- 1Department of Gastrointestinal Surgery / Department of Clinical Nutrition, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
- 2Beijing International Science and Technology Cooperation Base for Cancer Metabolism and Nutrition, Beijing, China
- 3Key Laboratory of Cancer FSMP for State Market Regulation, Beijing, China
- 4The Second Affiliated Hospital & Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Background: Non-small cell lung cancer (NSCLC) is among the most prevalent malignancies worldwide. Previous studies have shown that the status of inflammation, nutrition and immune are closely related to overall survival (OS) of patients with NSCLC, but little is known about their interactive and combined roles. Hence, we chose glucose to lymphocyte ratio (GLR) and modified Glasgow Prognosis Score (mGPS) as prognostic factors and assessed the prognostic values of them for patients with NSCLC.
Methods: Baseline clinicopathologic and laboratory characteristics of 862 patients with NSCLC were obtained from a multicenter prospective cohort. The Cox proportional hazard regression models were used to determine prognostic values of the clinical factors. A nomogram was also constructed integrating the clinical factors with clinical significance or independent prognostic values. Concordance index (C-index) was utilized to evaluate the prediction accuracy of the TNM stage and the nomogram.
Results: Multivariate analyses demonstrated that GLR [Hazard ratio (HR) = 1.029, 95% confidence interval (CI) = 1.004–1.056, P = 0.023] and mGPS (score of 1: HR = 1.404, 95% CI = 1.143–1.726, P = 0.001; score of 2: HR = 1.515, 95% CI = 1.159–1.980, P = 0.002) were independent prognostic factors for patients with NSCLC. The C-indexes of the TNM stage and the nomogram were 0.642 (95% CI = 0.620–0.663) and 0.694 (95% CI = 0.671–0.717), respectively.
Conclusion: GLR and mGPS were independent prognostic factors for patients with NSCLC. Moreover, our constructed nomogram might be superior in predicting prognosis of patients with NSCLC compared with the TNM stage.
Introduction
Non-small cell lung cancer (NSCLC), which is a common type of lung cancer, is the leading cause of cancer-related death worldwide, bringing a tremendous burden to families and society (1, 2). In previous studies, numerous prognostic factors were identified to better predict survival and inform clinical decisions for patients with NSCLC (3–10). Due to limitations of these studies, however, existing prognostic factors are inadequate to meet the growing needs for better prediction of survival and informing more effective treatment strategies for patients with NSCLC (11, 12). Therefore, development of better prediction models would result in better therapy decisions and would be beneficial to improve outcomes for patients with NSCLC.
Previous studies have shown that inflammation is an important promoting factor for the occurrence and development of lung cancer (13). The risk of death in patients with NSCLC with high levels of inflammation is much higher than those with low levels of inflammation. Elevated fasting blood glucose (FBG) is not only the direct embodiment of insulin resistance caused by inflammation, but also the direct cause of further inflammation. C-reactive protein (CRP), as a common clinical index, is very sensitive to the changes of inflammatory level.
On the other hand, the nutritional and immune status of patients with NSCLC are also crucial to their survival. Some studies suggested that the survival of patients with NSCLC, with poor nutritional and lymphocyte status, is worse than those with good status, and this gap can be corrected by nutritional supplement and immune intervention (14, 15). In addition to being an immune marker, recent studies have reported that lymphocytes are closely related to the nutritional status of the body (16). Moreover, serum albumin (Alb) has been used as a nutritional index in clinic for a long time.
Therefore, we identified glucose to lymphocyte ratio (GLR), which is a parameter that integrates both FBG levels and lymphocyte counts, and the modified Glasgow prognostic score (mGPS), which combines Alb and CRP, to be prognostic factors with high accuracy in patients with NSCLC. GLR and mGPS are previously reported prognostic indicators of a variety of cancers. We reasoned that a combination of both GLR and mGPS would be more promising in prediction of OS for patients with NSCLC. Thus, we established a nomogram model that combined GLR and mGPS. We showed that this nomogram was more accurate and specific in predicting prognosis for patients with NSCLC.
The current study aimed to evaluate the prognostic values of GLR, mGPS and a nomogram model that combined GLR and mGPS in patients with NSCLC.
Materials and Methods
Study Population
A total of 2,740 patients with NSCLC, who were diagnosed between 2012 and 2019, were enrolled from a multicenter prospective cohort which included patients from 14 hospitals (Figure 1). The inclusion criteria were as following: age ≥ 18 years at diagnosis, a pathological diagnosis of NSCLC, willing and able to provide written informed consent, and consciousness throughout treatment with no communication disorders. Patients with any of the following conditions will be excluded: acquired immune deficiency syndrome, mental or cognitive impairment, or receiving organ transplantation. Cases which patients were hospitalized for more than 2 times during the study were considered as single cases. 1,878 patients without reported data for one or more of above variables were excluded. Of these excluded patients, 59 were missing Alb levels, 1,272 were missing CRP levels, 133 were missing FBG levels, 117 were missing lymphocyte counts, 115 were missing age information, and 182 were missing TNM stage data (Figure 1). The study was approved by the Ethical Review Committees of the participating hospitals and was conducted in accordance with the guidelines of the Declaration of Helsinki. The study was registered with the Chinese Clinical Trial Registry (http://www.chictr.org.cn) and the registration number is ChiCTR1800020329.
