- 1Department of Respiratory Medicine, Xi’an Children’s Hospital, Xi’an Jiaotong University, Xi’an, China
- 2Department and Institute of Infectious Disease, Xi’an Children’s Hospital, Xi’an Jiaotong University, Xi’an, China
Background: Vitamin D, as an immunomodulator, may be related to the therapeutic effect of asthma patients, but the research in this area is still controversial. The aim of this meta-analysis was to analyze the role of vitamin D supplementation in the treatment of asthma patients.
Materials and Methods: Randomized Controlled Trials (RCTs) of vitamin D supplementation in asthma were searched in PubMed, EMBASE, and the Cochrane library. Primary outcomes were forced expiratory volume in one second (FEV1), asthma exacerbations, Asthma Control Test scores (ACT scores), and fractional exhaled nitric oxide (FENO).
Results: A total of 10 RCTs were included, including 1,349 patients. Vitamin D supplementation didn’t affect the ACT scores (SMD = 0.04, 95% CI = −0.13 to 0.21, P = 0.87), FEV1 (SMD = 0.04, 95% CI = −0.35 to 0.43, P < 0.01) and FENO (SMD = −0.01, 95% CI = −0.22 to 0.20, P = 0.27), but reduced the rate of asthma exacerbations (RR = 0.69, 95% CI = 0.41 to 0.88, P < 0.01), especially in subgroups of children (RR = 0.46, 95% CI = 0.30 to 0.70, P = 0.83) and follow up time less than 6 months (RR = 0.45, 95% CI = 0.32 to 0.63, P = 0.95). Additionally, though there was only one study included in the subgroup, it significantly enhanced FEV1 at the last visit for patients whose FEV1 baseline value was less than 70% (SMD = 0.94, 95% CI = 0.47 to 1.41).
Conclusion: Vitamin D supplementation can reduce asthma exacerbations, especially in children, and within 6 months of follow up time. In addition, vitamin D has a positive effect on improving FEV1 of patients whose FEV1 baseline value is less than 70%, but more RCTs are still needed to support this conclusion.
Systematic Review Registration: [https://inplasy.com], identifier [10.37766/inplasy20 22.6.0049].
Introduction
As one of the most common chronic, non-communicable diseases, asthma is a heterogeneous clinical syndrome affecting approximately 334 million people worldwide (1). It is defined by Expert Panel Report 3 (EPR-3) as “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role: in particular, mast cells, eosinophils, neutrophils (especially in sudden onset, fatal exacerbations, occupational asthma, and patients who smoke), T lymphocytes, macrophages, and epithelial cells. In susceptible individuals, this inflammation causes recurrent episodes of coughing (particularly at night or early in the morning), wheezing, breathlessness, and chest tightness. These episodes are usually associated with widespread but variable airflow obstruction that is often reversible either spontaneously or with treatment” (2). The global prevalence of asthma in adults is 4.3% (3) but varies in different countries, 7.8–11.9% in the United States (4–6), 10% in Japan (7), 2.38% in India (8), and 1.2–5.8% in China (9). More than 400 thousand people were estimated by the Global Burden of Disease collaboration to die from asthma, mainly in low- and middle-income countries (10). Airflow limitation, an important feature of asthma, is more common in low-and middle-income countries due to the higher prevalence of known risk factors and poor asthma management compared to high-income countries (11). Asthma in children is predominantly male, whereas in adults it is the opposite, probably due to the effects of sex hormones (12).
The existent evidence indicates that asthma is a disease associated with various factors, including environmental factors [air pollution (13), climate change, pollen (14), microbial exposure (15), and allergic triggers (16)], host factors [nutrition state (17) and infection (18)], and genetic factors [genetic susceptibility sites of asthma (19)]. Notably, many studies have shown that dietary factors could affect the course and development of asthma. High consumption of vegetables and fruits (20–23), especially apples and oranges, could reduce the risk of asthma. Pro-inflammatory cytokines associated with fruit and vegetable intake were simultaneously decreased and anti-inflammatory factors were increased (24, 25). In addition, there was a positive association between the frequent consumption of dairy products with asthma (26) and bronchial hyperreactivity (27). However, acute effects of milk ingestion were not significant in asthma patients (28–30). A diet that emphasizes fruits, vegetables, and whole grains, but not high-fat meat and dairy products, was related to reducing the risk of asthma (31–33).
As one of the fat-soluble vitamins required by the human body, vitamin D is obtained mainly through the skin synthesis pathway after ultraviolet B (UVB) radiation, and a small part from food (oily fish, egg yolk, mushroom, liver, or organ meat) and supplements. Cholecalciferol (vitamin D3) is derived from animals and ergocalciferol (vitamin D2) is derived from plants (34). Recently, vitamin D deficiency, one of the major risk factors in asthma, has triggered more and more interest in research, which was confirmed to involve in the development and prognosis of a variety of diseases, including cancer (35), inflammatory bowel disease (36), urinary tract infection (37), respiratory infections (38), and asthma (39). It was reported that the risk of acute respiratory infection (ARI) was reduced in individuals with high serum 25(OH)D levels (40). What’s more, a case-control study has reported that children who require hospitalization for acute respiratory infections had a significantly higher risk of vitamin D deficiency than children with mild acute respiratory infections (41). 1,25 (OH) 2D exerts antiviral activity and regulates inflammatory response to viral infection by stimulating cathelicidin release, regulating toll-like receptor expression, and inhibiting pro-inflammatory cytokines production (42). An RCT has proved that supplementation of vitamin D could protect against the development of acute respiratory tract infection (43). As for the rise of the COVID-19 pandemic, calcitriol non-significantly suppressed the expression of angiotensin II (Ang II) receptor type 1 (AT1) and angiotensin-converting enzyme (ACE), but markedly reduced Ang II formation, which acts as host cell receptors mediating SARS-CoV-2 infection (44). Evidence showed that vitamin D supplementation might reduce the risk of infection and death in COVID-19 (45, 46).
Furthermore, respiratory tract infection is the main cause of asthma aggravation (47). A great many studies have found that patients with low vitamin D levels were more likely to have asthma exacerbations (48–50). In addition, there is sufficient evidence that exposure to tobacco smoke and nicotine during the prenatal and postnatal periods impairs lung development, alters the immune response to viral infection, and increases the prevalence and severity of childhood wheezing (51). Chinellato I’s research demonstrated that vitamin D levels were significantly higher in children with non-smoking parents than those with both smoking parents, and were intermediate in those exposed to single maternal or paternal smoking (52). It has been reported that a modest reduction in 25- hydroxyvitamin D in pregnant women exposed to cigarette smoke, is probably because of the reduced ability of the placenta of women who smoke to transport vitamin D (53). In addition, smoking in adults was associated with osteopenia and decreased serum 25(OH)D and parathyroid hormone (PTH) concentrations (54, 55). While for smokers, Ben Michael Brumpton’s team found that Low serum 25(OH)D levels had a weaker correlation with greater decreases in lung function in adults with asthma, and a stronger correlation was observed in non-smokers, but not in ever smokers (56). As for the effect of vitamin D supplementation in smokers or non-smokers with asthma, Sluyter J. D.’s study demonstrated that vitamin D supplementation significantly improved the lung function of both ever-smokers and non-smokers with asthma. However, there is still a lack of RCTs on vitamin D supplementation in patients with asthma varying by smoking status (57).
However, there are contradictions between the mechanism research and clinical prognosis research on the effect of vitamin D supplementation on asthma. Some research has determined the relationship between vitamin D deficiency and the overall worsening of lung function and symptoms in patients with asthma (39, 58, 59). In terms of mechanism research, some asthma mouse model studies have indicated the protective effect of vitamin D supplementation. Serum IgE, whose elevated expression is the characteristic of active airway inflammation (60), could be reduced significantly via vitamin D supplementation. What’s more, vitamin D exerted a protective effect by reducing airway remodeling and inhibited airway inflammation by reducing oxidative stress and regulating the Th17/Treg balance and the NF-κB pathway (61). The classical Wnt/β-catenin pathway plays a key role in cell proliferation, cell migration, stem cell self-renewal, organogenesis, tissue homeostasis under physiological conditions, and damaged tissue repair (62). The intracellular accumulation and nuclear transfer of Wnt/β-catenin have a great impact on the maturation and structural adaptation of the lung, including the development of airway smooth muscle precursor cells, the maintenance of airway smooth muscle growth, and the regulation of its contraction, which was related to the pathogenesis of asthmatic airway remodeling (63–65). And the research showed that vitamin D improved airway remodeling in asthma by down-regulating the activity of the Wnt/β-catenin signaling pathway (66). In contrast, vitamin D deficiency aggravated the progression of asthma by increasing eosinophils, decreasing T regulatory cells, increasing NF-κB expression, and increasing pro-inflammatory cytokines (67). So far, there have been a number of meta-analyses regarding vitamin D supplementation in relation to asthma treatment. Some meta-analysis (68–72) have manifested that vitamin D supplementation reduced the rate of asthma exacerbations for patients with systemic corticosteroid treatment, especially in patients with vitamin D insufficiency, but didn’t affect the lung function (FEV1 or FENO) and ACT scores. However, there are still a few clinical studies manifesting that vitamin D supplementation in vitamin D-deficient patients didn’t improve the course and development of asthma (73, 74). Asthma control, asthma exacerbations, and lung function were all unaffected by vitamin D supplementation. The conclusions are not uniform, and some study populations only include children or adults. Therefore, a systematic meta-analysis of Randomized Controlled Trials (RCTs) was conducted to investigate the role of vitamin D supplementation and asthma treatment.
Objectives
The aim of this study was to evaluate the effect of vitamin D supplementation on clinical outcomes (Asthma Control Test scores, ACT scores; forced expiratory volume in 1 s, FEV1; fractional exhaled nitric oxide, FENO; asthma exacerbations) in asthma patients.
Methodology
Preferred reporting items (PRISMA) statements of systematic review and meta-analysis were used for the meta-analysis (75).
Search Strategy
A comprehensive literature search using predefined keywords from articles published over the last decade was conducted on PubMed, EMBASE, and the Cochrane library.
