- 1College of Medicine and Health Sciences, School of Public Health, University of Rwanda, Kigali, Rwanda
- 2Department of Public Health, School of Health Sciences, Mount Kenya University, Kigali, Rwanda
- 3Catholic Relief Services, Chief of Party, Kigali, Rwanda
Inadequate maternal nutrition before and during pregnancy is a principal risk factor for poor fetal development, resulting in low birth weight (LBW) and subsequently, poor child growth. Most studies focus on the impact of nutritional interventions after birth while only a few interventions consider integrated nutrition service packages. Therefore, there is limited evidence on whether integrated maternal nutrition interventions have a positive effect on birthweight. Thus, a post-program quasi-experimental study was carried out to determine the effectiveness of the integrated maternal nutrition intervention package on low birth weight in Rwanda. A total of 551 mother–baby pairs from the intervention and 545 controls were included in the analysis. Data regarding socio-demographic, maternal anthropometric parameters, and dietary diversity were collected using a structured questionnaire. Birth weight was assessed right after delivery, within 24 h. Logistic regression, linear regression, and path analysis were fitted to determine the effectiveness of the intervention on birth weight. The study found that the intervention reduced LBW by 66.99% (p < 0.001) and increased average birth weight by 219 g (p < 0.001). Logistic regression identified reduced risk of LBW among the intervention group (AOR = 0.23; 95%CI = 0.12–0.43; p < 0.001). It was also observed that the direct effect of the intervention on birth weight was 0.17 (β = 0.17; p < 0.001) and the main indirect mediator was maternal MUAC (β = 0.05; p < 0.001). Moreover, maternal passive smoking exposure and MUAC < 23 cm were found as risk factors for LBW. This study has demonstrated that an integrated maternal nutritional intervention package can significantly reduce LBW in low-income settings and should, therefore, be considered to improve birth weight.
Introduction
Birth weight is a significant predictor of the present and future health status of a newborn, and low birth weight (LBW <2,500 grams) is a major public health concern (1, 2). Globally, 15–20% of newborns have low birth weight (3), which represents approximately 30 million newborns (23.4% of all births) every year (4, 5). Almost half of all LBWs are from South Central Asia (27%) and sub-Saharan Africa (15%) (4, 6, 7). In some African countries, the prevalence of LBW has been reported to range between 10 and 15.7% (8). In Rwanda, according to the Demographic and Health Survey, it is estimated at 7% (9).
Low birth weight remains the single most important predictor of infant morbidity and mortality globally (10, 11). In African countries, 30% of all deaths of infants are attributed to LBW (12). Babies with LBW are about 40 times more likely to die within the first 30 days of life compared to their counterparts with normal birth weight (13). Furthermore, it is a significant factor associated with higher probabilities of infection; malnutrition; susceptibility to childhood illnesses; long-term physical and mental disorders; and problems related to behavior, learning, and psychosocial development during childhood (1, 13–15).
Maternal malnutrition during pregnancy is considered to be the main determinant of LBW (16, 17). However, the damage caused by maternal undernutrition can be prevented by improving the nutritional status of pregnant women. Appropriate nutrition intervention strategies during pregnancy are key to improving maternal nutrition and the birth weight of their newborns (18). The Lancet Maternal and Child Nutrition series in 2013 proposed nutrition-specific and nutrition-sensitive interventions to improve fetal nutrition and for optimal growth and child development (19, 20).
In line with the Lancet series and Rwanda National Food and Nutrition Policy for 2013–2018 (21), a program by the name Gikuriro meaning “good growth” was implemented between 2016 and 2020. The intervention was funded by USAID and implemented by Catholic Relief Services in partnership with the Government of Rwanda, the Netherlands Development Organization and other Rwandan Civil-Society Organizations. The program targeted five districts, namely, Kayonza and Ngoma from Eastern Province, Nyabihu from Western Province, and Kicukiro and Nyarugenge from Kigali City, and they were selected because of malnutrition and the absence of development partners, as of late 2014. The program implemented key nutrition-specific and nutrition-sensitive interventions to prevent maternal and early childhood malnutrition. Among the nutrition-specific interventions were nutrition education and counseling, and the key nutrition-sensitive interventions included the promotion of increased agricultural productivity; economic strengthening through social safety nets; and improved access to Water, Hygiene, and Sanitation (WASH) services. Adequate nutrition has been well recognized as important for maternal and fetal health. However, the effects of such integrated interventions during pregnancy on the child’s nutritional outcomes, including birth weight, are yet to be ascertained in the context.
The majority of the studies previously conducted to determine the effectiveness of individual interventions in the first 1,000 days window period revealed a modest impact on linear growth (22). For example, a single intervention during pregnancy showed a 50-g difference in birth weight and a 15% reduction of LBW, reported as the biggest effect (23, 24). Another study also showed that balanced energy and protein supplementation among pregnant women can increase birth weight by 41 g (25). A recent systematic review that included 16 high-quality randomized controlled trials demonstrated that improved maternal nutritional status leads to a significant reduction in LBW (3). This was particularly more effective when the intervention is a combination of nutrition education and multi-micronutrient supplements (3). However, scientific evidence on the effect of an integrated nutritional intervention package during pregnancy for improving birth weight is scarce. Therefore, the purpose of this study was to examine the effectiveness of an integrated maternal nutrition intervention package on low birth weight in Rwanda.
Materials and Methods
Study Design and Settings
The study adopted a quasi-experimental design and was conducted from November 2020 to June 2021. It was a post-intervention evaluation. The intervention group was drawn from two districts namely Kayonza District (rural area) and Kicukiro District (urban area), where the integrated nutrition intervention package (nutrition-specific and nutrition-sensitive) was implemented. The selection criteria for the two districts were based on the high proportion of food insecurity according to the Comprehensive Food Survey and Vulnerability Analysis (26) and locality (rural vs. urban). Similarly, in selecting the control districts three criteria were applied: high food insecurity, no existing nutrition-sensitive and nutrition-specific intervention package, and settlement pattern (rural vs. urban). After considering all the criteria, Gisagara District (rural area) and Gasabo District (urban area) were selected as the comparison control group. In a district, one public district hospital and all health centers were included in the study.
Study Interventions
Description of the Nutrition-Specific Intervention
The component of the intervention package, which is nutrition-specific, was nutrition education and counseling. The women received nutrition education and counseling by Community Health Workers (CHWs) and nutritionists. First, the nutritionists and CHWs in charge received training on the counseling guide module. Then, the CHWs in charge in turn trained the CHWs at the village level. The trained nutritionist counseled the pregnant women about nutrition during regular antenatal care visits and sessions lasted about 30 to 45 min each. Moreover, the nutritionists also trained the women through cooking demonstrations about a balanced diet through Village Nutrition School. In addition to these, the CHWs gave further nutrition education and counseling at the household level. The CHWs also received in-service training on a monthly basis. The main contents of the educational and counseling guide are indicated in Supplementary Material. The control group, on the other hand, only received routine nutritional education and counseling as per the Rwanda national ante-natal care guidelines adopted from WHO (27).
