- 1Changsha Social Work College, Changsha, China
- 2Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Objective: To comprehensively summarize the evidence on the associations of dietary copper, selenium, and manganese intake with depression based on a meta-analysis of observational studies.
Methods: The electronic database of PubMed, Web of Science, and Embase were searched up to January 7, 2022, for observational studies on the associations of dietary copper, selenium and manganese intake with depression (no restriction was set for the initiate time). The pooled relative risk (RR) of depression for the highest vs. lowest dietary copper, selenium, and manganese intake category were calculated.
Results: A total of 11 observational studies (61,430 participants) were identified as meeting the inclusion criteria. Specifically, five studies were related to the dietary copper intake. The overall multi-variable adjusted RR demonstrated that dietary copper intake was inversely associated with depression (RR = 0.63, 95% CI: 0.52–0.76; P < 0.001; I2 = 2.4%). With regard to the dietary selenium intake, six studies were identified for meta-analysis. The overall multi-variable adjusted RR showed that dietary selenium intake was also negatively associated with depression (RR = 0.63, 95% CI: 0.54–0.74; P < 0.001; I2 = 37.8%). In addition, four studies were specified for the dietary manganese intake, and the overall multi-variable adjusted RR indicated a negative relationship between dietary manganese intake and depression (RR = 0.71, 95% CI: 0.58–0.86; P < 0.001; I2 = 0.0%).
Conclusions: Our results suggest a negative relationship between dietary copper, selenium and manganese intake and depression, respectively. However, due to the limited prospective evidence, our results are restricted to cross-sectional design that precludes causal relationships. More well-designed prospective cohort studies are still needed.
Introduction
Depression is one of the most common global mental disorders (affecting females twice as much as males) (1), which usually presents as exhaustion, sadness, and lack of interest in daily activities (2). As a global burden of disease affecting ~300 million people (3), depression is estimated to be the leading cause of disability worldwide by 2030 (4). Nevertheless, the current treatment for depression may be limited to the following issues: costly pharmacotherapy, adverse side effects and unsatisfactory curative effect (5). Emerging evidence suggests that dietary factors are associated with depression (6, 7). Thus, the identification of modifiable dietary factors for depression appears to be an important step in its clinical prevention and management.
Micronutrients are important factors for cellular biochemical functions. Among them, copper, selenium, and manganese are considered to be significant ones. As a component of extracellular superoxide dismutase (8), copper is essential for iron uptake and signaling in energy metabolism, reactive oxygen species detoxification and eukaryotic organisms (9). In addition, copper plays a significant role in signaling involving mitophagy, bioenergetics, and dynamics and mitochondrial function, which determine cellular fate by metabolic reprogramming (9). In addition, selenium is severed as an essential micronutrient that maintain the different cellular functions, such as immune-endocrine function and signaling transduction pathways (10). Moreover, selenium incorporates into selenoproteins and selenium-dependent enzymes (e.g., glutathione peroxidases), which is closely related to intracellular redox regulation and modulation (11). On the other hand, as another essential nutrient for the body, manganese is an important component of manganese superoxide dismutase (MnSOD, SOD-2), which is the primary antioxidant enzyme that protects cells from oxidative stress (catalyze the dismutation of superoxide to hydrogen peroxide and oxygen in the mitochondria) (12). Since the oxidative stress is considered to play a significant role in the pathophysiology of depression (13, 14), the dietary copper, selenium, and manganese intake is considered to be beneficial to depression.
As far as we know, a number of observational studies have been employed to investigate the associations of dietary copper, selenium, and manganese intake with depression (15–25). However, their results are still conflicting. Thus, this meta-analysis of observational studies is employed to investigate the issues further. It is hypothesized that the dietary copper, selenium, and manganese intake is inversely associated with depression, respectively.
Materials and Methods
Search Strategy
Our meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (26). The electronic database of PubMed, Web of Science and Embase were searched up to January 7, 2022 (no restriction was set for the initiate time) by using a combination of keywords that related to depression (“depression,” “depressive”), copper (“copper”), selenium (“selenium”), and manganese (“manganese”). No language restriction was set in the search strategy. We screened the titles and abstracts of all articles, and then read the full articles to identify the eligible studies.
Study Selection
Two researchers reviewed the titles, abstracts, and full texts of all retrieved studies independently. Disagreements were resolved by discussions. The inclusion criteria were listed as follows: (1) observational studies; (2) the associations of dietary copper, selenium and manganese intake with depression; and (3) relative risk (RR) or odds ratio (OR) with 95% confidence interval (CI) was reported. The exclusion criteria were listed as follows: (1) duplicated or irrelevant articles; (2) reviews, letters, or case reports; (3) randomized controlled trials; and (4) non-human studies.
Data Extraction
The data was extracted by two researchers independently, and disagreements were resolved by discussions. The information about the first author and year of publication, location, age, sex, sample size, study design, adjustments, exposure assessment, category of exposure, effect estimates, and diagnostic criteria of depression, was collected. The corresponding effect estimates of depression with 95% CIs for the highest vs. lowest dietary copper, selenium and manganese intake category were extracted (adjusted for the maximum number of confounding variables).
Quality Assessment
The Newcastle-Ottawa (NOS) criteria for non-randomized studies was employed to assess the quality of each included study. NOS is based on three broad perspectives: (1) the selection process of study cohorts; (2) the comparability among different cohorts; (3) the identification of exposure or outcome of study cohorts. Disagreements with respect to the methodological quality were resolved by discussion.
Statistical Analyses
The RR for depression were the outcome measures in this meta-analysis. The I2 statistic, which measures the percentage of total variation across studies due to heterogeneity, was examined (I2 > 50% was considered heterogeneity). If significant heterogeneity was observed among the studies, the random-effects model was used; otherwise, the fixed effects model was accepted. Begg's test was employed to assess the publication bias (27). Moreover, subgroup analysis for sex, geographical region, sample size, diagnostic criteria of depression, exposure assessment, population, and study design were employed.
