- 1School of Nutrition and Food Sciences, Louisiana State University AgCenter, Baton Rouge, LA, United States
- 2School of Earth, Environmental and Marine Sciences & Department of Biology, University of Texas Rio Grande Valley, Edinburg, TX, United States
Fresh and fresh-cut fruits and vegetables have been associated in several foodborne illness outbreaks. Although investigations from those outbreaks reported that the contamination with pathogenic microorganisms may occur at any point in the farm to fork continuum, effective control strategies are still being widely investigated. In that direction, the concept of hurdle technology involving a sequence of different interventions have been widely explored. Among those interventions, ultraviolet (UV) light alone or in combination with other treatments such as use of organic acids or sanitizer solutions, has found to be a promising approach to maintain the microbiological safety and quality of fresh and fresh-cut produce. Recent advances in using UV as a part of hurdle technology on the safety of fresh produce at different stages are presented here. Furthermore, this review discusses the mechanism of UV induced antimicrobial activity, factors that influence antimicrobial efficacy and its effect on produce. In addition, the challenges, and prospects of using UV irradiation as an intervention treatment were also discussed.
Introduction
Consumer preference toward fresh-like, minimally processed foods with their natural nutritional, sensory and functional properties to prevent or control human diseases has seen meteoric rise over the past decades (1, 2). Minimally processed foods are usually subjected to mild processing or treatment with little to no preservatives (3). Fresh-cut fruits and vegetables are one such example of minimally processed healthful foods. However, fresh and fresh-cut produce have been associated in several foodborne illness outbreaks in recent years. A report by the Center for Science in the Public Interest (CSPI) revealed that fresh produce commodities (17 %) represent the highest number of outbreaks in the United States, during 2002–2011 (4). Between 2010 to 2020, a total of 3,223 foodborne outbreaks with a confirmed food vehicle and etiology occurred in the U.S., of which 13.5% were attributed to fresh produce (5). The available data on the food borne disease outbreak indicates that fresh produce is responsible for the majority of the number of illness and number of illness per outbreak (6).
Studies reported that fresh-cut fruits and vegetables are prone to faster physiological deterioration, biochemical changes and favorable for microbial growth than whole produce (6–8). Fresh-cut processing activities such as washing, peeling, cutting, shredding and/or grating manipulate the intact plant cells to break open and expose intracellular components such as oxidizing enzymes to the outside environment. These conditions accelerate decay (9) decrease the product shelf-life and provides favorable environments for proliferation of microorganisms (10, 11). An analysis of about 1,100 produce-related outbreaks in the United States where a pathogen was identified; majority were caused by bacteria (53%) and viruses (42.5%) and only 4.5% by parasites (12). Thus, it is a challenging task to ensure microbiological safety and quality of fresh produce that are minimally processed and consumed raw. To address these challenges, several chemical and physical interventions have been proposed and implemented with some success.
Most fresh produce packing houses use chemical sanitizers during mechanical washing followed by rinsing with potable water. Sanitizers like chlorine (as sodium or calcium hypochlorite), chlorine dioxide, acidified sodium chlorite, trisodium phosphate, peroxyacetic acid, organic acids (e.g., acetic, lactic, tartaric acid or citric, acetic), electrolyzed water and ozone are often used for this purpose (12). Despite their limited efficacy, some of these approaches are effective in minimizing microbial cross-contamination during washing. More importantly, the efficacy of these compounds depends on various factors like type of produce, target organism, the concentration of sanitizer, treatment time, presence of organic matter, etc. Alternatively, nonthermal and/or nonchemical disinfection technologies such as high-pressure processing, germicidal ultraviolet (UV-C) irradiation, pulsed UV treatment, cold plasma, and ultrasound are gaining increased popularity to reduce food safety risk or to extend the shelf-life of minimally processed foods (13, 14). Among these, germicidal UV treatment has shown promise to enhance microbial safety of fresh and fresh-cut produce at various stages of food production, processing, packaging, storage, and distribution.
Traditionally, UV irradiation has been used for water treatment, surface decontamination, and air disinfection with limited food-related applications (15). However, the use of UV irradiation for applications in the food industry has seen increased interest in the last two decades. Studies have demonstrated UV irradiation's potential to inactivate a wide range of microorganisms (16–18). UV irradiation was proven to be effective against viruses (19), parasites (20) and vegetative cells and fungi (21). Furthermore, UV irradiation was found to reduce the levels of mycotoxins (22) and allergens (23). The disinfection by UV irradiation is a physical method in which the energy is the germicidal medium (24). Minimal effect on quality, absence of residues, and low energy consumption are some advantages of UV irradiation treatment (16, 25). However, poor penetration power, irregular dose delivery, and long treatment times are major limitations of UV treatment (24). In the last decade, extensive research has been conducted in using UV irradiation treatment alone or in combination with other physical and chemical treatments to enhance the safety and quality of minimally processed fresh produce (26–29). However, for the successful application of UV treatment for fresh and fresh-cut produce safety; several important influencing factors need to be considered. In this paper, we present a concise review of the most significant findings on the efficacy of UV treatment alone or in combination with other treatment methods to destroy various foodborne pathogens focusing on fresh and fresh-cut fruits and vegetables. In addition, the effect of UV irradiation treatment on the shelf-life and quality of produce are outlined.
Principle of UV disinfection
Disinfection is the process of removing bacteria from surfaces. UV irradiation is a part of the electromagnetic spectrum that ranges from 200 to 400 nm. It is mainly subdivided into three regions by wavelength: UV-C (200–280 nm); UV-B (280–320 nm); and UV-A (320–400 nm). UV-C irradiation at a wavelength of about 254 nm has shown to be effective at damaging cells, with the highest DNA absorption indicating UV-C as the most germicidal region (30). The absorption of UV-C irradiation prompts the formation of DNA photoproducts like cyclobutane pyrimidine dimers and pyrimidine 6–4 pyrimidone photoproducts, which obstruct transcription and replication leading to mutagenesis and cell death (16). Low and medium pressure mercury vapor lamps are commonly used as a source of UV irradiation. More details on sources of UV irradiation can be found elsewhere (15). Disinfection efficacy of UV irradiation depends on its fluence or dose delivered. It is defined as the product of intensity (mW/cm2) and the exposure time (s) and is commonly expressed as mW-s/cm2 or mJ/cm2.
Food applications of UV irradiation treatment
UV irradiation has been used mainly for disinfection of liquid foods and beverages such as milk, juices, ciders, liquid egg, beverages, and honey (15). Also, its application was extended to disinfection of packaging materials, food contact surfaces, in-shell eggs, and surfaces of ready-to-eat meat and meat products (15). Other food processing applications of UV irradiation have been widely discussed (15, 17, 24). Recently, there is a growing body of evidence showing the effectiveness of UV irradiation treatment for the microbial decontamination of irrigation water, fresh and fresh-cut produce as well as process wash waters in the fresh produce industry (31, 32). Some of these studies and their findings were briefly described in the following sections.
