
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 04 March 2022
Sec. Nutritional Epidemiology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.847421
This article is part of the Research TopicThe Role of Soy in Human Health and DiseaseView all 5 articles
 Yahui Fan1,2,3
Yahui Fan1,2,3 Mingxu Wang3
Mingxu Wang3 Zhaofang Li3
Zhaofang Li3 Hong Jiang3
Hong Jiang3 Jia Shi3
Jia Shi3 Xin Shi3
Xin Shi3 Sijiao Liu3
Sijiao Liu3 Jinping Zhao3
Jinping Zhao3 Liyun Kong3,4*
Liyun Kong3,4* Wei Zhang1*
Wei Zhang1* Le Ma3,4,5*
Le Ma3,4,5*Background and Aims: Associations between soy intake and risk of cancer have been evaluated in prospective observational studies with inconsistent results. Whether the potential anticancer effects offered by soy were attributed to soy isoflavones and soy protein still needs to be elucidated. This study aimed to comprehensively quantify the association of soy, soy isoflavones and soy protein intake with risk of cancer incidence and cancer mortality by conducting a meta-analysis of all available studies.
Methods: PubMed, Embase, Web of Science, and Cochrane Library databases were searched up to 16 September 2021. Prospective cohort studies that examined the effect of soy, soy isoflavones and soy protein on cancer incidence and cancer mortality were identified. Random-effects models were used to pool the multivariable-adjusted relative risks (RRs) and corresponding 95% confidence intervals (CIs). The potential dose-response relations were explored by using generalized least-squares trend estimation.
Results: Eighty one prospective cohort studies were included in the meta-analysis. A higher intake of soy was significantly associated with a 10% reduced risk of cancer incidence (RR, 0.90; 95% CI, 0.83–0.96). Each additional 25 g/d soy intake decreased the risk of cancer incidence by 4%. Intake of soy isoflavones was inversely associated with risk of cancer incidence (RR, 0.94; 95% CI, 0.89–0.99), whereas no significant association was observed for soy protein. The risk of cancer incidence was reduced by 4% with each 10 mg/d increment of soy isoflavones intake. Similar inverse associations were also found for soy in relation to site-specific cancers, particularly lung cancer (RR, 0.67; 95%CI, 0.52–0.86) and prostate cancer (RR, 0.88; 95%CI, 0.78–0.99). However, high intake of soy, soy isoflavones and soy protein were not associated with cancer mortality.
Conclusions: Higher intake of soy and soy isoflavones were inversely associated with risk of cancer incidence, which suggested that the beneficial role of soy against cancer might be primarily attributed to soy isoflavones. These findings support recommendations to include soy as part of a healthy dietary pattern for the prevention of cancer.
Soybeans are considered as key components of plant-based dietary patterns and dietary guidelines from several organizations recommend their increasing consumption for the prevention of heart disease and other chronic conditions (1, 2). Such health benefits may be attributed to the multiple nutrients and associated phytochemicals of soy (3). As the major nutritional profile of soy, soy isoflavones and soy protein have been suggested to reduce oxidative stress and inflammation (4, 5), both of which are implicated in the pathogenesis of chronic non-communicable diseases (NCDs). Most studies over the past decades were designed to focus on the protective roles of soy in cardiovascular health, and had revealed that soy had favorable impacts on preventing the initiation and progression of cardiovascular disease (CVD) (6, 7). Our large prospective cohort studies have suggested that consumption of soy might reduce the risk of coronary heart disease (8). However, whether soy consumption is associated with risk of other major NCDs remains to be clarified.
Cancer and CVD potentially shared underlying biological mechanisms and common risk factors (9), indicating it seems plausible that soy intake may contribute to the decreased risk of cancer. Several prospective observational studies have been conducted to investigate soy intake in relation to risk of incident cancers, and yielded inconsistent results (10–12). Although some meta-analyses have reported inverse associations of soy with incidence of specific types of cancer, it is unclear whether the observed association still existed between soy and overall cancer (13–15). Furthermore, nutritional guidelines by the American Heart Association proposed that the cardiovascular protective effect offered by soy attributed to soy isoflavones and soy protein could be considered minimal (16). Little is known whether soy isoflavones or soy protein may impact the development of cancer. In addition, existing findings are largely lacking for the association of soy with prognosis of cancer.
Therefore, we conducted a meta-analysis of all available prospective cohort studies to assess the associations between intake of soy, soy isoflavones and soy protein and risk of cancer incidence as well as cancer mortality.
The present study was conducted in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (17).
The electronic databases PubMed, Embase, Web of Science, and Cochrane Library were utilized to systematically search up to 16 September 2021 for relevant prospective cohort studies examining the association between soy, soy isoflavones, soy protein and cancer incidence as well as cancer mortality. The following search items were included: (1) “soy” OR “soya” OR “bean” OR “soybean” OR “legume” OR “glycine max” OR “soy food” OR “tofu” OR “bean curd” OR “soymilk” OR “miso” OR “natto” OR “sufu” OR “tempeh” OR “genistein” OR “daidzein” OR “glycitein” OR “isoflavone” OR “phytoestrogen” OR “soy protein,” AND (2) “cancer” OR “neoplasm” OR “carcinoma” OR “tumor” OR “malignant,” AND (3) “morbidity” OR “incidence” OR “occurrence” OR “genesis” OR “occur” OR “mortality” OR “death” OR “fatal” OR “survival”. No restrictions were imposed with respect to publication language. Reference lists of retrieved articles and previous relevant reviews were scanned manually for pertinent studies. In addition, authors and experts of selected articles were contacted to identify any unpublished or ongoing studies that could fulfill inclusion criteria.
Studies were considered to be eligible if they satisfied the following criteria: (1) studies with prospective cohort design were published as original articles; (2) the exposures of interest were dietary intake of soy, soy products (such as tofu, soymilk, bean sprouts, natto, miso and tempeh), soy isoflavones and soy protein; (3) the outcomes of interest were the incidence of cancer and cancer mortality; (4) relative risks (RRs) along with corresponding 95% confidence intervals (95% CIs) were reported or could be calculated with sufficient data. In case of multiple publications on the same population or subpopulation, the estimates from the most recent or most informative report were considered in the meta-analysis. Studies measuring concentrations of isoflavones or their metabolites in plasma or urine samples were excluded due to the difficulty in converting biological concentration into amount of soy isoflavones intake. Two investigators (YHF and ZFL) independently assessed full text of those selected publications to determine potentially relevant articles for inclusion; any discrepancies or uncertainties on eligibility were resolved by consulting a third reviewer (LM).
A standardized predesigned data collection form was applied to extract the following data from each included study: first author name, year of publication, geographical location, duration of follow-up, cohort name, sample size, mean age or age range at baseline, sex, dietary assessment method, categories of soy consumption, type of cancer, number of cases or deaths, method of endpoints identification, RRs (95% CIS) from the multivariable model, and potential covariates in the maximally adjusted model. Where studies reported RRs with different degrees of adjustment for other risk factors, the maximum adjusted estimate was prioritized. In addition, if an article included data from multiple cohorts, we considered the analysis for each cohort as an independent report and extracted data separately. Whenever necessary, the original study authors were contacted to obtain additional information that was not available in the online publications or Supplementary Materials.
