- 1Department of Nutrition, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 2Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Department of Nutrition, Electronic Health and Statistics Surveillance Research Center, Science and Research Branch, Islamic Azad University, Tehran, Iran
- 4Cancer Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Nutrition, School of Public Health, International Campus, Iran University of Medical Sciences, Tehran, Iran
- 6Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
- 7Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 8Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 9Department of Clinical Nutrition, School of Nutritional Sciences and Dietetic, Tehran University of Medical Sciences, Tehran, Iran
- 10Department of Clinical Nutrition and Dietetics, Research Institute, Shahid Beheshti University of Medical Science, Tehran, Iran
- 11School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
- 12Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Science, Tehran, Iran
- 13Department of Community Nutrition, Faculty of Nutrition and Food Technology, National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: The association of dietary fat and colorectal cancer (CRC) was frequently reported. However, few studies assessed the effects of different types of dietary fats on CRC. This study aimed to investigate the association between intakes of different types of dietary fatty acids with colorectal cancer risk.
Methods: This case-control study was conducted on 480 participants including 160 CRC cases and 320 healthy controls in Firoozgar Hospital, Tehran, Iran. The intake of dietary fatty acids of the participants was assessed using a semi quantitative food frequency questionnaire (FFQ).
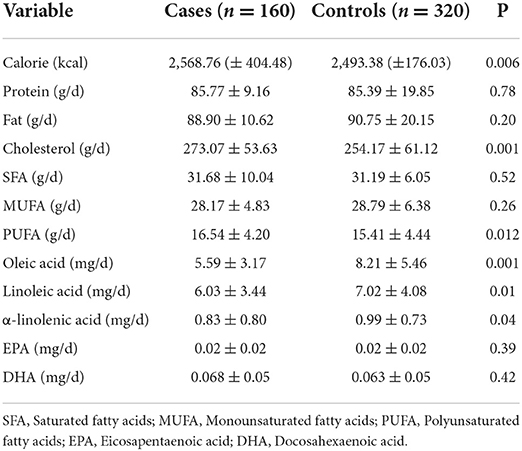
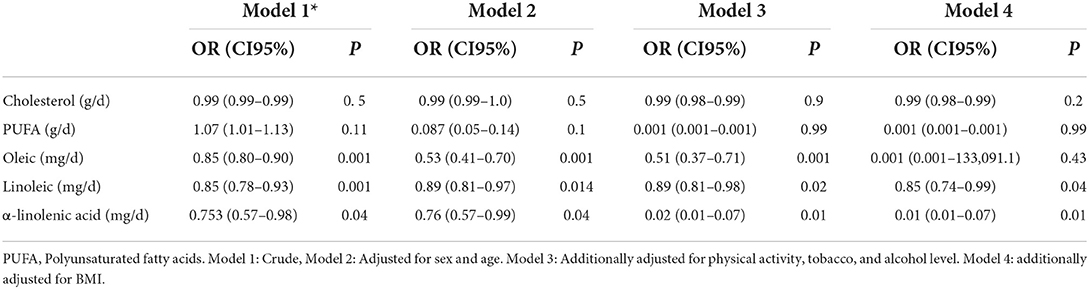
Results: The mean intake of cholesterol (273.07 ± 53.63 vs. 254.17 ± 61.12, P = 0.001), polyunsaturated fatty acids (PUFA) (16.54 ± 4.20 vs. 15.41 ± 4.44, P = 0.012), and calorie (2,568.76 ± 404.48 vs. 2,493.38 ± 176.03, P = 0.006) was higher and the mean intake of oleic acid (5.59 ± 3.17 vs. 8.21 ± 5.46) and linoleic acid (6.03 ± 3.44 vs. 7.02 ± 4.08, P = 0.01) was lower in the case group compared to the control group. An inverse association was found between colorectal cancer (CRC) and dietary intake of oleic acid (OR: 0.85, CI 95% 0.80–0.90, P = 0.001), linoleic acid (OR: 0.85, CI 95% 0.78–0.93, P = 0.001), and α-linolenic acid (OR: 0.75, CI 95% 0.57–0.98, P = 0.04). The association remained significant after adjusting for age and sex, sleep, smoking, and alcohol consumption, and BMI.
