
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr. , 09 May 2022
Sec. Clinical Nutrition
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.851590
 Tong Ji1
Tong Ji1 Li Zhang1
Li Zhang1 Rui Han1
Rui Han1 Linlin Peng2,3
Linlin Peng2,3 Shanshan Shen4
Shanshan Shen4 Xiaolei Liu5
Xiaolei Liu5 Yanqing Shi6
Yanqing Shi6 Xujiao Chen4
Xujiao Chen4 Qiong Chen2,3
Qiong Chen2,3 Yun Li1*
Yun Li1* Lina Ma1* on behalf of 3M Study Working Group
Lina Ma1* on behalf of 3M Study Working GroupBackground: In hospital settings, malnutrition affects 30–50% of aged inpatients and is related to a higher risk of hospital complications and death. This study aims to demonstrate the effectiveness of a tailored optimum nutritional therapy in malnourished, elderly inpatients based on multidisciplinary team recommendations in hopes of decreasing the incidence of deleterious clinical outcomes.
Methods and Design: This trial will be a multicenter, open-label, randomized control trial conducted in the geriatric wards of at least five hospitals in five different regions. We aim to include 500 inpatients over the age of 60 with or at risk of malnutrition based on a Mini Nutritional Assessment Short-Form (MNA-SF) score of ≤ 11 points and the Global Leadership Initiative on Malnutrition with an expected length of stay of ≥ 7 days. Eligible inpatients will be randomized into a 1:1 ratio, with one receiving a multidisciplinary team intervention and the other receiving standard medical treatment or care alone. A structured comprehensive assessment of anthropometry, nutritional status, cognition, mood, functional performance, and quality of life will be conducted twice. These assessments will take place on the day of group allocation and 1 year after discharge, and a structured screening assessment for elderly malnutrition will be conducted at 3 and 6 months after discharge using the MNA-SF. The primary outcome will be nutritional status based on changes in MNA-SF scores at 3, 6 months, and 1 year. The secondary outcome will be changes in cognition, mood, functional status, length of hospital stay, and all-cause mortality 1 year after discharge.
Discussion: Guided by the concept of interdisciplinary cooperation, this study will establish a multidisciplinary nutrition support team that will develop an innovative intervention strategy that integrates nutritional screenings, evaluations, education, consultation, support, and monitoring. Moreover, nutritional intervention and dietary fortification will be provided to hospitalized elderly patients with or at risk of malnutrition. The nutrition support team will formulate a clinical map for malnutrition in elderly patients with standardized diagnosis and treatment for malnutrition in this population.
Clinical Trial Registration: [www.ClinicalTrials.gov], identifier [ChiCTR2200055331].
Malnutrition is caused by nutrient deficiency, with or without inflammation, and results in increased metabolic demand and changes in body composition and cell mass, especially the reduction of fat-free weight (1). These changes negatively affect physical and psychological functions as well as clinical outcomes (2). Human organ structures degenerate naturally during the process of aging, and the functional reserve of the most organs of old age decreases, mainly due to decreased digestive capacity, poor dental status, taste alteration, and changes in human composition (reduction of lean tissue) (3, 4). This process results in insufficient food intake and absorption, metabolic disorders, and increased likelihood of malnutrition in aged populations (5). In addition, various comorbidities and polypharmacy play a role in malnutrition in the elderly. Malnutrition is associated with adverse clinical outcomes, such as a decline in physical and cognitive function, depression, frailty, sarcopenia, longer hospital stays, impaired quality of life, increased risk of infection, and mortality, which may result in heavy burdens on the individuals, their families, and society (6–8). Previous studies have revealed that malnutrition affects as many as 30–50% of elderly patients in nursing homes and acute care settings, highlighting the importance of assessing malnutrition in this population (9–11).
In the past, due to the lack of unified diagnostic criteria, it was difficult to standardize the diagnosis and treatment of malnutrition. In this context, the Global Leadership Initiative on Malnutrition (GLIM) developed the first international consensus, which was of great significance for standardizing the diagnosis, treatment, and the use of medical insurance for malnutrition (12). The GLIM criteria proposed a two-step approach: screening to identify the risk of malnutrition first and then assessing for a diagnosis and grading the severity of malnutrition. According to the GLIM criteria, the Mini Nutritional Assessment Short-Form (MNA-SF) is recommended for malnutrition risk screenings in older populations (12). The malnutrition diagnosis standard includes a combination of at least one of the phenotypic criteria (weight loss, reduced body mass index [BMI], or reduced muscle mass) and one of the etiologic criteria (reduced food intake/assimilation or disease burden/inflammation) recommended by the GLIM. Several clinical studies were conducted in both China and abroad as soon as the GLIM criteria were published. A prospective cohort study showed that the GLIM criteria for malnutrition diagnosis in inpatients was fully validated and could be applied in clinical practice (13). At present, there is a lack of interventional research to demonstrate the validity of the GLIM criteria in clinical applications.
Intervention strategies include comprehensive geriatric assessment, supportive interventions, nutritional counseling, food fortification, oral nutritional supplements (ONS), enteral nutrition (EN), parenteral nutrition (PN), and regular physical exercise for aged inpatients with malnutrition or at risk of malnutrition, according to the European Society for Parenteral and Enteral Nutrition consensus (14), American Society for Parenteral and Enteral Nutrition consensus (15), Integrated Caring for Older People (ICOPE) guidelines (16), and Chinese Society for Parenteral and Enteral Nutrition in geriatrics (17). Malnutrition management in the elderly is complicated and requires a multidisciplinary team. Johns Hopkins University established a multidisciplinary team to manage patients with malnutrition, which reduced the failure rate of nutritional screening by 47%, reduced inpatient expenses, and shortened hospital stays (18). Based on current literature, the establishment of a multidisciplinary and interdisciplinary nutrition support team (NST) and a multi-component malnutrition intervention strategy can ensure and improve the quality and safety of malnutrition treatment, ensure sufficient energy intake, and ameliorate nutritional condition and clinical outcomes (19, 20). Table 1 lists relevant studies (21–26).