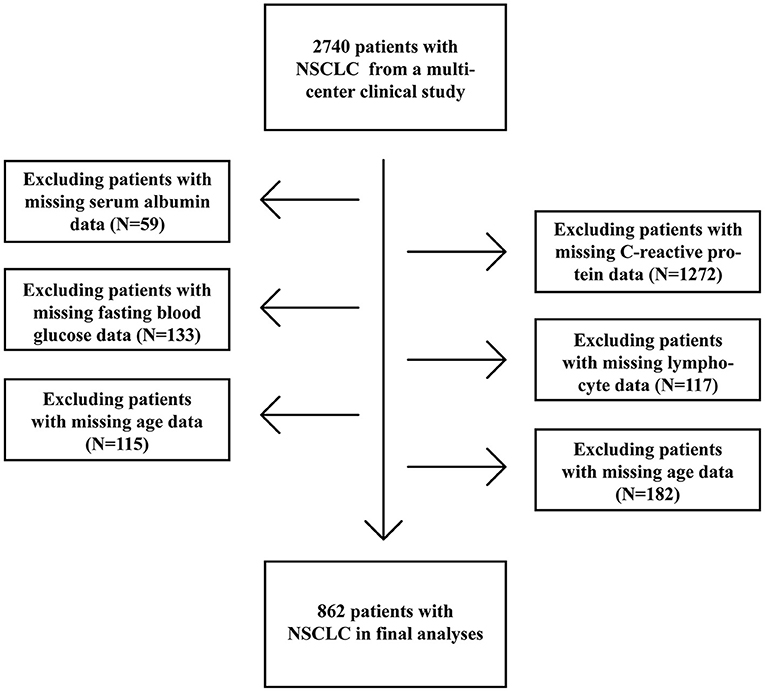
Figure 1. Procedures for selection of study participants with non-small cell lung cancer (NSCLC) from a multi-center clinical database.
Patient Characteristics and Outcomes
The following demographic and clinicopathological data were collected within 24 h of admission: sex, age, height, weight, smoking status, alcohol consumption, tea-drinking status, health information (hypertension, diabetes and coronary heart disease), TNM stage, FBG levels, lymphocyte counts, Alb and CRP levels. Smoking more than 20 cigarettes in a lifetime was regarded as smoking. Regular alcohol consumption in the past year was regarded as drinking. Regular tea drinking in the past year was regarded as tea drinking. TNM stages were determined according to the guidelines of the American Joint Committee on Cancer (17). Blood samples were taken at 6 a.m. the next day after the patient's admission. Before that, patients were required to be fasting for at least 8 h. The mGPS was defined as following: arbitrary Alb levels and CRP ≤ 10 mg/L were scored as 0; Alb ≥ 35 g/L or CRP > 10 mg/L were scored as 1; Alb <35 g/L and CRP > 10 mg/L were scored as 2. GLR was the level of FBG divided by the lymphocyte counts. Patient death due to NSCLC was defined as the primary endpoint of the clinical trials.
Statistical Analyses
Data were presented as simple percentages or medians and interquartile range (IQR). The Fisher's exact test or chi-square test was used for the assessment of baseline characteristics. Student's t test was utilized for the analyses of continuous variables with normal distributions. The Mann-Whitney test was used for the analyses of continuous variables with non-normal distributions.
The prognostic values of different variables were evaluated by the univariate and multivariate Cox proportional hazard regression models. Our proposed nomogram model was constructed based on these identified prognostic factors. Concordance index (C-index) and Area Under the Curve (AUC) were used to evaluate the accuracy of our nomogram model. The Cox regression models were used for hazard ratio (HR) and associated 95% confidence interval (CI). P < 0.05 was regarded as statistically significant. For all the analyses, either the R (version 4.0.1) or SPSS (version 26.0) software was employed.
Results
Patient Characteristics
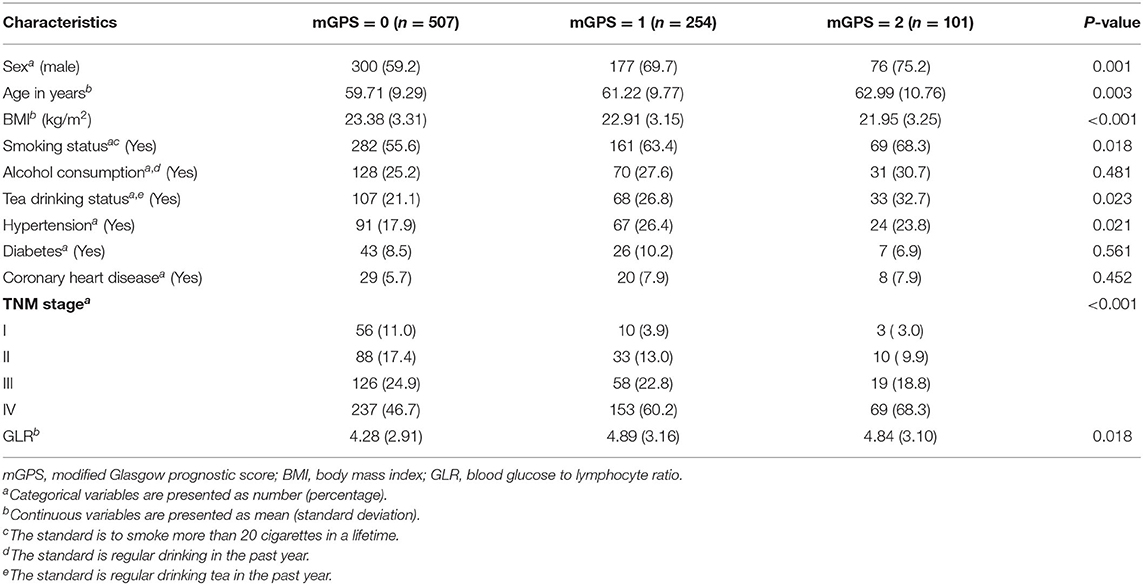
Of all the participants, the median age was 61 years (IQR, 54 to 67 years), the median BMI was 22.99 kg/m2 (IQR, 20.81 to 25.10 kg/m2), the median GLR was 3.66 (IQR, 2.76 to 5.18). 64.2% (553/862) of the patients were male. 8.0% (69/862), 15.2% (131/862), 23.5% (203/862) and 53.2% (459/862) of them were in stage I, II, III, and IV, respectively. According to the values of mGPS, the baseline characteristics were divided into three groups and were summarized in Table 1.
