
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 16 March 2022
Sec. Nutritional Immunology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.848400
This article is part of the Research Topic Dietary Polyphenols for Improving Gut Health, volume II View all 14 articles
 Youxia Wang1
Youxia Wang1 Changming Hong1
Changming Hong1 Zebiao Wu1
Zebiao Wu1 Shuwei Li2,3
Shuwei Li2,3 Yaoyao Xia1
Yaoyao Xia1 Yuying Liang1
Yuying Liang1 Xiaohua He1
Xiaohua He1 Xinyu Xiao1
Xinyu Xiao1 Wenjie Tang2,3*
Wenjie Tang2,3*The integrity of intestinal barrier determines intestinal homeostasis, which could be affected by various factors, like physical, chemical, and biological stimuli. Therefore, it is of considerable interest and importance to maintain intestinal barrier function. Fortunately, many plant polyphenols, including resveratrol, could affect the health of intestinal barrier. Resveratrol has many biological functions, such as antioxidant, anti-inflammation, anti-tumor, and anti-cardiovascular diseases. Accumulating studies have shown that resveratrol affects intestinal tight junction, microbial composition, and inflammation. In this review, we summarize the effects of resveratrol on intestinal barriers as well as the potential mechanisms (e.g., inhibiting the growth of pathogenic bacteria and fungi, regulating the expression of tight junction proteins, and increasing anti-inflammatory T cells while reducing pro-inflammatory T cells), and highlight the applications of resveratrol in ameliorating various intestinal diseases.
Gut is the main organ for digestion and absorption of nutrients, almost 95% of the nutrients are absorbed by small intestine (1, 2). Gut is the largest microecosystem of the body that exists 1,000 different bacterial species, as evidenced by the quantity reaches 1014 and 100-fold more genes than that found in the human genome (3). Notably, the gut is also considered to be the largest immune organ, which has a strong mucosal immune system and contains the largest library of immune cells in the body (4). Most importantly, the intestinal barrier, including microbial, chemical, physical, immune, and gut vascular barrier, plays a vital role in protecting intestinal health (Figure 1) (5). The rich species of microorganisms constitute the intestinal microbial barrier. Indeed, the largest number of phyla in the intestinal tract is Firmicutes, about 65%; the second is Bacteroidetes, about 25%; and a small number of Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia (6, 7). The chemical barrier consists of mucus, digestive juice, and bacteriostatic substances (8). The physical barrier, also known as mechanical barrier, has mucosal epithelium, lamina propria, and muscularis mucosae, which can prevent the invasion of bacteria and macromolecules (9). The mucosal epithelium has an orderly arrangement of epithelial cells. Actually, there are tight junctions, adhesive junctions, and desmosomes between adjacent epithelial cells. The intestinal immune barrier is mainly composed of lymphocytes and immune cells in mesenteric lymph nodes (MLNs) and intestinal lamina propria (LP) (10). In addition, gut is called “the second brain” of the host. The number of neurons distributed in the intestinal tract is second only to the brain, with about 100 million nerve cells, far more than the spinal cord and peripheral nervous system (11). Recently, study shown that gut vascular barrier (GVB) is the deepest protective layer of the intestinal tract, this is the last barrier that prevents microorganism and harmful toxins cross into the circulatory system and peripheral organs (12). Therefore, fine-tuning of intestinal health is the first element for body health.
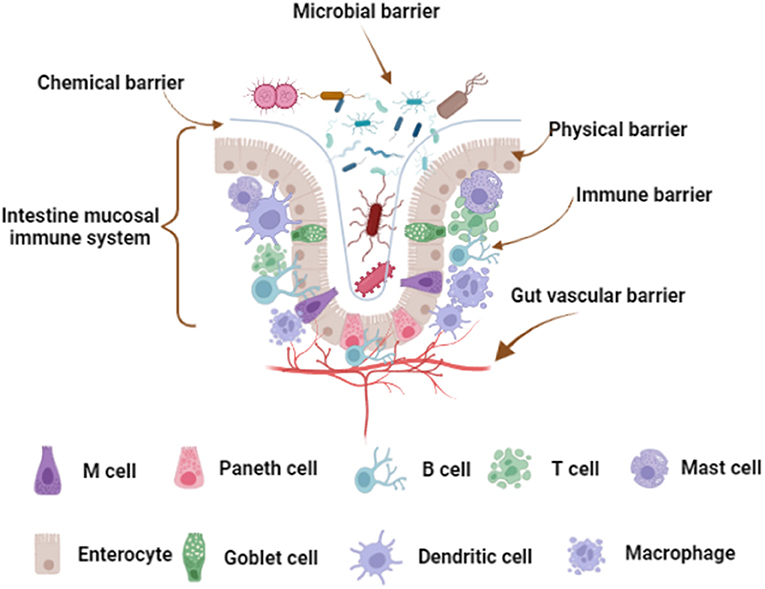
Figure 1. Intestinal barrier composition. The microbial barrier is the uppermost barrier of the intestinal tract. The chemical barrier is close to the microbial barrier and is a layer of mucous membrane. Physical barrier, also known as mechanical barrier, has mucosal epithelium, lamina propria and muscularis mucosae. Mucosal epithelium has an inherent epithelium, including immune cells and enterocytes, and there are tight junctions between the cells. The immune barrier is located below the lamina propria, including various immune cells scattered in the LP and MLNs. The gut vascular barrier is the deepest protective layer of the intestinal tract. The figure was created with Biorender.com.
In recent years, studies have found that many host diseases are related to intestinal health (13–15); and dietary polyphenols play important roles in regulating intestinal health (16–18). Resveratrol is a natural plant polyphenol, which was first isolated and purified from veratrum grandiflorum in 1940, and is widely found in grape, polygonum cuspidatum, berry, and peanut (19). Resveratrol has both cis and trans structures, and mainly exists in trans structure in the nature (20). Of note, resveratrol has many biological functions, such as antioxidant, anti-inflammation, anti-tumor, and anti-cardiovascular diseases, and with the particular interest to this article—protecting intestinal health (21–25). Notably, unless otherwise stated, all mentioned in the review are trans-resveratrol, and the trans-resveratrol is mainly grape extract. Herein, in this review, we summarize the effects of resveratrol on intestinal barrier, and highlight the applications of resveratrol in ameliorating various intestinal diseases.
The molecular weight of resveratrol is 228.25 g/mol, and resveratrol has benzene ring, hydroxyl groups, and C-C double bond, that could affect water solubility and absorption in intestinal tract (26, 27). In the intestinal tract, there are two ways to absorb and utilize resveratrol, including the absorption by enterocytes and the utilization by bacteria (Figure 2).
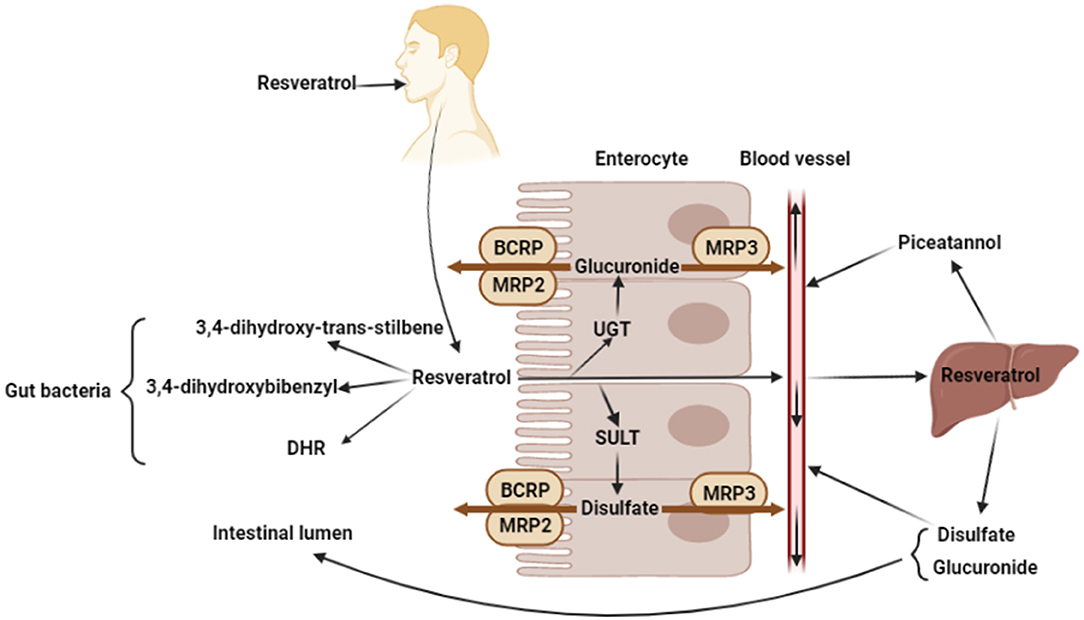
Figure 2. Absorption and metabolism of resveratrol. Oral resveratrol reaches the intestine and enters the enterocytes through passive diffusion. A small part of resveratrol can directly enter the blood circulation, while most resveratrol produces glucuronide and disulfate by the UDP-glucuronosyltransferases (UGT) and sulfotransferases (SULT) in enterocytes, and glucuronide and disulfate are transported back to intestinal lumen by the ABC transporter of enterocyte apical membrane (BCRP, MRP2), or transported into bloodstream by the ABC transporter of enterocyte basolateral membrane (MRP3). In addition, resveratrol produces 3,4-dihydroxybibenzyl, 3,4-dihydroxy-trans-stilbene, and dihydroresveratrol (DHR) by microorganisms. And in liver, resveratrol is metabolized to produce piceatannol, disulfate and glucuronide. The figure was created with Biorender.com.