Results
Study Identification and Selection
The detailed flow diagram of the study identification and selection were presented in Figure 1. Initially, a total of 755 potentially relevant articles (179 for PubMed, 198 for Embase, and 378 for Web of Science) were retrieved during the literature search. After eliminating 353 duplicated articles, 402 articles were screened according to the titles and abstracts, and then, 240 irrelevant studies were excluded. Thereafter, 79 reviews, case reports or letters, 61 non-human studies, 11 randomized controlled trials studies were removed. Eventually, 11 studies were selected for this meta-analysis (15–25).
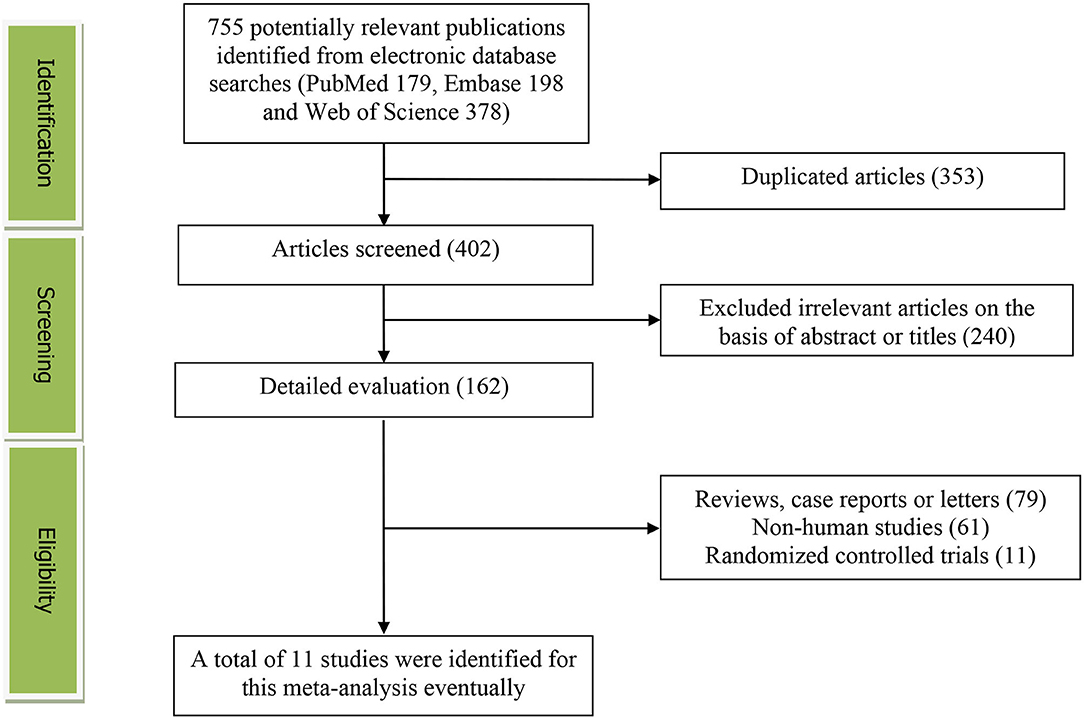
Figure 1. The detailed flow diagram of the study identification and selection in this meta-analysis.
Study Characteristics
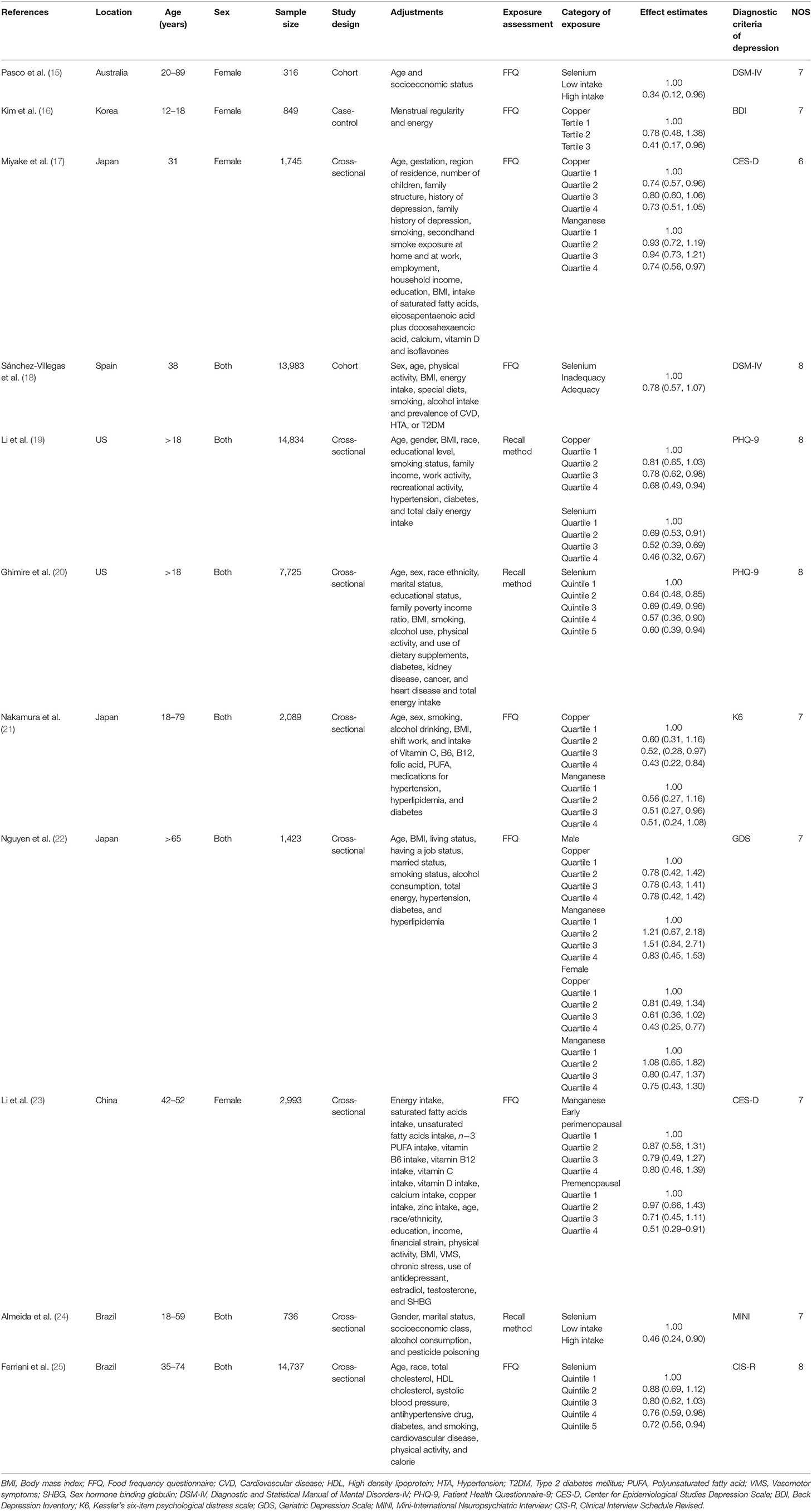
The main characteristics of the included studies were presented in Table 1. These studies were published between 2012 and 2022. Among them, five studies were performed in Asian countries [Japan (17, 21, 22), China (23) and Korea (16)], and the other six ones were from US (19, 20), Brazil (24, 25), Spain (18), and Australia (15). Four articles included only females (15–17, 23), and the other seven studies recruited both males and females (18–22, 24, 25). With regard to the study design, nine studies were cross-sectional/case-control (16, 17, 19–25) and two ones were prospective cohort (15, 18) studies. The sample size ranged from 316 to 14,834 for a total number of 61,430. The dietary micronutrients were assessed by food-frequency questionnaire (FFQ) in eight studies (15–18, 21–23, 25), and recall method in three studies (19, 20, 24). The diagnostic criteria of depression were Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (15, 18), Patient Health Questionnaire-9 (PHQ-9) (19, 20), Center for Epidemiological Studies Depression Scale (CES-D) (17, 23), Beck Depression Inventory (BDI) (16), Kessler's six-item psychological distress scale (K6) (21), Geriatric Depression Scale (GDS) (22), Mini-International Neuropsychiatric Interview (MINI) (24), and Clinical Interview Schedule Revised (CIS-R) (25), respectively.