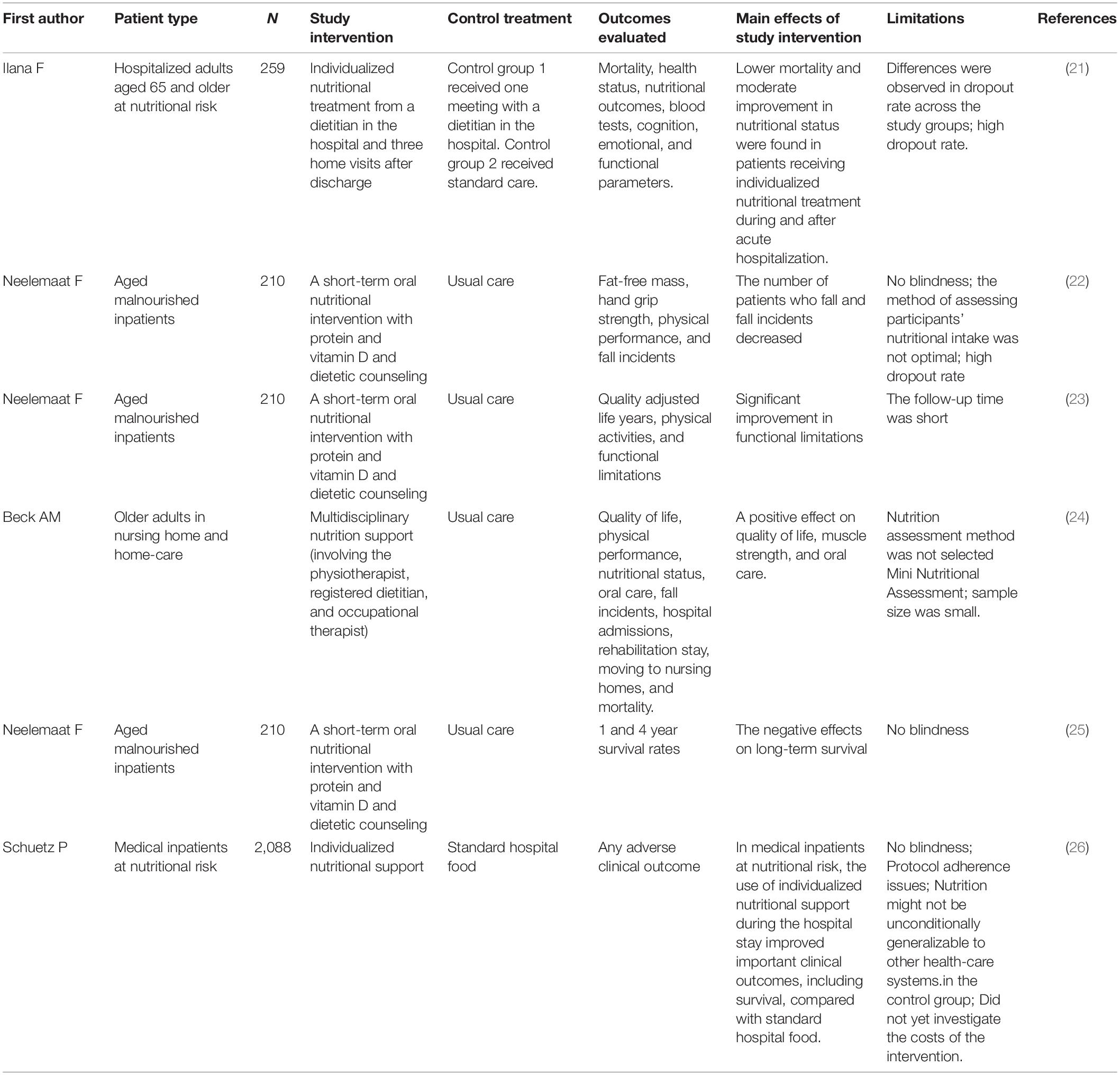
Table 1. Studies on multi-component nutrition intervention or multidisciplinary team intervention for malnutrition in older adults.
A recent systematic review of NST suggested that current studies lacked a robust evidence base because of the absence of well-designed research and good outcome measures (27). A meta-analysis on the efficacy of non-drug treatment in malnourished, aged patients indicated that there is a lack of high-quality evidence that shows which interventions are effective. There is an urgent requirement for high-quality research in this field (28).
This study aims to assess the efficacy of a multidisciplinary team decision-making model on multiple interventions for aged, malnourished inpatients. We hypothesize that the multidisciplinary team intervention strategies may improve nutritional status, depression, functional status, and quality of life; reduce the incidence of frailty; and shorten hospital stays.
This study is a prospective, multicenter, open-label randomized controlled trial. The multicenter trial includes at least five hospitals. The control group will undergo routine nursing and treatment management, in which the food served by the hospital cafeteria will depend on aged inpatient preferences, tastes, or abilities. The intervention group (hereafter also referred to as the case group) will be administered tailored optimum nutritional intervention for malnourished elderly patients based on multidisciplinary team recommendations that integrate nutrition education and counseling, food fortification, and nutritional support under NST guidance and supervision. Malnutrition risk screenings will be completed within 24 h of admission to the hospital. Follow-up investigations will be carried out at 3, 6 months and 1 year after discharge. The detailed flow diagram of the study is shown in Figure 1.
Medical inpatients will be screened for malnutrition risk by physician staff using the MNA-SF within 24 h of admission (12, 29). Inpatients who meet the following criteria will be included: (a) Age ≥ 60 years; (b) patients with clear consciousness who can communicate with researchers effectively; (c) malnutrition risk (MNA-SF ≤ 11 points; (d) length of stay ≥ 4 days; (e) patients who can be followed up for > 1 year; and (f) patients who can sign informed consent voluntarily and cooperate with the smooth implementation of the research.
Patients who meet the criteria listed below will be removed: (a) Mental and consciousness disorders, severe cognitive impairment (MMSE ≤ 9 points), and inability to communicate effectively; (b) poor patient compliance and inability to cooperate with the completion of evaluation and intervention; (c) patient or family refusal to participate in the study; and (d) end-stage disease, acute myocardial infarction, dissecting aortic aneurysm, severe aortic stenosis, endocarditis/acute pericarditis, acute thromboembolism, recent major surgery, or limb fracture in the past 1 month.
Eligible aged patients will be randomly 1:1 allocated into the control or intervention groups according to a pre-specified, computer-generated randomization scheme using SAS.
The NST is composed of multidisciplinary experts in geriatrics, nutrition, nursing, pharmacy, rehabilitation, stomatology, surgery, neurology, cardiology, and others who provide technical support (19, 20) (Figure 2). Geriatricians play a major role in assisting, establishing, and managing the teams; integrating different expert opinions; and providing tailored and comprehensive therapy in the NST. The primary members of NST are nutritionists, clinical pharmacists, and nursing teams who implement the program. Table 2 shows the roles and interventions of the members of the NST.
