- 1Laboratory Research of Medical and Molecular Parasitology and Mycology, LR12ES08, Faculty of Pharmacy, University of Monastir, Monastir, Tunisia
- 2Unit of Microbiology, Faculty of Dental Medicine, University of Monastir, Monastir, Tunisia
- 3Department of Dental Medicine, Fattouma Bourguiba University Hospital, University of Monastir, Monastir, Tunisia
- 4Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, ON, Canada
- 5Department of Health Sciences, Postgraduate School of Public Health, University of Genoa, Genoa, Italy
- 6NIHR Leeds Musculoskeletal Biomedical Research Unit, Section of Musculoskeletal Disease, Chapel Allerton Hospital, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, United Kingdom
- 7Research Laboratory “Heart Failure, LR12SP09”, Faculty of Medicine, Hospital Farhat Hached, University of Sousse, Sousse, Tunisia
The aim of this systematic review was to report the impacts of Ramadan intermittent fasting (RIF) on salivary flow-rate (SFR) and metabolic parameters. A thorough literature search was carried out using the databases PubMed and Scopus from their inception up to 15 July 2021. The Boolean connectors used in PubMed were (Saliva [Mesh] AND Fasting [Mesh]). The same keywords were used in Scopus. Inclusion criteria were defined using PICOS. The research included all original studies involving “healthy” adults and published in English. Methodological quality assessment was performed utilizing the Joanna Briggs Institute Critical Appraisal Tool, which allows attributing scores from 1 to 11 to the selected studies. Two authors carried out the literature search, study selection, and data extraction. Differences on issues were resolved by a third author if necessary. The systematic review protocol was registered within the “Open Science Framework” (Doi: 10.17605/OSF.IO/DE7BH). Six articles met the inclusion criteria. All studies were heterogeneous and had a high score of bias and several methodological differences. The following parameters were collected: SFR, melatonin, cortisol, glucose, immunoglobulin A (IgA), uric-acid, alkaline phosphatase (ALP), and aspartate aminotransferase (AST). The SFR decreased by 10% during Ramadan in fasting subjects. The circadian pattern of melatonin remained unchanged during Ramadan, but melatonin levels dropped significantly from baseline. The salivary cortisol levels were unchanged or increased during Ramadan. The salivary glucose levels were decreased. ALP increased significantly, whilst uric-acid and AST decreased significantly. Salivary IgA decreased during the last week of Ramadan. To conclude, there is a trend toward a decrease in SFR and the content of the majority of the biomarkers investigated, with the exception of ALP and uric-acid. These changes cannot be easily attributed to any single factor (hydration status, dietary habits, physical activity, or hygiene habits).
Systematic Review Registration: [https://osf.io/de7bh/], identifier [Doi: 10.17605/OSF.IO/DE7B].
Introduction
Human saliva is a biofluid produced and secreted by the major and minor salivary glands (1). The major salivary glands are the parotid, submandibular, and sublingual glands, responsible for more than 90% of salivary secretions, and the minor glands are distributed throughout the oral mucosa surfaces (1). Saliva plays an essential role in oral cavity maintenance and functionality (1), and it represents a mirror reflecting both oral and systemic health (2). Salivary secretions are composed of water, electrolytes, and several biomolecules, including proteins, enzymes, exosomes, nuclear acids, hormones, and cellular components (2). Many studies have demonstrated that the composition of saliva varies depending on the type of stimulation (2), the short-term acute mental stress (3), the taste and smell (4), and the daily and seasonal circadian rhythms (5). Hence, recurrent circadian fasting during Ramadan [i.e., Ramadan intermittent fasting (RIF)] may modify the salivary parameters.
Ramadan is the ninth month of the Muslim lunar calendar and it lasts 29 or 30 days depending on the actual observation of the moon’s crescent (6). The synodic nature of the Muslim calendar means that Ramadan occurs 10–11 days earlier each Gregorian year, migrating across all four seasons over approximately a 33-year cycle (6). Therefore, the fasting daytime duration can vary accordingly with longer fasting durations during summer. At any time point, the geographical situation will have an impact on the daylight. The higher the latitude is, the longer the fasting duration will be (7). Recurrent circadian fasting during Ramadan is practiced by around two billion Muslims every year (8), and healthy adult Muslims are asked to refrain from eating and drinking during this month between Sahur (dawn meal just before the start of fast) and Iftar (sunset meal marking the end of the fast) as a religious duty (6). Since food and water intake takes place from sunset to dawn, this modification in Muslims’ lifestyle for 1 lunar month may have an impact on oral health. A Muslim may be exempt from fasting during Ramadan (DR) for several reasons, including pregnancy, breastfeeding, diabetes mellitus, and mental disability, however; despite these exemptions, many Muslim patients with chronic medical conditions still choose to fast (9).
Several systematic reviews have studied the effects of RIF on general health (10–12), notably on the immune system (13), cardiovascular function (14), dietary intake and body composition or weight (15, 16), glycemic control (17), kidney function (18), and sleep (19). However, to the best of the authors’ knowledge, no previous systematic review has investigated the impacts of RIF on salivary secretion [e.g., salivary flow-rate (SFR)] and metabolic parameters such as cortisol, glucose, melatonin, and uric-acid. The aim of this paper was therefore to systematically review the impacts of RIF on SFR and saliva metabolic parameters.
Methods
Protocol and Eligibility Criteria
The systematic review protocol was registered within the “Open Science Framework” (OSF, DOI 10.17605/OSF.IO/DE7BH). This systematic review followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (20). The inclusion criteria were formulated based on the following PICOS tool questions (21): P (population) = healthy Muslim adults willing to fast DR; I (intervention/exposure) = exposure to RIF; C (Comparison): DR and outside Ramadan [i.e., before-Ramadan (BR) and after-Ramadan (AR)]; O (Outcome): SFR and saliva metabolic parameters; and S (Study design): all original articles written in English. No restrictions were applied in terms of study design, setting, country, or period. Publications not in compliance with the purpose of this systematic review as well as those not representing original research (i.e., reviews, editorials, qualitative papers, case reports, case series, and letters to editors) were not included.
Literature Search
An online literature search was performed using two databases: PubMed and Scopus from their inception up to 15 July 2021. For PubMed, the search was carried out using a strategy employing the combination of the following two “Medical Subject Headings” (MeSH) terms: Saliva AND Fasting. As for Scopus, the previous two terms were searched for in the article titles, abstracts, and/or keywords. In addition, the reference lists of the included articles were checked. All the authors involved in this review agreed on the articles to be included in this systematic review.
