
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 19 April 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.863391
Background and Aims: Despite accumulating evidence on the benefits of dietary fiber in the general population, there is a lack of representative data on mortality in patients with chronic kidney disease (CKD). This study examined the role of dietary fiber intake on all-cause and cardiovascular mortality in patients with CKD using representative Korean cohort data.
Methods: The study included 3,892 participants with estimated glomerular filtration rates <60 mL/min/1.73 m2 from the Korean Genome and Epidemiology Study. Mortality status was followed by data linkage with national data sources. Nutritional status was assessed using a validated food frequency questionnaire. Dietary fiber was categorized into quintiles (Q). A multivariable Cox proportional hazards regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and cardiovascular mortality.
Results: The average daily fiber intake of patients with CKD was 5.1 g/day. During the 10.1-year follow-up period, 602 (149 cardiovascular) deaths were documented. The HR (95% CI) for all-cause mortality in the highest quintile compared with that in the lowest quintile was 0.63 (0.46–0.87) after adjusting for age, sex, BMI, smoking, alcohol intake, exercise, total calorie intake, hypertension, diabetes, and dyslipidemia (P = 0.005). The HR (95% CI) for cardiovascular mortality in the highest quintile compared with that in the lowest quintile was 0.56 (0.29–1.08) after adjusting for same confounders (P = 0.082).
Conclusion: In conclusion, we observed an inverse association between dietary fiber intake and all-cause mortality in CKD patients. Small increments in fiber intake reduced the risk of all-cause mortality by 37%. This finding highlights the need for inexpensive but important dietary modification strategies for encouraging fiber intake in the Korean CKD population.
Chronic kidney disease (CKD) is a major health concern associated with premature mortality or poor quality of life (1). Globally, 10–15% of the population has CKD (2). Furthermore, the rising incidence of CKD is a leading public health burden in East Asia (3). In Korea, the prevalence of CKD in individuals aged >20 was 8.2% (4). Given the high incidence and prevalence of CKD and an urgent need for disease prevention and management strategies, appropriate dietary intake is essential for patients with CKD (5). Previous studies have suggested that unhealthy dietary patterns may contribute to kidney injury and metabolic derangements that may amplify the risks of cardiovascular disease (CVD), morbidity, and mortality (6, 7). Dietary interventions for CKD have been focused mainly on restricting dietary salt, phosphorus, potassium, and protein intake (8). The role of dietary fiber intake in patients with CKD is less well defined. Furthermore, there is no precise fiber intake recommendation for patients with CKD (9). Fiber is abundant in plant foods, including vegetables, fruits, whole grains, nuts, legumes, and seeds (10). Dietary fiber has various health benefits on gut motility, preventing constipation, lowering blood pressure and cholesterol, regulating blood sugar, reducing body weight, and improving gut microbiota (11–13). Adequate fiber intake could reduce the risk of non-communicable diseases (NCDs), including obesity, diabetes, and CVD, which are associated with CKD progression and mortality (14–16). Recently, several meta-analyses including clinical trials have shown that dietary fiber intake reduces the levels of uremic toxins and inflammatory markers and delays the progression of CKD (1, 17). In observational studies, a higher adherence to healthy dietary patterns rich in fiber, such as the Mediterranean diet is associated with a lower risk of incident CKD (18, 19) and poor adherence to a Dietary Approaches to Stop Hypertension (DASH) diets is associated with higher risk of end stage renal disease (ESRD) in patients with pre-existing CKD (20).
Dietary fiber can be divided into soluble dietary fiber and insoluble dietary fiber, which has different physical and functional properties (21). Soluble fiber has key effect on lowering postprandial glucose response and serum lipids. Whereas insoluble fiber has effects on increasing gut transit time and lowering insulin resistance (22). However, most fiber rich foods contain both soluble and insoluble fiber, which have synergic effects on metabolic health (21).
Patients with CKD have a higher mortality rate than the general population (23). CKD resulted in 1.2 million deaths in 2017 and the global all-age CKD mortality rate increased by 41.5% from 1990 to 2017 (2). Krishnamurthy et al. (8) found that higher dietary fiber intake was associated with decreased mortality in patients with CKD, but not in the non-CKD population. Recently, higher dietary fiber intake appeared to have a protective effect on all-cause mortality in non-diabetic peritoneal dialysis patients (24). However, the findings have been inconsistent among observational studies. Some studies have failed to prove any link between fiber intake and mortality in patients on dialysis (25). A more comprehensive study of the association between total fiber intake and all-cause and cause-specific mortality in patients with pre-existing CKD is needed.
Moreover, racial and ethnic disparities, which may influence disparities in clinical outcomes, are important and unresolved issues in patients with CKD (26). Although several populational studies have shown an association between dietary fiber intake and CKD progression and mortality (27, 28), it is unclear whether this association can be generalized to other ethnic groups, where dietary sources and ranges of fiber intake are considerably different. Therefore, this study aimed to comprehensively evaluate the association between dietary fiber intake and all-cause and CVD mortality in adult Korean patients with CKD using large-scale cohort data.