RR of Depression for the Highest vs. Lowest Dietary Copper Intake Category
The overall multi-variable adjusted RR showed that the dietary copper intake was inversely associated with depression (RR = 0.63, 95% CI: 0.52–0.76; P < 0.001; Figure 2). No substantial level of heterogeneity was obtained among various studies (P = 0.401, I2 = 2.4%). No evidence of publication bias existed according to the Begg's rank-correlation test (P = 0.707). Table 2 presented the results of subgroup analysis. The above findings were confirmed in female (RR = 0.60, 95% CI: 0.40–0.80; P < 0.001), but not in male (RR = 0.64, 95% CI: 0.36–1.11).

Figure 2. Forest plot of meta-analysis: overall multi-variable adjusted RR of depression for the highest vs. lowest category of dietary copper intake.
RR of Depression for the Highest vs. Lowest Dietary Selenium Intake Category
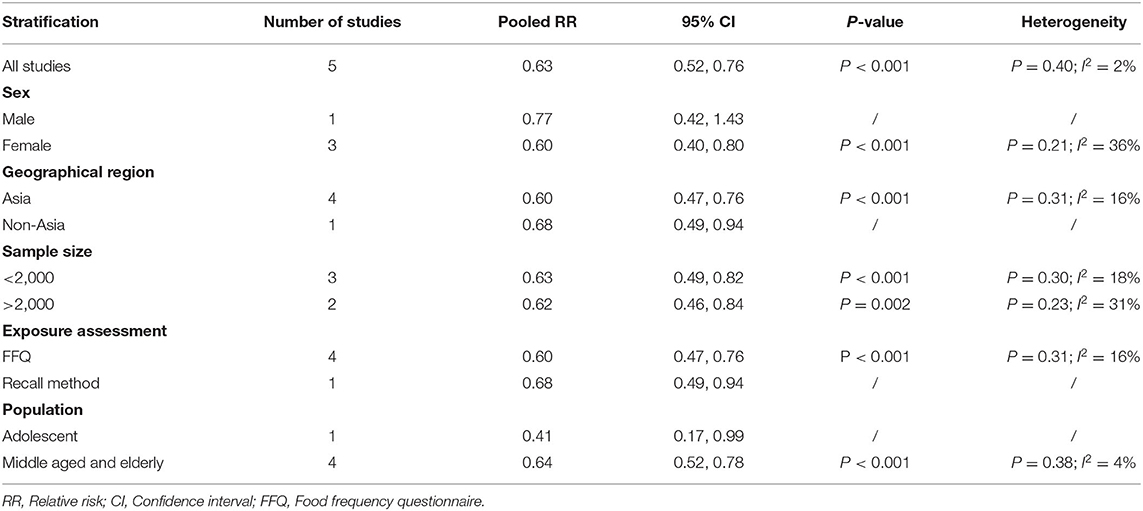
The overall multi-variable adjusted RR showed that the dietary selenium intake was inversely associated with depression (RR = 0.63, 95% CI: 0.54–0.74; P < 0.001) (Figure 3). No substantial level of heterogeneity was obtained among various studies (P = 0.154, I2 = 37.8%). No evidence of publication bias existed according to the Begg's rank-correlation test (P = 0.260). Table 3 presented the results of subgroup analysis. The above findings were confirmed in female (RR = 0.63, 95% CI: 0.47–0.85; P = 0.003), PHQ-9 (RR = 0.51, 95% CI: 0.39–0.68; P < 0.001), cross-sectional (RR = 0.60, 95% CI: 0.51–0.72; P < 0.001) studies, but not in male (RR = 0.77, 95% CI: 0.42–1.43; P = 0.001), DSM-IV (RR = 0.60, 95% CI: 0.28–1.28; P = 0.19) and prospective cohort studies (RR = 0.60, 95% CI: 0.28–1.28; P = 0.19).