Description of the Nutrition-Sensitive Intervention
In this intervention, three components were implemented, including promotion of increased agricultural productivity, promotion of financial literacy/economic strengthening, and improved access to WASH services. (1) Increased agricultural productivity: Beneficiaries were taught to practice nutrition-sensitive agriculture and to increase agriculture production using of Bio-Intensive Agriculture Techniques (BIATs). They were grouped into Farmer Field Learning School (FFLS) and advised on how to improve production mainly to attain food security at the household level. (2) Promotion of financial literacy/economic strengthening: The economic status of the women was improved by grouping them into Saving and Internal Lending Communities (SILC) as a way of responding to financial problems that prevent them from attaining better nutritional outcomes. The main methods of SILC were as follows: training those in charge of economic strengthening and project coordinators and district cooperative staff; then the trained staff train the sector cooperative officers, where, they in turn train field agents from the community. Then the field agents sensitize the people about SILC and form the groups. The goal was to help these women better manage their existing resources by teaching them basic financial management skills. This enabled the poor to build up useful lump sums without incurring excessive debt or interest charges. (3) Water, Sanitation, and Hygiene (WASH) interventions: Improved WASH services were implemented using Community Based Environmental Health Promotion Program (CBEHPP) approach through Community Health Clubs (CHC) at the village level. CBEHPP is a hygiene behavior change approach to reach communities and empower them to identify their personal and domestic hygiene needs. CHC and a demonstration site in every village were formed and initiated. The CHCs were responsible for ensuring that the levels of hygiene were monitored, together with the CHW facilitator, who visited each household to observe the household sanitation and environmental conditions. A detailed description of these interventions is presented in Supplementary Material. However, the control group did not receive any of these nutrition-sensitive interventions.
Study Population, Sampling, and Sample Size
The target population was mother–newborn pairs. All pregnant women who came for delivery to all public health facilities in the selected districts were recruited consecutively using the following inclusion criteria: (1) being a permanent resident in the study area and aged between 15 and 49 years, (2) having been enrolled in the selected nutrition intervention package at least 1 year before pregnancy and continued until delivery for intervention group but not for the control group, (3) belonging to wealth category (1 and 2, 4) those without any known medical, surgical, or obstetric problems/conditions, and (5) with live singleton babies and normal spontaneous delivery.
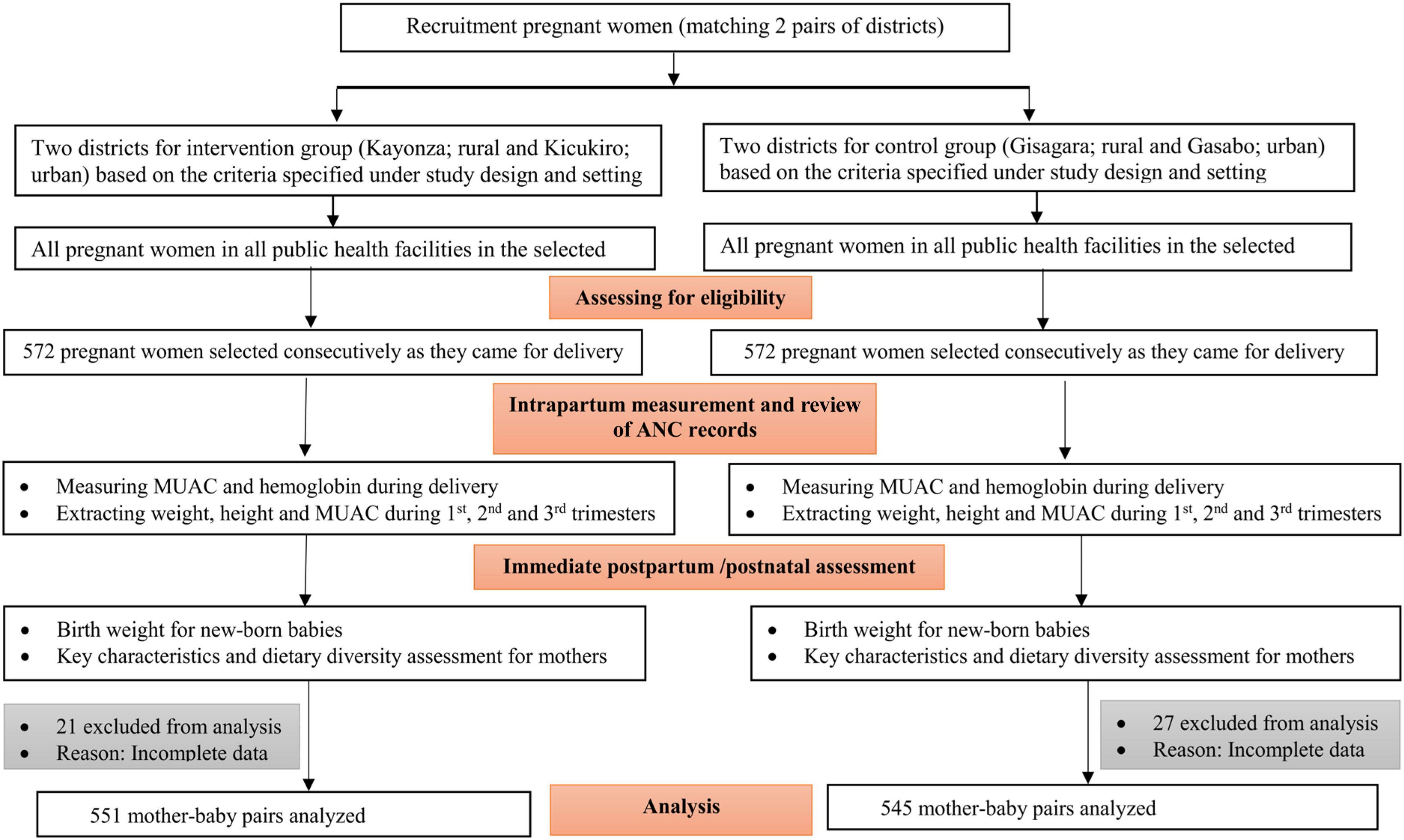
The sample size was justified based on a 3.5% effect size and LBW proportion difference between the intervention and control group. This was estimated to detect a reduction of low birth weight from an expected 7% (9) in the control group (general population) to 3.5% in the intervention group. A power of 80%, a confidence level of 95%, and a design effect of 1.25 were considered to achieve the desired sample size. Thus, a total sample size of 1,144 (572 mother–newborn pairs for each study group) was estimated. However, 21 from the intervention and 27 from the control group were excluded from the analysis due to incomplete data. The recruitment flow chart is shown in Figure 1.