Manually search to retrieve articles using keywords: {(Asthma [Title/Abstract]) OR (asthma exacerbations [Title/Abstract])} AND (vitamin D [Title/Abstract]) AND (supplementation [Title/Abstract]) AND (RCTs [Title/Abstract]).
Inclusion Criteria
Randomized Controlled Trials published in English were included, in which vitamin D was prospectively added after the diagnosis of asthma to explore the role of vitamin D supplementation in asthmatics. The intervention group consisted of asthma patients who received any form or dose of vitamin D supplementation in addition to standard treatment, while those who did not receive vitamin D formed the control group. Then, the asthma-related outcomes were analyzed, including lung function (FEV1), FENO, ACT scores, and the rate of asthma exacerbations.
Exclusion Criteria
Retrospective and observational studies, articles or preprints not published in peer-reviewed journals, articles that did not mention the results included in our study or for which the data were incomplete, and retrospective vitamin D supplementation studies were excluded.
Study Selection
All studies selected from the database were filtered by title and abstract to exclude unrelated or duplicate articles. Two authors screened independently, and a third co-author was involved in resolving differences that arose during the literature screening process.
Data Extraction
Two authors independently extracted the relevant data from the article, including study population (age, country), intervention measures (vitamin D administration method and dose), follow-up time and outcomes (FEV1, FENO, asthma exacerbations and ACT scores), and baseline data related to the results (mean age, FEV1, ACT scores and vitamin D content).
Quality Assessment
The two authors independently evaluated the methodological quality of the included studies based on Cochrane’s systematic review guidelines and resolved the differences through discussion with the third co-author. The risk of bias was plotted using Review Manager 5.4 and individual quality analysis was performed using the GRADE-PRO method.
Statistical Analysis
In this meta-analysis, we used risk ratio (RR) and standard error (SMD) as the impact measurement standards, R software version 4.1.1 (R project in Vienna, Austria) for statistical analysis and forest mapping. The methodological quality of the study was evaluated using Review Manager Version 5.4 following the Cochrane guidelines. A random effect model was used for statistical analysis due to differences in the mix of interventions and participants. The heterogeneity among studies was assessed by Cochran Q-test, and P < 0.05 was considered statistically significant. When data from three or more studies were available, results were summarized using either the standardized mean difference (SMD) for continuous variables or the risk ratio (RR) for dichotomized variables. Statistical analysis was performed using the Mann-Whitney U test, and a two-sided P-value of <0.05 was considered statistically significant. Using the I2 statistic to evaluate the degree of heterogeneity between included studies. I2 values of 25, 50, and 75% were considered low, medium, and high heterogeneity. In addition, in order to explore the impact and heterogeneity of each outcome, prespecified subgroup analyses were stratified by FEV1 baseline values (less than 70% or greater), age (children or adults), and follow up time. The use of funnel plots failed to demonstrate potential publication bias since each result did not reach 10 studies. Sensitivity analyses were performed to check the robustness of the results by omitting one study and analyzing the remainder in each round.
Results
Study Characteristics
In this review, we used database search and a comprehensive manual search strategy. A total of 259 studies was found in the initial search, and 49 RCTs were screened out. After manual deletion of duplicate references, the remaining 20 studies were selected by title and abstract. There were 15 eligible articles after excluding irrelevant articles. Among them, studies in which outcome indicators were variation quantity before and after intervention or the outcome indicators which had missing values were excluded. Eventually, 10 studies were included in the review and met the inclusion criteria through evaluating the full text (Figure 1 and Table 1).
Description of the Included Studies
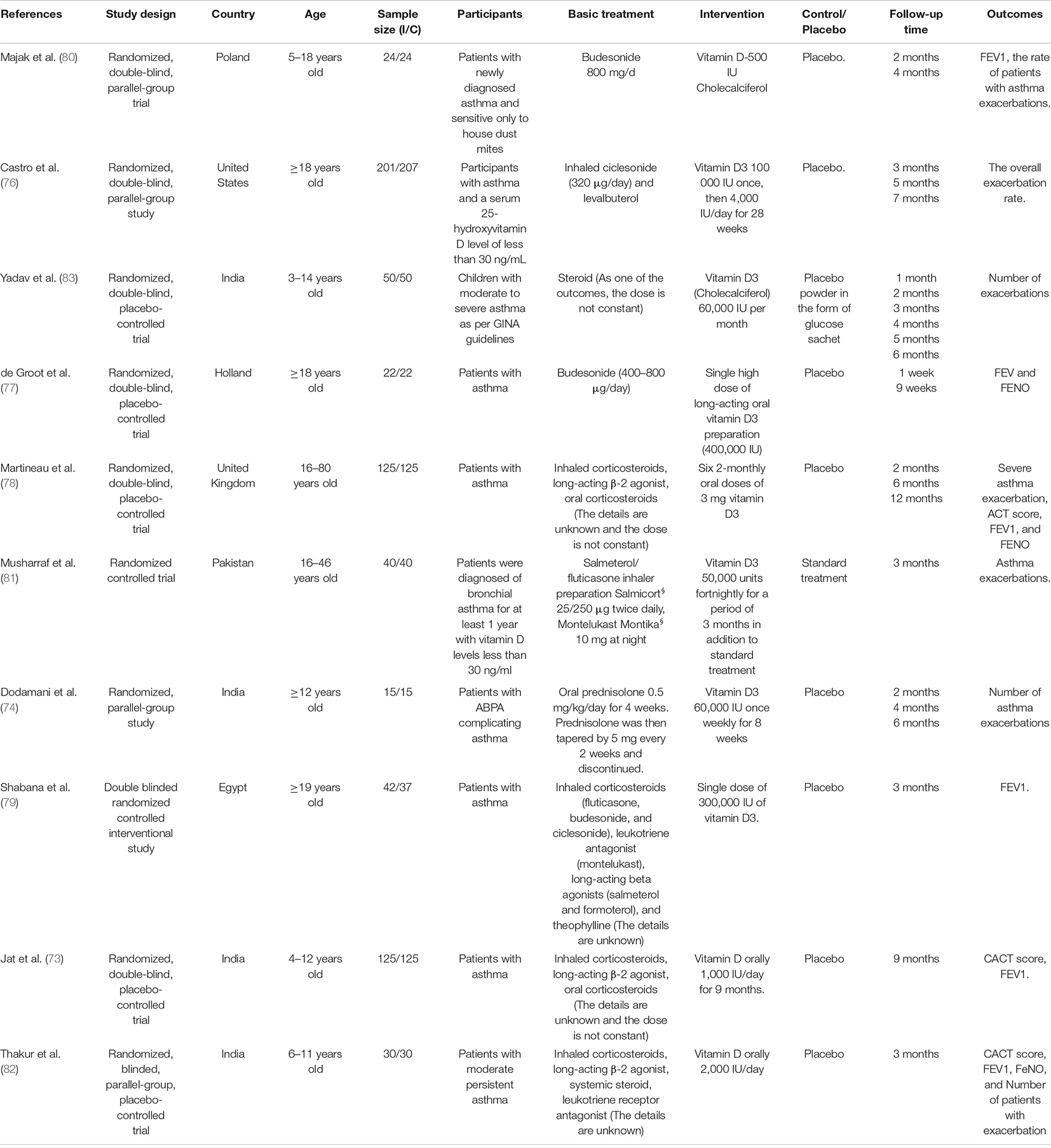
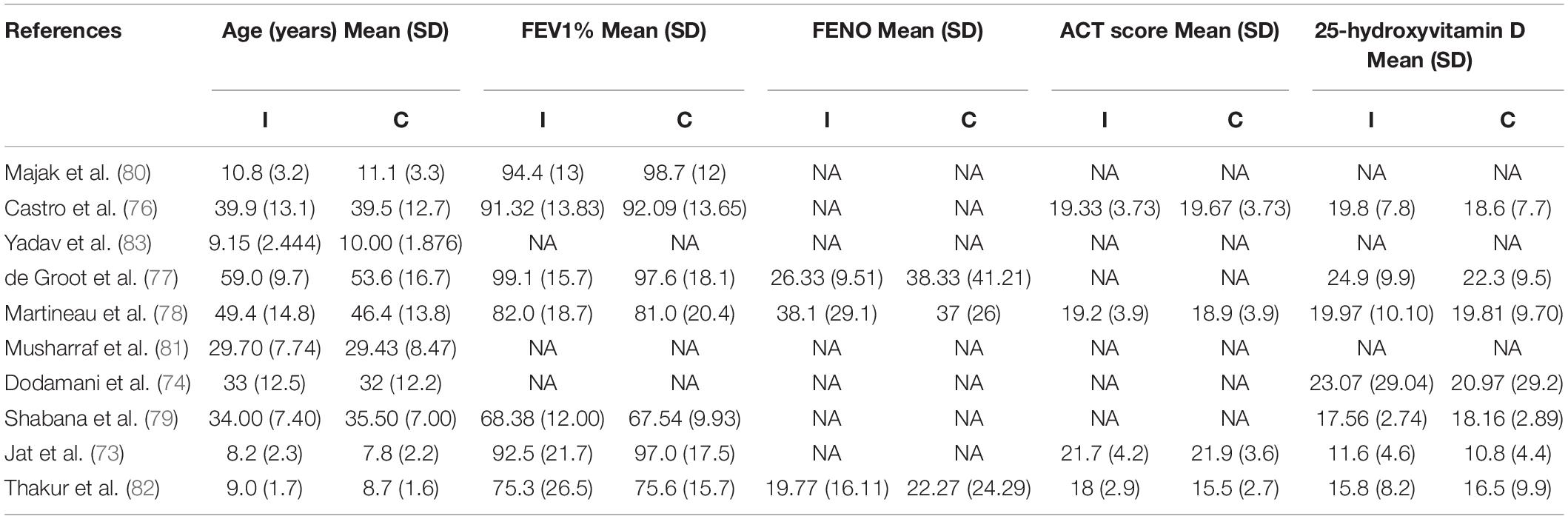
The characteristics and baseline data of included RCTs were presented in Tables 1, 2 In this review, all the included studies were RCTs, including the detailed information of 1,349 subjects, with the sample size ranging from 15 to 207, and the locations of the subjects involved in the United States (76), Holland (77), United Kingdom (78), Egypt (79), Poland (80), Pakistan (81), and India (73, 74, 82, 83).