Methodological quality of the included cohort study was assessed in accordance with the 9-star Newcastle-Ottawa scale (NOS), which focused on three domains: selection of participants (0–4 stars), comparability of cohorts (0–2 stars), and assessment of outcome (0–3 stars) (18). We assigned scores of 0–3, 4–6, and 7–9 for low, moderate, and high quality, respectively. Data extraction and quality assessment were conducted independently in a standardized manner by two researchers (YHF and JS), and the senior reviewer (LM) was involved to adjudicate any disagreements if necessary.
The pooled RRs were used as the common measures of association across studies. In order to take into account both within-study and between-study variability, random-effects models were applied to pool RRs and 95% CIs for the associations of soy, soy isoflavones and soy protein with cancer incidence and cancer mortality. For eligible studies that only reported stratified results by sex, cancer outcome or other variables, a within-study summary estimate was obtained using a fixed-effect model. Heterogeneity was quantified by Cochran's Q test (P < 0.10 for significance) and the I-square (I2) statistic with a value more than 50% signifying substantial heterogeneity (19). In further analysis, we explored the effect of individual soy foods in relation to cancer, including tofu, soy milk, miso and natto. Subgroup analysis and meta-regression were carried out by preset factors to explore the potential sources of heterogeneity, including gender (both, women or men), geographical location (the United States, Europe, or Asia), follow up periods (<10 y or ≥10 y), and adjustment for family history of cancer (yes or no). The potential dose-response associations were examined using the method proposed by Greenland and Longnecker, and only at least two studies with ≥3 exposures categories could be included (20). The mean or median soy, soy isoflavones and soy protein consumption per category was assigned to the corresponding RR estimate. For studies that continuous exposures were reported with a range, the midpoint of the upper and lower boundaries in each category was used to estimate median consumption. When the highest category was open-ended, the width of the adjacent interval was used to establish the highest cut-off value, and the midpoint of the lowest boundary and zero was defined as the amount of lowest quantile (21). For consistency, we converted different units (such as servings and times) to grams per day (g/d) according to standard conversions based on dietary guidelines and previous studies (22). Both non-linear and linear models were fitted and evaluated on the logarithm of the RR. Non-linear trends were examined using restricted cubic spline models with 3 knots at the 10, 50, and 90th percentiles, and the 2-stage generalized least-squares regression approach was used to estimate the linear dose-response slope (20). Sensitivity analysis by sequentially omitting individual study was performed to examine whether the pooled associations could be substantially affected by a single study, demonstrating the robustness of the findings. Publication bias was assessed by visual examination of funnel plots and statistically Egger's and Begg's Tests (23). The trim and fill method was applied to adjust for any observed publication bias via imputing additional studies. All statistical analyses were performed with Stata version 12.0 (Stata Corp, College Station, TX, USA). A two tailed P < 0.05 was deemed statistically significant, unless explicitly stated.
The search strategy yielded 10,731 records of potentially relevant articles from four databases after removing the duplicates, of which 261 full-text articles were retrieved for detailed evaluation after performing an initial screen of titles or abstracts. Hand searching of the bibliographic references of these articles identified 4 additional articles. Finally, 78 articles including 81 cohort studies were selected as appropriate for inclusion in the meta-analysis (Figure 1) (10–12, 15, 24–97).
The main characteristics of the included studies are summarized in Supplementary Table 1. Among included studies, 51 studies were from Asia, 20 from the United States, and 10 studies were from Europe. The number of participants from each selected study varied from 1,210 to 477,312, for a total of about 4.15 million participants across studies. The study population in 32 studies comprised both men and women, 36 studies consisted of only women, and 13 studies involved men only. The follow-up duration ranged from 2 to 19.4 years. In most of the studies, information on dietary intake of soy, soy isoflavones and soy protein derived from food frequency questionnaire (FFQ). The endpoint of cancer was primarily confirmed by examination of medical records, cancer registries, or death certificates for included studies. The majority of studies reported risk estimates adjusted for age (n = 78), smoking (n = 58) and body mass index (n = 57). Many studies also adjusted for alcohol (n = 45), total energy intake (n = 43), physical activity (n = 36) and family history of cancer (n = 26). Results from the assessment of study quality showed that most studies (n = 74) were rated as high quality, and the others (n = 7) were deemed to be of medium quality (Supplementary Table 2).
Forty seven studies examined the association between soy, soy isoflavones and soy protein intake and cancer risk (10–12, 15, 24–66). The pooled results showed that a higher intake of soy was significantly associated with a 10% reduced risk of overall cancer incidence when comparing extreme categories of soy intake (RR, 0.90; 95% CI, 0.83–0.96) (Figure 2). There was significant heterogeneity across the studies (I2 = 57.8%, P heterogeneity <0.001). The dose-response analysis revealed that each increase of 25 g/d in soy intake significantly decreased the risk of overall cancer incidence by 4% (RR, 0.96; 95%CI, 0.94–0.98) (Figure 3A). In the stratified analyses across study and participant characteristics, inconsistencies in these variables did not significantly alter the shape of association between soy intake and risk of overall cancer incidence (Supplementary Table 3). In terms of soy isoflavones and soy protein, participants in the highest category of soy isoflavones intake had 6% (95% CI, 1–11%) lower risk of overall cancer incidence, compared with those in the lowest category (Figure 2). Evidence of substantial heterogeneity existed among studies (I2 = 52.6%, P heterogeneity <0.001). No significant association between soy protein consumption and risk of overall cancer incidence was observed (RR, 0.95; 95% CI, 0.71–1.28). Each 10 mg/d increment of soy isoflavones intake was significantly associated with a 4% lower risk of overall cancer incidence (RR, 0.96; 95% CI, 0.94–0.99) in the dose-response analysis (Figure 3C). The association between soy isoflavones intake and risk of overall cancer incidence did not differ substantially by characteristics of study and participant examined (Supplementary Table 4).
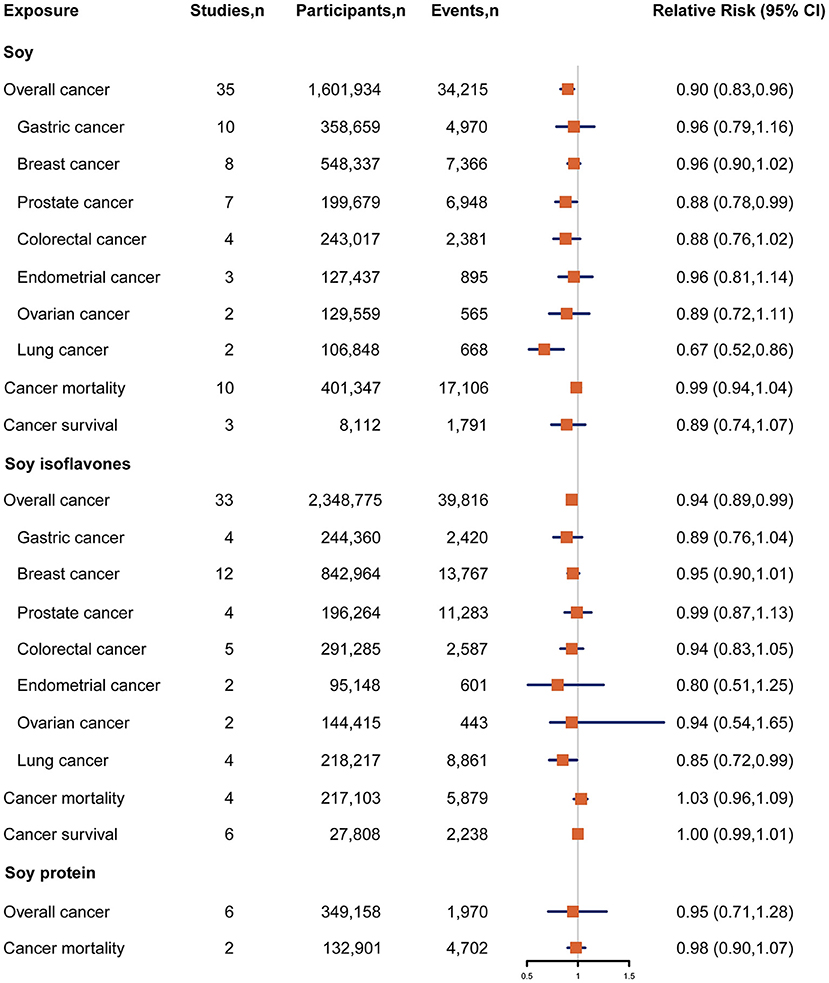
Figure 2. Forest plot for association between soy, soy isoflavones and soy protein intake and risk of cancer incidence and cancer mortality, expressed as comparison between highest and lowest categories of soy, soy isoflavones and soy protein intake. The size of the black squares reflects the relative statistical weight of study-specific estimate, horizontal lines indicate 95% CIs. The diamond indicates the pooled RR estimates with 95% CIs. CI, confidence interval; RR, relative risk.
