Abstract
The processing of medicinal and aromatic plants (MAPs) results in the production of a significant amount of plant by-products; herbal material of inferior quality and/or unusable plant parts that are not commercially exploitable. An extensive study of Greek native species was performed toward the production of innovative bioactive products using as raw materials the by-products obtained from the processing of cultivated MAPs. Origanum vulgare subsp. hirtum (oregano), Sideritis scardica (Greek mountain tea), Thymus vulgaris (thyme), and Matricaria recutita (chamomile) were selected due to their wide use for the preparation of beverages and culinary purposes. The determination of the percentage of the post-harvest processing by-products was performed for a 3 years period (2018–2020). Results showed that by-products derived from the above-mentioned species' processing constitute 64% (thyme), 54% (oregano), 37% (Greek mountain tea), and 24% (chamomile) of the total processed mass. To value the by-products as a potent source of bioactive ingredients, superior and inferior quality herbal material of the aforementioned plant species were extracted by an ultrasonic assisted extraction method. Hydroalcoholic extracts were chemically investigated using high-performance thin layer chromatography (HPTLC) and liquid chromatography-mass spectrometry (LC-MS) techniques. In addition, their free radical scavenging activity and total phenolic content (TPC) were estimated. Based on the results, herbs by-products revealed similar chemical content to the superior herbal material by the means of HPTLC and LC-MS analysis. In addition, strong free radical scavenging related to a high phenolic content was detected in the case of thyme, oregano, and Greek mountain tea. Moreover, the gas chromatography-mass spectrometry (GC-MS) analyses of the essential oils (EOs) of oregano and thyme by-products revealed the presence of carvacrol, thymol, γ-terpinene, and p-cymene among the major constituents. Finally, the LC-MS analyses of aqueous extracts of Greek mountain tea and chamomile by-products led to the identification of several bioactive compounds, such as flavonoids and phenylpropanoids. Overall, the presence of bioactive constituents in by-products, such as terpenes, phenolic compounds, and flavonoids underly their potent use as food antimicrobial and antioxidant additives, in the preparation of high added-value products, such as enriched aromatic edible oils, and innovative herbal teas, such as instant beverages.
Introduction
The processing procedure of medicinal and aromatic plants (MAPs) results in a significant amount of by-products, such as hydrolates and solid residues from the essential oils (EOs) process (1), or post-harvest by-products, such as branches and leaves of inferior quality (2), that are non-commercially acceptable (3). These residual biomasses are the potential sources of bioactive compounds, since they contain the same ingredients and properties as the final product (4, 5). Until now, these materials were treated as waste and disposed improperly to the fields or used as a burning material. However, MAPs by-products are a reservoir of valuable metabolites with important biological properties, which could add special value to the final products (6).
Oregano (Origanum spp.) and thyme (Thymus spp.) are among the world's most valued aromatic plants, not only for culinary purposes, but also for their EOs. The EO of thyme has a milder odor compared with oregano, mainly because it contains thymol in larger quantities compared with its isomer carvacrol, with major antimicrobial, antioxidant, and anti-inflammatory properties (7–9). Greek mountain tea (Sideritis spp.) possess antioxidant, anti-inflammatory, and antimicrobial properties (10, 11). Chamomile (Matricaria recutita L.) is a medicinal plant used traditionally as a mild sedative and to treat gastrointestinal problems. It has been shown to possess anti-inflammatory and antiviral properties (12, 13). These herbs possess the aforementioned biological properties due to the occurrence of several secondary metabolites, such as phenolic constituents, terpenoids, and flavonoids, in their extracts. Due to their rich chemical content, these herbs are the most frequent cultivated species in Greece, to produce EOs or they are sold as raw botanicals for the preparation of herbal teas. However, during the post-harvest processing for the selection of the marketed material, significant amount of herbal residues is produced. According to the farmers, limited part of these residues is utilized as fertilizer, but the massive production is rejected in the fields polluting the environment. Up to now, several studies have proven the presence of bioactive constituents in the selected plants by-products (14, 15). Toward this direction, the remaining herbal and/or hydrodistillation by-products constitute a promising source of bioactive compounds with potential applications in pharmaceutical, cosmeceutical, and food supplements industries. Hence, in continuation of our research efforts on MAPs by-products (16), the aim of this study was to assess in a systematic way, the amount of by-products generated after the harvesting and processing procedure of four Greek MAPs (thyme, oregano, Greek mountain tea, and chamomile). Moreover, the aim was to characterize the chemical profile and value the by-products as a potent source of bioactive ingredients for the production of innovative foods additives and high-added value products, such as instant beverages or enriched aromatic olive oils.
Materials and Methods
Analytical grade methanol (MeOH) for extraction, as well as acetonitrile (ACN), acetic acid (A.A), sulfuric acid (H2SO4), and vanillin for high-performance thin layer chromatography (HPTLC) analysis and ethanol for bioassays were purchased from Merck (Merck, Darmstadt, Germany). For free radical scavenging and total phenolic content assays, Folin–Ciocalteu solution, dimethylsulfoxide (DMSO), sodium carbonate (Na2CO3), gallic acid, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (Sigma-Aldrich, Steinheim, Germany).
Plant Material
The by–products production by the processing of four Greek cultivated species, Origanum vulgare subsp. hirtum L. (oregano, IPEN (International Plant Exchange Network) accession number GR-1-BBGK- 03,2107), Sideritis scardica L. (Greek mountain tea, IPEN accession number GR-1-BBGK- 13,5769), Thymus vulgaris L. var Varico 3 (thyme), and Matricaria recutita L. var Banatsa (chamomile, IPEN accession number GR-1-BBGK- 21,1) was studied. Plant propagating material was originated by mother plants maintained ex-situ, at the collection of Balkan Botanic Garden of Kroussia (41°05′44.3″N 23°06′33.7″E) of the Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization-DEMETER, in Greece.
Plant Material Propagation
Cultivated plants of oregano, Greek mountain tea, and thyme were propagated by cuttings (17, 18), while chamomile plants were produced by seeds (19). Cultivation was conducted during the period of 3 years (2018–2020) in the area of North-West Macedonia, Greece, from different farmers. Plants produced by cuttings were transplanted in field in the early spring of 2018. Plant density was 0.6 × 0.4 m for oregano, 0.8 × 0.6 m for Greek mountain tea and 0.8 × 0.35 m for thyme, while chamomile was sowed every year in October (400 g/acre). Harvest was conducted at full blossom (20–22) every year and post-harvest processing was undertaken by the Bagatzounis and Sons SA company, specialized on the commercialization of Greek MAPs.
The production of every farmer, every year was delivered to the company and considered as initial quantity of the lot. In particular, lot was considered for every harvest of plant material originated from the same plant species, same year of production, same farmer, same area of cultivation, and same plant material condition. The aerial parts of each plant were collected, dried, and processed in a grinding mill to separate leaves and flowers from branches. Simultaneously, the mill automatically separated the grated plant material in four grades determined according to Codex Alimentarius. The qualities A (4.5–1.0 mm), B (1.0–0.5 mm), and C (<0.5 for thyme, oregano, Greek mountain tea and <0.35 for chamomile) are commercially acceptable from the market (superior plant material) while the D quality (residual biomass) is considered non-commercial (inferior plant material).
Plant Sampling
The determination of the by-products percentage was conducted on samples of dry plant material of different lots during processing, in 3 years period (2018–2020). Samples were collected according to the standard sampling methods [ISO 948:1980, (23)]. The quantity of a sample that was processed (grated) depended on the quantity of initial lot. When the lot was: (i) 1–5 kg, all plant material was processed, (ii) 6–50 kg a sample of 5 kg was processed, (iii) 50–100 kg a sample of 10 kg was processed, and (iv) > 100 kg the processed sample was the square root of the quantity.
Preparation of Extracts and EOs From Plant Material
Hydroalcoholic extracts of superior (A, B, and C grade) and inferior quality (D grade) of the collected plant material (thyme, oregano, Greek mountain tea, and chamomile) were produced using the ultrasound assistant extraction (UAE), Elma S 100 H (Elmasonic, Singen, Germany), equipped with an ultrasonic frequency of 37 kHz. In each case, 5 g of each pulverized plant material were extracted with 200 ml of a hydroalcoholic mixture (H2O:MeOH 50:50) in 3 consecutive circles for 30 min at 35–40°C. Solvents were evaporated under reduced pressure (ca. 100 mbar) using rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) and percentage yield (w/w) for every extract was estimated. In total, 8 extracts were produced and subjected to further analysis.
The collected plant material (thyme, oregano, Greek mountain tea, and chamomile) was subjected to hydrodistillation to afford the respective EOs and aqueous extracts. For this reason, 100 g of aerial parts from the superior and inferior quality of plants were distilled using 1,000 ml water at 100°C for 3 h in a Clevenger apparatus. The percentage yield (v/w) of the produced EOs was estimated, they were dried over sodium sulfate anhydrous and stored at 4°C until they were analyzed. Furthermore, the remaining aqueous extracts were filtered, lyophilized to dryness (Zirbus Technology, Germany) and stored for further analysis.