Among the 10 included studies, one RCT (74) included patients with allergic bronchopulmonary aspergillosis (ABPA) complicating asthma, whereas the other nine RCTs included patients with asthma (73, 76–81) or moderate persistent asthma (82, 83). In addition, six studies in which participants were adults (74, 76–79, 81), while the other four RCTs were children (73, 80, 82, 83).
There was significant heterogeneity in the doses of vitamin D used in the intervention groups, with the control group receiving an equal dose of placebo, and both two groups receiving a standardized treatment, inhaled corticosteroid, according to the guidelines. The follow-up time ranged from 1 week (77) to 12 months (78).
Two RCTs (78, 82) analyzed ACT score, asthma exacerbations, FENO, and FEV1 as outcome measures. The other three studies all analyzed FEV1 as the outcome in addition to ACT scores (73), FENO (77), and asthma exacerbations (80), respectively. Asthma exacerbations were used as an outcome in Castro’s (76), Dodamani’s (74), Yadav’s (83), and Musharraf’s (81) studies. The rest of one RCT (79) used FEV1 to evaluate the outcome of the two groups.
Baseline FEV1 values were reported in seven studies in the two groups, six of which were greater than 70% (73, 76–78, 80, 82), whereas one of which was less than 70% (79). Three RCTs reported FENO baseline values, two of which were higher than those in the intervention group (77, 82), and the other was the opposite (78). Four RCTs counted the baseline values of ACT scores, among which the median value of three RCTs was greater than 19 points (73, 76, 78) and the other was less than 19 points (82). Baseline data for 25-hydroxyvitamin D were available for seven RCTs enrolled, with all the studies less than 30 ng/ml, and two of them more than 20 ng/ml (74, 77) and the others less than 20 ng/ml (73, 76, 78, 79, 82) (Table 2).
Methodological Quality of Study
According to Cochrane system evaluation guidelines, we conducted a risk bias assessment for each study included in this evaluation. A summary chart of bias risk was shown in Figure 2, in which red represents high deviation risk, green represents low deviation risk, and yellow represents ambiguous deviation risk. Figure 3 showed the risk of bias graph, in which the authors expressed our judgments on various risk items of bias in each study in percentage form.
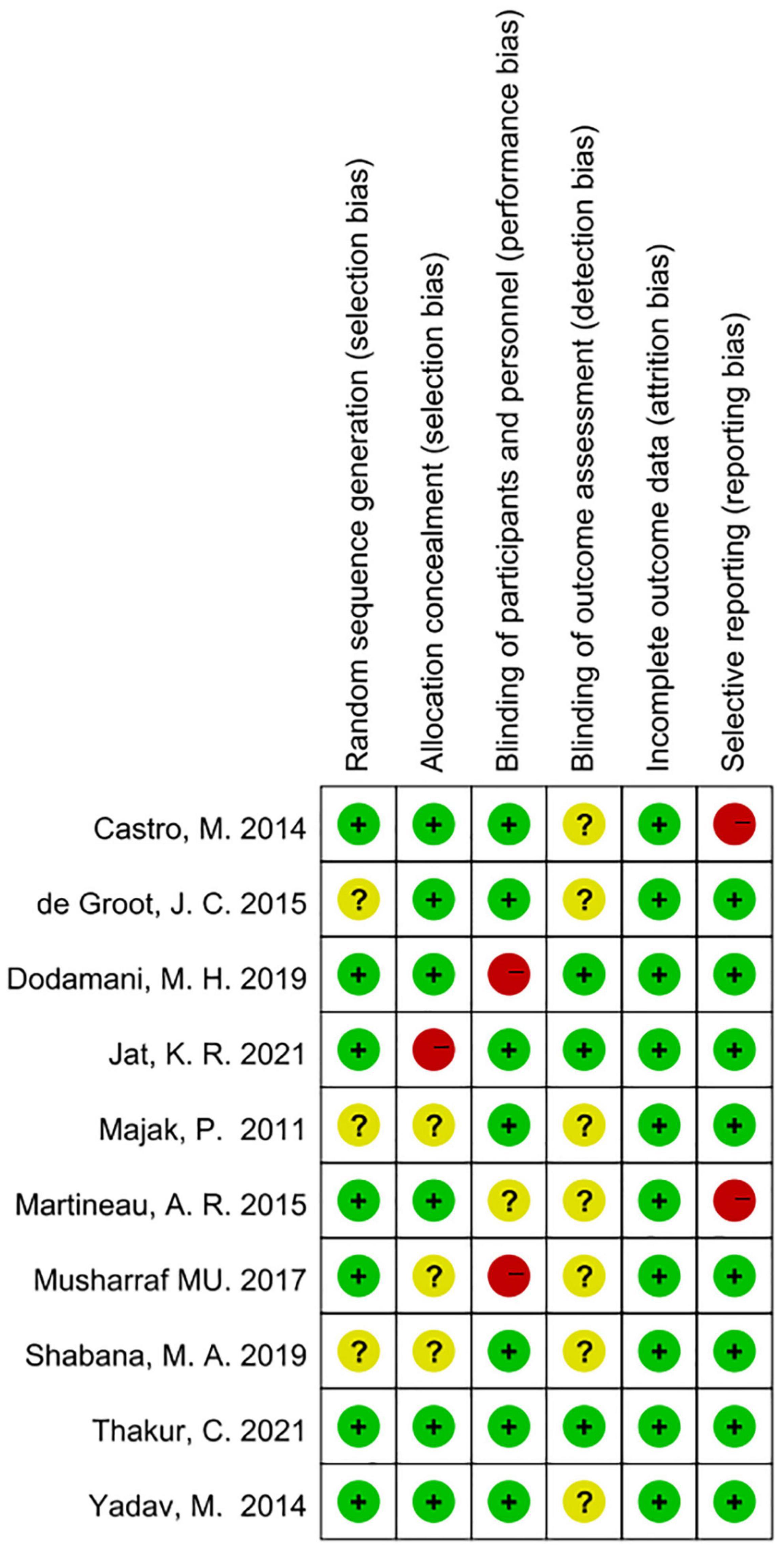
Figure 2. Risk of bias summary based on Cochrane Systematic Review Guidelines for each included study included in this review (green for low risk of bias, yellow for unclear risk of bias and red for high risk of bias).
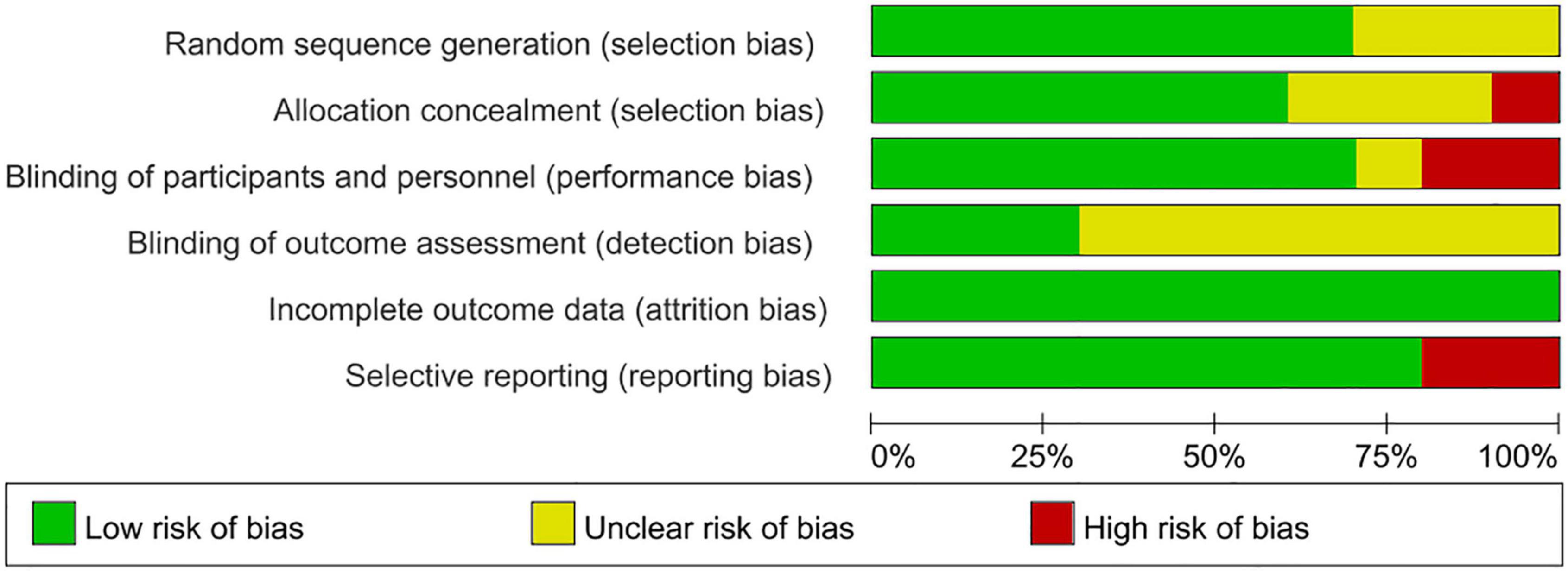
Figure 3. Risk of bias graph review authors judgments about each risk of bias item presented as percentages across various study designs.
Grade summary Table 3 gave an overall rating for the quality of evidence regarding the role of vitamin D supplementation in asthma patients. The GRADE summary demonstrated that the evidence for exacerbation of asthma (in the adult and over 6 months of follow-up subgroup) and FEV1 (in children, adults, and under 6 months of follow-up subgroup) were very low, meaning that the effect estimation was uncertain. It might be related to the significant difference in the dose and mode of vitamin D administration and the baseline data of patients across different RCTs.
