
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 10 May 2022
Sec. Food Chemistry
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.850730
This article is part of the Research Topic Chemical and Biological Changes of Polyphenols Caused by Food Thermal Processing View all 7 articles
 Nusrat Jan1
Nusrat Jan1 Sadaf Anjum2
Sadaf Anjum2 Sajad Mohd Wani1,2*
Sajad Mohd Wani1,2* Sajad Ahmad Mir2
Sajad Ahmad Mir2 A. R. Malik3
A. R. Malik3 Sajad Ahmad Wani4
Sajad Ahmad Wani4 Dina S. Hussein5
Dina S. Hussein5 Rabab Ahmed Rasheed6
Rabab Ahmed Rasheed6 Mansour K. Gatasheh7
Mansour K. Gatasheh7This study aimed to examine the effect of canning and storage on physicochemical, mineral, and antioxidant properties and phenolic composition of apricot wholes, halves, and pulp. The findings for physicochemical properties revealed that the total soluble solids, titratable acidity, total sugars, and ascorbic acid were found higher in apricot pulp (37.15, 1.39, and 20.74% and 7.21 mg/100 g FW, respectively) followed by apricot wholes and halves throughout the storage period. The remarkable contents of potassium, phosphorous, zinc, copper, iron, and manganese were found in the apricot pulp which revealed that canning and storage slightly affected the mineral composition. Bioactive substances were identified and quantified by reversed-phase high-performance liquid chromatography, which indicated a higher presence of chlorogenic acid (34.45 mg/kg FW), quercitin-3-glucoside (16.78 mg/kg FW), neochlorogenic acid (26.52 mg/kg FW), gallic acid (5.37 mg/kg FW), kaempferol (14.22 mg/kg FW), ellagic acid (6.02 mg/kg FW), procyanidin B2 (8.80 mg/kg FW), and epicatechin (9.87 mg/kg FW) in apricot pulp followed by apricot wholes and halves throughout the storage period. The total phenolic content was found highest in apricot pulp (13.76 GAE mg/100 g FW) followed by wholes (8.09 GAE mg/100 g FW) and halves (6.48 GAE mg/100 g FW) which decreased significantly throughout the storage period. Antioxidant properties were assessed by DPPH, ABTS+, MCA, and BCBA, which were found higher in the apricot pulp (92.23 TEAC μg/g DW, 92.33 TEAC μg/g DW, 33.80 TEAC μg/g DW, and 68.40 TEAC μg/g DW, respectively) that is correlated with the higher presence of bioactive compounds. Thus, apricot pulp containing excellent sources of nutrients, minerals, phytochemicals, and antioxidant components could be used for consumption purposes that provide nutraceuticals and antioxidants globally.
The imbalance between antioxidants (AOXs) and free radicals causes oxidative stress (1). Free radicals are molecules that have an unpaired electron (2). These molecules are extremely reactive and play a critical function in cell physiology, including regulation of life cycle, migration, development, activation of second messengers, signaling pathway stimulation, and AOX response triggering (3). Various disorders including cardiovascular disorder, hypertension, cancer, diabetes, atherosclerosis, and arthritis are linked to oxidative stress (3, 4). Fruits and vegetables (F&Vs) are natural sources of AOXs such as phenolic acids, including hydroxycinnamic acids (5) and hydroxybenzoic acids (6); flavonoids, including flavones (7), flavanols (8), flavonols (9), and flavanones (10); vitamin C (11, 12); and pigments like xanthophylls (13), betalain (14), carotenoids (15), chlorophyll a, chlorophyll b (16), and beta-carotene (17) that have high radical quenching ability (18). These are also the essential sources of minerals, such as macroelements (e.g., K, Ca, Mg, P, and S) (19) and microelements (e.g., Fe, Cu, Mn, Zn, Na, Mo, and B) (20), proteins (21), carbohydrates (22), and vitamins (23) for human nutrition. These chemicals are crucial in the production of functional foods. Fruits can be regarded as natural materials for preventing many diseases in humans, as they may lessen the risk of numerous age-related degenerative disorders (24).
Apricot (Prunus armeniaca L.) fruit is consumed as a fresh, dried, and processed product and has positive effects on human nutrition and health (25, 26). It is an essential food source for humans as it provides an optimal mix of bioactive phytochemicals, minerals (especially K, Fe, Mg, and P), fibers, sugars, and vitamins including A, C, riboflavin, thiamine, pantothenic acid, and niacin (27, 28). Nutritionally, apricots are chief sources of AOXs, carotenoids, and phenolics which are significant phytochemicals for their biological importance. The main sugar components are sucrose, glucose, and fructose (29). Lutein, β-carotene, γ-carotene, and β-cryptoxanthin are the most prevalent carotenoids contained in apricot fruit (28). The main phenolic substances present in apricots are catechin, neochlorogenic acid, epicatechin, and chlorogenic acid (30, 31). These compounds are antibacterial, anti-inflammatory, antimutagenic, and anti-allergic, and they have the potential to prevent cancer and coronary heart disease (32, 33). Therefore, apricots have been claimed to be a functional food for improving health and quality of life by boosting the body's defensive mechanism against free radicals, delaying aging, and protecting the body from diseases (25). However, apricots are climacteric in nature and highly perishable stone fruit; various postharvest conditions such as rapid ripening, weight loss, decay, and tissue softening limit their shelf life. In order to reduce its postharvest loss and avail its benefits round the year, apricots are processed in a variety of ways that include drying, freezing, and canning.
Among the successful operational processing techniques, canning is the most common method of food preservation which includes immersing food in acid brine, heating it, exhausting it, and closing it with hermetic seals. Although this technique ensures food safety, it can have an impact on the mechanical characteristics, taste, color, and nutritional quality of food (34). Furthermore, various changes occur in the phytochemical, nutritional, and AOX values during the processing of fruits. Several researchers have reported a decrease in phytochemical content during processing (35–37), while others have reported an increase (28, 38) or no change in phytochemicals (39). Conditions during the storage of food products like storage duration, light, and temperature affect phytochemical retention. Therefore, this study was undertaken to investigate the influence of canning and storage on physicochemical, mineral, AOX characteristics and polyphenolic substances of apricot wholes, halves, and pulp.