Data Collection and Measurements
Trained midwives/nurses collected the data using a structured quantitative questionnaire. It was composed of basic demographic, lifestyle and obstetric factors, anthropometric and biological measurements, and maternal dietary diversity. To make data collection practices consistent, a standardized operating procedure was developed for all measures. The mothers were interviewed face-to-face in the immediate postpartum period (within 24 h of delivery) using a questionnaire that was translated into the local language (Kinyarwanda). The data collectors were trained on the objectives of the study, participants’ recruitment, and anthropometric measurements.
Anthropometric measurements were taken for both the mothers and their newborns. The nutritional status of the mothers was assessed using mid-upper arm circumference (MUAC), body mass index (BMI), and weight gain. MUAC has been identified to have a strong relationship with LBW and it is not affected by any changes like edema common during pregnancy (28). It was measured using flexible non-elastic tape upon recruitment. On the basis of several studies in Africa and due to the need for international comparison, maternal undernutrition was defined as MUAC < 23 cm (28). In addition, antenatal care records were reviewed to retrieve MUAC, weight, and height which are measured during the ANC visits according to Rwandan Ministry of Health guidelines. Weight and height were used to assess body mass index (BMI) and weight gain.
Hemoglobin was measured upon recruitment before delivery using a portable HEMOCUE B-Hb photometer according to Rwandan Ministry of Health guidelines. For the newborn babies, weight was measured within 24 h of delivery. The primary outcome of the study was birth weight. It was measured to the nearest 100 g on digital scales at the health facilities. The scales were regularly calibrated as per the manufacturer’s recommendation. Low birth weight was defined as birth weight less than 2,500 g.
A food frequency questionnaire was also used to obtain dietary information. This tool included 9 food groups validated by the Food and Agriculture Organization (29). These food groups were cereals and tubers; pulses and legumes; vegetables; fruits; meat, fish and eggs; milk and milk products; oils and fats, and sweets and spices/beverages. Pregnant women presenting to the health facilities for delivery were asked about their frequency of food consumption based on these groups. They were asked to recall what they had eaten in the 24 h preceding the onset of labor. A dietary diversity score was then calculated according to the frequency of food groups consumed by women within the 24 h. A score “1” was assigned to each consumed food group and a score “0” was assigned if not consumed. The scores were aggregated to calculate the total maternal dietary diversity score (DDS) and those who scored below 5 were grouped as inadequate DDS whereas those who scored 5 and above were categorized as having adequate DDS.
Data Analysis
The analysis was performed using Statistical Package for the Social Sciences (SPSS) version 25.0 IBM New York. Means and percentages were used to summarize continuous and categorical data, respectively. Comparison of the explanatory variables between the intervention and control groups were conducted using an independent sample t-test (to compare means) or Chi-square test (to compare proportions). All statistical significance was set at a p-value less than 0.05.
The dependent variable was categorized into LBW (<2,500 g) and normal birth weight (≥2,500 g). To assess the effectiveness of the intervention, logistic regression was conducted by considering all variables with p < 0.2 in the bivariate analysis. The strength of association between LBW and the intervention was presented using an adjusted odds ratio with a corresponding 95% confidence interval. The model fitness was checked using the Hosmer and Lemeshow Test (p-value = 0.214), which indicated the model was an adequate fit. Model classification accuracy was also assured.
Linear regression was also carried out for some covariates associated with birth weight as continuous variables. These included maternal hemoglobin concentration (g/dl), maternal MUAC (cm) in the first or second trimester and delivery, BMI in the first trimester, and maternal DDS per 24 h. Multicollinearity, linearity, and interaction were checked among the variables considered in the model. The Scatter plot revealed linearity, and Durbin-Watson (<4) showed independence of the observations/data. The tolerance was greater than 0.1 and the variance of inflation was less than 10, indicating no multicollinearity.
Furthermore, fetal growth in the uterus strongly depends on maternal nutritional status and dietary practices, which could also be affected by the intervention. Considering this, a pathway and mediation analysis was conducted to assess the direct and indirect effects of the integrated nutrition intervention package on birth weight. In this case, the outcome variable is birth weight and the predictor for birth weight is the intervention. The nutritional status (MUAC) and dietary diversity score are mediators of the intervention impact. The pathway mediation was analyzed using PROCESS macro version 4.0 for SPSS designed by Hayes (30).
Results
Basic Socio-Demographic, Obstetric, and Lifestyle Characteristics of the Study Population
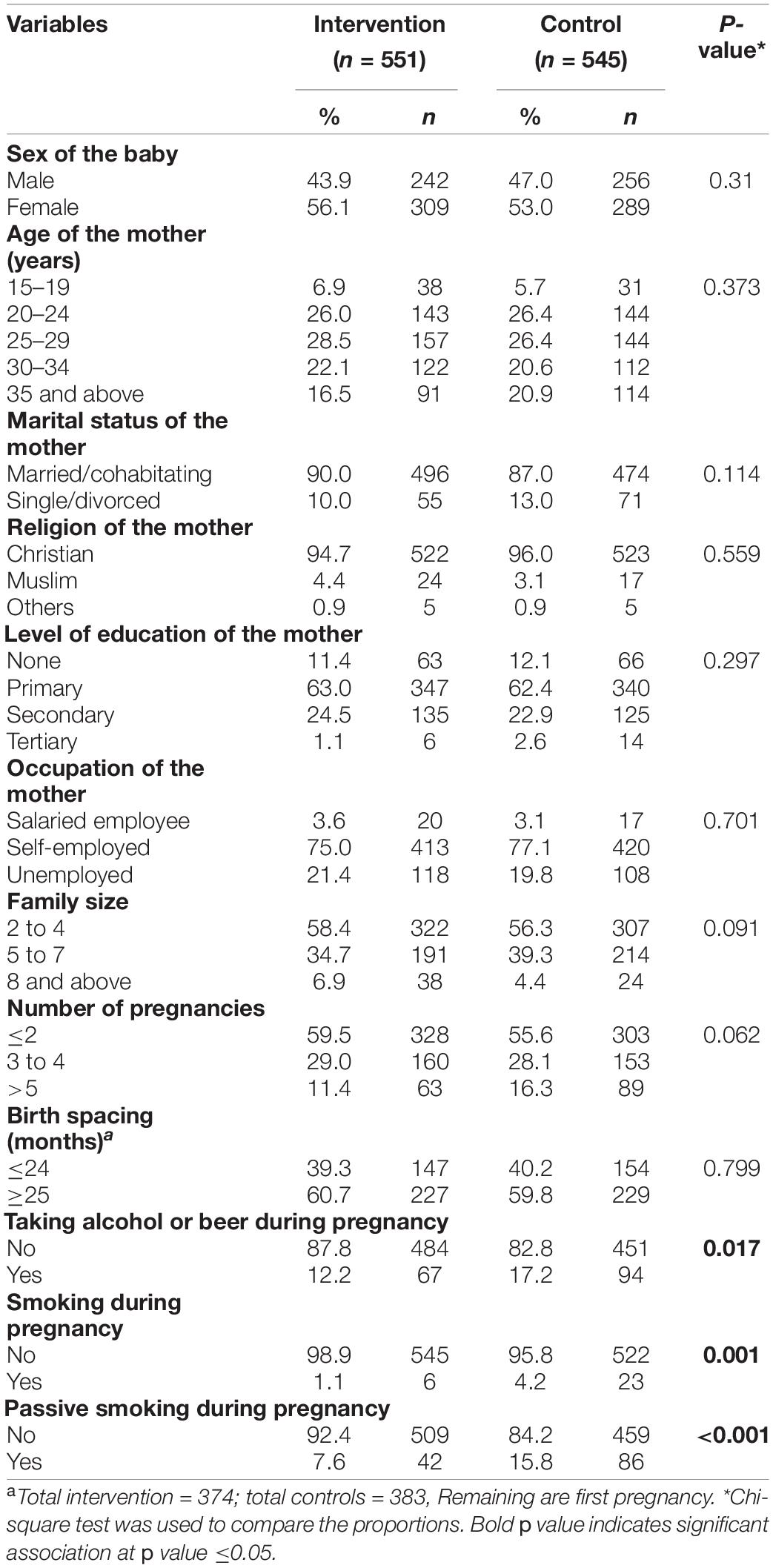
A total of 551 mother–baby pairs for intervention and 545 for controls were used in the analysis. Both groups were comparable in terms of the sex of the baby, maternal age, marital status, religion, education, occupation, family size, number of pregnancies, and birth spacing. However, the proportion of mothers taking alcohol, those who smoked, and those exposed to passive smoking during pregnancy were significantly higher in the control group than in the intervention group (Table 1).