Study Selection
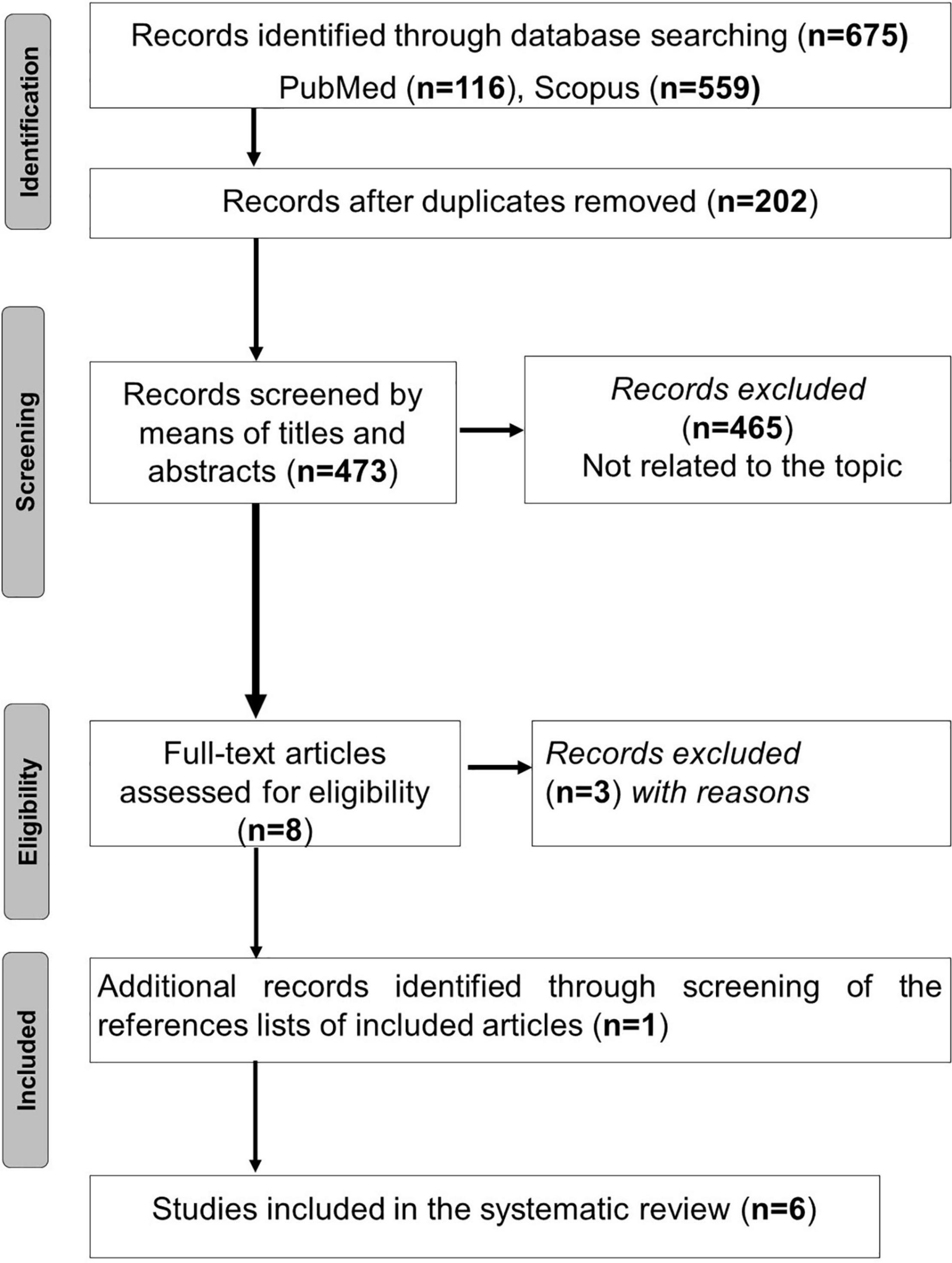
The process of articles selection is outlined in Figure 1. Duplicate articles were eliminated using End-Note X9 library. Titles of the remaining articles were independently appraised during the initial online literature search for studies by two of the authors (AB and MK in the authors’ list) to check for their relevance to the searched topics. Abstracts of these titles were then read to determine if the studies met the inclusion criteria. The studies whose abstracts met the inclusion criteria were then read in full-text format to determine their eligibility and therefore retention. Two authors (AB and MK in the authors’ list) conducted the study selection process for this review, with discrepancies being checked by a third author (HBS in the authors’ list), if necessary.
Data Extraction
Data from the retained studies were extracted using a format including the population, the parameters being investigated, the periods during which the parameters were collected, and the significant findings. Data were extracted, reviewed, and analyzed by two authors (AB and MK in the authors’ list). Extracted data were then verified by a third author (HBS in the authors’ list). Discrepancies in data collection were resolved through discussion.
Methodological Quality Assessment
Methodological quality assessment was performed using the Joanna Briggs Institute (JBI) critical appraisal tool, precisely the checklist for cohort studies (https://joannabriggs.org/last visit: 4 March 2022). The checklist appraises the following areas: recruitment, exposure measurement, reliability of exposure measurement, confounding factors identified, strategies to deal with confounding factors, participants free of outcome at the onset of the study, validity and reliability of outcome measurement, follow-up timeframe reported, follow-up completion, strategies utilized to deal with incomplete follow-up, and appropriate statistical analysis. The checklist included the following 11 items: 1. Were the two groups similar and recruited from the same population? 2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? 3. Was the exposure measured in a valid and reliable way? 4. Were the confounding factors identified? 5. Were the strategies to deal with confounding factors stated? 6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? 7. Were the outcomes measured in a valid and reliable way? 8. Was the follow-up time reported and sufficient to be long enough for outcomes to occur? 9. Was follow-up complete, and if not, were the reasons for loss to follow-up described and explored? 10. Were the strategies to address incomplete follow-up utilized? and 11. Was the appropriate statistical analysis used? These items are scored as either yes, no, unclear, or not applicable. Two reviewers (AB and MK in the authors’ list) independently scored the retained studies, with discrepancies being resolved through discussion. If discrepancies could not be resolved through discussion, a third author (HBS in the authors’ list) intervened to reach consensus. The risk of bias in the studies was judged to be low (“yes” scores > 70%), moderate (50 ≤ “yes” scores between ≤ 69%), and high (“yes” scores < 49%) (22).
Results
Search Results
The search process yielded 675 articles, of which 202 were duplicated. Among the 473 remaining papers, 465 were excluded based on the title and the abstract. When screening the references lists of the remaining eight articles (23–30), one additional paper was added (31). After assessing full-text articles for eligibility, three articles were excluded (23–25). Consequently, six articles were retained (26–31). The search results are presented in Figure 1.
Methodological Quality Assessment Results
The retained six studies were assessed for methodological quality (Table 1). All the studies have a high score of bias (i.e., final score ranging from 9.1 to 36.4%). Items 2, 5, and 10 were rated as not applicable for all the studies. No study reported data regarding items 4, 7, and 11. Six (26–31), five (26–29, 31), four (27–29, 31), one (29), and one (31) studies included information regarding items 6, 8, 3, 9, and 1, respectively.
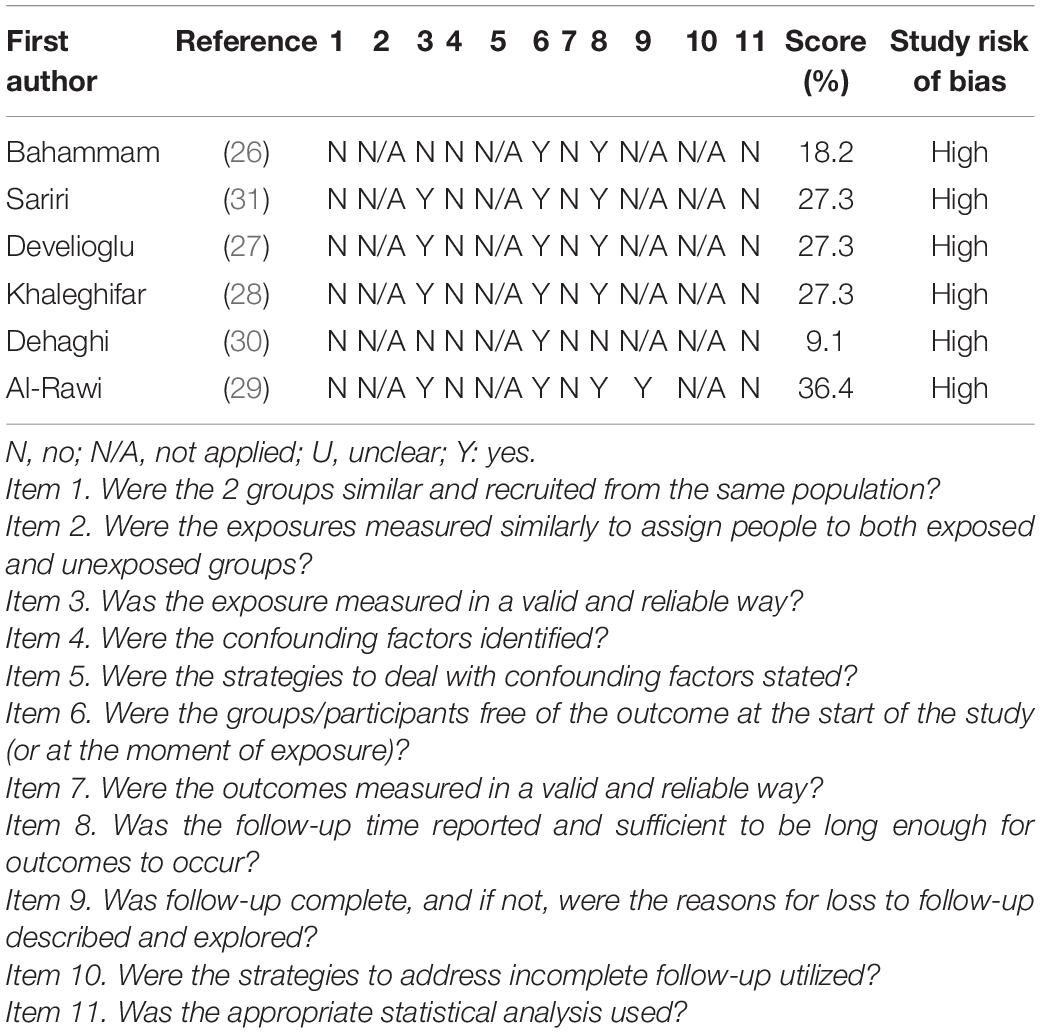
Table 1. Quality scoring of the retained articles according to Joanna Briggs Institute critical appraisal checklist.