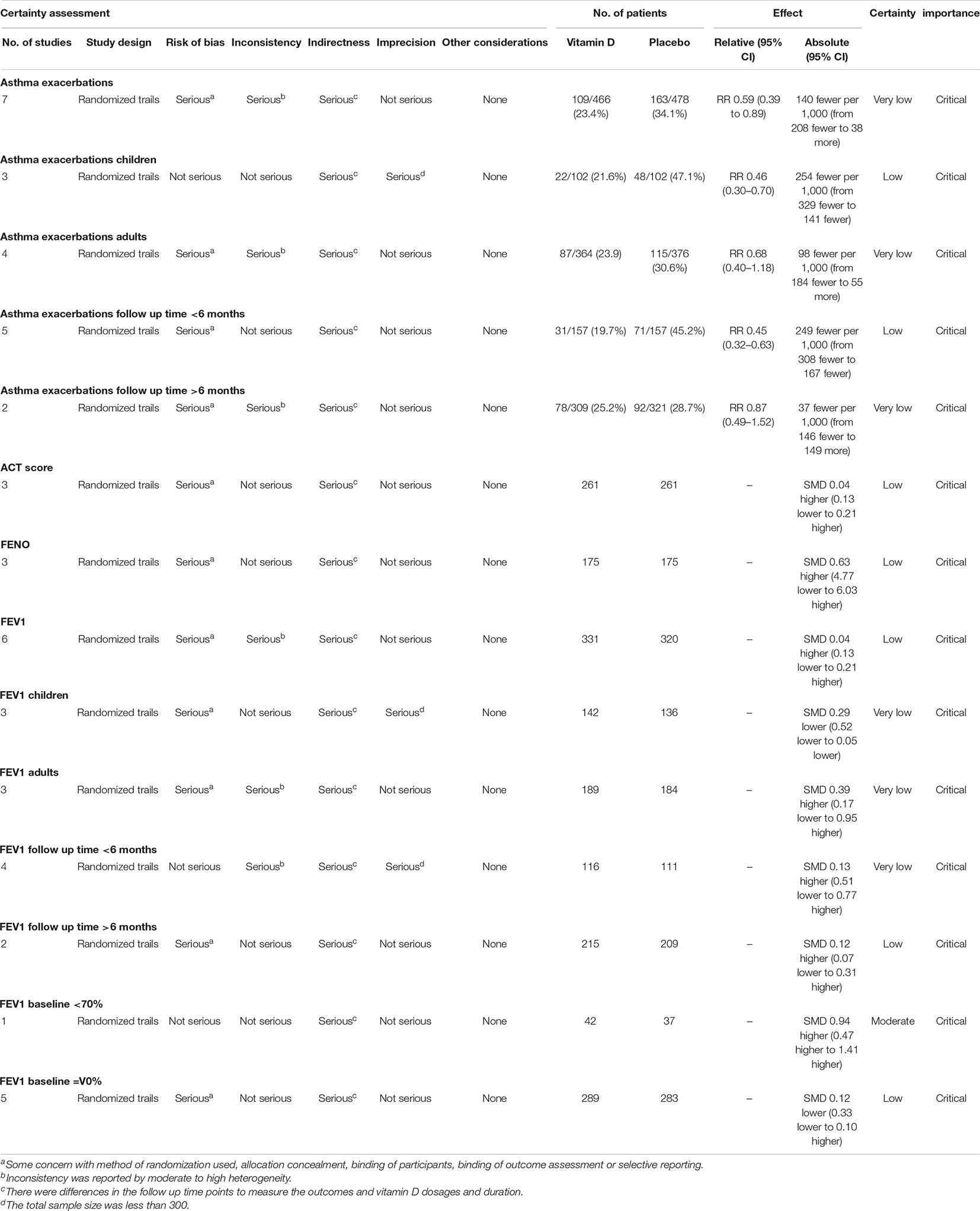
Table 3. The overall rating for the quality of evidence profile for asthma related health outcomes based on the grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working group methodology.
Efficacy Outcomes
Asthma Control Test Scores
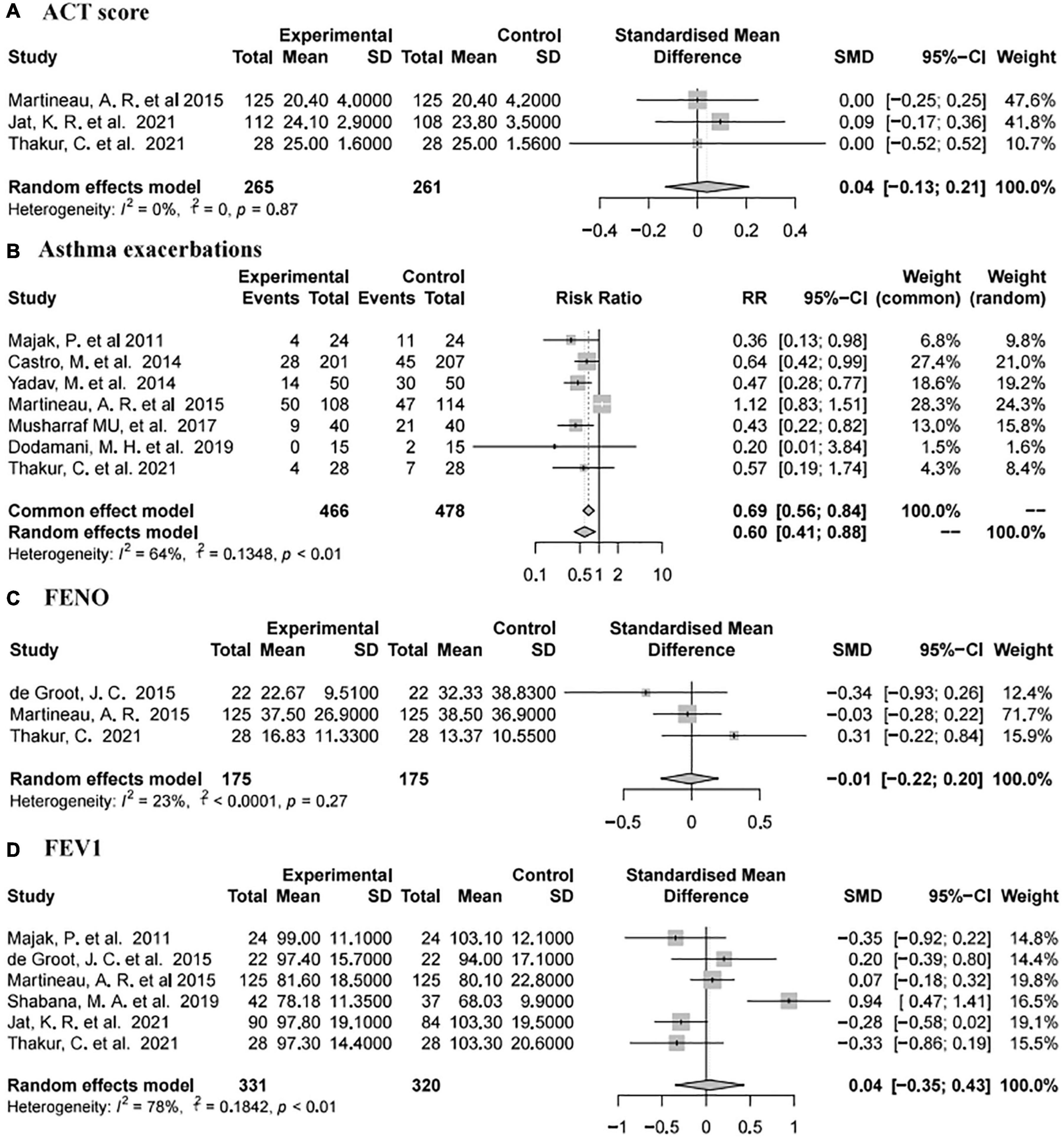
Asthma Control Test (ACT) scores were reported in three studies (73, 78, 82) involving 526 individuals (265 intervention and 261 placebo). The pooled data demonstrated that there was no significant difference between the placebo and vitamin D groups (SMD 0.04, 95% CI −0.13 to 0.21, low heterogeneity (I2 = 0%, P = 0.87; Figure 4A).
Forced Expiratory Volume in One Second
Forced expiratory volume in one second was reported in six studies (73, 77–80, 82) involving 651 subjects (331 intervention and 320 placebo). The summary data showed that there was no significant difference between the placebo group and vitamin D group [SMD 0.04, 95% CI −0.35 to 0.43, high heterogeneity (I2 = 78%, P < 0.01; Figure 4D)].
Subgroup analysis of the results for FEV1 was further performed (Figure 5). For the age subgroups, there was no significant difference between the placebo and vitamin D groups in adults [SMD 0.39, 95% CI −0.15 to 0.93, high heterogeneity (I2 = 81%, P < 0.01)], while vitamin D supplementation was associated with a reduction of FEV1 at the last visit in children [SMD −0.3, 95% CI −0.54 to −0.07, low heterogeneity (I2 = 0%, P = 0.97; Figure 5A)]. Regarding different FEV1 baseline values, there was no significant difference between the two groups for patients with FEV1 baseline values exceeding 70% [SMD −0.12, 95% CI −0.34 to 0.10, low heterogeneity (I2 = 31%, P = 0.22)], while vitamin D supplementation was related to the increase of FEV1 at last visit for patients with FEV1 baseline values less than 70% (SMD 0.94, 95% CI 0.47 to 1.41, without applicable heterogeneity; Figure 5B). For different follow-up times, vitamin D supplementation was not associated with FEV1 when the follow-up time was less than 6 months [SMD 0.13, 95% CI −0.48 to 0.74, high heterogeneity (I2 = 82%, P < 0.01)] or more than 6 months [SMD 0.11 95% CI −0.35 to 0.43, low heterogeneity (I2 = 39%, P = 0.53; Figure 5C)].