Treatment of irrigation water
Irrigation water is a major conduit of microbial contamination of fresh produce. Treatment of irrigation water with UV irradiation was found to be effective in the disinfection of various plant and environmental pathogens of human health concern. Scarlett et al. (32) compared the efficacy of UV treatment to disinfect several plant pathogens in irrigation water with chlorine and chlorine dioxide treatments. In their study, depending upon the type of plant pathogen, UV irradiation treatment of irrigation water at 250 mJ/cm2 and a turbidity of 20 NTU showed higher microbial population reductions than chlorine treatment at 5 ppm concentration. They found that the efficacy of disinfection treatments varied with type of pathogens, time of exposure, flow rate, and type of water. pH-independent disinfection efficacy without forming any known disinfection by-products is a major advantage of UV irradiation over chlorine treatments. Zhang et al. (33) reported that water flow rate, turbidity, organic matter content, the intensity of irradiation and treatment time have significant effects on the disinfection efficacy of UV treatment. Similar observations were also reported by others (34). Sprouts are high-risk food commodities with a history of several foodborne illness outbreaks. The sprouting conditions provide optimal temperature and humidity for any potential pathogens on the seeds or in the irrigation water to grow and survive. UV treatment of water used for sprout production shown to be effective in reducing microbial levels. Ge et al. (35) reported that UV-C irradiation treatment of contaminated irrigation water used for growing mung bean sprouts at 950 mJ/cm2 reduced internalized Salmonella Typhimurium by 1.84 log CFU/g. They found that the UV irradiation as a pre-harvest intervention significantly decreased Salmonella levels in the irrigation water and the internalized organisms in sprouts. Whereas the post-harvest treatment of sprouts with chlorine wash (500, 1,000, or 2,000 mg/L/min), UV treatment (from 78 to 778 mJ/cm2) and combined chlorine wash (2,000 mg/L/min) followed by UV irradiation (778 mJ/cm2) was found to be ineffective in eliminating internalized pathogens. Moreover, Salmonellae were able to recover in the spent irrigation water over a 24-h period and become more resistant to UV irradiation. Adhikari et al. (36) reported that water turbidity can affect the total microbial reduction. Escherichia coli in water at turbidity levels as high as 23.32 NTU and treated with UV-C irradiation (20–60 mJ/cm2) presented significant reductions. However, as the turbidity decreased to 10.93 NTU the reduction of E. coli increased by 2.15 Log MPN, indicating that water quality factors such as turbidity can have a major impact on the effectiveness of UV-C irradiation treatments on irrigation water (36). Studies reported that exposure of bacteria to UV irradiation may cause mutations and increase the UV repair mechanism, thus making the bacteria more resistant to subsequent UV exposures (37). This implies that UV irradiation can be used as a potential pre-harvest intervention to decontaminate irrigation water. Factors such as water type, quality, volume, flow rate, UV intensity, exposure time, and type of organism plays a significant role in the disinfection efficacy.
Treatment of fresh and fresh-cut produce
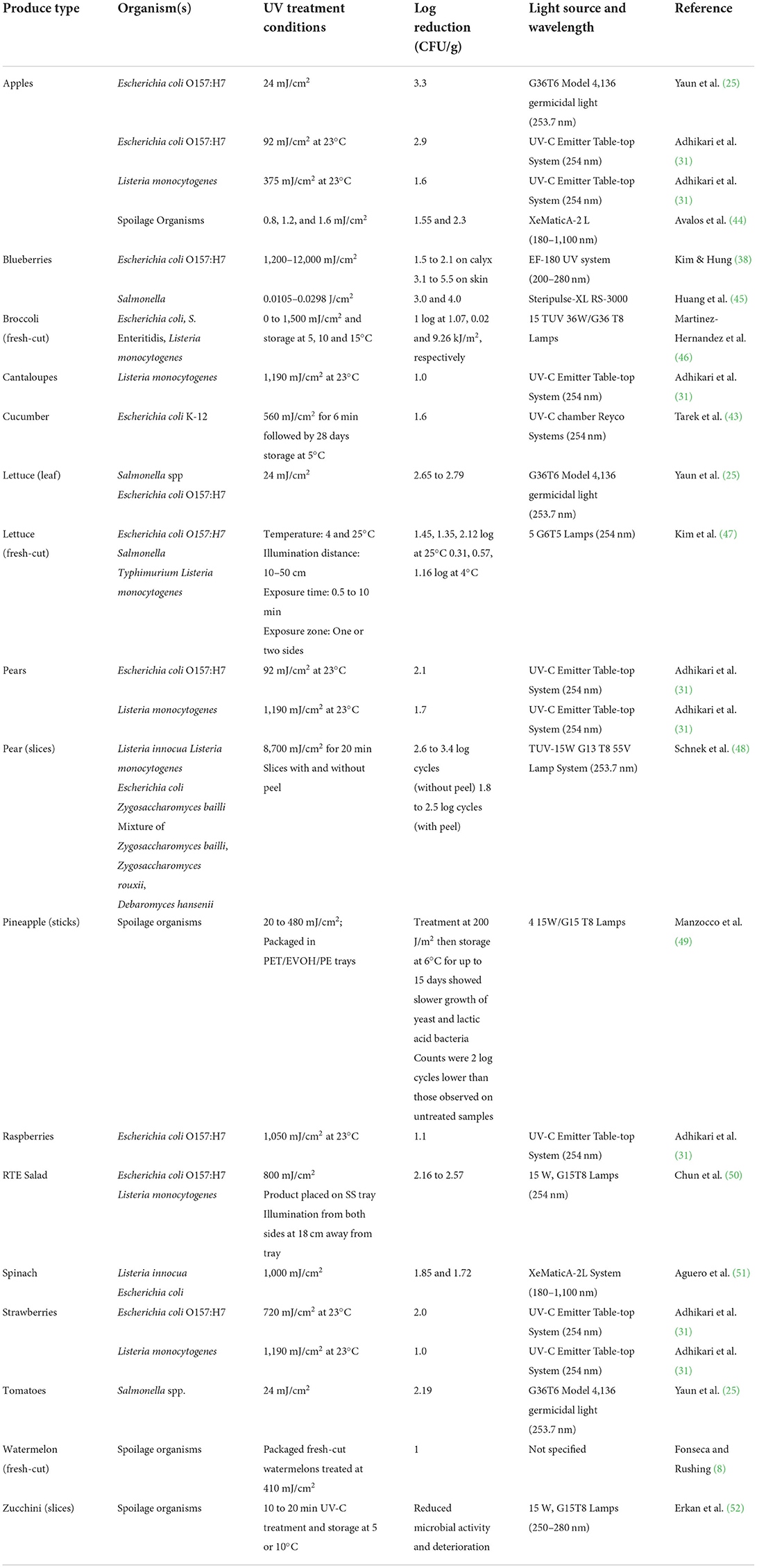
Contamination of fresh produce with pathogenic microorganisms during various pre- and post-harvest activities is widely reported. In general, contamination starts at the surface of intact produce and then spreads across interior portions during fresh-cut processing operations. Hence, surface decontamination of fresh produce using chemical sanitizers is a normal practice in the fresh produce industry. However, germicidal UV irradiation can be used as an alternative physical intervention treatment without causing undesirable quality changes and release of toxic disinfection by-products. Several studies have demonstrated that the UV irradiation treatment of fresh and fresh-cut produce is equally if not more efficient in reducing the growth and survival of spoilage and disease-causing organisms than several chemical sanitizers. Kim and Hung (38) found that UV-C irradiation treatment is more effective in reducing E. coli O157:H7 on blueberries compared to electrolyzed water and ozone treatments. Levels of E. coli O157:H7 were reduced by 1.5 to 2.1 log CFU/g on blueberry calyx and 3.1 to 5.5 log CFU/g on the blueberry skin following application of UV irradiation at 1,200–12,000 mJ/cm2. Ozone (4,000 mg/L) and EO water treatments showed only 0.7 log CFU/g on calyx and 0.1 to 1.1 log CFU/g on blueberry skins, respectively (38). Similarly, the UV-C irradiation was more effective in reducing E. coli O157:H7 levels on lettuce and apples as compared to 20–320 ppm of chlorine (25). Lower levels of UV-C irradiation treatment at wavelengths between 200 and 280 nm (<100 mJ/cm2) were able to achieve similar results on apple surfaces (>2.9 log CFU/g) as compare to ozonated water for 3 min (39), chlorinated water (200 ppm) (40), and ClO2 gas treatment at 1.1 mg/L for 10 min (41). Also, treatment of fresh produce with UV irradiation was found to significantly decrease internalized pathogens in lettuce, bean sprouts and other leafy greens (35, 42). Although UV irradiation is proven efficacious for surface decontamination of fresh produce, factors such as produce surface characteristics, UV fluence, method of irradiation delivery, and type and location of organisms were found to play significant role (43). Table 1 provides a summary of selected studies that have demonstrated the antimicrobial efficacy of UV-C irradiation on fresh and fresh-cut fruits and vegetables.