Commercial apricot fruits (Prunus armeniaca L.) were procured at the maturity stage in the month of July from ICAR-Central Institute of Temperate Horticulture, Srinagar, Jammu and Kashmir, India. Fruits having bruising, mechanical damage, or diseases were separated. Only those apricot fruits that were uniform in size, shape, and color were selected for the investigation. Standards for high-performance liquid chromatography (HPLC) were procured from Sigma-Aldrich (Steinheim Germany). All other chemicals and reagents used in the experiment had an analytical grade and were obtained from HiMedia India.
Apricots were canned following the technique illustrated by Wani et al. (28) with minor modifications. The apricots were categorized into three lots, each containing 100 apricots. One lot was canned whole, the second lot was canned in halves after the stones were removed, and the third lot was canned as pulp. Bajaj pulper was used to turn the apricots into fine pulp after destoning. For canning of apricot wholes and halves, 40 Brix sugar syrup was boiled, filtered, and poured into the cans, while for canning of apricot pulp, the pulp obtained was filled into cans. The tin cans were then steam exhausted. After that, the cans were autoclaved for 30 min at 121°C. The cans were then cooled in running tap water to 38°C, cleaned, tagged, and kept at room temperature. Analysis of processed apricot treatments was performed for 12 months at an interval of 4 months.
Moisture content, titratable acidity, reducing sugars, total soluble solids, and total sugars of the apricot wholes, halves, and pulp were performed using the standard protocols of AOAC (40). The results were represented as follows: titratable acidity as % malic acid per 100 g, reducing sugars as % glucose, and total soluble solids as degrees Brix (°Bx). Ascorbic acid (AA) content was estimated based on the oxidation of AA by 2, 6-dichlorophenol indophenol dye, and the result was represented as mg per 100 g FW (41).
Minerals were extracted from the apricot treatments by the dry ashing process as elucidated by Sarkar et al. (9) with slight modifications. We determined potassium, phosphorous, zinc, copper, iron, and manganese. Digestion of the sample (2 g) was carried out in a di-acid blend (HNO3:HClO4, 5:1, v/v). The digested material was dissolved in double-distilled water, which was then filtered using Whatman no. 42. Finally, the volume was made up to 50 ml before subjecting it to mineral estimation. Mineral profile analysis of apricot wholes, halves, and pulp was assessed using Atomic Absorption Spectrophotometer (Labtronics, Model LT-2100), and the absorbance was measured at a wavelength of 766.5 nm (Potassium), 880 nm (Phosphorous), 324.8 nm (Copper), 248.3 nm (Iron), 279.5 nm (Manganese), and 213.9 nm (Zinc). The results were represented as mg/100 g FW.
A sample weight (500 g) of each apricot treatment was taken. Methanol (80% v/v) was used as an extraction solvent. Extraction was performed three times with a sample to solution ratio of 1:3. The extracts were then filtered and pooled together. In a rotary vacuum evaporator, the pooled extract was concentrated at 40°C. Prior to HPLC analysis, the concentrate was kept in a desiccator at a low temperature.
The phenolic compounds of apricot wholes, halves, and pulp were identified and quantified using reversed-phase high-performance liquid chromatography (RP-HPLC) equipment (Agilent Technologies, 1,260 Infinity, Germany) equipped with a manual injector, degasser, quaternary pump, and a diode array detector. In HPLC grade methanol solvent, concentrated extracts of all the treatments [1.0% solution (w/v)] were prepared and filtered through Millipore membrane filters (0.22 mm) and Whatman filter paper no. 42; 20 μl of sample volume was loaded into the C-18 column (Agilent Eclipse Plus, C18 3.5 μm, 4.6 × 100 mm, USUXR16700, USA), which was thermostatically operated at 35°C. The mobile phase consisted of 0.1% formic acid in methanol and H2O, and the HPLC gradient was as follows: 5 to 15% in 15 min, 15 to 30% in 20 min, 30 to 40% in 5 min, 40 to 50% in 10 min, 50 to 60% in 5 min, 60 to 75% in 5 min, and finally reaching 95% in a total time of 65 min employed at a flow rate of 1.0 ml/min. Detection and quantification were carried out at 280 nm (for kaempferol, quercetin-3-glucoside, epicatechin, and procyanidin B2) and 320 nm (for ellagic acid, gallic acid, chlorogenic acid, and neochlorogenic acid). The retention time and the characteristic UV spectra of the standard compounds were used to identify the phenolic compounds. Results were reported as milligram per kilogram (mg/kg FW). The protocol was followed as elucidated by Wani et al. (42).
The total phenolic content (TPC) of treatments was assayed following the protocol as described by Wani et al. (43), and the content was evaluated using the Folin–Ciocalteu colorimetric assay. Absorbance readings were taken at 725 nm using a spectrophotometer (UV-2450, Shimadzu, Japan), and the results were represented as GAE mg/100 g of FW.
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of apricot treatments was performed as elucidated by Wani et al. (43). On a UV–vis spectrophotometer (UV-2450, Shimadzu, Japan), the absorbance reading was taken at 517 nm, and the radical scavenging activity was reported as percent inhibition as follows:
where AA = absorbance after sample incubation and AB = absorbance before sample addition.
The metal chelating activity (MCA) of apricot treatments was assessed using the protocol of Sharma and Gujral (44). On a UV–vis spectrophotometer (UV-2450, Shimadzu, Japan), the absorbance reading was taken at 562 nm, and the percentage chelation of iron (Fe2+) was used to determine the metal chelation activity as follows:
where AA = absorbance after sample incubation and AB = absorbance before sample addition.
The 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS+) radical cation method of apricot treatments was performed following the protocol illustrated by Tembo et al. (45). On a UV–vis spectrophotometer (UV-2450, Shimadzu, Japan), the absorbance reading was taken at 734 nm, and % inhibition was computed as follows:
where A0 = absorbance of the control and A1 = absorbance of the sample.
The β-carotene bleaching assay (BCBA) of apricot treatments was performed by measuring the coupled autoxidation of linoleic acid and β-carotene following the protocol of Ueno et al. (46). On a UV–vis spectrophotometer (UV-2450, Shimadzu, Japan), the absorbance reading was taken at 460 nm, and % inhibition of bleaching was computed as follows:
Trolox was used as the reference standard for all the AOX properties, and the results were expressed as μg Trolox equivalent/g DW.