Conclusions: The results of this study support a protective effect of oleic acid, linoleic acid, and α-linolenic acid against CRC. Further longitudinal studies are warranted to confirm these results.
Introduction
Colorectal cancer is the second leading cause of cancer death and the third most common cancer in the world. Keum and Giovannucci (1) Over the past 27 years, the incidence cases of CRC have doubled worldwide Keum and Giovannucci (1) and its incidence is steadily increasing in developing countries, with 2 million new cases in 2018 (1, 2). More than 700,000 people die annually due to CRC (3). The highest incidence of colorectal cancer was reported in North America, Australia, New Zealand, Western Europe, and Japan. Moreover, the prevalence of CRC has been increasing rapidly in Asian countries, including South Korea and Iran, in recent decades (3). Cancer is the third most common mortality factor in Iran after cardiovascular diseases and accidents. Also, findings show that colorectal cancer is the third most common cancer in Iranian men and fourth most common among Iranian women (4). CRC develops through the synergistic effects of several genetic and epigenetic factors that lead to transformation of the normal intestinal epithelium into invasive adenocarcinoma (5, 6). In order to reduce the mortality rate from colorectal cancer, prevention and early detection are key strategies (7). Reducing the incidence and mortality of CRC requires concerted efforts to reduce modifiable risk factors, and promote population-wide and targeted screening (1). Some prevalent risk factors for colorectal cancer include inflammatory bowel diseases such as Crohn's disease and ulcerative colitis, family history, genetics, and lifestyle factors such as lack of physical exercise, smoking, alcohol consumption, high fat and low fiber diets, high consumption of processed meats, and obesity (8–11). Among the factors associated with the development of CRC, modifiable risk factors such as unhealthy dietary intake, lack of physical exercise, alcohol consumption and smoking are of great importance 2018.
The effect of dietary intake on chronic diseases was frequently reported (12–15). Also, the association between colorectal cancer risk and diet has been studied in many studies (16–18). The findings show that the global spread of unhealthy, westernized diets high in red, processed meat and saturated fats (19). Epidemiological studies suggest that dietary fats may play important roles in colorectal cancer (20). Dietary fats can change the structure of the gut microbiota community and affect its function by modulating the production of metabolites (21). On the other hand, there is mounting evidence that consumption of excess dietary fats can enhance intestinal permeability differentially and induces IEC oxidative stress and apoptosis. In addition, a high-fat diet (HFD) enhances intestinal permeability directly by stimulating pro-inflammatory signaling cascades and indirectly via increasing TNFα, interleukin (IL) 1B, IL6, and interferon γ (IFNγ) and decreasing IL10, IL17, and IL22 (22). For example, omega-6 fatty acids are precursor to the production of inflammatory eicosanoids (23) and can be converted into various pro-inflammatory mediators such as prostaglandin E2 by the cyclooxygenase pathway (24). In addition, up-regulation of cyclooxygenase 2, which is involved in fatty acids metabolism, occurs in 50% of colon adenomas and 85% of colon cancers (25). Recent evidence indicates that PUFAs, such as docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) can significantly affect the epigenome status of cells (26). It was also established that in CRC cells, PUFAs may affect the activity of certain microRNAs through alteration in promoter DNA methylation (27). An increased risk of colorectal cancer has been found to be associated with high dietary intake of n-6 Polyunsaturated fatty acids (PUFAs) such as arachidonic acid (28). On the other hand, Among specific types of PUFA, the preventive effects of total n-3 PUFA and marine-derived n-3 PUFA on CRC risk was reported (29). A prospective study reported that fish consumption protected women from colorectal cancer (30). A Case-control study on French Canadians identified an inverse association between dietary intake of n-3 PUFA and colorectal cancer (31). Furthermore, high saturated fatty acids intake may increase colorectal cancer risk (32). However, some studies reported contradictory results and found no associations of dietary fat or fatty acids with the risk of CRC (33–40). For example, a cohort study on dietary intake of fatty acids reported that there was no association between fatty acid intake and colorectal cancer risk (41). A systematic review of research papers published since the 1990s reported that there is no significant association between CRC risk with total fat intake, SFAs, MUFAs, and PUFAs (42). However, in Iran, so far, no study has been conducted on the relationship between dietary fats and the risk of colon cancer. So, the present case-control study aimed to investigate the association between intakes of different types of dietary fatty acids with colorectal cancer risk in Iranian adults.