Chemical Evaluation of Extracts and Essential Oils
HPTLC Analysis
The chemical profile of the obtained extracts was determined using an HPTLC system, purchased from Camag (CAMAG, Muttenz, Switzerland). Samples were applied on silica gel F254-precoated plates from Merck (Merck, Darmstadt, Germany) using an automated sample applicator ATS4 and the chromatograms were developed in an ADC2 automated development chamber with the appropriate mobile phase. The plates were documented under UV 254 and 366 nm and after spraying with sulfuric vanillin using the TLC Visualizer 2. The system was operating under the VisionCats 2.2 and WinCats 1.4.9 software. For the HPTLC fingerprinting of hydroalcoholic and aqueous extracts, 100 μg of each sample were loaded on a normal and reversed phase TLC plate and the solvent mixture EtOAC:MeOH:A.A (70:30:1) and H2O:ACN:A.A (80:20:1) were used as a mobile phase, respectively.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The identification of the chemical composition of the EOs was performed with an Agilent 7820A Gas Chromatograph System linked to an Agilent 5977B mass spectrometer system (Agilent Technologies, Santa Clara, CA, USA) equipped with a HP5-MS capillary column (30 m × 0.25 mm and 0.25 μm film thickness). The initial column temperature was 60°C and then increased at a rate of 3°C/min to a maximum temperature of 300°C, where it remained for 10 min. The total analysis time was 90 min. Helium was used as a carrier gas at a flow rate of 1.0 ml/min, split ratio 1:10, injector temperature 220°C, and ionization voltage 70 eV. The compound identification was conducted using the NIST14 and ADAMS 07 libraries, bibliographic data, and the comparison of the kovats (IK) and Adams indices. The Kovats indices compare the retention time of a product with a linear alkane of the same number of carbons and were determined by injecting a mixture of alkanes (standard C9–C30) under the same operating conditions. The chromatograms were processed with Agilent MSD ChemStation Data Analysis software.
Ultra-High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) Analysis
The ultra-high-performance liquid chromatography was performed employing a Vanquish UHPLC system (Thermo Scientific, Bremen, Germany) equipped with a binary pump, an autosampler, an online vacuum degasser, and a temperature-controlled column compartment. LC-MS grade methanol (MeOH) and formic acid (FA) were purchased from Fisher Scientific (Fisher Optima, Loughborough, UK) and LC-MS water was produced from a Barnstead MicroPure Water Purification System (Thermo Scientific, Bremen, Germany). An Accucore Vanquish UPLC C18 (2.1 mm × 50 mm, 1.5 μm) reversed phased column (Thermo Scientific, Bremen, Germany) was used for the analysis. The high-resolution mass spectrometry (HRMS) was performed on an Orbitrap Exactive Plus Mass Spectrometer (Thermo Scientific, Bremen, Germany).
Samples were prepared in duplicates and injected two times at a concentration of 100 ppm diluted in MeOH:H2O 50:50. The mobile phase consisted of solvents A: aqueous 0.1% (v/v) formic acid and B: acetonitrile. Different gradient elutions were performed for positive and negative ion mode detection and after optimization of the chromatography, the gradient applied was: T = 0 min, 5% B; T = 3 min, 5% B; T = 21 min, 95% B; T = 26 min, 95% B; T = 26.1 min, 5% B; and T = 30 min, 95% B. The flow rate was 0.3 ml/min and the injection volume was 5 μl. The column temperature was kept at 40°C while the sample tray temperature was set at 10°C. The ionization was performed at HESI, for both positive and negative modes. The conditions for the HRMS for both negative and positive ionization modes were set as follow: capillary temperature, 320°C; spray voltage, 2.7 kV; S-lens Rf level, 50 V; sheath gas flow, 40 arb. units; aux gas flow, 8 arb. units; aux. gas heater temperature, 50°C. The analysis was performed using the Fourier transform mass spectrometry mode (FTMS) in the full scan ion mode, applying a resolution of 70,000, while the acquisition of mass spectra was performed in every case using the centroid mode. The data dependent acquisition capability has been also used at 35,000 resolution, allowing for the tandem mass spectrometry (MS/MS) fragmentation of the three most intense ions of every peak exceeding the predefined threshold applying a 10 s dynamic exclusion. Normalized collision energy was set at 35. Data acquisition and analysis has been completed employing Xcalibur 2.1 and MZmine (24).
DPPH Free Radical Scavenging Assay
Evaluation of the free radical scavenging activity of the produced hydroalcoholic and aqueous extracts was performed using the free radical DPPH assay as described previously (25). Extracts were prepared using DMSO as a solvent in an initial concentration of 4 mg/ml (stock solution) and dilutions were made to reach the tested concentrations (200 and 100 μg/ml). Then, 10 μl of extract in DMSO and 190 μl of DPPH solution (12.4 mg/100 ml in ethanol) were mixed in a 96-well plate and then subsequently incubated, at room temperature, for 30 min in darkness. Finally, the absorbance was measured at 517 nm in a microplate reader (Tecan, Männedorf, Switzerland). All evaluations were performed in triplicates, gallic acid was used as positive control and the percentage inhibition of the DPPH radical was estimated by the following equation:
where A: Control (w/o sample), B: Blank (w/o sample, w/o DPPH), C: sample, D: Blank sample (w/o DPPH).
Total Phenolic Content (TPC) Determination
The phenolic content of the extracts was determined by using a Folin–Ciocalteu colorimetric method (26). Folin–Ciocalteu solution was prepared with 10% dilution in distilled water and the alkaline environment was achieved with the addition of 7.5% sodium carbonate in distilled water. Extracts were prepared using DMSO as a solvent in stock concentrations and dilutions were made if necessary. In 96 well plates, 25 μl of extract in DMSO, 125 μl Folin–Ciocalteu solution and 100 μl Na2CO3 solution were mixed. The plates were incubated for 30 min at ambient temperature in dark. Absorbance was measured at 765 nm, using a microplate reader (Tecan, Männedorf, Switzerland). The total phenolic content (TPC) of the extracts was determined by a standard curve of absorbance values derived from standard concentration solutions of gallic acid (GA, 1.25, 2.5, 5, 10, 20, 30, 40, 50, and 100 μg/ml final concentrations). TPC was expressed as milligram of gallic acid equivalent per gram of dried extract (mg GAE/g dry weight). Each sample was tested in triplicate.
Results
Determination of the Percentage of MAPs By-Products
Processing of over 10 tons of oregano reveal that only 46% of the produced product was commercially acceptable, while 54% was considered as by-product (Table 1). The respective percentage for chamomile, after the processing of 0.5 tons of dried plant material was 75 and 25% by-products, for Greek mountain tea after processing of ~0.9 tons was 65 and 35% by-product and for thyme after processing of nearly 0.3 tons- was 36 and 64% by-product (Table 1). In our effort to assess MAPs by-products as a potent source of bioactive ingredients, we proceeded to the preparation of extracts and EOs from the aerial parts of superior and inferior quality of the selected herbs; thyme, oregano, Greek mountain tea, and chamomile. To this direction, eight hydroalcoholic extracts using the superior and inferior quality of the selected herbs were produced, and their percentage extraction yields were compared (samples were prepared in triplicates). Based on the results (Table 2), in the case of Greek mountain tea (MT vs. MTW) and chamomile (CH vs. CHW), both extracts of commercial and non-commercial herbal materials were characterized by similar extraction yields, whereas in the case of thyme (THV vs. THVW) and oregano (ORV vs. ORVW), by-products' extracts were characterized by lower extraction yields.