Resveratrol is perceived as a xenobiotics by gastrointestinal tract and crosses the intestinal epithelium to the blood through transcellular pathway (28). In the intestine, resveratrol binds to the proteins and lipids which will influence its absorption or elimination in feces (27). Enterocytes play an important role in the transcellular pathway absorption of resveratrol, which enters enterocytes through passive diffusion and forms complexes with intestinal membrane transporters (29). The ATP-binding cassette (ABC) family is one of the largest transporter families of lipid membranes. So far, 49 ABC transporter subtypes have been identified, which can be divided into 7 subfamilies (30, 31). Oral resveratrol reaches the intestine and enters the enterocytes through passive diffusion. Then, resveratrol is metabolized or directly into the bloodstream. However, only a small part of resveratrol can directly enter the blood circulation and be transported to peripheral organs, for example, the liver. Most resveratrol produces glucuronide and disulfate by the UDP-glucuronosyltransferases (UGT) and sulfotransferases (SULT) in enterocytes (32), and glucuronide and disulfate are transported back to intestinal lumen by the ABC transporter of enterocyte apical membrane (BCRP, MRP2), or transported into bloodstream by the ABC transporter of enterocyte basolateral membrane (MRP3) (33, 34). Glucuronide and disulfate are the major circulating forms of resveratrol, which could be detected in liver, adipose tissue, or the heart, after oral administration (35, 36). Resveratrol or its metabolites enter the bloodstream and bind proteins and fats to circulate throughout the body. In addition, resveratrol entering the bloodstream can be metabolized in the liver, for instance, resveratrol is hydroxylated to form piceatannol by the liver cytochrome P450 (34, 37, 38). Besides, resveratrol, which is not passively absorbed by intestinal epithelial cells, would be degraded by some intestinal bacteria. Slackia equolifaciens and Adlercreutzia equolifaciens have been proved to convert resveratrol to dihydroresveratrol (DHR) through hydrogenation (39). Another study shown that resveratrol can be metabolized by gut bacteria into other metabolites, 3,4-dihydroxy-trans-stilbene and 3,4-dihydroxybibenzyl (lunularin). However, the exact bacteria that produce these two metabolites are still unknown (40). Besides, the piceid, which is resveratrol precursor, is metabolized to produce dihydropiceid and DHR by the gut bacteria (41).
The intestinal barrier, which is useful for nutrient absorption and protection from external invasion, is composed of five layers: microbial barrier, chemical barrier, physical barrier, immune barrier, and gut vascular barrier (42). In this section, we discuss the associated investigations about how resveratrol affects the aformentioned intestinal barriers.
The rapid metabolism in the intestinal tract and the characteristic of poor water solubility may lead to low bioavailability of resveratrol. After oral administration of resveratrol, 18% of resveratrol enters into the bloodstream and 25% of resveratrol is excreted, while the rest could be metabolized by liver, intestine, and gut microbiota (29). In addition, some gut microbiota could use resveratrol precursors to produce resveratrol and its derivatives (41, 43). In recent years, many studies have shown that resveratrol and its derivatives could modulate gut microbiota composition and affect gut barrier function.
Resveratrol performs its antibacterial and antifungal activities through inhibiting the growth of bacteria and fungi (44). Resveratrol inhibits the growth of Vibrio cholerae through inhibiting the formation of its biofilm, and the minimum inhibitory concentration (MIC) is 60 μg/ml, which is dose-dependent (45). Resveratrol also inhibits the growth of Campylobacter jejuni and Campylobacter coli (which are the major cause of bacterial gastroenteritis) through the formation of its biofilm, and the MIC are 100 and 50 μg/ml, respectively (46). Moreover, resveratrol inhibits the growth of Escherichia coli in three ways. First, resveratrol binds reversibly to ATP synthase of Escherichia coli that affects the ATP hydrolysis and synthesis. Second, resveratrol induces DNA fragmentation and concomitant upregulation of the stress-response regulon in Escherichia coli. Finally, resveratrol treatment has been correlated with membrane damage (47, 48). Resveratrol also has inhibitory effect on other Gram-positive pathogens, such as S. aureus, Enterococcus faecalis, and Streptococcus pyogenes, and the MIC is 100–200 μg/ml (44).
Additionally, resveratrol also has inhibitory effect on some fungi, such as Candida albicans, Saccharomyces cerevisiae, and Trichosporon beigelii, and the inhibitory activity is 10–20 μg/ml (49). Interestingly, in weaned piglets, resveratrol exhibits significant inhibiting ability against Enterococcus and Clostridium (50). However, the inhibitory effect of resveratrol on Candida albicans remains controversial. Collado-González and Weber et al. failed to detect the antifungal effect of resveratrol against Candida albicans (51, 52). Intriguingly, Houillé et al. (53) found that resveratrol derivates has inhibiting effect on Candida albicans and non-albicans Candida (NAC) species in structure- and/or dose-dependent manner. Afterward, studies have revealed that resveratrol could induce Candida albicans apoptosis and have synergistic effect on azoles (an antifungal medicine) against Candida albicans (54, 55). These findings suggest that it might be structure- and dose-dependent on the antimicrobial effect of resveratrol and its derivates. Nevertheless, the above findings still need experimental confirmation.
Resveratrol not only inhibits the growth of intestinal pathogens, but also affects the abundance of intestinal dominant flora. Studies have found that resveratrol increases the abundance of Bacteroides, Lactobacillus, and Bifidobacterium in the intestines of mice and rats (56, 57). In DSS-induced colitic mice model, dietary resveratrol significantly enriches the gut microbiota, and restores bacterial community diversity and rebalances the probiotics and pathobionts (58). In db/db mice, resveratrol treatment decreases the relative abundance of Firmicutes and increases abundance of Bacteroidetes. Importantly, Bacteroides, Alistipes, and Parabacteroides, which exhibit anti-inflammatory properties, are markedly increased in the resveratrol-treated db/db mice group (59). In high-fat diet (HFD)-fed mice, resveratrol treatment significantly increases Lactobacillus and Bifidobacterium, whereas Enterococcus faecalis is significantly decreased, and resveratrol supplemented diets cause a higher abundance of Bacteroidetes while a lower abundance of Firmicutes (60). Additionally, the number of Lactobacillus and Bifidobacterium is significantly increased in resveratrol-fed animals (61).
Together, resveratrol improves intestinal microbial barrier by inhibiting the growth of pathogenic bacteria and fungi and modulating the composition of intestinal dominant flora. Although the mechanism of resveratrol in inhibiting the growth of Escherichia coli has been identified, the inhibitory effects of resveratrol on other pathogens still need to be further explored.
Intestinal chemical barrier plays an important role in resisting and killing pathogenic bacteria. Various mucins of intestinal chemical barrier can protect intestinal epithelia cells from pathogen invasion (62). Resveratrol has been found that it well orchestrates the intestinal chemical barrier. For instance, oxyresveratrol significantly increases the expression of mucin 2 (MUC2) through the increasing level of NAD+ in human LS174 goblet cells (63, 64). Moreover, the mRNA level of trefoil factor 3 (TFF3), which could increase mucosal integrity and mucus viscosity, is also increased (63). Subsequently, a recently study demonstrated that oxyresveratrol triggers the endoplasmic reticulum (ER) stress and promotes the expression levels of autophagy-related genes and ultimately induces MUC2 formation in human LS174 goblet cells (65). The increasing expression of MUC2 and TFF3 also occurs in the HFD-induced mice with resveratrol treatment (66).