Maternal Nutritional and Birth Weight Status by Study Group
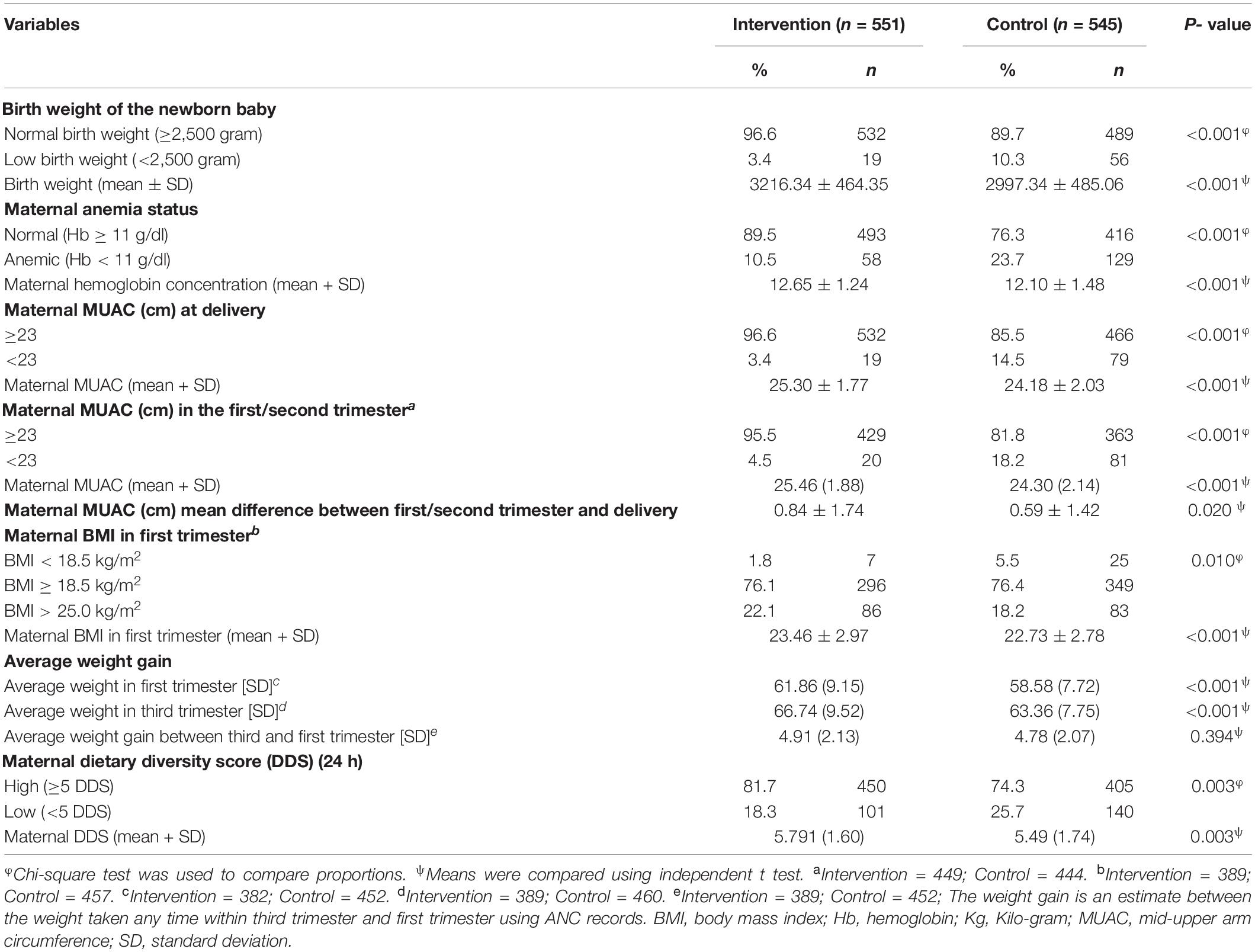
Table 2 shows the nutritional status of the mothers during pregnancy and the birth weight of their newborns. A significantly higher proportion of low birth weight (p < 0.001) was observed among the control group (10.3%) compared to the intervention group (3.4%). There was a significant variation in maternal anemia status, where anemia was more in the control group than intervention group (23.7% vs. 10.5%; p < 0.001). The proportion of maternal MUAC less than 23 cm at delivery was significantly higher (p < 0.001) among the control group (14.5%) compared to the intervention group (3.4%). Similarly, MUAC less than 23 cm during first/second trimester was significantly higher (p < 0.001) in the control group (18.2%) compared to the intervention group (4.5%). The average MUAC difference was significantly more among the intervention group compared to the control group (p = 0.020). BMI less than 18.5 kg/m2 in the first trimester was also significantly lower (p = 0.010) in the intervention (1.8%) than the control (5.5%) group. The average weight gain between the first and third trimester was significantly higher among the intervention group than that in the control group (p < 0.001). Although the average weight gain between the first and third trimesters was higher in the intervention group, there was no statistically significant difference (p = 0.119). Similarly, the proportion of maternal dietary diversity scores less than 5 out of 9 food groups was higher in the control group than the intervention group (25.7 vs. 18.3%; p = 0.003).