Study Selection and Characteristics
Table 2 exposes the main characteristics and methodological points of the retained studies. The latter were published between 2004 (26) and 2020 (29, 30). The studies were conducted in Saudi Arabia (26), Turkey (27), United Arab Emirates (29), and Iran (28, 30, 31). The study design was not reported in three studies (27–29). In the remaining studies, three designs were applied: observational design with repeated measures (26), case-control (31), and descriptive analytical research design (30). All the studies (26–31) opted for convenience samples. The Ramadan year was omitted in three studies (28, 30, 31). Only one study (29) mentioned the number of fasting days DR. Only one study (28) reported the average ambient temperature, which was around 15°C. Only three studies reported the mean fasting duration [i.e., 12 h (26), 15 h (29), 17 h (27)]. No study reported data with regard to the Ramadan season, the average ambient pressure, or the average ambient humidity.
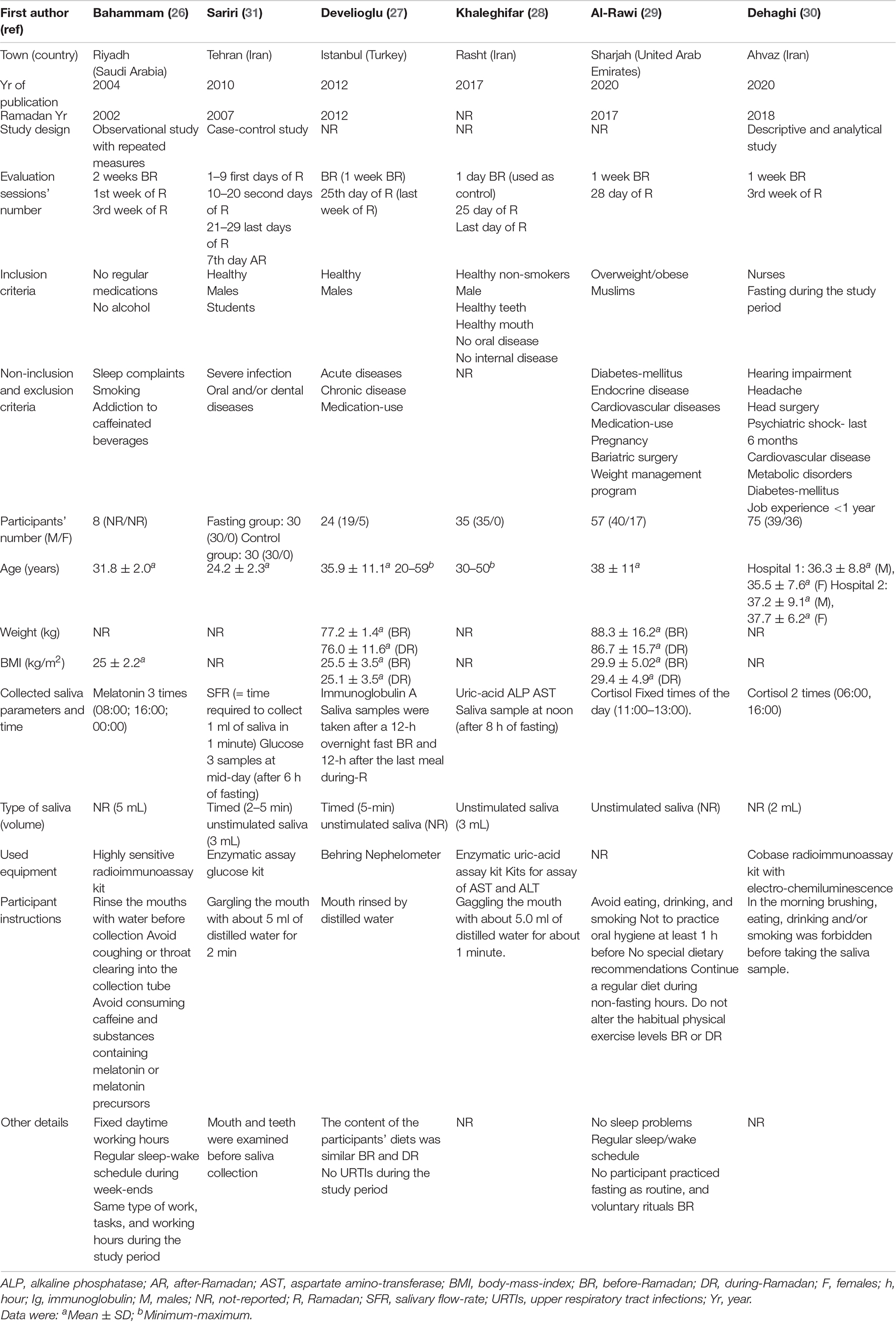
Table 2. Main characteristics and methodology points of the published studies aiming to evaluate the impacts of Ramadan intermittent fasting (RIF) on saliva parameters.
The number of evaluation sessions was two (27, 29, 30), three (26, 28), and four (31). Five studies (26–30) opted for a session BR with different periods applied [i.e., 1 day BR (28), 1 week BR (27, 29, 30), 2 weeks BR (26)]. Only one study opted for a session AR (i.e., 7 days AR) (31). The number of sessions DR was one (27, 29, 30), two (26, 28), and four (31), and different periods were retained [i.e., 1 week (26), first 10 days (31), 10–20 second days (31), third week (26, 30), 21–29 last days (31), 25th day of Ramadan (27, 28), last day of Ramadan (28, 29)].
Two-hundred twenty-nine participants fasting DR were included. The sample sizes varied from 8 (26) to 75 (30). Three studies included mixed population of males and females (27, 28, 30), two studies included only males (28, 31), and the participants’ sex was not reported in one study (26). Four studies included healthy participants (26–28, 31), one study involved both overweight and obese participants (29), and one study omitted to report the health status of the included participants (30). The included participants were: students (31), employees in a factory (28), staff of a training and research hospital (27), and nurses (30). Several non-inclusion/exclusion criteria were applied. They were related to habits [e.g., smoking (26, 28), alcohol-use (26), addiction to caffeinated beverages (26)], medication-use (26, 27, 29), some health complaints [e.g., sleep complaints (26)], acute diseases [e.g., upper respiratory tract infections (27), severe infections (31)], chronic conditions [e.g., unhealthy teeth or mouth (28), oral diseases (28, 31), internal diseases (28), endocrine diseases (29), diabetes mellitus (29, 30), metabolic disorders (30), cardiovascular diseases (29, 30), hearing impairment (30), headache (30), psychiatric shock (30), unspecified (27)], previous surgeries [e.g., bariatric surgery (29), head surgery (30)], pregnancy (29), weight management program (29), and job experience <1 year (30). Only one study highlighted that no participant practiced fasting as routine and voluntary rituals before the month of Ramadan (29). In one study (29), participants were asked to continue their regular diet during non-fasting hours, and not to alter their habitual physical exercise levels BR or DR. Participants’ ages varied from 24.2 ± 2.3 (31) to 59 (27) years. Participants’ weight and body mass index were reported in two (27, 29) and three (26, 27, 29) studies, respectively.