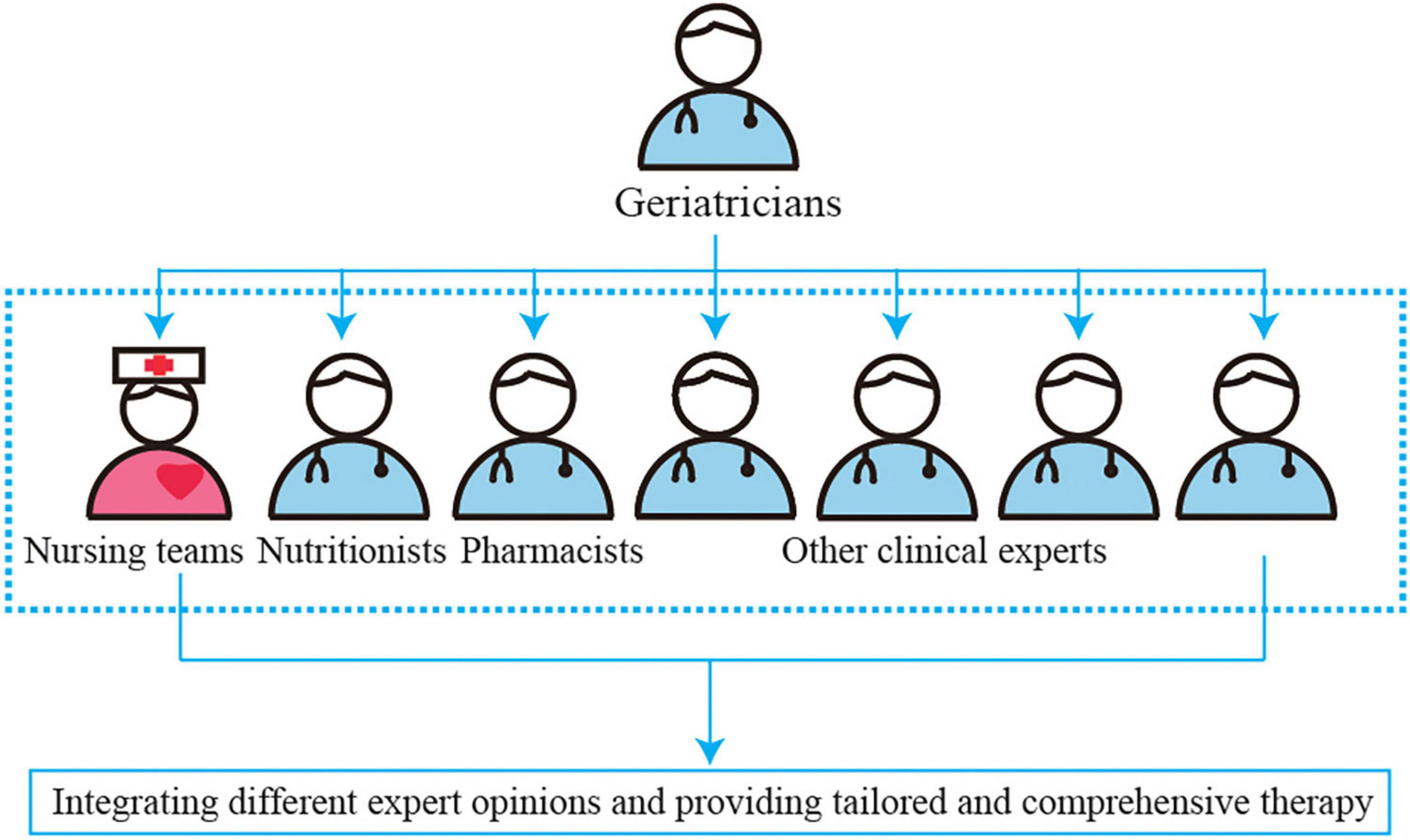
Figure 2. The clinical treatment model of nutrition support team (NST) to provide the tailored optimum nutritional intervention in malnourished aged patients based on multidisciplinary team recommendations.
We designed a practical nutritional intervention process by consensus and according to current guidelines (14–17) which clarified the nutritional strategies for the aged inpatients (Figure 3). We will apply the standard malnutrition clinical strategies for the elderly to the intervention patients. Different ladder treatments can be used simultaneously during the nutritional interventions.
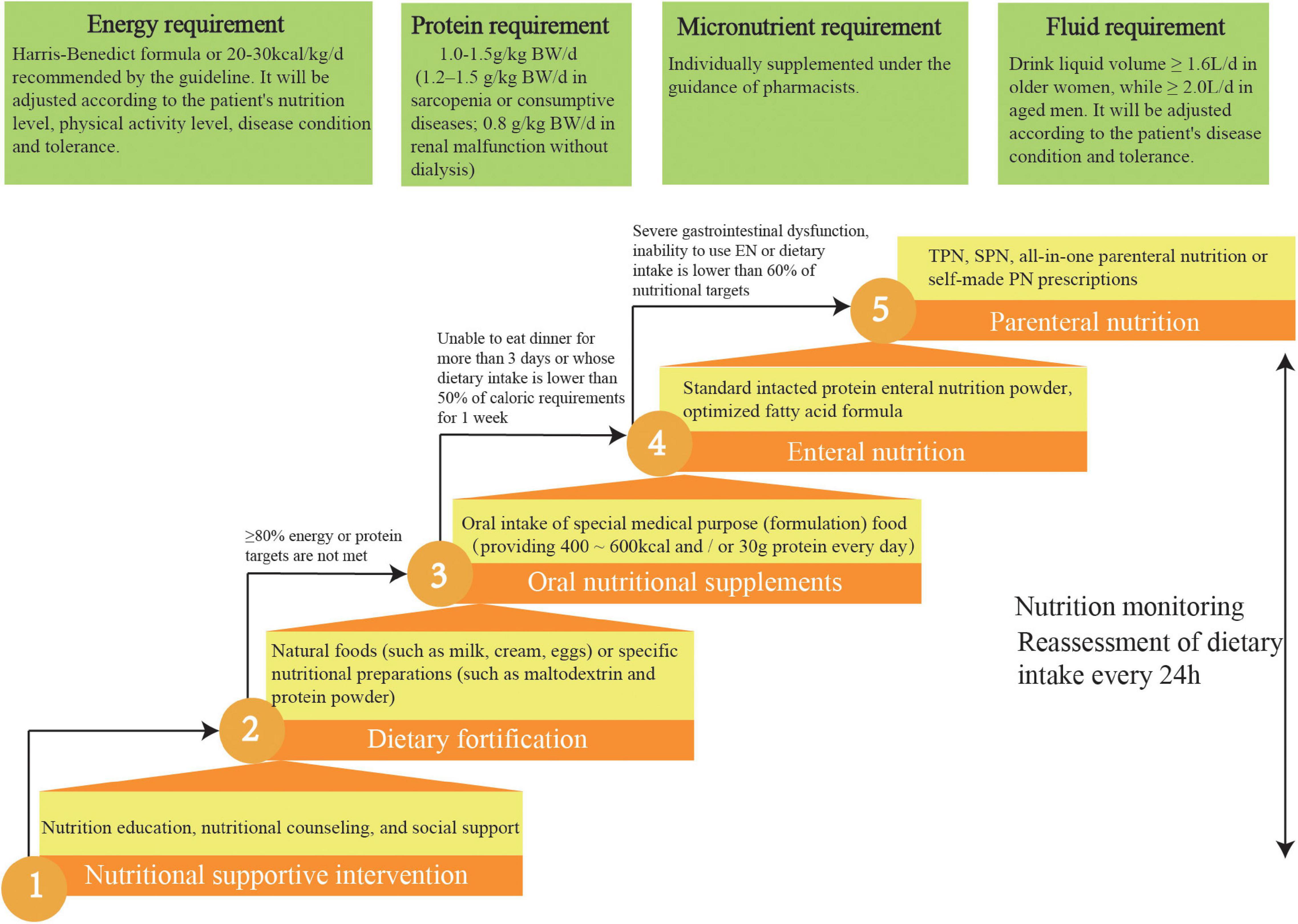
Figure 3. Five-steps-ladder nutritional approach used for the intervention group patients. TPN, total parenteral nutrition; SPN, supplementary parenteral nutrition.