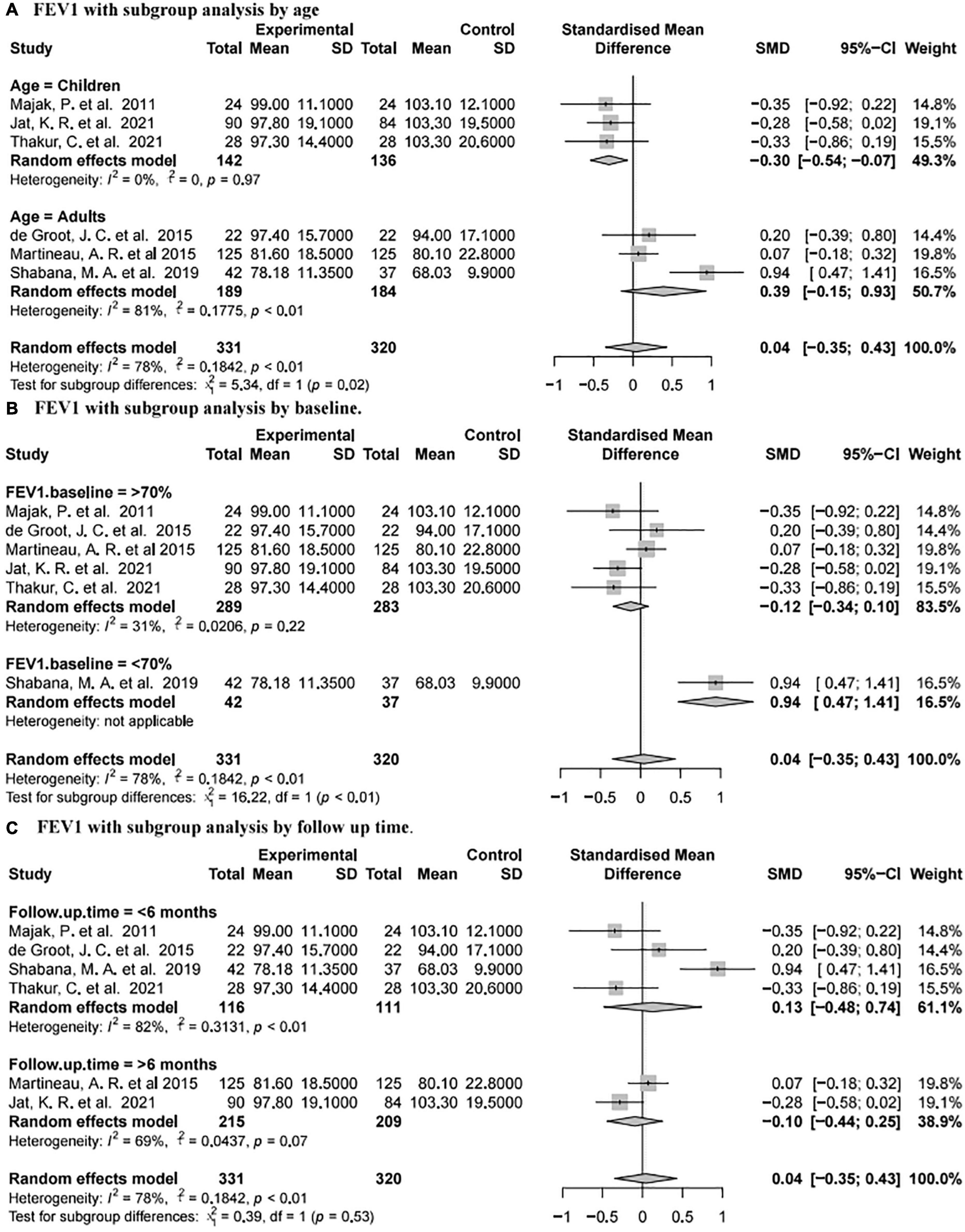
Figure 5. Forest plot random effect model for vitamin D supplementation for FEV1 with subgroup by various factors.
Asthma Exacerbations
Seven studies (74, 76, 78, 80–83) reported asthma exacerbations involving 944 subjects (466 intervention and 478 placebo). The pooled data showed that vitamin D supplementation was associated with a reduced rate of asthma exacerbations (RR 0.60, 95% CI 0.41–0.88, high heterogeneity (I2 = 64%, P < 0.01; Figure 4B)].
Subgroup analysis of asthma exacerbation results was complicated (Figure 6). In terms of different age groups, there was no significant difference between the placebo and vitamin D groups in adults [RR 0.69, 95% CI 0.40 to 1.17, high heterogeneity (I2 = 71%, P = 0.02)], while vitamin D supplementation was related to reducing the rate of asthma exacerbations in children [RR 0.46, 95% CI 0.30 to 0.70, low heterogeneity (I2 = 0%, P = 0.83; Figure 6A)]. According to different follow-up time, vitamin D supplementation was related to the reduction of asthma exacerbations with less than 6 months of follow-up [RR 0.45, 95% CI 0.32 to 0.63, low heterogeneity (I2 = 0%, P = 0.95)], but not with more than 6 months of follow-up [RR 0.87, 95% CI 0.50 to 1.50, high heterogeneity (I2 = 77%, P = 0.04; Figure 6B)].
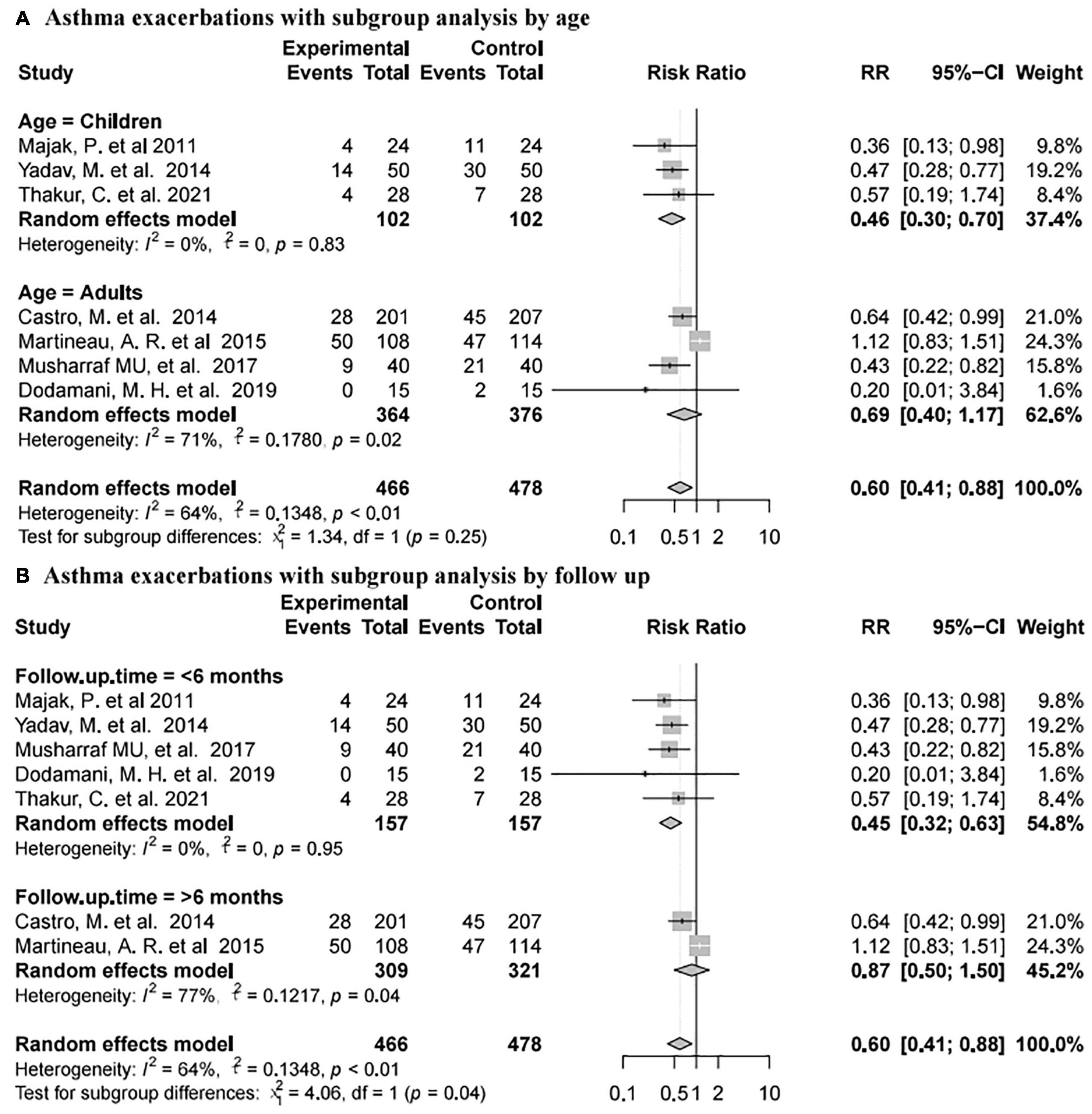
Figure 6. Forest plot random effect model for vitamin D supplementation for asthma exacerbations with subgroup by various factors.
Fractional Exhaled Nitric Oxide
Fractional exhaled nitric oxide was reported in three studies (77, 78, 82) involving 350 subjects (175 intervention and 175 placebo). The pooled data indicated that there was no significant difference between the placebo and vitamin D groups [SMD −0.01, 95% CI −0.22 to 0.2, low heterogeneity (I2 = 23%, P = 0.27; Figure 4C)].
Sensitivity Analysis
Sensitivity analysis of the outcomes using R language software (4.1.1) indicated that, after omitting each individual study, our results were consistent with the complete analysis of all endpoints, and that there was no significant correlation between vitamin D supplementation and the prognosis of patients with asthma (Figure 7).