Surface characteristics of fresh produce
Surface characteristics of fresh produce were found to have a significant effect on the disinfection efficacy of UV irradiation treatment (Table 1). Produce with smoother, and even surfaces such as pears, apples and tomatoes were more receptive to UV irradiation (48, 53–55) while rougher or uneven surfaces limit UV exposure for microbial inactivation. Yaun et al. (25) observed the higher effectiveness of UV-C treatment in reducing bacterial populations on the surface of apples than on tomatoes and lettuce. A study by Adhikari et al. (31) reported that UV-C irradiation treatment of organic apples and pears showed a 2.1 to 2.9 log CFU/g reduction of E. coli O157:H7 at 92 mJ/cm2 whereas strawberries and raspberries required a much higher UV fluency (720 to 1,050 mJ/cm2) to achieve only 1.1 to 2 log CFU/g reduction. They found higher inactivation rates on fruits with smoother surfaces such as apples and noticeably lower for fruits that have uneven surfaces, dimples or seeds (strawberries), or druplets (raspberry) that are impermeable to UV-C irradiation (31). In another study by Syamaladevi et al. (56) UV-C treatment at 756 mJ/cm2 showed a 3.7 log CFU/g reduction of generic E. coli on intact pear surfaces while a 3.1 and 2.91 log CFU/g reductions were observed on wounded pear and peach surfaces, respectively. They concluded that abrasion on the pear and trichomes on peach surfaces protected the microorganisms by shielding from UV-C radiation. Similar results were observed for the inactivation of Penicillium expansum on fruit surfaces (56) and inactivation of E. coli O157:H7 on blueberry skin and calyx (38).
Manzocco et al. (49) studied the efficacy of UV-C treatment to reduce the microbial load on fresh-cut pineapple sticks. UV-C irradiation did not significantly affect total viable bacteria, yeast and molds. However, the growth of yeast and lactic acid bacteria was slower after UV-C treatment at 20 mJ/cm2 and storage at 6°C for up to 15 days. They concluded that the rough surface of pineapple sticks with multiple fruitlets possibly helped microorganisms to avoid UV irradiation exposure. Similarly, Durak et al. (57) reported differences in the surface decontamination efficacy among baby spinach and green onions when subjected to UV-C, acidified sodium hypochlorite (ASC) and a combination of treatments. These differences were mainly attributed to the dissimilarities in surface topographies of each respective fresh produce. They reported that the surface inoculated E. coli O157:H7 was likely sheltered and protected from the germicidal effects of UV and ASC treatments on baby spinach. Green onions have smoother surfaces and possess mucus-like compounds that may have helped to interfere with the surface attachment and/or sheltering of the pathogen from UV and ASC treatments.
UV dose and method of delivery
Several studies reported that the disinfection efficacy of UV irradiation treatment depends on the method of delivery and the dose delivered. Cairns (58) compiled a comprehensive list of lethal UV doses required to achieve different magnitudes of log reduction in various vegetative cells of bacteria (1–7 log), spores (1–4 log), protozoa (1–4 log) and viruses (1–6 log), respectively. Depending on the nature of the organism, the required UV dose or fluency ranged from 0.4 to 235 mJ/cm2. It was reported that the degree of cross-linking between thymine and cytosine in the same DNA strand of microbial cells, which is a basis for UV disinfection, is proportional to the amount of UV-C irradiation exposure (59, 60). Allende et al. (10) conducted in vitro studies on the inactivation of 20 bacterial strains associated with fresh fruits and vegetables. The UV dose required to completely inhibit the tested strains ranged from 3 to 8.5 mJ/cm2. In vivo tests in the same study on Red Oak leaf lettuce showed the greatest reductions of natural microflora at a higher dose of 711 mJ/cm2. However, treatment at higher doses showed a negative effect on the quality of packaged product upon storage at 5°C for 7 days (10). Another study by Chun et al. (50) reported that the efficacy of UV-C radiation to inactivate E. coli O157:H7 and Listeria monocytogenes on fresh-cut salad increased with increasing UV dose from 100 to 800 mJ/cm2. UV doses of 800 mJ/cm2 reduced E. coli and L. monocytogenes counts on fresh-cut salad by 2.16 and 2.57 log CFU/g, respectively. Fino and Kniel (61) investigated the UV inactivation of three feline calcivirus (a surrogate for norovirus) and two piconavirus (hepatitis A virus and Aichi virus) on green onions, lettuce, and strawberries. They reported a reduction of 1.9–5.6 log TCID50/ml on the tested produce and the inactivation of viruses varied depending on the UV dose and the type of produce.