Independent Prognostic Factors for NSCLC
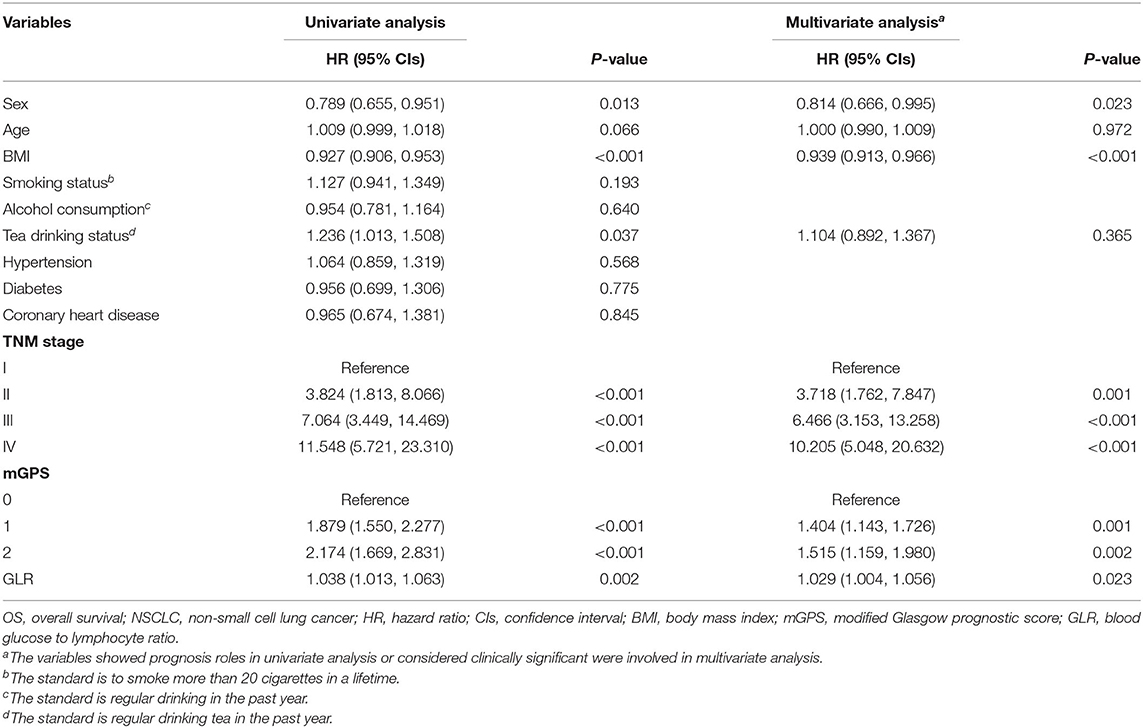
Univariate analyses indicated that sex, BMI, tea drinking status, TNM stage, mGPS and GLR were prognostic factors for patients with NSCLC, while age, smoking status, alcohol consumption, and health status (hypertension, diabetes and coronary heart disease) were not. Multivariate analyses further indicated that sex (HR = 0.814, 95% CI = 0.666–0.995, P = 0.023), BMI (HR = 0.939, 95% CI = 0.913–0.966, P < 0.001), TNM stage (stage II: HR = 3.718, 95% CI = 1.762–7.847, P = 0.001; stage III: HR = 6.466, 95% CI = 3.153–13.258, P < 0.001; stage IV: HR = 10.205, 95% CI = 5.048–20.632, P < 0.001), mGPS (score of 1: HR = 1.404, 95% CI = 1.143-1.726, P = 0.001; score of 2: HR = 1.515, 95% CI = 1.159-1.980, P = 0.002) and GLR (HR = 1.029, 95% CI = 1.004–1.056, P = 0.023) were independent prognostic factors. The detailed results of these analyses were summarized in Table 2.
GLR and mGPS Prognostic Roles
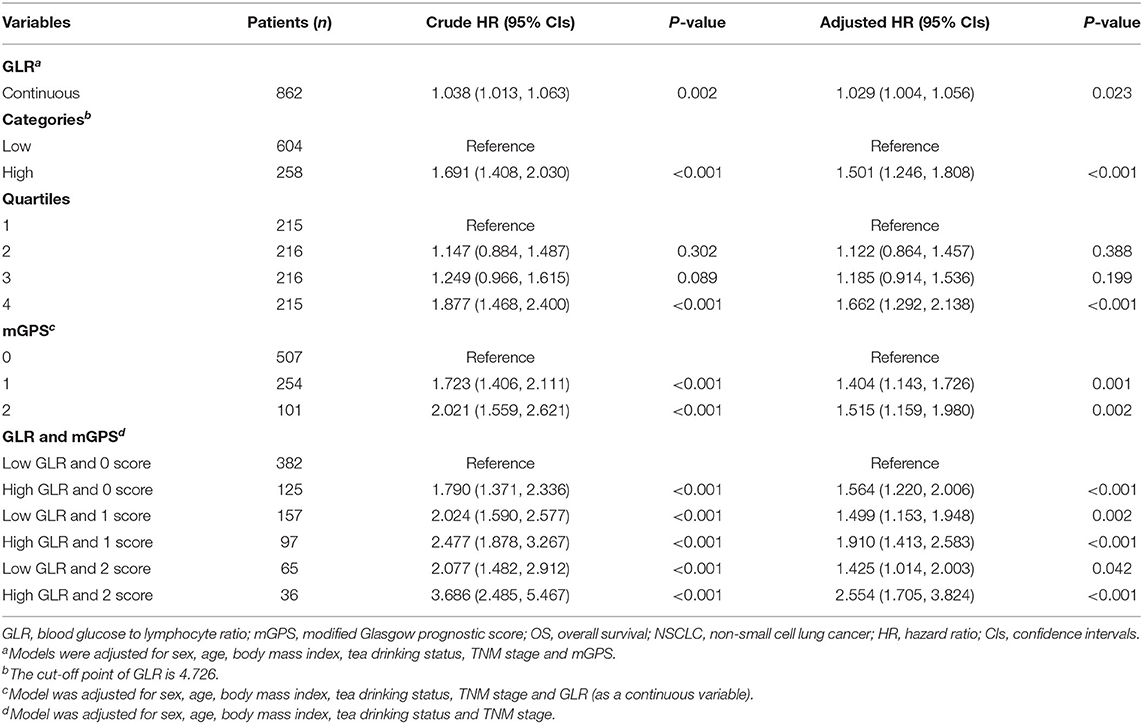
Correlation analyses indicated that the risk of death was positively related to GLR levels (Table 3, Supplementary Figure 1). Receiver operating characteristic curve (ROC) analyses determined that the optimal cut-off value for GLR was 4.726. Patients with a GLR > 4.726 had a lower OS compared with patients who had a GLR smaller than or equal to 4.726 (HR = 1.501, 95% CI = 1.246–1.808, P < 0.001; Supplementary Figures 2, 3). When GLR was divided into 4 quartiles (1st quartile: GLR <2.760; 2nd quartile: 2.760 ≤ GLR <3.662; 3rd quartile: 3.662 ≤ GLR <5.194; 4th quartile: GLR ≥ 5.194), patients in the 4th quartile had a significantly higher risk of death (HR = 1.662, 95% CI = 1.292–2.138, P < 0.001) compared to those in the 1st quartile. For mGPS, patients with a score of 1 or 2 had a significantly decreased survival time compared to those with a score of 0. Detailed associations between mGPS and OS in patients with NSCLC were presented in Table 3, Supplementary Figure 4.