All the experiments were carried out in triplicates, and the mean values were calculated. Data were assessed statistically by one-way ANOVA using SPSS, Version 22 (IBM Statistics 21.0, Chicago, IL, USA). To measure the significant differences between sample means, Duncan's test was used at 5% level of significance.
The effect of canning and storage on physicochemical characteristics of apricot treatments (wholes, halves, and pulp) is given in Table 1. The data revealed that moisture content varied non-significantly among the apricot treatments throughout the storage period. However, AA, titratable acidity, total sugars, reducing sugars, and total soluble solids (TSS) significantly varied across the treatments. TSS was found highest in canned apricot pulp (37.15 °Bx) followed by apricot wholes (35.59 °Bx) and apricot halves (32.12 °Bx) at 0 month of storage. Apricot pulp showed the highest titratable acidity (1.39%) followed by apricot wholes (1.14%), whereas apricot halves had the lowest titratable acidity (0.81%) at 0 month of storage. In case of sugars, apricot pulp had the highest total sugars (20.74%) and lowest reducing sugars (5.74%) followed by apricot wholes and halves at 0 month of storage. AA content was noticed highest in apricot pulp (7.21 mg/100 g FW) followed by apricot wholes (6.9 mg/100 g FW) and lowest in apricot halves (5.39 mg/100 g FW) at 0 month of storage. The data further revealed that TSS, reducing sugars, and total sugars inclined in all three treatments of apricot during 12 months of storage significantly (p < 0.05); however, a decline in titratable acidity and AA was noticed during 12 months of storage in all three forms of apricot treatments significantly (p < 0.05).
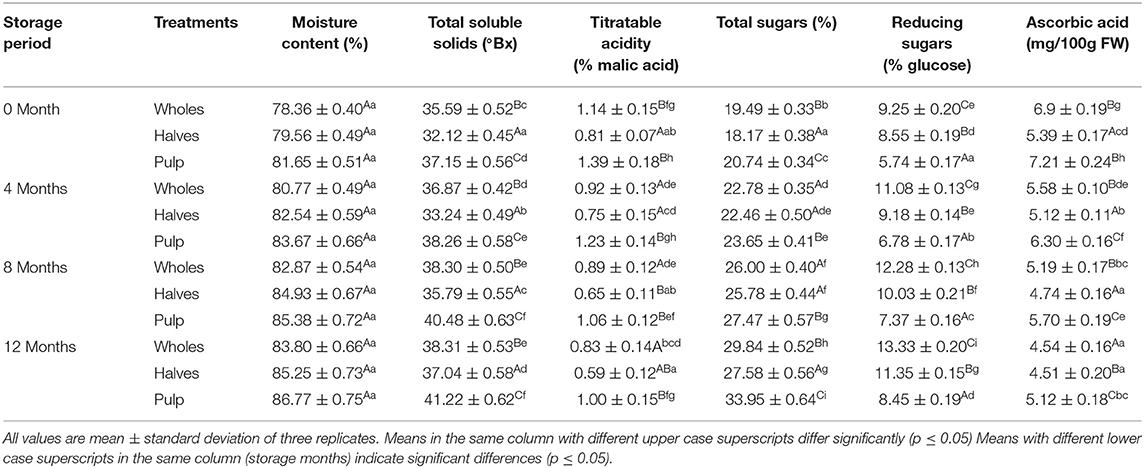
Table 1. Effect of canning and storage on physiochemical properties of canned apricot wholes, halves and pulp.
The influence of canning and storage on the mineral composition of apricot wholes, halves, and pulp is summarized in Table 2. The data indicated that canning affected the mineral composition of all the treatments of apricot significantly (p < 0.05). In this study, apricot pulp had the highest potassium content (1,508 mg/100 g FW), while apricot halves had the lowest potassium content (1,377 mg/100 g FW) at 0 month of storage. Iron content was found highest in apricot pulp (9.01 mg/100 g FW), while it was found lowest in apricot halves (3.02 mg/100 g FW). In contrast, phosphorous and copper were found highest in apricot halves (51.84 mg/100 g FW; 2.84 mg/100 g FW), respectively. Zinc and manganese were noticed highest in apricot wholes (19.21 mg/100 g FW and 0.79 mg/100 g FW, respectively) at 0 month of storage. However, the data further revealed that mineral content decreased in all the treatments during the 12 months of storage period significantly (p < 0.05).
The amount of phenolic compounds differ significantly among the three apricot treatments (wholes, halves, and pulp) throughout the storage period. HPLC detected eight phenolic compounds in all the apricot treatments, namely, chlorogenic acid, neochlorogenic acid, quercitin-3-glucoside, gallic acid, kaempferol, ellagic acid, procyanidin B2, and epicatechin (Table 3). The data indicated that across the treatments, the content of chlorogenic acid (34.45 mg/kg FW), neochlorogenic acid (26.52 mg/kg FW), quercitin-3-glucoside (16.78 mg/kg FW), gallic acid (5.37 mg/kg FW), kaempferol (14.22 mg/kg FW), ellagic acid (6.02 mg/kg FW), procyanidin B2 (8.80 mg/kg FW), and epicatechin (9.87 mg/kg FW) was observed to be significantly higher in apricot pulp followed by apricot wholes and halves at 0 month of storage. The data further revealed that phenolic composition was observed to be declined significantly (p < 0.05) during 12 months of storage in all the treatments.
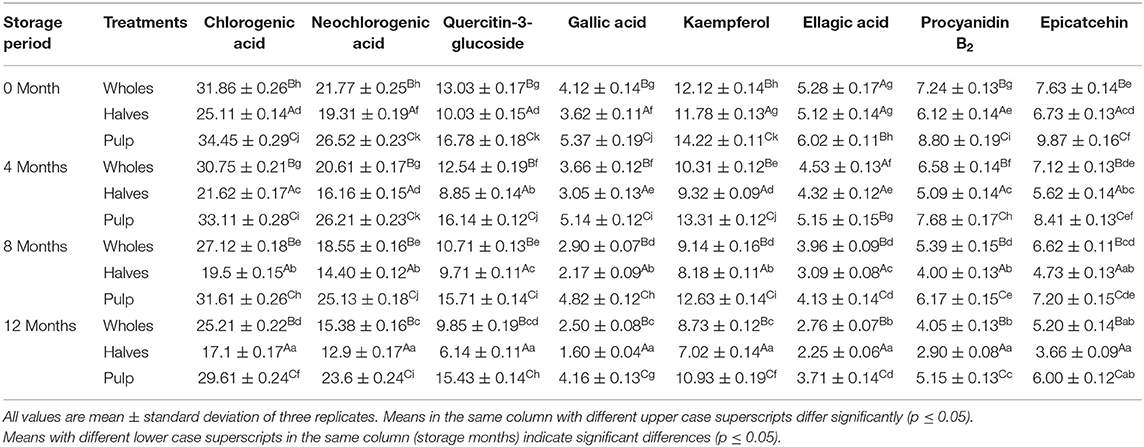
Table 3. Effect of canning and storage on phenolic composition (mg/kg FW) of apricot wholes, halves and pulp.