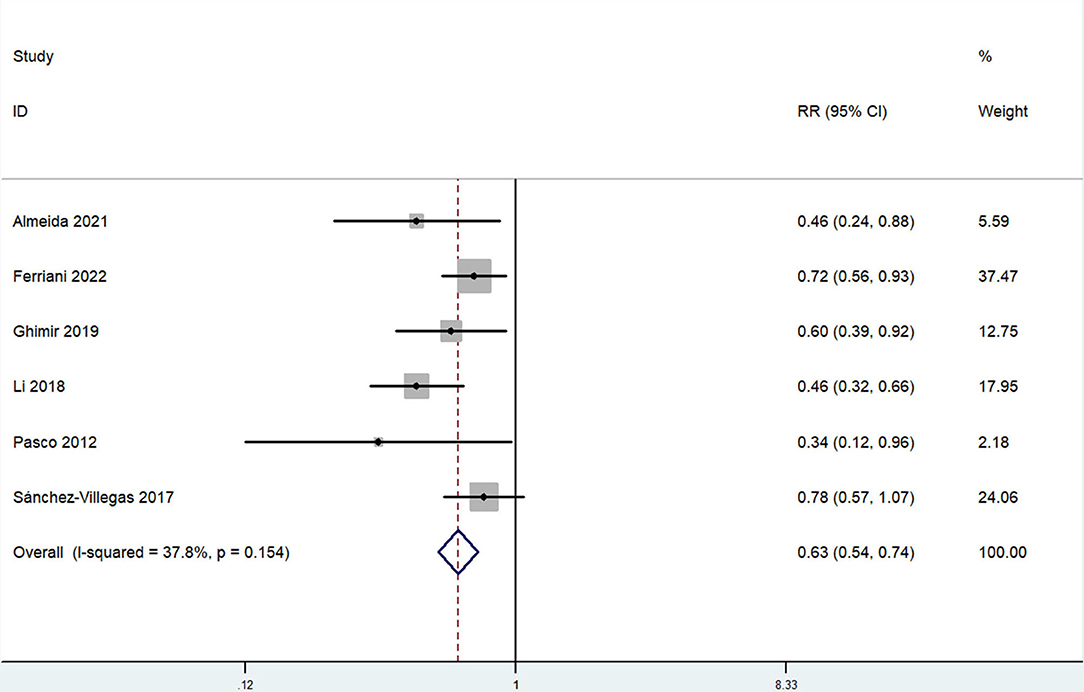
Figure 3. Forest plot of meta-analysis: overall multi-variable adjusted RR of depression for the highest vs. lowest category of dietary selenium intake.
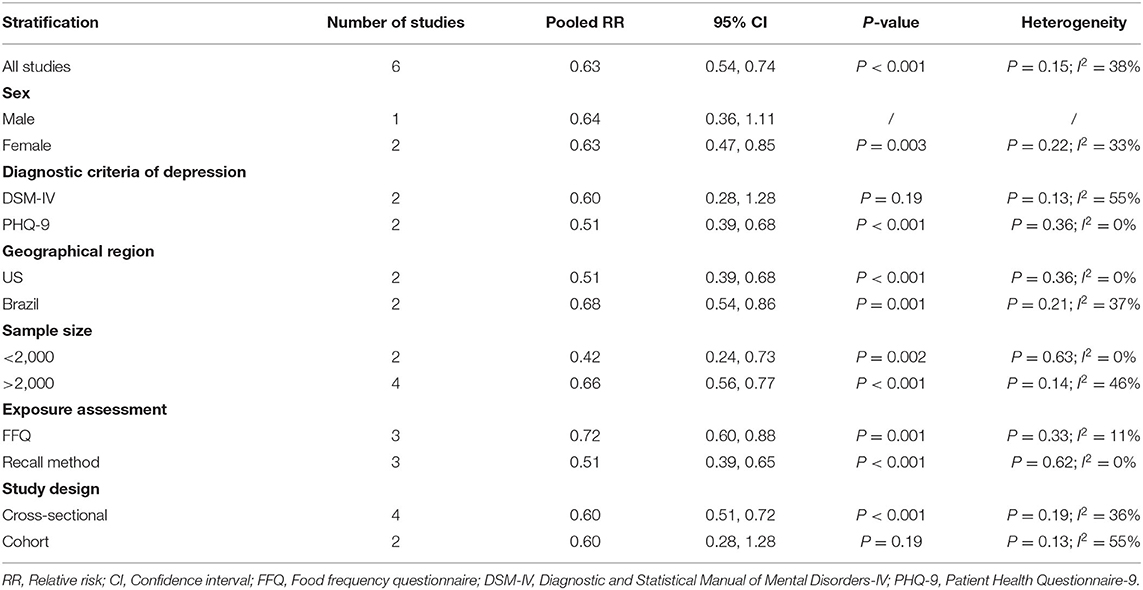
Table 3. Subgroup analysis of depression for the highest vs. lowest dietary selenium intake category.