Intervention patients are required to meet four individual nutrition targets: ≥ 70% energy requirement, 90% fluid requirement, 100% protein requirement, and 100% micronutrient intake.
Daily energy requirements of patients are estimated before nutritional interventions. Currently, indirect calorimetry is recognized as the gold standard for assessing resting energy expenditure. Because of costly instruments and complicated operations, this study chose the most frequently used and accurate alternative method, the Harris–Benedict formula (30).
where, wt is weight and ht is height.
According to current guidelines, the energy target for aged patients is 20–30 kcal/kg/d. Current literature indicates that aged, malnourished inpatients who meet this energy target can improve their prognoses and reduce mortality (14, 17). The energy target will be adjusted by the experts in the NST according to the patient’s nutrition level, physical activity level, disease condition, and tolerance. It is recommended that elderly patients with malnutrition consume larger amounts of high-quality protein (1–1.2 g/kg/d) (14, 17), with an even higher intake (1.2–1.5 g/kg/d) recommended for elderly patients with sarcopenia or consumptive diseases (31). However, patients with renal malfunction should reduce their protein intake.
In this study, micronutrients, such as serum ferritin, folic acid, vitamin B12, and serum 25 hydroxyvitamin D, for elderly patients at risk of and with malnutrition will be determined (4). According to these test results, they will be individually supplemented under the guidance of pharmacists. Clinical guidelines state that older women should drink ≥ 1.6 L, whereas older men should drink ≥ 2.0 L daily under the appropriate ambient temperature and general activity intensity, in the absence of special clinical conditions (such as heart failure or renal failure). Patients can choose drinks according to their preferences, such as water, tea, or coffee (14, 17).
Nutritional education and supportive interventions for elderly patients are as follows: first, informing patients about the purpose of nutritional screenings and evaluations; second, answering questions from patients and their families about malnutrition; third, providing nutritional knowledge and a nutrient package for patients; and fourth, creating a soothing dining environment and encouraging patients to dine with others (14, 17). The pamphlets on nutrition jointly prepared by geriatricians and nutritionists will be delivered to the intervention group. On this basis, nutritionists will also carry out individualized nutrition education for patients in the intervention group. The intervention group will be provided nutritional counseling outside the hospital.
A fortified diet refers to using natural foods (such as milk, cream, or eggs) or specific nutritional preparations (such as maltodextrin or protein powder) to strengthen the diet. Studies have shown that dietary fortification can boost energy and protein intake as well as improve clinical outcomes in the elderly. For aged patients, when food intake cannot meet 60% of the caloric requirement, ONS is the preferred nutritional intervention. The standard nutritional treatment for ONS is oral administration several times between meals, providing 400–600 kcal and/or 30 g protein/day (14, 17).
EN should be initiated immediately for patients who are expected to be unable to eat dinner for more than 3 days or whose dietary intake is lower than 50% of caloric requirements for 1 week. The standard intact protein powder is suitable for EN in most older inpatients. Long-term application of optimized fatty acid formulas can improve lipid metabolism and reduce cardiovascular events. Dietary fiber intake of ≥ 25 g/day is helpful in reducing constipation and diarrhea and in improving clinical outcomes (14, 17).
Current guidelines recommend total PN for older patients with severe gastrointestinal dysfunction or the inability to use EN. For example, in aged inpatients where the energy and protein levels provided by EN are lower than 60% of the target energy requirement of the body, supplementary PN should be provided. All-in-one PN is recommended in elderly patients. This method can meet the physiological needs of the elderly, reduce the burden on the liver and kidney, reduce the concentration and osmotic pressure of a single nutrient, and reduce adverse events. For the elderly with special nutritional needs, self-made PN prescriptions can be issued to meet individual needs (14, 17).
Nutrition monitoring will be performed in patients in the intervention group. The detection indicators include clinical symptoms and signs, energy supply compliance, laboratory examination (regular tests for electrolytes, blood glucose, blood lipid, plasma prealbumin, albumin, and C-reactive protein (CRP), according to the needs of the condition), and monitoring and management of EN and PN complications (14, 17).
The control group will undergo routine nursing and treatment management, in which the food served by the hospital cafeteria will depend on aged inpatient preferences, tastes, or abilities. The control group will receive pharmacological and non-pharmacological treatment according to the principles of “evidence-based medicine” and “clinical practice guidelines.” Nutritional treatment may be initiated in control patients at any time, but our nutritional support team will not take the initiative to provide multidisciplinary nutritional support.
The primary efficacy outcome concerns the nutritional status of the participants. The MNA-SF score can be used to evaluate nutritional condition in individuals with various comorbidities (32). The MNA-SF is a screening tool with sensitivity and specificity in the highest quartile (>83 and >90%, respectively) for aged, malnourished inpatients (33). MNA-SF scores higher than 11 indicate that the nutritional status is considered normal and there is no risk of malnutrition. MNA-SF scores less than 11, however, indicate patients that are at risk of malnutrition (34). Investigators from the clinical staff will be trained to ensure the standardization and accuracy of data input, and follow-up assessments will be conducted at 3, 6, and 24 months after patient discharge through phone calls and at 12 months via face-to-face communication. The values during follow-up will be compared with those at baseline.
The following outcomes will be the secondary outcomes:
1. Anthropometric measurements [i.e., handgrip strength, calf circumference, weight, BMI, and fat-free mass index (FFMI)], blood biochemical analysis (i.e., routine blood tests, biochemical indexes, glycosylated hemoglobin, serum 25- hydroxyvitamin D concentration, folic acid, vitamin B12, ferritin, CRP), hospital costs and stay, hospital readmissions and all-cause mortality rate will be assessed.
2. Functional status using the Activities of Daily Living (ADL) scale (35) and functional impairment screening tool (FIST) (36); cognition using the Mini Mental State Examination (MMSE) score (37); depression via the 15-item Geriatric Depression Scale (GDS-15) (38); quality of life using the 12-item Short Form Survey (SF-12) score (39); physical function status using the short physical performance battery protocol (SPPB) (40); frailty via frailty phenotype (41) and frailty screening questionaire (FSQ) (42); social frailty (SF) via HALFT scale (43) and intrinsic capacity (IC) via WHO ICOPE screening tool (44) will be evaluated, as presented in the following section.
A structured comprehensive assessment of patient functional status, cognition, mood, physical performance, quality of life, and the condition of frailty will be performed twice during the course of the study. The first baseline assessment is to be conducted on the day of group allocation, and a reassessment will be performed 12 months after discharge. Current guidelines recommend that the nutritional condition of aged inpatients be comprehensively evaluated based on clinical severity stratification, food intake, weight and body composition, and comprehensive geriatric assessment.
The MNA-SF is a highly sensitive and specific screening tool for malnutrition in the elderly. It determines the nutritional status of patients according to six questions regarding medical history, weight, eating status, and simple physical examination, and has a total possible score of 14 points. The scoring criteria are as follows: malnutrition (0–7 points), at risk of malnutrition (8–11 points), and normal nutritional status (12–14 points) (45).
