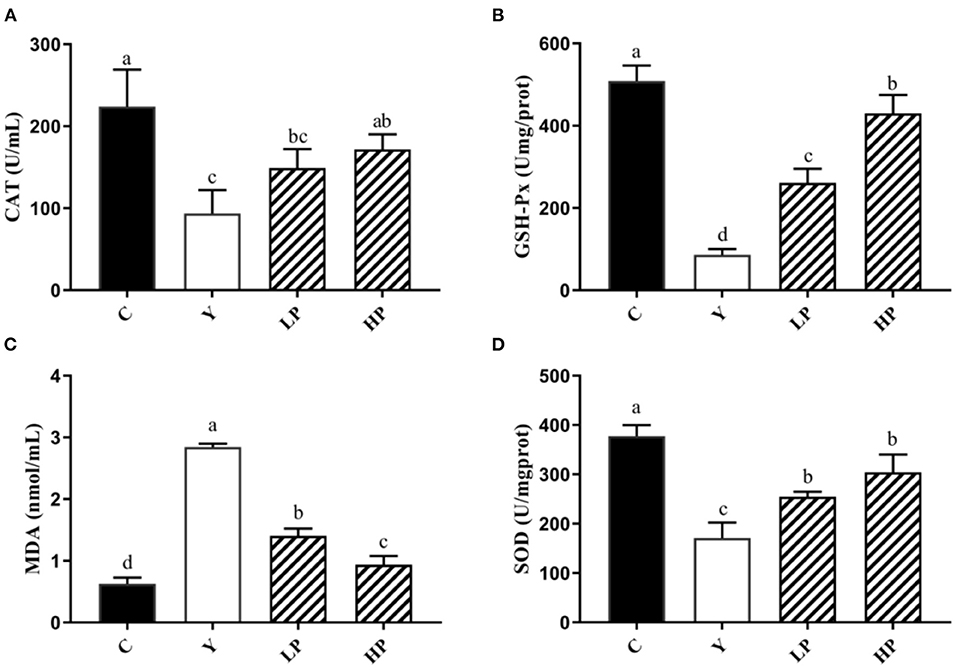
Figure 5. Effects of OP on the contents of CAT (A), GSH-Px (B), MDA (C), and SOD (D) in liver of oxidative injured mice. Different letters in the figure represent significant differences (P < 0.05). C: the normal group; Y: the Cy-induced group; LP: Cy+200 mg/kg OP; HP: Cy+400 mg/kg OP.
The effect of OP on the relative expression of antioxidant related genes in the ileum of Cy-induced mice was also examined. The experimental results have been shown as in Figure 6. It was noted that compared with the normal group, intraperitoneal injection of Cy significantly reduced the relative mRNA expression of SOD, GSH-Px, HO-1 and NQO1 (P < 0.05). Moreover, compared with the Cy treated group, the relative expression of SOD, GSH-Px, HO-1 and NQO1 genes were significantly increased upon prior intake of OP (P < 0.05) in a dose-dependent manner. These results suggested that OP can regulate the expression of various antioxidant genes and exert substantial protective effect on intestinal oxidation in immunosuppressed mice, which can partially recover the decreased antioxidant capacity caused by Cy.