Furthermore, studies reported that the method of UV irradiation delivery onto fruit and vegetable surfaces plays an important role in disinfection. Kim et al. (47) examined the effect of UV-C treatment conditions such as time, intensity, method of exposure, space between sample and UV source, and temperature (4 and 25°C) for inactivating bacterial pathogens such as E. coli O157:H7, Salmonella spp. and L. monocytogenes on fresh-cut lettuce. Treatment at 25°C for 1 min showed a reduction of 1.35 to 2.12 log while at 4°C, only 0.31 to 1.16 log for the tested pathogens. Decreasing the distance between the sample and the lamp to 10 cm and exposing the sample from both sides significantly increased the log reduction (47). Similarly, Lim and Harrison (62) studied the efficacy of UV-C irradiation (0 to 223.1 mJ/cm2) to reduce Salmonella contamination at various locations on green tomatoes. They reported that regardless of the location of the tomatoes, UV-C treatment was shown to be effective in reducing the levels of Salmonella. Liu et al. (63) compared the decontamination efficacy of direct UV exposure with water-assisted UV exposure on blueberries contaminated with E.coli O157:H7 or Salmonella. They found that water-assisted UV treatment in general showed higher efficacies than direct UV treatment. Method of inoculation affected the inactivation rate with higher reduction (>1.4 log) in blueberries that were spot inoculated than inoculated with dipping technique. As per Fan et al. (64) water assisted, two-sided exposure and tumbling motion during UV-C treatment may minimize the shadowing effect and help increase disinfection efficacy.
More recently, Pulsed UV (PUV) treatment showed promise to reduce microbial populations on the surfaces of fresh produce. Aguero et al. (51) evaluated the efficacy of pulsed UV treatmenton the surface of spinach and reported 1.85 Log CFU/g (Listeria innocua) and 1.72 Log CFU/g (E. coli) reductions with just two light pulses at fluences lower than 1,000 mJ/cm2. However, the authors found that a gradual increase in fluence did not resultedgradual population decrease instead it increased CO2 levels and decreased O2 in the headspace of treated samples (51). Avalos et al. (44) studied PUV fluences of 0.8, 1.2, and 1.6 mJ/cm2 against apple slices and found a 1.55 log CFU/g reduction of mesophilic and psychrophilic bacteria and 2.3 log CFU/g reductions of yeast and mold populations. Another study by Huang et al. (45) tested PUV at 0.0105–0.0298 J/cm2 on berries during washing (water turbidity 63.7 NTU) and observed a 3 log CFU/g reduction of Salmonella and 4 Log CFU/g when PUV combined with 1% hydrogen peroxide.
Type of organisms
UV sensitivity of microorganisms varies significantly due to the differences in cellular components such as cell wall structure, thickness, composition, structure of nucleic acid, type of cellular proteins, photoproducts, physiological state of microorganism and the ability of the cell to repair UV damage (15). In addition, the efficacy of UV radiation may vary between species to species, growth media, stage of culture, density of organisms and surface characteristic of the food may also affect (24, 65, 66). Martinez-Hernandez et al. (46) observed high sensitivity of Salmonella Enteritidis to UV-C radiation while L. monocytogenes was significantly resistant, requiring 2 and 926 mJ/cm2 UV doses, respectively when tested on fresh-cut broccoli. Kim et al. (67) studied the bactericidal effect of UVC-LEDs (at four peak wavelengths from 266 to 279 nm) against foodborne pathogens and spoilage microorganisms. They reported that the UV sensitivities of gram-positive, gram-negative bacteria and yeasts differed from each other. For each microorganism groups, higher doses of irradiation resulted in higher reduction levels. Gram-negative organisms showed the lowest resistance while yeasts showed the highest resistance to UVC-LEDs.
UV-C irradiation produces DNA mutations in injured organisms (59, 60). Studies reported that the damage occurred at the DNA level can be repaired by the injured organism when exposed to wavelengths higher than 330 nm (68, 69). Sommer et al. (37) investigated the efficacy of UV-C treatment to disinfect seven pathogenic E. coli O157:H7 and one non-pathogenic strain of E. coli (ATCC 11229) in water. They found that a UV fluency of up to 30 mJ/cm2 is required depending on the strain to achieve a 6-log reduction and that all the strains demonstrated photo repair ability (37). Guerrero-Beltran and Barbosa-Canovas (24) presented a list of photo reactivated microorganisms with higher resistance to UV-C irradiation than non-reactivated microorganisms while Fan et al. (64) discussed the fate of pathogens and potential induction of viable but nonculturable (VBNC) state during post UV-C treatment storage period.
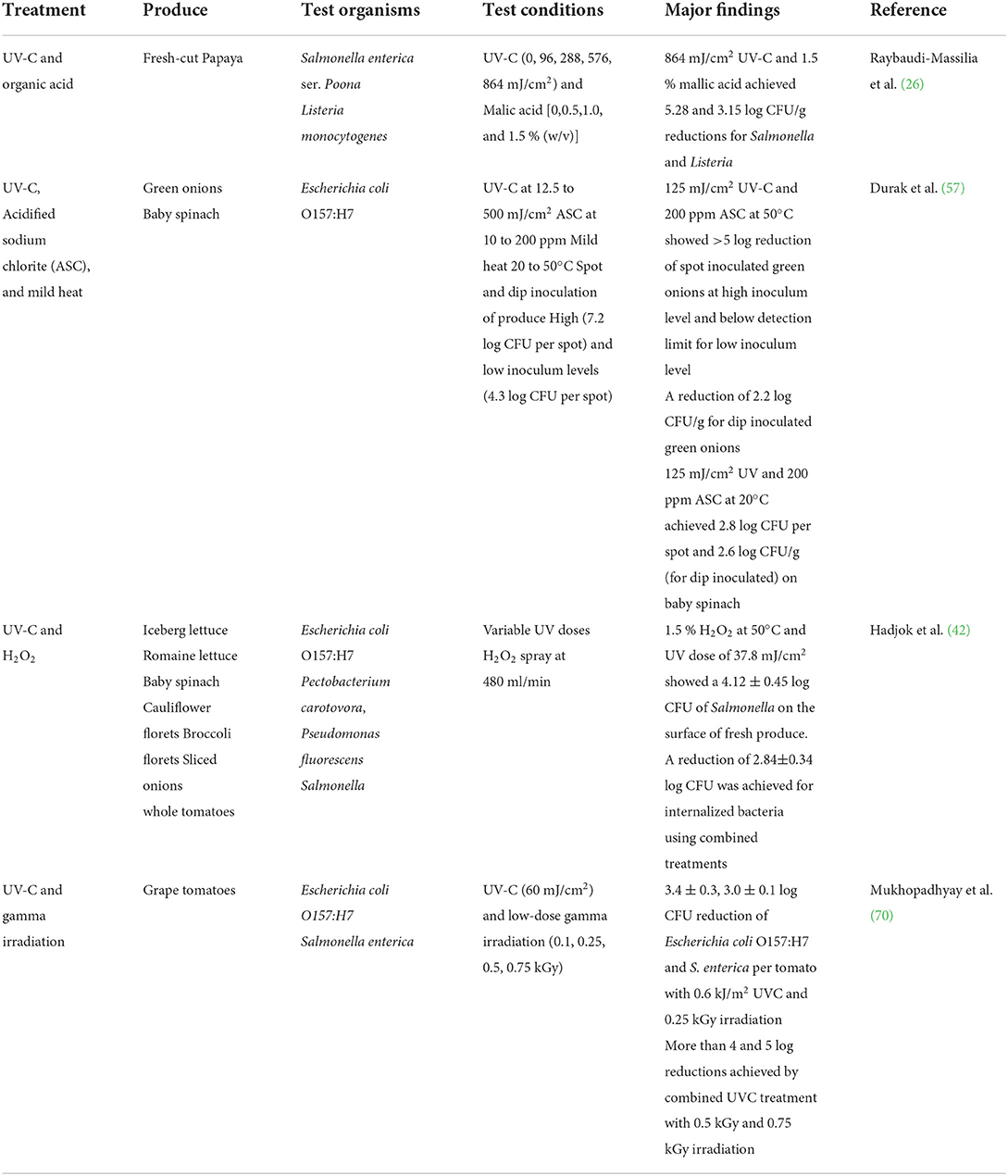
Combination of treatments
Due to inherent complexity of food matrices and limited penetration depth, the disinfection efficacy of UV irradiation is mostly confined to the surface of the product. Several studies investigated the efficacy of UV irradiation treatment in combination with other treatments to increase overall log reductions (Table 2). UV irradiation combined with laser irradiation was effective against Bacillus cereus, compared to UV or laser irradiation alone (71, 72). Durak et al. (57) reported that a combination treatment of UV (125 mJ/cm2), acidified sodium hypochlorite (ASC; 200 ppm) and mild heat (50°C) showed more than 5-log reductions of E. coli O157:H7 on green onions. While in the same study, a reduction of 2.6 log CFU/g was observed on baby spinach with the combination treatments at 20°C. They concluded that when microorganisms come in contact with produce; depending upon the surface characteristics of the produce, they may infiltrate or internalize, firmly attach to the surface, or become localized into rough surfaces which may protect against the UV radiation. Their results indicate limited effectiveness of individually used UV, ASC, and mild-heat application on both green onions and baby spinach (<3 log) while