This study used data from population-based cohorts in the Korean Genome and Epidemiology Study (KoGES): KoGES_Ansan and Ansung study (community-based cohort in urban and rural counites), the KoGES_health examinee study (national health examinee registry), and the KoGES_cardiovascular disease association study (community-based cohort in rural counties). Participants were recruited through on-site invitation, mailed letters, telephone calls, media campaigns, or community leader-mediated conferences. In KoGES_Ansan and Ansung study, a total of 10,030 participants aged 40–69 years was voluntarily enrolled at baseline between 2001 and 2002. In the KoGES_health examinee study, a total of 173,357 participants was enrolled at baseline from 2004 to 2013. In the KoGES_cardiovascular disease association study, a total of 28,338 participants was enrolled at baseline from 2005 to 2011. A total of 211,571 participants aged over 40 years was enrolled at the baseline KoGES survey. We excluded participants with missing lifestyle (n = 2,231), laboratory test (n = 5,853), and nutritional intake (n = 14,007) data and implausible daily total calorie intakes (<500 kcal or >6,000 kcal). Excluding 54,530 participants with missing mortality data and 63 participants who died in their year of enrollment, a total of 143,050 participants were selected. Finally, a total of 3,892 participants with an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 were included in the analysis. Among the 3,892 participants, 602 deaths were documented during the follow-up time. The study population selection process is depicted in Supplementary Figure 1. Written informed consent documents were signed by all participants. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. This study was approved by the institutional review boards of Yongin Severance Hospital (IRB Number: 9-2021-0066).
The body mass index (BMI) was calculated as the body weight (in kilograms) divided by the square of the height (in meters). Waist circumference (WC) was measured at the midline level between the inferior margin of the ribs and the superior border of the iliac crest. Anthropometric measurements were performed using validated and standardized protocols. Smoking status was categorized as current smoker, former smoker, and non-smoker. A current smoker was defined as any participant who currently smoked and had smoked at least 100 cigarettes during their lifetime. A former smoker was defined as an adult who had smoked at least 100 cigarettes in their lifetime and had quit smoking. Never smokers were defined as adults who had never smoked or had smoked <100 cigarettes in their lifetime. Alcohol intake status was categorized as current drinker, former drinker, and non-drinker. A regular exerciser was defined as a person who regularly exercised more than once a week. Diabetes was defined as a glycated hemoglobin level of 6.5% or higher, a fasting plasma glucose concentration ≥126 mg/dL after overnight fasting, or the taking of anti-diabetic medications. Dyslipidemia was defined as a total cholesterol level ≥200 mg/dL, triglyceride level ≥150 mg/dL, or the taking of anti-dyslipidemic medications. Hypertension was defined as a systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or the taking of antihypertensive medications. Blood concentrations of creatinine, blood urea nitrogen, glucose, glycated hemoglobin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were analyzed at the central laboratory. Detailed information on the study protocol is available at the following website: https://www.kdca.go.kr/contents.es?mid=a40504100100.
The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) Study equation (29) as follows:
GFR (mL/min/1.73 m2) = 175 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female) × (1.212 if African American).
CKD was defined as an eGFR of <60 mL/min/1.73 m2.
Mortality status was followed by data linkage with national data sources based on the unique personal identification key code system. The KoGES data have been linked to national data sources (Korea National Statistical Office), including death records for evaluating mortality. Participant deaths were tracked to December 2019. The underlying causes of death were based on Korean Standard Classification of Disease (KCD) codes listed in the National Death Index. All-cause mortality included all deaths of specified and unknown causes.
A food frequency questionnaire (FFQ) consisting of 103 food items was developed for the KoGES survey (30). The FFQ addresses the diet of the past 12 months and has long been the instrument of choice in epidemiologic studies. Carbohydrate, fat, and protein intake were recorded as g/day. Dietary fiber intake (g/day) was classified in quintiles (Q). The protocol and results of a validation study for the FFQ are detailed in previous studies (30, 31).
Data were analyzed from 2001 through 2013. Participant deaths were tracked to December 2019. The follow-up period for each study participant was calculated as the time from their KoGES initial assessment to either a mortality event or the censoring date. Data are presented as mean ± standard deviation (SD), for continuous variables, or number (%), for categorical variables. Dietary fiber intake was divided into quintiles. Baseline characteristics of the study population according to dietary fiber intake quintiles were compared using one-way analysis of variance, for continuous variables, and the chi-square test, for categorical variables. A Cox proportional hazard spline curve was used to assess the association between dietary fiber intake and all-cause mortality risk. The incidence per 1,000 person-years was calculated for each group. The Kaplan–Meier method with the log-rank test was used to compare cumulative incidence rates for all-cause mortality according to dietary fiber intake quintiles. A multivariable Cox proportional hazards regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality and CVD mortality in Q2, Q3, Q4, and Q5 with reference to Q1, after adjusting for potential confounding factors. Model 1 was adjusted for age, sex, and BMI. Model 2 was additionally adjusted for smoking, alcohol intake, exercise, and total calorie intake. Model 3 was additionally adjusted for history of hypertension, diabetes, and dyslipidemia. Model 4 was additionally adjusted for baseline eGFR. We performed subgroup analysis to investigate the dietary fiber intake and mortality in CKD patients according to CKD stages, sex and BMI criteria 25 kg/m2.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R package version 4.0.3 (http://www.R-project.org). All statistical tests were two sided, and P < 0.05 was considered statistically significant.