Table 1
| Before processing | After processing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acceptable in market | Non-commercially acceptable | ||||||||
| Initial plant material | Lot No | Initial weight (kg) | A grade (kg) |
B grade
(kg) |
C grade
(kg) |
Total weight
(kg) |
Total per initial weight (%) | By-products (kg) | By- products per initial weight (%) |
| Thymus vulgaris | |||||||||
| Chopped plant | 8008-01 | 18.5 | 2.1 | 6.2 | 1.0 | 8.3 | 45% | 10.2 | 55% |
| Chopped plant | 1100-01 | 98.0 | 11.5 | 34.5 | 0.0 | 46.1 | 47% | 51.9 | 53% |
| Grated plant | 8049-01 | 164.0 | 0.0 | 0.0 | 24.6 | 24.6 | 15% | 139.4 | 85% |
| Total Thymus vulgaris | 280.5 | 13.6 | 40.8 | 25.6 | 79.0 | 36% | 201.5 | 64% | |
| Origanum vulgare subsp. hirtum | |||||||||
| Whole plant | 7969-1 | 300.0 | 58.8 | 58.8 | 29.4 | 147.0 | 49% | 153.0 | 51% |
| Whole plant | 7969-1 | 345.0 | 64.9 | 64.9 | 32.4 | 162.2 | 47% | 182.9 | 53% |
| Whole plant | 7969-1 | 300.0 | 58.8 | 58.8 | 29.4 | 147.0 | 49% | 153.0 | 51% |
| Whole plant | 7992-1 | 300.0 | 56.4 | 56.4 | 28.2 | 141.0 | 47% | 159.0 | 53% |
| Whole plant | 7992-1 | 309.0 | 60.6 | 60.6 | 30.3 | 151.4 | 49% | 157.6 | 51% |
| Whole plant | 8037-1 | 1,014.0 | 186.6 | 186.6 | 93.3 | 466.4 | 46% | 547.6 | 54% |
| Whole plant | 8014-01 | 694.0 | 136.0 | 136.0 | 68.0 | 340.1 | 49% | 353.9 | 51% |
| Whole plant | 8052-01 | 430.0 | 77.4 | 77.4 | 38.7 | 193.5 | 45% | 236.5 | 55% |
| Whole plant | 8037-01 | 1,230.0 | 241.1 | 241.1 | 120.5 | 602.7 | 49% | 627.3 | 51% |
| Chopped plant | 7976-1 | 650.0 | 96.2 | 96.2 | 48.1 | 240.5 | 37% | 409.5 | 63% |
| Chopped plant | 8044-01 | 371.0 | 56.4 | 56.4 | 28.2 | 141.0 | 38% | 230.0 | 62% |
| Chopped plant | 8092-1 | 1,020.0 | 183.6 | 183.6 | 91.8 | 459.0 | 45% | 561.0 | 55% |
| Chopped plant | 8121-01 | 17.0 | 3.0 | 3.0 | 1.5 | 7.5 | 44% | 9.5 | 56% |
| Chopped plant | 8135-01 | 650.0 | 101.4 | 101.4 | 50.7 | 253.5 | 39% | 396.5 | 61% |
| Chopped plant | 8139-01 | 1,224.0 | 220.3 | 220.3 | 110.2 | 550.8 | 45% | 673.2 | 55% |
| Stems | 8044-02 | 211.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0% | 211.0 | 100% |
| Stems | 8135-02 | 350.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0% | 350.0 | 100% |
| Grated plant | 8130-01 | 225.0 | 74.7 | 74.7 | 37.4 | 186.8 | 83% | 38.3 | 17% |
| Grated plant | 8129-01 | 630.0 | 199.1 | 199.1 | 99.5 | 497.7 | 79% | 132.3 | 21% |
| Grated plant | 8129-01 | 52.0 | 16.2 | 16.2 | 8.1 | 40.6 | 78% | 11.4 | 22% |
| Total Origanum vulgare subsp. hirtum | 10,322.0 | 1,891.4 | 1,891.4 | 945.7 | 4,728.5 | 46% | 5,593.5 | 54% | |
| Sideritis scardica | |||||||||
| Grated plant | 8139-04 | 60.0 | 44.4 | 44.4 | 74% | 15.6 | 26% | ||
| Whole plant | 8009-01 | 130.0 | 40.3 | 40.3 | 80.6 | 62% | 49.4 | 38% | |
| Whole plant | 8009-01 | 175.5 | 42.1 | 42.1 | 21.1 | 105.3 | 60% | 70.2 | 40% |
| Whole plant | 002-2018 | 514.0 | 119.8 | 204.0 | 323.8 | 63% | 190.2 | 37% | |
| Total Sideritis scardica | 879.5 | 202.2 | 286.4 | 65.5 | 554.1 | 65% | 325.4 | 35% | |
| Matricaria recutita | |||||||||
| Flower/stem | 7973-1 | 210.0 | 37.0 | 18.5 | 129.4 | 184.8 | 88% | 25.2 | 12% |
| Flower/stem | 7974-1 | 150.0 | 26.7 | 26.7 | 80.1 | 133.5 | 89% | 16.5 | 11% |
| Stem | 8030-01 | 80.0 | 0.0 | 0% | 80.0 | 100% | |||
| Flower/stem | 8113-01 | 40.0 | 9.1 | 5.5 | 21.8 | 36.4 | 91% | 3.6 | 9% |
| Flower/stem | 8121-02 | 10.0 | 1.3 | 1.5 | 6.4 | 9.2 | 92% | 0.8 | 8% |
| Flower/stem | 8139-03 | 50.0 | 6.2 | 7.0 | 30.8 | 44.0 | 88% | 6.0 | 12% |
| Total Matricaria recutita | 540.0 | 80.2 | 59.2 | 268.5 | 407.9 | 75% | 132.1 | 25% | |
Percentages of by-products derived from the cultivated Greek medicinal and aromatic plants' (MAPs) processing of different lot (between 2018 and 2000 years).
Table 2
| Plant species | Plant material | Code | % extraction yield (w/w) |
|---|---|---|---|
|
Thymus vulgaris
(thyme) |
Superior | THV | 28.0 |
| Inferior | THVW | 18.6 | |
|
Origanum vulgare subsp. hirtum (oregano) |
Superior | ORV | 30.6 |
| Inferior | ORVW | 4.10 | |
|
Sideritis scardica
(Greek mountain tea) |
Superior | MT | 11.8 |
| Inferior | MTW | 10.8 | |
|
Matricaria recutita
(chamomile) |
Superior | CH | 21.8 |
| Inferior | CHW | 21.2 |
Percentage extraction yields of hydroalcoholic extracts deriving from superior and inferior plant material.
Chemical Investigation and Free Radical Scavenging Activity of MAPs By-Products Extracts
Chemical Investigation of Hydroalcoholic Extracts
The chemical profile of hydroalcoholic extracts was investigated using HPTLC and LC-MS techniques. HPTLC chromatograms (Figure 1) revealed the presence of similar secondary metabolites in the extracts obtained from both superior and inferior raw materials for all the studied herbs. In the case of chamomile (CH vs. CHW) and Greek mountain tea (MT vs. MTW), flavonoids were detected (absorbance at 254 nm and orange/pink spots after spraying with vanillin-sulfuric acid solution and heating), phenylpropanoids (light absorbance at 254 nm and gray spots after spraying and heating), as well as sugars (no absorbance, dark gray spots after spraying and heating, increased polarity). Thyme (THV vs. THVW) and oregano extracts (ORV vs. ORVW) were mostly characterized by the presence of terpenoids (no absorbance under UV light at 254 nm and blue-purple spots after spraying and heating), flavonoids and sugars (27). The chemical content of MAP extracts as determined by LC-MS analyses is shown in Table 3. Identification of compounds was based on LC-MS data compared with the data from literature (28) and particularly, for thyme (29, 30), oregano (31, 32), Greek mountain tea (33), and chamomile (34, 35), as well as from databases, such as Dictionary of Natural Products and GNPS Public Spectral Libraries (36). Overall, the majority of identified compounds of the hydroalcoholic extracts of superior quality material were present in lower amounts or in traces in the inferior plant extracts as shown in Table 3. In particular, 29 compounds were tentatively identified for thyme, 40 for oregano, 28 for Greek mountain tea, and 31 for chamomile. The presence of phenolic acids as well as mono and disaccharides of flavonoids in the hydroalcoholic extracts of all plant species comes in agreement with literature data.
Figure 1
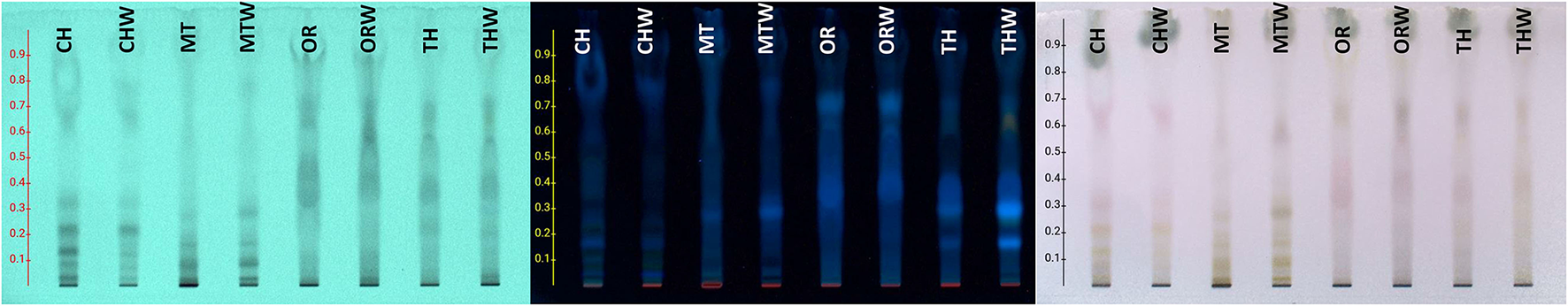
High-performance thin layer chromatography (HPTLC) of hydroalcoholic extracts of chamomile (CH, superior plant material; CHW, inferior plant material), Greek mountain tea (MT, superior plant material; MTW, inferior plant material), oregano (ORV, superior plant material; ORVW, inferior plant material), and thyme (THV, superior plant material; THVW, inferior plant material), on a reversed phase TLC plate and H2O:ACN:A.A: (80:20:1) as a mobile phase.