The mean birth weight was 3,216.34 g in the intervention group while it was 2,997.34 g in the control group. This difference was significant by 219 g more in the intervention group (p < 0.001). It was also observed that mean maternal hemoglobin concentration, maternal MUAC during the first/second trimester and during delivery, BMI in the first trimester, and dietary diversity scores, were significantly (p < 0.01) higher among the intervention group compared to the control group (Table 2).
Bivariate and Multivariate Analysis: Socio-Demography, Obstetric, Lifestyle, and Other Characteristics Associated With Low Birth Weight
The following variables were found to be significant in bivariate analysis: level of education of the mothers (p = 0.001), passive smoking during pregnancy (p < 0.001), and maternal MUAC (p < 0.001). Although the proportion of LBW was lower (5.8%) among those aged 15–19 years compared to those 35 years and above (10.2%), there was no significant difference (p = 0.274). After considering/adjusting all variables with a p-value less than 0.2 together in multivariate analysis, passive smoking during pregnancy (AOR = 4.34; 95%CI = 2.64–7.15; p < 0.001) and maternal MUAC less than 23 cm (AOR = 2.95; 95%CI = 1.66–5.24; p < 0.001) were found to be independent risk factors for LBW (Table 3).
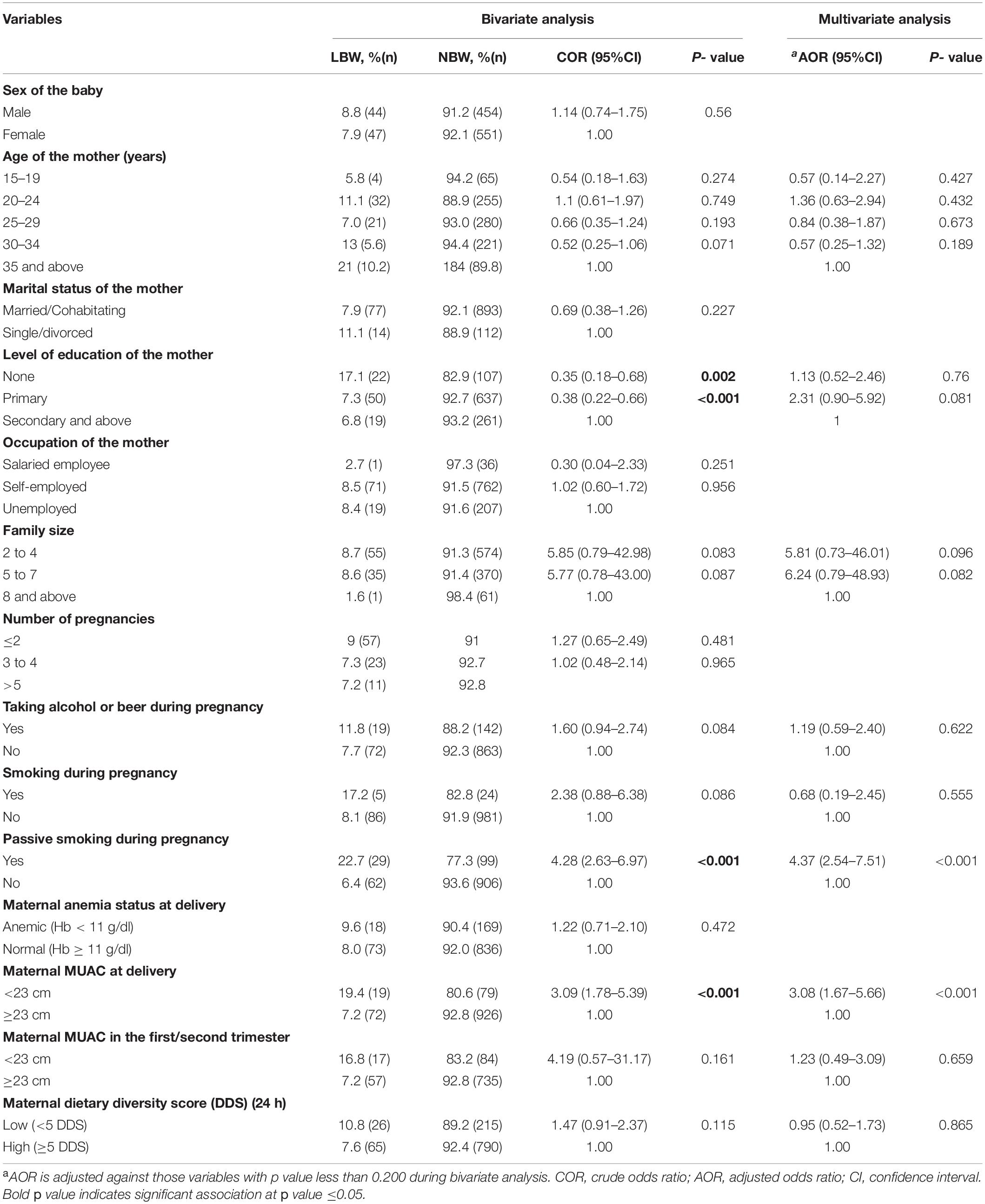
Table 3. Bivariate and multivariate analysis: Socio-demography, obstetric, lifestyle, and nutritional status associated with LBW.
Effectiveness of Integrated Nutrition Intervention on Low Birth Weight
After controlling for the potential confounding against maternal nutritional status using multivariable logistic regression (backward conditional method), newborns in the intervention group were found to have a significantly lower risk of low birth weight (AOR = 0.23; 95%CI = 0.12–0.43; p < 0.001). Moreover, the multiple linear regression confirmed that the intervention group was significantly associated with higher birth weight (β = 0.16; 95%CI = 0.09–0.22; p < 0.001) (Table 4).