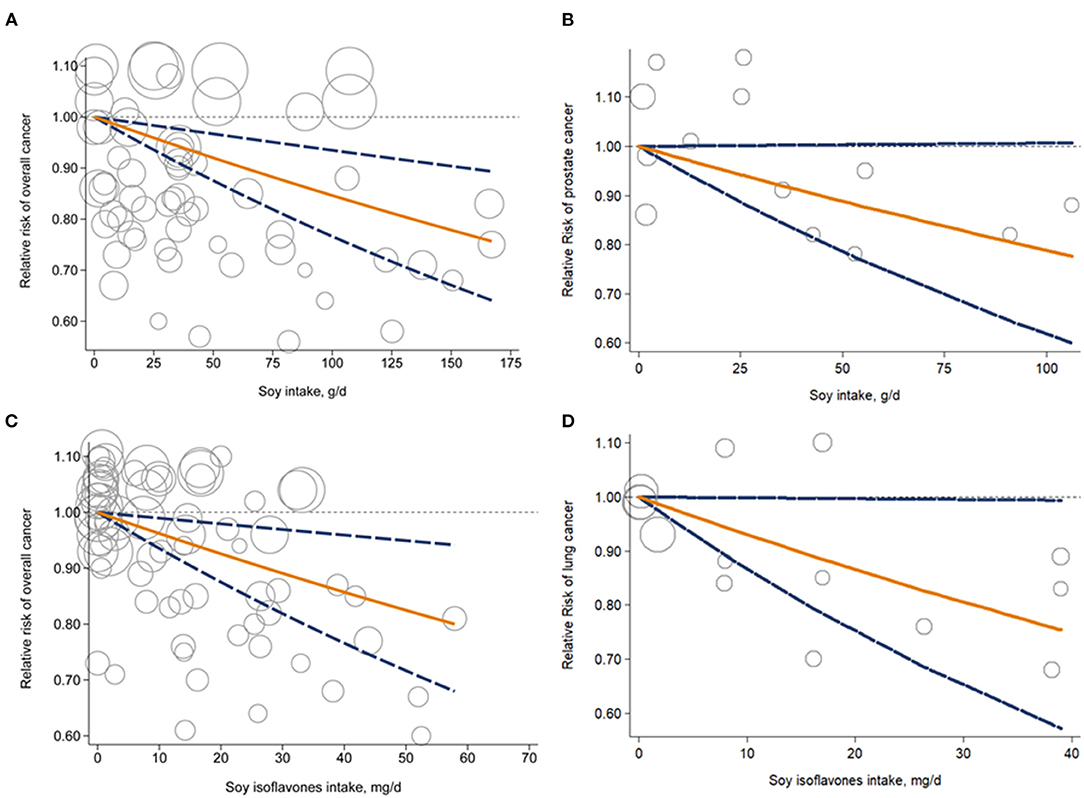
Figure 3. Dose-response analysis for liner association of soy intake with overall cancer risk (A) and prostate cancer risk (B); soy isoflavones intake and overall cancer risk (C) and lung cancer risk (D). Circles represent point estimates plotted over precision measures. Solid lines represent summary RRs; dashed lines are the corresponding 95%CIs. CI, confidence interval; RR, relative risk.
Associations between intake of soy, soy isoflavones and soy protein and risk of specific cancer sites were investigated in 54 studies {breast cancer (n = 15) (24–26, 29, 33, 38, 41, 46, 48, 51, 53, 56, 59, 61, 67), gastric cancer (n = 10) (31, 32, 36, 37, 54, 62, 64, 65, 68), prostate cancer (n = 9) (28, 34, 47, 50, 55, 57, 63, 66, 69), colorectal cancer (n = 5) (30, 43, 44, 52, 70), bladder cancer (n = 4) (11, 35, 58, 60), lung cancer (n = 4) (15, 42, 45, 71), endometrial cancer (n = 3) (26, 39, 72), ovarian cancer (n = 3) (26, 40, 49), cervical cancer (n = 1) (27), pancreatic cancer (n = 1) (73), liver cancer (n = 1) (74)}. Participants in the highest category of soy intake had a 33% lower risk of lung cancer compared with those in the lowest category (RR, 0.67; 95% CI, 0.52–0.86) (Figure 2). A similar inverse association was also observed between soy intake and risk of prostate cancer (RR, 0.88; 95% CI, 0.78–0.99), with low heterogeneity detected among studies (I2 = 12.3%; P heterogeneity = 0.34) (Figure 2). The dose-response analysis showed that each 25 g/d increment of soy intake was marginally associated with 6% (RR, 0.94; 95% CI, 0.89–1.00) lower risk of prostate cancer (Figure 3B). Regarding the association of soy isoflavones with lung cancer, the pooled RR of lung cancer was 0.85 (95% CI, 0.72–0.99) when comparing the extreme categories of soy isoflavones intake. Association of soy protein intake and risk of most individual cancers could not be evaluated due to the limited number of studies. The dose-response analysis revealed a significant 6% decrease in risk of lung cancer for a 10 mg/d increase in soy isoflavones intake (RR, 0.94; 95% CI, 0.89–0.98) (Figure 3D).
The associations between soy, soy isoflavones and soy protein intake and risk of cancer mortality in general population were reported in 17 studies (75–90). Among these studies, most did not report a significant inverse association except one study (88). The pooled RR for cancer mortality comparing the extreme categories of soy intake was 0.99 (95% CI, 0.94–1.04) (Figure 2). No heterogeneity was found between these studies (I2 = 0.0%, P heterogeneity = 0.64). Both soy isoflavones and soy protein intake were not associated with risk of cancer mortality, with a pooled RR of 1.03 (95% CI, 0.96–1.09; I2 = 0.0, P heterogeneity = 0.46) and 0.98 (95% CI, 0.90–1.07; I2 = 0.0%, P heterogeneity =0.81), respectively (Figure 2).
Eight studies were included in the analysis of soy and soy isoflavones intake with risk of cancer mortality in cancer patients (i.e., cancer survival) (91–97). All these studies reported non-significant associations. The pooled results showed that a higher intake of soy was not significantly associated with a lower risk of cancer mortality in cancer patients when comparing extreme categories of soy intake (RR, 0.89; 95% CI, 0.74–1.07) (Figure 2). Little evidence of heterogeneity was found (I2 = 0.0%, P heterogeneity = 0.39). No statistically significant association was also found between soy isoflavones intake and risk of cancer mortality among cancer patients (RR, 1.00; 95% CI, 0.99–1.01) (Figure 2).