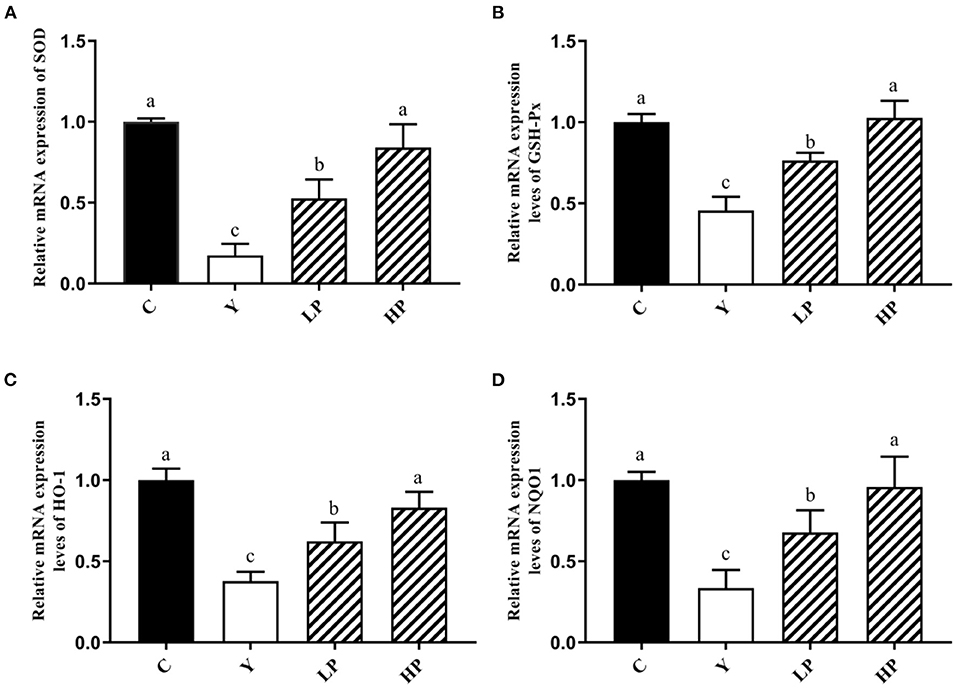
Figure 6. Effects of OP on the relative mRNA expression levels of intestinal antioxidant factors SOD (A), GSH-Px (B), HO-1 (C), and NQO1 (D) in oxidative injured mice. Different letters in the figure represent significant differences (P < 0.05). C: the normal group; Y: the Cy-induced group; LP: Cy+200 mg/kg OP; HP: Cy+400 mg/kg OP.
Finally, we investigated the dose dependent effect of OP on the relative expression of major proteins of Nrf2-Keap1 pathway in the mice ileum. The result has been shown in Figure 7. The relative expression level of Nrf2 protein in ileum tissue of mice after Cy treatment was found to be significantly reduced (P < 0.05), but the relative expression level of Keap1 protein was significantly increased (P < 0.05) as compared with the normal group. Interestingly, prior intake of OP significantly upregulated Nrf2 protein expression level in ileum of the mice (P < 0.05), while it significantly decreased Keap1 protein expression in a dose-dependent manner (P < 0.05), as compared with the Cy group. These findings suggested that OP might play a protective role on intestinal oxidative stress in immunosuppressed mice through activating Nrf2-Keap1 pathway.
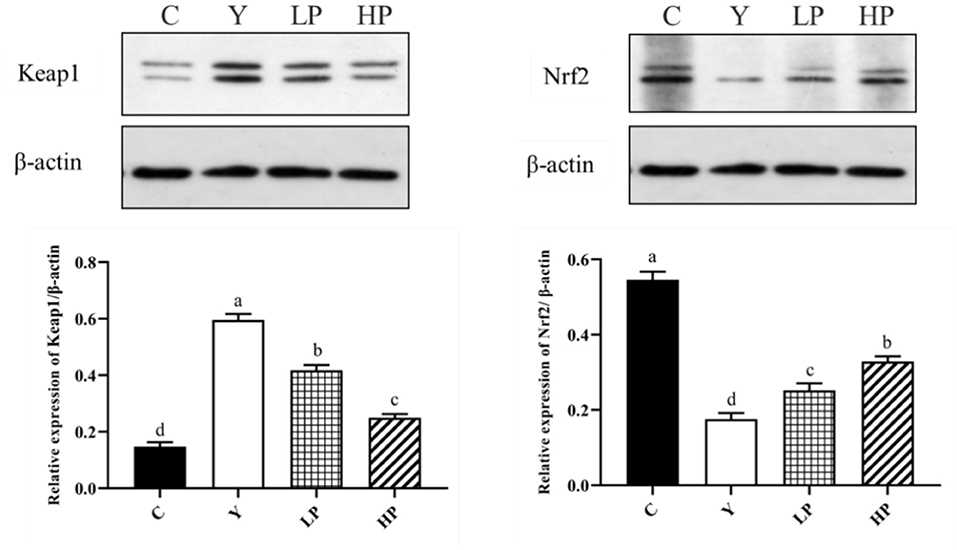
Figure 7. Effects of OP on Keap1-Nrf2 signaling pathway in the ileum of oxidative injured mice. Different letters in the figure represent significant differences (P < 0.05). C: the normal group; Y: the Cy-induced group; LP: Cy+200 mg/kg OP; HP: Cy+400 mg/kg OP.