Collectively, resveratrol is helpful for the maintenance and repairment of intestinal chemical barrier via increasing the expression and secretion of mucin. The possible mechanism is largely associated with the triggering ER stress in goblet cells by resveratrol treatment. Additionally, it is worthy of exploring whether there are other links such as bile salts and mucoitins between resveratrol and intestinal chemical barrier.
The physical barrier is the largest and most important barrier in the intestine, and the tight junction (TJ) (e.g., ZO-1, ZO-2, and occludin) is the most important factor that contributes to the integrity of intestinal physical barrier (67). In the model of cyclophosphamide-induced immunosuppressed mice, the expression of ZO-1, ZO-2, and occludin proteins are significantly decreased, whereas resveratrol treatment can increase the expression of those TJ-associated proteins (68). Another study found that the mRNA levels of TJ-associated proteins were down-regulated in HFD-induced mice, which can be reversed after resveratrol treatment (66). In addition, resveratrol can prevent porcine intestinal epithelial cells from deoxynivalenol-induced damage through the Nrf2 signaling pathway (69), and decrease radiation-induced damage in intestinal epithelial cells via facilitating autophagy and preventing apoptosis by the activation of SIRT1 (70). Meanwhile, resveratrol can alleviate H2O2-induced damage through upregulating the expression of tight-junction proteins (occludin, claudin-1, and ZO-1), which depends on the PI3K/Akt-mediated Nrf2 signaling pathway (71).
In conclusion, resveratrol could protect the intestinal physical barrier from damage, which might be related to the regulation of tight junction protein expression and mitigation of oxidative stress.
The intestinal immune barrier plays a vital role in protecting body health. Indeed, there are a large number of immune cells and lymphocytes in the MLNs and LP, which play important roles in the intestinal immunity (72). Resveratrol is an antitoxin produced by plant, and its anti-inflammatory function has been well documented. Several studies have shown that resveratrol could improve intestinal immune barrier. For example, resveratrol affects intestinal immune cells and lymphoid tissue. In colitis mice, resveratrol treatment increases the number of anti-inflammatory regulatory T cells (CD4+FOXP3+ and CD4+IL-10+) and down-regulates the number of inflammatory T cells, such as Th1 (CD4+IFN-γ+) and Th17 (CD4+IL-17+) cells in MLNs (73). In IL-10(–/–) chronic colitis mice, resveratrol treatment decreases the quantity of CXCR3+ T cells in MLNs and LP, and increases the percentage and absolute numbers of CD11b+ and Gr-1+ myeloid derived suppressor cells (MDSCs) in LP (74). Low dose resveratrol regulates Treg/Th17 balance through reducing the number of Th17 cells, while high dose resveratrol shapes Treg/Th17 balance through down-regulating the number of Th17 cells and up-regulating the number of Treg cells in ulcerative colitis mice (75). Moreover, resveratrol facilitates Th1/Th2 balance towards Th2 polarization and enhances Treg/Th17 balance towards Treg in the small intestine LP in mice (76). Intriguingly, in colitis mice, after resveratrol treatment, the percentage of CD4+ T cells in MLNs is restored to normal level, but decreases these cells in the colon LP. Likewise, the percentages of macrophages in MLN and the LP of colitis mice are decreased after resveratrol treatment. And resveratrol reverses the increased levels of tumor necrosis factor-α (TNF-α), interleukin (IL-6), and interleukin 1β (IL-1β) (77). Furthermore, resveratrol could raise the IgG concentration in serum of weaning piglets (78). Moreover, resveratrol inhibits degranulation and expression of CXCL8, CCL2, CCL3, and CCL4 in a dose-dependent manner in human intestinal mast cells. Resveratrol blocks the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and signal transducer and activator of transcription (STAT) 3. Mitochondrial STAT3 is phosphorylated by ERK1/2 and contributes to mast cell degranulation (79). In OVA-induced mouse model, the percentage of mast cells is significantly increased in the MLNs, which could be reversed by resveratrol treatment (80).
Taken together, resveratrol increases anti-inflammatory T cells while reduces pro-inflammatory T cells. In addition, resveratrol also affects the number of macrophages and mast cells. Most importantly, resveratrol inhibits mast cell degranulation by blocking the phosphorylation of ERK1/2. Therefore, resveratrol can improve the intestinal immune barrier.
The gut vascular barrier (GVB) is composed of intestinal vessel endothelium, which is an important valve to control the entry of pathogenic microorganisms and molecular substances into bloodstream, liver, brain, and other organs (12, 81). GVB has many characteristics in common with the blood-brain barrier (BBB), but GVB has more tolerant permeability, which allows for the diffusion of larger molecules (up to 4 kDa) (82). Therefore, if molecules (>4 kDa) and microorganisms cross mucous membrane and epithelial barrier, it will retain the LP, unless GVB is destroyed resulting in a change in permeability (83). Studies have shown that resveratrol reduces vascular oxidative stress, relieves vascular inflammation, and improves vascular function (84, 85). Besides, resveratrol decreases small intestinal pro-inflammatory cytokines and gut vascular permeability, which could attenuate the blood levels of pro-inflammatory cytokines from small intestine and alleviate cytokines-mediated BBB disruption and neuroinflammation (76). Thus, resveratrol may affect intestinal vascular permeability and improve GVB, but more researches are needed to prove the aforementioned function by resveratrol.
Given the above, the intestine is not only important for nutrition absorption, but also a key line of defense against the invasion of exogenous pathogenic microorganisms, and the damage of intestinal barrier is closely related to many diseases (86). Resveratrol has a positive effect on all intestinal barriers, this means that resveratrol can regulate many diseases related to intestinal barrier damage. Subsequently, in this part, we continue to explore the effects of resveratrol on several intestinal diseases (Table 1).
Inflammatory bowel diseases (IBD), mainly includes Crohn's disease (CD) and ulcerative colitis (UC), are a group of chronic and relapsing inflammatory diseases in the digestive tract, which could be affected by the genetic, environment, and immune factors. However, it remains to know the precise aetiology of IBD (101). It is worth noting that mucosal injury caused by high level oxidative stress plays a key role in the pathogenesis of IBD, and the activation of nuclear factor κ light-chain-enhancer of activated B cells (NF-κB) also regulates the process of IBD (102). In heat-stressed rats, resveratrol reverses the cortisol level and diamine oxidase (DAO) activity increasing in serum, decreases malondialdehyde (MDA), and increases the mRNA expression of cytokines and antioxidant enzymes (89). Resveratrol (20 mg/kg/day) decreases MDA content, increases glutathione (GSH) level and catalase (CAT) activity in intestine of irradiated rats. Furthermore, resveratrol reduces the contents of inflammatory cytokines, including TNF-α, NF-κB, and IL-1β in intestine (87). In colitis mice, 100 mg/kg resveratrol significantly decreases inflammatory cytokines level of TNF-α, IFN-γ, and IL-17 (90); 2 and 10 mg/kg resveratrol decreases the ulcerative area and colon mass index (88). Likewise, resveratrol suppresses the activation of NF-κB in Caco-2 cells and SW480 human colon cancer cells (103). Sabzevary-Ghahfarokhi et al. (104) used TNF-α-stimulated Caco-2 cells to simulate UC in vitro, and it was found that the protein expressions of IL-1β and p-Nrf2 are increased, however, this was reversed by resveratrol treatment. In addition, the destruction of intestinal epithelial cells tight junctions increases the interactions between intestinal microbiota and the host immune system, which also affects the course of IBD (105). In IBD patients, the biodiversity of commensal bacteria is decreased (especially Bacteroidetes and Firmicutes, including the clinically relevant Faecalibacterium prausnitzii); nevertheless, the Escherichia coli abundance is increased (106). As mentioned earlier, resveratrol regulates the expression of TJ proteins to improve the physical barrier. In addition, resveratrol regulates the abundance of commensal bacteria and inhibits the growth of Escherichia coli. Therefore, it is likely that resveratrol might alleviate IBD.