The association of at least one phenotypic criterion and one etiologic criterion can suffice the diagnostic requirements of malnutrition based on the GLIM criteria (12). Table 3 shows the diagnostic criteria for malnutrition based on the GLIM criteria. The phenotypic criteria include non-volitional weight loss, low BMI, and reduced muscle mass. Fat-free mass by bioelectrical impedance analysis (BIA) is measured using a body composition analyzer (Hongshentai, IOI353, China). The fat-free mass is divided by the squared height (kg/m2) to obtain the FFMI.
Considering the equipment conditions in different clinical research centers and the safety of patients with respect to cardiac implantation equipment (such as cardiac pacemakers), if BIA is not available, physical testing or standard anthropometric measurements (e.g., calf circumference) and functional assessments (e.g., handgrip strength) recommended by the GLIM may be considered supportive measures (11). The thresholds values for calf circumference and handgrip strength recommended by Asian Working Group for Sarcopenia (46) are used: calf circumference of < 34 cm and handgrip strength of < 28 kg in men, calf circumference of < 33 cm and handgrip strength of < 18 kg in women. Handgrip strength, expressed in kilograms, will be measured using a handheld dynamometer (CAMRY, EH101, China).
The etiologic criteria include reduced food intake or assimilation, disease burden, and inflammation. The first MNA-SF item is employed to determine reduced food intake, and asks if food intake has declined over the past 3 months due to loss of appetite, digestive problems, chewing, or swallowing difficulties, with scores of “0” or “1” considered as positive (47). Gastrointestinal symptoms (such as dysphagia, nausea, vomiting, diarrhea, constipation, or abdominal pain) or chronic conditions (such as short bowel syndrome, pancreatic insufficiency, post-bariatric surgery, esophageal strictures, gastroparesis, or intestinal pseudo-obstruction) that detrimentally impact food assimilation or absorption will also be considered. Disease burden includes acute disease or injury-related conditions (including major infection, burns, trauma, or closed head injuries) and chronic disease-related conditions (including malignant disease, chronic obstructive pulmonary disease, congestive heart failure, chronic renal disease, or any disease with chronic or recurrent inflammation). CRP is selected as a biomarker to assess inflammation, as recommended by the GLIM. CRP levels of > 10 mg/L are considered positive (48).
Functional performance will be assessed using the ADL scale (35), which comprises domains such as independence in feeding, bathing, dressing, functional mobility, bladder control, and bowel movements. Using the ADL scale, functional impairment will be assessed as follows: mild (61–99); moderate (41–60); and severe (< 40). FIST will be used to evaluate physical function. It includes three domains: ability to perform activities of daily living, ability to engage in domestic life, and ability to engage in social activities (36). The FIST total score is 0–16. The higher the points are, the better the physical function is.
The MMSE was invented by Folstein et al. (37) and is now applied widely and successfully in cognitive screenings for the elderly. It primarily evaluates orientation, registration and recall, language ability, calculation, and attention. The patients are scored between 0 and 30 points. MMSE scores of greater than 27 indicate normal cognitive function, while scores less than 27 indicate cognitive impairment. According to the scoring standard, the patient may also be classified as having severe dementia (≤10 points), moderate dementia (10–20 points), or mild dementia (≥21 points).
The GDS-15 scale will be used to evaluate depressive status in the last week, with scores ranging from 0 to 15 (38). GDS-15 scores greater than 5 indicate that the patient may be prone to depression.
The SF-12 is a simplified alternative version of the SF-36 health survey, originally developed in the United States (39). The higher the SF-12 score, the better the quality of life.
The WHO recommends using the SPPB test to assess functional capacity in the elderly (40). There are three parts to the test: balance assessment, 4-m walking assessment, and the get-up and sit test. The three parts together add up to the final score. According to the score standard, patients may be regarded as having serious limitation (0–4 points), moderate limitation (5–6 points), slight limitation (7–9 points), and minimal limitation (10–12 points).
The frailty phenotype assessment was developed by Fried et al. (41) and comprises the following criteria: weakness, slow gait speed, low physical activity, exhaustion, and unintentional weight loss. According to the assessment, aged patients may be identified as frail (≥3 criteria present), pre-frail (1–2 criteria present), or robust (0 criteria present). The FSQ scale was developed by Ma et al. (42). It consists of 4 components: slowness, weakness, inactivity, and exhaustion, with scores ranging from 0 to 4. A score of 0 may be indicated robust; 1–2 may be indicated pre-frail; and a score of ≥ 3 may be considered frailty. SF will be assessed by HALFT scale (43). The HALFT scale includes the following 5 items: inability to help others, limited social participation, loneliness, financial difficultly, and not having anyone to talk to, with scores ranging from 0 to 5. According to the score standard, patients may be regarded as non-SF (0 score), pre-SF (1–2 scores), and SF (≥3 scores).
IC will be evaluated by WHO ICOPE screening tool (44) and consists of the following five items: cognitive decline, limited mobility, malnutrition, sensory loss, and depressive symptoms, with scores ranging from 0 to 6. Higher score indicates better IC.
A diagram of the schedule of aged patient enrolment, allocation, and assessment is shown in Figure 4. In this study, sociodemographic and clinical data of the patients as well as information on various comorbidities and polypharmacy will be collected during patient hospitalization.