Table 4 presented the TPC and AOX properties of apricot wholes, halves, and pulp, namely, DPPH radical scavenging activity, MCA, ABTS+, and BCBA. TPC and AOX capacity (AC) varied significantly (p < 0.05) among the studied apricot treatments. The data indicated that TPC was observed highest in apricot pulp (13.76 GAE mg/100 g FW) followed by apricot wholes (8.09 GAE mg/100 g FW), while the lowest was noticed in apricot halves (6.48 GAE mg/100 g FW) at 0 month of storage. Apricot pulp (92.23 TEAC μg/g DW) exhibited the highest AC (DPPH) followed by apricot wholes (88.16 TEAC μg/g DW) and apricot halves (77.37 TEAC μg/g DW). Highest AC (ABTS+) was noticed in apricot pulp (92.33 TEAC μg/g DW), while the lowest AC was noticed in apricot halves (84.21 TEAC μg/g DW). Apricot pulp (33.80 TEAC μg/g DW) showed the highest AC (MCA) followed by apricot wholes (11.97 TEAC μg/g DW) and apricot halves (10.54 TEAC μg/g DW). Highest AC (BCBA) was observed in apricot pulp (68.40 TEAC μg/g DW), while the lowest AC was observed in apricot halves (TEAC 50.63 μg/g DW) at 0 month of storage. However, TPC and AOX properties were observed to be declined significantly (p < 0.05) during 12 months of storage in all the treatments.
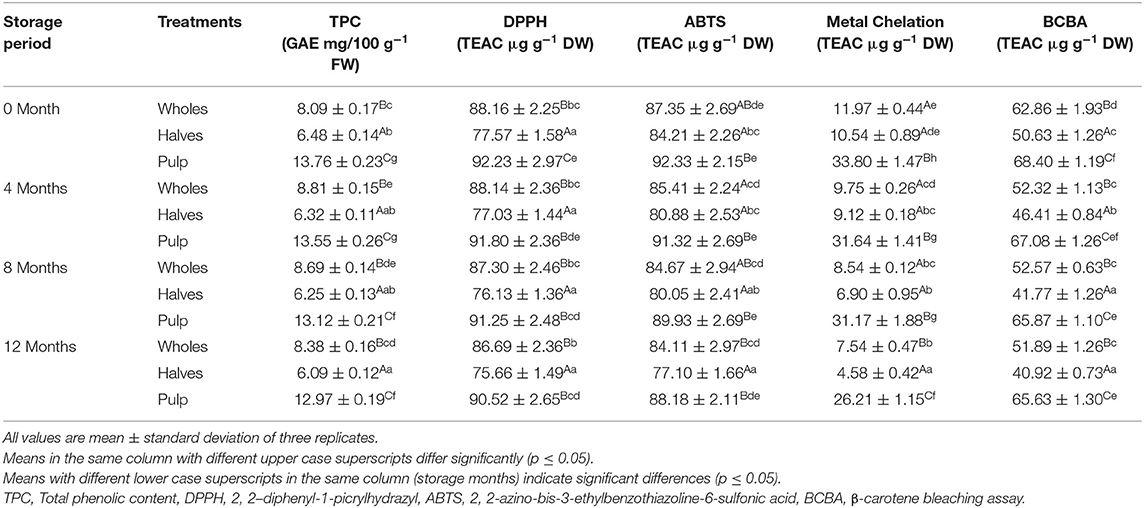
Table 4. Effect of canning and storage on total phenolic content and antioxidant properties of apricot wholes, halves and pulp.
The moisture content varied non-significantly across the treatments. However, the highest TSS was noticed in canned apricot pulp followed by apricot wholes and apricot halves at 0 month of storage. The highest TSS in canned apricot pulp is likely due to the conversion of complex substances into soluble forms at higher temperatures which are retained in the pulp (28). Further, TSS was positively correlated with total sugars. However, TSS was increased significantly in all three treatments of apricot during 12 months of storage possibly due to the hydrolysis of complex carbohydrates into simpler ones during storage (47). The findings obtained in this research were corroborated to the results of canned apricots by Wani et al. (28). The titratable acidity of the fruit is directly related to its organic acid content. During storage and ripening, its concentration usually reduces due to enzyme-catalyzed processes, which makes the fruit taste sweeter (41). Organic acids are also commonly utilized as preservatives in a variety of fruit products. The results for titratable acidity showed the highest value for the apricot pulp followed by apricot wholes and apricot halves at 0 month of storage. The highest titratable acidity value of apricot pulp may be because of the delaying of the ripening process due to pulping. However, a decline in titratable acidity was noticed during 12 months of storage in all three forms of apricot treatments significantly (p < 0.05). This could be because organic acids are used in respiratory metabolism during storage (48). Vijayanand et al. (49) also found that after 2 months of storage at ambient temperature, the acidity of canned mango slices decreased gradually.
Sugars are a vital component of the diet and a quick energy source for the body. Fruit with elevated sugar content can also be used to determine its maturity (2). The results depicted that at 0 month of storage, apricot pulp showed the highest total sugars and lowest reducing sugars. Sugar utilization in browning reactions may account for the lowest reducing sugars in apricot pulp (28). However, reducing sugars and total sugars enhanced in all three treatments of apricot during 12 months of storage significantly. This increase in reducing sugars may be because of the breakdown of complex carbohydrates and non-reducing sugars, while the breakdown of complex carbohydrates into simpler sugars may be the reason for the increase in total sugars (28). Vijayanand et al. (49) found a comparable rise in reducing sugars over a 2-month storage period in canned mango slices. Gowda and Huddar (50) observed a similar rise in total sugars during storage in canned mango slices.