Colorectal cancer (CRC) is one of the three most common cancers in the world, which is a serious threat to human health (107). Chronic inflammation of the intestine is one of the main determinants of CRC. Therefore, anti-inflammatory compounds may play a beneficial role in the treatment of CRC (108). As previously mentioned, resveratrol has anti-inflammation and anti-tumor functions, and it can regulate the proportion of anti-inflammatory and inflammatory immune cells. Moreover, resveratrol decreases pro-inflammatory factors, such as TNF-α and IL-1β, pro-inflammatory enzymes such as iNOS and COX-2 and inflammatory signaling pathways, such as NF-kB. Resveratrol can reduce the number of aberrant crypt foci (ACF) on azoxymethane (AOM)-induced colon carcinogenesis in F344 rats by regulating the expression of Bax and p21 (109). In addition, oxidative stress is also considered to be one of the factors that aggravate cancer, and resveratrol also inhibits lipid peroxidation and scavenge ROS (110, 111). Furthermore, resveratrol has anticancer function mainly through inhibiting proliferation and inducing apoptosis of tumor cell (112, 113). Some studies have found that resveratrol inhibits the tumor cell cycle at the transition S to G2/M which is associated with cyclin and cyclin-dependent kinase activities (114–117). Hence, resveratrol may be a potential compound for the treatment and prevention of CRC. In fact, several studies have shown that resveratrol can ameliorate CRC. Murugan et al. found that resveratrol significantly reduces tumor incidence, histological lesions, and tumor size in 1,2-dimethylhydrazine (DMH)-induced colon cancer of mice, and resveratrol inhibits the proliferation of tumor cells. Resveratrol regulates the oxidative imbalance caused by DMH treatment, which has been shown that the activities of some antioxidant enzymes are increased (92). Yuan et al. (94) shown that resveratrol may suppress the invasion and metastasis of colon cancer through reversal of epithelial mesenchymal transition (EMT) markers via the AKT/GSK3β/Snail signaling pathway. Constanze et al. simulated of tumor microenvironment (TME) by multicellular culture in vitro, they found that multicellular-TME, similar to TNF-β-TME, promotes proliferation, colony formation, invasion of CRC cells and enables activation of cancer stem cells (CSC). However, resveratrol reduces the secretion of T-lymphocyte/fibroblast (TNF-β, TGF-β3) proteins, antagonizes the T-lymphocyte/fibroblast-promoting NF-κB activation, and NF-κB nuclear translocation. Thus, fibroblasts and T-lymphocytes are promising targets for resveratrol in the prevention of CRC metastasis (93). In addition, in colorectal cancer patients, resveratrol reduces tumor cell proliferation by 5% (91), low dosages of resveratrol combination with other bioactive compounds in freeze-dried grape powder (GP) inhibit Wnt signaling pathway in normal colonic mucosa, that indicated a reduction in the expression of a panel of Wnt target genes, suggesting that resveratrol or GP may play a beneficial role in colon cancer prevention (118).
Altogether, resveratrol can be used as a compound for the treatment or prevention of CRC, perhaps better in combination with chemotherapy. Moreover, resveratrol improves CRC by antagonizing chronic inflammation, oxidative stress, and inhibiting the growth of tumor cells.
Irritable bowel syndrome (IBS) is a functional bowel disorder that causes chronic and recurrent pain (119), and the pathogenesis is still unclear. In recent year, altered gut immune activation, intestinal permeability, and gut microbiome have been identified in some IBS patients (120). Moreover, most IBS patients are accompanied by depression (121), and the Gut-Brain Axis plays an important role in IBS (122). As mentioned earlier, resveratrol could affect microbial and immune barriers. Therefore, we speculate that resveratrol plays an important role in the treatment of IBS. In fact, several studies have shown that resveratrol could ameliorate IBS. Xu et al. utilized chronic-acute combined stress (CACS)-induced IBS-like symptoms (depression, anxiety, and intestinal dysfunction) in ICR male mice, trans-resveratrol treatment significantly reverses CACS-induced depression- and anxiety-like behaviors and intestinal dysfunction in mice, including improves hippocampal neuronal remodeling, protects ileal and colonic epithelial barrier structure against CACS insults. The underlying mechanism may be related to normalization of PDE4A expression and CREB-BDNF signaling both in the central nervous and peripheral systems (95). Yu et al. utilized the CACS-induced IBS-like symptoms in male Sprague-Dawley (SD) rats, resveratrol treatment improves anti-IBS-like effects on depression, anxiety, visceral hypersensitivity, and intestinal motility abnormality through regulating 5-HT1A-dependent PKA-CREB-BDNF signaling in the gut-brain axis (96). Thus, resveratrol has an alleviating effect on IBS, and gut-brain axis plays a vital role. In addition, microbiota is the key factor affecting IBS, resveratrol has been shown to affect the intestinal microbial barrier. Thus, resveratrol may also alleviate IBS by affecting the composition of microbiota, and the specific mechanism needs to be further studied.
Some pathogenic microorganisms could cause intestinal infectious diseases and seriously affect intestinal health. As mentioned before, resveratrol inhibits the growth of many pathogens, we speculate that resveratrol can resist intestinal infectious diseases caused by some pathogens. Escherichia coli is Gram-negative bacterium as a family member of Enterobacteriaceae, which predominantly colonize in intestine of warm-blooded animals such as humans (123). Escherichia coli could be divided into commensal and pathogenic strains. The latter [e.g., enterotoxigenic E. coli (ETEC), enterohemorrhagic E. coli (EHEC), and enteropathogenic E. coli (EPEC)] would induce a wide variety of intestinal infections (124). For example, ETEC, the most common cause of bacterial diarrhea, enters the gut and adheres to the small intestinal epithelium through the colonization factors to cause diseases (125). It has been reviewed that the adhesion of pathogenic bacteria on intestinal epithelium is related to the formation of bacteria biofilm (126). Interestingly, resveratrol restrains the growth of avian pathogenic E. coli through inhibiting the formation of bacterial biofilm, and the MIC is 128 μg/ml (97). It has been found that the adhesion inhibition of E. coli O157:H7 to HT-29 colonic cells is more than 60% with resveratrol and its derivatives treatments (127). Overall, the inhibiting adhesion and biofilm formation of E. coli on intestinal epithelium might be a target of resveratrol to offer treatment of E. coli infection. However, the function of resveratrol on various E. coli is still unclear.
Campylobacter jejuni (C. jujuni) is a pathogen of human, which could cause bacterial diarrheal disease by destroying epithelial barrier, for example, tight-junction disruption and epithelial apoptosis. In addition, occludin and claudin-5 in colonic epithelial cells were redistributed after infection of C. jujuni (128). As previous studies shown, resveratrol can attenuate intestinal epithelial damage by C. jujuni infection (98) and also alleviate inflammation via decreasing inflammatory factors such as TNF-α or C-reactive protein (CRP) levels (129). However, Lobo et al. shown that the anti-C. jujuni effect of resveratrol might base on improving barrier function at the epithelial level instead of decreasing cytokine release. Thus, resveratrol might be a promising compound for the treatment and prevention of C. jujuni infection. They also demonstrated that resveratrol can rescues colonic epithelial barrier function through evaluating the intestinal epithelial leakiness in the C. jujuni-infected mice (130, 131).
Many studies have stressed the antiviral functions of resveratrol both in vivo and in vitro (132). Xu et al. (100) has revealed that resveratrol alleviates duck viral enteritis (an acute, contagious and herpesvirus infection of poultry intestine) through suppressing the multiplication of duck enteritis virus in host cells. Meantime, some studies have revealed that resveratrol could inhibit the replication of rotavirus (the main cause of acute severe viral diarrhea of infant animals) in Caco-2 cell lines and ameliorate the severity of diarrhea (133, 134). In addition, resveratrol also plays an important role in the treatment of parasitic infections. Resveratrol could alleviate the oxidative stress caused by Trichinella spiralis infection in small intestine (99). Altogether, resveratrol plays an important role in resisting intestinal infection, and the realization of this function depends on the strong antibacterial activity of resveratrol.