Eight different saliva parameters were evaluated: SFR (31), glucose (31), melatonin (26), cortisol (29, 30), immunoglobulin A (IgA) (27), uric-acid (28), alkaline phosphatase (ALP) (28), and aspartate amino-transferase (AST) (28). The numbers of saliva sampling were one (27, 28), two (29, 30), and three (26, 31). Different times of saliva sampling were chosen. In some studies, fixed times were applied [e.g., mid-night (26), 6h00 (30), 8h00 (26), between 11h00 and 13h00 (29), 16h00 (26)]. In some other studies, a minimum of hours of fasting was needed [e.g., 6 (31), 8 (28), 12 (27)]. Four studies reported that they opted for unstimulated saliva (27–29, 31), and only two studies reported the duration of saliva collection [e.g., 2–5 (31) and 5 (27) min]. The volume of the collected saliva (in mL) was highlighted in four studies [e.g., 2 (30), 3 (28, 31), and 5 (26)]. One study omitted to report the equipment used to analyze the saliva outcomes (29). Before saliva collection, participants were asked to rinse their mouths with water (26–31) and to avoid: (i) coughing or throat clearing into the collection tube (26), (ii) consuming caffeine and substances containing melatonin or melatonin precursors (26), (iii) eating, drinking, and smoking (29, 30), (iv) brushing (30), and (v) using oral hygiene products (29).
Impact of Ramadan Intermittent Fasting on the Salivary Flow-Rate and Saliva Metabolites
Table 3 presents the main results of the six retained studies.
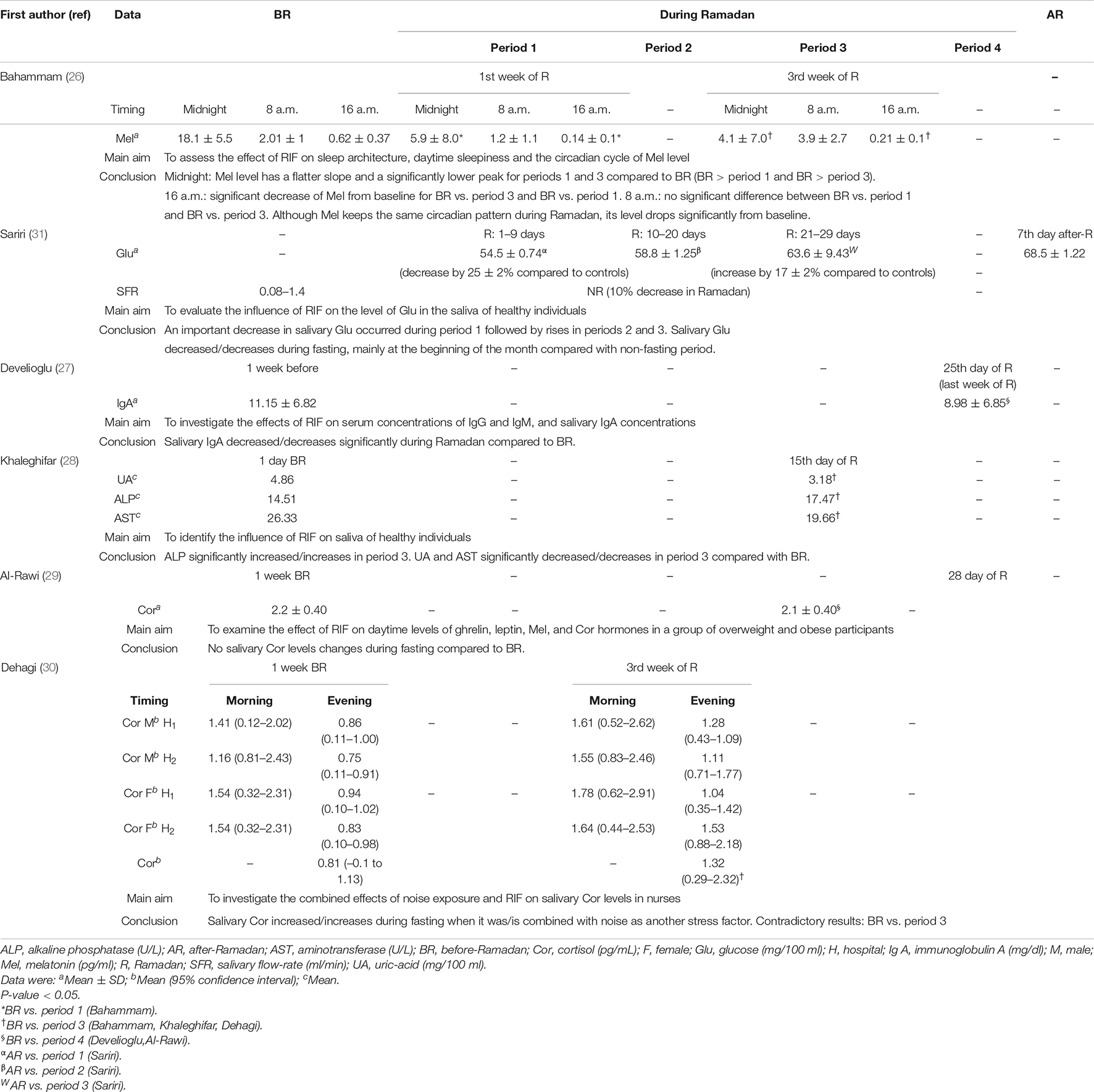
Table 3. Main results of the published studies aiming to evaluate the impacts of Ramadan intermittent fasting (RIF) on saliva parameters.
Salivary Flow-Rate
The only study evaluating the SFR reported its decrease by 10% DR compared to controls (31).
Salivary Hormones: Melatonin and Cortisol
Khaleghifar et al. (28) reported that melatonin keeps the same circadian pattern DR, but its level drops significantly from baseline. At midnight, melatonin level has a flatter slope and a significantly lower peak in the first and the third weeks of Ramadan compared to BR. At 8 a.m., there is no significant difference between BR and the first or third weeks of Ramadan. At 16 a.m., there is a significant decrease of melatonin from baseline for BR vs. the first or third weeks of Ramadan.
Regarding salivary cortisol levels, studies reported different results (29, 30). One study reported no change in salivary cortisol levels DR compared to BR (29). Another study reported that RIF has a significant effect on salivary cortisol secretory levels (30). The latter increases during fasting when it is combined with noise as another stress factor (30).
Salivary Metabolic and Immunologic Data
Sariri et al. (31) reported a significant decrease in salivary glucose during the first 10 days of Ramadan (by 25% compared to controls), the 10–20 days of Ramadan, and 21–29 days of Ramadan (by 17% compared to controls). Khaleghifar et al. (28) reported that compared to BR, on the 15th day of Ramadan, ALP significantly increases, and uric-acid and AST significantly decrease. Develioglu et al. (27) noted that salivary IgA decreases significantly during the last week of Ramadan compared to BR.
Discussion
The present systematic review included six studies, all having a high score of bias (26–31). In these studies, eight saliva parameters were evaluated (SFR, melatonin, cortisol, glucose, IgA, uric-acid, ALP, and AST). The main results were: (i) the SFR decreased by 10% DR in fasting participants compared to controls (31), (ii) the circadian pattern of melatonin was unchanged DR, but melatonin level dropped significantly from baseline (28), (iii) the salivary cortisol levels were unchanged DR compared to BR (29), or increased DR (30), (iv) the salivary glucose levels were decreased DR (31), (v) compared to BR, on the 15th day of Ramadan, ALP significantly increased, and uric-acid and AST significantly decreased (28); (vi) the salivary IgA decreased during the last week of Ramadan compared to BR (27). All the retained studies were heterogeneous and had several methodological differences. This heterogeneity limited the ability of the present review to perform any data synthesis via meta-analysis. It also challenged the researchers’ ability to identify trends in the data. Research reports in this area are few and they were almost limited to the changes of glucose concentrations in plasma (31). To the best of the authors’ knowledge, this is the first systematic review investigating the effects of RIF on SFR and saliva parameters.