Methods and materials
Study population
This case-control study was conducted from July 2020 to May 2021 on 480 randomly selected participants using the simple random sampling method including 160 patients with stage 3 and 4 colorectal cancer as the case group and 320 healthy people as the control group in Firoozgar Hospital in Tehran, Iran. The sample size was calculated using OpenEPI software (http://www.openepi.com/) and the odds ratios obtained in previous studies (43). The age-matched control group was selected from non-colorectal cancer patients referred to Firoozgar hospital for general check-ups. The inclusion criteria for the cases included histologically confirmed colorectal cancer by pathology, consent to participate in the study, no more than 3 months after diagnosis of cancer, age between 35 and 70 years, absence of other diseases affecting food intake, and not treated with drugs that affect food intake. Inclusion criteria for the control group included consent to participate in the study, age between 35 to 70 years, no history of cancer or other diseases that affect dietary intake, and no use of drugs that affect dietary intake.
Data collection
Data of anthropometric measurements including weight, height, body mass index, and data on socio-demographic factors including age, sex, education, income level, marital status, health status, smoking status, and alcohol consumption were collected using a general questionnaire. All participants were assessed for dietary intake and physical activity through a face-to face interview with a trained researcher as follows.
Dietary assessment
The dietary intake of the participants in the study was assessed using a validated semi-quantitative food frequency questionnaire (FFQ) (44). Data collected from FFQ were converted to grams using household measures and were analyzed using Nutritionist IV software (Sousa). The intake of total fat, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, linoleic acid, α-linolenic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) during the year before diagnosis was assessed.
Assessment of physical activity
Information on the level of physical activity was collected by the International Physical Activity Questionnaire (IPAQ), which has already been validated in Iran (45). Using this questionnaire, the amount of activity of people at home, at work, and during exercise was determined. Individuals were evaluated and compared in terms of activity using the metabolic equivalent of task (MET).
Statistical analysis
Chi-square and independent t-test were used to compare qualitative and quantitative variables between the case and control groups, respectively. The logistic regression analysis method was used to investigate the relationship between CRC and dietary fatty acids. A stepwise (forward) selection procedure was used for modeling and variables were selected based on significance and background knowledge as crude regression model (Model 1), adjusted for age and sex (Model 2), further adjustment for physical activity, smoking, and alcohol consumption (Model 3), and additionally adjustment for BMI (Model 4). Statistical analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, USA) and P-value < 0.05 was considered as significant in all analyses.
Results
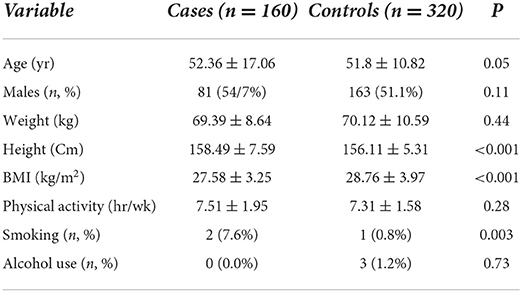
Distribution of the characteristics of the cases and controls is presented in Table 1. The cases had higher height (158.49 ± 7.59 vs. 156.11 ± 5.31 cm, P < 0.001), lower body mass index (BMI) (27.58 ± 3.25 vs. 28.76 ± 3.97 kg/m2, P < 0.001), and higher smoking (7.6 vs. 0.8%, P = 0.001). The amount of dietary fat intake of the participants is presented in Table 2. The mean intake of cholesterol (273.07 ± 53.63 vs. 254.17 ± 61.12 mg/d, P = 0.001), PUFA (16.54 ± 4.20 vs. 15.41 ± 4.44 g/d, P = 0.012), and calorie (2,568.76 ± 404.48 vs. 2,493.38 ± 176.03 kcal/d, P = 0.006) in the case group was significantly higher than the control group. The mean intake of oleic acid (5.59 ± 3.17 vs. 8.21 ± 5.46 g/d), linoleic acid (6.03 ± 3.44 vs. 7.02 ± 4.08 g/d, P = 0.01), and α-linolenic acid (0.83 ± 0.80 vs. 0.99 ± 0.73 g/d, P = 0.04) in the case group was significantly lower than the control group.