Sensitivity analyses showed that no individual study excluded changed the combined RR substantially between soy, soy isoflavones and soy protein and any of the outcomes. The Egger's (P > 0.05) or Begg's (P > 0.05) did not indicate the presence of publication bias for most associations examined in the current meta-analysis, except for the associations between soy protein intake and risk of overall cancer incidence (P = 0.03 for Egger's test and P = 0.06 for Begg's test); soy intake and risk of prostate cancer (P = 0.03 for Egger's test and P = 0.07 for Begg's test). Furthermore, the trim and fill correction procedures indicated that the pooled RRs were not affected by publication bias (data not shown).
The present meta-analysis suggested that increasing intake of soy was significantly associated with a lower risk of overall cancer incidence as well as site-specific cancers, particularly lung and prostate cancer. Similar risk reduction was also observed for intake of soy isoflavones, rather than soy protein. In contrast, no significant association was detected for dietary intake of soy, soy isoflavones and soy protein in relation to cancer mortality for both general population and cancer patients.
Numerous prospective observational studies had been conducted to examine the association of soy intake with cancer risk. In a cohort of 30,817 Japanese men and women, a significant decreased risk of bladder cancer was seen among men who had highest category of soy intake compared with those in the lowest category (11). Among 68,412 women free of cancer from Shanghai Women's Health Study, each 5 g/d increment in soy intake was associated with an 8% reduction in colorectal cancer risk for postmenopausal women (15). However, in the Women's Health Study consisting of 38,408 US women, higher intake of tofu was not significantly associated with total cancer as well as site-specific cancer risk during over 10-year follow up period (10). Although previous meta-analyses reported that soy consumption was inversely associated with 15–37% lower risks of cancer in different sites, such as stomach, prostate and lung cancer (13–15), no meta-analysis has summarized the association for soy intake in relation to overall cancer risk. Taking cancer together is a matter of vital importance for a more comprehensive way to assess the prevention of cancer morbidity and mortality for both general population and cancer patients. Our results showed that a higher intake of soy was associated with a decreased risk of overall cancer incidence. Furthermore, this result was also supported by our dose-response finding in which the risk of overall cancer decreased with increment in soy intake. Of note, the direction of the inverse association between soy intake and risk of cancer was generally similar to those in results of soy isoflavones, suggesting that the protective effects of soy on cancer may be primarily attributed to soy isoflavones.
Several biological mechanisms have been proposed for the potential health benefits of soy, especially isoflavones on preventing cancer, involving an increased antioxidant effect and reduced inflammation. It has been well-established that elevated levels of reactive oxygen species could damage DNA, drive gene mutations, and contribute to an increase in cell proliferation or a decrease in cell apoptosis, subsequently leading to mutagenesis and carcinogenesis. Soy and soy isoflavones have been suggested to counteract oxidative stress by activation of nuclear factor erythroid 2-related factor 2 (Nrf2) (4, 98) and modulation of genes expression involved in cell proliferation and cell apoptosis (99). In a 1,2-dimethyl hydrazine-induced colon cancer model in Wistar rats, genistein treatment for 6 weeks increased Nrf2 and hemoxygenase-1 protein expression and maintained glutathione levels (100). In vivo, isoflavones could enhance glutathione S-transferase and quinone reductase activity in SD rats fed soy diet for 2 weeks (101). Using human MCF-7 breast cancer cell line, Jin et al. revealed that treatment with daidzein for 24 h could induce cell apoptosis via down-regulation of B cell leukemia/lymphoma2 (BCL2) expression and up-regulation of BCL2-associated X protein expression in the mitochondrial caspase-dependent cell death pathway (102). Takahashi et al. also reported isoflavones exhibited an inhibitory effect on mitogen-activated protein kinase phosphatase 2 in human prostate cancer cell LNCaP, which blocked mitogenic signal transduction (103). Moreover, soy isoflavones were thought to suppress the expression of inflammatory mediators and block sustained damage-induced cellular proliferation through activation of nuclear factor kappa-B (NF-κB) (104, 105). In activated macrophage-Like RAW 264.7 cells, 8-Hydroxydaidzein treatment diminished expression of nitric oxide synthase, cyclooxygenase-2 and tumor necrosis factor-&alpha by inactivating the transcriptional activities of NF-κB and activator protein 1 (106). Also, a 5-month diet with genistein could downregulate pro-inflammatory responses and ameliorate liver damage by phosphorylation of adenosine monophosphate-activated protein kinase for C57BL/6 mices injected with diethylnitrosamine (107). As a critical inhibitor of protein-tyrosine kinases (PTK), isoflavones may also exert anti-mitotic and anti-angiogenic effects by inhibiting endothelial cell proliferation, migration, and capillary structure formation in response to vascular endothelial growth factor (VEGF) (108). Yu et al. found that the genistein could interrupt VEGF-stimulated human umbilical vein endothelial cells activation by inhibition of PTK activity and decreasing matrix metalloproteinases production that promoted angiogenesis (109).
In contrast with the favorable impact of soy on cancer incidence, our findings suggested that soy intake was not associated with the risk of cancer mortality for both general population and cancer patients. The discrepancies appear to be explained by the fact that cancer incidence and cancer mortality are two very distinct outcomes, with cancer mortality being greatly determined by the treatment approaches individual receives (110). It is well-known that cancer treatments induce many adverse symptoms including nausea/vomiting, impairment of taste and smell and bowel changes, which could interfere with the ability to eat, digest, or absorb soy (111). In a comparison of blood daidzein levels between prostate cancer patients and controls, the proportion of equol producers was significantly lower among prostate cancer patients, indicating that conversion of daidzein into equol is regulated by isoflavones ingestion and intestinal metabolism (112). Alternatively, approximately three out of four cancer patients suffer from at least one coexisting chronic disease, sharing biological mechanisms and common risk factors with cancer. Most comorbidities may alter the risk of cancer mortality because multiple pathological processes combined occur simultaneously (113). Lane et al. found that 29% of 537 renal tumor patients who received active treatment, died mostly of cardiovascular causes compared to 4% of those who died of cancer (114). Consistently, in a recent meta-analysis of 28 prospective and retrospective cohort studies on breast, lung, prostate, and colorectal cancer, and glioma, increased dietary intake of isoflavone was only significantly associated with risk of total mortality, rather than cancer-specific mortality in patients with breast cancer, which further reflected the potential prevalence of coexisting chronic diseases in cancer patients (115). Furthermore, it should be noted that an increased prevalence of competing risk factors, for example the adverse effects of obesity, physical inactivity, smoking, and high alcohol consumption, may result in the reduction of the proportional effect of dietary soy intake.