During the median 10.1 (min-max, 0.2–15.9) years of follow-up, there were 602 deaths. Detailed information on the study population according to mortality status is presented in Supplementary Table 1 (according to all-cause mortality). The proportion of men was higher among all-cause mortality cases. The mean age was 68.5 ± 7.3 years for participants with a death event and 61.8 ± 8.0 years for participants without death event, respectively. Participants with death events were more likely to be older, to have a lower BMI, higher WC, higher SBP, lower total cholesterol levels, lower HDL-C levels, higher blood glucose and HbA1c levels, smoke more, and have hypertension and diabetes; however, they were less likely to be non-smokers, exercise regularly, and live in urban areas. Participants with death events consumed less total energy, carbohydrates (g/day), fat (g/day), protein (g/day), minerals, and vitamins. However, the carbohydrate intake proportion was higher than that of those without death events.
Table 1 presents the baseline characteristics of the study population according to quintiles of dietary fiber intake. The mean age of study population was 62.9 ± 8.3 years, and the proportion of men was 38.4% in this study. The mean dietary fiber intake was 5.1 ± 2.6 g/day. Participants with higher dietary fiber intakes were more likely to be men, younger, have a higher BMI, higher WC, higher HDL-C levels, drink more alcohol, exercise more regularly, and live in urban areas; however, they were less likely to smoke. Regarding nutritional status, participants with higher dietary fiber intakes were more likely to have higher total energy intakes and consume carbohydrate (g/day), fat (g/day and %), protein (g/day and %), minerals, and vitamins; however, the carbohydrate proportion (%) of their diet was likely to be lower. Post-hoc comparison P-values between the five categories are presented in Supplementary Table 2.
As shown in Figure 1A, Kaplan–Meier curves revealed a significantly lower cumulative incidence of total mortality in Q5, followed by that from Q4 to Q1 (log-rank test P < 0.001). Figure 1B shows the all-cause mortality incidence rates (per 1,000 person-years) and their 95% CIs. Participants with higher fiber intakes had a lower incidence per 1,000 person years. Cox proportional hazards spline curves showed inverse association between dietary fiber intake and total mortality in patients with CKD (Figure 2A). There were similar inverse association between dietary fiber intake and CVD mortality in patients with CKD (Figure 2B).
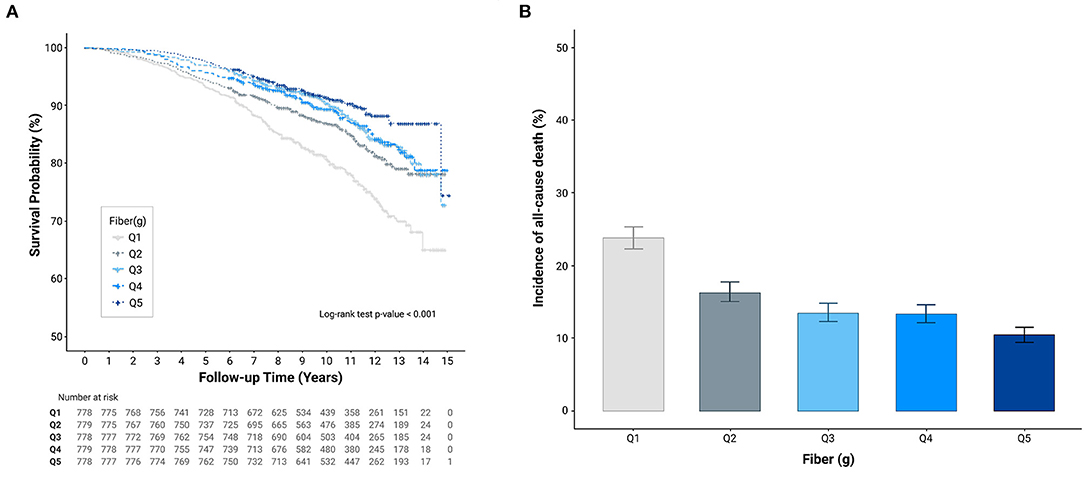
Figure 1. Cumulative incidence of all-cause mortality according to quintiles of dietary fiber intake (g/day). (A) Kaplan-Meier curves (B) All-cause mortality incidence rates.
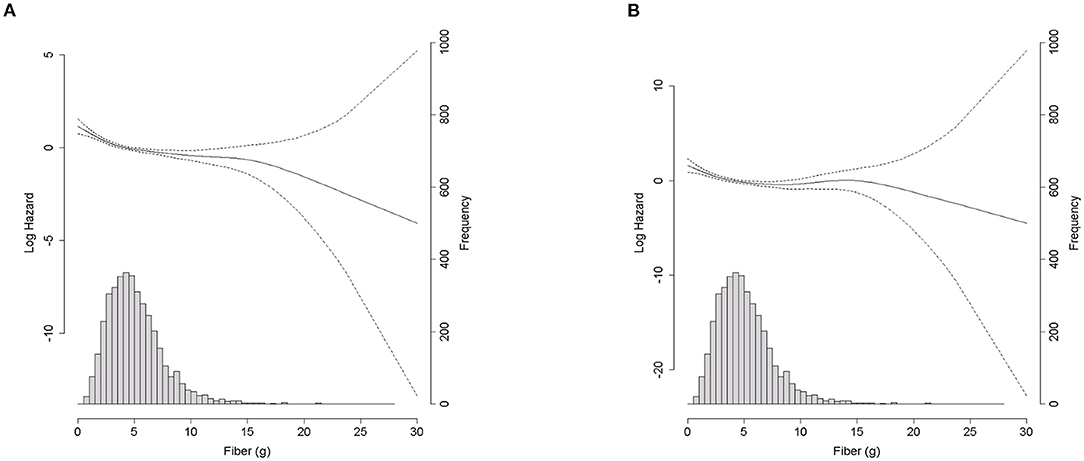
Figure 2. Density plot of fiber intake (g/day) and spline curve for all-cause mortality and CVD mortality according to dietary fiber intake (g/day). (A) All-cause mortality. (B) Cardiovascular motality.