Impacts of Ramadan Intermittent Fasting on Salivary Flow-Rate
SFR decreased by 10% DR (31). DR, the lack of gustatory stimulation decreases the stimulation of salivary glands, therefore, SFR may decline. The autonomic nervous system controls SFR and the secretion of various salivary compounds (32). Stimulation of this system induces modifications in salivary secretions and SFR (33). In Ramadan, sedentary activity with minimal orofacial movement and metabolism slowing down in body tissues cells, including oral cavity cells, may explain the low stimulation of the autonomic nervous system (28). This hyposalivation can cause malodor, especially DR (34). Since saliva works to moisten the mouth, to neutralize acids produced by plaque, and to clean bacteria and food particles from the mouth, any salivary modifications create a suitable environment for aerobic and anaerobic bacteria that coat several sites in the oral cavity, notably the dorsum of the tongue (35). Overall, it has been shown that oral microflora modifications taking place DR may lead to malodor, even if other factors are involved (36).
Impact of Ramadan Intermittent Fasting on Salivary Hormones: Melatonin and Cortisol
Melatonin in saliva or plasma is an indicator of the timing of the circadian clock (37). According to Bahammam (26), the sleep hormone follows the same circadian rhythm both BR and DR. This means that melatonin secretion is low during the daytime, while the highest levels are released at night, but its level drops significantly from baseline (26). This variation may be due to the sleep habits modification DR (36). Nevertheless, this outcome should be considered with caution because of the small sample size in the study (n = 8) (26).
Cortisol is a hormone produced by the adrenal glands (38). Cortisol plays an essential role in balancing blood glucose and releasing sugar from the body’s stores in response to increased energy demands (39). Cortisol has an important role in the metabolism of fats and proteins as well as in the circadian rhythm regulation (38). This hormone is usually measured in the morning (7–9 a.m.) because it reaches a peak at this time (40). DR, external sources of glucose are reduced due to fasting. Consequently, salivary glucose concentration drops significantly (31). Thus, we can “speculate” that cortisol levels in saliva may rise to regulate glucose levels, however; the latter mechanism is not that straightforward and has to be elucidated by further research.
Dehagi et al. (30) reported that when participants are exposed to RIF and noise, which is another stress source, salivary cortisol levels increase. In addition to its glycemic effects, cortisol is also liberated during the stress periods in order to allow the body to adapt to an emotional or physical shock by mobilizing additional energy sources. The contradictory results of the studies of Al-Rawi et al. (29) and Dehagi et al. (30) may be due to methodological reasons, notably the study design and population, and the lack of information about the timing and duration of sleep in one study (30). It should be highlighted that many people in various Islamic countries may change their sleep rhythm during the Holy month. Indeed, their nighttime sleep duration is reduced compared to non-fasting days (41), in addition to the dietary patterns’ changes (42).
Impact of Ramadan Intermittent Fasting on Salivary Metabolic and Immunologic Data
Alkaline phosphatase and aminotransferase are usually measured together to investigate the hepatic, cardiovascular, and renal functions (43). ALP is a protein produced by various cell types (e.g., polymorphonuclear leukocytes, osteoblasts, macrophages, and fibroblasts) within the alveolar bone and/or the salivary glands (44, 45). ALP can be a salivary biomarker of periodontal diseases and caries (46), as it interferes in the balance of the remineralization-demineralization cycle since it is primarily involved in calcium and phosphate binding (47). It seems that the function of ALP relatively depends on the salivary pH and buffering capacity (48). Khalighefar et al. (28) reported that ALP rebounds during the middle of Ramadan compared to BR. Although ALP increase may suggest much more susceptibility to dental caries and/or oral diseases, it is believed that this fluctuation is not so critical to lead to an illness. AST is an enzyme involved in the metabolism of several tissues and organs (49). Khaleghifar et al. (28) indicated that AST activity in fasting volunteers decreases significantly DR. This decrease can be related to the fact that fasting reduces the metabolism of body tissues cells, including oral cavity cells, thus leading to reduced SFR during fasting (28). Uric-acid is the ultimate product of the metabolic breakdown of purines, which are the nitrogenous bases in DNA and RNA (50). It is involved in healing and defense (50). Khaleghifar et al. (28) reported that uric-acid decreases DR since the metabolism is reduced (28). In contrast, several studies have shown that blood uric-acid increases during RIF (51–54). According to studies reported in the literature, despite the shifts in metabolic interactions among the organs producing uric-acid, AST or ALP, we cannot conclude on the effects of RIF on these enzymes because of the scarcity of these studies in addition to the limitations of the unique retained study investigating those parameters (28).
Salivary glucose DR plunges from baseline, especially in the first 10 days (31). First, this is expected because of food restriction for 4 weeks. Secondly, this fact is interesting and beneficial for oral health. Actually, both cariogenic bacteria and Candida use glucose for their development and survival (55, 56). This dysbiosis enhances the proliferation of these bacteria and dental biofilm development (46, 57). A recent study investigated the effect of different salivary glucose concentrations on dual-species biofilms of Candida albicans and Streptococcus mutans (58). The authors reported that higher salivary glucose increases counts of Candida albicans (58). It is possible that the higher levels of IgA detected in saliva BR can be attributed to the greater colonization of the oral cavity by Candida albicans due to the higher salivary glucose levels during that period compared to DR.
Salivary IgA has an important role in mucosal immunity. Its levels increase in case of oral mucosa infection, such as candidiasis. It allows inhibiting the adherence of candida to epithelial cells (59, 60). In contrast, the decrease in those salivary IgA levels does not necessarily suggest that the participant is more susceptible to oral infection onset, since a salivary IgA concentration threshold is absent (27). Subsequently, authors suggested that RIF results in neither severe immunological disturbances nor adverse impact on health (27). Some remarks related to the usefulness of salivary IgA in real practice should be highlighted. First, there are some concerns regarding the usefulness of salivary IgA as a biomarker in the detection of respiratory tract infection due to lack of reproducibility, low specificity, and sensitivity (61). Secondly, there are conflicting data in the literature regarding salivary IgA levels induced by exercise, with some studies reporting a decrease whilst others have reported an increase or no change (62). Thirdly, previous studies have reported a decrease in systemic IgA levels without leading to an increase in infection (63). Fourthly, exposure to pathogenic microbes may be reduced DR, possibly due to consumption of more fresh foods DR compared to other months (64). It is possible that oral health and microbial exposure from foods are poorer BR, which may explain the higher IgA levels detected in the saliva DR (65). In this context, a recent study involving mice reported that oral colonization by Candida albicans increases IgA production (65). Another study suggested that an increase in salivary IgA is an attempt by the immune system to counter the accumulation of microorganisms (64). Considering the aforementioned studies (63–65), the decrease in IgA levels DR may reflect a lower microbial colonization of the oral cavity DR. This is plausible since the number of hours when the mouth is exposed to foods and beverages is reduced DR compared to other periods when one considers the number of hours spent fasting and sleeping.
Overall, it seems that fluctuations in salivary parameters in Ramadan are not as significant as blood changes. These alterations are not enough to cause diseases in healthy participants. Nevertheless, we believe that further studies using other salivary biomarkers are needed in order to investigate correlations with the risk of oral disturbances or infections, such as caries, malodor, periodontal disease, or candidiasis in Ramadan.
In view of the absence of evidence about the impacts of RIF on oral health, we recommend the following four advices for people observing Ramadan: (i) adopt a well-balanced diet with sufficient hydration before Sahur and after Iftar; (ii) brush teeth, at least after Iftar and just after Sahur, before the dawn; (iii) rinse mouth without swallowing water for a better biofilm control and reduction of halitosis; and (iv) take care of the oral cavity, particularly for patients with chronic systemic diseases, especially with metabolic disorders (e.g., diabetes mellitus) in order to avoid the progression of a preexistent pathology (e.g., periodontal disease, dental caries). Finally, it is recommended that dentists carry out “dental procedures” with special precautions [e.g., administer intramuscular or trans-dermal treatment instead of oral agents] (36).