Table 3
| No. | Rt (min) | m/z | M. Formula | Ion mode | MS/MS fragments | Tentative identification | Hydroalcoholic extracts of inferior plant material |
|---|---|---|---|---|---|---|---|
| Thymus vulgaris (thyme) | |||||||
| 1 | 0.41 | 341.1092 | C12H22O11 | [M-H]− | 89, 59 | bis-hexoses | y* |
| 2 | 0.50 | 191.0190 | C6H8O7 | [M-H]− | 111 | Citric acid | y |
| 3 | 0.65 | 359.0989 | C15H20O10 | [M-H]− | 197, 179 | methoxy hydroxyphenylglycol glucorinide | tr |
| 4 | 3.2 | 305.0704 | C15H14O7 | [M-H]− | 225, 97 | gallocatechin | y |
| 5 | 4.71 | 387.1667 | C18H28O9 | [M-H]− | 207 | hydroxyjasmonic acid hexoside | y |
| 6 | 5.36 | 593.1522 | C27H30O15 | [M-H]− | 353, 383, 473 | apigenin-6,8-di-C-hexoside | y |
| 7 | 6.03 | 447.0939 | C21H20O11 | [M-H]− | 285 | luteolin hexoside | y |
| 8 | 6.15 | 355.1039 | C16H20O9 | [M-H]− | 193 | ferulic acid hexoside | tr |
| 9 | 6.25 | 463.0887 | C21H20O12 | [M-H]− | 300, 285 | quercetin hexoside | y |
| 10 | 6.3 | 595.1679 | C27H32O15 | [M-H]− | 151, 287, 135 | eriodictyol disaccharide | tr |
| 11 | 6.41 | 461.0733 | C21H18O12 | [M-H]− | 285, 300 | luteolin glucuronide | y |
| 12 | 6.45 | 447.0939 | C21H20O11 | [M-H]− | 285 | luteolin hexoside | y |
| 13 | 6.65 | 521.1306 | C24H26O13 | [M-H]− | 323, 161, 179 | rosmarinic acid hexoside | y |
| 14 | 6.82 | 193.0500 | C10H10O4 | [M-H]− | 161, 137 | ferulic acid | y |
| 15 | 6.88 | 471.1876 | C22H32O11 | [M-H]− | 165, 99, | Unidentified | y |
| 16 | 6.90 | 553.0997 | C27H22O13 | [M-H]− | 135, 161, 179 | Caffeoyl feruloylquinic acid | y |
| 17 | 6.93 | 445.0778 | C21H18O11 | [M-H]− | 269 | apigenin glucuronide | y |
| 18 | 7.14 | 359.0779 | C18H16O8 | [M-H]− | 161, 197 | rosmarinic acid | y |
| 19 | 7.2 | 555.1151 | C27H24O13 | [M-H]− | 161, 135, 197 | salvianolic acid derivative (K) | y |
| 20 | 7.26 | 493.1149 | C26H22O10 | [M-H]− | 161, 135, 197 | salvianolic acid derivative (A) | y |
| 21 | 7.34 | 371.1352 | C17H24O9 | [M-H]− | 191 | sinapyl alcohol monosaccharide derivative (syringin) | tr |
| 22 | 7.36 | 717.1482 | C36H30O16 | [M-H]− | 357, 283, 339 | salvianolic acid derivative (B) | y |
| 23 | 7.44 | 315.0515 | C16H12O7 | [M-H]− | 300 | tetrahydroxy-7-methoxyflavone | y |
| 24 | 7.50 | 537.1047 | C27H22O12 | [M-H]− | 197, 239, 137 | salvianolic acid derivative (H, I) | y |
| 25 | 7.93 | 373.0934 | C19H18O8 | [M-H]− | 135, 175, 197, 160 | rosmarinic acid methylester | y |
| 26 | 7.98 | 285.0409 | C15H10O6 | [M-H]− | kaempferol/luteolin | y | |
| 27 | 8.18 | 299.0564 | C16H12O6 | [M-H]− | 284 | methyl kaempferol/ methyl luteolin | y |
| 28 | 8.65 | 271.0617 | C15H12O5 | [M-H]− | 151, 119 | naringenin | y |
| 29 | 9.25 | 327.2180 | C18H32O5 | [M-H]− | 211, 229, 183 | trimethoxy hydroxyflavone | y |
| Origanum vulgare subsp. hirtum (oregano) | |||||||
| 30 | 0.41 | 179.0553 | C6H12O6 | [M-H]− | 75 | hexoses | y |
| 31 | 0.46 | 341.1092 | C12H22O11 | [M-H]− | 89, 59 | bis-hexose | y |
| 32 | 0.42 | 191.0554 | C7H12O6 | [M-H]− | 111 | quinic acid | y |
| 33 | 0.52 | 191.019 | C6H8O7 | [M-H]− | 111 | Citric acid | y |
| 34 | 0.77 | 197.0449 | C9H10O5 | [M-H]− | 72, 135 | syringic acid | y |
| 35 | 4.82 | 387.1633 | C18H28O9 | [M-H]− | 207, 163 | hydroxyjasmonic acid hexoside | y |
| 36 | 4.91 | 609.0897 | C27H30O16 | [M-H]− | 369, 399, 489 | luteolin disaccharide | y |
| 37 | 5.3 | 593.1521 | C27H30O15 | [M-H]− | 353, 383, 474 | apigenin disaccharide | y |
| 38 | 5.55 | 637.1058 | C27H26O18 | [M-H]− | 285, 113, 351 | luteolin diglucuronide | y |
| 39 | 5.78 | 447.094 | C21H20O11 | [M-H]− | 327, 357, 285 | luteoline hexoside | y |
| 40 | 6.01 | 621.1106 | C27H26O17 | [M-H]− | 113, 269, 285. 193 | apigenin diglucuronide | y |
| 41 | 6.22 | 431.0988 | C21H20O10 | [M-H]− | 311, 283 | apigenin hexoside | y |
| 42 | 6.3 | 799.1382 | C36H32O21 | [M-H]− | 285, 179, 135, 351 | unidentified | y |
| 43 | 6.38 | 461.0733 | C21H18O12 | [M-H]− | 285, 300 | luteolin glucuronide | y |
| 44 | 6.49 | 715.1321 | C36H28O16 | [M-H]− | 321, 295, 339 | unidentified | y |
| 45 | 6.66 | 537.1046 | C27H22O12 | [M-H]− | 295, 399 | salvianolic acid derivative (J/I/H) | y |
| 46 | 6.69 | 717.1475 | C36H30O16 | [M-H]− | 339, 243, 135 | salvianolic acid derivative (E/B) | y |
| 47 | 6.89 | 719.1634 | C36H32O16 | [M-H]− | 169, 197 | salvianolic acid derivative | y |
| 48 | 6.93 | 445.0782 | C21H18O11 | [M-H]− | apigenin glucuronide | y | |
| 49 | 7.01 | 717.147 | C36H30O16 | [M-H]− | 313, 295, 321 | salvianolic acid derivative (E/B) | y |
| 50 | 7.1 | 359.0773 | C18H16O8 | [M-H]− | 161, 197 | rosmarinic acid isomer | y |
| 51 | 7.2 | 493.1149 | C26H22O10 | [M-H]− | 109, 185, 295 | salvianolic acid derivative (A) | y |
| 52 | 7.28 | 537.1046 | C27H22O12 | [M-H]− | 321, 295, 339 | salvianolic acid derivative (J/I/H) | y |
| 53 | 7.57 | 717.1475 | C36H30O16 | [M-H]− | 321, 295 | salvianolic acid derivative (E/B) | y |
| 54 | 7.73 | 343.0827 | C18H16O7 | [M-H]− | 145, 197 | dihydroxy trimethoxy flavone | y |
| 55 | 7.79 | 287.0565 | C15H12O6 | [M-H]− | 151 | eriodictyol | y |
| 56 | 7.89 | 491.0991 | C26H20O10 | [M-H]− | 311 | salvianolic acid derivative (C) | y |
| 57 | 7.95 | 285.0409 | C15H10O6 | [M-H]− | 151 | luteolin | y |
| 58 | 8.05 | 717.1477 | C36H30O16 | [M-H]− | 339, 321 | salvianolic acid derivative (iso E/B) | y |
| 59 | 8.13 | 299.0564 | C16H12O6 | [M-H]− | 284 | trihydroxy methoxy flavone (methyl kaempferol) | y |
| 60 | 8.19 | 493.1148 | C26H22O10 | [M-H]− | 109, 295, 185 | salvianolic acid derivative (A) | y |
| 61 | 8.39 | 329.0671 | C17H14O7 | [M-H]− | 314, 299 | trihydroxy dimethoxy flavone | y |
| 62 | 8.49 | 373.0933 | C19H18O8 | [M-H]− | 135, 175, 197 | rosmarinic acid methyl ester | y |
| 63 | 8.64 | 271.0615 | C15H12O5 | [M-H]− | 151 | trihydroxyflavanone (naringenin) | y |
| 64 | 8.67 | 329.0671 | C17H14O7 | [M-H]− | 299, 314 | trihydroxy dimethoxy flavone | y |
| 65 | 8.72 | 269.046 | C15H10O5 | [M-H]− | 151, 119 | apigenin | y |
| 66 | 8.74 | 717.1478 | C36H30O16 | [M-H]− | 339, 311, 353 | salvianolic acid derivative | y |