Combination Prognostic Roles
Based on different combinations of GLR and mGPS, all patients were assigned into six groups: group 1 (Low GLR and mGPS = 0), group 2 (High GLR and mGPS = 0), group 3 (Low GLR and mGPS = 1), group 4 (High GLR and mGPS = 1), group 5 (Low GLR and mGPS = 2), and group 6 (High GLR and mGPS = 2). Compared with patients in group 1, lower OS was observed for patients in group 2 (HR = 1.564, 95% CI = 1.220–2.006, P < 0.001), group 3 (HR = 1.499, 95% CI = 1.153–1.948, P = 0.002), group 4 (HR = 1.910, 95% CI = 1.413–2.583, P < 0.001), group 5 (HR = 1.425, 95% CI = 1.014–2.003, P = 0.042), and group 6 (HR = 2.554, 95% CI = 1.705–3.824, P < 0.001). Detailed results were summarized in Table 2.
Evaluation of the Constructed Nomogram
Variables, with clinical significance (age) or with independent prognostic value (TNM stage, BMI, GLR, mGPS, sex), were included in the constructed nomogram (Figure 2). The calibration curves for 1-, 2- and 3-year OS were in good agreement with prediction from our nomogram (Supplementary Figure 5). C-indexes of the TNM stage and the nomogram were 0.642 (95% CI, 0.620–0.663) and 0.694 (95% CI, 0.671–0.717), respectively. Time-dependent ROCs were generated based on the GLR, mGPS, TNM stage and our nomogram. AUCs of ROCs generated from the TNM stage and our nomogram were 68.48 and 74.54% at 1 year, 67.74 and 73.27% at 2 years, 73.16 and 76.82% at 3 years, and 77.59 and 81.69% at 4 years, respectively (Supplementary Figure 6). These data suggested that our nomogram model might performs better in predicting OS compared with the classical TNM stage classification in patients with NSCLC (Supplementary Figure 7).
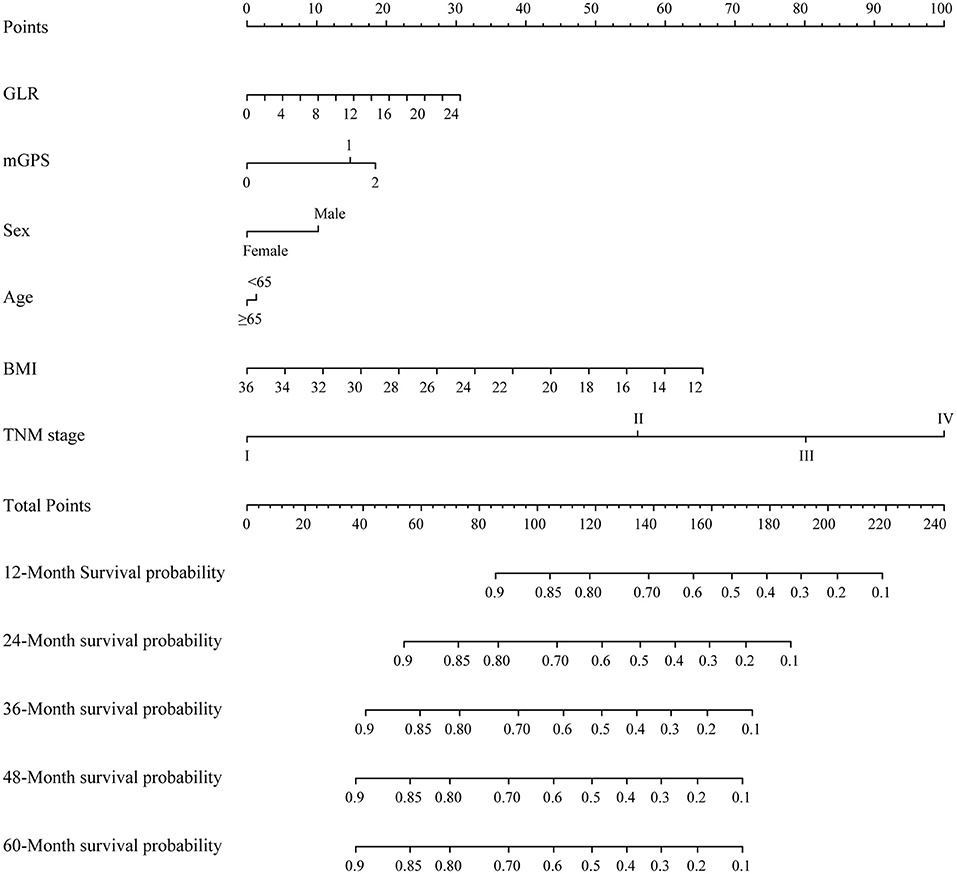
Figure 2. A proposed nomogram for predicting median survival time and survival probability of patients with non-small cell lung cancer (NSCLC). To use the nomogram, a line is drawn upward to the Points axis to determine the number of points received for each variable. Sum of these points makes the total points. For total points, a line is drawn from the Total Points axis downward to the survival axes to determine the estimated median survival time and survival probability.