The HRs and 95% CIs for all-cause mortality across the dietary fiber intake quintiles are presented in Table 2. Compared to Q1, the HRs (95% CI) for all-cause mortality in Q2, Q3, Q4, and Q5 were 0.71 (0.57–0.90), 0.66 (0.52–0.84), 0.69 (0.54–0.88), and 0.55 (0.42–0.72), respectively, after adjusting for age, sex, and BMI. In the isocaloric model, compared to Q1, the HRs (95% CI) for all-cause mortality in Q2, Q3, Q4, and Q5 were 0.75 (0.65–0.95), 0.72 (0.55–0.93), 0.75 (0.57–0.98), and 0.61 (0.45–0.85), respectively, after adjusting for age, sex, BMI, smoking, alcohol consumption, exercise, and total calorie intake. After additionally adjusting for history of hypertension, diabetes, dyslipidemia, and baseline eGFR, a significant inverse association between dietary fiber intake and all-cause mortality was noted in patients with CKD [Q5 vs. Q1; 0.67 (0.48–0.93), P = 0.016].
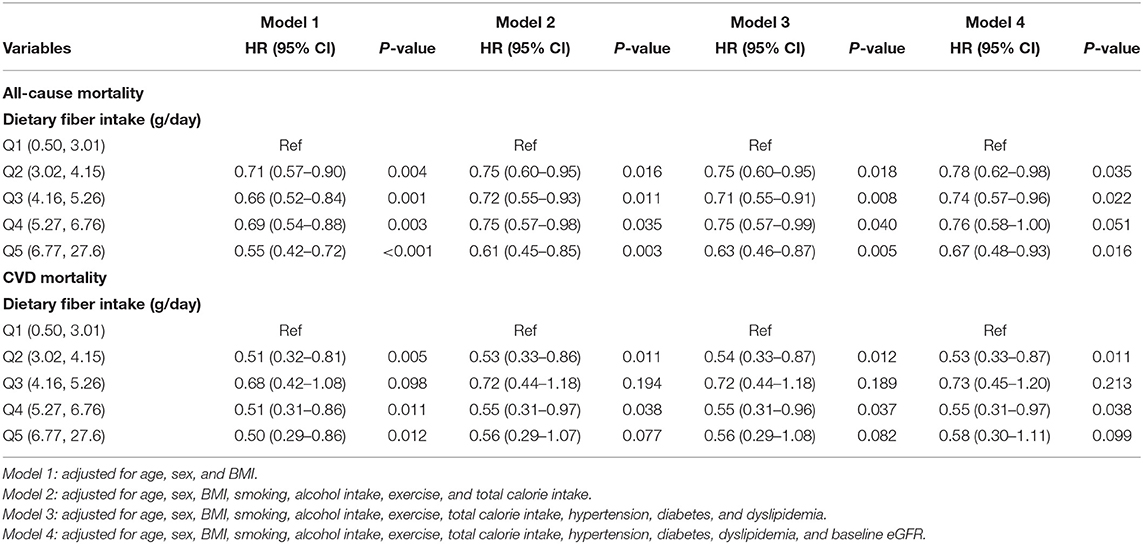
Table 2. Multiple cox proportional hazard regression analysis of dietary fiber intake quintiles for all-cause mortality and cardiovascular mortality.
Additionally, we examined the association between dietary fiber intake and CVD mortality in patients with CKD. There were 149 CVD death events during the follow-up period (Supplementary Table 3).
Compared to Q1, the HR and 95% CI for CVD mortality in Q5 was 0.50 (0.29–0.86) after adjusting for age, sex, and BMI (P = 0.012). Compared to Q1, the HR and 95% CI for CVD mortality in Q5 was 0.58 (0.30–1.11) after adjusting for age, sex, BMI, smoking, alcohol consumption, exercise, total calorie intake, hypertension, diabetes, dyslipidemia, and baseline eGFR (P = 0.099).
Each 1 g/day increase of total dietary fiber was associated with HR 0.92, 95% CI 0.88–0.95 for all-cause mortality and HR 0.91, 95% CI (0.84–0.99) for CVD mortality. With further adjustment for age, sex, BMI, smoking, alcohol consumption, exercise, and total calorie intake, hypertension, diabetes, dyslipidemia, and eGFR, the corresponding HR and 95% CI for all-cause mortality and CVD mortality were 0.94, 0.90–0.99 (P = 0.014) and 0.93, (0.84–1.03) (P = 0.161), respectively (data not shown).