| 67 | 8.93 | 359.0778 | C18H16O8 | [M-H]− | 329, 344 | jaseidin isomer | y |
| 68 | 9.25 | 327.218 | C18H32O5 | [M-H]− | 211, 229 | trihydroxy octadecadienoic acid | y |
| 69 | 9.96 | 313.0722 | C17H14O6 | [M-H]− | 283, 298 | dimethoxy dihydroxyflavone | y |
| Sideritis scardica (Greek mountain tea) | |||||||
| 70 | 0.3 | 162.0528 | C6H10O5 | [M-H]− | polysacharides residues | y | |
| 71 | 0.41 | 341.1089 | C12H22O11 | [M-H]− | 89, 59 | bis-hexose | y |
| 72 | 0.45 | 191.0552 | C7H12O6 | [M-H]− | 111 | quinic acid | y |
| 73 | 1.8 | 353.0882 | C16H18O9 | [M-H]− | 191 | Caffeoylquinic acid derivative | y |
| 74 | 5.33 | 435.1512 | C16H24O10 | [M+Hac-H] | 341, 321 | fatty acyl dissaccharide | y |
| 75 | 6.19 | 625.1414 | C27H30O17 | [M-H]− | 301, 445 | hypolaetin disaccharide | y |
| 76 | 6.29 | 785.2523 | C35H46O20 | [M-H]− | 179, 161 | phenylethanoid trisaccharide (echinacoside) | y |
| 77 | 6.31 | 521.2034 | C26H34O11 | [M-H]− | 329 | dihydrodehydrodiconiferyl alcohol hexoside | y |
| 78 | 6.4 | 667.1528 | C29H32O18 | [M-H]− | 301, 139 | hypolaetin acetyl-hexoside | y |
| 79 | 6.46 | 755.2416 | C34H44O19 | [M-H]− | 161, 461 | phenylethanoid trisaccharide (lavandulifolioside) | y |
| 80 | 6.55 | 623.1955 | C29H36O15 | [M-H]− | 161, 113, 461 | phenylethanoid disaccharide (verbascoside isomer) | y |
| 81 | 6.63 | 609.1471 | C27H30O16 | [M-H]− | 447 | isoscutellarein disaccharide | y |
| 82 | 6.75 | 623.1991 | C29H36O15 | [M-H]− | 161, 299, 284 | phenylethanoid disaccharide (verbascoside isomer) | y |
| 83 | 6.87 | 431.0983 | C21H20O10 | [M-H]− | 268 | apigenin hexoside | y |
| 84 | 6.91 | 641.1722 | C28H32O17 | [?+?]+ | 317 | tetrahydroxy flavone dissacharide | y |
| 85 | 6.98 | 769.2564 | C35H46O19 | [M-H]− | 161, 175 | phenylethanoid disaccharide (sideritiside) | y |
| 86 | 7.12 | 637.2148 | C30H38O15 | [M-H]− | 175, 160 | phenylethanoid disaccharide (leucoseptoside) | y |
| 87 | 7.32 | 651.1579 | C29H32O17 | [M-H]− | 285 | isoscutellarein acetyl dissacharide | y |
| 88 | 7.54 | 681.1683 | C30H34O18 | [M-H]− | 315, 300 | methyl hupolaetin acetyl dissacharide | y |
| 89 | 7.83 | 623.1627 | C28H32O16 | [M-H]− | 299, 284, 161 | methyl isoscutellarein dissacharide | y |
| 90 | 8.57 | 577.1359 | C30H26O12 | [M-H]− | 269 | apigenin coumaroyl hexoside | y |
| 91 | 8.6 | 665.1735 | C30H34O17 | [M-H]− | 299 | methyl isoscutellarein acetyl dissacharide | y |
| 92 | 8.65 | 269.0459 | C15H10O5 | [M-H]− | 151 | apigenin | y |
| 93 | 8.77 | 723.1796 | C32H36O19 | [M-H]− | 315, 300 | methyl hupolaetin diacetyl dissacharide | y |
| 94 | 9.1 | 577.1359 | C30H26O12 | [M-H]− | 269 | apigenin coumaroyl hexoside | y |
| 95 | 9.79 | 707.1841 | C32H36O18 | [M-H]− | 299, 284 | methyl isoscutellarein diacetyl dissacharide | y |
| 96 | 10.55 | 343.0822 | C18H16O7 | [M-H]− | 313, 328 | dihydroxy trimethoxyflavone | y |
| 97 | 11.76 | 395.2442 | C22H35O6 | [M-H]− | 165, 90 | sideripullol derivative | |
| Matricaria recutita (chamomile) | |||||||
| 98 | 0.41 | 179.0553 | C6H12O6 | [M-H]− | 75 | hexoses | y |
| 99 | 0.48 | 191.019 | C6H8O7 | [M-H]− | 111 | Citric acid | y |
| 100 | 0.50 | 341.1092 | C12H22O11 | [M-H]− | 89, 59 | bis-hexoses | y |
| 101 | 2.01 | 353.0881 | C16H18O9 | [M-H]− | 191 | Chlorogenic acid | y |
| 102 | 4.38 | 355.1039 | C16H20O9 | [M-H]− | 149, 193 | hydroxy methoxycinnamic acid hexoside isomer 1 | y |
| 103 | 5.45 | 323.0776 | C15H16O8 | [M-H]− | 121 | umbellliferone hexoside | y |
| 104 | 5.48 | 367.1036 | C17H20O9 | [M-H]− | 93, 173 | feruloylquinic acid | y |
| 105 | 5.55 | 479.0837 | C21H20O10 | [M-H]− | 317, 165 | myricetin hexoside | y |
| 106 | 5.75 | 337.0933 | C16H18O8 | [M-H]− | 87, 219, 201 | daphnetin hexoside | y |
| 107 | 5.80 | 463.0889 | C21H20O12 | [M-H]− | 301, 151 | quercetin hexoside 1 | y |
| 108 | 5.92 | 355.1039 | C16H20O9 | [M-H]− | 149, 193 | hydroxy methoxycinnamic acid hexoside isomer 2 | y |
| 109 | 6.20 | 463.0885 | C21H20O12 | [M-H]− | 301, 151 | quercetin hexoside 2 | y |
| 110 | 6.30 | 593.1521 | C27H30O15 | [M-H]− | 285 | luteolin disaccharide | y |
| 111 | 6.37 | 447.0938 | C21H20O11 | [M-H]− | 285 | luteolin hexoside | y |
| 112 | 6.49 | 493.0994 | C22H22O13 | [M-H]− | 331, 168, 316 | patuletin hexoside | y |
| 113 | 6.65 | 515.1199 | C25H24O12 | [M-H]− | 191, 179 | dicaffeylquinic acid derivative | y |
| 114 | 6.75 | 515.1198 | C25H24O12 | [M-H]− | 191, 179 | dicaffeylquinic acid derivative | y |
| 115 | 6.79 | 577.1563 | C27H30O15 | [M-H]− | 269 | apigenin disaccharide | y |
| 116 | 6.92 | 431.0989 | C21H20O10 | [M-H]− | 268 | apigenin hexoside | y |
| 117 | 7.09 | 479.1189 | C22H22O12 | [?+?]+ | 317 | isorhamnetin hexoside | y |
| 118 | 7.09 | 515.1198 | C25H24O12 | [M-H]− | 173, 179, 191 | dicaffeylquinic acid derivative | y |
| 119 | 7.52 | 517.1357 | C24H22O13 | [M-H]− | 271 | apigenin malonylhexoside | tr |
| 120 | 7.61 | 473.1094 | C23H22O11 | [M-H]− | 286 | apigenin acetylhexoside | tr |
| 121 | 7.74 | 473.1096 | C23H22O11 | [M-H]− | 286 | apigenin acetylhexoside | tr |
| 122 | 8.00 | 593.1313 | C30H26O13 | [M-H]− | 269 | apigenin caffeylhexoside | tr |
| 123 | 8.06 | 517.1356 | C25H26O12 | [M-H]− | 269 | apigenin malonylhexoside | tr |
| 124 | 8.27 | 473.1088 | C23H22O11 | [M-H]− | 473, 268 | apigenin acetyl- malonyl- hexoside | tr |
| 125 | 8.39 | 515.1200 | C25H24O12 | [M-H]− | 173, 179, 191 | dicaffeylquinic acid derivative | y |
| 126 | 8.68 | 269.0458 | C15H10O5 | [M-H]− | 151 | trihydroxyflavone | tr |
| 127 | 9.54 | 305.1385 | C17H20O5 | [M+H]+ | 245 | sesquiterpene lactone (matricarin) | tr |
| 128 | 10.26 | 373.0928 | C19H18O8 | [M-H]− | dihydroxy tetramethoxyflavone | y | |
Liquid chromatography-mass spectrometry (LC-MS) based characterization of Thymus vulgaris (thyme), Origanum vulgare subsp. hirtum (oregano), Sideritis scardica (Greek mountain tea), and Matricaria recutita (chamomile) extracts obtained from the plant material of superior and inferior quality.
y = presence of compound in the inferior plant material extracts, tr = traces of the identified compound in the inferior plant material extracts.