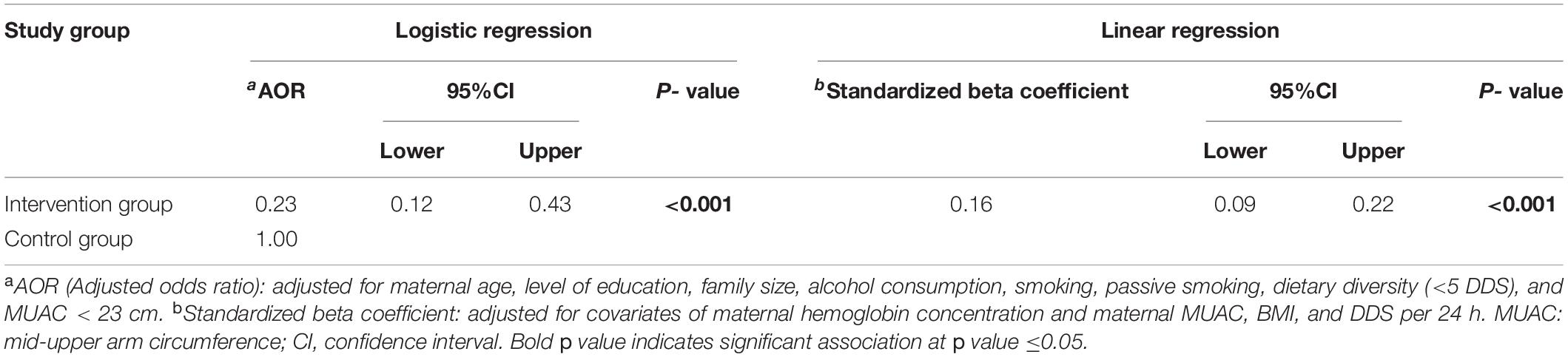
Table 4. Regression analysis for the effectiveness of integrated nutrition intervention on low birth weight.
Pathway and Mediation Analysis for the Direct and Indirect Effects of the Intervention on Birth Weight
The path analysis also showed similar results with multiple linear regression. The average birth weight in the intervention group was significantly higher compared to the control group (β = 0.23; 95%CI = 0.18–0.28; p < 0.001). The direct effect of the intervention on birth weight was 0.17 (β = 0.17; 95%CI = 0.10–0.22; p < 0.001) while the indirect effect was 0.06 (β = 0.06; 95%CI = 0.04–0.10; p < 0.001). The main indirect mediator among the maternal nutritional indicators was MUAC (β = 0.05; 95%CI = 0.03–0.07; p < 0.001) (Table 5).
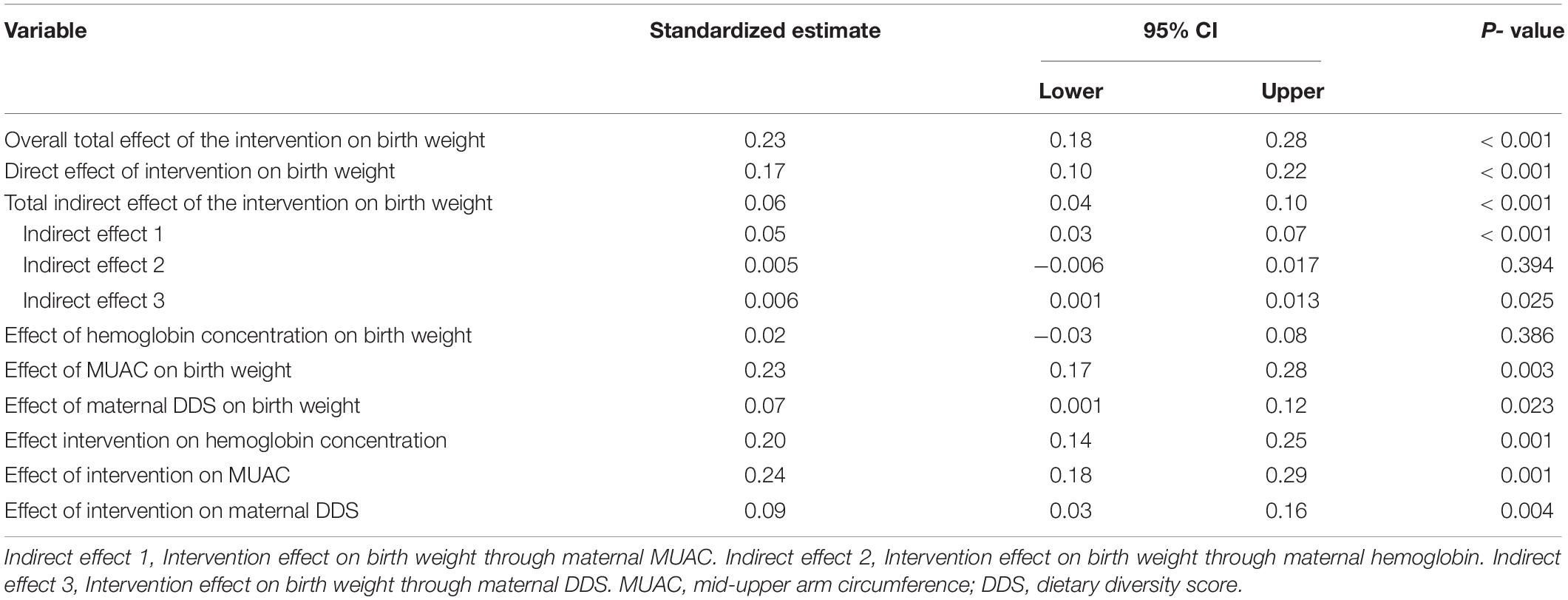
Table 5. Pathway and mediation analysis for the direct and indirect effects of the intervention on birth weight.
Discussion
The findings of this study revealed a significant improvement in birth weight among babies born to pregnant women who participated in this integrated maternal nutrition intervention program. The prevalence of low birth weight was decreased by 66.99% in the intervention group compared to the control group. Similarly, the mean birth weight of the newborn babies in the intervention group was 219 g higher compared to the control group. This result is similar to the recent studies done on nutrition education and counseling intervention in Ethiopia (31) and Kenya (32). However, it is challenging to compare it with other studies due to the combined interventions used in this study.
After adjusting for potential confounders, multiple logistic and linear regression demonstrated that the intervention was significantly associated with a reduction in low birth weight. This could be the result of the different combined interventions that contributed to an improved maternal nutritional status that in turn reduced low birth weight. However, some reviews and studies found mixed or varying results regarding the effectiveness of standalone interventions on maternal nutritional status and birth weight. For example, some reviews on maternal nutrition education and multiple micronutrient interventions showed a reduced risk of low birth weight (23, 25, 33, 34), while other reviews on only nutrition education intervention showed no or limited effects (3, 35–37). In addition, other single vitamin or mineral supplementation interventions, including vitamin A (38), folic acid supplementation (39, 40), iron (24), iodine (41), and zinc (42), marine oil, and fatty acid supplementation (43), were not found to be significantly associated with a reduction in LBW.
Generally, WASH intervention, as a combined intervention, is found to be more effective on infant nutritional status than the single WASH intervention (44, 45). Women’s economic empowerment (social safety nets) and cash transfer were associated with improved nutritional status and birth weight (37, 46). Though there is limited reporting of the impact of the agricultural intervention on birth weight (47), it is believed to produce a high effect when implemented within the other components (social safety nets and educational programs) (20).
In light of the above, this study confirms that there could be a synergic effect of the integrated nutrition intervention package. There was a significant direct effect of the intervention on birth weight (β = 0.16; p = 0.001). Maternal nutrition education and counseling significantly lead to increased and improved dietary practices among women (48, 49). The economic strengthening compounded with nutrition education may contribute to enabling women to use and readjust resources to take adequate and good quality diet, which in turn improves gestational weight gain and growth of the fetus (50). This was more effective when it is combined with agricultural intervention and nutrition education (20).