Discussion of Methodology
According to the JBI critical appraisal tool, precisely the checklist for cohort studies, the methodological quality is considered as “low.” In fact, no study succeeded to get the average score and items related to “confounding factors” and “sample size calculation.” Moreover, “salivary collection methods” were not reported in any of the six retained studies (Table 1). First, non-inclusion of a non-fasting control group can be considered as a “bias” since the variations in the assessed parameters cannot be exclusively attributed to RIF. However, it is important to note that including non-fasting participants is still problematic, due to religious considerations in Muslim countries. For that reason, the non-fasting control groups could be the participants themselves outside the Ramadan period (e.g., BR and/or AR). Given the circumstances of the Ramadan observance, and for practical reasons, the authors think that is more feasible and easier to control the parameters than to arrange a separate group of participants who do not observe Ramadan. Secondly, selecting participants by a convenience sample may be considered as a major confounding factor (66). Convenience sampling is a type of non-probability sampling methods based on the judgment of the investigator (66). Its low cost and comfort of use make it an easy choice for investigators. Nevertheless, it can lead to under/over representation of specific groups inside the sample (66). Thus, it may be impossible to make generalizations in the whole population. For these reasons, convenience sampling should be treated with caution. Thirdly, calculation of an optimal size is a crucial point since it helps avoid an inadequate power to detect statistical effects (67). Using few participants in a study may lead to lower “precision” in findings. A large sample size is, however, expensive and exposes more participants to procedures (67). Fourthly, the procedure of saliva collection was not well-described (Table 1). In fact, it is very important to standardize saliva sampling in order to make comparison between studies possible. Since saliva collection should be made at least one time DR, unstimulated saliva might be preferred. In fact, stimulated saliva must be collected by chewing sterile paraffin (68). Then, a minimum duration for sufficient saliva collection may be defined to ensure efficient analysis.
Additional limitations should be highlighted. For example, information about the season, the average ambient pressure, and/or the average ambient humidity was lacking in the included studies (Table 1). The average ambient temperature as well as the fasting duration were mentioned in some studies (26–29) (Table 1). Consequently, both climatic conditions and geographical locations strongly influence RIF (69). Also, the inclusion of patients with obesity (i.e., body mass index ≥ 30 kg/m2) may be considered as a limitation. In fact, a lower SFR was observed among obese compared to non-obese participants (70, 71). In addition, the inclusion of females and old participants could complicate the interpretation of saliva parameters (72, 73). Indeed, Mahesh et al. (72) reported significant changes in the pH and the buffer-capacity in post-menopausal females’ saliva compared to regularly menstruating ones. Besides, it is known that females do not fast all the month of Ramadan. Subsequently, the comparison with males may not be valid because they are not exposed to the same fasting period. With regard to age, changes in salivary pH, buffering-capacity, calcium, and proteins concentrations were reported (73). Finally, the number of evaluation sessions was heterogeneous. Therefore, saliva collection should be performed at least three times as follows: BR (e.g., 1 week BR), DR (e.g., during the last 7–10 days of Ramadan), and AR (e.g., 7–10 days AR). In future studies aiming to evaluate the effects of RIF on oral health, three important points should be reported. The first is related to the practice of fasting as a routine (e.g., some Muslims fast on Mondays and Thursdays during all the year). The inclusion of some participants who practice this ritual may influence some saliva parameters. The second point concerns the chewing stick, called “Miswak,” which is widely used in some Arab states of the Persian Gulf (74, 75). In fact, it seems that “Miswak” use increases SFR (76). The third point concerns the hydration status. The role of the hydration status BR and DR were not considered in the six retained studies and the differences observed in the concentrations of the different metabolites may be partly due to the hydration status, which can alter the salivary composition and SFR (77). The six studies involved in this review did not adjust the concentrations of the different salivary biomarkers before comparing the data obtained BR and DR. Therefore, in the future, it would be interesting to see if the differences observed are still present after adjusting the metabolites by factors, such as total protein content, saliva osmolality, SFR, and saliva secretion rate (78).
The critical limitation of this Systematic Review is our inability to make a strong clinical case for the impacts of RIF on saliva parameters. Table 4 summarizes some recommendations for designing future studies aiming to investigate the impacts of RIF on saliva parameters. It is recommended that researchers assess the antimicrobial, anticancer, and wound healing properties of fasting saliva (collected just before iftar) and compare it with non-fasting saliva. Moreover, it would be great to compare the fasting saliva proteome with the non-fasting saliva, and to see if the fasting saliva can be a source of novel peptides that display health benefits (79). This will address the “myth/superstition” in medieval Europe where fasting saliva was used as a medicine (80, 81).

Table 4. Some recommendations for designing future studies related to the impact of Ramadan intermittent fasting on salivary parameters.
Conclusion
There is a general trend toward a decrease in SFR and a decrease in the content of the majority of the biomarkers investigated, with the exception of ALP and uric-acid. These changes cannot be easily attributed to any single factor, especially because of the lack of information on the hydration status, dietary habits, physical activity, and hygiene habits. Although the findings of this systematic review are interesting, scientific evidence should be interpreted carefully because studies of the impact of RIF on saliva parameters are scarce. This is mostly due to the lack of accurate methodological details or variations in the investigated saliva parameters and the employed methodologies. Furthermore, the authors have provided some recommendations for designing future studies related to the impact of RIF on salivary parameters.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
AB, MK, and HB performed bibliographic research, collected published manuscripts, and helped to draft the manuscript. NB helped draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank Prof. Samir Boukattaya for his invaluable contribution to the improvement of the quality of the writing in the present manuscript.
Abbreviations
ALP, alkaline phosphatase; AR, after-Ramadan; AST, aspartate amino transferase; BR, before-Ramadan; DR, during-Ramadan; IgA, immunoglobulin A; JBI, Joanna Briggs Institute; RIF, Ramadan intermittent fasting; SFR, salivary flow-rate.
References
1. Porcheri C, Mitsiadis TA. Physiology, pathology and regeneration of salivary Glands. Cells. (2019) 8:976. doi: 10.3390/cells8090976
2. Han P, Ivanovski S. Effect of saliva collection methods on the detection of periodontium-related genetic and epigenetic biomarkers-A pilot study. Int J Mol Sci. (2019) 20:4729. doi: 10.3390/ijms20194729
3. Naumova EA, Sandulescu T, Al Khatib P, Thie M, Lee WK, Zimmer S, et al. Acute short-term mental stress does not influence salivary flow rate dynamics. PLoS One. (2012) 7:e51323. doi: 10.1371/journal.pone.0051323
4. Carpenter GH. The secretion, components, and properties of saliva. Annu Rev Food Sci Technol. (2013) 4:267–76. doi: 10.1146/annurev-food-030212-182700
5. Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Invest. (2008) 68:448–58. doi: 10.1080/00365510701819127
6. Ahmed SH, Chowdhury TA, Hussain S, Syed A, Karamat A, Helmy A, et al. Ramadan and diabetes: A narrative review and practice update. Diabetes Ther. (2020) 11:2477–520. doi: 10.1007/s13300-020-00886-y
7. Chamari K, Roussi M, Bragazzi NL, Chaouachi A, Abdul RA. Optimizing training and competition during the month of Ramadan: recommendations for a holistic and personalized approach for the fasting athletes. Tunis Med. (2019) 97:1095–103.
8. Kettani H. World Muslim population: 1950-2020. Int J Environ Sci Technol. (2010) 10:154–64. doi: 10.7763/ijesd.2010.v1.29
9. Abolaban H, Al-Moujahed A. Muslim patients in Ramadan: a review for primary care physicians. Avicenna J Med. (2017) 7:81–7. doi: 10.4103/ajm.AJM_76_17
10. Leiper JB, Molla AM, Molla AM. Effects on health of fluid restriction during fasting in Ramadan. Eur J Clin Nutr. (2003) 57(Suppl. 2):S30–8. doi: 10.1038/sj.ejcn.1601899
12. Alkandari JR, Maughan RJ, Roky R, Aziz AR, Karli U. The implications of Ramadan fasting for human health and well-being. J Sports Sci. (2012) 30(Suppl. 1):S9–19. doi: 10.1080/02640414.2012.698298
13. Adawi M, Watad A, Brown S, Aazza K, Aazza H, Zouhir M, et al. Ramadan fasting exerts immunomodulatory effects: insights from a systematic review. Front Immunol. (2017) 8:1144. doi: 10.3389/fimmu.2017.01144
14. Salim I, Al Suwaidi J, Ghadban W, Alkilani H, Salam AM. Impact of religious Ramadan fasting on cardiovascular disease: a systematic review of the literature. Curr Med Res Opin. (2013) 29:343–54. doi: 10.1185/03007995.2013.774270
15. Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients. (2019) 11:478. doi: 10.3390/nu11020478
16. Osman F, Haldar S, Henry CJ. Effects of time-restricted feeding during Ramadan on dietary intake, body composition and metabolic outcomes. Nutrients. (2020) 12:2478. doi: 10.3390/nu12082478
17. Tahapary DL, Astrella C, Kristanti M, Harbuwono DS, Soewondo P. The impact of Ramadan fasting on metabolic profile among type 2 diabetes mellitus patients: a meta-analysis. Diabetes Metab Syndr Clin Res Rev. (2020) 14:1559–70. doi: 10.1016/j.dsx.2020.07.033