Annotation table was constructed based on compounds present in superior plant material extracts.
Evaluation of TPC and Free Radical Scavenging Activity of Hydroalcoholic Extracts
The TPC of plant extracts, measured by Folin–Ciocalteu method ranged from 20.3 to 177.2 mg GAE/g dry weight (Table 4) with the highest phenolic content found in thyme and oregano extracts. More specifically in the case of thyme, both the categories of plant material were characterized by similar levels of phenols (THV:177.2 mg GAE/g dw, and THW:166.4 mg GAE/g dw), followed by oregano extracts where no statistically significant differences were detected (ORV:160.1 mg GAE/g dw, and ORVW:143.8 mg GAE/g dw). The phenolic content of Greek mountain tea extracts ranged from 58 (MT) to 68.3 (MTW) mg GAE/g dw, while chamomile was characterized by the lowest phenolic content (<35 mg GAE/g dw) for both superior (CH) and inferior (CHW) plant material.
Table 4
| Plant species | Plant material | Code | % DPPH inhibition | TPC | |
|---|---|---|---|---|---|
| 200 μg/mL | 100 μg/mL | mg GAE/g dry weight | |||
| Thymus vulgaris (thyme) | Superior | THV | 85.9 ± 0.6 | 66.6 ± 0.8 | 177.2 ± 8.2 |
| Inferior | THVW | 82.7 ± 0.7 | 78.6 ± 2.7 | 166.4 ± 4.7 | |
| Origanum vulgare subsp. hirtum (oregano) | Superior | ORV | 91.5 ± 0.1 | 87.1 ± 0.2 | 160.1 ± 8.0 |
| Inferior | ORVW | 78.1 ± 1.0 | 78.1 ± 1.0 | 143.8 ± 7.0 | |
| Sideritis scardica (Greek mountain tea) | Superior | MT | 74.8 ± 5.9 | 40.9 ± 3.3 | 68.3 ± 8.4 |
| Inferior | MTW | 58.2 ± 2.4 | 29.0 ± 0.3 | 58.0 ± 2.0 | |
| Matricaria recutita (chamomile) | Superior | CH | 66.6 ± 1.0 | 38.2 ± 5.0 | 34.9 ± 2.9 |
| Inferior | CHW | 28.3 ± 1.6 | 13.1 ± 0.8 | 20.3 ± 3.1 | |
Total phenolic content (TPC) and antioxidant capacity of hydroalcoholic extracts.
The DPPH inhibition at 200 μg/ml final concentration was stronger for oregano (78.1% ORVW−91.5% ORV) and thyme (82.7% THVW−85.9% THV), with minor differences detected among the different plant material (Table 4). Greek mountain tea extract was characterized as a potent antioxidant factor (37), in comparison with literature (10), at 200 μg/ml (MT:74.8% inhibition) whereas its by-product extract, revealed slightly reduced activity at the same concentration (MTW: 58.2% inhibition). Finally, chamomile extracts, exhibited moderate antioxidant activity. It is noteworthy, that the comparison between DPPH and TPC methods, revealed strong correlations (r 0.8177, p < 0.013) between phenolic content and scavenging properties in all tested extracts.
Based on the above results, the primary evaluation of oregano, thyme, Greek mountain tea, and chamomile by-products revealed similar chemical content to the superior herbal material and strong free radical scavenging capacity related to a high phenolic content. Therefore, since they constitute a very promising source of bioactive compounds, further investigation regarding their potent exploitation was followed.
Chemical Investigation and Evaluation of EOs and Aqueous Extracts
Taking into consideration the main use of thyme and oregano for culinary purposes, their EOs were produced via hydrodistillation to evaluate the volatile content of the herbs. As expected, oregano afforded the best yield in EO production (ORV_HDEO: 4%, ORVW_HDEO: 0.8 %) followed by thyme (THV_HDEO: 1.2%, THVW_HDEO: 0.15%). Results are shown in Table 5. It is worth noticing that in both cases the by-products afforded even a small percentage of EO. In the case of chamomile only superior quality (CH_HDEO) afforded 0.4% EO, whereas Greek mountain tea did not produce EO at all. However, considering the wide use of Greek mountain tea and chamomile as infusions, the remaining aqueous extracts of superior (MT_HDAQ and CH_HDAQ) and inferior (MTW_HDAQ and CHW_HDAQ) qualities from the hydrodistillation process were selected for further evaluation, regarding their chemical content and free radical scavenging activity.
Table 5
| Plant species | Plant material | Code | % EO yield | Code | % extraction yield (v/w) |
|---|---|---|---|---|---|
| Thymus vulgaris (thyme) | Superior | THV_HDEO | 1.2 | 15.3 | |
| Inferior | THVW_HDEO | 0.15 | 6.0 | ||
| Origanum vulgare subsp. hirtum (oregano) | Superior | ORV_HDEO | 4.0 | 6.1 | |
| Inferior | ORVW_HDEO | 0.8 | 5.6 | ||
| Sideritis scardica (Greek mountain tea) | Superior | - | MT_HDAQ | 13.7 | |
| Inferior | - | MTW_HDAQ | 13.5 | ||
| Matricaria recutita (chamomile) | Superior | CH_HDEO | 0.4 | CH_HDAQ | 12.1 |
| Inferior | - | CHVW_HDAQ | 12.6 |
Percentage yields of essential oil and aqueous extracts deriving from superior and inferior plant material.
GC-MS Analysis of EO of Thyme and Oregano
Based on the results of GC-MS analyses, in total 20 constituents were identified in oregano superior plant material (ORV_HDEO) representing 99.98% of the total content (Table 6), 14 of which were detected in the inferior quality as well (ORVW_HDEO). The major constituents of oregano EO were carvacrol, thymol, p-cymene, and γ-terpinene which were detected in both plant materials in corresponding amounts. Especially in the case of carvacrol—which was found to be the predominant constituent—and p-cymene, their percentage in the inferior plant material were slightly higher (78.20 and 6.68%, respectively) compared with the superior plant material (64.78 and 4.29%, respectively). Other compounds present in oregano by-product were: δ-2-carene, β-myrcene, terpinen-4-ol, trans-caryophyllene, borneol, caryophyllene oxide, β-phellandrene, and trans-sabinene hydrate. Surprisingly, eugenol was detected only in the by-product in a percentage of 0.21%.