Ascorbic acid is known as a potent free radical trapping agent because it prevents the degradation of fruit during ripening. Apart from that, AA provides an indication of the nutritional quality of F&Vs (41). According to several studies, AA is an extremely heat-labile compound that is readily destroyed when exposed to heat (45). Thus, the influence of canning and storage on the AA content of apricot wholes, halves, and pulp was evaluated. The data revealed that the AA content was noticed highest in the apricot pulp. Higher solute concentrations in apricot pulp, particularly sugars and organic acids, may protect against AA oxidation by chelating metal ions and lowering their catalytic efficacy (51). However, AA was noticed to be declined in all three treatments of apricot during 12 months storage period significantly. The higher degradation of AA in all the treatments was due to higher storage temperatures. Further, because enzymatic degradation is eliminated during processing at higher temperatures, non-enzymatic mechanisms could be the reason for AA loss during 12 months storage period (45). The formation of dicarbonyl compounds and dehydroascorbic acid during its degradation (diketogulonic acid, xylosone, 3-deoxythreosone, and erythrulose) can experience AA browning (non-enzymatic browning) through a Strecker-like decomposition process with amino acids resulting in brown pigments formation (51), including hydroxymethylfurfural. These results coincide well with the results recorded by Cao et al. (52) for cucumber.
Canning affected the mineral composition of all the treatments significantly. Copper, iron, zinc, and manganese were found in lesser quantities in all the treatments and were classified as microelements, while phosphorous and potassium levels were much higher and were classified as macroelements. In this study, we found that among the macrominerals, potassium content was found highest in all three treatments at 0 month of storage. These values were comparable with the previous reports on mineral analysis conducted by Akin et al. (29) on various apricot varieties. The data further revealed that potassium and iron were found highest in apricot pulp, phosphorous and copper in apricot halves, and zinc and manganese in apricot wholes at 0 month of storage. Minerals are not affected as much due to canning as minerals are thermally stable during normal processing conditions. However, minerals decreased during the storage period due to the leaching of minerals to the syrup, while the concentration of iron gradually increased with storage of canned treatments which may be attributed to the possible deposition of iron from the iron lids used for canning. These results are in line with the previous results recorded by Rickman et al. (53) for canned F&Vs. Hence, the apricot pulp could contribute to high minerals than that of apricot wholes and halves.
The RP-HPLC analysis was performed in order to identify major polyphenol compounds and account for the elevated AC and TPC values noticed in apricot wholes, halves, and pulp. At 0 month of storage, the content of chlorogenic acid, neochlorogenic acid, quercitin-3-glucoside, gallic acid, kaempferol, ellagic acid, procyanidin B2, and epicatechin was observed to be significantly higher in apricot pulp followed by apricot wholes and halves. Canning of apricot pulp was carried out without a medium, and thus, the polyphenolic components that would have leached into the canning medium were retained in the slurry. It can, therefore, be envisaged that chlorogenic acid, quercitin-3-glucoside, gallic acid, neochlorogenic acid, kaempferol, ellagic acid, procyanidin B2, and epicatechin contribute significantly to the AC and TPC found in the apricot pulp. The results were comparable with Wani et al. (42) for canned apricot pulp. Hence, these AOX phytochemicals found in apricot pulp could be an essential parameter for consumers, as they play a key role in the detoxification of ROS in the human body and in combating anti-aging and many degenerative diseases (9). However, these polyphenolic compounds were observed to be declined significantly during 12 months of storage in all the treatments. According to Chen et al. (54), processing at higher temperatures can result in complex chemical and physical reactions that affect phenolic profile, such as the liberation of bound phenolic substances, polyphenols decomposition, and the breakdown and conversion of phenolic substances. Earlier, Rababah et al. (37) investigated that storing apricot jam at 25°C for 5 months resulted in a significant loss in polyphenols. Aaby et al. (35) reported a 20% decline in ellagitannin and 17% decline in ellagic acid derivatives during the processing of strawberries. Odriozola-Serrano et al. (39) noticed that the concentration of quercetin and kaempferol in strawberry juice is unaffected by thermal pasteurization (90°C, 60 s); however, Igual et al. (36) found that the concentration of quercetin, naringenin, naringin, and narirutin in grapefruit juice is reduced.
The TPC varied significantly across the treatments. The data revealed that TPC was observed highest in apricot pulp followed by wholes and halves at 0 month of storage. Higher phenolic content in apricot pulp is attributed to factors such as increased leaching of soluble chemicals and the liberation of phenolics from their bonded forms to the pulp due to cell injury (28). The findings were corroborated to the findings of Stevanato et al. (55). However, during 12 months of storage, TPC was declined significantly in all three apricot treatments. Polyphenols interacting with sugars and sugar metabolites could be the possible justification behind a reduction in TPC during storage (56). It is also likely that complex formation occurs between phenolic substances and proteins resulting in changes in the nutritional, functional, and structural attributes of both substances. The association may be because the phenolic group is a good hydrogen donor, forming hydrogen bonds with the carboxyl group of the protein (45). Furthermore, higher storage temperatures can cause phenolic compounds to undergo chemical oxidation, resulting in the formation of polymers and quinones (57), thereby resulting in a decrease in phenolic compounds during storage. Similar reports were demonstrated by Wani et al. (28) for dried, frozen, and canned apricots.
Preservation techniques are expected to be the reason for the significant loss of natural AOXs. When compared with fresh fruits, processed fruit items are considered to provide lesser health-promoting protection, and yet, the functional characteristics of the latter may remain stable throughout the storage (45). The effect of canning and storage period on AOX stability was studied in apricot wholes, halves, and pulp during 12 months of storage. Four techniques (MCA, DPPH, BCBA, and ABTS+) were employed due to numerous reaction features and processes involved in a complex or mixed system (58, 59) in order to reflect the overall AOX potential of apricots. For instance, ABTS+ measures both lipophilic and hydrophilic AOXs, while the DPPH method only considers lipophilic AOXs (58). The BCBA is among the most widely used method for measuring AOX activity in the area of food chemistry (46).