18. Bragazzi NL. Ramadan fasting and chronic kidney disease: a systematic review. J Res Med Sci. (2014) 19:665–76.
19. Faris MAE, Jahrami HA, Alhayki FA, Alkhawaja NA, Ali AM, Aljeeb SH, et al. Effect of diurnal fasting on sleep during Ramadan: a systematic review and meta-analysis. Sleep Breath. (2020) 24:771–82. doi: 10.1007/s11325-019-01986-1
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89.
21. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. (2018) 106:420–31. doi: 10.5195/jmla.2018.345
22. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
23. Vasaghi-Gharamaleki B, Mirzaii-Dizgah I. Unstimulated whole saliva cortisol levels during Ramadan in Iranian Muslims. J Contemp Dent Pract. (2014) 15:341–4. doi: 10.5005/jp-journals-10024-1540
24. Rahim ZH, Yaacob HB. Effects of fasting on saliva composition. J Nihon Univ Sch Dent. (1991) 33:205–10. doi: 10.2334/josnusd1959.33.205
25. Bahijri S, Borai A, Ajabnoor G, Abdul Khaliq A, AlQassas I, Al-Shehri D, et al. Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS One. (2013) 8:e60917. doi: 10.1371/journal.pone.0060917
26. Bahammam A. Effect of fasting during Ramadan on sleep architecture, daytime sleepiness and sleep pattern. Sleep Biol Rhythms. (2004) 2:135–43. doi: 10.1111/j.1479-8425.2004.00135.x
27. Develioglu ON, Kucur M, Ipek HD, Celebi S, Can G, Kulekci M. Effects of Ramadan fasting on serum immunoglobulin G and M, and salivary immunoglobulin A concentrations. J Int Med Res. (2013) 41:463–72. doi: 10.1177/0300060513476424
28. Khaleghifar NSR, Aghamaali M, Ghafoori H. The effect of ramadan fasting on biochemistry of saliva. Appl Biotechnol Report. (2017) 4:583–6.
29. Al-Rawi N, Madkour M, Jahrami H, Salahat D, Alhasan F, BaHammam A, et al. Effect of diurnal intermittent fasting during Ramadan on ghrelin, leptin, melatonin, and cortisol levels among overweight and obese subjects: a prospective observational study. PLoS One. (2020) 15:e0237922. doi: 10.1371/journal.pone.0237922
30. Dehaghi B, Norei M, Angali K. The influence of Ramadan fasting and equivalent noise exposure on salivary cortisol levels. PJMHS. (2020) 14:989–92.
31. Sariri RVA, Erfani A. Alterations in salivary glucose during ramadan fasting. Health. (2010) 2:769–72. doi: 10.4236/health.2010.27116
32. Leicht CA, Goosey-Tolfrey VL, Bishop NC. Exercise intensity and its impact on relationships between salivary immunoglobulin A, saliva flow rate and plasma cortisol concentration. Eur J Appl Physiol. (2018) 118:1179–87. doi: 10.1007/s00421-018-3847-6
33. Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. (2007) 133:3–18. doi: 10.1016/j.autneu.2006.10.006
34. Eldarrat A, Alkhabuli J, Malik A. The prevalence of self-reported halitosis and oral hygiene practices among libyan students and office workers. Libyan J Med. (2008) 3:170–6. doi: 10.4176/080527
35. Alqumber MA, Arafa KA. Site-specific mouth rinsing can improve oral odor by altering bacterial counts. Blind crossover clinical study. Saudi Med J. (2014) 35:1412–6.
36. Bragazzi N. Ramadan and oral pathologies. In: H Chtourou editor. Effects of Ramadan Fasting on Health and Athletic Performance: OMICS Group eBooks. (2015). p. 86–90. Available online at: https://www.academia.edu/19555725/Ramadan_and_Oral_Pathologies (accessed February 15, 2022).
37. Waller KL, Mortensen EL, Avlund K, Fagerlund B, Lauritzen M, Gammeltoft S, et al. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat Sci Sleep. (2016) 8:47–53. doi: 10.2147/NSS.S75946
38. Thau L, Gandhi J, Sharma S. Physiology, Cortisol. StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
39. Qaid MM, Abdelrahman MM, Gonzlez-Redondo P. Role of insulin and other related hormones in energy metabolism. A review. Cogent Food Agric. (2016) 2:1267691.
40. Sherman B, Pfohl B, Winokur G. Circadian analysis of plasma cortisol levels before and after dexamethasone administration in depressed patients. Arch Gen Psychiatry. (1984) 41:271–5. doi: 10.1001/archpsyc.1984.01790140061007
41. Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. (2017) 21:577–86. doi: 10.1007/s11325-017-1473-x
42. Shadman Z, Akhoundan M, Khoshniat Nikoo M. A review of Ramadan fasting and diabetes mellitus: controversies regarding the effects of Ramadan fasting on diabetic patients. J Fasting Health. (2014) 2:119–30.
43. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. (2010) 16:84–90. doi: 10.1016/j.cardfail.2009.08.002
44. Todorovic T, Dozic I, Vicente-Barrero M, Ljuskovic B, Pejovic J, Marjanovic M, et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. (2006) 11:E115–9.