Table 6
| Origanum vulgare subsp. hirtum | Thymus vulgaris | ||||
|---|---|---|---|---|---|
| Superior (ORV_HDEO) | Inferior (ORVW_HDEO) | Superior (THV_HDEO) | Inferior (THVW_HDEO) | ||
| KI | Constituents | Area % | |||
| 952 | α-pinene | 0.40 | - | 0.90 | - |
| 962 | camphene | 0.09 | - | 0.77 | - |
| 981 | 1-octen-3-ol | 0.25 | - | 0.60 | - |
| 992 | β-myrcene | 0.57 | 0.65 | 0.34 | 0.44 |
| 1003 | α-phellandrene | 0.09 | 0.10 | - | - |
| 1014 | δ-2-carene | 0.71 | 0.72 | 0.79 | 0.68 |
| 1022 | p-cymene | 4.29 | 6.68 | 22.50 | 21.88 |
| 1025 | limonene | - | - | 0.33 | 0.21 |
| 1025 | β-phellandrene | 0.23 | 0.28 | - | - |
| 1027 | 1,8-cineol | - | - | 0.61 | 0.43 |
| 1055 | γ-terpinene | 4.00 | 2.13 | 4.43 | 2.00 |
| 1070 | trans-sabinene hydrate | 0.22 | 0.17 | 0.15 | 0.16 |
| 1088 | terpinolene | - | - | 0.15 | 0.12 |
| 1099 | linalool | 0.13 | - | 1.30 | 1.62 |
| 1138 | camphor | - | - | 0.51 | 1.73 |
| 1160 | borneol | 0.39 | 0.30 | 1.71 | 1.69 |
| 1173 | terpinen-4-ol | 0.47 | 0.61 | 0.66 | 0.72 |
| 1186 | α-terpineol | - | - | 0.22 | 0.23 |
| thymol methyl ether | - | - | 1.23 | 1.36 | |
| 1239 | carvacrol methyl ether | 0.27 | - | 0.84 | 0.40 |
| 1291 | thymol | 20.14 | 7.19 | 53.04 | 56.50 |
| 1306 | carvacrol | 64.78 | 78.20 | 5.15 | 7.25 |
| 1351 | eugenol | - | 0.21 | 0.12 | 0.09 |
| 1412 | trans-caryophyllene | 1,26 | 0.80 | 1.07 | 0.86 |
| 1446 | α-humulene | 0.16 | 0.10 | - | - |
| 1471 | Trans-muurola-3,5-diene | - | - | 0.09 | - |
| 1472 | Geranyl propanoate | - | - | 0.12 | - |
| 1505 | β-bisabolene | 1.19 | - | - | - |
| 1507 | γ-cadinene | - | - | 0.18 | - |
| 1573 | caryophyllene oxide | 0.34 | 0.43 | 1.33 | - |
| Total % | 99.98 | 98.57 | 99.14 | 98.37 | |
Chemical composition of the essential oils (EOs) of superior and inferior plant material of T. vulgaris (thyme) and O. vulgare subsp. hirtum (oregano).
Oregano (ORV_HDEO, superior plant material; ORVW_HDEO, inferior plant material) and thyme (THV_HDEO, superior plant material; THVW_HDEO, inferior plant material).
Correspondingly, in the case of the chromatographic analyses of thyme EO, 26 constituents were identified in thyme superior plant material (THV_HDEO) representing 99.14% of the total content (Table 6), 19 of which were detected in the inferior quality as well (THVW_HDEO). The major constituents detected were thymol (>50%), p-cymene (>20%), carvacrol (5–7%), γ-terpinene (2.0–4.4%), linalool (1.3–1.6%), and borneol (1.7%), and were present in both plant materials in similar amounts. In this case, thymol was found to be the predominant constituent of thyme followed by p-cymene, with slightly higher percentages detected in the inferior plant material compared with the superior, as depicted in Table 6. Other common constituents were carvacrol methyl ether, thymol methyl ether, trans-caryophyllene, β-myrcene, limonene, δ-2-carene, 1,8-cineol, and camphor, terpinen-4-ol. On the other hand, α-pinene, camphene and 1-octen-3-ol were only detected in the inferior quality of both studied herbs.
The remaining aqueous extracts from the hydrodistillation process of Greek mountain tea (MTW_HDAQ) and chamomile (CHW_HDAQ) by-products, were chemically investigated using HPTLC and LC-MS techniques. The aqueous extracts of superior and inferior plant material were lyophilized and no significant differences were noted regarding their percentage extraction yield (Table 5). Their chemical profile was investigated using HPTLC and LC-MS techniques. The results revealed that all by-products extracts showed identical chemical profile compared with the superior quality extracts, characterized by the presence of phenolic compounds, flavonoids, and sugars. Analysis by LC-MS confirmed the similar profile of aqueous extracts of superior and by-products material. However, their chemical content was not as rich as the hydroalcoholic ones. In particular, Greek mountain tea (MT_HDAQ, MTW_HDAQ) was rich in phenylethanoid disaccharides and more specifically compounds 74, 79, 80, 82, 83, and 86–91 (Table 3) were detected, while in the case of chamomile (CH_HDAQ, CHW_HDAQ) cinnamic acid, caffeoylquinic acid derivatives were present along with some flavone and flavonol derivatives; compounds 102, 105, 108, 116–118, 124, and 125 were detected as shown in Table 3.
Evaluation of TPC and Free Radical Scavenging Activity
All extracts were characterized by the similar levels of phenols ranging from 12.4 to 21.5 mg GAE/g dw while not statistically significant differences (p > 0.3260 for Greek mountain tea and p > 0.2655 for chamomile) were detected between superior and inferior plant material in both cases (Table 7). Regarding the free radical scavenging activity, Greek mountain tea extract was characterized as a moderate antioxidant factor at 200 g/ml concentration (MT_HDAQ: 31.4% inhibition) whereas its by-product extract, revealed slightly increased activity at the same concentration (MTW_HDAQ: 55.3% inhibition). Finally, chamomile extracts (CH_HDAQ and CHW_HDAQ) exhibited low antioxidant activity. The reduced free radical scavenging activity and the lower phenolic content exhibited by the aqueous extracts compared with the hydroalcoholic ones, are attributed to the lower chemical profile as described above.
Table 7
| Plant species | Plant material | Code | % DPPH Inhibition 200 μg/mL |
TPC
mg GAE/g dry weight |
|---|---|---|---|---|
| Sideritis scardica (Greek mountain tea) | Superior | MT_HDAQ | 31.4 ± 2.5 | 14.8 ± 0.5 |
| Inferior | MTW_HDAQ | 55.3 ± 0.6 | 21.5 ± 1.8 | |
| Matricaria recuita (chamomile) | Superior | CH_HDAQ | 12.94 ± 1.4 | 14.4 ± 0.8 |
| Inferior | CHW_HDAQ | 7.5 ± 1.2 | 12.3 ± 0.3 |
The TPC and antioxidant capacity of hydroalcoholic extracts.
Discussion
The goal of this research was to compare the chemical content and antioxidant activity, as well as to value the potent exploitation of the current post-harvest processing by-products toward the development of innovative “food products.” The presence of phenolic acids, as well as mono- and disaccharides of flavonoids in the hydroalcoholic and aqueous extracts of all plant species comes in agreement with literature data. Moreover, evidence of the presence of these substances in the respective by-products extracts, justifies the high TPC and free scavenging activity as determined by the DPPH assay. Hence, inferior plant material not intended for the market could be utilized for the production of instant beverages. In addition, the hydroalcoholic extracts of inferior plant material and aqueous extracts remaining after hydrodistillation can serve as a source of bioactive ingredients to fortify food products and supplements. The EOs of aromatic plants, especially from thyme and oregano, are used as food additives due to their antibacterial properties. In this study, it is evident that the presence of thymol and carvacrol in the EOs of by-products, known for their antimicrobial activity (38). Hence, thyme and oregano by-products could be exploited as food antimicrobial additives, due to their potential bacteriostatic activity. Moreover, the infusion of oils with aromatic plants has proven to increase their oxidative stability and shelf-life (39). Hence, the presence of terpenes and other volatile constituents in the studied inferior plant material could be further exploited for the production of enriched aromatic edible oils and especially of functional olive oils.
In conclusion, taking into consideration all the aforementioned results, it is obvious that the non-commercially acceptable plant material is a valuable source of bioactive compounds and could be further exploited as food antimicrobial and/or antioxidant additives, for the production of innovative nutritional products, such as herbal instant beverages or enriched aromatic olive oils.
Funding
This research has been co-financed by Greece and the European Union (European Regional Development Fund) in context Research—Create—Innovate within the Operational Program Competitiveness, Entrepreneurship, and Innovation (EPANEK) of the NSRF 2014-2020. Project Code: T1EΔK-05041. Acronym FeedMAP.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
IG, KG, and NA conceived and designed the experiment. ED, AV, AC, PB, EA, and KG collected raw materials. ED, AV, AC, KG, and NA performed the analytical experiments. ED, AV, AC, and NA analyzed the data and interpreted the results. ED, AV, AC, IG, KG, and NA wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
PB was employed by Bagatzounis & Sons S.A. EA was employed by ELVIZ Hellenic Feedstuff Industry S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Saha A Tripathy V Basak BB Kumar J . Entrapment of distilled palmarosa (Cymbopogon martinii) wastes in alginate beads for adsorptive removal of methylene blue from aqueous solution. Environ Prog Sustain Energy. (2018) 37:1942–53. 10.1002/ep.12872
2.
Routray W Orsat V . Plant By-Products and Food Industry Waste: A Source of Nutraceuticals and Biopolymers, In: GrumezescuMHolbanAM, editors. Handbook of Food Bioengineering, Food Bioconversion. (2017). p. 279–315.
3.
Joint FAO/WHO Codex Alimentarius Commission . Codex Alimentarius: CX/SCH 21/5/3 On Spices and Culinary Herbs. Rome: World Health Organization: Food and Agriculture Organization of the United Nations (2021).
4.
Navarrete A Herrero M Martin A Cocero MJ . Ibanez, E. Valorization of solid wastes from essential oil industry. J Food Eng. (2011) 104:196–201. 10.1016/j.jfoodeng.2010.10.033
5.