combination treatments showed a reduction of >5 log on green onions. Hadjok et al. (42) found that fresh produce (such as iceberg lettuce, romaine lettuce, cauliflower florets, baby spinach, sliced Spanish onions, broccoli florets, and ripened whole tomatoes) subjected to a combination of UV-C and H2O2 treatments yielded higher overall reductions (E. coli O157:H7, Pseudomonas fluorescens, Pectobacterium carotovora, and Salmonella) compared to individual treatments. For example, Salmonella counts on lettuce were reduced by 4.12 log CFU with 1.5 % H2O2 at 50°C and 37.8 mJ/cm2 UV fluency while the individual treatments showed only around 2 log reductions (42). In another study by Kim et al. (73) A reduction of 1.8–2.8 log CFU/g bacterial pathogens was achieved on iceberg lettuce by photocatalytic disinfection using TiO2 and UV-C irradiation while treatment with UV alone and NaOCl resulted only 1.4 and 1.1 log reductions, respectively.
Process wash water
Postharvest processing of fresh produce requires extensive amount of water to cool, hydrate, wash, and transport products which are considered as high-risk activities. As such, the quality of water is very important and any contamination in water can lead to produce contamination (74). Furthermore, water can serve as a route of cross-contamination and in absence of proper mitigation techniques in place the extended use of the same processing water may result in the build-up of microbial loads, and reduce the effectiveness of chemical sanitizers used in wash water (75). Selma et al. (11) reported that UV treatment of fresh-cut onion, carrot, escarole, and spinach wash waters for 60 min showed a 4 log CFU/mL reduction of microflora while UV, in combination with ozone treatment, showed 6.6 log CFU/mL reduction. They found that UV treatment itself did not change the physicochemical properties of water, but ozone-UV treatment significantly reduced the turbidity of wash water, which helped to increase the disinfection efficacy. Their study concluded that UV treatment could be used as cost-effective intervention only when the levels of undesirable microbial and chemical components in the wash water are at a minimum (11). Millan-Sango et al. (76) studied the efficacy of UV-C (164 mJ/cm2) and ultrasound (US; 26 kHz) treatments alone and in-combination for the disinfection of natural microflora in fresh produce wash water. They found that the combination treatment is most efficient and achieved a reduction of 3.57 log CFU/mL. The energy requirements of US, UV and US+UV were 0.107, 0.040, 0.114 kW/h, respectively and the resultant microbial reduction in relation to the energy spent was 4.15, 21.53, and 8.72 × 10−6 CFU/mL/J, respectively (76).
Effect on the quality
Use of UV irradiation treatment has been incorrectly associated with loss of nutritional value and sensory quality (77). However, studies revealed that pre-storage exposure of fresh produce to UV irradiation was effective in minimizing the development of postharvest diseases (78). Studies showing the effect of UV irradiation treatment on the quality of fresh-cut fruits and vegetables were presented in Table 3. Castagna et al. (79) reported that UV-B treatment of two varieties of tomatoes was found to increase phenolic, flavonoid and flavonol concentrations in both peel and flesh. UV-C irradiation activates several biological processes and increases respiratory rate. Erkan et al. (52) reported increase in respiration rates of squash slices with UV treatment and was correlated with the increase in UV-C intensity. In contrast, Vicente et al. (81) found lower respiration rate on UV-C treated peppers than untreated control fruits. Thus, the effect of UV treatment on the quality of whole and fresh-cut fruits and vegetables should be considered on a case-by-case basis with several influencing factors. Though PUV treatment of packaged spinach showed a reduction of L. innocua and E. coli, shelf-life of product was reduced due to increased CO2 and decreased O2 levels in the headspace of the package (51). Mukhopadhyay et al. (70) reported no significant changes in the visual and firmness quality of the spinach upon PUV treatment.
Concluding remarks
The present review discussed the application of UV radiation during pre/post-harvest application to maintain the safety of fresh produce. Fresh and fresh-cut fruits and vegetables are prone to microbial contamination during various pre- and post-harvest activities. To ensure the safety of these minimally processed produce for human consumption, effective preventive controls should be introduced at various pre- and post-harvest stages. The newly enacted U.S. Food Safety Modernization Act (FSMA) Produce Safety Rule requires all agricultural water must be safe for its intended use. Critical knowledge gap exists on identifying proper disinfecting technique for agricultural water. Any chemical residues in agricultural water would adversely affects crop production or soil quality. This has increased the potential application of UV irradiation at the preharvest level. At post-harvest level, producers are investigating extensively on technologies that are environmentally friendly and could be applied in combination with other methods. This is in fact because of the growing interest of consumer in fresh produce that receives minimal chemical treatments. The use of UV irradiation on post-harvest processing is limited because of the complexity of food matrices. However, recent studies indicated the potential of using UV irradiation in combination with other methods to get similar or even higher efficacy as compared to chemical sanitizers. UV irradiation being a simple and low-cost approach has shown promise as an efficient surface decontamination technique on fresh produce with smoother surfaces. Future studies, should focus on application of UV radiation as part of hurdle technology with other treatments that has the ability to penetrate the surface of fresh produce to achieve an additive or synergistic effect. The effect of UV treatment on the quality of produce needs to be studied on a case-by-case basis.