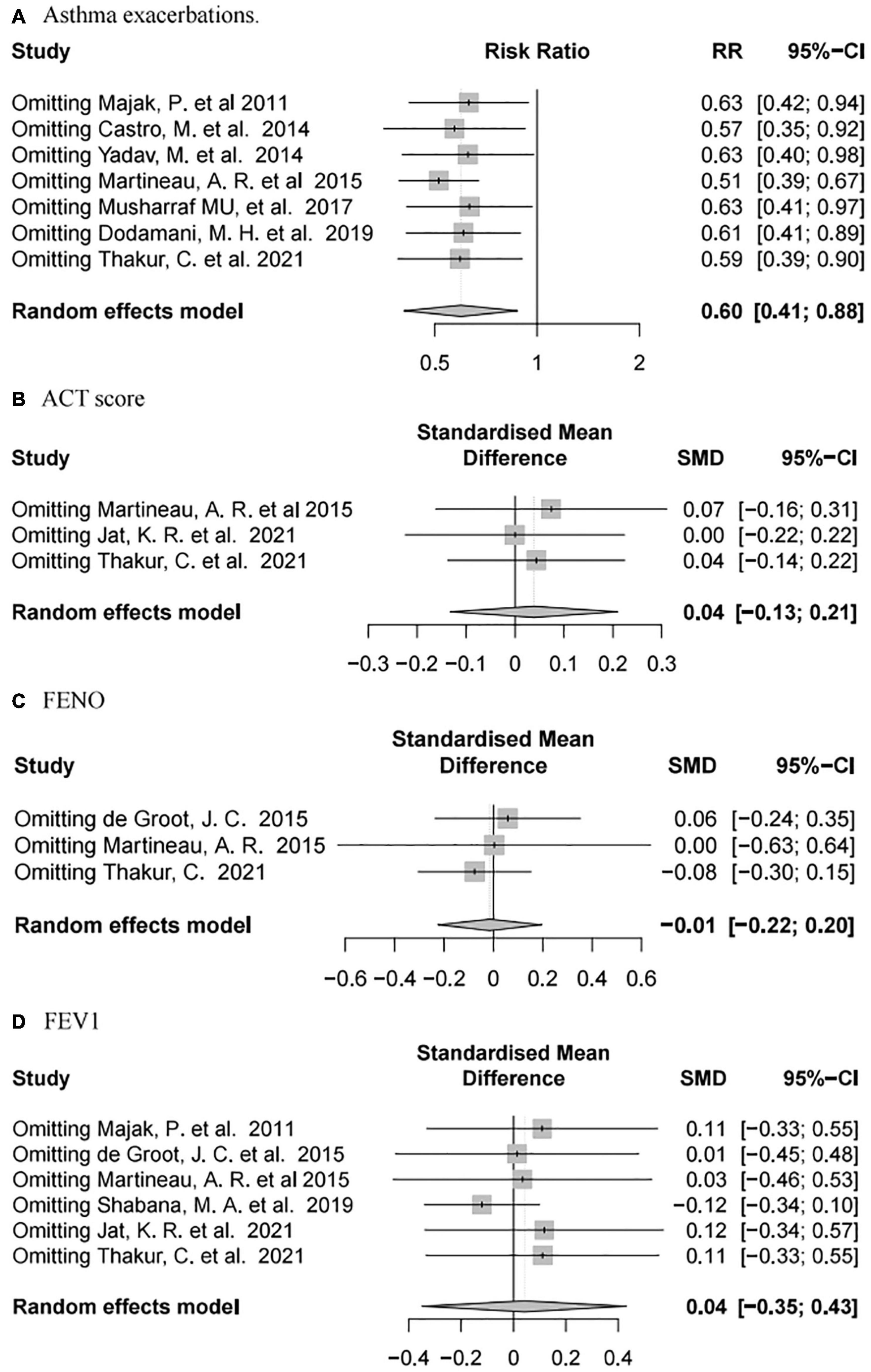
Figure 7. Forest plot random effect model of sensitivity analysis for vitamin D supplementation for various outcomes.
Discussion
In this systematic meta-analysis, vitamin D supplementation in asthmatics did not improve major health outcomes including ACT scores, FEV1, FENO, and asthma exacerbations, but contributed to increased FEV1 in subgroups with less than 70% of FEV1 baseline. In addition, enrolled RCTs showed significant baseline heterogeneity in both vitamin D dose and demographic characteristics.
There are still no objective markers to assess asthma severity. Although asthma patients have a certain degree of the inflammatory response, some severe patients may also develop exacerbation and deterioration of asthma after inflammation is controlled (84). The Primary Care Asthma Control Screening tool (adult) (85) or the Asthma Control Test [adults (86) or children (87)] can be used to quickly assess control of asthma symptoms with questionnaires. Clinical efficacy results indicated a cutoff point of 19 or lower for C-ACT or ACT (86, 87), indicating incomplete asthma control. Over the years, the definition of acute asthma or exacerbation has varied. Currently, severe exacerbation is defined as requiring corticosteroid use for at least 3 days or as an inpatient or emergency room visit due to the need for corticosteroids for asthma. Moderate deterioration was defined as an event that required modification of treatment to prevent it from becoming severe and not so severe as to require oral corticosteroids (OCS) by the American Thoracic Society/European Respiratory Society (88). The transcriptomic profile of bronchoscopy has identified high and low type 2 immunity asthma and other molecular phenotypes (89, 90). Adaptive T helper 2 cell activation produces a series of cytokines following allergen sensitization and stimulation of dendritic cells. Eosinophils are recruited to the lung mucosa by chemokine receptors and other eosinophil chemo-attractants (3). In non-eosinophilic asthma, innate lymphoid cells, macrophages, and neutrophils have an important role in stimulating the release of cytokines (interleukin-33 and interleukin-25) or chemokines (C–X–C motif chemokine ligand 8), to regulate the immune response (91, 92).
With the development of economy and medical level, vitamin D, a proline obtained from skin exposure to ultraviolet B (UVB) light and dietary intake from the liver, fish, egg yolk, and other sources, is transformed to 25-hydroxyvitamin D [25(OH)D] in liver (93, 94), which has gradually attracted the attention of the majority of domestic and foreign research scholars. Several studies have demonstrated a correlation between vitamin D deficiency and asthma prevalence and severity. Patients with vitamin D deficiency have a higher prevalence of asthma, which could be a strong prediction factor of asthma (95–97). Additionally, vitamin D deficiency was also associated with severe asthma exacerbations in multiple prospective and retrospective (98–100). Compared with children with insufficient or sufficient vitamin D, there was a correlation between vitamin D deficiency and pulmonary dysfunction in asthmatic children treated with inhaled corticosteroids (101). Although as a nutrient that regulates metabolism, vitamin D has been shown to immunomodulate various immune cells and structural cells in the airway, by activating vitamin D receptors (VDR) (102–105). Several in vitro and in vivo studies using asthma murine models have also shown that vitamin D modulated the inflammatory response. In vitamin D-treated asthmatic mice, the Penh values, type 2 cytokines, perivascular and peribronchial inflammation, goblet cell proliferation, total IgE and histamine, and mucus hypersecretion were all significantly reduced (106). Vitamin D deficiency also potentiated oxidative stress and corticosteroid resistance in severe asthma exacerbations. Vitamin D3 supplementation significantly increased the change of FEV1, and effectively alleviated ROS and DNA damage, which were related to a decrease in TNF-α and NF-κB in epithelial cells (107). Oxidative stress-activated transcription factors (TF) and signaling pathways, and partly activated the innate immune response through toll-like receptors 2 (TLR-2) and toll-like receptors 4 (TLR-4), thus promoting the release of cytokines and chemokines. In addition, oxidative stress had an important role in affecting corticosteroid insensitivity by inhibiting the activity and expression of HDAC-2 via serine hyperphosphorylation (108). Although there has been sufficient evidence that vitamin D deficiency was associated with progression and exacerbation of asthma, there are many inconsistencies in multiple prospective clinical studies. The researches indicated that vitamin D supplementation was not of use in preventing severe asthma exacerbations or control of asthma in children (73, 82, 109) or adults (76, 78). High-dose vitamin D supplementation during pregnancy did not reduce the risk and improve the allergy outcomes of asthma in children (under 6 years of age) compared to standard doses (110).
Additionally, we further confirmed that it could effectively alleviate the probability of asthma exacerbations in children and when follow-up time was less than 6 months (Figure 6). Noticeably, it significantly enhanced FEV1 in patients whose FEV1 baseline value was less than 70%, though there was only one study included in the subgroup. Only one former meta-analysis (111) demonstrated that vitamin D supplementation couldn’t reduce asthma exacerbations and FeNO, nor could it improve lung function and asthma symptoms. Our meta-analysis offers several advantages over previous meta-analyses. First, all included studies were RCTs, and the studies with incomplete data were strictly excluded according to the standard. Second, subgroup analyses of included studies were performed to minimize heterogeneity for baseline values including age, FEV1 values, and follow-up time in our analysis (Table 2). Finally, the sensitivity analysis was similar to the above results, indicating that the results of this meta-analysis were reliable (Figure 7). However, there are still many defects in our meta-analysis. First, heterogeneity in dose and mode of administration of vitamin D in enrolled studies was unavoidable, and not all subjects enrolled in various studies received consistent basic anti-asthma therapy. Some studies standardized the therapeutic dose of glucocorticoids for asthma (74, 76, 77, 80, 81), some observed it as an outcome variable (73, 78, 82, 83). And most studies didn’t mention whether the hormone dose was changed during the follow up (73, 78, 79, 82), so we are not sure whether this will affect the accuracy of the results of RCTs. Second, the sample size of several studies included in this analysis was too small to demonstrate the reliability of the results. Finally, not all subjects enrolled in the study were asthmatics of the same severity or etiology.
In conclusion, our meta-analysis demonstrated that there was high heterogeneity in RCTs regarding improvement in exacerbation of asthma and FEV1 with vitamin D supplementation. Vitamin D supplementation led to a reduction of asthma exacerbations, especially in children and with a follow-up period of less than 6 months. In addition, it played an important role in improving FEV1 in patients with FEV1 baseline values below 70%. Though evaluating the ACT scores and FENO, we found that vitamin D worked the same way as a placebo. Based on the results of the GRADE analysis, all major findings were low or very low except for the FEV1 subgroup with baseline values below 70%. Therefore, a larger and well-designed RCT is needed to evaluate the effect of vitamin D in the treatment of asthma, including uniform vitamin D dosing and administration mode, follow-up time, and strict inclusion and exclusion criteria. Furthermore, whether basic asthma treatment should be standardized during follow-up or used as an outcome measure of asthma treatment efficacy still needs to be further explored.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
ML: data selection, data extraction, quality assessm statistical analysis, and writing – original draft. JW: data selection, data extraction, and quality assessment. XS: conceptualization, writing – review, and supervision.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledged the support of the Infectious Diseases Department of Children’s Hospital Affiliated to Xi’an Jiaotong University. We also appreciated editors and proofreaders for their assistance.