Wang Q Rehman M Peng D Liu L . Antioxidant capacity and α-glucosidase inhibitory activity of leaf extracts from ten ramie cultivars. Ind Crop Prod. (2018) 122:430–7. 10.1016/j.indcrop.2018.06.020
6.
Sahaa A Basaka BB . Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind Crops Prod. (2020) 145:111979. 10.1016/j.indcrop.2019.111979
7.
Kosakowska O Weglarz Z Pióro-Jabrucka E Przybył JL Kraśniewska K Gniewosz M et al . Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules. (2021) 26:988. 10.3390/molecules26040988
8.
Marrelli M Statti GA Conforti F . Origanum spp: an update of their chemical and biological profiles. Phytochem Rev. (2018) 17:873–88. 10.1007/s11101-018-9566-0
9.
Hosseinzadeh S Jafarikukhdan A Hosseini A Armand R . The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int J Clin Exp. (2015) 6:635–42. 10.4236/ijcm.2015.69084
10.
Koleva II van Beek TA Linssen JPH Groot AD Evstatieva LN . Screening of Plant Extracts for Antioxidant Activity: a Comparative Study on Three Testing Methods. Phytochem Anal. (2002) 13:8–17. 10.1002/pca.611
11.
Todorova M Trendafilova A . Sideritis scardica Griseb, an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J Ethnopharmacol. (2014) 152:256–65. 10.1016/j.jep.2014.01.022
12.
McKay DL Blumberg JB . A Review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L). Phytother Res. (2006) 20:519–30. 10.1002/ptr.1900
13.
Singh O Khanam Z Misra N Srivastava MK . Chamomile (Matricaria chamomilla L): An overview. Pharmacogn Rev. (2011) 5:82–95. 10.4103/0973-7847.79103
14.
Bouloumpasi E Hatzikamari M Lazaridou A Chatzopoulou P Biliaderis CG Irakli M . Antibacterial and Antioxidant Properties of Oregano and Rosemary Essential Oil Distillation By-Products. In: Proceedings of the 2nd International Electronic Conference on Foods - “Future Foods and Food Technologies for a Sustainable World”. Basel:MDPI (2021). 10.3390/Foods2021-11020
15.
Tzima K Brunton NP Rai DK . Evaluation of the impact of chlorophyll removal techniques on polyphenols in rosemary and thyme by-products. J Food Biochem. (2020) 44:e13148. 10.1111/jfbc.13148
16.
Dina E Sklirou AD Chatzigeorgiou S Manola MS Cheilari A Louka XP et al . An enriched polyphenolic extract obtained from the by-product of Rosa damascena hydrodistillation activates antioxidant and proteostatic modules. Phytomed. (2021) 93:153757. 10.1016/j.phymed.2021.153757
17.
Lapichino G Arnone C Bertolino M Amico Roxas U . Propagation of three Thymus species by stem cuttings. Acta Hortic. (2006) 723:411–4. 10.17660/ActaHortic.2006.723.57
18.
Skoufogianni E Solomou AD Danalatos NG . Ecology, cultivation and utilization of the aromatic greek Oregano (Origanum vulgare L): a review. Not Bot Horti Agrobot Cluj Napoca. (2019) 47:545–52. 10.15835/nbha47311296
19.
Timothy KK Mwangi M . Studies on German Chamomile (Matricaria recutita L.) Propagation and The Effect of Light and Age on Seed Viability. J Anim Plant Sci. (2015) 24:2, 3781–6.
20.
Senatore F . Influence of harvesting time on yield and composition of the essential oil of a thyme (Thymus pulegioides L.) growing wild in Campania (Southern Italy). J Agric Food Chem. (1996) 44:1327–32. 10.1021/jf950508z
21.
Gasic O Lukic V Adamovic D . The influence of the sowing and harvest time of the essential oils of Chamomilla recutita (L) Rausch. J Essent Oil Res. (1991) 3:295–302. 10.1080/10412905.1991.9697947
22.
Baranauskiene R Venskutonis PR Dambrauskiene E Viškelis P . Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp vulgare and ssp hirtum. Ind Crop Prod. (2013) 49:43–51. 10.1016/j.indcrop.2013.04.024
23.
International Organization for Standardization. Spices and Condiments – Sampling Method (ISO/DIS Standard No. 948) (1980). Available online at: https://www.iso.org/standard/5369.html (accessed February 17, 2022).
24.
Pluskal T Castillo S Villar-Briones A Oresic M . MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC bioinformatic. (2010) 11:395. 10.1186/1471-2105-11-395
25.
Stagos D Portesis N Spanou C Mossialos D Aligiannis N Chaita E et al . Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem Toxicol. (2012) 50:4115–24. 10.1016/j.fct.2012.08.033
26.
Singleton VL Orthofer R Lamuela-Raventos RM . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. (1999) 299:152–78. 10.1016/S0076-6879(99)99017-1
27.
Pascual ME Carretero ME Slowing KV Villar A . Simplified Screening by TLC of Plant Drugs. Pharm Biol. (2002) 40:139–43. 10.1076/phbi.40.2.139.5849
28.
Iqbal Y Ponnampalam EN Suleria HAR Cottrell JJ Dunshea FR . LC-ESI/QTOF-MS Profiling of Chicory and Lucerne Polyphenols and Their Antioxidant Activities. Antioxidant. (2021) 10:932. 10.3390/antiox10060932
29.
Fecka I Turek S . Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. (2008)108:1039–53. 10.1016/j.foodchem.2007.11.035
30.
Bendif H Peron G Miara MD Sut S Dall'Acqua S Flamini G et al . Total phytochemical analysis of Thymus munbyanus subsp coloratus from Algeria by HS-SPME-GC-MS, NMR and HPLC-MSn studies. J Pharm Biomed Anal. (2020) 186:113330. 10.1016/j.jpba.2020.113330
31.
Grevsen K Fretté X Christensen LP . Content and composition of volatile terpenes, flavonoids and phenolic acids in Greek oregano (Origanum vulgare L. ssp hirtum) at different development stages during cultivation in cool temperate climate. Eur J Hortic Sci. (2009) 74:193–203.
32.
Taamalli A Arráez-Román D Abaza L Iswaldi I Fernández-Gutiérrez A Zarrouk M et al . LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem Anal. (2015) 26:320–30. 10.1002/pca.2566
33.
Petreska J Stefkov G Kulevanova S Alipieva K Bankova V Stefova M . Phenolic Compounds of Mountain Tea from the Balkans: LC/DAD/ESI/MSn Profile and Content. Nat Prod Commun. (2011) 6:21–30. 10.1177/1934578X1100600107
34.
Sotiropoulou NS Megremi SF Tarantilis P . Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L) and Sage (Salvia officinalis L) Prepared at Different Temperatures. Appl Sci. (2020) 10:2270. 10.3390/app10072270
35.
Tsivelika N Irakli M Mavromatis A Chatzopoulou P Karioti A . Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Food. (2021) 10:2345. 10.3390/foods10102345
36.
Wang M Carver JJ Phelan VV Sanchez LM Garg N Peng Y et al . Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat Biotech. (2016) 34:828–37. 10.1038/nbt.3597
37.
Lee SK Mbwambo ZH Chung H Luyengi L Gamez EJ Mehta RG et al . Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. (1998) 1:35–46. 10.2174/138620730101220118151526
38.
Magi G Marini E Facinelli B . Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front Microbiol. (2015) 6:165. 10.3389/fmicb.2015.00165
39.
Reboredo-Rodríguez P Figueiredo-González M González-Barreiro C Simal-Gándara J Salvador MD Cancho-Grande B et al . State of the Art on Functional Virgin Olive Oils Enriched with Bioactive Compounds and Their Properties. Int J Mol Sci. (2017) 18:668. 10.3390/ijms18030668
Summary
Keywords
MAPs' by-products, Origanum vulgare subsp. hirtum, Sideritis scardica , Thymus vulgaris , Matricaria recutita
Citation
Dina E, Vontzalidou A, Cheilari A, Bagatzounis P, Agapidou E, Giannenas I, Grigoriadou K and Aligiannis N (2022) Sustainable Use of Greek Herbs By-Products, as an Alternative Source of Biologically Active Ingredients for Innovative Products. Front. Nutr. 9:867666. doi: 10.3389/fnut.2022.867666
Received
01 February 2022
Accepted
08 March 2022
Published
07 April 2022
Volume
9 - 2022
Edited by
Miguel Angel Prieto Lage, University of Vigo, Spain
Reviewed by
Javier E. Alvarez, University of Vigo, Spain; Antía González Pereira, University of Vigo, Spain; Aurora Silva, Chemistry and Technology Network (REQUIMTE), Portugal
Updates
Copyright
© 2022 Dina, Vontzalidou, Cheilari, Bagatzounis, Agapidou, Giannenas, Grigoriadou and Aligiannis.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nektarios Aligiannis aligiannis@pharm.uoa.gr
This article was submitted to Nutrition and Food Science Technology, a section of the journal Frontiers in Nutrition
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.