Author contributions
Conceptualization, investigation, resources, data curation and writing—original draft preparation, and writing—review and editing: VY, JM, and AA. Visualization, supervision, project administration: VY and AA. Funding acquisition: AA. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huxley RR, Lean M, Crozier A, John JH, Neil A, Oxford Fruit Vegetable Study G. Effect of dietary advice to increase fruit and vegetable consumption on plasma flavonol concentrations: results from a randomised controlled intervention trial. J Epidemiol Commun Health. (2004) 58:288–9. doi: 10.1136/jech.2003.014274
2. Monteiro CA, Levy R, Claro RM, de Castro IR, Cannon G. Increasing Consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. (2011) 14:5–13. doi: 10.1017/S1368980010003241
3. Elizabeth L, Machado P, Zinocker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: A narrative review. Nutrients. (2020) 12:1955–87. doi: 10.3390/nu12071955
4. CSPI. A Review of Foodborne Illness in America from 2002–2011. Washington, DC: Center for Science in the Public Interest (2014).
5. CDC. National Outbreak Reporting System (Nors). (2022). Available online at: https://wwwn.cdc.gov/norsdashboard/ (accessed June 20, 2022).
6. Hussain MA, Gooneratne R. Understanding the fresh produce safety challenges. Foods. (2017) 6:23–4. doi: 10.3390/foods6030023
7. O'Beirne D, Francis GA. Reducing pathogen risks in map-prepared produce. In: Ahvenainen R, editor. Novel Food Packaging Techniques. Cambridge: Woodhead Publishing Ltd (2003). p. 231–75. doi: 10.1533/9781855737020.2.231
8. Fonseca JM, Rushing JW. Effect of ultraviolet-c light on quality and microbial population of fresh-cut watermelon. Postharvest Biol Technol. (2006) 40:256–61. doi: 10.1016/j.postharvbio.2006.02.003
9. Laurila E, Ahvenainen R. Minimal processing of fresh fruits and vegetables. In: Jongen W, editor, Fruit and Vegetable Processing. Cambridge/Boca Raton, FL: Woodhead Publishing Limited/CRC Press. (2002), p. 288–309. doi: 10.1533/9781855736641.3.288
10. Allende A, McEvoy JL, Luo Y, Artes F, Wang CY. Effectiveness of two-sided UV-C treatments in inhibiting natural microflora and extending the shelf-life of minimally processed 'red oak leaf' lettuce. Food Microbiol. (2006) 23:241–9. doi: 10.1016/j.fm.2005.04.009
11. Selma MV, Allende A, Lopez-Galvez F, Conesa MA, Gil MI. Disinfection potential of ozone, ultraviolet-c and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. (2008) 25:809–14. doi: 10.1016/j.fm.2008.04.005
12. Ramos B, Miller FA, Brandão TRS, Teixeira P, Silva CLM. Fresh fruits and vegetables—an overview on applied methodologies to improve its quality and safety. Innov Food Sci Emerg Technol. (2013) 20:1–15. doi: 10.1016/j.ifset.2013.07.002
13. Kramer B, Wunderlich J, Muranyi P. Recent findings in pulsed light disinfection. J Appl Microbiol. (2017) 122:830–56. doi: 10.1111/jam.13389
14. Sari LK, Setha S, Naradisorn M. Effect of UV-C irradiation on postharvest quality of ‘phulae’ pineapple. Sci Hortic. (2016) 213:314–20. doi: 10.1016/j.scienta.2016.09.049
15. Koutchma T. Advances in ultraviolet light technology for non-thermal processing of liquid foods. Food Bioproc Tech. (2009) 2:138–55. doi: 10.1007/s11947-008-0178-3
16. Gayan E, Condon S, Alvarez I. Biological aspects in food preservation by ultraviolet light: a review. Food Bioproc Tech. (2014) 7:1–20. doi: 10.1007/s11947-013-1168-7
17. Bintsis T, Litopoulou-Tzanetaki E, Robinson RK. Existing and potential applications of ultraviolet light in the food industry - a critical review. J Sci Food Agric. (2000) 80:637–45. doi: 10.1002/(SICI)1097-0010(20000501)80:6<637::AID-JSFA603>3.0.CO;2-1
18. Xu F, Wang S, Xu J, Liu S, Li G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol Technol. (2016) 117:125–31. doi: 10.1016/j.postharvbio.2016.01.012
19. Eischeid AC, Meyer JN, Linden KG. Uv Disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl Environ Microbiol. (2009) 75:23–8. doi: 10.1128/AEM.02199-08
20. Hijnen WAM, Beerendonk EF, Medema GJ. Inactivation credit of UV radiation for viruses, bacteria and protozoan (Oo)cysts in water: a review. Water Res. (2006) 40:3–22. doi: 10.1016/j.watres.2005.10.030
21. Gomez-Lopez VM, Devlieghere F, Bonduelle V, Debevere J. Intense light pulses decontamination of minimally processed vegetables and their shelf-life. Int J Food Microbiol. (2005) 103:79–89. doi: 10.1016/j.ijfoodmicro.2004.11.028
22. Dong QF, Manns DC, Feng GP, Yue TL, Churey JJ, Worobo RW. Reduction of patulin in apple cider by UV radiation. J Food Prot. (2010) 73:69–74. doi: 10.4315/0362-028X-73.1.69
23. Shriver SK, Yang WW. Thermal and nonthermal methods for food allergen control. Food Eng Rev. (2011) 3:26–43. doi: 10.1007/s12393-011-9033-9
24. Guerrero-Beltran JA, Barbosa-Canovas GV. Advantages and limitations on processing foods by UV light. Food Sci Technol Int. (2004) 10:137–47. doi: 10.1177/1082013204044359
25. Yaun BR, Sumner SS, Eifert JD, Marcy JE. Inhibition of pathogens on fresh produce by ultraviolet energy. Int J Food Microbiol. (2004) 90:1–8. doi: 10.1016/S0168-1605(03)00158-2
26. Raybaudi-Massilia R, Calderón-Gabaldón MI, Mosqueda-Melgar J, Tapia MS. Inactivation of Salmonella enterica Ser. Poona and Listeria monocytogenes on fresh-cut “Maradol” Red Papaya (Carica papaya L) treated with UV-C light and malic acid. J Verbrauch Lebensm. (2013) 8:37–44. doi: 10.1007/s00003-013-0808-1
27. Wu J, Liu W, Yuan L, Guan WQ, Brennan CS, Zhang YY, et al. The influence of postharvest UV-C treatment on anthocyanin biosynthesis in fresh-cut red cabbage. Sci Rep. (2017) 7:5232. doi: 10.1038/s41598-017-04778-3
28. Chisari M, Barbagallo RN, Spagna G, Artes F. Improving the quality of fresh-cut melon through inactivation of degradative oxidase and pectinase enzymatic activities by UV-C treatment. Int J Food Sci Technol. (2011) 46:463–8. doi: 10.1111/j.1365-2621.2010.02466.x
29. George DS, Razali Z, Santhirasegaram V, Somasundram C. Effects of ultraviolet light (UV-C) and heat treatment on the quality of fresh-cut Chokanan mango and Josephine pineapple. J Food Sci. (2015) 80:426–34. doi: 10.1111/1750-3841.12762
30. Kowalski W. Ultraviolet Germicidal Irradiation Handbook. UVGI for Air and Surface Disinfection. New York, NY: Springer (2009). doi: 10.1007/978-3-642-01999-9