The association between dietary intake of fatty acid and the risk of colorectal cancer is presented in Table 3. A negative association was found between CRC and dietary intake of linoleic acid (OR: 0.85, CI 95% 0.78–0.93, P = 0.001), oleic acid (OR: 0.85, CI 95% 0.80–0.90, P = 0.001), and α-linolenic acid (OR: 0.75, CI 95% 0.57–0.98, P = 0.04). The association remained significant after adjusting for age and sex (Model 2) and further adjustments for physical activity, smoking, and alcohol consumption (Model3). The association between CRC and oleic acid was disappeared after additional adjustment for BMI (Model 4).
Discussion
The results of the present study indicated that there was an inverse association between colorectal cancer and dietary intake of oleic acid, linoleic acid and, α-linolenic acid. Epidemiological and experimental studies reported contradictory results on the association of dietary fatty acids and colorectal cancer. Butler et al. performed a nested case-control study of 350 colorectal cancer cases and an equal number of individually matched control subjects within the Singapore Chinese Health cohort Study to evaluate the associations between plasma fatty acid composition and colorectal cancer risk and reported a statistically significant inverse associations with colon cancer risk for higher levels of the essential PUFAs (i.e., α-linolenic and linoleic acids), oleic acid, and also found that the oleic: stearic acid ratio was positively associated with reduced frequency of colon cancer. The protective effects of alpha-linolenic acid may be due to its role as a precursor to the biosynthesis of EPA and DHA and their effects on increasing apoptosis and reducing the proliferation of cancer cells (46, 47).
Regarding the association of CRC with oleic acid, the consumption of an olive oil rich diet has been linked to a reduction in the incidence of cardiovascular disease and cancer (38), which was in line with the present study. Carrillo et al. reported that an oleic acid-enriched diet improved animal survival with lung tumor and confirm the anti-cancer properties of oleic acid (49). On the other hand, Bull et al. found that a high olive oil diet stimulated cervical cancer carcinogenesis, and oleic acid was considered to be associated with migration and invasion of cancer cells (58). Some experimental findings suggest that oleic acid consumption may induce colorectal cancer (CRC) whereas extra virgin olive oil minor compounds modulate mitogenic action of oleic acid on colon cancer and in this way, it has protective effects against this cancer (48). The exact mechanism of the association between cancer and oleic acid is not yet clear. Some studies reported an inhibition in cell proliferation induced by oleic acid in different tumor cell lines (49, 50). Oleic acid may suppress the over-expression of HER2 (erbB-2), an oncogene which plays a key role in the invasive progression and metastasis in several human cancers. In addition, oleic acid may induce apoptosis in carcinoma cells through increase in intracellular ROS production or caspase 3 activity (49). Oleic acid may induce NADPH oxidase 4 (NOX4) expression, resulting in an elevation of reactive oxygen species levels that can lead to promoted CRC metastasis (51). While, Carolina E. Storniolo et al., reported that the consumption of seed oils, high oleic acid seed oils, or olive oil will probably have different effects on CRC.
The results of the present study indicated an inverse association between CRC and omega-6 fatty acids. A cohort study in the United States observed no association between intake of omega-6 and omega-3 fatty acids and colorectal cancer (52). Another study in Canada found no association between omega-6 PUFA intake and colorectal cancer risk. The reason for these different results may be the different biological functions of different types of omega-6 fatty acids (53). For example, linoleic acid and arachidonic acid are both omega-6 fatty acids that may have different effects on carcinogenesis (54–56). One study reported a positive association with colon cancer for the desaturase indices reflecting increased synthesis of arachidonic acid (57). Eicosanoids derived from omega-6 may enhance the risk of CRC through production of pro inflammatory factors (58). For example, arachidonic acid is converted to proinflammatory factors such as prostaglandin E2 through the cyclooxygenase pathway and higher serum levels of prostaglandin E2 may promote carcinogenesis (24). However, some studies have indicated that some derivatives of linoleic acid may have anticancer effects. 15-LOX-1 is the principle enzyme for converting colonic linoleic acid into 13-S-hydroxyoctadecadienoic acid (13-S-HODE), which induces apoptosis (59). Additionally, peroxisome proliferator activated receptors (PPARs) are nuclear receptors for linoleic acid and arachidonic acid metabolites which increase colonic tumorigenesis. In a recent study 13-S-HODE was reported to bind to PPAR, inhibit its activation and decrease the expression of PPAR in CRC cells (58). These derivatives regulate a number of critical cancer-related signaling pathways through their effects on the activity of transcription factors, estrogen metabolism, lipid peroxidation, gene expression, insulin sensitivity, membrane fluid, cell proliferation, apoptosis, angiogenesis, and metastasis (58). Therefore, examining the association of fatty acids with colorectal cancer in terms of omega classification may be misleading. Moreover, several genetic, demographic, and environmental factors may influence the association between fatty acids and colorectal cancer (53). Cheng et al. reported that PUFA omega-6 was associated with increased risk of CRC in women and decreased CRC risk in men (60).