Apricot pulp exhibited the highest DPPH radical scavenging potential followed by apricot wholes and apricot halves at 0 month of storage. The findings of DPPH scavenging potential are in line with the ABTS+, MCA, and BCBA indicating the maximum AC of apricot pulp followed by apricot wholes and apricot halves. Generally, treatments with high TPCs exhibited higher DPPH scavenging activity, whereas chelating agents can prevent the peroxidation of lipids and suppress the generation of free radicals by deactivating the transition metals (2). As expected, apricot pulp exhibited the highest AOX properties (DPPH, ABTS+, and MCA), which may be due to its higher AA and phenolic compounds. Besides this, non-enzymatic browning occurs during the processing of pulp, which may further contribute to its higher AOX properties (57). AA browning is considered to be the cause of non-enzymatic browning of pulp during thermal processing or storage (51). Similar reports were demonstrated by Bof et al. (60) for the AOX potential of fruit pulp and jelly. AA and phenolics showed higher AOX activity in Amaranthus hypochondriacus (61), Amaranthus tricolor (62), Amaranthus blitum (63), weedy species (64), stem amaranth (65), green morph amaranth (66), and red morph amaranth (67), which are corroborative to the present findings. Furthermore, apricot pulp significantly (p < 0.05) interrupted the β-carotene bleaching in comparison with apricot wholes and halves. Discoloration of β-carotene occurs in the absence of AOXs because it binds with linoleic acid and develops free radicals. As a result, the β-carotene bleaching rate can be delayed in the presence of AOXs (2). Several studies investigated that the BCBA is linked to flavonoids and polyphenols components, which can prevent linoleic acid oxidation and the generation of hydroperoxides (68, 69), thereby supporting our results. However, the AOX activity decreased during 12 months of storage owing to the diffusion of hydrophilic compounds, including AOXs and phenolics, to syrup (70). Furthermore, depletion in AOX activity noticed during storage could be related to a decline in thermally susceptible phenolic compounds and vitamin C and the generation of melanoidins with pro-oxidant characteristics (71). Similar reports were demonstrated by Tembo et al. (45) for baobab fruit pulp.
Apricot fruit is rich in AA, organic acids, and phenolic compounds, which can be utilized in a variety of food and pharmaceutical applications. This study revealed that eight polyphenolic compounds were detected by RP-HPLC in all the treatments and were declined throughout the storage period. AOX properties (DPPH, ABTS+, MCA, and BCBA) and TPC were investigated, which were found higher in apricot pulp throughout the storage period. Furthermore, apricot pulp exhibited excellent sources of nutrients and minerals compared with apricot wholes and halved that declined during the storage period. It can be concluded that apricot pulp retained most of its nutrients, minerals, AOX phytochemicals, and AOX activity during canning and 12 months of storage that offered huge prospects for nutritional and health-boosting effects and that consuming it can reduce the danger of numerous oxidative stress-related disorders such as diabetes, cancer, aging, and cardiovascular disorders.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
NJ: Writing original draft, review, and editing. SA: Investigation. SMW: Conceptualization. SM: Formal Analysis. AM: Supervision. SAW: Critically reviewed the manuscript. DH, RR, and MG: Interpreted the data and analyzed the samples. All authors have read and approved the final version of the manuscript.
SMW acknowledges the Department of Biotechnology, GOI for providing the financial support. The authors extend their appreciation to the researchers supporting project number (RSP-2021/393), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Meda NTR, Bangou MJ, Bakasso S, Millogo-Rasolodimby J, Nacoulma OG. Antioxidant activity of phenolic and flavonoid fractions of cleome gynandra and maerua angolensis of burkina faso. J App Pharm Sci. (2013) 3:36–42. doi: 10.7324/JAPS.2013.30207
2. Soulef S, Seddik K, Nozha M, Smain A, Saliha D, Hosni K. Phytochemical screening and in vivo and in vitro evaluation antioxidant capacity of Fargaria ananassa, Prunus armeniaca, and Prunus persica fruits growing in algeria. Prog Nutr. (2020) 22:236–52.
3. Duran-Bedolla J, Rodriguez MH, Navor VS, Arancibia SR, Cerbon M, Rodriguez MC. Oxidative stress: production in several processes and organelles during plasmodium sp development. Oxid Antioxid Med Sci. (2013) 2:93–100. doi: 10.5455/oams.130413.rv.007
4. Ahmed D, Munim F, Saeed S. Phenolic and flavonoid contents and anti-oxidative potential of epicarp and mesocarp of Lagenaria sicerariafruit: a comparative study. Asian Pac J Trop Med. (2014) 7:249–55. doi: 10.1016/S1995-7645(14)60241-8
5. Sarker U, Oba S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci Rep. (2018) 8:12349. doi: 10.1038/s41598-018-30897-6
6. Sarker U, Oba S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci Rep. (2019) 9:18233. doi: 10.1038/s41598-019-52033-8
7. Sarker U, Oba S. Phenolic profiles and antioxidant activities in selected drought-tolerant leafy vegetable amaranth. Sci Rep. (2020) 10:18287 doi: 10.1038/s41598-020-71727-y
8. Sarker U, Oba S. Nutraceuticals, phytochemicals, and radical quenching ability of selected drought-tolerant advance lines of vegetable amaranth. BMC Plant Biol. (2020) 20:564. doi: 10.1186/s12870-020-02780-y
9. Sarker U, Hossain MN, Iqbal MA, Oba S. Bioactive components and radical scavenging activity in selected advance lines of salt-tolerant vegetable Amaranth. Front. nutr. (2020) 7:587257. doi: 10.3389/fnut.2020.587257
10. Sarker U, Oba S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. (2020) 20:499. doi: 10.1186/s12870-020-02700-0
11. Sarker U, Oba S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. (2018) 252:72–83. doi: 10.1016/j.foodchem.2018.01.097
12. Sarker U, Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep. (2018) 8:16496. doi: 10.1038/s41598-018-34944-0
13. Sarker U, Oba S. Color attributes, betacyanin, and carotenoid profiles, bioactive components, and radical quenching capacity in selected Amaranthus gangeticus leafy vegetables. Sci Rep. (2021) 11:11559. doi: 10.1038/s41598-021-91157-8
14. Sarker U, Oba S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J Sci Food Agric. (2018) 8:12349. doi: 10.1002/jsfa.9423