The potential limitations of the present meta-analysis are worth discussion in interpreting our findings. First, although most studies controlled for a wide range of potential socio-demographic, lifestyle, and dietary factors, the possibility of residual confounding by unmeasured or imprecisely measured factors cannot be completely ruled out, which is inherent to observational studies. Second, because self-reported dietary intake through FFQ is subject to bias, some measurement errors or misclassifications in soy intake assessment are inevitable. By prospectively collecting diet data before cancer occurrence or cancer death in most studies, any misclassification of soy intake would be non-differential and thus tend to bias results toward the null, resulting in the underestimation of true associations. Third, the observed relationship may be influenced by synergistic or additive effects of soy isoflavones and soy protein with other nutrients and non-nutrient constituents from soy. More future studies with larger sample sizes are warranted to examine whether these interactions may modify the associations between soy, soy isoflavones and soy protein intake and cancer risk. Fourth, different methods used in the cooking and processing of soy might influence the nutraceutical values of soy and bioavailability of isoflavones and soy protein. In our study, whether the individual soy foods were non-fermented or fermented did not exert significant changes in risk of cancer. Likewise, an intervention trial conducted among free-living individuals showed that consumption of different types of soy products did not alter urinary isoflavone excretion, indicating that the cooking or preparation methods might not appreciably affect the associations observed (116). Fifth, limited data was available for the association between soy protein and cancer despite a comprehensive literature research and inclusion of a considerable number of studies, more future large-scale prospective studies, particularly evaluating soy protein, are warranted to clarify the effects of soy protein on cancer incidence and cancer mortality. Finally, publication bias could be of concern in all meta-analyses. Although there was no evidence of significant publication bias for the outcomes in our study, the possibility of the undetected selection bias introduced because of exclusion of gray literature and unpublished data cannot be fully eliminated.
Our findings of the current meta-analysis suggested that higher intake of soy and soy isoflavones were inversely associated with risk of cancer in a dose-response manner, indicating that the beneficial role of soy against cancer may be primarily attributed to soy isoflavones. These findings support recommendations to include soy as part of a healthy dietary pattern for the prevention of cancer.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
LM, WZ, and LK conceived the study and formulated an analytical plan and supervised the study. YF and ZL designed the search strategy, performed the literature search, and screened studies for eligibility. YF, HJ, and XS extracted the data. YF and JS contributed to the study quality assessment. YF, HJ, SL, and JZ performed the statistical analysis. YF, MW, LM, WZ, and LK drafted the original manuscript and all other authors reviewed and revised the draft of the manuscript. All authors approved the final manuscript for submission.
This study was partially supported by grants from the National Natural Science Foundation of China (NSFC-82022062; NSFC-81973025; NSFC-81473059); Nutrition Science Research Foundation of BY-HEALTH (TY0181101); the Natural Science Foundation of Shaanxi Province of China (2017JM8041); New-star Plan of Science and Technology of Shaanxi Province (2015LJXX-07); the Nutrition Research Foundation Fund of the Chinese Nutrition Society-DSM Special Research Foundation (CNSDSM2016-041); the Fundamental Research Funds for the Central Universities (qngz2016004; xzy032019008). The funders had no role in the study design, implementation, analysis, decision to publish, or reparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.847421/full#supplementary-material
1. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. (2016) 8:754. doi: 10.3390/nu8120754
2. Benkhedda K, Boudrault C, Sinclair SE, Marles RJ, Xiao CW, Underhill L. Food risk analysis communication. Issued by health Canada's food directorate. Health Canada's proposal to accept a health claim about soy products and cholesterol lowering. Int Food Risk Anal J. (2014) 4:22. doi: 10.5772/59411
3. Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients. (2018) 10:43. doi: 10.3390/nu10010043
4. Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. (2009) 9:139–45. doi: 10.1016/j.coph.2008.12.012
5. Chakrabarti S, Jahandideh F, Wu J. Food-derived bioactive peptides on inflammation and oxidative stress. Biomed Res Int. (2014) 2014:608979. doi: 10.1155/2014/608979
6. Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, et al. Association of dietary intake of soy. Circulation. (2007) 116:2553–62. doi: 10.1161/CIRCULATIONAHA.106.683755
7. Ho SY, Schooling M, Hui LL, McGhee SM, Mak KH, Lam TH. Soy consumption and mortality in Hong Kong: proxy-reported case-control study of all older adult deaths in 1998. Prev Med. (2006) 43:20–6. doi: 10.1016/j.ypmed.2006.03.007
8. Ma L, Liu G, Ding M, Zong G, Hu FB, Willett WC, et al. Isoflavone intake and the risk of coronary heart disease in US men and women results from 3 prospective cohort studies. Circulation. (2020) 141:1127–37. doi: 10.1161/CIRCULATIONAHA.119.041306
9. Masoudkabir F, Sarrafzadegan N, Gotay C, Ignaszewski A, Krahn AD, Davis MK, et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention. Atherosclerosis. (2017) 263:343–51. doi: 10.1016/j.atherosclerosis.2017.06.001
10. Wang L, Lee IM, Zhang SM, Blumberg JB, Buring JE, Sesso HD. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr. (2009) 89:905–12. doi: 10.3945/ajcn.2008.26913
11. Wada K, Tsuji M, Tamura T, Konishi K, Goto Y, Mizuta F, et al. Soy isoflavone intake and bladder cancer risk in Japan: from the takayama study. Cancer Epidemiol Biomarkers Prev. (2018) 27:1371–5. doi: 10.1158/1055-9965.EPI-18-0283
12. Nozue M, Shimazu T, Charvat H, Mori N, Mutoh M, Sawada N, et al. Fermented soy products intake and risk of cardiovascular disease and total cancer incidence: the Japan public health center-based prospective study. EurJ Clin Nutr. (2021) 75:954–68. doi: 10.1038/s41430-020-00732-1
13. Lu D, Pan C, Ye C, Duan H, Xu F, Yin L, et al. Meta-analysis of soy consumption and gastrointestinal cancer risk. Sci Rep. (2017) 7:4048. doi: 10.1038/s41598-017-03692-y
14. Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. (2018) 10:40. doi: 10.3390/nu10010040
15. Yang G, Shu XO, Chow WH, Zhang X, Li HL, Ji BT, et al. Soy food intake and risk of lung cancer: evidence from the Shanghai women's health study and a meta-analysis. Am J Epidemiol. (2012) 176:846–55. doi: 10.1093/aje/kws168
16. Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston Me, t al. Soy protein, isoflavones, and cardiovascular health: an American heart association science advisory for professionals from the nutrition Committee. Circulation. (2006) 113:1034–44. doi: 10.1161/CIRCULATIONAHA.106.171052
17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
18. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online at: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed September, 14 2021).