In this review, we pay more attention to the therapeutic and preventive effects of resveratrol on intestinal diseases, and we find that this can be achieved by regulating the intestinal barrier integrality. As mentioned earlier, resveratrol does affect the composition of intestinal microorganisms, and resveratrol is considered as a potential prebiotic candidate to promote changes in bacterial composition associated with a healthy phenotype (135). Song et al. (68) found that resveratrol regulates the function of gut microbiota to resist immunosuppression. However, few mechanistic clues as to how the resveratrol-gut microbiota-metabolism axis could be functioning, including resveratrol-gut microbiota-brain axis, resveratrol-gut microbiota-liver axis, and resveratrol-gut microbiota-kidney axis. Thus, resveratrol plays a health-promoting role through the connection between intestinal microorganisms and extraintestinal target organs, which has become a focus of future research. In addition, resveratrol may have another potential mechanism for intestinal barrier modulation. It has been proposed that long non-coding RNAs (lncRNAs) might be a potential regulatory factor for intestinal barrier. Although the influences of lncRNAs are still require a broaden experimental confirmation in intestinal barrier, resveratrol has been reported that it can modulate lncRNAs to combat different diseases, such as insulin resistance (136), lung cancer (137), and prostate cancer (138). Thus, it would be very interesting to explore whether and how resveratrol shapes intestinal barrier via lncRNAs. In addition, a novel experiment has been well demonstrated that the ovarian tumor deubiquitinase 1 (OTUD1) alleviates IBD through inhibiting receptor-interacting serine/threonine-protein kinase 1 (RIPK1)-mediated NF-κB activation (139). Therefore, whether and how resveratrol interacts with OTUD1 to mediate intestinal barrier and ultimately regulate IBD should also be taken into consideration. However, there are many difficulties to overcome with lncRNAs. Firstly, it is still lack studies on the effects of lncRNAs in intestinal barrier. Secondly, it is difficult to select sensitive and specific lncRNAs as clinical biomarkers. Finally, it is still unclear which targeting methods and drug vectors are suitable for lncRNA-targeted therapy (140). Generally, there is still a long way to find more underlying relationships between resveratrol and intestinal barrier.
The intestinal barrier is a complex network, which is composed of microbial, chemical, physical, immune, and vascular barrier. Intestinal barrier not only affects the absorption of nutrients, but also is closely related to body health. Resveratrol is a natural plant polyphenol, which has many biological functions, such as antioxidant, anti-inflammatory, anti-cancer, and cardiovascular protection. In this review, we have introduced the absorption and metabolism of resveratrol by gastrointestinal tract, we also summarize the effects of resveratrol on five intestinal barriers (Figure 3), and it can resist intestinal barrier damage and maintain intestinal barrier function. In addition, resveratrol could treat and/or prevent a variety of intestinal-related diseases, such as IBD, CRC, IBS, and intestinal infectious diseases. In general, our review illustrates that resveratrol can affect intestinal health and disease by regulating the intestinal barrier.
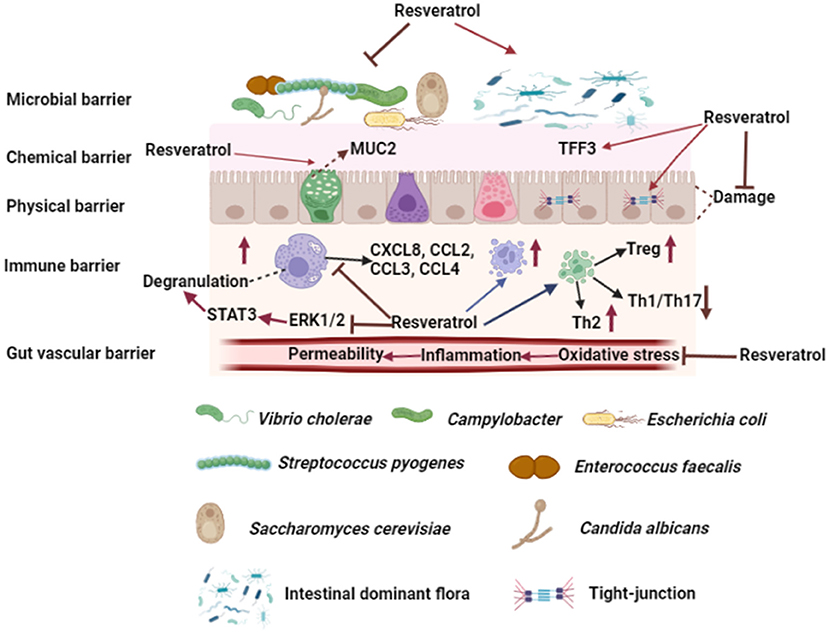
Figure 3. Effects of resveratrol on intestinal barrier. Resveratrol could improve intestinal microbial barrier by inhibiting the growth of pathogens and modulating the composition of intestinal dominant flora. Resveratrol promotes goblet cells to secrete MUC2 and increases TFF3 in mucous layer. Moreover, resveratrol increases the expression of tight junction protein between enterocytes and protects enterocytes from injury. Resveratrol affects T cell differentiation and increases the number of Treg and Th2 cells in intestinal lymph nodes and lamina propria, while decreases the number of Th1 and Th17 cells. Furthermore, resveratrol increases the number of mast cells and macrophages in intestinal lymph nodes and lamina propria, and inhibits mast cell degranulation by inhibiting the phosphorylation of ERK1/2 and STAT3 and affecting mast cell chemokine secretion. Resveratrol improves gut vascular barrier by reducing vascular permeability. The figure was created with Biorender.com.
YW, CH, ZW, SL, YX, YL, and XH: writing-review and editing. WT: supervision. All authors have read and agreed to the published version of the manuscript.
This work was financially supported by the Sichuan Science and Technology Programmes (2021JDYZ0001 and 2021ZDZX0009).
WT and SL were employed by Sichuan Animtech Feed Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Goodman BE. Insights into digestion and absorption of major nutrients in humans. Adv Physiol Educ. (2010) 34:44–53. doi: 10.1152/advan.00094.2009
2. Greenwood-Van Meerveld B, Johnson AC, Grundy D. Gastrointestinal physiology and function. Handb Exp Pharmacol. (2017) 239:1–16. doi: 10.1007/164_2016_118
3. Candela M, Biagi E, Maccaferri S, Turroni S, Brigidi P. Intestinal microbiota is a plastic factor responding to environmental changes. Trends Microbiol. (2012) 20:385–91. doi: 10.1016/j.tim.2012.05.003
4. Olivares-Villagómez D, van Kaer L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. (2018) 39:264–75. doi: 10.1016/j.it.2017.11.003
5. Julio-Pieper M, Bravo JA. Intestinal barrier and behavior. Int Rev Neurobiol. (2016) 131:127–41. doi: 10.1016/bs.irn.2016.08.006
6. Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. (2011) 332:970–4. doi: 10.1126/science.1198719
7. Possemiers S, Grootaert C, Vermeiren J, Gross G, Marzorati M, Verstraete W, et al. The intestinal environment in health and disease—recent insights on the potential of intestinal bacteria to influence human health. Curr Pharm Des. (2009) 15:2051–65. doi: 10.2174/138161209788489159
8. Gao Y, Meng L, Liu H, Wang J, Zheng N. The compromised intestinal barrier induced by mycotoxins. Toxins. (2020) 12:toxins12100619. doi: 10.3390/toxins12100619
9. Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. (2018) 10:a006338. doi: 10.1101/cshperspect.a029314
10. Soderholm AT, Pedicord VA. Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunology. (2019) 158:267–80. doi: 10.1111/imm.13117
11. Avetisyan M, Schill EM, Heuckeroth RO. Building a second brain in the bowel. J Clin Invest. (2015) 125:899–907. doi: 10.1172/JCI76307
12. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. (2015) 350:830–4. doi: 10.1126/science.aad0135
13. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. (2021) 19:55–71. doi: 10.1038/s41579-020-0433-9
14. Liang J, Zhang M, Wang X, Ren Y, Yue T, Wang Z, et al. Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr Polym. (2021) 273:118558. doi: 10.1016/j.carbpol.2021.118558
15. Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut. (2016) 65:330–9. doi: 10.1136/gutjnl-2015-309990
16. Bianchi MG, Chiu M, Taurino G, Brighenti F, Del Rio D, Mena P, et al. Catechin and procyanidin B2 modulate the expression of tight junction proteins but do not protect from inflammation-induced changes in permeability in human intestinal cell monolayers. Nutrients. (2019) 11:2271. doi: 10.3390/nu11102271
17. Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, et al. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients. (2019) 11:2216. doi: 10.3390/nu11092216
18. Shimizu M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J Food Drug Anal. (2017) 25:93–9. doi: 10.1016/j.jfda.2016.12.003