References
1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 15:2163–96. doi: 10.1016/S0140-6736(12)61729-2
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma–summary report 2007. J Allergy Clin Immunol. (2007) 120:S94–138.
4. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. (2020) 42:5–15.
5. Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. (2012) 35:1–58.
6. Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. (2012) 94:1–8.
7. Nakamura Y, Tamaoki J, Nagase H, Yamaguchi M, Horiguchi T, Hozawa S, et al. Japanese guidelines for adult asthma 2020. Allergol Int. (2020) 69:519–48. doi: 10.1016/j.alit.2020.08.001
8. Singh SK, Gupta J, Sharma H, Pedgaonkar SP, Gupta N. Socio-economic correlates and spatial heterogeneity in the prevalence of asthma among young women in India. BMC Pulm Med. (2020) 20:190. doi: 10.1186/s12890-020-1124-z
9. Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. (2019) 394:407–18. doi: 10.1016/S0140-6736(19)31147-X
10. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet Respir Med. (2017) 5:691–706. doi: 10.1016/S2213-2600(17)30293-X
11. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. (2008) 31:143–78.
12. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
14. D’Amato G, Chong-Neto HJ, Monge Ortega OP, Vitale C, Ansotegui I, Rosario N, et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy. (2020) 75:2219–28. doi: 10.1111/all.14476
15. Holt PG, Sly PD. Environmental microbial exposure and protection against asthma. N Engl J Med. (2015) 373:2576–8. doi: 10.1056/NEJMcibr1511291
16. D Amato M, Cecchi L, Annesi-Maesano I, D Amato G. News on climate change, air pollution, and allergic triggers of asthma. J Investig Allergol Clin Immunol. (2018) 28:91–7. doi: 10.18176/jiaci.0228
17. Alwarith J, Kahleova H, Crosby L, Brooks A, Brandon L, Levin SM, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev. (2020) 78:928–38.
18. Kumar S, Roy RD, Sethi GR, Saigal SR. Mycoplasma pneumoniae infection and asthma in children. Trop Doct. (2019) 49:117–9.
19. Bonnelykke K, Ober C. Leveraging gene-environment interactions and endotypes for asthma gene discovery. J Allergy Clin Immunol. (2016) 137:667–79. doi: 10.1016/j.jaci.2016.01.006
20. Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. (2002) 76:560–8. doi: 10.1093/ajcn/76.3.560
21. Seyedrezazadeh E, Pour Moghaddam M, Ansarin K, Reza Vafa M, Sharma S, Kolahdooz F. Fruit and vegetable intake and risk of wheezing and asthma: a systematic review and meta-analysis. Nutr Rev. (2014) 72:411–28. doi: 10.1111/nure.12121
22. Hosseini B, Berthon BS, Wark P, Wood LG. Effects of fruit and vegetable consumption on risk of asthma, wheezing and immune responses: a systematic review and meta-analysis. Nutrients. (2017) 9:341. doi: 10.3390/nu9040341
23. Shaheen SO, Sterne JA, Thompson RL, Songhurst CE, Margetts BM, Burney PG. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. (2001) 164:1823–8. doi: 10.1164/ajrccm.164.10.2104061
24. Wood LG, Garg ML, Powell H, Gibson PG. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res. (2008) 42:94–102. doi: 10.1080/10715760701767307
25. Baines KJ, Wood LG, Gibson PG. The nutrigenomics of asthma: molecular mechanisms of airway neutrophilia following dietary antioxidant withdrawal. Omics. (2009) 13:355–65. doi: 10.1089/omi.2009.0042
26. Han YY, Forno E, Brehm JM, Acosta-Pérez E, Alvarez M, Colón-Semidey A, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. (2015) 115:288–293.e1. doi: 10.1016/j.anai.2015.07.020
27. Woods RK, Walters EH, Raven JM, Wolfe R, Ireland PD, Thien FC, et al. Food and nutrient intakes and asthma risk in young adults. Am J Clin Nutr. (2003) 78:414–21. doi: 10.1093/ajcn/78.3.414
28. Haas F, Bishop MC, Salazar-Schicchi J, Axen KV, Lieberman D, Axen K. Effect of milk ingestion on pulmonary function in healthy and asthmatic subjects. J Asthma. (1991) 28:349–55. doi: 10.3109/02770909109089462
29. Woods RK, Weiner JM, Abramson M, Thien F, Walters EH. Do dairy products induce bronchoconstriction in adults with asthma? J Allergy Clin Immunol. (1998) 101:45–50. doi: 10.1016/S0091-6749(98)70192-7
30. Nguyen MT. Effect of cow milk on pulmonary function in atopic asthmatic patients. Ann Allergy Asthma Immunol. (1997) 79:62–4. doi: 10.1016/S1081-1206(10)63086-4
31. Rice JL, Romero KM, Galvez Davila RM, Meza CT, Bilderback A, Williams DL, et al. Association between adherence to the mediterranean diet and asthma in Peruvian children. Lung. (2015) 193:893–9. doi: 10.1007/s00408-015-9792-9
32. Guglani L, Joseph CL. Asthma and diet: could food be thy medicine? Indian Pediatr. (2015) 52:21–2.
33. Lv N, Xiao L, Ma J. Dietary pattern and asthma: a systematic review and meta-analysis. J Asthma Allergy. (2014) 7:105–21. doi: 10.2147/JAA.S49960
34. Chang SW, Lee HC. Vitamin D and health – the missing vitamin in humans. Pediatr Neonatol. (2019) 60:237–44. doi: 10.1016/j.pedneo.2019.04.007
35. de La Puente-Yague M, Cuadrado-Cenzual MA, Ciudad-Cabanas MJ, Hernandez-Cabria M, Collado-Yurrita L. Vitamin D: and its role in breast cancer. Kaohsiung J Med Sci. (2018) 34:423–7. doi: 10.1016/j.kjms.2018.03.004
36. Bendix M, Dige A, Jorgensen SP, Dahlerup JF, Bibby BM, Deleuran B, et al. Seven weeks of high-dose vitamin D treatment reduces the need for infliximab dose-escalation and decreases inflammatory markers in Crohn’s disease during one-year follow-up. Nutrients. (2021) 13:1083. doi: 10.3390/nu13041083
37. Qadir S, Memon S, Chohan MN, Memon Y. Frequency of vitamin-D deficiency in children with urinary tract infection: a descriptive cross-sectional study. Pak J Med Sci. (2021) 37:1058–62. doi: 10.12669/pjms.37.4.3896
38. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. (2019) 23:1–44.
39. Liu J, Dong YQ, Yin J, Yao J, Shen J, Sheng GJ, et al. Meta-analysis of vitamin D and lung function in patients with asthma. Respir Res. (2019) 20:161. doi: 10.1186/s12931-019-1072-4
40. Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. (2010) 5:e11088. doi: 10.1371/journal.pone.0011088
41. Ingham TR, Jones B, Camargo CA Jr., Kirman J, The Whiti Te Ra Study Group. Association of vitamin D deficiency with severity of acute respiratory infection: a case-control study in New Zealand children. Eur Respir J. (2014) 44:439. doi: 10.1186/s13054-016-1208-6
42. Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. (2011) 50:194–200. doi: 10.1016/j.jcv.2010.12.006
43. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. (2017) 356:i6583. doi: 10.1136/bmj.i6583
44. Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. (2019) 26:101295. doi: 10.1016/j.redox.2019.101295
45. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. (2020) 12:988.
46. Mendy A, Apewokin S, Wells AA, Morrow AL. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv. (2020) [Preprint]. doi: 10.1101/2020.06.25.20137323
47. Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. (2017) 140:895–906. doi: 10.1016/j.jaci.2017.08.003
48. Turkeli A, Ayaz O, Uncu A, Ozhan B, Bas VN, Tufan AK, et al. Effects of vitamin D levels on asthma control and severity in pre-school children. Eur Rev Med Pharmacol Sci. (2016) 20:26–36.
49. Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. (2017) 195:854–9.
50. Maes K, Serré J, Mathyssen C, Janssens W, Gayan-Ramirez G. Targeting vitamin D deficiency to limit exacerbations in respiratory diseases: utopia or strategy with potential? Calcif Tissue Int. (2020) 106:76–87. doi: 10.1007/s00223-019-00591-4
51. Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest. (2016) 149:552–61. doi: 10.1378/chest.15-1858
52. Chinellato I, Piazza M, Sandri M, Paiola G, Tezza G, Boner AL. Correlation between vitamin D serum levels and passive smoking exposure in children with asthma. Allergy Asthma Proc. (2018) 39:8–14. doi: 10.2500/aap.2018.39.4124
53. Banihosseini SZ, Baheiraei A, Shirzad N, Heshmat R, Mohsenifar A. The effect of cigarette smoke exposure on vitamin D level and biochemical parameters of mothers and neonates. J Diabetes Metab Disord. (2013) 12:19. doi: 10.1186/2251-6581-12-19
54. Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. (1999) 53:920–6. doi: 10.1038/sj.ejcn.1600870
55. Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosén T, Lindstedt G, Lundberg PA, et al. Serum intact parathyroid hormone in a random population sample of men and women: relationship to anthropometry, life-style factors, blood pressure, and vitamin D. Calcif Tissue Int. (1995) 56:104–8. doi: 10.1007/BF00296339
56. Brumpton BM, Langhammer A, Henriksen AH, Camargo CA Jr., Chen Y, Romundstad PR, et al. Vitamin D and lung function decline in adults with asthma: the HUNT study. Am J Epidemiol. (2016) 183:739–46. doi: 10.1093/aje/kwv243
57. Alsharairi NA. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: a review of the literature. Nutrients. (2019) 11:725. doi: 10.3390/nu11040725
58. Yawn J, Lawrence LA, Carroll WW, Mulligan JK. Vitamin D for the treatment of respiratory diseases: is it the end or just the beginning? J Steroid Biochem Mol Biol. (2015) 148:326–37. doi: 10.1016/j.jsbmb.2015.01.017
59. Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. (2012) 67:10–7. doi: 10.1111/j.1398-9995.2011.02711.x
60. Casaro M, Souza VR, Oliveira FA, Ferreira CM. OVA-induced allergic airway inflammation mouse model. Methods Mol Biol. (2019) 1916:297–301.