Discussion
In the current study, we concluded that GLR and mGPS were negatively correlated with OS in patients with NSCLC. We also confirmed that clinical factors, such as sex, BMI and TNM stage, were independent risk factors for patients with NSCLC. Further, we developed a nomogram model that incorporated NSCLC prognostic factors, which might provide more accurate prediction of OS than the TNM staging system or other traditional indicators included in the nomogram in patients with NSCLC. Using c-indexes and AUC, we showed that our nomogram model was superior in predicting outcomes of patients with NSCLC compared to the classical TNM stage classification method.
GLR, which is the ratio of FBG levels and lymphocyte counts, was shown to be a good predictor of OS in several malignancies, such as pancreatic and gallbladder tumors (18, 19). Level of FBG was also found to be independent predictor for OS in patients with NSCLC (20). Diabetes can lead to hyperinsulinemia and insulin resistance, which can promote tumor growth (21, 22). Moreover, previous studies showed that hyperglycemia, which could form irreversible glycation end-products, could change the tumor microenvironment and contribute to tumor development (23). Previous studies also demonstrated that circulating lymphocytes can contribute to improved outcomes in cancer patients by enhancing cancer immune-surveillance (13, 24). In the tumor microenvironment, lack of T cells indicated impaired anti-tumor immunity (25). More than that, lymphocytes, which are important cells in immunity, also indicate patient's nutritional status (16). Research indicated that nutrition education and oral supplementation can both improve nutritional status as well as the activity of lymphocytes, which will improve the prognosis of patients with NSCLC (14, 15, 26). In conclusion, the elevated GLR, representing hyperglycemia and low lymphocyte count, is closely related to cancer progression and poor prognosis, which is consistent with our results. Interestingly, regulatory T cells were activated under low glucose levels, countering anti-tumor immunity (27–29). Thus, the potential synergism between hypoglycemia and immunosuppression might block cancer progression. Further studies could be carried out to verify this hypothesis and explore the possible mechanisms.
It was reported that mGPS, combining Alb levels and CRP levels, was identified as a prognostic marker for patients with NSCLC (30). As an inflammatory index, decreased Alb levels indicated malnutrition and systemic inflammation (31). Studies had shown that malnutrition and systemic inflammation are closely related to the adverse outcomes of patients with NSCLC (32). Moreover, increased CRP levels were linked to lymphocytopenia and impaired T cell response in tumors, which can further promote cancer progression (33). It is recognized that ongoing systemic inflammatory responses (indicated by an elevated CRP) in cancer patients can lead to poor survival due to reasons such as increased protein breakdown (34–37). Hence, higher mGPS, meaning hypoalbuminemia and elevated CRP levels, is associated with a poor prognosis in patients with NSCLC, which is consistent with our conclusion.
In this study, we explored how a combination of GLR and mGPS would relate to OS of patients with NSCLC. A higher death risk was found in patients with high GLR and mGPS = 2, compared with those who have a low GLR and mGPS = 0. Higher GLR and mGPS mean higher FBG and CRP levels, and lower lymphocyte count and albumin levels, which are associated with high inflammatory status, malnutrition and immune insufficiency. Studies had shown that systemic inflammation, malnutrition and immune dysfunction are related to the progression of cancer, which will lead to worse survival (38, 39). More than that, these indicators and states will also affect each other. Prior studies identified an association between inflammatory status and the occurrence of diabetes. Inflammatory responses likely contribute to diabetes occurrence by regulating insulin resistance, which in turn intensifies the symptoms of hyperglycemia and further promotes long-term complications of diabetes (40). Moreover, by activating macrophages, hypoalbuminemia can impair the immune response within tumors (41). Hence, we reasoned that integration of systemic inflammatory state, malnutrition and inhibition of immune function could provide a more comprehensive and accurate prediction of OS, compared to any of those single factors.
In conclusion, lowering FBG levels, activating immune system, improving systemic inflammation levels, and maintaining adequate nutrition could improve the prognosis of patients with NSCLC. It should be noted that while parenteral and enteral nutrition was an important way of nutritional support for patients with NSCLC, it could also potentially lead to hyperglycemia (42). Therefore, it would be advisable to maintain an appropriate FBG levels while actively carry out nutritional interventions (43).
In addition to GLR and mGPS, our model also included sex, age, BMI and TNM stage. Previous studies had shown that age, TNM stage and BMI are predictors for OS of patients with NSCLC (44–46). In this study, we showed that our constructed nomogram might be superior in predicting OS of patients with NSCLC compared with any of above factors included in the nomogram, providing a basis for personalized treatment and clinical applications.
However, our study might have flaws in several ways. The sample sizes were relatively small, which might affect the statistical power. It is worth mentioning that in Figure 3, when GLR is higher than 6.25, the association between GLR and risk of death for patients with NSCLC was no longer significant. Based on threshold analyses, piecewise regression analyses and population distribution analyses, we suspected that this was due to the small sample size (Supplementary Figure 8, Supplementary Table 1). In addition, laboratory data were determined using standard laboratory tests, which were limited in scope compared to more advanced testing techniques. Studies with larger sample size and more clinical factors should be carried out in the future to further improve OS prediction for patients with NSCLC. Because the records of NSCLC treatment in our database were not detailed enough, such as the interval between admission and operation, chemotherapy or radiotherapy, the operation mode of operation, the scheme and specific dose of chemotherapy, the radiation dose of radiotherapy, etc., we believed that adding these treatment data to the multivariate analysis might affect the reliability and stability of the results. Therefore, we did not adjust for the treatments. In future research, we will improve the deficiencies mentioned above in the database and record the treatment data of patients in more detail.