Overall, the combined intervention may enhance access and use of different health services, increase food security in the household, improve maternal nutrition knowledge, and safe food preparation, improve access to clean water and sanitation, empower women and enhance behavior change toward healthy practices. All of these may be among the possible reasons for the direct effect of the combined intervention toward improving birth weight. Therefore scaling up a range of multi-sectoral interventions during pregnancy could lead to increased birth weight (19, 20).
The study also found that the indirect effect of the intervention through maternal MUAC and maternal dietary intake practices significantly improved birth weight. Similarly, a systematic review on the effect of a nutrition intervention during pregnancy indicated an indirect effect of nutrition intervention on birth weight (3). A few other studies also reveal that dietary diversity among pregnant women can be improved through nutrition education, and this can in turn can reduce the risk of low birth weight (31, 51). Various studies have reported a strong relationship between maternal MUAC and birth weight, whereby women who delivered LBW babies were associated with low MUAC values (1, 28, 52).
Strengths and Limitations
The strengths of this study are adequate sample size, well-organized post-intervention quasi-experimental design, and long duration impact of program evaluation (5 years). Moreover, to the best of our knowledge, this is the first study in Rwanda to report the effectiveness of integrated nutrition intervention on birth weight. However, the study had some limitations. First, we used only end-line post-program evaluation, which limits the ability to appreciate the trend of the nutritional indicators during and before pregnancy. Secondly, the lack of randomization to minimize some concealed confounding bias. Thirdly, it is difficult to know which component of the combined intervention contributes more to improving birth weight as the intervention was delivered as a package. Lastly, the 24-h food frequency before labor started was assessed after delivery, which could have been subject to some recall bias.
Conclusion
The results of this study have shown that an integrated nutritional intervention package, including nutrition education/counseling, agriculture enhancement, economic strengthening through social safety nets, and access to WASH services, can significantly improve birth weight. Therefore, multiple sectors and stakeholders should intensify the different strategies mentioned above to address the diverse and complex determinants of maternal malnutrition to improve their nutritional status and in turn reduce the risk of low birth weight.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the University of Rwanda College of Medicine and Health Sciences (CMHS). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
MH designed and carried out the research and analyzed and wrote the manuscript. CM, AA, and MU participated in the design, discussion, and provided critical comments on the manuscript. MM provided critical comments on the manuscript. All authors have read and approved the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Rwanda Ministry of Health for allowing us to conduct the study. We would also like to thank all research assistants and respondents for their time during data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.874714/full#supplementary-material
Abbreviations
AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; DDS, dietary diversity score; Hb, hemoglobin; LBW, low birth weight; MUAC, mid-upper arm circumference; SPSS, Statistical Package for the Social Sciences; USAID, United States Agency for International Development; WASH, Water, Sanitation, and Hygiene.
References
1. Assefa N, Berhane Y, Worku A. Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One. (2012) 7:e39957. doi: 10.1371/journal.pone.0039957
2. Chrisman JR, Mattos IE, Koifman RJ, Koifman S, Boccolini PMM, Meyer A. Prevalence of very low birthweight, malformation, and low Apgar score among newborns in Brazil according to maternal urban or rural residence at birth. J Obstet Gynaecol Res. (2016) 42:496–504.
3. da Silva Lopes K, Ota E, Shakya P, Dagvadorj A, Balogun OO, Peña-Rosas JP, et al. Effects of nutrition interventions during pregnancy on low birth weight: an overview of systematic reviews. BMJ Glob Health. (2017) 2:e000389.
4. Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. (2019) 7:e849–60. doi: 10.1016/S2214-109X(18)30565-5
5. Hughes MM, Black RE, Katz J. 2500-g Low birth weight cutoff: history and implications for future research and policy. Matern Child Health J. (2017) 21:283–9. doi: 10.1007/s10995-016-2131-9
6. Barros FC, Barros AJD, Villar J, Matijasevich A, Domingues MR, Victora CG. How many low birthweight babies in low- and middle-income countries are preterm? Rev Saude Publica. (2011) 45:607–16.
7. Mahumud RA, Sultana M, Sarker AR. Distribution and determinants of low birth weight in developing countries. J Prev Med Pub Health. (2017) 50:18.
8. He Z, Bishwajit G, Yaya S, Cheng Z, Zou D, Zhou Y. Prevalence of low birth weight and its association with maternal body weight status in selected countries in Africa: a cross-sectional study. BMJ Open. (2018) 8:e020410. doi: 10.1136/bmjopen-2017-020410
9. NISR and ICF. National Institute of Statistics of Rwanda (NISR) [Rwanda], Ministry of Health (MOH) [Rwanda], and ICF. Rwanda Demographic and Health Survey 2019-20 Final Report. Kigali: NISR and ICF (2021).
10. Ghimire R, Phalke DB, Phalke VD, Banjade B, Singh AK. Determinants of low birth weight: a case control study in Pravara Rural hospital in Western Maharashtra, India. Int J Sci Res. (2014) 3:243–5.
11. Khan A, Nasrullah FD, Jaleel R. Frequency and risk factors of low birth weight in term pregnancy. Pak J Med Sci. (2016) 32:138.
12. Grady SC, Frake AN, Zhang Q, Bene M, Jordan DR, Vertalka J, et al. Neonatal mortality in East Africa and West Africa: a geographic analysis of district-level demographic and health survey data. Geospatial Health. (2017) 12:501. doi: 10.4081/gh.2017.501
13. Metgud CS, Naik VA, Mallapur MD. Factors affecting birth weight of a newborn–a community based study in rural Karnataka, India. PLoS One. (2012) 7:e40040. doi: 10.1371/journal.pone.0040040
14. Christian P, Lee SE, Donahue Angel M, Adair LS, Arifeen SE, Ashorn P, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low-and middle-income countries. Int J Epidemiol. (2013) 42:1340–55.
15. Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. (2013) 382:417–25.
16. Figueiredo ACMG, Gomes-Filho IS, Silva RB, Pereira PPS, Mata FAFD, Lyrio AO, et al. Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. (2018) 10:601.
17. Mosha D, Canavan CR, Bellows AL, Blakstad MM, Noor RA, Masanja H, et al. The impact of integrated nutrition-sensitive interventions on nutrition and health of children and women in rural Tanzania: study protocol for a cluster-randomized controlled trial. BMC Nutr. (2018) 4:29. doi: 10.1186/s40795-018-0238-7
18. Sharma M, Mishra S. Effects of maternal health and nutrition on birth weight of infant. Int J Sci Res. (2014) 3:855–8.
19. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet Lond Engl. (2013) 382:452–77.