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
20. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
21. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. (2016) 353:i2716. doi: 10.1136/bmj.i2716
22. Tang J, Wan Y, Zhao M, Zhong H, Zheng JS, Feng F. Legume and soy intake and risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr. (2020) 111:677–88. doi: 10.1093/ajcn/nqz338
23. Namazi N, Saneei P, Larijani B, Esmaillzadeh A. Soy product consumption and the risk of all-cause, cardiovascular and cancer mortality: a systematic review and meta-analysis of cohort studies. Food Funct. (2018) 9:2576–88. doi: 10.1039/c7fo01622k
24. Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer : those confounded milks. Int J Epidemiol. (2020) 49:1526–37. doi: 10.1093/ije/dyaa007
25. Wei Y, Lv J, Guo Y, Bian Z, Gao M, Du H, et al. Soy intake and breast cancer risk: a prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur J Epidemiol. (2020) 35:567–78. doi: 10.1007/s10654-019-00585-4
26. Dunneram Y, Greenwood DC, Cade JE. Diet and risk of breast, endometrial and ovarian cancer: UK women's cohort study. Br J Nutr. (2019) 122:564–74. doi: 10.1017/S0007114518003665
27. Paul P, Koh WP, Jin A, Michel A, Waterboer T, Pawlita M, et al. Soy and tea intake on cervical cancer risk: the Singapore Chinese health study. Cancer Causes Control. (2019) 30:847–57. doi: 10.1007/s10552-019-01173-3
28. Reger MK, Zollinger TW, Liu Z, Jones JF, Zhang J. Dietary intake of isoflavones and coumestrol and the risk of prostate cancer in the prostate, lung, colorectal and ovarian cancer screening trial. Int J Cancer. (2018) 142:719–28. doi: 10.1002/ijc.31095
29. Baglia ML, Zheng W, Li H, Yang G, Gao J, Gao YT, et al. The association of soy food consumption with the risk of subtype of breast cancers defined by hormone receptor and HER2 status. Int J Cancer. (2016) 139:742–8. doi: 10.1002/ijc.30117
30. Hedelin M, Löf M, Sandin S, Adami HO, Weiderpass E. Prospective study of dietary phytoestrogen intake and the risk of colorectal cancer. Nutr Cancer. (2016) 68:388–95. doi: 10.1080/01635581.2016.1152380
31. Umesawa M, Iso H, Fujino Y, Kikuchi S, Tamakoshi A, JACC Study Group. Salty food preference and intake and risk of gastric cancer: the JACC Study. J Epidemiol. (2016) 26:92–7. doi: 10.2188/jea.JE20150023
32. Wada K, Tsuji M, Tamura T, Konishi K, Kawachi T, Hori A, et al. Soy isoflavone intake and stomach cancer risk in Japan: from the takayama study. Int J Cancer. (2015) 137:885–92. doi: 10.1002/ijc.29437
33. Morimoto Y, Maskarinec G, Park SY, Ettienne R, Matsuno RK, Long C, et al. Dietary isoflavone intake is not statistically significantly associated with breast cancer risk in the multiethnic cohort. Br J Nutr. (2014) 112:976–83. doi: 10.1017/S0007114514001780
34. Wang Y, Stevens VL, Shah R, Peterson JJ, Dwyer JT, Gapstur SM, et al. Dietary flavonoid and proanthocyanidin intakes and prostate cancer risk in a prospective cohort of US men. Am J Epidemiol. (2014) 179:974–86. doi: 10.1093/aje/kwu006
35. Zamora-Ros R, Sacerdote C, Ricceri F, Weiderpass E, Roswall N, Buckland G, et al. Flavonoid and lignan intake in relation to bladder cancer risk in the European prospective investigation into cancer and nutrition (EPIC) study. Br J Cancer. (2014) 111:1870–80. doi: 10.1038/bjc.2014.459
36. Kweon SS, Shu XO, Xiang Y, Cai H, Yang G, Ji BT, et al. Intake of specific nonfermented soy foodsmay be inversely associated with risk of distal gastric cancer in a Chinese population. J Nutr. (2013) 143:1736–42. doi: 10.3945/jn.113.177675
37. Ko KP, Park SK, Yang JJ, Ma SH, Gwack J, Shin A, et al. Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol. (2013) 23:337–43. doi: 10.2188/jea.je20120232
38. Wada K, Nakamura K, Tamai Y, Tsuji M, Kawachi T, Hori A, et al. Soy isoflavone intake and breast cancer risk in Japan: from the takayama study. Int J Cancer. (2013) 133:952–60. doi: 10.1002/ijc.28088
39. Olberding NJ, Lim U, Wilkens LR, Setiawan VW, Shvetsov YB, Henderson BE, et al. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst. (2012) 104:67–76. doi: 10.1093/jnci/djr475
40. Hedelin M, Löf M, Andersson TM, Adlercreutz H, Weiderpass E. Dietary phytoestrogens and the risk of ovarian cancer in the women's lifestyle and health cohort study. Cancer Epidemiol Biomarkers Prev. (2011) 20:308–17. doi: 10.1158/1055-9965.EPI-10-0752
41. Butler LM, Wu AH, Wang R, Koh WP, Yuan JM, Yu MC. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. (2010) 91:1013–9. doi: 10.3945/ajcn.2009.28572
42. Seow A, Koh WP, Wang R, Lee HP, Yu MC. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. (2009) 18:821–7. doi: 10.1158/1055-9965.EPI-08-0892
43. Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, et al. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. (2009) 89:577–83. doi: 10.3945/ajcn.2008.26742
44. Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer. (2008) 99:1511–6. doi: 10.1038/sj.bjc.6604678
45. Cutler GJ, Nettleton JA, Ross JA, Harnack LJ, Jacobs DR Jr, Scrafford CG, et al. Dietary flavonoid intake and risk of cancer in postmenopausal women: the Iowa women's health study. Int J Cancer. (2008) 123:664–71. doi: 10.1002/ijc.23564
46. Hedelin M, Löf M, Olsson M, Adlercreutz H, Sandin S, Weiderpass E. Dietary phytoestrogens are not associated with risk of overall breast cancer but diets rich in coumestrol are inversely associated with risk of estrogen receptor and progesterone receptor negative breast tumors in Swedish women. J Nutr. (2008) 138:938–45. doi: 10.1093/jn/138.5.938
47. Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN, Multiethnic Cohort Study. Legume and isoflavone intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer. (2008) 123:927–32. doi: 10.1002/ijc.23594
48. Travis RC, Allen NE, Appleby PN, Spencer EA, Roddam AW, Key TJ. A prospective study of vegetarianism and isoflavone intake in relation to breast cancer risk in British women. Int J Cancer. (2008) 122:705–10. doi: 10.1002/ijc.23141
49. Chang ET, Lee VS, Canchola AJ, Clarke CA, Purdie DM, Reynolds P, et al. Diet and risk of ovarian cancer in the California teachers study cohort. Am J Epidemiol. (2007) 165:802–13. doi: 10.1093/aje/kwk065
50. Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, et al. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. (2007) 99:1200–9. doi: 10.1093/jnci/djm065
51. Nishio K, Niwa Y, Toyoshima H, Tamakoshi K, Kondo T, Yatsuya H, et al. Consumption of soy foods and the risk of breast cancer: findings from the Japan collaborative cohort (JACC) study. Cancer Causes Control. (2007) 18:801–8. doi: 10.1007/s10552-007-9023-7
52. Oba S, Nagata C, Shimizu N, Shimizu H, Kametani M, Takeyama N, et al. Soy product consumption and the risk of colon cancer: a prospective study in Takayama, Japan. Nutr Cancer. (2007) 57:151–7. doi: 10.1080/0163558070127447
53. Touillaud MS, Thiébaut AC, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F. No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiol Biomarkers Prev. (2006) 15:2574–6.
54. Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan). Cancer Causes Control. (2005) 16:773–80. doi: 10.1007/s10552-005-5385-x
55. Allen NE, Sauvaget C, Roddam AW, Appleby P, Nagano J, Suzuki G, et al. A prospective study of diet and prostate cancer in Japanese men. Cancer Causes Control. (2004) 15:911–20. doi: 10.1007/s10552-004-1683-y
56. Keinan-Boker L, van Der Schouw YT, Grobbee DE, Peeters PH. Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr. (2004) 79:282–8. doi: 10.1093/ajcn/79.2.282
57. Nomura AM, Hankin JH, Lee J, Stemmermann GN. Cohort study of tofu intake and prostate cancer: no apparent association. Cancer Epidemiol Biomarkers Prev. (2004) 13:2277–9.