15. Sarker U, Oba S. Leaf pigmentation, its profiles and radical scavenging activity in selected Amaranthus tricolor leafy vegetables. Sci Rep. (2020) 10:18233. doi: 10.1038/s41598-020-66376-0
16. Sarker U, Islam MT, Rabbani MG, Oba S. Antioxidant leaf pigments and variability in vegetable amaranth. Genetika. (2018) 50:209–20. doi: 10.2298/GENSR1801209S
17. Sarker U, Islam MT, Rabbani MG, Oba S. Variability in total antioxidant capacity, antioxidant leaf pigments and foliage yield of vegetable amaranth. J Integr Agric. (2018) 17:1145–53. doi: 10.1016/S2095-3119(17)61778-7
18. Sarker U, Oba S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. (2018) 18:258. doi: 10.1186/s12870-018-1484-1
19. Sarker U, Islam MT, Oba S. Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE. (2018) 13:e0206388. doi: 10.1371/journal.pone.0206388
20. Sarker U, Islam MT, Rabbani MG, Oba S. Genotypic diversity in vegetable amaranth for antioxidant, nutrient and agronomic traits. Indian J Genet. (2017) 77:173–6. doi: 10.5958/0975-6906.2017.00025.6
21. Sarker U, Oba S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl Biochem Biotechnol. (2018) 186:999–1016. doi: 10.1007/s12010-018-2784-5
22. Sarker U, Islam MT, Rabbani MG. Oba S. Variability, heritability and genetic association in vegetable amaranth (Amaranthus tricolor L). Span J Agric Res. (2015) 13:e0702. doi: 10.5424/sjar/2015132-6843
23. Sarker U, Islam MT. Rabbani MG, Oba S. Genotype variability in composition of antioxidant vitamins and minerals in vegetable amaranth. Genetika. (2015) 47:85–96. doi: 10.2298/GENSR1501085S
24. Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, et al. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci Technol. (2016) 65:1025–30. doi: 10.1016/j.lwt.2015.09.038
25. Karatas N, Sengul M. Some important physicochemical and bioactive characteristics of the main apricot cultivars from Turkey. Turk J Agric. (2020) 44:651–61. doi: 10.3906/tar-2002-95
26. Gecer MK, Kan T, Gundogdu M, Ercisli S, Ilhan G. Sagbas HI. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L) from aras valley in Turkey. Genet Resour Crop Evol. (2020) 67:935–45. doi: 10.1007/s10722-020-00893-9
27. Karatas N. Evaluation of nutritional content in wild apricot fruits for sustainable apricot production. Sustainability. (2022) 14:1063. doi: 10.3390/su14031063
28. Wani SM, Masoodi FA, Ahmad M, Mir SA. Processing and storage of apricots: effect on physicochemical and antioxidant properties. J Food Sci Technol. (2018) 55:4505–14. doi: 10.1007/s13197-018-3381-x
29. Akin EB, Karabulut I, Topcu A. Some compositional properties of main Malatya apricot (Prunus armeniaca L) varieties. Food Chem. (2008) 107:939–48. doi: 10.1016/j.foodchem.2007.08.052
30. Karatas N, Ercisli S, Bozhuyuk MR. Assessment of morphological traits, nutritional and nutraceutical composition in fruits of 18 apricot cv. sekerpare clones. Sustainability. (2021) 13:11385. doi: 10.3390/su132011385
31. Huang W, Bi X, Zhang X, Liao X, Hu X, Wu J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov Food Sci Emerg Technol. (2013) 18:74–82. doi: 10.1016/j.ifset.2013.01.001
32. Rampackova E, Gottingerova M, Gala P, Kiss T, Ercisli S, Necas T. Evaluation of protein and antioxidant content in apricot kernels as a sustainable additional source of nutrition. Sustainability. (2021) 13:4742. doi: 10.3390/su13094742
33. Chang SK, Alasalvar C, Shahidi F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J Funct Foods. (2016) 21:113–32. doi: 10.1016/j.jff.2015.11.034
34. Hungaro HM, Alvarenga VOO, Pena WEL. de Souza Sant'Ana A. Hearts of palms preserves and botulism in Brazil: an overview of outbreaks, causes and risk management strategies trends. Food Sci Technol. (2013) 34:80–95. doi: 10.1016/j.tifs.2013.07.008
35. Aaby K, Wrolstad RE, Ekeberg D, Skrede G. Polyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J Agric Food Chem. (2007) 55:5156–66. doi: 10.1021/jf070467u
36. Igual M, Garcia-Martinez E, Camacho MM, Martinez-Navarrete N. Changes in flavonoid content of grapefruit juice caused by thermal treatment and storage. Innov Food Sci Emerg Technol. (2011) 12:153–62. doi: 10.1016/j.ifset.2010.12.010
37. Rababah TM, Majdi A, Al-Mahasneh Kilani I, Yang W, Alhamad MN, Ereifeja K. Al-U'datt M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J Sci Food Agric. (2010) 91:1096–102. doi: 10.1002/jsfa.4289
38. Patras A, Brunton NP, Butler F, Downey G. Effect of thermal and high pressure processing on antioxidant activity and instrumental color of tomato and carrot purees. Innov Food Sci Emerg Technol. (2009) 10:16–22. doi: 10.1016/j.ifset.2008.09.008
39. Odriozola-Serrano I, Soliva-Fortuny R, Martin-Belloso O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur Food Res Technol. (2008) 228:239–48. doi: 10.1007/s00217-008-0928-5
40. AOAC. Association of Official Analytical Chemists. 18th ed. Arlington. AOAC Official Methods of Analysis (2005).
41. Gull A, Bhat N, Wani SM, Masoodi FA, Amin T, Ganai SA. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. (2021) 349:129149. doi: 10.1016/j.foodchem.2021.129149
42. Wani SM, Masoodi FA, Yousuf S, Dar BN, Rather SA. Phenolic compounds and antiproliferative activity of apricots: influence of canning, freezing, and drying. J Food Process Preserv. (2020) 44:e14887. doi: 10.1111/jfpp.14887
43. Wani SM, Jan N, Wani TA, Ahmad M, Masoodi FA. Gani A. Optimization of antioxidant activity and total polyphenols of dried apricot fruit extracts (Prunus armeniaca L) using response surface methodology. J Saudi Soc Agric Sci. (2017) 16:119–26. doi: 10.1016/j.jssas.2015.03.006
44. Sharma P, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res Int. (2011) 44:235–40. doi: 10.1016/j.foodres.2010.10.030
45. Tembo DT, Holmes MJ, Marshall LJ. Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from malawi. J Food Compost Anal. (2017) 58:40–51. doi: 10.1016/j.jfca.2017.01.002
46. Ueno H, Yamakura S, Arastoo RS, Oshima T, Kokubo K. Systematic evaluation and mechanistic investigation of antioxidant activity of fullerenols using β-carotene bleaching assay. J Nanomater. (2014) 2014:1–7. doi: 10.1155/2014/802596
47. Jain SK, Mukherjee S. Enhancing keeping quality of fruits in mango cv. Langra Ind J Hortic. (2011) 68:142–4.
48. Valero D, Serrano M. Postharvest Biology and Technology for Preserving Fruit Quality. Boca Raton, NY: CRC Press-Taylor and Francis (2010).