We found an inverse association between CRC and the intake of omega-3 fatty acids. Omega-3 may be a protective factor against CRC by reducing the level of pro-inflammatory cytokines produced by the cyclooxygenase pathway. The inefficient metabolic conversion of ALA to very long-chain omega-3 fatty acids including EPA and DHA (61) can be linked to adverse health outcomes (62). Several studies indicated that omega-3 PUFAs are crucial for inhibiting various types of tumors (33, 63). Angiogenesis mediators such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and prostaglandin E2 (PGE2) may be inhibited by omega-3 PUFAs (46).
However, some other studies reported contradictory results. One study on Chinese women found no association between omega-3 intake and colorectal cancer (28). In addition, Reddy et al. found that intake of ALA omega-3 fatty acids was associated with an increased risk of CRC in women (64). Future studies on the effects of omega-3 fatty acids on CRC are required.
The strengths of this study were the adequate sample size, focus on diet prior to diagnosis, and highlighting the differences between different types of dietary fatty acids. However, this study had some limitations. One limitation of assessing dietary intake in case-control studies is the using of self-report FFQ which has potentially a recall bias. In addition, there are several other factors that may affect the results. However, the effects of some confounders such as age, sex, smoking, tobacco, and BMI were adjusted, but the effects of other factors, such as genetics and stress were not assessed.
Conclusion
In the present study, the patients with CRC had higher intake of cholesterol and lower intake of oleic acid, linoleic acid, and α-linolenic acid than the control group. Higher intake of oleic acid, linoleic acid, and α-linolenic acid was associated with a reduced frequency of CRC. Dietary modification in terms of receiving rich sources of unsaturated fatty acids may be effective in reducing the risk of colorectal cancer. Further longitudinal studies are warranted to examine the effects of different types of fatty acids on colorectal cancer and to discover the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by (Code: IR.SBMU.CRC.REC.1398.028). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SS, SF, ST, NH, ZR, and MG designed the study and carried out the data collection. MA, MEA, FB, SK, HS, MS, SN, AA, and SD were involved in the design of the study, analysis of the data, and critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by Cancer Research Center (CRC), Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code:15784).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Przeglad gastroenterologiczny. (2019) 14:89–103. doi: 10.5114/pg.2018.81072
3. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
4. Simonian M, Khosravi S, Mortazavi D, Bagheri H, Salehi R, Hassanzadeh A, et al. Environmental risk factors associated with sporadic colorectal Cancer in Isfahan, Iran. Middle East J Cancer. (2018) 9:318–22. doi: 10.30476/MEJC.2018.42144
5. Carter JV, Galbraith NJ, Yang D, Burton JF, Walker SP, Galandiuk S, et al. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Br J Cancer. (2017) 116:762–74. doi: 10.1038/bjc.2017.12
6. Shaw E, Farris MS, Stone CR, Derksen JWG, Johnson R, Hilsden RJ, et al. Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: a systematic review and meta-analysis. BMC Cancer. (2018) 18:71. doi: 10.1186/s12885-017-3970-5