We also examined the association between total fiber intake and all-cause mortality in patients with CKD according to the CKD stages, sex, BMI criteria 25kg/m2 (Supplementary Table 4). The numbers (%) of CKD 3, 4, and 5 were 3,717 (95.5%), 122 (3.1%), and 53 (1.4%), respectively. There were significant association between dietary fiber intake and all-cause mortality in the only CKD stage 3, female, and BMI <25 kg/m2.
Despite the accumulating evidence on the benefits of dietary fiber in the general population, there is a lack of representative data on mortality in patients with CKD, especially in East Asia. In this study, we observed a significant and inverse relationship between dietary fiber intake and mortality risk in patients with CKD in a Korean prospective cohort followed up for 10 years. A similar trend was noted in the association between dietary fiber intake and CVD mortality.
The American Dietetic Association recommended a daily dietary fiber intake of 25–30 g for adults in the general population (32). Similarly, the 2015 Dietary Reference Intake for Koreans (KDRI) suggested that the sufficient daily fiber intake for Korean adults is 25 g/day for men and 20 g/day for women, regardless of age (33). However, to our knowledge, there are no specific guidelines for dietary fiber intake in the CKD population in the available nephrology guidelines.
Dietary recommendations for patients with CKD are different from those for healthy people. Patients with advanced CKD are frequently advised to restrict the intake of vegetables, fruits, legumes, nuts, and whole-grains due to their high potassium content and the risk of hyperkalemia and hyperphosphatemia (34). Therefore, fiber intake in patients with CKD is generally lower than that in the general population (8). Data from the National Health and Nutrition Examination Survey (NHANES) in the US showed that total dietary fiber intake in the CKD population averaged 15.4 g/day, which is far below the recommended 25–30 g/day intake for the general population (8). In this study, the average daily fiber intake of patients with CKD was only 5.1 g/day, which was extremely low. This result may be due to the competing dietary potassium and phosphorus concerns. However, a high-fiber diet increases fecal potassium excretion by increasing stool volume (35), and the bioavailability of potassium and phosphorus in high-fiber foods is lower than that in other phosphorus and potassium foods, especially processed foods (1). Supporting this, there were weak or no correlations between dietary potassium and serum potassium in advanced CKD (36, 37), and cohort studies fail to prove a close relationship between diets rich in fibers and serum potassium levels (38). Considering these results, the recent Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommended “adjusting” potassium intake “when needed” to maintain potassium levels in the normal range, but not routinely, as this may result in deficiencies in other healthy nutrients, such as fiber (39). Interestingly, low potassium and phosphorus intake was associated with increased odds of advanced stage CKD in Korean hypertensive patients (40). In this study, we observed that higher dietary fiber intake was associated with a reduced risk of death in patients with CKD. When comparing persons with dietary fiber intakes in the top and bottom quintiles, we found a statistically significant inverse association between fiber intake and all-cause mortality, with an overall relative risk of 0.63 (95% CI: 0.46–0.87). Together, these studies suggest that, regardless of the risk of hyperkalemia and hyperphosphatemia, a higher fiber intake may be beneficial for Koreans with CKD. However, there has been economic growth and lifestyle changes in Korea over the past decades. Consequently, there has been a noticeable shift in nutrient intake from grain-based foods to western style meals focused on protein and lipid (41). Although rice consumption still constitutes the largest proportion, it has continuously declined to one third of total energy intake. This is much lower than the intake of other Asians, whose trends are opposite to the current situation in Western countries where the consumption of rice is increasing (41). A national dietary plan and strategy should be set to establish nutritional transition toward higher fiber intake for Koreans with CKD.
Several mechanisms support the inverse association between dietary fiber intake and mortality in patients with CKD. The bulking effect of dietary fiber is important for colonic health and gut motility. Dietary fiber leads to uremic toxin excretion by increasing stool output (42), secondary to increased proteolytic activities by protein fermenting bacteria (43). A systemic review including 203 patients with CKD from seven reports showed that dietary fiber reduced p-cresyl sulfate, thereby protecting the intestinal epithelial barrier of patients with CKD (44). Favorable alteration in the gut microbiome not only modulates uremic toxins (45) but also chronic inflammatory pathways (11). Compared with the healthy population, patients with CKD exhibited increased serum levels of CRP, IL-6, interferon-γ, and tumor necrosis factor-α (46). However, increased dietary fiber intake and supplementation were associated with a decreased systemic inflammatory state, including the C-reactive protein level (8, 47). Because uremic toxins and chronic inflammation are central to the progression of CKD, some have hypothesized that fiber may slow the progression of CKD. Emerging studies have shown that high dietary fiber intake is associated with the primary prevention of CKD and delaying of CKD progression (48, 49). The Tehran Lipid and Glucose Study, including 1 630 participants with 6.1 years of follow-up, showed an 11% lower risk of incident CKD per 5-g/d increase in total fiber intake (48). In this study, compared to the lowest tertile groups, the highest tertile vegetable fiber and legume fiber groups were significantly associated with lower risk of CKD incidence (48). In the Blue Mountains Eye Study, after 5 years of follow-up, high cereal fiber intake was associated with a 50% reduction in the incidence of CKD (49). A meta-analysis of 14 controlled feeding trials including patients with CKD demonstrated that dietary fiber can reduce serum urea and creatinine levels with a dose dependent response for serum creatinine (1). No specific recommendations have been made in relation to the preferred types of dietary fiber consumption, although it is recommended that ≥50% of all grains consumed should be whole grains (50). Although we could not distinguish fiber types, the main sources of fiber intake in Koreans are grains and vegetables (51).