RR of Depression for the Highest vs. Lowest Dietary Manganese Intake Category
The overall multi-variable adjusted RR showed that the dietary manganese intake was inversely associated with depression (RR = 0.71, 95% CI: 0.58–0.86; P < 0.001; Figure 4). No substantial level of heterogeneity was obtained among various studies (P = 0.778, I2 = 0.0%). No evidence of publication bias existed according to the Begg's rank-correlation test (P = 1.000). Table 4 presented the results of subgroup analysis. The above findings were confirmed in female (RR = 0.71, 95% CI: 0.58–0.88; P = 0.002) and CES-D (RR = 0.71, 95% CI: 0.56–0.89; P = 0.003) studies, but not in male (RR = 0.83, 95% CI: 0.45–1.53) and other criteria studies (RR = 0.71, 95% CI: 0.49–1.02; P = 0.06).
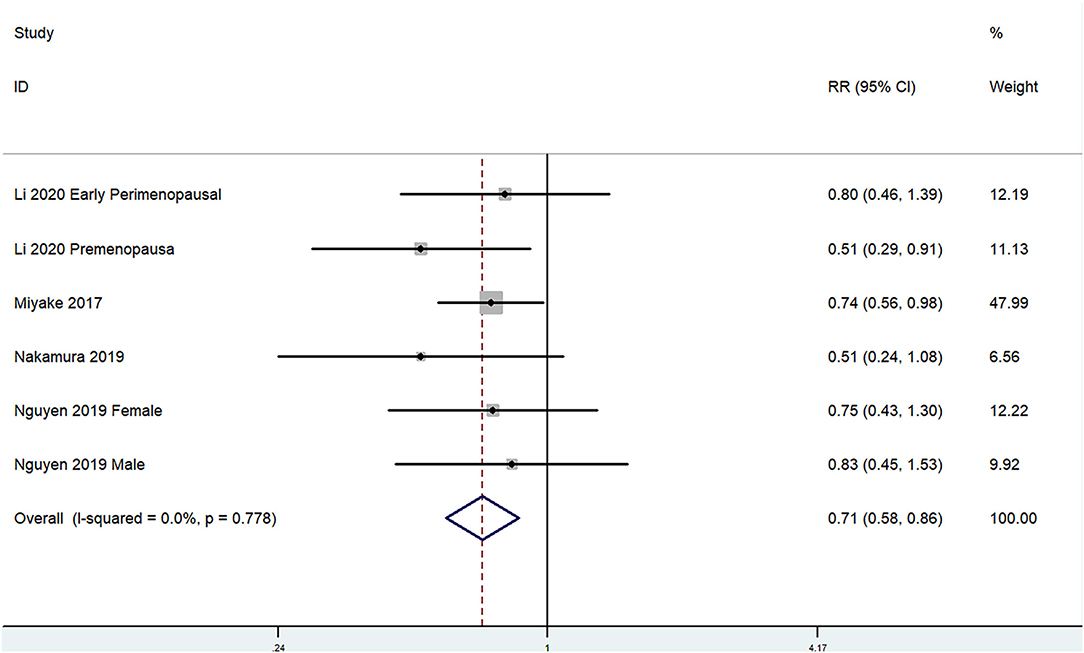
Figure 4. Forest plot of meta-analysis: overall multi-variable adjusted RR of depression for the highest vs. lowest category of dietary manganese intake.
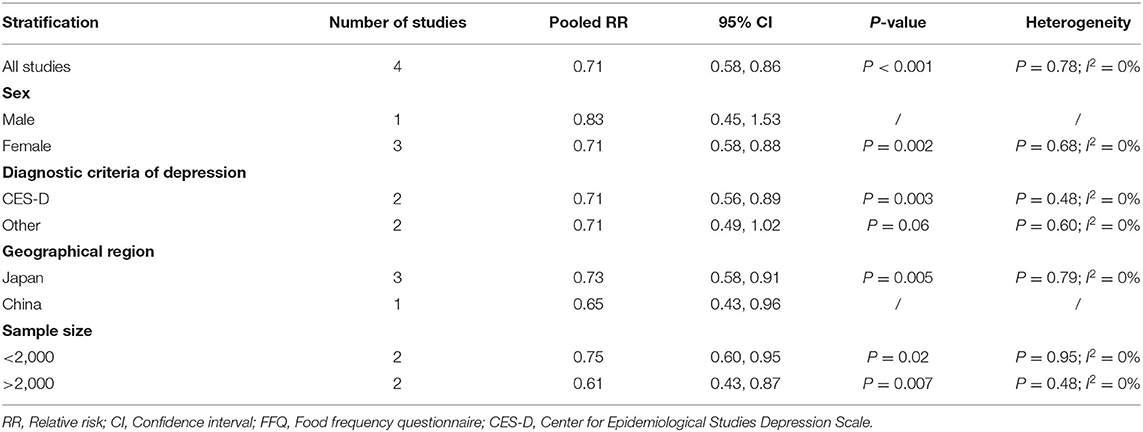
Table 4. Subgroup analysis of depression for the highest vs. lowest dietary manganese intake category.
Discussion
A total of 11 observational studies were identified in the present meta-analysis. The pooled results demonstrated a negative relationship between dietary copper, selenium, and manganese intake and depression, respectively.
The pathophysiology of depression is involved in oxidative stress, whereas copper, selenium, and manganese are served as important antioxidants that act against oxidative stress. Copper is a cofactor of the copper/zinc superoxide dismutase, a protein located in both the cytosol and mitochondrial inner membrane space to relieve the electron transport chain-generated reactive oxygen species (9). On the other hand, copper may drive the activity of the two neurotrophic factors Brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (28), which further influence the activity-dependent neural plasticity and neural network (29). Indeed, experimental evidence demonstrates that low-dose copper exacerbates depression-like behavior in ApoE4 transgenic mice (30). Differently from other metals, selenium incorporates into selenoproteins (glutathione per-oxidases and thioredoxin reductases) and protects from lipoperoxidation and oxidative cell damage (the glutathione antioxidant system is implicated in the pathophysiology of mood disorders) (31). Consistently, the selenocompound 1-methyl-3-(phenylselany1)-1H-indole attenuates depression-like behavior, oxidative stress, and neuroinflammation in streptozotocin-treated mice (32). Moreover, manganese is an important component of MnSOD, an antioxidant mitochondrial metalloenzyme that protects cells from oxidative stress (12, 33). Reduced MnSOD activity due to manganese deficiency might contribute to the development of depression. These above may significantly account for the major findings of our study.