45. Rodrigues WC, Fabris AL, Hassumi JS, Goncalves A, Sonoda CK, Okamoto R. Kinetics of gene expression of alkaline phosphatase during healing of alveolar bone in rats. Br J Oral Maxillofac Surg. (2016) 54:531–5. doi: 10.1016/j.bjoms.2016.02.018
46. Uppu K, Sahana S, Madu GP, Vasa AA, Nalluri S, Raghavendra KJ. Estimation of salivary glucose, calcium, phosphorus, alkaline phosphatase, and immunoglobulin a among diabetic and nondiabetic children: A case-control study. Int J Clin Pediatr Dent. (2018) 11:71–8. doi: 10.5005/jp-journals-10005-1488
47. Vimalraj S. Alkaline phosphatase: structure, expression and its function in bone mineralization. Gene. (2020) 754:144855. doi: 10.1016/j.gene.2020.144855
48. Prakash AR, Nahar P, Ashtekar M, Natarajan S, Singh R, Kulkarni G. Detection of salivary alkaline phosphatase levels in smokers, diabetic patients, potentially malignant diseases and oral malignant tumours. J Pharm Bioallied Sci. (2020) 12(Suppl. 1):S430–5. doi: 10.4103/jpbs.JPBS_129_20
49. Otto-Slusarczyk D, Grabon W, Mielczarek-Puta M. Aspartate aminotransferase–key enzyme in the human systemic metabolism. Postepy Hig Med Dosw. (2016) 70:219–30. doi: 10.5604/17322693.1197373
50. El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. (2017) 8:487–93. doi: 10.1016/j.jare.2017.03.003
51. Fedail SS, Murphy D, Salih SY, Bolton CH, Harvey RF. Changes in certain blood constituents during Ramadan. Am J Clin Nutr. (1982) 36:350–3. doi: 10.1093/ajcn/36.2.350
52. El Ati J, Beji C, Danguir J. Increased fat oxidation during Ramadan fasting in healthy women: an adaptative mechanism for body-weight maintenance. Am J Clin Nutr. (1995) 62:302–7. doi: 10.1093/ajcn/62.2.302
53. Gumaa KA, Mustafa KY, Mahmoud NA, Gader AM. The effects of fasting in Ramadan. 1. Serum uric acid and lipid concentrations. Br J Nutr. (1978) 40:573–81. doi: 10.1079/bjn19780161
54. Nomani MZ, Hallak MH, Siddiqui IP. Effects of Ramadan fasting on plasma uric acid and body weight in healthy men. J Am Diet Assoc. (1990) 90:1435–6. doi: 10.1016/s0002-8223(21)01813-7
55. Marsh PD. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health. (2006) 6(Suppl. 1):S14. doi: 10.1186/1472-6831-6-S1-S14
56. Man A, Ciurea CN, Pasaroiu D, Savin AI, Toma F, Sular F, et al. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem Inst Oswaldo Cruz. (2017) 112:587–92. doi: 10.1590/0074-02760170098
57. Du Q, Fu M, Zhou Y, Cao Y, Guo T, Zhou Z, et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Sci Rep. (2020) 10:2961. doi: 10.1038/s41598-020-59733-6
58. Brito ACM, Bezerra IM, Borges MHS, Cavalcanti YW, Almeida LFD. Effect of different salivary glucose concentrations on dual-species biofilms of Candida albicans and Streptococcus mutans. Biofouling. (2021) 37:615–25. doi: 10.1080/08927014.2021.1946519
59. Epstein JB, Kimura LH, Menard TW, Truelove EL, Pearsall NN. Effects of specific antibodies on the interaction between the fungus Candida albicans and human oral mucosa. Arch Oral Biol. (1982) 27:469–74. doi: 10.1016/0003-9969(82)90086-3
60. Challacombe SJ. Immunologic aspects of oral candidiasis. Oral Surg Oral Med Oral Pathol. (1994) 78:202–10. doi: 10.1016/0030-4220(94)90148-1
61. Turner SEG, Loosemore M, Shah A, Kelleher P, Hull JH. Salivary IgA as a potential biomarker in the evaluation of respiratory tract infection risk in athletes. J Allergy Clin Immunol. (2021) 9:151–9. doi: 10.1016/j.jaip.2020.07.049
62. Yasuda N, Yamamoto K, Iwashita N. Concurrent evaluation of salivary and urinary alpha-amylase activity following prolonged exercise with or without carbohydrate solution in aerobically active men. Neuro Endocrinol Lett. (2021) 42:265–76.
63. Tong TK, Baker JS, Henriquez FL, Shi Q, Zhang H, Kong Z, et al. A Combined approach for health assessment in adolescent endurance runners. Healthcare (Basel Switzerl). (2021) 9:163. doi: 10.3390/healthcare9020163
64. Haeri-Araghi H, Zarabadipour M, Safarzadeh-Khosroshahi S, Mirzadeh M. Evaluating the relationship between dental caries number and salivary level of IgA in adults. J Clini Exp Dent. (2018) 10:e66–9. doi: 10.4317/jced.54271
65. Millet N, Solis NV, Swidergall M. Mucosal IgA prevents commensal Candida albicans dysbiosis in the oral cavity. Front Immunol. (2020) 11:555363. doi: 10.3389/fimmu.2020.555363
66. Sousa VD, Zauszniewski JA, Musil CM. How to determine whether a convenience sample represents the population. Appl Nurs Res. (2004) 17:130–3. doi: 10.1016/s0897-1897(04)00029-1
67. Mascha EJ, Vetter TR. Significance, errors, power, and sample size: the blocking and tackling of statistics. Anesth Analg. (2018) 126:691–8. doi: 10.1213/ane.0000000000002741
68. Gomar-Vercher S, Simon-Soro A, Montiel-Company JM, Almerich-Silla JM, Mira A. Stimulated and unstimulated saliva samples have significantly different bacterial profiles. PLoS One. (2018) 13:e0198021. doi: 10.1371/journal.pone.0198021
69. Fenneni MA, Latiri I, Aloui A, Rouatbi S, Saafi MA, Bougmiza I, et al. Effects of Ramadan on physical capacities of North African boys fasting for the first time. Libyan J Med. (2014) 9:25391. doi: 10.3402/ljm.v9.25391
70. Modeer T, Blomberg CC, Wondimu B, Julihn A, Marcus C. Association between obesity, flow rate of whole saliva, and dental caries in adolescents. Obesity (Silver Spring Md). (2010) 182367–73. doi: 10.1038/oby.2010.63
71. Yamashita JM, Moura-Grec PG, Freitas AR, Sales-Peres A, Groppo FC, Ceneviva R, et al. Assessment of oral conditions and quality of life in morbid obese and normal weight individuals: a cross-sectional study. PLoS One. (2015) 10:e0129687. doi: 10.1371/journal.pone.0129687
72. Mahesh DR, Komali G, Jayanthi K, Dinesh D, Saikavitha TV, Dinesh P. Evaluation of salivary flow rate, pH and buffer in pre, post & post menopausal women on HRT. J Clin Diagn Res. (2014) 8:233–6.
73. Pandey P, Reddy NV, Rao VA, Saxena A, Chaudhary CP. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. (2015) 6(Suppl. 1):S65–71. doi: 10.4103/0976-237X.152943
74. Chaouachi K. Re: Tobacco smoking and periodontal health in a Saudi Arabian population. Natto S, Baljoon M, Bergstrom J (2005;76:1919-1926). J Periodontol. (2006) 77:929–30; author reply 30. doi: 10.1902/jop.2006.065001
75. Haque MM, Alsareii SA. A review of the therapeutic effects of using miswak (Salvadora Persica) on oral health. Saudi Med J. (2015) 36:530–43. doi: 10.15537/smj.2015.5.10785
76. Mortazavi S, Aslani A, Babaee M, Hajiahmadi M. Persica chewing gum effects on saliva fluoride concentration and flow rate: A triple-blind randomized clinical trial. Contemp Clin Dent. (2019) 10:117–22. doi: 10.4103/ccd.ccd_509_18
77. Allgrove JE, Oliveira M, Gleeson M. Stimulating whole saliva affects the response of antimicrobial proteins to exercise. Scand J Med Sci Sports. (2014) 24:649–55. doi: 10.1111/sms.12056
78. Lindsay A, Costello JT. Realising the Potential of urine and saliva as diagnostic tools in sport and exercise medicine. Sports Med. (2017) 47:11–31. doi: 10.1007/s40279-016-0558-1
79. Pasha S, Inui T, Chapple I, Harris S, Holcombe L, Grant MM. The saliva proteome of dogs: variations within and between breeds and between species. Proteomics. (2018) 18:1700293. doi: 10.1002/pmic.201700293
80. Furner-Pardoe J, Anonye BO, Cain R, Moat J, Ortori CA, Lee C, et al. Anti-biofilm efficacy of a medieval treatment for bacterial infection requires the combination of multiple ingredients. Sci Rep. (2020) 10:12687. doi: 10.1038/s41598-020-69273-8
Keywords: cortisol, oral health, melatonin, Ramadan fasting, salivary biomarkers, salivary flow rate
Citation: Besbes A, Khemiss M, Bragazzi N and Ben Saad H (2022) The Impacts of Ramadan Intermittent Fasting on Saliva Flow-Rate and Metabolic Data: A Systematic Review. Front. Nutr. 9:873502. doi: 10.3389/fnut.2022.873502
Received: 10 February 2022; Accepted: 08 March 2022;
Published: 06 April 2022.
Edited by:
Ellen E. Blaak, Maastricht University, NetherlandsReviewed by:
F. Capela e Silva, University of Evora, PortugalReza Rastmanesh, American Physical Society, United States
Copyright © 2022 Besbes, Khemiss, Bragazzi and Ben Saad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Bragazzi, cm9iZXJ0b2JyYWdhenppQGdtYWlsLmNvbQ==
†ORCID: Amira Besbes, orcid.org/0000-0003-3742-8687; Mehdi Khemiss, orcid.org/0000-0003-4502-0374; Nicola Bragazzi, orcid.org/0000-0001-8409-868X; Helmi Ben Saad, orcid.org/0000-0002-7477-2965
 Amira Besbes
Amira Besbes Mehdi Khemiss
Mehdi Khemiss Nicola Bragazzi
Nicola Bragazzi Helmi Ben Saad
Helmi Ben Saad