Cy is widely employed in the treatment of different cancers as well as autoimmune diseases (31), and can cause oxidative stress and intestinal mucosal damage while exerting anti-tumor effects (32). It has been established that administration of large doses of Cy can cause severe side effects such as intestinal barrier disruption, increased intestinal permeability, gastrointestinal mucosal destruction, immune deficiency as well as secondary infections, and the production of excessive free oxygen radicals. In recent years, several studies have found that diverse bioactive peptides isolated from the different sources can exhibit strong antioxidant activity (23), which can improve or restore the body's immunity. Solenocera Crassicornis head protein Hydrolysates-Fraction 1 (SCHPS-F1) is mainly composed of different low molecular weight peptides. It has been reported that Cy-induced nephrotoxicity could be alleviated by restoring antioxidant enzyme activity and Nrf2-Keap1 antioxidant pathway-related protein expression. In addition, Fontoura et al. isolated a novel antioxidant peptide from the protease hydrolysates of the feather keratin from corn germ which caused substantial hydroxyl radical scavenging and lipid peroxidation inhibition (33). Here, we investigated the potential protective effects of OP on intestinal mucosal loss and oxidative stress in immunosuppressed mice upon intraperitoneal injection of Cy and pre-gavage administration of OP.
The experimental results showed that most of the mice exhibited decrease in weight, loss of appetite and mental malaise after the treatment with Cy. However, when OP was administered, the appetite began to recover and weight loss in mice was significantly reduced. Intestinal tract not only plays a vital role in nutrient digestion and absorption, but also is the biggest immune organ. The results of HE staining and electron microscopy suggested that the intestinal villi of Cy treated mice was loose, some villi were broken and their structural integrity was destroyed. The villi of LP as well as HP groups were found to be compact, the intestinal injury was improved, and the villi length was close to the normal group. SOD), CAT) and GSH-Px are the key enzymes and regulatory factors involved in the body's antioxidant defense system. For instance, SOD can effectively catalyze superoxide anion and remove free radicals, thereby reducing the lipid peroxidation and maintaining the balance between oxidative and anti-oxidative states (34–36). Meanwhile, GSH-Px and CAT can functionally remove H2O2 and reduce ·OH level, thus reducing the damage caused by free radicals to the body (37, 38). MDA is the final by-product of endogenous lipid peroxidation, which can lead to DNA rearrangement and even apoptosis (39, 40). Both the ileum and liver are important immune organs of the body. When the intestinal environment is affected by inflammation, oxidative stress and disorder of bacterial community structure, the liver can also produce corresponding adverse reactions. Therefore, the relative expression level of anti-oxidant genes, and the content of antioxidant enzymes in the serum and liver were also measured in this study. The results indicated that the contents of SOD, CAT and GSH-Px in the serum and mice liver were decreased markedly, whereas the content of MDA was substantially increased after the treatment with Cy, thus suggesting that Cy caused oxidative stress in mice. Moreover, compared with Cy treated group, gavage of OP significantly increased the mRNA expression contents of SOD and GSH-Px in the mice ileum, decreased the MDA content and enhanced the secretion of the SOD, CAT, and GSH-Px in the mice serum and liver. These findings suggested that early intake of OP can significantly reduce the lipid peroxidation in the serum, ileum and liver tissues, and alleviate the oxidative lesions induced by Cy. The possible mechanisms contributing to these protective effects may be due to improvement in the contents of antioxidant enzymes by up-regulation of their relative mRNA expression. A number of previous studies have reported many similar results. For example, fucoidan has been shown to significantly reduce the contents of MDA, IL-6, IL-1β, and TNF-α in the liver and kidney tissues of mice induced by Cy (41), and it also increased the activities of SOD, GSH-Px and CAT. In addition, fucoidan can also activate Nrf2/HO-1 pathway, by increasing Nrf2, GCLM, HO-1 and NQO1 protein levels. Sulfate polysaccharide AL1-1 in sea cucumber can significantly increase intestinal total antioxidant capacity (T-AOC) and decrease intestinal MDA level (42). AL1-1 can also increase the levels of CAT and GSH-Px, and significantly enhance the immune response. These studies are consistent with the conclusion of our experiment results.