7. Hanna K, Coussens C, Jones L, Wilson S. Cancer and the Environment: Gene-environment Interaction (2002).
8. Amersi F, Agustin M, Ko CY. Colorectal Cancer: Epidemiology, risk factors, and health services. Clin Colon Rectal Surg. (2005) 18:133–40. doi: 10.1055/s-2005-916274
9. Ashkar F, Rezaei S, Salahshoornezhad S, Vahid F, Gholamalizadeh M, Dahka SM, et al. The Role of medicinal herbs in treatment of insulin resistance in patients with Polycystic Ovary Syndrome: a literature. Review. (2019) 11:57–75. doi: 10.1515/bmc-2020-0005
10. Doaei S, Jarrahi SM, Moghadam AS, Akbari M, Kooshesh SJ, Badeli M, et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: a meta-analysis. Bji. (2019) 10:237–42. doi: 10.1515/bmc-2019-0025
11. Vahid F, Hekmatdoost A, Mirmajidi S, Doaei S, Rahmani DZJTAJ, Faghfoori MS. Association between index of nutritional quality and nonalcoholic fatty liver disease: the role of vitamin D and B group. Plos one. (2019) 358:212–8. doi: 10.1016/j.amjms.2019.06.008
12. Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA. Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol Prevent Biomark. (2014) 23:255–73. doi: 10.1158/1055-9965.EPI-13-0515
13. Kolahdouz-Mohammadi R, Malekahmadi M, Clayton ZS, Sadat SZ, Pahlavani N, Sikaroudi MK, et al. Effect of egg consumption on blood pressure: a systematic review and meta-analysis of randomized clinical trials. Curr Hypertens Rep. (2020) 22:1–9. doi: 10.1007/s11906-020-1029-5
14. Pahlavani N, Rostami D, Ebrahimi F, Azizi-Soleiman F. Nuts effects in chronic disease and relationship between walnuts and satiety: review on the available evidence. Obesity Med. (2020) 17:100173. doi: 10.1016/j.obmed.2019.100173
15. Movahed S, Tabrizi FV, Pahlavani N, Toussi MS, Motlagh A, Eslami S, et al. Comprehensive assessment of nutritional status and nutritional-related complications in newly diagnosed esophageal cancer patients: a cross-sectional study. Clin Nutr. (2021) 40:4449–55. doi: 10.1016/j.clnu.2021.01.003
16. Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutri Cancer. (2008) 61:47–69. doi: 10.1080/01635580802395733
17. Doaei S, Hajiesmaeil M, Aminifard A, Mosavi-Jarrahi S, Akbari M, Gholamalizadeh M, et al. Effects of gene polymorphisms of metabolic enzymes on the association between red and processed meat consumption and the development of colon cancer; a literature review. J Nutr Sci. (2018) 7:17. doi: 10.1017/jns.2018.17
18. Gholamalizadeh M, Behrad Nasab M, Ahmadzadeh M, Doaei S, Jonoush M, Shekari S, et al. The association among calorie, macronutrient, and micronutrient intake with colorectal cancer: A case–control study. Food Science and Nutri. (2022) 22:3775 doi: 10.1002/fsn3.2775
19. Yang J, McDowell A, Kim EK, Seo H, Lee WH, Moon C, et al. Development of a colorectal cancer diagnostic model and dietary risk assessment through gut microbiome analysis. Experiment Mol Med. (2019) 51:1–15. doi: 10.1038/s12276-019-0313-4
20. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC, et al. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. (1994) 54:2390–7.
21. Yang J, Yu J. The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein and Cell. (2018) 9:474–87. doi: 10.1007/s13238-018-0543-6
22. Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutri. (2019) 11:77–91. doi: 10.1093/advances/nmz061
23. Calder PC. Dietary modification of inflammation with lipids. Proceed Nutri Soc. (2002) 61:345–58. doi: 10.1079/PNS2002166
24. Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. (2010) 10:181–93. doi: 10.1038/nrc2809
25. Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. (2001) 1:11–21. doi: 10.1038/35094017
26. Burdge GC, Lillycrop KA. Fatty acids and epigenetics. Curr Opin Clin Nutr Metab Care. (2014) 17:156–61. doi: 10.1097/MCO.0000000000000023
27. Boigues JFH, Mach N. The effect of polyunsaturated fatty acids on obesity through epigenetic modifications. Endocrinología y Nutrición (English Edition). (2015) 62:338–49. doi: 10.1016/j.endoen.2015.08.003
28. Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, et al. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Prevent Biomark. (2009) 18:2283–91. doi: 10.1158/1055-9965.EPI-08-1196
29. Chen GC, Qin LQ, Lu DB, Han TM. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control. (2015) 26:133–41. doi: 10.1007/s10552-014-0492-1
30. Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E, et al. Prospective study of diet and female colorectal cancer: the New York University Women's Health Study (1997).