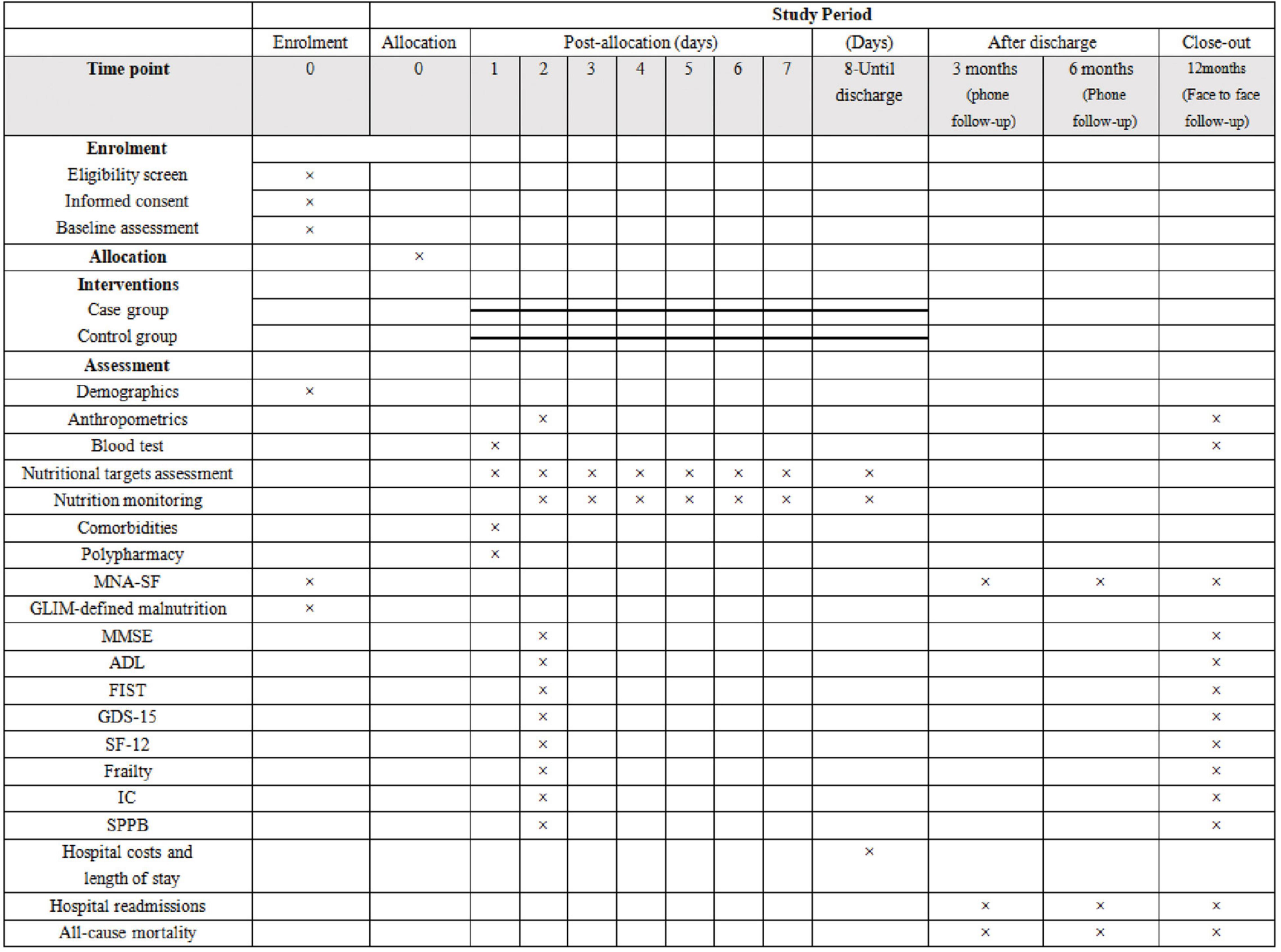
Figure 4. Diagram for time schedule of enrolment, allocation, and assessment. MNA-SF, Mini Nutritional Assessment Short Form; GLIM, the Global Leadership Initiative on Malnutrition; MMSE, Mini-Mental State Examination; ADL, Activities of Daily Living scale; FIST, functional impairment screening tool; GDS-15, the 15-item geriatric depression scale; SF-12, the 12-item short form health survey; IC, intrinsic capacity; SPPB, short physical performance battery.
The 3M study is a parallel, randomized controlled study. It will adopt a ratio of 1:1 between the case and control groups. The MNA-SF score is the primary outcome index. According to the literature, the mean MNA-SF value in the control group was estimated to be 12.1 ± 1.9 (49). After the intervention, the expected average difference in MNA-SF scores between the two groups will be 1.5 (50). The sample size was estimated using a bilateral test. The calculation is based on an alpha level of 0.05 and a desired power of 90%. The sample size of N1 and N2 was 34 cases in the case and control groups, and was calculated using the PASS (version 11.0.7; PASS, NCSS, LLC., Kaysville, Utah, United States). Assuming that the dropout rate is 20%, the sample size is as follows: N1 = N2 = 34 ÷ 0.8 = 43. Considering the long follow-up time, we will enroll at least 50 participants in each group. A total of 500 patients will be included.
Clinical trial supervisors will periodically scrutinize the extracted data for validity and accuracy. All clinical data collection and comprehensive assessments will be performed by uniformly trained investigators. The investigators will faithfully fill clinical report forms according to study protocol requirements. All participant information will be kept strictly confidential and not divulged to any individual or organization.
Epidate 3.1 will be used for data entry, and Excel will be used to establish the database. All data will be checked, numbered, and entered by two researchers. The primary outcome, that is, the MNA-SF score, will be compared between the intervention and control groups using analysis of repeated measurements of variance. The Student’s t-test or Mann–Whitney U-test will be performed to evaluate the statistical differences between the intervention and control groups in the matter of functional status, cognition, quality of life, mood level, physical functional capacity, and length of stay, and the normality of the distributions will be tested using the Shapiro–Wilk test. The incidence of malnutrition and frailty will be compared between the intervention and control groups using chi-square statistics. Survival analyses will be conducted using intention-to-treat analysis by log-rank tests and Cox proportional-hazards model adjusted for confounders. P-value of < 0.05 will be considered for statistical significance. All statistical analyses will be performed using SPSS.
This study was approved by the ethics review board of Xuanwu Hospital of Capital Medical University (2021-060) and all the patients will sign written informed consent.
As the population ages, society faces enormous challenges related to advancing age, deteriorating health, various comorbidities, and polypharmacy, which can lead to disability, dependence, and economic burden (51, 52). One significant challenge is malnutrition. Malnutrition is common but easily ignored in older, hospitalized patients. Malnutrition is reversible, but older adults may experience adverse consequences if it is not diagnosed and treated at an early stage. Early detection of individual nutritional risks and timely initiation of appropriately tailored nutritional therapy are essential in the elderly. The etiology of malnutrition is often multifactorial in aged patients (53), and multi-professional nutritional support teams are required to ensure and improve the quality and safety of nutritional therapy.
The first report of NST in organizing nutritional support was published in 1972, in response to the high incidence of sepsis in hospitalized patients with PN (54). According to current literature, large-scale, multicenter, high-quality clinical trials are insufficient in various fields of clinical nutrition, including NSTs. Most of the current evidence is based on observational studies and the clinical experience of doctors and nutritionists. Recently, some new and important clinical trials have provided strong evidence in favor of NST (26, 55).
Thorne and Baldwin attempted to verify the theory that using multidisciplinary interventions may improve the nutritional status, quality of life, functional status, and survival in malnourished inpatients or in those at risk of malnutrition, through a meta-analysis (56). However, the research involved a broad group of patients and could not therefore be proven as valid for aged inpatients specifically. A recent systematic review showed that multidisciplinary nutritional support was associated with lower mortality and higher quality of life in older patients (57). However, the number of studies included was relatively small. Thus, a more high-quality randomized control study is required to verify these findings and provide a more reliable basis for clinical practice.
In this 3M study, the GLIM criteria will be used for the diagnosis of malnutrition in the elderly and to guide clinical nutritional intervention to compensate for the lack of interventional research. Guided by the concept of interdisciplinary cooperation, the 3M study will establish a multidisciplinary NST; develop an innovative intervention strategy, integrating nutritional education and consultation, dietary fortification, and nutrition support and monitoring; carry out nutrition screenings, comprehensive nutrition evaluations, and nutrition therapy for hospitalized aged inpatients with malnutrition or those at risk of malnutrition; and formulate the clinical pathway for malnutrition, standardized diagnosis, and treatment of malnutrition in older inpatients. If this project is successful, we hope to promote clinical pathways in hospitals nationwide in order to improve the nutritional status of aged patients and reduce poor prognoses.