Dietary fiber intake may modify the cardio-metabolic risk profile by lowering blood pressure (52) and LDL-C levels (53), ameliorating postprandial hyperglycemia, and enhancing peripheral insulin sensitivity (54), which may contribute to complications and mortality related to CVD. Based on the available published literature, there appear to be an association between dietary fiber intake and mortality from CVD, including coronary heart disease and cerebrovascular disease (55, 56). Threapleton et al. published a systematic review and meta-analysis including 22 prospective cohort studies reporting on inverse associations between dietary fiber intake and coronary heart disease or CVD (55). A dose–response meta-analysis from 15 prospective cohort studies also revealed a pooled relative risk reduction of 9% in CVD mortality from a dietary fiber intake increment of 10 g/day (56). Of great importance is the fact that the leading cause of death in patients with CKD is CVD rather than progression to end-stage renal disease (57). In line with previous studies, we observed a borderline statistical significance in the association between dietary fiber intake and CVD mortality, with 149 CVD death events in patients with CKD. Further prospective studies including larger numbers of deaths would be needed to confirm the association between dietary fiber intake and CVD mortality in Korean patients with CKD.
In the current study, non-significant associations was noted between dietary fiber intake and all-cause mortality in CKD stage 4 and 5, men and participants with BMI ≥ 25 kg/m2. There were very few patients with CKD stages 4 and 5 in the dataset. A small dataset could lead to overfitting problems in logistic regression models. Although the exact reasons for the different association between dietary fiber intake and mortality in men and overweight individuals are unclear, dietary fiber sources might lead to different results. Further research is needed on the relationship between fiber intake and mortality according to sex and obesity.
Our study has several limitations. First, foods containing high fiber are commonly plant-based foods, such as vegetables, fruits, nuts, and seeds. These foods contain not only dietary fiber but also various vitamins, minerals, and polyphenols. Therefore, it is unclear whether the dietary fiber itself had beneficial effects or whether other nutrients in the food containing dietary fiber had positive effects on mortality in patients with CKD. Therefore, caution is needed in interpreting these results. Second, we could not obtain detailed information on the dietary fiber sources. In future study, we will attempt to investigate the association between fiber type and mortality in CKD patients. Third, previous studies using the same data (KoGES) showed a low dietary fiber intake (mean 6.3 ± 3.6 g/day) in the general middle-aged Korean adult population (30, 58). However, the mean average dietary fiber intake among patients with CKD was too low compared with the recommended dietary fiber intake for Koreans (59). Therefore, it is unclear whether our results could be generalized to other countries with different food habits. Forth, we did not consider the effects of changes of fiber intake during the follow-up period. The dataset is only composed of baseline survey data and data linkage with national mortality data. The consideration of changes in dietary fiber intake during the follow-up period will be incorporated into future study. Finally, we could not obtain information on urine albumin and creatinine levels or dialysis status.
Despite these limitations, our study has several strengths. The present study provided data based on a validated 103-question FFQ in a population-based sample and had a rather long follow-up duration. In addition, this is the first study to examine the association between dietary fiber intake and all-cause mortality in a Korean population with CKD.
In this study, dietary fiber intake was associated with a lower risk of all-cause mortality in patients with CKD. The average daily fiber intake of Korean patients with CKD was extremely low; however, we found that small increments in the fiber intake reduced the risk of premature death by 37% and had desirable effects on cardiovascular mortality. This finding highlights the need for encouraging adequate fiber intake through consumption of whole grains, vegetables, and legumes in CKD patients. Further study is needed to define the recommended fiber intake in patients with CKD and the health impacts of different types of fiber on CKD related complications and mortality.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.kdca.go.kr/contents.es?mid=a40504010000.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Yongin Severance Hospital (IRB Number: 9-2021-0066). The patients/participants provided their written informed consent to participate in this study.
Y-JK, HL, GP, and J-WL contributed to the conception or design of the work, contributed to the acquisition, analysis, or interpretation of the data, and drafting of the manuscript. All authors critically revised the manuscript, provided final approval and agree to be accountable for all aspects of the work, and ensuring integrity and accuracy.