19. Huang X-T, Li X, Xie M-L, Huang Z, Huang Y-X, Wu G-X, et al. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem Biol Interact. (2019) 306:29–38. doi: 10.1016/j.cbi.2019.04.001
20. Tian B, Liu J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J Sci Food Agric. (2020) 100:1392–404. doi: 10.1002/jsfa.10152
21. Carter LG, D'Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. (2014) 21:R209–25. doi: 10.1530/ERC-13-0171
22. Dull A-M, Moga MA, Dimienescu OG, Sechel G, Burtea V, Anastasiu CV. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules. (2019) 24:667. doi: 10.3390/molecules24040667
23. Huang D-D, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. (2020) 125:109767. doi: 10.1016/j.biopha.2019.109767
24. Li H, Xia N, Hasselwander S, Daiber A. Resveratrol and vascular function. Int J Mol Sci. (2019) 20:2155. doi: 10.3390/ijms20092155
25. Moore A, Beidler J, Hong MY. Resveratrol and depression in animal models: a systematic review of the biological mechanisms. Molecules. (2018) 23:2197. doi: 10.3390/molecules23092197
26. Biasutto L, Marotta E, Mattarei A, Beltramello S, Caliceti P, Salmaso S, et al. Absorption and metabolism of resveratrol carboxyesters and methanesulfonate by explanted rat intestinal segments. Cell Physiol Biochem. (2009) 24:557–66. doi: 10.1159/000257512
27. Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. (2015) 2015:837042. doi: 10.1155/2015/837042
28. Delmas D, Aires V, Limagne E, Dutartre P, Mazué F, Ghiringhelli F, et al. Transport, stability, and biological activity of resveratrol. Ann N Y Acad Sci. (2011) 1215:48–59. doi: 10.1111/j.1749-6632.2010.05871.x
29. Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. (2011) 1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x
30. Liu X. ABC Family transporters. Adv Exp Med Biol. (2019) 1141:13–100. doi: 10.1007/978-981-13-7647-4_2
31. Theodoulou FL, Kerr ID. ABC Transporter research: going strong 40 years on. Biochem Soc Trans. (2015) 43:1033–40. doi: 10.1042/BST20150139
32. Wang P, Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors. (2018) 44:16–25. doi: 10.1002/biof.1410
33. Planas JM, Alfaras I, Colom H, Juan ME. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch Biochem Biophys. (2012) 527:67–73. doi: 10.1016/j.abb.2012.06.004
34. Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release. (2012) 158:182–93. doi: 10.1016/j.jconrel.2011.09.083
35. Andres-Lacueva C, Macarulla MT, Rotches-Ribalta M, Boto-Ordóñez M, Urpi-Sarda M, Rodríguez VM, et al. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J Agric Food Chem. (2012) 60:4833–40. doi: 10.1021/jf3001108
36. Bresciani L, Calani L, Bocchi L, Delucchi F, Savi M, Ray S, et al. Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats. Nutr Metab Cardiovasc Dis. (2014) 24:408–15. doi: 10.1016/j.numecd.2013.09.008
37. Jarosova V, Vesely O, Doskocil I, Tomisova K, Marsik P, Jaimes JD, et al. Metabolism of cis- and trans-resveratrol and dihydroresveratrol in an intestinal epithelial model. Nutrients. (2020) 12:595. doi: 10.3390/nu12030595
38. Potter GA, Patterson LH, Wanogho E, Perry PJ, Butler PC, Ijaz T, et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br J Cancer. (2002) 86:774–8. doi: 10.1038/sj.bjc.6600197
39. Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Wozniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. (2020) 60:626–59. doi: 10.1080/10408398.2018.1546669
40. Bode LM, Bunzel D, Huch M, Cho G-S, Ruhland D, Bunzel M, et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. (2013) 97:295–309. doi: 10.3945/ajcn.112.049379
41. Wang D, Zhang Z, Ju J, Wang X, Qiu W. Investigation of piceid metabolites in rat by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. (2011) 879:69–74. doi: 10.1016/j.jchromb.2010.11.012
42. Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal barrier in human health and disease. Int J Environ Res Public Health. (2021) 18:12836. doi: 10.3390/ijerph182312836
43. Basholli-Salihu M, Schuster R, Mulla D, Praznik W, Viernstein H, Mueller M. Bioconversion of piceid to resveratrol by selected probiotic cell extracts. Bioprocess Biosyst Eng. (2016) 39:1879–85. doi: 10.1007/s00449-016-1662-1
44. Vestergaard M, Ingmer H. Antibacterial and antifungal properties of resveratrol. Int J Antimicrob Agents. (2019) 53:716–23. doi: 10.1016/j.ijantimicag.2019.02.015
45. Augustine N, Goel AK, Sivakumar KC, Kumar RA, Thomas S. Resveratrol—a potential inhibitor of biofilm formation in Vibrio cholerae. Phytomedicine. (2014) 21:286–9. doi: 10.1016/j.phymed.2013.09.010
46. Duarte A, Alves AC, Ferreira S, Silva F, Domingues FC. Resveratrol inclusion complexes: antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res Int. (2015) 77:244–50. doi: 10.1016/j.foodres.2015.05.047
47. Dadi PK, Ahmad M, Ahmad Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int J Biol Macromol. (2009) 45:72–9. doi: 10.1016/j.ijbiomac.2009.04.004
48. Hwang D, Lim Y-H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci Rep. (2015) 5:10029. doi: 10.1038/srep10029
49. Jung HJ, Hwang IA, Sung WS, Kang H, Kang BS, Seu YB, et al. Fungicidal effect of resveratrol on human infectious fungi. Arch Pharm Res. (2005) 28:557–60. doi: 10.1007/BF02977758
50. Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. (2019) 24:1220. doi: 10.3390/molecules24071220
51. Collado-González M, Guirao-Abad JP, Sánchez-Fresneda R, Belchí-Navarro S, Argüelles J-C. Resveratrol lacks antifungal activity against Candida albicans. World J Microbiol Biotechnol. (2012) 28:2441–6. doi: 10.1007/s11274-012-1042-1
52. Weber K, Schulz B, Ruhnke M. Resveratrol and its antifungal activity against Candida species. Mycoses. (2011) 54:30–3. doi: 10.1111/j.1439-0507.2009.01763.x
53. Houillé B, Papon N, Boudesocque L, Bourdeaud E, Besseau S, Courdavault V, et al. Antifungal activity of resveratrol derivatives against Candida species. J Nat Prod. (2014) 77:1658–62. doi: 10.1021/np5002576
54. Lee J, Lee DG. Novel antifungal mechanism of resveratrol: apoptosis inducer in Candida albicans. Curr Microbiol. (2015) 70:383–9. doi: 10.1007/s00284-014-0734-1
55. Wang J, Zhang X, Gao L, Wang L, Song F, Zhang L, et al. The synergistic antifungal activity of resveratrol with azoles against Candida albicans. Lett Appl Microbiol. (2021) 72:688–97. doi: 10.1111/lam.13458
56. Chen M-l, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. (2016) 7:e02210–15. doi: 10.1128/mBio.02210-15
57. Larrosa M, Yañéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, et al. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem. (2009) 57:2211–20. doi: 10.1021/jf803638d
58. Li F, Han Y, Cai X, Gu M, Sun J, Qi C, et al. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. (2020) 11:1063–73. doi: 10.1039/C9FO01519A
59. Cai T-T, Ye X-L, Li R-R, Chen H, Wang Y-Y, Yong H-J, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. (2020) 11:1249. doi: 10.3389/fphar.2020.01249
60. Yang C, Deng Q, Xu J, Wang X, Hu C, Tang H, et al. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res Int. (2019) 116:1202–11. doi: 10.1016/j.foodres.2018.10.003
61. Bervoets L, van Hoorenbeeck K, Kortleven I, van Noten C, Hens N, Vael C, et al. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. (2013) 5:10. doi: 10.1186/1757-4749-5-10
62. Hao W, Hao C, Wu C, Xu Y, Jin C. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere. (2022) 288(Pt 2):132556. doi: 10.1016/j.chemosphere.2021.132556
63. Hwang D, Jo H, Kim J-K, Lim Y-H. Oxyresveratrol-containing Ramulus mori ethanol extract attenuates acute colitis by suppressing inflammation and increasing mucin secretion. J Funct Foods. (2017) 35:146–58. doi: 10.1016/j.jff.2017.05.042