31. Adhikari A, Syamaladevi RM, Killinger K, Sablani SS. Ultraviolet-C light inactivation of Escherichia coli O157:H7 and Listeria monocygotenes on organic fruit surfaces. Int J Food Microbiol. (2015) 210:136–42. doi: 10.1016/j.ijfoodmicro.2015.06.018
32. Scarlett K, Collins D, Tesoriero L, Jewell L, Ogtrop V, Daniel R. Efficacy of chlorine, chlorine dioxide and ultraviolet radiation as disinfectants against plant pathogens in irrigation water. Eur J Plant Pathol. (2016) 145:27–38. doi: 10.1007/s10658-015-0811-8
33. Zhang J, Yuan L, Liu W, Lin Q, Wang Z, Guan W. Effects of UV-C on antioxidant capacity, antioxidant enzyme activity and colour of fresh-cut red cabbage during storage. Int J Food Sci Technol. (2017) 52:626–34. doi: 10.1111/ijfs.13315
34. Jones LA, Worobo RW, Smart CD. UV light inactivation of human and plant pathogens in unfiltered surface irrigation water. Appl Environ Microbiol. (2014) 80:849–54. doi: 10.1128/AEM.02964-13
35. Ge C, Bohrerova Z, Lee J. Inactivation of internalized salmonella typhimurium in lettuce and green onion using ultraviolet C irradiation and chemical sanitizers. J Appl Microbiol. (2013) 114:1415–24. doi: 10.1111/jam.12154
36. Adhikari A, Parraga KJ, Chhetri V, Jane M, Fontenot K, Beaulieu JC. Evaluation of ultraviolet (UV-C) light treatment for microbial inactivation in agricultural waters with different levels of turbidity. Food Sci Nutr. (2019) 8:1237–43. doi: 10.1002/fsn3.1412
37. Sommer R, Lhotsky M, Haider T, Cabaj A. UV inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli O157 and other pathogenic Escherichia coli strains in water. J Food Prot. (2000) 63:1015–20. doi: 10.4315/0362-028X-63.8.1015
38. Kim C, Hung YC. Inactivation of E. coli O157:H7 on blueberries by electrolyzed water, ultraviolet light, and ozone. J Food Sci. (2012) 77:M206–11. doi: 10.1111/j.1750-3841.2011.02595.x
39. Achen M, Yousef AE. Efficacy of ozone against Escherichia coli O157: H7 on apples. J Food Sci. (2001) 66:1380–4. doi: 10.1111/j.1365-2621.2001.tb15218.x
40. Beuchat LR, Nail BV, Adler BB, Clavero MRS. Efficacy of spray application of chlorinated water in killing pathogenic bacteria on raw apples, tomatoes, and lettuce. J Food Prot. (1998) 61:1305–11. doi: 10.4315/0362-028X-61.10.1305
41. Du J, Han Y, Linton RH. Efficacy of chlorine dioxide gas in reducing Escherichia coli O157: H7 on apple surfaces. Food Microbiol. (2003) 20:583–91. doi: 10.1016/S0740-0020(02)00129-6
42. Hadjok C, Mittal GS, Warriner K. Inactivation of human pathogens and spoilage bacteria on the surface and internalized within fresh produce by using a combination of ultraviolet light and hydrogen peroxide. J Appl Microbiol. (2008) 104:1014–24. doi: 10.1111/j.1365-2672.2007.03624.x
43. Tarek AR, Rasco BA, Sablani SS. Ultraviolet-C light sanitization of English cucumber (Cucumis sativus) packaged in polyethylene film. J Food Sci. (2016) 81:1419–30. doi: 10.1111/1750-3841.13314
44. Avalos K, Marsellés-Fontanet A, Martín-Belloso O, Soliva-Fortuny R. Impact of pulsed light treatments on antioxidant characteristics and quality attributes of fresh-cut apples. Innov Food Sci Emerg Tech. (2016) 33:206–15. doi: 10.1016/j.ifset.2015.10.021
45. Huang Y, Sido R, Huang R, Chen H. Application of water-assisted pulsed light treatment to decontaminate raspberries and blueberries from Salmonella. Int J Food Microbiol. (2015) 208:43–50. doi: 10.1016/j.ijfoodmicro.2015.05.016
46. Martinez-Hernandez GB, Huertas JP, Navarro-Rico J, Artes F, Palop A, Artes-Hernandez F. Inactivation kinetics of foodborne pathogens by Uv-C radiation and its subsequent growth in fresh-cut Kailan-Hybrid Broccoli. Food Microbiol. (2015) 46:263–71. doi: 10.1016/j.fm.2014.08.008
47. Kim YH, Jeong SG, Back KH, Park KH, Chung MS, Kang DH. Effect of various conditions on inactivation of Escherichia Coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in fresh-cut lettuce using ultraviolet radiation. Int J Food Microbiol. (2013) 166:349–55. doi: 10.1016/j.ijfoodmicro.2013.08.010
48. Schnek M, Guerrero S, Alzamore SM. Response of some microorganisms to ultraviolet treatment on fresh-cut pear. Food Bioproc Tech. (2008) 1:384–92. doi: 10.1007/s11947-007-0029-7
49. Manzocco L, Plazzotta S, Maifreni M, Calligaris S, Anese M, Nicoli MC. Impact of UV-C light on storage quality of fresh-cut pineapple in two different packages. LWT - Food Sci Technol. (2016) 65:1138–43. doi: 10.1016/j.lwt.2015.10.007
50. Chun H-H, Kim J-Y, Song KB. Inactivation of foodborne pathogens in ready-to-eat salad using UV-C irradiation. Food Sci Biotechnol. (2010) 19:547–51. doi: 10.1007/s10068-010-0076-0
51. Agüero V, Jagus R, Martín-Belloso O. Surface decontamination of spinach by intense pulsed light treatments: impact on quality attributes. Postharv Biol Technol. (2016) 121:118–25. doi: 10.1016/j.postharvbio.2016.07.018
52. Erkan M, Wang CY, Krizek DT. UV-C radiation reduces microbial populations and deterioration in Cucurbita pepo fruit tissue. Environ Exp Bot. (2001) 45:1–9. doi: 10.1016/S0098-8472(00)00073-3
53. Mukhopadhyay S, Ukuku D, Fan X, Juneja VK. Efficacy of integrated treatment of UV light and low-dose gamma irradiation on inactivation of Escherichia coli O157:H7 and Salmonella enterica on grape tomatoes. J Food Sci. (2013) 78:1049–56. doi: 10.1111/1750-3841.12154
54. Gómez PL, Schenk ML, Salvatori DM, Alzamora SM. Potential of UV-C light for preservation of cut apples fortified with calcium: Assessment of optical and rheological properties and native flora dynamics. Food Bioproc Tech. (2015) 8:1890–903. doi: 10.1007/s11947-015-1545-5
55. Lante A, Tinello F, Nicoletto M. UV-a light treatment for controlling enzymatic browning of fresh-cut fruits. Innov Food Sci Emerg Technol. (2016) 34:141–7. doi: 10.1016/j.ifset.2015.12.029
56. Syamaladevi RM, Adhikari A, Lupien SL, Dugan F, Bhunia K, Dhingra A, et al. Ultraviolet-C light inactivation of Penicillium expansum on fruit surfaces. Food Control. (2015) 50:297–303. doi: 10.1016/j.foodcont.2014.09.006
57. Durak MZ, Churey JJ, Worobo RW. Efficacy of UV, acidified sodium hypochlorite, and mild heat for decontamination of surface and infiltrated Escherichia coli O157:H7 on green onions and baby spinach. J Food Prot. (2012) 75:1198–206. doi: 10.4315/0362-028X.JFP-12-009
58. Cairns B. Uv dose required to achieve incremental log inactivation of bacteria, protozoa and viruses. IUVA News. (2006) 8:38–45.