58. Sun CL, Yuan JM, Wang XL, Gao YT, Ross RK, Yu MC. Dietary soy and increased risk of bladder cancer: a prospective cohort study of men in Shanghai, China. Int J Cancer. (2004) 112:319–23. doi: 10.1002/ijc.20384
59. Horn-Ross PL, Hoggatt KJ, West DW, Krone MR, Stewart SL, Anton H, et al. Recent diet and breast cancer risk: the California teachers study (USA). Cancer Causes Control. (2002) 13:407–15. doi: 10.1023/a:1015786030864
60. Sun CL, Yuan JM, Arakawa K, Low SH, Lee HP, Yu MC. Dietary soy and increased risk of bladder cancer: the Singapore Chinese health study. Cancer Epidemiol Biomarkers Prev. (2002) 11:1674–7.
61. Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. (1999) 81:1248–56. doi: 10.1038/sj.bjc.669083
62. Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. (1998) 27:173–80. doi: 10.1093/ije/27.2.173
63. Jacobsen BK, Knutsen SF, Fraser GE. Does high soy milk intake reduce prostate cancer incidence? The adventist health study (United States). Cancer Causes Control. (1998) 9:553–7. doi: 10.1023/a:1008819500080
64. Inoue M, Tajima K, Kobayashi S, Suzuki T, Matsuura A, Nakamura T, et al. Protective factor against progression from atrophic gastritis to gastric cancer-data from a cohort study in Japan. Int J Cancer. (1996) 66:309–14. doi: 10.1002/(SICI)1097-0215(19960503)66:3<309::AID-IJC7>3.0.CO;2-2
65. Nomura A, Grove JS, Stemmermann GN, Severson RK. A prospective study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption. Cancer Res. (1990) 50:627–31.
66. Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. (1989) 49:1857–60.
67. Shirabe R, Saito E, Sawada N, Ishihara J, Takachi R, Abe SK, et al. Fermented and nonfermented soy foods and the risk of breast cancer in a Japanese population-based cohort study. Cancer Med. (2021) 10:757–71. doi: 10.1002/cam4.3677
68. Hara A, Sasazuki S, Inoue M, Iwasaki M, Shimazu T, Sawada N, et al. Isoflavone intake and risk of gastric cancer: a population-based prospective cohort study in Japan. Am J Clin Nutr. (2012) 95:147–54. doi: 10.3945/ajcn.111.020479
69. Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S, et al. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. (2007) 16:538–45. doi: 10.1158/1055-9965.EPI-06-0517
70. Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S, et al. Dietary soy and isoflavone intake and risk of colorectal cancer in the Japan public health center-based prospective study. Cancer Epidemiol Biomarkers Prev. (2008) 17:2128–35. doi: 10.1158/1055-9965.EPI-08-0182
71. Shimazu T, Inoue M, Sasazuki S, Iwasaki M, Sawada N, Yamaji T, et al. Isoflavone intake and risk of lung cancer: a prospective cohort study in Japan. Am J Clin Nutr. (2010) 91:722–8. doi: 10.3945/ajcn.2009.28161
72. Budhathoki S, Iwasaki M, Sawada N, Yamaji T, Shimazu T, Sasazuki S, et al. Soy food and isoflavone intake and endometrial cancer risk: the Japan public health center-based prospective study. BJOG. (2015) 122:304–11. doi: 10.1111/1471-0528
73. Yamagiwa Y, Sawada N, Shimazu T, Yamaji T, Goto A, Takachi R, et al. Soy food intake and pancreatic cancer risk: the Japan public health center-based prospective study. Cancer Epidemiol Biomarkers Prev. (2020) 29:1214–21. doi: 10.1158/1055-9965.EPI-19-1254
74. Abe SK, Sawada N, Ishihara J, Takachi R, Mori N, Yamaji T, et al. Comparison between the impact of fermented and unfermented soy intake on the risk of liver cancer: the JPHC study. Eur J Nutr. (2021) 60:1389–401. doi: 10.1007/s00394-020-02335-9
75. Katagiri R, Sawada N, Goto A, Yamaji T, Iwasaki M, Noda M, et al. Association of soy and fermented soy product intake with total and cause specific mortality: prospective cohort study. BMJ. (2020) 368:m34. doi: 10.1136/bmj.m34
76. Sawada N, Iwasaki M, Yamaji T, Shimazu T, Inoue M, Tsugane S, et al. Soy and isoflavone consumption and subsequent risk of prostate cancer mortality: the Japan public health center-based prospective study. Int J Epidemiol. (2020) 49:1553–61. doi: 10.1093/ije/dyaa177
77. Tang WG, Shu XO, Gao J, Li HL, Zhang W, Fang J, et al. A prospective cohort study on the relationship between soy food intake and risk of total cancer mortality. Oncology. (2017) 37:350–8. doi: 10.3781/j.issn.1000-7431.2017.22.058
78. Yamasaki K, Kayaba K, Ishikawa S. Soy and soy products intake, all-cause mortality, and cause-specific mortality in Japan: the Jichi medical school cohort study. Asia Pac J Public Health. (2015) 27:531–41. doi: 10.1177/1010539514539545
79. Zamora-Ros R, Jiménez C, Cleries R, Agudo A, Sánchez MJ, Sánchez-Cantalejo E, et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology. (2013) 24:726–33. doi: 10.1097/EDE.0b013e31829d5902
80. Iso H, Kubota Y, JACC Study Group. Nutrition and disease in the Japan collaborative cohort study for evaluation of cancer (JACC). Asian Pac J Cancer Prev. (2007) 8:S35–80.
81. Sakauchi F, Khan MM, Mori M, Kubo T, Fujino Y, Suzuki S, et al. Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan. Nutr Cancer. (2007) 57:138–45. doi: 10.1080/01635580701274178
82. Kurosawa M, Kikuchi S, Xu J, Inaba Y. Highly salted food and mountain herbs elevate the risk for stomach cancer death in a rural area of Japan. J Gastroenterol Hepatol. (2006) 21:1681–6. doi: 10.1111/j.1440-1746.2006.04290.x
83. Tokui N, Yoshimura T, Fujino Y, Mizoue T, Hoshiyama Y, Yatsuya H, et al. Dietary habits and stomach cancer risk in the JACC study. J Epidemiol. (2005) 15:S98–108. doi: 10.2188/jea.15.s98
84. Khan MM, Goto R, Kobayashi K, Suzumura S, Nagata Y, Sonoda T, et al. Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev. (2004) 5:58–65.