Interestingly, our findings are only confirmed in females, but not males. It suggests that some genetic sexual differences with the diet-related pathology of depression should be considered. For example, the genetic contributions of the serotonin transporter in depression may be different (34), and the process of some serotonin systems may be more apparent in females than that in males either (35). Importantly, the inverse relationship between dietary selenium intake and depression is lost in prospective cohort study, which might be attributed to the potential reversed causality (e.g., depressive subjects may consume less dietary copper, selenium and manganese due to the reduced appetite). Moreover, the diagnostic criteria of depression vary greatly among individuals, which may also influence the reliability of subgroup analysis. Overall, very small number of studies are qualified for subgroup analysis, and the corresponding results should be considered very carefully. More well-designed prospective cohort studies with sexual specification are still needed.
It should also be noted that a very recent meta-analysis study has investigated the role of selenium in depression (36). The authors fail to demonstrate any significant differences in serum selenium levels between depressive and healthy subjects. On the contrary, they find the selenium supplementation significantly reduces depressive symptoms. The inconsistent results may be explained as follow: (1) The selenium in serum may not reflect the issues of dietary selenium intake (37). In fact, demographic variables, health status, and some other factors may also influence serum selenium levels (38), and only one study has adjusted these confounding variables (39). (2) Given that the long-term exposure to low serum selenium level may impair brain function (40), the duration of low selenium intake is ignored in most studies. Importantly, their overall OR result shows no significant relationship between dietary selenium intake and depression. However, their search was performed on June 30, 2020 (updated on April 12, 2021) and two recent published studies were not specified for analysis (24, 25). Moreover, the category of exposure was unclear in one included study either (excluded in the present meta-analysis) (41). Most importantly, the effect estimates for the highest vs. lowest and lowest vs. highest (inadequate vs. adequate) exposure category was also pooled directly. Above all, our study is an important advance and supplement to their study.
Another relevant meta-analysis study has also comprehensively evaluated the relationship between body burden of copper and depression (42). They demonstrate that the blood copper level in depressive subjects is higher than that in controls, which implies that blood copper may be served as a biomarker for depression. On this basis, our study further demonstrates that dietary copper intake is inversely associated with depression either. Interestingly, Johnson et al. further found an inverse relationship between selenium level in household groundwater and depression, and GPX1 gene is related to depression risk and significantly influences the protective impact of selenium (43), which indicates a gene-environment interaction.
Although our findings may encourage to build an awareness with the collaboration between physicians and nutritionists, our results might be influenced by environmental and medical treatment factors, the interaction of multiple dietary factors, and the reversed causality (depressed individuals may have irregular/inadequate nutrition patterns that lead to nutritional inadequacy of these micronutrients intake). Moreover, the toxicity of these micronutrients should also be recognized. For instance, excess copper intake is reported to induce oxidative stress, damage to the mitochondrial, and leads to apoptosis, DNA damage and inflammatory responses (44, 45). In addition, selenium exposure is associated with increased risk for type 2 diabetes (46). Elevated selenium exposure has also been suspected to be a risk factor for the development of several neurodegenerative and neuropsychiatric diseases (47, 48). Moreover, long-term exposure to manganese may have adverse effects on mood state, neurobehavior, and peripheral neurotransmitters (49). Therefore, a careful validation by high-quality randomized controlled trial/prospective cohort study is still needed.
Our study has several strengthens. First, this is the first meta-analysis of observational studies on the associations of dietary copper, selenium, and manganese intake with depression. In addition, the included studies are analyzed based on the adjusted results and large samples. Moreover, the limited heterogeneity level may reflect a decent reliability of our results. Finally, our findings may provide significant information to better consider the dietary effects on depression. The limitations of our study should also be acknowledged. First, only two prospective cohort studies were identified due to the limited relevant literature, which precludes causal relationships (depressive subjects may consume less dietary copper, selenium, and manganese due to the reduced appetite). Second, the classification of exposure and diagnostic criteria of depression varies greatly among individuals. Third, the adjusted factors were not uniform. Fourth, the environmental and medical treatment factors are considered in few studies, their impact cannot be clearly clarified and our topic might be over-simplified (the interaction of multiple dietary factors). Last but not the lease, the circulating level of these micronutrients is not considered due to the limited evidence, and the issue of microelement deficiency cannot be addressed. These limitations may weaken the significance of our study.
Conclusions
Our results suggest a negative relationship between dietary copper, selenium, and manganese intake and depression, respectively. However, due to the limited prospective evidence, our results are restricted to cross-sectional design that precludes causal relationships. More well-designed prospective cohort studies with sexual specification are still needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YZ and JD conceived the idea, drafted this manuscript, selected and retrieved relevant papers, and assessed each study. JD performed the statistical analysis. YZ was the guarantor of the overall content. All authors revised and approved the final manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82102581), National Postdoctoral Science Foundation of China (2021M693562), Provincial Outstanding Postdoctoral Innovative Talents Program of Hunan (2021RC2020), Young Investigator Grant of Xiangya Hospital, Central South University (2020Q14), and FuQing Postdoc Program of Xiangya Hospital, Central South University (176).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.854774/full#supplementary-material
Supplementary Figure 1. Funnel plot with pseudo 95% confidence limits for the analysis of dietary copper intake and depression.
Supplementary Figure 2. Funnel plot with pseudo 95% confidence limits for the analysis of dietary selenium intake and depression.
Supplementary Figure 3. Funnel plot with pseudo 95% confidence limits for the analysis of dietary manganese intake and depression.