There are some limitations to the study. First, due to the nutritional intervention strategies of this study, including nutrition education and consultation, it is impossible to blind the participants and investigators. Second, the study strategy is based on the use of individualized comprehensive therapies based on the NST. It may not be possible to analyze the impact of each intervention on the clinical outcome of elderly patients, but we will record the nutritional therapy of each patient in the case group. Finally, patients in the intervention group may have poor compliance. The NST provides individual nutritional therapy for patients, but not all patients can reach the therapeutic target (including energy, fluid, protein, and micronutrient targets). We will record the actual nutritional intake in the form of a nutrition diary.
Currently, there is shortage of clinical standard pathways and management modes for malnourished elderly patients in China. We propose a comprehensive and pragmatic study protocol that aims to assess the effectiveness of tailored optimum nutritional intervention in malnourished elderly patients. These nutritional interventions will be based on multidisciplinary team recommendations that may further develop standardized strategies. We hope that the implementation of this protocol would improve the nutritional, functional, and mood status, as well as reduce the incidence of frailty, enhance quality of life, shorten hospital stays, and improve mortality in elderly patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
This study was approved by the ethics review board of Xuanwu Hospital of Capital Medical University (2021-060). The patients/participants provided their written informed consent to participate in this study.
TJ wrote the manuscript. LM and YL designed the study, revised the manuscript, and supervised the work. TJ, LZ, RH, LP, SS, XL, YS, XC, QC, and 3M Study Working Group collected and collated data. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R&D Program of China (2020YFC2008604 and 2020YFC2008600).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge all the people who participated in the 3M Study.
1. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. (2017) 36:49–64. doi: 10.1016/j.clnu.2016.09.004
2. Corish CA, Bardon LA. Malnutrition in older adults: screening and determinants. Proc Nutr Soc. (2019) 78:372–9. doi: 10.1017/S0029665118002628
3. Black M, Bowman M. Nutrition and healthy aging. Clin Geriatr Med. (2020) 36:655–69. doi: 10.1016/j.cger.2020.06.008
4. Norman K, Hass U, Pirlich M. Malnutrition in older adults-recent advances and remaining challenges. Nutrients. (2021) 13:2764. doi: 10.3390/nu13082764
5. O’Keeffe M, Kelly M, O’Herlihy E, O’Toole PW, Kearney PM, Timmons S, et al. Potentially modifiable determinants of malnutrition in older adults: a systematic review. Clin Nutr. (2019) 38:2477–98. doi: 10.1016/j.clnu.2018.12.007
6. Lorenzo-Lopez L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodriguez-Villamil JL, Millan-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. (2017) 17:108. doi: 10.1186/s12877-017-0496-2
7. Felder S, Lechtenboehmer C, Bally M, Fehr R, Deiss M, Faessler L, et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. (2015) 31:1385–93. doi: 10.1016/j.nut.2015.06.007
8. Khalatbari-Soltani S, Marques-Vidal P. The economic cost of hospital malnutrition in Europe; a narrative review. Clin Nutr ESPEN. (2015) 10:e89–94. doi: 10.1016/j.clnesp.2015.04.003
9. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. (2010) 58:1734–8. doi: 10.1111/j.1532-5415.2010.03016.x
10. van Zwienen-Pot JI, Visser M, Kuijpers M, Grimmerink M, Kruizenga HM. Undernutrition in nursing home rehabilitation patients. Clin Nutr. (2017) 36:755–9. doi: 10.1016/j.clnu.2016.06.003
11. Rojer AG, Kruizenga HM, Trappenburg MC, Reijnierse EM, Sipila S, Narici MV, et al. The prevalence of malnutrition according to the new ESPEN definition in four diverse populations. Clin Nutr. (2016) 35:758–62. doi: 10.1016/j.clnu.2015.06.005
12. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
13. Brito JE, Burgel CF, Lima J, Chites VS, Saragiotto CB, Rabito EI, et al. GLIM criteria for malnutrition diagnosis of hospitalized patients presents satisfactory criterion validity: a prospective cohort study. Clin Nutr. (2021) 40:4366–72. doi: 10.1016/j.clnu.2021.01.009
14. Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. (2019) 38:10–47. doi: 10.1016/j.clnu.2018.05.024
15. White JV, Guenter P, Jensen G, Malone A, Schofield M, et al. Academy Malnutrition Work Group Consensus statement: academy of nutrition and dietetics and American society for parenteral and enteral nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. (2012) 36:275–83. doi: 10.1177/0148607112440285
16. WHO.Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity. Geneva: WHO (2017).
17. Geriatric Nutrition Support Group, Society of Parenteral and Enteral Nutrition, Chinese Medical Association. Guidelines for parenteral and enteral nutrition support in geriatric patients in China. Chin J Geriatr. (2020) 02:119–20. doi: 10.6133/apjcn.2015.24.2.11
18. Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN J Parenter Enteral Nutr. (2011) 35:209–16. doi: 10.1177/0148607110392234
19. Lee JS, Kang JE, Park SH, Jin HK, Jang SM, Kim SA, et al. Nutrition and clinical outcomes of nutrition support in multidisciplinary team for critically ill patients. Nutr Clin Pract. (2018) 33:633–9. doi: 10.1002/ncp.10093
20. Reber E, Strahm R, Bally L, Schuetz P, Stanga Z. Efficacy and efficiency of nutritional support teams. J Clin Med. (2019) 8:1281. doi: 10.3390/jcm8091281
21. Feldblum I, German L, Castel H, Harman-Boehm I, Shahar DR. Individualized nutritional intervention during and after hospitalization: the nutrition intervention study clinical trial. J Am Geriatr Soc. (2011) 59:10–7. doi: 10.1111/j.1532-5415.2010.03174.x
22. Neelemaat F, Lips P, Bosmans JE, Thijs A, Seidell JC, van Bokhorst-de VDSM. Short-term oral nutritional intervention with protein and vitamin D decreases falls in malnourished older adults. J Am Geriatr Soc. (2012) 60:691–9. doi: 10.1111/j.1532-5415.2011.03888.x
23. Neelemaat F, Bosmans JE, Thijs A, Seidell JC, van Bokhorst-de VDSM. Oral nutritional support in malnourished elderly decreases functional limitations with no extra costs. Clin Nutr. (2012) 31:183–90. doi: 10.1016/j.clnu.2011.10.009
24. Beck AM, Christensen AG, Hansen BS, Damsbo-Svendsen S, Moller TK. Multidisciplinary nutritional support for undernutrition in nursing home and home-care: a cluster randomized controlled trial. Nutrition. (2016) 32:199–205. doi: 10.1016/j.nut.2015.08.009
25. Neelemaat F, van Keeken S, Langius J, de van der Schueren M, Thijs A, Bosmans JE. Survival in malnourished older patients receiving post-discharge nutritional support; long-term results of a randomized controlled trial. J Nutr Health Aging. (2017) 21:855–60. doi: 10.1007/s12603-017-0939-7
26. Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. (2019) 393:2312–21. doi: 10.1016/S0140-6736(18)32776-4
27. Mistiaen P, Van den Heede K. Nutrition support teams: a systematic review. JPEN J Parenter Enteral Nutr. (2020) 44:1004–20. doi: 10.1002/jpen.1811
28. Correa-Perez A, Abraha I, Cherubini A, Collinson A, Dardevet D, de Groot L, et al. Efficacy of non-pharmacological interventions to treat malnutrition in older persons: a systematic review and meta-analysis. The SENATOR project ONTOP series and MaNuEL knowledge hub project. Ageing Res Rev. (2019) 49:27–48. doi: 10.1016/j.arr.2018.10.011
29. Xu JY, Zhu MW, Zhang H, Li L, Tang PX, Chen W, et al. A cross-sectional study of GLIM-defined malnutrition based on new validated calf circumference cut-off values and different screening tools in hospitalised patients over 70 years old. J Nutr Health Aging. (2020) 24:832–8. doi: 10.1007/s12603-020-1386-4
30. Flack KD, Siders WA, Johnson L, Roemmich JN. Cross-validation of resting metabolic rate prediction equations. J Acad Nutr Diet. (2016) 116:1413–22. doi: 10.1016/j.jand.2016.03.018
31. Trouwborst I, Verreijen A, Memelink R, Massanet P, Boirie Y, Weijs P, et al. Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients. (2018) 10:605. doi: 10.3390/nu10050605
32. Carrazco-Pena KB, Tene CE, Elizalde AM. [Family dysfunction and malnutrition in the elderly]. Rev Med Inst Mex Seguro Soc. (2015) 53:14–9.