64. Hwang D, Jo H, Ma S-H, Lim Y-H. Oxyresveratrol stimulates mucin production in an NAD+-dependent manner in human intestinal goblet cells. Food Chem Toxicol. (2018) 118:880–8. doi: 10.1016/j.fct.2018.06.039
65. Yeom J, Ma S, Lim Y-H. Oxyresveratrol induces autophagy via the ER stress signaling pathway, and oxyresveratrol-induced autophagy stimulates MUC2 synthesis in human goblet cells. Antioxidants. (2020) 9:214. doi: 10.3390/antiox9030214
66. Wang P, Wang J, Li D, Ke W, Chen F, Hu X. Targeting the gut microbiota with resveratrol: a demonstration of novel evidence for the management of hepatic steatosis. J Nutr Biochem. (2020) 81:108363. doi: 10.1016/j.jnutbio.2020.108363
67. Lee SH. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest Res. (2015) 13:11–8. doi: 10.5217/ir.2015.13.1.11
68. Song X, Liu L, Peng S, Liu T, Chen Y, Jia R, et al. Resveratrol regulates intestinal barrier function in cyclophosphamide-induced immunosuppressed mice. J Sci Food Agric. (2021). doi: 10.1002/jsfa.11458
69. Yang J, Zhu C, Ye J, Lv Y, Wang L, Chen Z, et al. Protection of porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway. J Agric Food Chem. (2019) 67:1726–35. doi: 10.1021/acs.jafc.8b03662
70. Qin H, Zhang H, Zhang X, Zhang S, Zhu S, Wang H. Resveratrol protects intestinal epithelial cells against radiation-induced damage by promoting autophagy and inhibiting apoptosis through SIRT1 activation. J Radiat Res. (2021) 62:574–81. doi: 10.1093/jrr/rrab035
71. Zhuang Y, Wu H, Wang X, He J, He S, Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid Med Cell Longev. (2019) 2019:7591840. doi: 10.1155/2019/7591840
72. Kong C, Faas MM, Vos P de, Akkerman R. Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier. Food Funct. (2020) 11:9445–67. doi: 10.1039/d0fo01700k
73. Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J Leukoc Biol. (2019) 106:467–80. doi: 10.1002/JLB.3A1218-476RR
74. Singh UP, Singh NP, Singh B, Hofseth LJ, Taub DD, Price RL, et al. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav Immun. (2012) 26:72–82. doi: 10.1016/j.bbi.2011.07.236
75. Yao J, Wei C, Wang J-Y, Zhang R, Li Y-X, Wang L-S. Effect of resveratrol on Treg/Th17 signaling and ulcerative colitis treatment in mice. World J Gastroenterol. (2015) 21:6572–81. doi: 10.3748/wjg.v21.i21.6572
76. Dou Z, Rong X, Zhao E, Zhang L, Lv Y. Neuroprotection of resveratrol against focal cerebral ischemia/reperfusion injury in mice through a mechanism targeting gut-brain axis. Cell Mol Neurobiol. (2019) 39:883–98. doi: 10.1007/s10571-019-00687-3
77. Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, et al. Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. (2010) 332:829–39. doi: 10.1124/jpet.109.160838
78. Chen X, Zeng Z, Huang Z, Chen D, He J, Chen H, et al. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim Biotechnol. (2021) 32:240–5. doi: 10.1080/10495398.2019.1683022
79. Bilotta S, Paruchuru LB, Feilhauer K, Köninger J, Lorentz A. Resveratrol is a natural inhibitor of human intestinal mast cell activation and phosphorylation of mitochondrial ERK1/2 and STAT3. Int J Mol Sci. (2021) 22(14). doi: 10.3390/ijms22147640
80. Zhang Y-F, Liu Q-M, Gao Y-Y, Liu B, Liu H, Cao M-J, et al. Attenuation of allergic responses following treatment with resveratrol in anaphylactic models and IgE-mediated mast cells. Food Funct. (2019) 10:2030–9. doi: 10.1039/c9fo00077a
81. Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. (2017) 17:761–73. doi: 10.1038/nri.2017.100
82. Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. (2021) 27:844–55. doi: 10.1016/j.molmed.2021.06.007
83. Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. (2019) 71:1216–28. doi: 10.1016/j.jhep.2019.08.005
84. Man AW, Li H, Xia N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients. (2020) 12(1). doi: 10.3390/nu12010119
85. Wu CW, Nakamoto Y, Hisatome T, Yoshida S, Miyazaki H. Resveratrol and its dimers ε-viniferin and δ-viniferin in red wine protect vascular endothelial cells by a similar mechanism with different potency and efficacy. Kaohsiung J Med Sci. (2020) 36:535–42. doi: 10.1002/kjm2.12199
86. Silen W, Machen TE, Forte JG. Acid-base balance in amphibian gastric mucosa. Am J Physiol. (1975) 229:721–30. doi: 10.1152/ajplegacy.1975.229.3.721
87. Radwan RR, Karam HM. Resveratrol attenuates intestinal injury in irradiated rats via PI3K/Akt/mTOR signaling pathway. Environ Toxicol. (2020) 35:223–30. doi: 10.1002/tox.22859
88. Abdallah DM, Ismael NR. Resveratrol abrogates adhesion molecules and protects against TNBS-induced ulcerative colitis in rats. Can J Physiol Pharmacol. (2011) 89:811–8. doi: 10.1139/y11-080
89. Cheng K, Song Z, Li S, Yan E, Zhang H, Zhang L, et al. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J Therm Biol. (2019) 85:102415. doi: 10.1016/j.jtherbio.2019.102415
90. Sharma M, Mohapatra J, Wagh A, Patel HM, Pandey D, Kadam S, et al. Involvement of TACE in colon inflammation: a novel mechanism of regulation via SIRT-1 activation. Cytokine. (2014) 66:30–9. doi: 10.1016/j.cyto.2013.12.010
91. Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. (2010) 70:7392–9. doi: 10.1158/0008-5472.CAN-10-2027
92. Dariya B, Behera SK, Srivani G, Farran B, Alam A, Nagaraju GP. Computational analysis of nuclear factor-κB and resveratrol in colorectal cancer. J Biomol Struct Dyn. (2021) 39:2914–22. doi: 10.1080/07391102.2020.1757511
93. Buhrmann C, Shayan P, Brockmueller A, Shakibaei M. Resveratrol suppresses cross-talk between colorectal cancer cells and stromal cells in multicellular tumor microenvironment: a bridge between in vitro and in vivo tumor microenvironment study. Molecules. (2020) 25:4292. doi: 10.3390/molecules25184292
94. Yuan L, Zhou M, Huang D, Wasan HS, Zhang K, Sun L, et al. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial-mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Mol Med Rep. (2019) 20:2783–95. doi: 10.3892/mmr.2019.10528
95. Xu Y, Cui S-Y, Ma Q, Shi J, Yu Y, Li J-X, et al. Trans-resveratrol ameliorates stress-induced irritable bowel syndrome-like behaviors by regulation of brain-gut axis. Front Pharmacol. (2018) 9:631. doi: 10.3389/fphar.2018.00631
96. Yu Y-C, Li J, Zhang M, Pan J-C, Yu Y, Zhang J-B, et al. Resveratrol improves brain-gut axis by regulation of 5-HT-dependent signaling in the rat model of irritable bowel syndrome. Front Cell Neurosci. (2019) 13:30. doi: 10.3389/fncel.2019.00030
97. Ruan X, Deng X, Tan M, Wang Y, Hu J, Sun Y, et al. Effect of resveratrol on the biofilm formation and physiological properties of avian pathogenic Escherichia coli. J Proteomics. (2021) 249:104357. doi: 10.1016/j.jprot.2021.104357
98. Lobo de Sá FD, Heimesaat MM, Bereswill S, Nattramilarasu PK, Schulzke JD, Bücker R. Resveratrol prevents campylobacter jejuni-induced leaky gut by restoring occludin and Claudin-5 in the paracellular leak pathway. Front Pharmacol. (2021) 12:640572. doi: 10.3389/fphar.2021.640572
99. Elgendy DI, Othman AA, Hasby Saad MA, Soliman NA, Mwafy SE. Resveratrol reduces oxidative damage and inflammation in mice infected with Trichinella spiralis. J Helminthol. (2020) 94:e140. doi: 10.1017/S0022149X20000206
100. Xu J, Yin Z, Li L, Cheng A, Jia R, Song X, et al. Inhibitory effect of resveratrol against duck enteritis virus in vitro. PLoS ONE. (2013) 8:e65213. doi: 10.1371/journal.pone.0065213
101. Brakenhoff LK, van der Heijde DM, Hommes DW, Huizinga TW, Fidder HH. The joint-gut axis in inflammatory bowel diseases. J Crohns Colitis. (2010) 4:257–68. doi: 10.1016/j.crohns.2009.11.005