61. Ma JG, Wu GJ, Xiao HL, Xiao YM, Zha L. Vitamin D has an effect on airway inflammation and Th17/Treg balance in asthmatic mice. Kaohsiung J Med Sci. (2021) 37:1113–21. doi: 10.1002/kjm2.12441
62. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. (2017) 169:985–99.
63. Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. (2009) 119:2538–49. doi: 10.1172/JCI38079
64. Huo Y, Guan L, Xu J, Zhou L, Chen R. Tiotropium inhibits methacholine-induced extracellular matrix production via β-catenin signaling in human airway smooth muscle cells. Int J Chron Obstruct Pulmon Dis. (2018) 13:1469–81. doi: 10.2147/COPD.S158552
65. Hussain M, Xu C, Lu M, Wu X, Tang L, Wu X. Wnt/β-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:3226–42. doi: 10.1016/j.bbadis.2017.08.031
66. Huang Y, Wang L, Jia XX, Lin XX, Zhang WX. Vitamin D alleviates airway remodeling in asthma by down-regulating the activity of Wnt/β-catenin signaling pathway. Int Immunopharmacol. (2019) 68:88–94. doi: 10.1016/j.intimp.2018.12.061
67. Hall SC, Agrawal DK. Vitamin D and bronchial asthma: an overview of data from the past 5 years. Clin Ther. (2017) 39:917–29. doi: 10.1016/j.clinthera.2017.04.002
68. Jolliffe DA, Greenberg L, Hooper RL, Griffiths CJ, Camargo CA, Kerley CP, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. (2017) 5:881–90. doi: 10.1016/S2213-2600(17)30306-5
69. Wang M, Liu M, Wang C, Xiao Y, An T, Zou M, et al. Association between vitamin D status and asthma control: a meta-analysis of randomized trials. Respir Med. (2019) 150:85–94. doi: 10.1016/j.rmed.2019.02.016
70. Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS One. (2015) 10:e0136841. doi: 10.1371/journal.pone.0136841
71. Jaura JKG, Safranek S. Does vitamin D supplementation reduce asthma exacerbations? J Fam Pract. (2020) 69:E4–6.
72. Chen Z, Peng C, Mei J, Zhu L, Kong H. Vitamin D can safely reduce asthma exacerbations among corticosteroid-using children and adults with asthma: a systematic review and meta-analysis of randomized controlled trials. Nutr Res. (2021) 92:49–61. doi: 10.1016/j.nutres.2021.05.010
73. Jat KR, Goel N, Gupta N, Gupta CP, Datta S, Lodha R, et al. Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: a randomized controlled trial (ESDAC trial). Pediatr Allergy Immunol. (2021) 32:479–88. doi: 10.1111/pai.13415
74. Dodamani MH, Muthu V, Thakur R, Pal A, Sehgal IS, Dhooria S, et al. A randomised trial of vitamin D in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Mycoses. (2019) 62:320–7. doi: 10.1111/myc.12879
75. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
76. Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. (2014) 311:2083–91. doi: 10.1001/jama.2014.5052
77. de Groot JC, van Roon EN, Storm H, Veeger NJ, Zwinderman AH, Hiemstra PS, et al. Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. J Allergy Clin Immunol. (2015) 135:670–5.e3.
78. Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax. (2015) 70:451–7. doi: 10.1136/thoraxjnl-2014-206449
79. Shabana MA, Esawy MM, Ismail NA, Said AM. Predictive role of IL-17A/IL-10 ratio in persistent asthmatic patients on vitamin D supplement. Immunobiology. (2019) 224:721–7. doi: 10.1016/j.imbio.2019.09.005
80. Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. (2011) 127:1294–6. doi: 10.1016/j.jaci.2010.12.016
81. Musharraf MU, Sandhu GA, Mumtaz MU, Rashid MF. Role of vitamin d in prevention of acute exacerbation of bronchial asthma in aduLTS. J Postgrad Med Inst. (2017) 31:310–3. doi: 10.1002/rmv.1909
82. Thakur C, Kumar J, Kumar P, Goyal JP, Singh K, Gupta A. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: a randomized controlled trial (ViDASTA Trial). Pediatr Pulmonol. (2021) 56:1427–33. doi: 10.1002/ppul.25287
83. Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr. (2014) 81:650–4.
84. Wilson SJ, Ward JA, Sousa AR, Corfield J, Bansal AT, De Meulder B, et al. Severe asthma exists despite suppressed tissue inflammation: findings of the U-BIOPRED study. Eur Respir J. (2016) 48:1307–19. doi: 10.1183/13993003.01129-2016
85. LeMay KS, Armour CL, Reddel HK. Performance of a brief asthma control screening tool in community pharmacy: a cross-sectional and prospective longitudinal analysis. Prim Care Respir J. (2014) 23:79–84. doi: 10.4104/pcrj.2014.00011
86. Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. (2006) 117:549–56. doi: 10.1016/j.jaci.2006.01.011
87. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the childhood asthma control test. J Allergy Clin Immunol. (2007) 119:817–25. doi: 10.1016/j.jaci.2006.12.662
88. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American thoracic society/European respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. (2009) 180:59–99. doi: 10.1164/rccm.200801-060ST
89. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. (2009) 180:388–95. doi: 10.1164/rccm.200903-0392OC
90. Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. A transcriptome-driven analysis of epithelial brushings and bronchial biopsies to define asthma phenotypes in U-BIOPRED. Am J Respir Crit Care Med. (2017) 195:443–55. doi: 10.1164/rccm.201512-2452OC
91. Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. (2010) 138:1140–7. doi: 10.1378/chest.09-3058
94. Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 1: a review of potential mechanisms. Pulm Pharmacol Ther. (2015) 32:60–74. doi: 10.1016/j.pupt.2015.02.004
95. Walsh LJ, Wong CA, Oborne J, Cooper S, Lewis SA, Pringle M, et al. Adverse effects of oral corticosteroids in relation to dose in patients with lung disease. Thorax. (2001) 56:279–84. doi: 10.1136/thorax.56.4.279
96. Kaufman K, Sorensen R. An explorative study of low levels of allergen-specific IgE and clinical allergy symptoms during early childhood. Pediatrics. (2012) 130:S5–6. doi: 10.1111/j.1398-9995.2011.02578.x
97. Checkley W, Robinson CL, Baumann LM, Hansel NN, Romero KM, Pollard SL, et al. 25-hydroxy vitamin D levels are associated with childhood asthma in a population-based study in Peru. Clin Exp Allergy. (2015) 45:273–82. doi: 10.1111/cea.12311
98. Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the childhood asthma management program study. J Allergy Clin Immunol. (2010) 126:52–8.e5. doi: 10.1016/j.jaci.2010.03.043
99. Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. (2012) 186:140–6.
100. Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. (2009) 179:765–71. doi: 10.1164/rccm.200808-1361OC
101. Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A, et al. Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med. (2012) 186:508–13. doi: 10.1164/rccm.201202-0351OC
102. Hall SC, Fischer KD, Agrawal DK. The impact of vitamin D on asthmatic human airway smooth muscle. Expert Rev Respir Med. (2016) 10:127–35. doi: 10.1586/17476348.2016.1128326
103. Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. (2015) 7:8127–51. doi: 10.3390/nu7095383
104. Berraies A, Hamzaoui K, Hamzaoui A. Link between vitamin D and airway remodeling. J Asthma Allergy. (2014) 7:23–30. doi: 10.2147/JAA.S46944
105. Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy. (2015) 45:114–25. doi: 10.1111/cea.12430
106. Feng L, Meng T, Qi Y, Athari SS, Chen X. Study effect of vitamin d on the immunopathology responses of the bronchi in murine model of asthma. Iran J Allergy Asthma Immunol. (2021) 20:509–19. doi: 10.18502/ijaai.v20i5.7399
107. Lan N, Luo G, Yang X, Cheng Y, Zhang Y, Wang X, et al. 25-Hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS One. (2014) 9:e111599. doi: 10.1371/journal.pone.0111599
108. Chung KF, Marwick JA. Molecular mechanisms of oxidative stress in airways and lungs with reference to asthma and chronic obstructive pulmonary disease. Ann N Y Acad Sci. (2010) 1203:85–91. doi: 10.1111/j.1749-6632.2010.05600.x
109. Forno E, Bacharier LB, Phipatanakul W, Guilbert TW, Cabana MD, Ross K, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA. (2020) 324:752–60. doi: 10.1001/jama.2020.12384
110. Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-dose vitamin D supplementation during pregnancy and asthma in offspring at the age of 6 years. JAMA. (2019) 321:1003–5. doi: 10.1001/jama.2019.0052
Keywords: vitamin D, asthma, FEV1, asthma exacerbations, children
Citation: Liu M, Wang J and Sun X (2022) A Meta-Analysis on Vitamin D Supplementation and Asthma Treatment. Front. Nutr. 9:860628. doi: 10.3389/fnut.2022.860628
Received: 23 January 2022; Accepted: 25 May 2022;
Published: 06 July 2022.
Edited by:
Lilia Castillo-Martinez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, MexicoReviewed by:
Naser Alsharairi, Griffith University, AustraliaWenting Luo, First Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2022 Liu, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinrong Sun, MTM3MjA1MzM5MTZAMTYzLmNvbQ==
 Meiqi Liu
Meiqi Liu Jun Wang
Jun Wang Xinrong Sun1*
Xinrong Sun1*