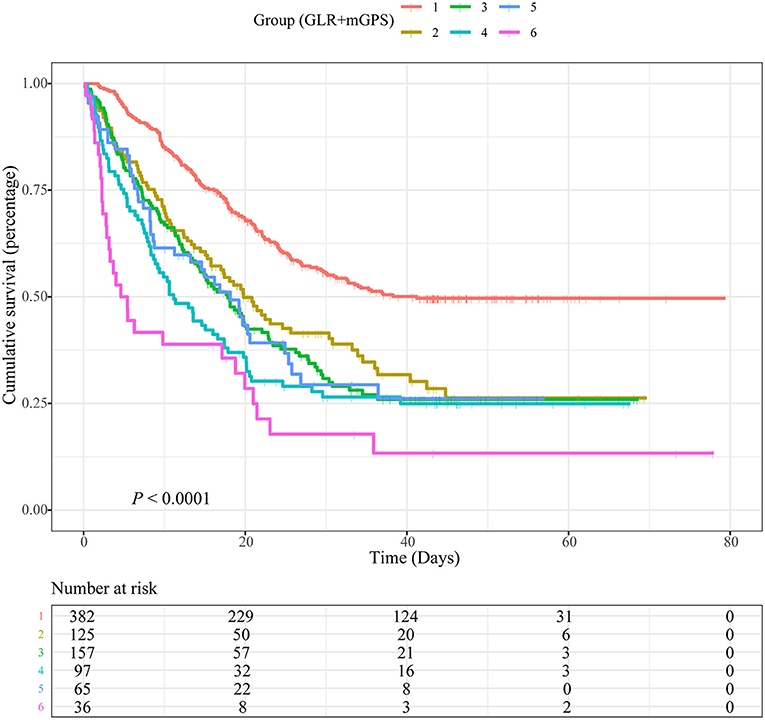
Figure 3. Kaplan-Meier curves showing associations between a combination of blood glucose to lymphocyte ratio (GLR) and modified Glasgow prognostic score (mGPS) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC). Group 1: Low GLR and mGPS = 0; Group 2: High GLR and mGPS = 0; Group 3: Low GLR and mGPS = 1; Group 4: High GLR and mGPS = 1; Group 5: Low GLR and mGPS = 2; Group 6: High GLR and mGPS = 2.
Conclusion
In summary, GLR and mGPS could be used as independent prognostic factors for survival of patients with NSCLC. The proposed nomogram could predict OS of patients with NSCLC with good sensitivity and specificity. Compared to the TNM staging system or other traditional indicators included in the nomogram, our proposed nomogram might provide a more accurate and specific tool for facilitating clinical decisions, personalized treatment, and disease management in patients with NSCLC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Review Committees of the participating hospitals. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-PS: conceptualization and methodology. MY: data curation and writing- original draft preparation. QZ: visualization, investigation, and data curation. Y-ZG, TL, and K-PZ: software. MT: validation and visualization. C-LH, XZ, M-MS, H-LX, and X-YL: writing-reviewing and editing. Z-WW, G-TR, X-WZ, H-YZ, Q-QL, X-RL, and S-QL: supervision and investigation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program (2017YFC1309200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.871301/full#supplementary-material
Supplementary Figure 1. Associations between blood glucose to lymphocyte ratio (GLR) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC). Model was adjusted for sex, age, BMI, tea drinking status, TNM stage and modified Glasgow prognostic score (mGPS).
Supplementary Figure 2. Receiver operating characteristic curve (ROC) for determining the cut-off point of blood glucose to lymphocyte ratio (GLR).
Supplementary Figure 3. Kaplan-Meier curves showing associations between blood glucose to lymphocyte ratio (GLR) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC).
Supplementary Figure 4. Kaplan-Meier curves showing associations between modified Glasgow prognostic score (mGPS) and overall survival (OS) in patients with non-small cell lung cancer (NSCLC).
Supplementary Figure 5. Calibration curves for predicting survival probability of patients with non-small cell lung cancer (NSCLC).
Supplementary Figure 6. Area Under the Curves (AUCs) of time-dependent receiver operating characteristic curves (ROCs) generated based on the TNM stage and the nomogram for patients with non-small cell lung cancer (NSCLC).
Supplementary Figure 7. Area Under the Curves (AUCs) of TNM stage and the nomogram in patients with non-small cell lung cancer (NSCLC).
Supplementary Figure 8. Associations between blood glucose to lymphocyte ratio (GLR <6.25 or ≥ 6.25) and OS in patients with non-small cell lung cancer (NSCLC). The models were adjusted for sex, age, BMI, tea drinking status, TNM stage and modified Glasgow prognostic score (mGPS).
Supplementary Table 1. Associations between GLR and OS in patients with NSCLC. GLR, blood glucose to lymphocyte ratio; OS, overall survival; NSCLC, non-small cell lung cancer; HR, hazard ratio; CIs, confidence intervals. a Models were adjusted for sex, age, body mass index, tea drinking status, TNM stage and mGPS.