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through High Value-Added Food Technology Development Program funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (321030051HD030). The grant was awarded to J-WL and Y-JK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was conducted using data from the Korean Genome and Epidemiology Study (KoGES; 4851-302) by the National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea. We thank Dr. Woojin Park for helping with illustrating the figures.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.863391/full#supplementary-material
1. Chiavaroli L, Mirrahimi A, Sievenpiper JL, Jenkins DJ, Darling PB. Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. (2015) 69:761–8. doi: 10.1038/ejcn.2014.237
2. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
3. Song Y, Lobene AJ, Wang Y, Hill Gallant KM. The DASH diet and cardiometabolic health and chronic kidney disease: a narrative review of the evidence in East Asian Countries. Nutrients. (2021) 13:984. doi: 10.3390/nu13030984
4. Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: the Korean national health and nutritional examination survey 2011-2013. J Korean Med Sci. (2016) 31:915–23. doi: 10.3346/jkms.2016.31.6.915
5. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. (2019) 322:1294–304. doi: 10.1001/jama.2019.14745
6. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
7. Khan SS, Kazmi WH, Abichandani R, Tighiouart H, Pereira BJ, Kausz AT. Health care utilization among patients with chronic kidney disease. Kidney Int. (2002) 62:229–36. doi: 10.1046/j.1523-1755.2002.00432.x
8. Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. (2012) 81:300–6. doi: 10.1038/ki.2011.355
9. Molitch ME, Adler AI, Flyvbjerg A, Nelson RG, So WY, Wanner C, et al. Diabetic kidney disease: a clinical update from kidney disease: improving global outcomes. Kidney Int. (2015) 87:20–30. doi: 10.1038/ki.2014.128
10. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. (2012) 3:506–16. doi: 10.3945/an.112.002154
11. Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The health benefits of dietary fibre. Nutrients. (2020) 12:209. doi: 10.3390/nu12103209
12. Ma W, Nguyen LH, Song M, Wang DD, Franzosa EA, Cao Y, et al. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. (2021) 13:102. doi: 10.1186/s13073-021-00921-y
13. Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. (2021) 18:101–16. doi: 10.1038/s41575-020-00375-4
14. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. (2019) 393:434–45. doi: 10.1016/S0140-6736(18)31809-9
15. Katagiri R, Goto A, Sawada N, Yamaji T, Iwasaki M, Noda M, et al. Dietary fiber intake and total and cause-specific mortality: the Japan Public Health Center-based prospective study. Am J Clin Nutr. (2020) 111:1027–35. doi: 10.1093/ajcn/nqaa002
16. Song S, Song Y. Dietary fiber and its source are associated with cardiovascular risk factors in Korean adults. Nutrients. (2021) 13:160. doi: 10.3390/nu13010160
17. Yang HL, Feng P, Xu Y, Hou YY, Ojo O, Wang XH. The role of dietary fiber supplementation in regulating uremic toxins in patients with chronic kidney disease: a meta-analysis of randomized controlled trials. J Ren Nutr. (2021) 31:438–47. doi: 10.1053/j.jrn.2020.11.008
18. Asghari G, Farhadnejad H, Mirmiran P, Dizavi A, Yuzbashian E, Azizi F. Adherence to the Mediterranean diet is associated with reduced risk of incident chronic kidney diseases among Tehranian adults. Hypertens Res. (2017) 40:96–102. doi: 10.1038/hr.2016.98
19. Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Zeimbekis A, Kastorini CM, et al. Adherence to the Mediterranean diet is associated with renal function among healthy adults: the ATTICA study. J Ren Nutr. (2010) 20:176–84. doi: 10.1053/j.jrn.2009.08.006
20. Banerjee T, Crews DC, Tuot DS, Pavkov ME, Burrows NR, Stack AG, et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. (2019) 95:1433–42. doi: 10.1016/j.kint.2018.12.027
21. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. (2010) 2:1266–89. doi: 10.3390/nu2121266
22. Weickert MO, Pfeiffer AFH. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J Nutr. (2018) 148:7–12. doi: 10.1093/jn/nxx008
23. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. (2006) 17:2034–47. doi: 10.1681/ASN.2005101085
24. Xu X, Li Z, Chen Y, Liu X, Dong J. Dietary fibre and mortality risk in patients on peritoneal dialysis. Br J Nutr. (2019) 122:996–1005. doi: 10.1017/S0007114519001764
25. Lin Z, Qin X, Yang Y, Huang Y, Wang J, Kong Y, et al. Higher dietary fibre intake is associated with lower CVD mortality risk among maintenance haemodialysis patients: a multicentre prospective cohort study. Br J Nutr. (2021) 126:1510–8. doi: 10.1017/S0007114521000210
26. Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. (2005) 68:914–24. doi: 10.1111/j.1523-1755.2005.00485.x
27. Buil-Cosiales P, Zazpe I, Toledo E, Corella D, Salas-Salvadó J, Diez-Espino J, et al. Fiber intake and all-cause mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. (2014) 100:1498–507. doi: 10.3945/ajcn.114.093757
28. Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch Intern Med. (2011) 171:1061–8. doi: 10.1001/archinternmed.2011.18
29. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. (2006) 145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004
30. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. (2007) 61:1435–41. doi: 10.1038/sj.ejcn.1602657
31. Kim Y, Han BG. Cohort profile: the Korean genome and epidemiology study (KoGES) Consortium. Int J Epidemiol. (2017) 46:e20. doi: 10.1093/ije/dyv316
32. Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. (2008) 108:1716–31. doi: 10.1016/j.jada.2008.08.007
33. Ministry Ministry of Health and Welfare The The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2015. Ministry of Health and Welfare Sejong (2015).