Nrf2 is a critical transcription factor which can effectively regulate oxidative stress in the body and restore oxidative damage by modulating the secretion of antioxidant enzymes (43, 44). Under regular physiological conditions, Nrf2 remains bound to Keap1 (Kelch-like ECH-associated protein 1), in the cytoplasm, which can also regulate degradation of Nrf2 by the ubiquitin–proteasome pathway. In response to oxidative stress, the continuous production and accumulation of ROS causes Nrf2 to dissociate from Keap1 and migrate into the nucleus to bind to antioxidant response elements (ARE) and regulate the secretion of various cytoprotective genes (HO-1, NQO1) downstream of Nrf2-Keap1 signaling pathway (45). The experimental results showed that intake of OP significantly increased the gene expression levels of major detoxifying enzymes, HO-1 and NQO1 in the Nrf2 signaling pathway of mice ileum. Interestingly, the Western-blot results also indicated that the expression of Keap1 protein in ileum mice induced by Cy was substantially reduced and the expression of Nrf2 protein was up-regulated by OP intake in a dose-dependent manner as compared with the Cy treatment group. Overall, the findings suggest that OP can regulate oxidative stress in Cy treated mice, and the mechanism may be relevant to the activation of pathway of Nrf2-Keap1 to combat the oxidative stress. OP also showed potent antioxidant activity in vitro. By regulating Nrf2-Keap1 pathway in CPP-treated mice, the expression of upstream factor Keap1 was decreased, whereas that of Nrf2 was increased, and then the expression of downstream factors (NQO1, HO-1, GSH-Px, SOD and CAT) was found to be enhanced. It prevented oxidative damage induced by Cy in mice. As an effective antioxidant, OP can significantly reduce reproductive oxidative stress damage associated with the Nrf2-Keap1/ARE pathway.
This finding may be some potential limitations. First, Cy-induced long-term intestinal oxidative damage is not equivalent to ordinary oxidative damage. The Cy modeling method has indeed increased the success rate and achieved excellent results in vivo, but at the same time, it is necessary to ignore the interference effects of other negative effects. In addition, peptides are hydrolyzed in the gastrointestinal tract into free amino acids, which also have the antioxidant capacity in the body. However, it is worth noting that it is also due to the characteristic products of different peptide sequences that cause the different results.
In this study, intragastric administration of OP was found to significantly increase the average daily gain of Cy-induced oxidative stress mice. OP treatment also improved SOD, CAT and GSHPx activity levels in the serum and liver, and significantly reduced MDA content. Moreover, OP increased the relative mRNA expression of SOD, GSH-Px, Nrf2, HO-1 and NQO1 in ileum significantly, up-regulated Nrf2 protein expression but down-regulated Keap1 protein expression. Overall, OP can regulate the intestinal oxidative damage induced by Cy in mice, and its mechanism may be primarily mediated by enhancing the expression of various antioxidant genes, antioxidant enzyme activities and activating Nrf2-Keap1 pathway. The results showed that OP might have useful potential in the treatment of intestinal oxidative damage and other similar related diseases.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals, which is approved by the Animal Ethics Committee of Zhejiang University of Technology (20210308038).