References
1. Wu Z, Liu Z, Ge W, Shou J, You L, Pan H, et al. Analysis of potential genes and pathways associated with the colorectal normal mucosa-adenoma-carcinoma sequence. Cancer Med. (2018) 7:2555–66. doi: 10.1002/cam4.1484
2. Deng H, Hang Q, Shen D, Zhang Y, Chen M. Low expression of chrdl1 and sparcl1 predicts poor prognosis of lung adenocarcinoma based on comprehensive analysis and immunohistochemical validation. Cancer Cell Int. (2021) 21:259. doi: 10.1186/s12935-021-01933-9
3. Li Y, Tian X, Gao L, Jiang X, Fu R, Zhang T, et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. (2019) 8:3782–92. doi: 10.1002/cam4.2286
4. Wang LK, Wu TJ, Hong JH, Chen FH, Yu J, Wang CC. Radiation induces pulmonary fibrosis by promoting the fibrogenic differentiation of alveolar stem cells. Stem Cells Int. (2020) 2020:6312053. doi: 10.1155/2020/6312053
5. Russo A, Saide A, Smaldone S, Faraonio R, Russo G. Role of Ul3 in multidrug resistance in P53-mutated lung cancer cells. Int J Mol Sci. (2017) 18:547. doi: 10.3390/ijms18030547
6. Kwiecien I, Rutkowska E, Polubiec-Kownacka M, Raniszewska A, Rzepecki P, Domagala-Kulawik J. Identification of Pd-1 ligands: Pd-L1 and Pd-L2 on macrophages in lung cancer milieu by flow cytometry. Transl Lung Cancer Res. (2021) 10:1679–89. doi: 10.21037/tlcr-20-1103
7. Li S, Li B, Zheng Y, Li M, Shi L, Pu X. Exploring functions of long noncoding rnas across multiple cancers through co-expression network. Sci Rep. (2017) 7:754. doi: 10.1038/s41598-017-00856-8
8. Zhang C, Pang G, Ma C, Wu J, Wang P, Wang K. Preoperative risk assessment of lymph node metastasis in ct1 lung cancer: a retrospective study from eastern China. J Immunol Res. (2019) 2019:6263249. doi: 10.1155/2019/6263249
9. Li R, Wu H, Jiang H, Wang Q, Dou Z, Ma H, et al. Fbln5 Is targeted by microrna27a3p and suppresses tumorigenesis and progression in highgrade serous ovarian carcinoma. Oncol Rep. (2020) 44:2143–51. doi: 10.3892/or.2020.7749
10. Wu Y, Zhang Y, Wang D, Zhang Y, Zhang J, Zhang Y, et al. Usp29 enhances chemotherapy-induced stemness in non-small cell lung cancer via stabilizing snail1 in response to oxidative stress. Cell Death Dis. (2020) 11:796. doi: 10.1038/s41419-020-03008-5
11. Xu L, Liao WL, Lu QJ, Li CG, Yuan Y, Xu ZY, et al. Ang promotes proliferation and invasion of the cell of lung squamous carcinoma by directly up-regulating Hmga2. J Cancer. (2016) 7:862–71. doi: 10.7150/jca.14440
12. Liu M, Wang X, Li W, Yu X, Flores-Villanueva P, Xu-Monette ZY, et al. Targeting Pd-L1 in non-small cell lung cancer using Car T Cells. Oncogenesis. (2020) 9:72. doi: 10.1038/s41389-020-00257-z
13. Matiello J, Dal Pra A, Zardo L, Silva R, Berton DC. Impacts of post-radiotherapy lymphocyte count on progression-free and overall survival in patients with stage iii lung cancer. Thorac Cancer. (2020) 11:3139–44. doi: 10.1111/1759-7714.13621
14. Pivi GA, da Silva RV, Juliano Y, Novo NF, Okamoto IH, Brant CQ, et al. A prospective study of nutrition education and oral nutritional supplementation in patients with alzheimer's disease. Nutr J. (2011) 10:98. doi: 10.1186/1475-2891-10-98
15. Hu Y, Sun L, Yuan Z, Xu Y, Cao J. High throughput data analyses of the immune characteristics of microtus fortis infected with schistosoma japonicum. Sci Rep. (2017) 7:11311. doi: 10.1038/s41598-017-11532-2
16. Sim JH, Bang JY, Kim SH, Kang SJ, Song JG. Association of preoperative prognostic nutritional index and postoperative acute kidney injury in patients with colorectal cancer surgery. Nutrients. (2021) 13:1604. doi: 10.3390/nu13051604
17. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition ajcc cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. (2017) 67:93–9. doi: 10.3322/caac.21388
18. Zhong A, Cheng CS, Kai J, Lu R, Guo L. Clinical significance of glucose to lymphocyte ratio (Glr) as a prognostic marker for patients with pancreatic cancer. Front Oncol. (2020) 10:520330. doi: 10.3389/fonc.2020.520330
19. Navarro J, Kang I, Hwang HK, Yoon DS, Lee WJ, Kang CM. Glucose to lymphocyte ratio as a prognostic marker in patients with resected Pt2 gallbladder cancer. J Surg Res. (2019) 240:17–29. doi: 10.1016/j.jss.2019.02.043
20. Yang JR, Chen GC, Xu JY, Ling CJ, Yu N, Yang J, et al. Fasting blood glucose levels and prognosis in patients with non-small-cell lung cancer: a prospective cohort study in China. Onco Targets Ther. (2019) 12:5947–53. doi: 10.2147/OTT.S210103
21. Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. (1999) 155:1271–9. doi: 10.1016/S0002-9440(10)65229-7
22. Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. (2001) 61:7318–24. Available online at: https://aacrjournals.org/cancerres/article/61/19/7318/508097
23. Rojas A, Gonzalez I, Morales E, Perez-Castro R, Romero J, Figueroa H. Diabetes and cancer: looking at the multiligand/rage axis. World J Diabetes. (2011) 2:108–13. doi: 10.4239/wjd.v2.i7.108
24. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. (2004) 21:137–48. doi: 10.1016/j.immuni.2004.07.017
25. Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. (2015) 36:257–64. doi: 10.1016/j.it.2015.02.007
26. Lee SC, Lee JG, Lee SH, Kim EY, Chang J, Kim DJ, et al. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (conut) score in patients with resectable non-small cell lung cancer. Sci Rep. (2020) 10:12385. doi: 10.1038/s41598-020-68929-9
27. Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, et al. Rapid effector function of memory cd8+ t cells requires an immediate-early glycolytic switch. Nat Immunol. (2013) 14:1064–72. doi: 10.1038/ni.2687
28. Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (Glut1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. (2014) 289:7884–96. doi: 10.1074/jbc.M113.522037
29. Kishton RJ, Sukumar M, Restifo NP. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. (2017) 26:94–109. doi: 10.1016/j.cmet.2017.06.016
30. Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer–a prospective study examining key clinicopathological factors. Lung Cancer. (2015) 88:304–9. doi: 10.1016/j.lungcan.2015.03.020
31. Sun H, Hu P, Shen H, Dong W, Zhang T, Liu Q, et al. Albumin and neutrophil combined prognostic grade as a new prognostic factor in non-small cell lung cancer: results from a large consecutive cohort. PLoS ONE. (2015) 10:e0144663. doi: 10.1371/journal.pone.0144663
32. Cehreli R, Yavuzsen T, Ates H, Akman T, Ellidokuz H, Oztop I. Can inflammatory and nutritional serum markers predict chemotherapy outcomes and survival in advanced stage nonsmall cell lung cancer patients? Biomed Res Int. (2019) 2019:1648072. doi: 10.1155/2019/1648072
33. Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. (2012) 7:655–62. doi: 10.1097/JTO.0b013e318244ffe1
34. Fearon KC, Barber MD, Falconer JS, McMillan DC, Ross JA, Preston T. Pancreatic cancer as a model: inflammatory mediators, acute-phase response, and cancer cachexia. World J Surg. (1999) 23:584–8. doi: 10.1007/PL00012351
35. McMillan DC, Scott HR, Watson WS, Preston T, Milroy R, McArdle CS. Longitudinal study of body cell mass depletion and the inflammatory response in cancer patients. Nutr Cancer. (1998) 31:101–5. doi: 10.1080/01635589809514687
36. McMillan DC, Watson WS, O'Gorman P, Preston T, Scott HR, McArdle CS. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. (2001) 39:210–3. doi: 10.1207/S15327914nc392_8
37. Meek CL, Wallace AM, Forrest LM, McMillan DC. The relationship between the insulin-like growth factor-1 Axis, weight loss, an inflammation-based score and survival in patients with inoperable non-small cell lung cancer. Clin Nutr. (2010) 29:206–9. doi: 10.1016/j.clnu.2009.08.013
38. Wang A, He Z, Cong P, Qu Y, Hu T, Cai Y, et al. Controlling nutritional status (conut) score as a new indicator of prognosis in patients with hilar cholangiocarcinoma is superior to nlr and pni: a single-center retrospective study. Front Oncol. (2020) 10:593452. doi: 10.3389/fonc.2020.593452
39. Fenoglio D, Belgioia L, Parodi A, Missale F, Bacigalupo A, Tarke A, et al. Development of exhaustion and acquisition of regulatory function by infiltrating Cd8+Cd28- T lymphocytes dictate clinical outcome in head and neck cancer. Cancers. (2021) 13:2234. doi: 10.3390/cancers13092234
40. Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. (2013) 13:435–44. doi: 10.1007/s11892-013-0375-y
41. Rivadeneira DE, Grobmyer SR, Naama HA, Mackrell PJ, Mestre JR, Stapleton PP, et al. Malnutrition-induced macrophage apoptosis. Surgery. (2001) 129:617–25. doi: 10.1067/msy.2001.112963
42. Davidson P, Kwiatkowski CA, Wien M. Management of hyperglycemia and enteral nutrition in the hospitalized patient. Nutr Clin Pract. (2015) 30:652–9. doi: 10.1177/0884533615591057
43. Drincic AT, Knezevich JT, Akkireddy P. Nutrition and hyperglycemia management in the inpatient setting (meals on demand, parenteral, or enteral nutrition). Curr Diab Rep. (2017) 17:59. doi: 10.1007/s11892-017-0882-3
44. Salmeron D, Chirlaque MD, Isabel Izarzugaza M, Sanchez MJ, Marcos-Gragera R, Ardanaz E, et al. Lung cancer prognosis in spain: the role of histology, age and sex. Respir Med. (2012) 106:1301–8. doi: 10.1016/j.rmed.2012.06.006
45. Tie H, Luo J, Shi R, Li Z, Chen D, Wu Q. Characteristics and prognosis of synchronous multiple primary lung cancer after surgical treatment: a systematic review and meta-analysis of current evidence. Cancer Med. (2021) 10:507–20. doi: 10.1002/cam4.3614
Keywords: glucose to lymphocyte ratio, modified Glasgow Prognosis Score, non-small cell lung cancer, inflammation, nutrition, immune
Citation: Yang M, Zhang Q, Ge Y-Z, Tang M, Hu C-L, Wang Z-W, Zhang X, Song M-M, Ruan G-T, Zhang X-W, Liu T, Xie H-L, Zhang H-Y, Zhang K-P, Li Q-Q, Li X-R, Liu X-Y, Lin S-Q and Shi H-P (2022) Prognostic Roles of Glucose to Lymphocyte Ratio and Modified Glasgow Prognosis Score in Patients With Non-small Cell Lung Cancer. Front. Nutr. 9:871301. doi: 10.3389/fnut.2022.871301
Received: 08 February 2022; Accepted: 04 April 2022;
Published: 10 May 2022.
Edited by:
Kalliopi-Anna Poulia, Agricultural University of Athens, GreeceReviewed by:
Hao Chen, Guangdong Academy of Medical Sciences, ChinaCheng-Le Zhuang, Tongji University, China
Copyright © 2022 Yang, Zhang, Ge, Tang, Hu, Wang, Zhang, Song, Ruan, Zhang, Liu, Xie, Zhang, Zhang, Li, Li, Liu, Lin and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Ping Shi, c2hpaHBAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Ming Yang
Ming Yang Qi Zhang
Qi Zhang Yi-Zhong Ge
Yi-Zhong Ge Meng Tang
Meng Tang Chun-Lei Hu
Chun-Lei Hu Zi-Wen Wang
Zi-Wen Wang Xi Zhang
Xi Zhang Meng-Meng Song
Meng-Meng Song Guo-Tian Ruan
Guo-Tian Ruan Tong Liu
Tong Liu Hai-Lun Xie
Hai-Lun Xie He-Yang Zhang
He-Yang Zhang Kang-Ping Zhang
Kang-Ping Zhang Qin-Qin Li
Qin-Qin Li Xiang-Rui Li
Xiang-Rui Li Xiao-Yue Liu
Xiao-Yue Liu Shi-Qi Lin
Shi-Qi Lin Han-Ping Shi
Han-Ping Shi