References
1. Kessler RC. Epidemiology of women and depression. J Affect Disord. (2003) 74:5–13. doi: 10.1016/S0165-0327(02)00426-3
2. Yary T, Aazami S. Dietary intake of zinc was inversely associated with depression. Biol Trace Elem Res. (2012) 145:286–90. doi: 10.1007/s12011-011-9202-y
3. Jung A, Spira D, Steinhagen-Thiessen E, Ilja Demuth I, Norman K. Zinc deficiency is associated with depressive symptoms-results from the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci. (2017) 72:1149–54. doi: 10.1093/gerona/glw218
4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to (2030). PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
5. Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. (2009) 26:83–97. doi: 10.1002/da.20505
6. Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. (2014) 99:181–97. doi: 10.3945/ajcn.113.069880
7. Zhang Y, Yang Y, Xie MS, Ding X, Li H, Liu ZC, et al. Is meat consumption associated with depression? A meta-analysis of observational studies. BMC Psychiatry. (2017) 17:409. doi: 10.1186/s12888-017-1540-7
8. Ma XY, Jiang S, Yan SM, Li M, Wang CG, Pan YG, et al. Association between copper, zinc, iron, and selenium intakes and TC/HDL-C ratio in US adults. Biol Trace Elem Res. (2020) 197:43–51. doi: 10.1007/s12011-019-01979-x
9. Ruiz LM, Libedinsky A, Elorza AA. Role of copper on mitochondrial function and metabolism. Front Mol Biosci. (2021) 8:711227. doi: 10.3389/fmolb.2021.711227
10. Gorini F, Sabatino L, Pingitore A, Vassalle C. Selenium: an element of life essential for thyroid function. Molecules. (2021) 26:7084. doi: 10.3390/molecules26237084
11. Ding J, Zhang Y. Relationship between the circulating selenium level and stroke: a meta-analysis of observational studies. J Am Coll Nutr. (2021). 1–9. doi: 10.1080/07315724.2021.1902880
12. Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. (2013) 454–455:562–77. doi: 10.1016/j.scitotenv.2013.03.047
13. Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. (2014) 8:CC04–7. doi: 10.7860/JCDR/2014/10258.5292
14. Liu T, Zhong SM, Liao XX, Chen J, He TT, Lai SK. A meta-analysis of oxidative stress markers in depression. PLoS One. (2015) 10:e0138904. doi: 10.1371/journal.pone.0138904
15. Pasco JA, Jacka FN, Williams LJ, Evans-Cleverdon M, Brennan SL, Kotowicz MA, et al. Dietary selenium and major depression: a nested case-control study. Complement Ther Med. (2012) 20:119–23. doi: 10.1016/j.ctim.2011.12.008
16. Kim TH, Choi JY, Lee HH, Park YS. Associations between dietary pattern and depression in Korean adolescent girls. J Pediatr Adolesc Gynecol. (2015) 28:533–7. doi: 10.1016/j.jpag.2015.04.005
17. Miyake Y, Tanaka K, Okubo H, Sasaki S, Furukawa S, Arakawa M. Manganese intake is inversely associated with depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J Affect Disord. (2017) 211:124–29. doi: 10.1016/j.jad.2017.01.016
18. Sánchez-Villegas A, Pérez-Cornago A, Zazpe I, Santiago S, Lahortiga F, Martínez-González MA. Micronutrient intake adequacy and depression risk in the SUN cohort study. Eur J Nutr. (2018) 57:2409–19. doi: 10.1007/s00394-017-1514-z
19. Li ZY, Wang WJ, Xin XL, Song XX, Zhang DF. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J Affect Disord. (2018) 228:68–74. doi: 10.1016/j.jad.2017.12.004
20. Ghimire S, Baral BK, Feng D, Sy FC, Rodriguez R. Is selenium intake associated with the presence of depressive symptoms among US adults? Findings from National Health and Nutrition Examination Survey (NHANES) 2011-2014. Nutrition. (2019) 62:169–76. doi: 10.1016/j.nut.2018.12.007
21. Nakamura M, Miura A, Nagahata T, Shibata Y, Okada E, Ojima T. Low zinc, copper, and manganese intake is associated with depression and anxiety symptoms in the Japanese working population: findings from the eating habit and well-being study. Nutrients. (2019) 11:847. doi: 10.3390/nu11040847
22. Nguyen T, Miyagi S, Tsujiguchi H, Kambayashi Y, Hara A, Nakamura H, et al. Association between lower intake of minerals and depressive symptoms among elderly Japanese women but not men: findings from Shika study. Nutrients. (2019) 11:389. doi: 10.3390/nu11020389
23. Li D, Wu Q, Xu WZ, Zheng HY, Tong YQ, Li Y. Dietary manganese intake is inversely associated with depressive symptoms in midlife women: a cross-sectional study. J Affect Disord. (2020) 276:914–9. doi: 10.1016/j.jad.2020.07.070
24. Almeida TLF, Petarli GB, Cattafesta M, Zandonade E, Bezerra OMPA, Tristão KG, et al. Association of selenium intake and development of depression in Brazilian farmers. Front Nutr. (2021) 8:671377. doi: 10.3389/fnut.2021.756637
25. Ferriani LO, Silva DA, Molina M, Mill JG, Brunoni AR, da Fonseca M, et al. Associations of depression and intake of antioxidants and vitamin B complex: Results of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J Affect Disord. (2022) 297:259–68. doi: 10.1016/j.jad.2021.10.027
26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
28. Travaglia A, Mendola DL, Magrì A, Nicoletti VG, Pietropaolo A, Rizzarelli E. Copper, BDNF and Its N-terminal domain: inorganic features and biological perspectives. Chemistry. (2012) 18:15618–31. doi: 10.1002/chem.201202775