HC, XX, and SL provided the project administration and funding acquisition. HZ and XZ designed the research and wrote the manuscript. HZ, TL, and QJ executed the experiments and analyzed the data. HC, XX, and YD reviewed and edited this manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by grants from the National Key Research and Development Program of China (2020YFD0900902) and the National Natural Science Foundation of China (32101947).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. (2004) 55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701
2. Scherz-Shouval R, Elazar Z. ROS mitochondria and the regulation of autophagy. Trends Cell Biol. (2007) 17:422–7. doi: 10.1016/j.tcb.2007.07.009
3. Sakai Y, Yamamori T, Yoshikawa Y, Bo T, Suzuki M, Yamamoto K, et al. Oxidase 4 mediates ROS production in radiation-induced senescent cells and promotes migration of inflammatory cells. Free Radic Res. (2018) 52:92–102. doi: 10.1080/10715762.2017.1416112
4. Li L, Feng L, Jiang WD, Jiang J, Wu P, Kuang SY, et al. Dietary pantothenic acid deficiency and excess depress the growth, intestinal mucosal immune and physical functions by regulating NF-κB, TOR, Nrf2 and MLCK signaling pathways in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. (2015) 45:399–413. doi: 10.1016/j.fsi.2015.04.030
5. Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: role of oxygen free radicals. Hepatology. (2002) 35:622–9. doi: 10.1053/jhep.2002.31656
6. Szelenyi I, Brune K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. (1988) 33:865–71. doi: 10.1007/BF01550977
7. Chung CH, Jung W, Keum H, Kim TW, Jon S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. (2020) 14:6887–96. doi: 10.1021/acsnano.0c01018
8. Tian T, Wang Z, Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev. (2017) 2017:1–18. doi: 10.1155/2017/4535194
9. Guan G, Lan S. Implications of antioxidant systems in inflammatory bowel disease. Biomed Res Int. (2018) 2018:1–7. doi: 10.1155/2018/1290179
10. Stallmach A, Wittig BM, Moser C, Fischinger J, Duchmann R, Zeitz M. Safety and efficacy of intravenous pulse cyclophosphamide in acute steroid refractory inflammatory bowel disease. Gut. (2003) 52:377–82. doi: 10.1136/gut.52.3.377
11. Martí JL, Mayordomo JI, Isla MD, Saenz A, Escudero P, Tres A, et al. Autotransplant for inflammatory bowel disease (IBD): a case of ulcerative colitis. Bone Marrow Transplant. (2001) 28:109–10. doi: 10.1038/sj.bmt.1703103
12. Miao M, Cheng B, Guo L, Shi J. Effects of fuzheng paidu tablet on peripheral blood T lymphocytes, intestinal mucosa T lymphocytes, and immune organs in cyclophosphamide-induced immunosuppressed mice. Hum Vaccin Immunother. (2015) 11:2659–63. doi: 10.1080/21645515.2015.1082694
13. Russo F, Linsalata M, Clemente C, D'Attoma B, Orlando A, Campanella G, et al. The effects of fluorouracil, epirubicin, and cyclophosphamide (FEC60) on the intestinal barrier function and gut peptides in breast cancer patients: an observational study. BMC Cancer. (2013) 13:56. doi: 10.1186/1471-2407-13-56
14. Yan S, Wang K, Wang X, Ou A, Wang F, Wu L, et al. Effect of fermented bee pollen on metabolic syndrome in high-fat diet-induced mice. Food Sci Hum Wellness. (2021) 10:345–55. doi: 10.1016/j.fshw.2021.02.026
15. Truong V-L, Jeong W-S. Antioxidant and anti-inflammatory roles of tea polyphenols in inflammatory bowel diseases. Food Sci Hum Wellness. (2022) 11:502–11. doi: 10.1016/j.fshw.2021.12.008