33. Skipper A, Ferguson M, Thompson K, Castellanos VH, Porcari J. Nutrition screening tools: an analysis of the evidence. JPEN J Parenter Enteral Nutr. (2012) 36:292–8. doi: 10.1177/0148607111414023
34. Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. (2009) 13:782–8. doi: 10.1007/s12603-009-0214-7
35. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist. (1970) 10:20–30. doi: 10.1093/geront/10.1_part_1.20
36. Zhang Y, Liu P, Pan Y, Li Y, Zhang L, Li Y, et al. Reliability and validity of the function impairment screening tool in Chinese older adults. Front Med (Lausanne). (2021) 8:720607. doi: 10.3389/fmed.2021.720607
37. Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
38. Shin C, Park MH, Lee SH, Ko YH, Kim YK, Han KM, et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord. (2019) 259:370–5. doi: 10.1016/j.jad.2019.08.053
39. Ware JJ, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34:220–33. doi: 10.1097/00005650-199603000-00003
40. Izquierdo M, Casas-Herrero A, Zambom-Ferraresi F, Martínez-Velilla N, Alonso-Bouzón C, Rodriguez-Mañas L. Multicomponent Physical Exercise Program Vivifrail. A Practical Guide for Prescribing a Multicomponent Physical Training Program to Prevent Weakness and Falls in People Over 70 [Internet]. (2017). Available online at: http://vivifrail.com/wp-content/uploads/2019/11/VIVIFRAIL-ENG-Interactivo.pdf (accessed Aug 4, 2019).
41. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–56. doi: 10.1093/gerona/56.3.m146
42. Ma L, Tang Z, Chan P, Walston JD. Novel frailty screening questionnaire (FSQ) predicts 8-year mortality in older adults in China. J Frailty Aging. (2019) 8:33–8. doi: 10.14283/jfa.2018.38
43. Ma L, Sun F, Tang Z. Social frailty is associated with physical functioning, cognition, and depression, and predicts mortality. J Nutr Health Aging. (2018) 22:989–95. doi: 10.1007/s12603-018-1054-0
44. Ma L, Chhetri JK, Zhang Y, Liu P, Chen Y, Li Y, et al. Integrated care for older people screening tool for measuring intrinsic capacity: preliminary findings from ICOPE pilot in China. Front Med (Lausanne). (2020) 7:576079. doi: 10.3389/fmed.2020.576079
45. Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. (2001) 56:M366–72. doi: 10.1093/gerona/56.6.m366
46. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7. doi: 10.1016/j.jamda.2019.12.012
47. Sanchez-Rodriguez D, Locquet M, Reginster JY, Cavalier E, Bruyere O, Beaudart C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J Cachexia Sarcopenia Muscle. (2020) 11:1200–11. doi: 10.1002/jcsm.12574
48. Merker M, Felder M, Gueissaz L, Bolliger R, Tribolet P, Kagi-Braun N, et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: a secondary analysis of a randomized clinical trial. JAMA Netw Open. (2020) 3:e200663. doi: 10.1001/jamanetworkopen.2020.0663
49. Zhang XL, Zhang Z, Zhu YX, Tao J, Zhang Y, Wang YY, et al. Comparison of the efficacy of nutritional risk screening 2002 and mini nutritional assessment short form in recognizing sarcopenia and predicting its mortality. Eur J Clin Nutr. (2020) 74:1029–37. doi: 10.1038/s41430-020-0621-8
50. Yang PH, Lin MC, Liu YY, Lee CL, Chang NJ. Effect of nutritional intervention programs on nutritional status and readmission rate in malnourished older adults with pneumonia: a randomized control trial. Int J Environ Res Public Health. (2019) 16:164758. doi: 10.3390/ijerph16234758
51. Beard JR, Officer AM, Cassels AK. The world report on ageing and health. Gerontologist. (2016) 56(Suppl. 2):S163–6. doi: 10.1093/geront/gnw037
52. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. (2009) 374:1196–208. doi: 10.1016/S0140-6736(09)61460-4
53. Volkert D, Kiesswetter E, Cederholm T, Donini LM, Eglseer D, Norman K, et al. Development of a model on determinants of malnutrition in aged persons: a MaNuEL project. Gerontol Geriatr Med. (2019) 5:798990387. doi: 10.1177/2333721419858438
54. Allison SP. The uses and limitations of nutritional support the arvid wretlind lecture given at the 14th espen congress in vienna, 1992. Clin Nutr. (1992) 11:319–30. doi: 10.1016/0261-5614(92)90082-2
55. Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. (2016) 35:18–26. doi: 10.1016/j.clnu.2015.12.010
56. Thorne F, Baldwin C. Multimodal interventions including nutrition in the prevention and management of disease-related malnutrition in adults: a systematic review of randomised control trials. Clin Nutr. (2014) 33:375–84. doi: 10.1016/j.clnu.2013.12.018
Keywords: malnutrition, older adults, multidisciplinary team, randomized control study, protocol
Citation: Ji T, Zhang L, Han R, Peng L, Shen S, Liu X, Shi Y, Chen X, Chen Q, Li Y and Ma L (2022) Management of Malnutrition Based on Multidisciplinary Team Decision-Making in Chinese Older Adults (3M Study): A Prospective, Multicenter, Randomized, Controlled Study Protocol. Front. Nutr. 9:851590. doi: 10.3389/fnut.2022.851590
Received: 10 January 2022; Accepted: 16 March 2022;
Published: 09 May 2022.
Edited by:
Harriët Jager-Wittenaar, Hanze University of Applied Sciences, NetherlandsReviewed by:
Marian A. E. De Van Der Schueren, Independent Researcher, NetherlandsCopyright © 2022 Ji, Zhang, Han, Peng, Shen, Liu, Shi, Chen, Chen, Li and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, bGl5X3h3QHNpbmEuY29t; Lina Ma, bWFsaW5hMDg4M0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.