49. Vijayanand P, Deepu E, Kulkarni SG. Physico chemical characterization and the effect of processing on the quality characteristics of Sindura, Mallika, and Totapuri mango cultivars. J Food Sci Technol. (2015) 52:1047–53. doi: 10.1007/s13197-013-1041-8
50. Gowda IND, Huddar AG. Investigations on processing quality of some mango varieties, hybrids and their blends. J Food Sci Technol. (2004) 41:154–9.
52. Cao SF, Hu ZC, Wang HO. Effect of salicylic acid on the activities of antioxidant enzymes and phenylalanine ammonia-lyase in cucumber fruit in relation to chilling injury. J Hortic Sci Biotechnol. (2009) 84:125–30. doi: 10.1080/14620316.2009.11512492
53. Rickman JC, Bruhn CM, Barrett DM. Nutritional comparison of fresh, frozen, and canned fruits and vegetables II. Vitamin A and carotenoids, vitamin E, minerals and fiber J Sci Food Agric. (2007) 87:1185–96. doi: 10.1002/jsfa.2824
54. Chen Y, Yu L, Rupasinghe H. Effect of thermal and non-thermal pasteurization on the microbial inactivation and phenolic degradation in fruit juice: a mini-review. J Sci Food Agric. (2013) 93:981–6. doi: 10.1002/jsfa.5989
55. Stevanato N, Ribeiro TH, Giombelli C, Cardoso T, Wojeicchowski JP, Danesi EDG, Bolanho Barros BC. Effect of canning on the antioxidant activity, fiber content, and mechanical properties of different parts of peach palm heart. J Food Process Preserv. (2020) 44:1. doi: 10.1111/jfpp.14554
56. Agbenorhevi J, Marshall LJ. Investigation into the total phenols and antioxidant activity during storage of fruit smoothies. J Food Sci Eng. (2012) 2:72–9. doi: 10.17265/2159-5828/2012.02.002
57. Nayak B, Liu RH, Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit Rev Food Sci Nutr. (2015) 55:887–919. doi: 10.1080/10408398.2011.654142
58. Apak R, Ozyurek M, Guçlu K, Capanolu E. Antioxidant activity/capacity measurement. 1 classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem. (2016) 64:997–1027. doi: 10.1021/acs.jafc.5b04739
59. Du G, Li M, Ma F, Liang D. Antioxidant capacity and the relationship with polyphenol and vitamin C in actinidia fruits. Food Chem. (2009) 113:557–62. doi: 10.1016/j.foodchem.2008.08.025
60. Bof CMJ, Fontana RC, Piemolini-Barreto LT, Sandri IG. Effect of freezing and processing technologies on the antioxidant capacity of fruit pulp and jelly. Braz Arch Biol Technol. (2012) 55:107–14. doi: 10.1590/S1516-89132012000100014
61. Sarker U, Oba S. Nutritional and bioactive constituents and scavenging capacity of radicals in amaranthus hypochondriacus. Sci Rep. (2020) 10:19962. doi: 10.1038/s41598-020-71714-3
62. Sarker U, Oba S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. (2020) 11: 559876. doi: 10.3389/fpls.2020.559876
63. Sarker U, Oba S. Nutrients, minerals, pigments, phytochemicals, and radical scavenging activity in Amaranthus blitum leafy vegetables. Sci Rep. (2020) 10:3868. doi: 10.1038/s41598-020-59848-w
64. Sarker U, Oba S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci Rep. (2019) 9:20413. doi: 10.1038/s41598-019-50977-5
65. Sarker U, Oba S, Daramy MA. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci Rep. (2020) 10:3892. doi: 10.1038/s41598-020-60252-7
66. Sarker U, Hossain MM, Oba S. Nutritional and antioxidant components and antioxidant capacity in green morph Amaranthus leafy vegetable. Sci Rep. (2020) 10:1336. doi: 10.1038/s41598-020-57687-3
67. Sarker U, Oba S. Protein, dietary fiber, minerals, antioxidant pigments and phytochemicals, and antioxidant activity in selected red morph Amaranthus leafy vegetable. PLoS ONE. (2019) 14:e0222517. doi: 10.1371/journal.pone.0222517
68. Duh PD, Yen GC. Antioxidative activity of three herbal water extracts. Food Chem. (1997) 60:639–45. doi: 10.1016/S0308-8146(97)00049-6
69. Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. (2005) 90:743–9. doi: 10.1016/j.foodchem.2004.04.035
70. Campbell OE, Padilla-zakour OI. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res Int. (2013) 54:448–55. doi: 10.1016/j.foodres.2013.07.016
Keywords: apricots, canning, storage, antioxidants, bioactive compounds, HPLC
Citation: Jan N, Anjum S, Wani SM, Mir SA, Malik AR, Wani SA, Hussein DS, Rasheed RA and Gatasheh MK (2022) Influence of Canning and Storage on Physicochemical Properties, Antioxidant Properties, and Bioactive Compounds of Apricot (Prunus armeniaca L.) Wholes, Halves, and Pulp. Front. Nutr. 9:850730. doi: 10.3389/fnut.2022.850730
Received: 08 January 2022; Accepted: 28 March 2022;
Published: 10 May 2022.
Edited by:
Huilin Liu, Beijing Technology and Business University, ChinaReviewed by:
Umakanta Sarker, Bangabandhu Sheikh Mujibur Rahman Agricultural University, BangladeshCopyright © 2022 Jan, Anjum, Wani, Mir, Malik, Wani, Hussein, Rasheed and Gatasheh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sajad Mohd Wani, d2FuaXNhamFkODJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.