16. Hao L, Wang X, Cao Y, Xu J, Xue C. A comprehensive review of oyster peptides: preparation, characterisation and bioactivities. Rev Aquac. (2022) 14:120–38. doi: 10.1111/raq.12588
17. Jo C, Khan FF, Khan MI, Iqbal J. Marine bioactive peptides: types, structures, and physiological functions. Food Rev Int. (2017) 33:44–61. doi: 10.1080/87559129.2015.1137311
18. Miao J, Liao W, Kang M, Jia Y, Wang Q, Duan S, et al. Anti-fatigue and anti-oxidant activities of oyster (Ostrea rivularis) hydrolysate prepared by compound protease. Food Funct. (2018) 9:6577–85. doi: 10.1039/C8FO01879K
19. Shiozaki K, Shiozaki M, Masuda J, Yamauchi A, Ohwada S, Nakano T, et al. Identification of oyster-derived hypotensive peptide acting as angiotensin-I-converting enzyme inhibitor. Fish Sci. (2010) 76:865–72. doi: 10.1007/s12562-010-0264-0
20. Umayaparvathi S, Arumugam M, Meenakshi S, Dräger G, Kirschning A, Balasubramanian T. Purification and characterization of antioxidant peptides from oyster (Saccostrea cucullata) hydrolysate and the anticancer activity of hydrolysate on human colon cancer cell lines. Int J Pept Res Ther. (2014) 20:231–43. doi: 10.1007/s10989-013-9385-5
21. Wang X, Yu H, Xing R, Liu S, Chen X, Li P. Effect and mechanism of oyster hydrolytic peptides on spatial learning and memory in mice. RSC Adv. (2018) 8:6125–35. doi: 10.1039/C7RA13139A
22. Xiang XW, Zheng HZ, Wang R, Chen H, Xiao JX, Zheng B, et al. Ameliorative effects of peptides derived from oyster (Crassostrea gigas) on immunomodulatory function and gut microbiota structure in cyclophosphamide-treated mice. Mar Drugs. (2021) 19:456. doi: 10.3390/md19080456
23. Yang Q, Cai X, Yan A, Tian Y, Du M, Wang S, et al. Specific antioxidant peptide: its properties in controlling oxidation and possible action mechanism. Food Chem. (2020) 327:126984. doi: 10.1016/j.foodchem.2020.126984
24. Park YR, Park CI, Soh Y. Antioxidant and anti-inflammatory effects of NCW peptide from clam worm (Marphysa sanguinea). J Microbiol Biotechnol. (2020) 30:1387–94. doi: 10.4014/jmb.2003.03050
25. Mirzapour-Kouhdasht A, Moosavi-Nasab M, Kim YM, Eun JB. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. (2021) 342:128339. doi: 10.1016/j.foodchem.2020.128339
26. Senisterra G, Wu H, Allali-Hassani A, Wasney GA, Barsyte-Lovejoy D, Dombrovski L, et al. Small-molecule inhibition of MLL activity by disruption of its interaction with WDR5. Biochem J. (2013) 449:151–9. doi: 10.1042/BJ20121280
27. D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. (1998) 114:262–7. doi: 10.1016/S0016-5085(98)70476-7
28. Sawczyn T, Zimmermann J, Stygar D, Kukla M, Nabrdalik K, Matysiak N, et al. Ileal transposition (IT) surgery changing the ultrastructure of the transposed segment as well as jejunum. Histomorphometric and electron microscopy analysis. Obes Surg. (2018) 28:1232–9. doi: 10.1007/s11695-017-2992-z
29. Mio K, Yamanaka C, Matsuoka T, Kobayashi T, Aoe S. Effects of β-glucan rich barley flour on glucose and lipid metabolism in the ileum, liver, and adipose tissues of high-fat diet induced-obesity model male mice analyzed by DNA microarray. Nutrients. (2020) 12:1–18. doi: 10.3390/nu12113546
30. Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr Polym. (2020) 235:115957. doi: 10.1016/j.carbpol.2020.115957