29. Stuke H, Hellweg R, Bermpohl F. The development of depression: the role of brain-derived neurotrophic factor. Nervenarzt. (2012) 83:869–77. doi: 10.1007/s00115-011-3374-8
30. Xu J, He KW, Zhang KQ, Yang C, Nie LL, Dan D, et al. Low-dose copper exposure exacerbates depression-like behavior in ApoE4 transgenic mice. Oxid Med Cell Longev. (2021) 2021:6634181. doi: 10.1155/2021/6634181
31. Roman M, Jitaru P, Barbante C. Selenium biochemistry and its role for human health. Metallomics. (2014) 6:25–54. doi: 10.1039/C3MT00185G
32. Bampi SR, Casaril AM, Fronza MG, Domingues M, Vieira B, Begnini KR, et al. The selenocompound 1-methyl-3-(phenylselanyl)-1H-indole attenuates depression-like behavior, oxidative stress, and neuroinflammation in streptozotocin-treated mice. Brain Res Bull. (2020) 161:158–65. doi: 10.1016/j.brainresbull.2020.05.008
33. Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese is essential for neuronal health. Annu Rev Nutr. (2015) 35:71–108. doi: 10.1146/annurev-nutr-071714-034419
34. Chang CC, Chang HA, Fang WH, Chang TC, Huang SY. Gender-specific association between serotonin transporter polymorphisms (5-HTTLPR and rs25531) and neuroticism, anxiety and depression in well-defined healthy Han Chinese. J Affect Disord. (2017) 207:422–8. doi: 10.1016/j.jad.2016.08.055
35. Wurtman JJ. Depression and weight gain: the serotonin connection. J Affect Disord. (1993) 29:183–92. doi: 10.1016/0165-0327(93)90032-F
36. Sajjadi SS, Foshati S, Haddadian-Khouzani S, Rouhani MH. The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Rep. (2022) 12:1045. doi: 10.1038/s41598-022-05078-1
37. Duffield AJ, Thomson CD. A comparison of methods of assessment of dietary selenium intakes in Otago, New Zealand. Br J Nutr. (1999) 82:131–8. doi: 10.1017/S0007114599001282
38. Conner TS, Richardson AC, Miller JC. Optimal serum selenium concentrations are associated with lower depressive symptoms and negative mood among young adults. J Nutr. (2015) 145:59–65. doi: 10.3945/jn.114.198010
39. Ekramzadeh M, Mazloom Z, Sagheb M. Association of depression with selenium deficiency and nutritional markers in the patients with end-stage renal disease on hemodialysis. J Ren Nutr. (2015) 25:381–7. doi: 10.1053/j.jrn.2014.12.005
40. Gao SJ, Jin YL, Unverzagt FW, Liang CK, Hall KS, Cao JX, et al. Selenium level and depressive symptoms in a rural elderly Chinese cohort. BMC Psychiatry. (2012) 12:72. doi: 10.1186/1471-244X-12-72
41. Banikazemi Z, Mirzaei H, Mokhber N, Mobarhan MG. Selenium intake is related to Beck's depression score. Iran Red Crescent Med J. (2016) 18:e21993. doi: 10.5812/ircmj.21993
42. Ni MM, You YP, Chen JY, Zhang LS. Copper in depressive disorder: a systematic review and meta-analysis of observational studies. Psychiatry Res. (2018) 267:506–15. doi: 10.1016/j.psychres.2018.05.049
43. Johnson LA, Phillips JA, Mauer C, Edwards M, Balldin VH, Hall JR. The impact of GPX1 on the association of groundwater selenium and depression: a Project FRONTIER study. BMC Psychiatry. (2013) 13:7. doi: 10.1186/1471-244X-13-7
44. Huo HH, Wang SZ, Bai YM, Liao JZ, Li XR, Zhang H, et al. Copper exposure induces mitochondrial dynamic disorder and oxidative stress via mitochondrial unfolded protein response in pig fundic gland. Ecotoxicol Environ Saf. (2021) 223:112587. doi: 10.1016/j.ecoenv.2021.112587
45. Guo HR, Wang YQ, Cui HM, Ouyang YJ, Yang TY, Liu CY, et al. Copper induces spleen damage through modulation of oxidative stress, apoptosis, DNA damage, and inflammation. Biol Trace Elem Res. (2022) 200:669–77. doi: 10.1007/s12011-021-02672-8
46. Vinceti M, Filippini T, Wise LA, Rothman KJ. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ Res. (2021) 197:111210. doi: 10.1016/j.envres.2021.111210
47. Naderi M, Puar P, Zonouzi-Marand M, Chivers DP, Niyogi S, Kwong R. A comprehensive review on the neuropathophysiology of selenium. Sci Total Environ. (2021) 767:144329. doi: 10.1016/j.scitotenv.2020.144329
48. Adani G, Filippini T, Michalke B, Vinceti M. Selenium and other trace elements in the etiology of Parkinson's disease: a systematic review and meta-analysis of case-control studies. Neuroepidemiology. (2020) 54:1–23. doi: 10.1159/000502357
Keywords: dietary copper intake, dietary selenium intake, dietary manganese intake, depression, meta-analysis, observational studies
Citation: Ding J and Zhang Y (2022) Associations of Dietary Copper, Selenium, and Manganese Intake With Depression: A Meta-Analysis of Observational Studies. Front. Nutr. 9:854774. doi: 10.3389/fnut.2022.854774
Received: 14 January 2022; Accepted: 17 February 2022;
Published: 15 March 2022.
Edited by:
Surasak Saokaew, University of Phayao, ThailandReviewed by:
Zbigniew Stanislaw Krejpcio, Poznan University of Life Sciences, PolandTommaso Filippini, University of Modena and Reggio Emilia, Italy
Monica Cattafesta, Federal University of Espirito Santo, Brazil
Copyright © 2022 Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhang, emhhbmd5aTAyMDVAY3N1LmVkdS5jbg==
 Jun Ding1
Jun Ding1 Yi Zhang
Yi Zhang