102. Shi Y, Zhou J, Jiang B, Miao M. Resveratrol and inflammatory bowel disease. Ann N Y Acad Sci. (2017) 1403:38–47. doi: 10.1111/nyas.13426
103. Panaro MA, Carofiglio V, Acquafredda A, Cavallo P, Cianciulli A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br J Nutr. (2012) 108:1623–32. doi: 10.1017/S0007114511007227
104. Sabzevary-Ghahfarokhi M, Soltani A, Luzza F, Larussa T, Rahimian G, Shirzad H, et al. The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol Biol Rep. (2020) 47:6941–7. doi: 10.1007/s11033-020-05753-4
105. Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr Res Rev. (2018) 31:85–97. doi: 10.1017/S095442241700021X
106. Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. (2009) 22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d
107. Reijonen P, Österlund P, Isoniemi H, Arola J, Nordin A. Histologically verified biliary invasion was associated with impaired liver recurrence-free survival in resected colorectal cancer liver metastases. Scand J Surg. (2019) 108:201–9. doi: 10.1177/1457496918812237
108. Honari M, Shafabakhsh R, Reiter RJ, Mirzaei H, Asemi Z. Resveratrol is a promising agent for colorectal cancer prevention and treatment: focus on molecular mechanisms. Cancer Cell Int. (2019) 19:180. doi: 10.1186/s12935-019-0906-y
109. Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans-resveratrol–a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. (2006) 27:1038–46. doi: 10.1093/carcin/bgi286
110. Omidian K, Rafiei H, Bandy B. Increased mitochondrial content and function by resveratrol and select flavonoids protects against benzoapyrene-induced bioenergetic dysfunction and ROS generation in a cell model of neoplastic transformation. Free Radic Biol Med. (2020) 152:767–75. doi: 10.1016/j.freeradbiomed.2020.01.021
111. Yang G, Chang C-C, Yang Y, Yuan L, Xu L, Ho C-T, et al. Resveratrol alleviates rheumatoid arthritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J Agric Food Chem. (2018) 66:12953–60. doi: 10.1021/acs.jafc.8b05047
112. Namasivayam N. Chemoprevention in experimental animals. Ann N Y Acad Sci. (2011) 1215:60–71. doi: 10.1111/j.1749-6632.2010.05873.x
113. Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. (2011) 1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x
114. Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: Implications for colon cancer prevention. Mol Nutr Food Res. (2008) 52 Suppl 1:S52–61. doi: 10.1002/mnfr.200700448
115. Liang Y-C, Tsai S-H, Chen L, Lin-Shiau S-Y, Lin J-K. Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem Pharmacol. (2003) 65:1053–60. doi: 10.1016/S0006-2952(03)00011-X
116. Schneider Y, Vincent F, Duranton B, Badolo L, Gossé F, Bergmann C, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. (2000) 158:85–91. doi: 10.1016/s0304-3835(00)00511-5
117. Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. (2010) 10:238. doi: 10.1186/1471-2407-10-238
118. Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, et al. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. (2009) 1:25–37. doi: 10.2147/CMAR.S4544
119. Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. (2016) 1:133–46. doi: 10.1016/S2468-1253(16)30023-1
120. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. (2015) 313:949–58. doi: 10.1001/jama.2015.0954
121. Surdea-Blaga T, Baban A, Nedelcu L, Dumitrascu DL. Psychological interventions for irritable bowel syndrome. J Gastrointestin Liver Dis. (2016) 25:359–66. doi: 10.15403/jgld.2014.1121.253.ibs
122. Raskov H, Burcharth J, Pommergaard H-C, Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. (2016) 7:365–83. doi: 10.1080/19490976.2016.1218585
123. Jang J, Hur H-G, Sadowsky MJ, Byappanahalli MN, Yan T, Ishii S. Environmental Escherichia coli: ecology and public health implications-a review. J Appl Microbiol. (2017) 123:570–81. doi: 10.1111/jam.13468
124. Riley LW. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect. (2014) 20:380–90. doi: 10.1111/1469-0691.12646
125. Mirhoseini A, Amani J, Nazarian S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microb Pathog. (2018) 117:162–9. doi: 10.1016/j.micpath.2018.02.032
126. Béchon N, Ghigo J-M. Gut biofilms: bacteroides as model symbionts to study biofilm formation by intestinal anaerobes. FEMS Microbiol Rev. (2021) 2021:fuab054. doi: 10.1093/femsre/fuab054
127. Selma MV, Larrosa M, Beltrán D, Lucas R, Morales JC, Tomás-Barberán F, et al. Resveratrol and some glucosyl, glucosylacyl, and glucuronide derivatives reduce Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes Scott A adhesion to colonic epithelial cell lines. J Agric Food Chem. (2012) 60:7367–74. doi: 10.1021/jf203967u
128. Bücker R, Krug SM, Moos V, Bojarski C, Schweiger MR, Kerick M, et al. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol. (2018) 11:474–85. doi: 10.1038/mi.2017.66
129. Koushki M, Dashatan NA, Meshkani R. Effect of resveratrol supplementation on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Clin Ther. (2018) 40:1180–1192.e5. doi: 10.1016/j.clinthera.2018.05.015
130. Chen ML, Ge Z, Fox JG, Schauer DB. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect Immun. (2006) 74:6581–9. doi: 10.1128/IAI.00958-06
131. Rees LE, Cogan TA, Dodson AL, Birchall MA, Bailey M, Humphrey TJ. Campylobacter and IFNgamma interact to cause a rapid loss of epithelial barrier integrity. Inflamm Bowel Dis. (2008) 14:303–9. doi: 10.1002/ibd.20325
132. Benedetti F, Sorrenti V, Buriani A, Fortinguerra S, Scapagnini G, Zella D. Resveratrol, rapamycin and metformin as modulators of antiviral pathways. Viruses. (2020) 12:1458. doi: 10.3390/v12121458
133. Cui Q, Fu Q, Zhao X, Song X, Yu J, Yang Y, et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS ONE. (2018) 13:e0192692. doi: 10.1371/journal.pone.0192692
134. Huang H, Liao D, Zhou G, Zhu Z, Cui Y, Pu R. Antiviral activities of resveratrol against rotavirus in vitro and in vivo. Phytomedicine. (2020) 77:153230. doi: 10.1016/j.phymed.2020.153230
135. Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. (2018) 10:1651. doi: 10.3390/nu10111651
136. Shu L, Hou G, Zhao H, Huang W, Song G, Ma H. Long non-coding RNA expression profiling following treatment with resveratrol to improve insulin resistance. Mol Med Rep. (2020) 22:1303–16. doi: 10.3892/mmr.2020.11221
137. Yang Q, Xu E, Dai J, Liu B, Han Z, Wu J, et al. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol Appl Pharmacol. (2015) 285:79–88. doi: 10.1016/j.taap.2015.04.003
138. Al Aameri RF, Sheth S, Alanisi EM, Borse V, Mukherjea D, Rybak LP, et al. Tonic suppression of PCAT29 by the IL-6 signaling pathway in prostate cancer: reversal by resveratrol. PLoS ONE. (2017) 12:e0177198. doi: 10.1371/journal.pone.0177198
139. Wu B, Qiang L, Zhang Y, Fu Y, Zhao M, Lei Z, et al. The deubiquitinase OTUD1 inhibits colonic inflammation by suppressing RIPK1-mediated NF-κB signaling. Cell Mol Immunol. (2021). doi: 10.1038/s41423-021-00810-9
Keywords: resveratrol, intestinal barrier, antioxidant, anti-inflammation, anti-tumor
Citation: Wang Y, Hong C, Wu Z, Li S, Xia Y, Liang Y, He X, Xiao X and Tang W (2022) Resveratrol in Intestinal Health and Disease: Focusing on Intestinal Barrier. Front. Nutr. 9:848400. doi: 10.3389/fnut.2022.848400
Received: 04 January 2022; Accepted: 07 February 2022;
Published: 16 March 2022.
Edited by:
Guan Yang, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Liang Zhao, Beijing Technology and Business University, ChinaCopyright © 2022 Wang, Hong, Wu, Li, Xia, Liang, He, Xiao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjie Tang, d2VuaGFuMjhAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.