31. Floudas CS, Brar G, Mabry-Hrones D, Duffy AG, Wood B, Levy E, et al. A pilot study of the PD-1 targeting agent AMP-224 used with low-dose cyclophosphamide and stereotactic body radiation therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. (2019) 18:e349–60. doi: 10.1016/j.clcc.2019.06.004
32. Lorinczy D. Cyclophosphamide treatment evoked side effects on skeletal muscle monitored by DSC. J Therm Anal Calorim. (2020) 142:1897–901. doi: 10.1007/s10973-020-09388-2
33. Fontoura R, Daroit DJ, Corrêa APF, Moresco KS, Santi L, Beys-da-Silva WO, et al. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. N Biotechnol. (2019) 49:71–6. doi: 10.1016/j.nbt.2018.09.003
34. Yim MB, Chock PB, Stadtman ER. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. (1990) 87:5006–10. doi: 10.1073/pnas.87.13.5006
35. Klug-Roth D, Rabani J, Fridovich I. Pulse radiolytic investigations of superoxide catalyzed disproportionation. Mechanism for bovine superoxide dismutase. J Am Chem Soc. (1973) 95:2786–90. doi: 10.1021/ja00790a007
36. Liochev SI, Fridovich I. Copper- and zinc-containing superoxide dismutase can act as a superoxide reductase and a superoxide oxidase. J Biol Chem. (2000) 275:38482–5. doi: 10.1074/jbc.M007891200
37. Wang W, Zhang F, Li Q, Chen H, Zhang W, Yu P, et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp, and its antioxidant activity and protective effect against H2 O2-induced injury RAW264.7 cells. Int J Biol Macromol. (2018) 118:816–33. doi: 10.1016/j.ijbiomac.2018.06.117
38. Hao W, Guo H, Zhang J, Hu G, Yao Y, Dong J. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in salvia miltiorrhiza cell cultures. Sci World J. (2014) 2014:843764. doi: 10.1155/2014/843764
39. Masiello MG, Cucina A, Proietti S, Palombo A, Coluccia P, D'Anselmi F, et al. Phenotypic switch induced by simulated microgravity on MDA-MB-231 breast cancer cells. Biomed Res Int. (2014) 2014:652434. doi: 10.1155/2014/652434
40. Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. (2008) 14:665–74. doi: 10.2119/2008-00102.Catera
41. Tian S, Jiang X, Tang Y, Han T. Laminaria japonica fucoidan ameliorates cyclophosphamide-induced liver and kidney injury possibly by regulating Nrf2/HO-1 and TLR4/NF-κB signaling pathways. J Sci Food Agric. (2021) 102:2604–12. doi: 10.1002/jsfa.11602
42. Feng G, Laijin S, Chen S, Teng W, Dejian Z, Yin C, et al. In vitro and in vivo immunoregulatory activity of sulfated fucan from the sea cucumber A. leucoprocta. Int J Biol Macromol. (2021) 187:931–8. doi: 10.1016/j.ijbiomac.2021.08.008
43. Kang KW, Lee SJ, Kim SG. Molecular mechanism of Nrf2 activation by oxidative stress. Antioxid Redox Signal. (2005) 7:1664–73. doi: 10.1089/ars.2005.7.1664
44. Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. (2013) 53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320
Keywords: oyster peptides, cyclophosphamide, oxidative stress, intestinal mucosa, Nrf2-Keap1 antioxidant pathway
Citation: Chen H, Zheng H, Li T, Jiang Q, Liu S, Zhou X, Ding Y and Xiang X (2022) Protective Effect of Oyster Peptides Derived From Crassostrea gigas on Intestinal Oxidative Damage Induced by Cyclophosphamide in Mice Mediated Through Nrf2-Keap1 Signaling Pathway. Front. Nutr. 9:888960. doi: 10.3389/fnut.2022.888960
Received: 03 March 2022; Accepted: 08 April 2022;
Published: 16 May 2022.
Edited by:
Demin Cai, Yangzhou University, ChinaCopyright © 2022 Chen, Zheng, Li, Jiang, Liu, Zhou, Ding and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingwei Xiang, eHh3MTEwODZAemp1dC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.