20. Ruel MT, Alderman H. Maternal and child nutrition study group. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet Lond Engl. (2013) 382:536–51. doi: 10.1016/S0140-6736(13)60843-0
21. Ministry of Health. Rwanda National Food and Nutrition Policy 2013-2018. Rwanda: Ministry of Health (MoH) (2014). Available online at: https://www.moh.gov.rw/fileadmin/user_upload/policies/National_Food_and_Nutrition_Policy_.pdf
22. Taneja S, Chowdhury R, Dhabhai N, Mazumder S, Upadhyay RP, Sharma S, et al. Impact of an integrated nutrition, health, water sanitation and hygiene, psychosocial care and support intervention package delivered during the pre- and peri-conception period and/or during pregnancy and early childhood on linear growth of infants in the first two years of life, birth outcomes and nutritional status of mothers: study protocol of a factorial, individually randomized controlled trial in India. Trials. (2020) 21:127. doi: 10.1186/s13063-020-4059-z
23. Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochr Datab Syst Rev. (2017) 4:CD004905.
24. Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. (2015) 2015:CD004736.
25. Ota E, Hori H, Mori R, Tobe-Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. (2015) 2015:CD000032.
26. World Food Programme. Comprehensive Food Security and Vulnerability Analysis, 2018. Kigali: World Food Programme (2018).
27. WHO. WHO Recommendations on Intrapartum Care for a Positive Childbirth Experience. Geneva: World Health Organization (2018).
28. Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. (2013) 5:54a8b618c1bc031ea140e3f2934599c8. doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8
30. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis Second Edition: A Regression-Based Approach. New York, NY: Guilford Press (2018).
31. Demilew YM, Alene GD, Belachew T. Effects of guided counseling during pregnancy on birth weight of newborns in West Gojjam Zone, Ethiopia: a cluster-randomized controlled trial. BMC Pediatr. (2020) 20:466. doi: 10.1186/s12887-020-02363-8
32. Nyamasege CK, Kimani-Murage EW, Wanjohi M, Kaindi DWM, Ma E, Fukushige M, et al. Determinants of low birth weight in the context of maternal nutrition education in urban informal settlements, Kenya. J Dev Orig Health Dis. (2019) 10:237–45. doi: 10.1017/S2040174418000715
33. Girard AW, Olude O. Nutrition education and counselling provided during pregnancy: effects on maternal, neonatal and child health outcomes. Paediatr Perinat Epidemiol. (2012) 26:191–204.
34. Ramakrishnan U, Grant F, Goldenberg T, Zongrone A, Martorell R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: a systematic review. Paediatr Perinat Epidemiol. (2012) 26:285–301.
35. East CE, Biro MA, Fredericks S, Lau R. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database Syst Rev. (2019) 4:CD000198.
36. Park JJH, Fang ML, Harari O, Dron L, Siden EG, Majzoub R, et al. Association of early interventions with birth outcomes and child linear growth in low-income and middle-income countries: bayesian network meta-analyses of randomized clinical trials. JAMA Netw Open. (2019) 2:e197871. doi: 10.1001/jamanetworkopen.2019.7871
37. Victora CG, Barros FC, Assunção MC, Restrepo-Méndez MC, Matijasevich A, Martorell R. Scaling up maternal nutrition programs to improve birth outcomes: a review of implementation issues. Food Nutr Bull. (2012) 33:S6–26.
38. McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. (2015) 2015:CD008666.
39. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. (2015) 2015:CD007950.
40. Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst Rev. (2013) 2013:CD006896.
41. Harding KB, Peña-Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev. (2017) 3:CD011761.
42. Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. (2015) 2015:CD000230.
43. Salvig JD, Lamont RF. Evidence regarding an effect of marine n-3 fatty acids on preterm birth: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2011) 90:825–38.
44. Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, et al. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev. (2013) 2013:CD009382.
45. Gizaw Z, Worku A. Effects of single and combined water, sanitation and hygiene (WASH) interventions on nutritional status of children: a systematic review and meta-analysis. Ital J Pediatr. (2019) 45:77. doi: 10.1186/s13052-019-0666-2
46. Taukobong HFG, Kincaid MM, Levy JK, Bloom SS, Platt JL, Henry SK, et al. Does addressing gender inequalities and empowering women and girls improve health and development programme outcomes? Health Policy Plan. (2016) 31:1492–514. doi: 10.1093/heapol/czw074
47. Masset E, Haddad L, Cornelius A, Isaza-Castro J. Effectiveness of agricultural interventions that aim to improve nutritional status of children: systematic review. BMJ. (2012) 344:d8222.
48. Demilew YM, Alene GD, Belachew T. Effect of guided counseling on dietary practices of pregnant women in West Gojjam Zone, Ethiopia. PLoS One. (2020) 15:e0233429. doi: 10.1371/journal.pone.0233429
49. Nikièma L, Huybregts L, Martin-Prevel Y, Donnen P, Lanou H, Grosemans J, et al. Effectiveness of facility-based personalized maternal nutrition counseling in improving child growth and morbidity up to 18 months: a cluster-randomized controlled trial in rural Burkina Faso. PLoS One. (2017) 12:e0177839. doi: 10.1371/journal.pone.0177839
50. von Salmuth V, Brennan E, Kerac M, McGrath M, Frison S, Lelijveld N. Maternal-focused interventions to improve infant growth and nutritional status in low-middle income countries: a systematic review of reviews. PLoS One. (2021) 16:e0256188. doi: 10.1371/journal.pone.0256188
51. Goodarzi-Khoigani M, Baghiani Moghadam MH, Nadjarzadeh A, Mardanian F, Fallahzadeh H, Mazloomy-Mahmoodabad S. Impact of nutrition education in improving dietary pattern during pregnancy based on pender’s health promotion model: a randomized clinical trial. Iran J Nurs Midwifery Res. (2018) 23:18–25. doi: 10.4103/ijnmr.IJNMR_198_16
Keywords: birth weight, effectiveness, integrated nutrition intervention, low birth weight, Rwanda
Citation: Habtu M, Agena AG, Umugwaneza M, Mochama M and Munyanshongore C (2022) Effectiveness of Integrated Maternal Nutrition Intervention Package on Birth Weight in Rwanda. Front. Nutr. 9:874714. doi: 10.3389/fnut.2022.874714
Received: 12 February 2022; Accepted: 23 June 2022;
Published: 22 July 2022.
Edited by:
Annalisa Terranegra, Sidra Medicine, QatarReviewed by:
Felix Sayinzoga, Rwanda Biomedical Center, RwandaGrant Murewanhema, University of Zimbabwe, Zimbabwe
Copyright © 2022 Habtu, Agena, Umugwaneza, Mochama and Munyanshongore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Habtu, bWlrZWwuaGFidHVAZ21haWwuY29t
 Michael Habtu
Michael Habtu Alemayehu Gebremariam Agena3
Alemayehu Gebremariam Agena3