31. Nkondjock A, Shatenstein B, Maisonneuve P, Ghadirian P. Assessment of risk associated with specific fatty acids and colorectal cancer among French-Canadians in Montreal: a case-control study. Int J Epidemiol. (2003) 32:200–9. doi: 10.1093/ije/dyg048
32. Hill M. Bile acids, colorectal cancer: hypothesis. Euro J Cancer Prevent. (1991) 1:69–74. doi: 10.1097/00008469-199110002-00012
33. Fernández-Bañares F, Esteve M, Navarro E, Cabre E, Boix J, Abad-Lacruz A, et al. Changes of the mucosal n3 and n6 fatty acid status occur early in the colorectal adenoma-carcinoma sequence. Gut. (1996) 38:254–9. doi: 10.1136/gut.38.2.254
34. Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, et al. Associations of red meat, fat, and protein intake with distal colorectal cancer risk. Nutr Cancer. (2010) 62:701–9. doi: 10.1080/01635581003605938
35. Sun Z, Liu L, Wang PP, Roebothan B, Zhao J, Dicks E, et al. Association of total energy intake and macronutrient consumption with colorectal cancer risk: results from a large population-based case-control study in Newfoundland and Labrador and Ontario, Canada. Nutr J. (2012) 11:1–9. doi: 10.1186/1475-2891-11-18
36. Zhong X, Fang YJ, Pan ZZ, Li B, Wang L, et al. Dietary fat, fatty acid intakes and colorectal cancer risk in Chinese adults. Euro J Cancer prevent. (2013) 22:438–47. doi: 10.1097/CEJ.0b013e32835e88c4
37. Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutrition cancer. (2014) 66:716–27. doi: 10.1080/01635581.2013.804101
38. Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, Mozaffarian D, et al. Dietary intake of fish, ω-3, ω-6 fatty acids and risk of colorectal cancer: a prospective study in US men and women. Int J Cancer. (2014) 135:2413–23. doi: 10.1002/ijc.28878
39. Kraja B, Muka T, Ruiter R, Keyser CE, Hofman A, Franco OH, et al. Dietary fiber intake modifies the positive association between n−3 PUFA intake and colorectal cancer risk in a caucasian population. J Nutr. (2015) 145:1709–16. doi: 10.3945/jn.114.208462
40. Navarro SL, Neuhouser ML, Cheng TYD, Tinker LF, Shikany JM, et al. The interaction between dietary fiber and fat and risk of colorectal cancer in the women's health initiative. Nutrients. (2016) 8:779. doi: 10.3390/nu8120779
41. Deschner EE, Lytle JS, Wong G, Ruperto JF, Newmark HL. The effect of dietary omega-3 fatty acids (fish oil) on azoxymethanol-induced focal areas of dysplasia and colon tumor incidence. Cancer. (1990) 66:2350–6.
42. Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. (2018) 10:1963. doi: 10.3390/nu10121963
43. Nkondjock A, Ghadirian P. Dietary carotenoids and risk of colon cancer: case-control study. Int J Cancer. (2004) 110:110–6. doi: 10.1002/ijc.20066
44. Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. (2010) 13:654–62. doi: 10.1017/S1368980009991698
45. Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S, et al. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. (2012) 18:1073–80. doi: 10.5829/idosi.wasj.2012.18.08.754
46. Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation, endothelial activation in women. J Nutr. (2004) 134:1806–11. doi: 10.1093/jn/134.7.1806
47. Xu Y, Qian SY. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed J. (2014) 37:112. doi: 10.4103/2319-4170.131378
48. Storniolo CE, Martínez-Hovelman N, Martínez-Huélamo M, Lamuela-Raventos RM, Moreno JJ. Extra virgin olive oil minor compounds modulate mitogenic action of oleic acid on colon cancer cell line. J Agric Food Chem. (2019) 67:11420–7. doi: 10.1021/acs.jafc.9b04816
49. Carrillo Pérez CM, Cavia Camarero M, Alonso de la Torre S. Antitumor effect of oleic acid; mechanisms of action. a review. Nutrición Hospitalaria. (2012) 27:1860–5. doi: 10.3305/nh.2012.27.6.6010
50. Jiang L, Wang W, He Q, Wu Y, Lu Z, Sun J, et al. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci Rep. (2017) 7:1–11. doi: 10.1038/s41598-017-11842-5
51. Shen CJ, Chang KY, Lin BW, Lin WT, et al. Oleic acid-induced NOX4 is dependent on ANGPTL4 expression to promote human colorectal cancer metastasis. Theranostics. (2020) 10:7083–99. doi: 10.7150/thno.44744
52. Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, et al. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. (1994) 107:1709–18. doi: 10.1016/0016-5085(94)90811-7
53. Oh K, Willett WC, Fuchs CS, Giovannucci E. Dietary marine n-3 fatty acids in relation to risk of distal colorectal adenoma in women. Cancer Epidemiolo Prevent Biomark. (2005) 14:835–41. doi: 10.1158/1055-9965.EPI-04-0545
54. Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr. (1998) 68:142–53. doi: 10.1093/ajcn/68.1.142
55. Lu XF, He H, Yu Q, Ma S, Shen UN. Colorectal cancer cell growth inhibition by linoleic acid is related to fatty acid composition changes. J Zhejiang Univ Sci B. (2010) 11:923–30. doi: 10.1631/jzus.B1000125
56. Cockbain A, Toogood G, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. (2012) 61:135–49. doi: 10.1136/gut.2010.233718
57. Butler LM, Yuan JM, Huang JY, Su J, Wang R, et al. Plasma fatty acids and risk of colon, rectal cancers in the Singapore Chinese Health Study. npj Precision Oncol. (2017) 1:38. doi: 10.1038/s41698-017-0040-z
58. Bull AW, Steffensen KR, Leers J, Rafter JJ. Activation of PPAR γ in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR γ. Carcinogenesis. (2003) 24:1717–22. doi: 10.1093/carcin/bgg131
59. Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, et al. 15.-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Nat Cancer Inst. (2000) 92:1136–42. doi: 10.1093/jnci/92.14.1136
60. Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, et al. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. (2003) 193:17–24. doi: 10.1016/S0304383502007176
61. Williams CM, Burdge G. Long-chain n– 3 PUFA: plant v. marine sources. Proceed Nutri Soc. (2006) 65:42–50. doi: 10.1079/PNS2005473
62. Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n– 3, n– 6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. (2004) 80:204–16. doi: 10.1093/ajcn/80.1.204
63. Marshall LA, Johnston PV. Modulation of tissue prostaglandin synthesizing capacity by increased ratios of dietary alpha-linolenic acid to linoleic acid. Lipids. (1982) 17:905–13. doi: 10.1007/BF02534586
Keywords: dietary intake, fatty acids, colorectal cancer risk, fat, cancer
Citation: Shekari S, Fathi S, Roumi Z, Akbari ME, Tajadod S, Afsharfar M, Hasanpour Ardekanizadeh N, Bourbour F, Keshavarz SA, Sotoudeh M, Gholamalizadeh M, Nemat Gorgani S, Shafaei Kachaei H, Alizadeh A and Doaei S (2022) Association between dietary intake of fatty acids and colorectal cancer, a case-control study. Front. Nutr. 9:856408. doi: 10.3389/fnut.2022.856408
Received: 17 January 2022; Accepted: 12 September 2022;
Published: 03 October 2022.
Edited by:
Marilia Seelaender, University of São Paulo, BrazilReviewed by:
Omid Sadeghi, Isfahan University of Medical Sciences, IranMonica Trif, Centre for Innovative Process Engineering, Germany
Copyright © 2022 Shekari, Fathi, Roumi, Akbari, Tajadod, Afsharfar, Hasanpour Ardekanizadeh, Bourbour, Keshavarz, Sotoudeh, Gholamalizadeh, Nemat Gorgani, Shafaei Kachaei, Alizadeh and Doaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeid Doaei, c2RvYWVlQHlhaG9vLmNvbQ==
 Soheila Shekari1
Soheila Shekari1 Maryam Gholamalizadeh
Maryam Gholamalizadeh Saeid Doaei
Saeid Doaei