59. Snowball MR, Hornsey IS. Purification of water supplies using ultraviolet light. Dev Food Microbiol. (1988) 3:171–91. doi: 10.1007/978-1-4613-1085-3_7
60. Sastry SK, Datta AK, Worobo RW. Ultraviolet light. J Food Sci. (2000) 65:90–2. doi: 10.1111/j.1750-3841.2000.tb00623.x
61. Fino VR, Kniel KE. UV light inactivation of hepatitis a virus, Aichi virus, and feline Calicivirus on strawberries, green onions, and lettuce. J Food Prot. (2008) 71:908–13. doi: 10.4315/0362-028X-71.5.908
62. Lim W, Harrison MA. Effectiveness of UV light as a means to reduce Salmonella contamination on tomatoes and food contact surfaces. Food Control. (2016) 66:166–73. doi: 10.1016/j.foodcont.2016.01.043
63. Liu C, Huang Y, Chen H. Inactivation of Escherichia coli O157:H7 and Salmonella enterica on blueberries in water using ultraviolet light. J Food Sci. (2015) 80:1532–7. doi: 10.1111/1750-3841.12910
64. Fan X, Huang R, Chen H. Application of ultraviolet C technology for surface decontamination of fresh produce. Trends Food Sci Technol. (2017) 70:9–19. doi: 10.1016/j.tifs.2017.10.004
67. Kim DK, Kim SJ, Kang DH. Bactericidal effect of 266 to 279nm wavelength Uvc-Leds for inactivation of gram positive and gram negative foodborne pathogenic bacteria and yeasts. Food Res Int. (2017) 97:280–7. doi: 10.1016/j.foodres.2017.04.009
68. Liltved H, Landfald B. Effects of high intensity light on ultraviolet-irradiated and non-irradiated fish pathogenic bacteria. Water Res. (2000) 34:481–6. doi: 10.1016/S0043-1354(99)00159-1
69. Yajima H, Yasuhira S, Zhao JH, Ishii C, Inoue H, Yasui A, et al. Eukaryotic gene encoding an endonuclease that specifically repairs DNA damage by ultraviolet light. EMBO J. (1995) 14:2393–9. doi: 10.1002/j.1460-2075.1995.tb07234.x
70. Mukhopadhyay S, Sokorai K, Ukuku D, Fan X, Olanya M, Juneja V. Effects of pulsed light and sanitizer wash combination on inactivation of Escherichia coli O157:H7, microbial loads and apparent quality of spinach leaves. Food Microbiol. (2019) 82:127–34. doi: 10.1016/j.fm.2019.01.022
71. Ward GD, Watson IA, Stewart-Tull DE, Wardlaw AC, Wang RK, Nutley MA, et al. Bactericidal action of high-power Nd:Yag laser light on Escherichia Coli in saline suspension. J Appl Microbiol. (2000) 89:517–25. doi: 10.1046/j.1365-2672.2000.01144.x
72. Maktabi S, Watson I, Parton R. Synergistic effect of UV, laser and microwave radiation or conventional heating on E. coli and on some spoilage and pathogenic bacteria. Innov Food Sci Emerg Technol. (2011) 12:129–34. doi: 10.1016/j.ifset.2010.12.011
73. Kim Y, Choi Y, Kim S, Park J, Chung M, Song K, et al. Disinfection of iceberg lettuce by titanium dioxide-UV photocatalytic reaction. J Food Prot. (2009) 72:1916–22. doi: 10.4315/0362-028X-72.9.1916
74. Gombas D, Luo Y, Brennan J, Shergill G, Petran R, Walsh R, et al. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J Food Prot. (2017) 80:312–30. doi: 10.4315/0362-028X.JFP-16-258
75. Gil MI, Selma MV, Lopez-Galvez F, Allende A. Fresh-cut product sanitation and wash water disinfection: problems and solutions. Int J Food Microbiol. (2009) 134:37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021
76. Millan-Sango D, Allende A, Spiteri D, Van I, Jan F. Treatment of fresh produce water effluents by non-thermal technologies. J Food Eng. (2017) 199:77–81. doi: 10.1016/j.jfoodeng.2016.12.006
77. Sharma GA. Modeling UV-induced inactivation of microorganisms on surfaces. J Food Protect. (2000) 63:63–70. doi: 10.4315/0362-028X-63.1.63
78. Turtoi M. Ultraviolet light treatment of fresh fruits and vegetables surface: a review. J Agroaliment Processes Technol. (2013) 19:325–37.
79. Castagna A. Dall'Asta C, Chiavaro E, Galaverna G, Ranieri A. Effect of post-harvest UV-B irradiation on polyphenol profile and antioxidant activity in flesh and peel of tomato fruits. Food Bioproc Tech. (2013) 7:2241–50. doi: 10.1007/s11947-013-1214-5
80. Kim HJ, Fonseca JM, Kubota C, Kroggel M, Choi JH. Quality of fresh-cut tomatoes as affected by salt content in irrigation water and post-processing ultraviolet-C treatment. J Sci Food Agri. (2008) 88:1969–74. doi: 10.1002/jsfa.3305
Keywords: UV irradiation, fresh produce, pre- and post-harvest contamination, microbiological safety, quality
Citation: Yemmireddy V, Adhikari A and Moreira J (2022) Effect of ultraviolet light treatment on microbiological safety and quality of fresh produce: An overview. Front. Nutr. 9:871243. doi: 10.3389/fnut.2022.871243
Received: 07 February 2022; Accepted: 04 July 2022;
Published: 22 July 2022.
Edited by:
Marco Iammarino, Experimental Zooprophylactic Institute of Puglia and Basilicata (IZSPB), ItalyReviewed by:
Tatiana Koutchma, Agriculture and Agri-Food Canada (AAFC), CanadaRavi Jadeja, Oklahoma State University, United States
Copyright © 2022 Yemmireddy, Adhikari and Moreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Achyut Adhikari, YWNhZGhpa2FyaUBhZ2NlbnRlci5sc3UuZWR1
 Veerachandra Yemmireddy
Veerachandra Yemmireddy Achyut Adhikari
Achyut Adhikari Juan Moreira
Juan Moreira