85. Kurozawa Y, Ogimoto I, Shibata A, Nose T, Yoshimura T, Suzuki H, et al. Dietary habits and risk of death due to hepatocellular carcinoma in a large scale cohort study in Japan. Univariate analysis of JACC study data. Kurume Med J. (2004) 51:141–9. doi: 10.2739/kurumemedj.51.141
86. Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. (2002) 87:37–42. doi: 10.1038/sj.bjc.6600415
87. Nagata C, Takatsuka N, Shimizu H. Soy and fish oil intake and mortality in a Japanese community. Am J Epidemiol. (2002) 156:824–31. doi: 10.1093/aje/kwf118
88. Nagata C, Takatsuka N, Kawakami N, Shimizu H. A prospective cohort study of soy product intake and stomach cancer death. Br J Cancer. (2002) 87:31–6. doi: 10.1038/sj.bjc.6600349
89. Ozasa K, Watanabe Y, Ito Y, Suzuki K, Tamakoshi A, Seki N, et al. Dietary habits and risk of lung cancer death in a large-scale cohort study (JACC Study) in Japan by sex and smoking habit. JPN J Cancer Res. (2001) 92:1259–69. doi: 10.1111/j.1349-7006.2001.tb02148.x
90. Kato I, Tominaga S, Matsumoto K. A prospective study of stomach cancer among a rural Japanese population: a 6-year survey. JPN J Cancer Res. (1992) 83:568–75. doi: 10.1111/j.1349-7006.1992.tb00127.x
91. Ho SC, Yeo W, Goggins W, Kwok C, Cheng A, Chong M, et al. Pre-diagnosis and early post-diagnosis dietary soy isoflavone intake and survival outcomes: a prospective cohort study of early stage breast cancer survivors. Cancer Treat Res Commun. (2021) 27:100350. doi: 10.1016/j.ctarc.2021.100350
92. Minami Y, Kanemura S, Oikawa T, Suzuki S, Hasegawa Y, Nishino Y, et al. Associations of Japanese food intake with survival of stomach and colorectal cancer: a prospective patient cohort study. Cancer Sci. (2020)111:2558–69. doi: 10.1111/cas.14459
93. Leo QJ, Ollberding NJ, Wilkens LR, Kolonel LN, Henderson BE, Le Marchand L, et al. Nutritional factors and non-Hodgkin lymphoma survival in an ethnically diverse population: the multiethnic cohort. Eur J Clin Nutr. (2016) 70:41–6. doi: 10.1038/ejcn.2015.139
94. Kyrø C, Zamora-Ros R, Scalbert A, Tjønneland A, Dossus L, Johansen C, et al. Pre-diagnostic polyphenol intake and breast cancer survival: the European prospective investigation into cancer and nutrition (EPIC) cohort. Breast Cancer Res Treat. (2015) 154:389–401. doi: 10.1007/s10549-015-3595-9
95. Conroy SM, Maskarinec G, Park SY, Wilkens LR, Henderson BE, Kolonel LN. The effects of soy consumption before diagnosis on breast cancer survival: the multiethnic cohort study. Nutr Cancer. (2013) 65:527–37. doi: 10.1080/01635581.2013.776694
96. Nechuta SJ, Caan BJ, Chen WY, Lu W, Chen Z, Kwan ML, et al. Soy food intake after diagnosis of breast cancer and survival: an in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am J Clin Nutr. (2012) 96:123–32. doi: 10.3945/ajcn.112.035972
97. Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Gaudet MM, et al. Dietary flavonoid intake and breast cancer survival among women on long island. Cancer Epidemiol Biomarkers Prev. (2007) 16:2285–92. doi: 10.1158/1055-9965.EPI-07-0245
98. Zhai X, Lin M, Zhang F, Hu Y, Xu X, Li Y, et al. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol Nutr Food Res. (2013) 57:249–59. doi: 10.1002/mnfr.201200536
99. Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. (2002) 21:265–80. doi: 10.1023/a:1021210910821
100. Sekar V, Anandasadagopan SK, Ganapasam S. Genistein regulates tumor microenvironment and exhibits anticancer effect in dimethyl hydrazine-induced experimental colon carcinogenesis. Biofactors. (2016) 42:623–37. doi: 10.1002/biof.1298
101. Appelt LC, Reicks MM. Soy induces phase II enzymes but does not inhibit dimethylbenz[a]anthracene-induced carcinogenesis in female rats. J Nutr. (1999) 129:1820–6. doi: 10.1093/jn/129.10.1820
102. Jin S, Zhang QY, Kang XM, Wang JX, Zhao WH. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann Oncol. (2010) 21:263–8. doi: 10.1093/annonc/mdp499
103. Takahashi Y, Lavigne JA, Hursting SD, Chandramouli GV, Perkins SN, Kim YS, et al. Molecular signatures of soy-derived phytochemicals in androgen-responsive prostate cancer cells: a comparison study using DNA microarray. Mol Carcinog. (2006) 45:943–56. doi: 10.1002/mc.20247
104. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. (2006) 4:221–33. doi: 10.1158/1541-7786.MCR-05-0261
105. Fang HY, Greten FR. Cell autonomous and non-autonomous functions of IKKβ and NF-κB during the pathogenesis of gastrointestinal tumors. Cancers (Basel). (2011) 3:2214–22. doi: 10.3390/cancers3022214
106. Kim E, Kang YG, Kim JH, Kim YJ, Lee TR, Lee J, et al. The antioxidant and anti-inflammatory activities of 8-Hydroxydaidzein (8-HD) in activated Macrophage-Like RAW264.7 Cells. Int J Mol Sci. (2018) 19:1828. doi: 10.3390/ijms19071828
107. Lee SR, Kwon SW, Lee YH, Kaya P, Kim JM, Ahn C, et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer. (2019) 19:6. doi: 10.1186/s12885-018-5222-8
108. Lee SR, Kwon SW, Lee YH, Kaya P, Kim JM, Ahn C, et al. Barbigerone, an isoflavone, inhibits tumor angiogenesis and human non-small-cell lung cancer xenografts growth through VEGFR2 signaling pathways. Cancer Chemother Pharmacol. (2012) 70:425–37. doi: 10.1007/s00280-012-1923-x
109. Yu X, Zhu J, Mi M, Chen W, Pan Q, Wei M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med Oncol. (2012) 29:349–57. doi: 10.1007/s12032-010-9770-2
110. St-Hilaire S, Mannel S, Commendador A, Mandal R, Derryberry D. Correlations between meteorological parameters and prostate cancer. Int J Health Geogr. (2010) 9:19. doi: 10.1186/1476-072X-9-19
111. Hoq M, Ali M, Islam A, Banerjee C. Risk factors of acute malnutrition among children aged 6-59 months enrolled in a community-based programme in Kurigram, Bangladesh: a mixed-method matched case-control study. J Health Popul Nutr. (2019) 38:36. doi: 10.1186/s41043-019-0192-2
112. Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. JPN J Clin Oncol. (2004) 34:86–9. doi: 10.1093/jjco/hyh015
113. Wong CS, Chen TT, Chang WP, Wong HS, Wu MY, Adikusuma W, et al. Prognostic effect of comorbid disease and immune gene expression on mortality in kidney cancer-a population-based study. Cancers. (2020) 12:1654. doi: 10.3390/cancers12061654
114. Lane BR, Abouassaly R, Gao T, Weight CJ, Hernandez AV, Larson BT, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer. (2010) 116:3119–26. doi: 10.1002/cncr.25184
115. Micek A, Godos J, Brzostek T, Gniadek A, Favari C, Mena P, et al. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: a comprehensive systematic review with meta-analysis. Nutr Rev. (2021) 79:42–65. doi: 10.1093/nutrit/nuaa043
Keywords: soy, soy isoflavones, soy protein, cancer, meta-analysis
Citation: Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, Liu S, Zhao J, Kong L, Zhang W and Ma L (2022) Intake of Soy, Soy Isoflavones and Soy Protein and Risk of Cancer Incidence and Mortality. Front. Nutr. 9:847421. doi: 10.3389/fnut.2022.847421
Received: 02 January 2022; Accepted: 09 February 2022;
Published: 04 March 2022.
Edited by:
Justyna Godos, University of Catania, ItalyReviewed by:
Agnieszka Micek, Jagiellonian University, PolandCopyright © 2022 Fan, Wang, Li, Jiang, Shi, Shi, Liu, Zhao, Kong, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Ma, bWFsZUBtYWlsLnhqdHUuZWR1LmNu; Wei Zhang, emhhbmd3ZWkxNDA0MDhAMTYzLmNvbQ==; Liyun Kong, a29uZ2x5QHhqdHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.