34. Wang AY, Sea MM, Ng K, Wang M, Chan IH, Lam CW, et al. Dietary fiber intake, myocardial injury, and major adverse cardiovascular events among end-stage kidney disease patients: a prospective cohort study. Kidney Int Rep. (2019) 4:814–23. doi: 10.1016/j.ekir.2019.03.007
35. Cummings JH, Hill MJ, Jenkins DJ, Pearson JR, Wiggins HS. Changes in fecal composition and colonic function due to cereal fiber. Am J Clin Nutr. (1976) 29:1468–73. doi: 10.1093/ajcn/29.12.1468
36. Noori N, Kalantar-Zadeh K, Kovesdy CP, Murali SB, Bross R, Nissenson AR, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. (2010) 56:338–47. doi: 10.1053/j.ajkd.2010.03.022
37. St-Jules DE, Goldfarb DS, Sevick MA. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. (2016) 26:282–7. doi: 10.1053/j.jrn.2016.02.005
38. González-Ortiz A, Xu H, Ramos-Acevedo S, Avesani CM, Lindholm B, Correa-Rotter R, et al. Nutritional status, hyperkalaemia and attainment of energy/protein intake targets in haemodialysis patients following plant-based diets: a longitudinal cohort study. Nephrol Dial Transplant. (2021) 36:681–8. doi: 10.1093/ndt/gfaa194
39. Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2020) 97:42–61. doi: 10.1016/j.kint.2019.09.018
40. Kim J, Lee J, Kim KN, Oh KH, Ahn C, Lee J, et al. Association between dietary mineral intake and chronic kidney disease: the health examinees (HEXA) study. Int J Environ Res Public Health. (2018) 15:1070. doi: 10.3390/ijerph15061070
41. Kim J-G, Kim J-S, Kim J-G. Trends of food supply and nutrient intake in South Korea over the past 30 years. Curr Res Nutr Food Sci J. (2019) 7:85–95. doi: 10.12944/CRNFSJ.7.1.09
42. Camerotto C, Cupisti A, D'Alessandro C, Muzio F, Gallieni M. Dietary fiber and gut microbiota in renal diets. Nutrients. (2019) 11:2149. doi: 10.20944/preprints201906.0216.v1
43. Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. (2015) 30:924–33. doi: 10.1093/ndt/gfu287
44. Wu M, Cai X, Lin J, Zhang X, Scott EM, Li X. Association between fibre intake and indoxyl sulphate/P-cresyl sulphate in patients with chronic kidney disease: meta-analysis and systematic review of experimental studies. Clin Nutr. (2019) 38:2016–22. doi: 10.1016/j.clnu.2018.09.015
45. Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. (2021) 398:786–802. doi: 10.1016/S0140-6736(21)00519-5
46. Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. (2009) 24:1445–52. doi: 10.1007/s00467-008-1046-0
47. Xie LM, Ge YY, Huang X, Zhang YQ, Li JX. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med. (2015) 8:1363–9.
48. Mirmiran P, Yuzbashian E, Asghari G, Sarverzadeh S, Azizi F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br J Nutr. (2018) 119:479–85. doi: 10.1017/S0007114517003671
49. Gopinath B, Harris DC, Flood VM, Burlutsky G, Brand-Miller J, Mitchell P. Carbohydrate nutrition is associated with the 5-year incidence of chronic kidney disease. J Nutr. (2011) 141:433–9. doi: 10.3945/jn.110.134304
50. Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. (2014) 37(Suppl. 1):S120–43. doi: 10.2337/dc14-S120
51. Lee H-J, Kim Y-A, Lee H-S. The estimated dietary fiber intake of Korean by age and sex. J Kor Soc Food Sci Nutr. (2006) 35:1207–14. doi: 10.3746/jkfn.2006.35.9.1207
52. Anderson JW, Baird P, Davis RH Jr., Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutr Rev. (2009) 67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x
53. Satija A, Hu FB. Cardiovascular benefits of dietary fiber. Curr Atheroscler Rep. (2012) 14:505–14. doi: 10.1007/s11883-012-0275-7
54. Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr. (1990) 52:524–8. doi: 10.1093/ajcn/52.3.524
55. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Bmj. (2013) 347:f6879. doi: 10.1136/bmj.f6879
56. Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. (2016) 109:39–54. doi: 10.1016/j.acvd.2015.09.005
57. Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J Am Soc Nephrol. (2017) 28:2167–79. doi: 10.1681/ASN.2016050562
58. Ahn Y, Park SJ, Kwack HK, Kim MK, Ko KP, Kim SS. Rice-eating pattern and the risk of metabolic syndrome especially waist circumference in Korean Genome and Epidemiology Study (KoGES). BMC Public Health. (2013) 13:61. doi: 10.1186/1471-2458-13-61
Keywords: dietary fiber, chronic kidney diseases, mortality, Korean Genome and Epidemiology Study, cardiovascular mortality
Citation: Kwon Y-J, Lee HS, Park GE and Lee J-W (2022) Association Between Dietary Fiber Intake and All-Cause and Cardiovascular Mortality in Middle Aged and Elderly Adults With Chronic Kidney Disease. Front. Nutr. 9:863391. doi: 10.3389/fnut.2022.863391
Received: 27 January 2022; Accepted: 25 March 2022;
Published: 19 April 2022.
Edited by:
Zeljko Krznaric, University Hospital Centre Zagreb, CroatiaReviewed by:
Frank Thielecke, Swiss Distance University of Applied Sciences, SwitzerlandCopyright © 2022 Kwon, Lee, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Won Lee, aW5kaTU2NDVAeXVocy5hYw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.