
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 18 July 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.825897
 Tingting Liang1,2,3†
Tingting Liang1,2,3† Xinqiang Xie2†
Xinqiang Xie2† Lei Wu1,2†
Lei Wu1,2† Longyan Li2
Longyan Li2 Lingshuang Yang2
Lingshuang Yang2 He Gao2
He Gao2 Zhenshan Deng3
Zhenshan Deng3 Xiangqian Zhang3
Xiangqian Zhang3 Xuefeng Chen1
Xuefeng Chen1 Jumei Zhang2
Jumei Zhang2 Yu Ding4*
Yu Ding4* Qingping Wu2*
Qingping Wu2*The aim of this systematic review and meta-analysis was to evaluate the effects of probiotics and glucose-lowering drugs (thiazolidinedione [TZD], glucagon-like pep-tide-1 receptor agonists [GLP-1 RA], dipeptidyl peptidase IV inhibitors, and sodium glucose co-transporter 2 inhibitors [SGLT-2i]) in patients with type 2 diabetes from randomized con-trolled trials (RCTs). The PubMed, Web of science, Embase, and Cochrane Library databases were searched on the treatment effects of probiotics and glucose-lowering drugs on glycemia, lipids, and blood pressure metabolism published between Jan 2015 and April 2021. We performed meta-analyses using the random-effects model. We included 25 RCTs (2,843 participants). Overall, GLP-1RA, SGLT-2i, and TZD significantly reduce fasting blood sugar (FBS) and glycated hemoglobin (HbA1c), whereas GLP-1 RA increased the risk of hypoglycaemia. Multispecies probiotics decrease FBS, total cholesterol (TC), and systolic and diastolic blood pressure (SBP, DBP). Moreover, subgroup analyses indicated that participants aged >55 years, BMI ≥30 kg/m2, longer duration of intervention, and subjects from Eastern countries, showed significantly higher reduction in FBS and HbA1c, TC, TG and SBP. This meta-analysis revealed that including multiple probiotic rather than glucose-lowering drugs might be more beneficial regarding T2D prevention who suffering from simultaneously hyperglycemia, hypercholesterolemia, and hypertension.
According to the data released by the International Diabetes Federation in 2017, 425 million people (8.8%) (age 20–79 years) have type 2 diabetes worldwide, whereas 114.4 million people (10.9%) in China have the disease (1). Type 2 diabetes most often accompanied with hyperglycaemia, hypertension, and abnormal blood lipid profiles (2), which frequently occur simultaneously and affect human health (3, 4). Therefore, it is important to determine effective methods for treating these comorbid diseases simultaneously.
Accumulating evidence indicates that there is a relationship between antihyperglycemic agents (e.g., thiazolidinedione [TZD] (5), glucagon-like peptide-1 receptor agonists [GLP-1 RA] (6–8), dipeptidyl peptidase IV inhibitors [DPP-4i] (9–11), sodium glucose co-transporter 2 inhibitors [SGLT-2i] (12)) and hyperglycaemia, hyperlipidaemia, and hypertension. Although these drugs improve glycaemic control in patients with type 2 diabetes, it has been indicated that different classes of antidiabetic drugs differ in glycaemic efficacy (13), and that different glucose-lowering agents can have varying impacts on a patient's lipid profile (14). Analyses of the effects of these anti-diabetic drugs on clinical outcomes have yielded conflicting results, and the differences across these classes of drugs have not been investigated. Additionally, despite having a comparable efficacy in glucose control, these drugs differ in their tolerability profiles: sulfonylureas induce hypoglycaemia; pioglitazone is associated with weight gain, fluid retention, and bone fractures; and acarbose is associated with gastrointestinal side effects (15). Hypoglycaemia, diarrhea, and urinary tract infections have also been associated with the use of glucose-lowering drugs (16, 17). Thus, avoidance of these adverse reactions is recommended as an important therapeutic consideration when selecting treatments and individualizing treatment goals. Moreover, whether a new drug is superior to another is interesting to clinicians, as well as patients.
The results of several studies have suggested a close association between probiotic administration and hyperglycaemia, lipid abnormalities, and hypertension (18–23). The findings of a previous study indicated that supplementation of multispecies probiotics can regulate glycaemic and lipid indicators (fasting blood sugar [FBS] and high-density lipoprotein cholesterol [HDL-C]); however, the authors noted no significant changes in other indices (24). Another study showed that there were significant reductions in FBS, insulin, and the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) index after intake of a single species probiotic (lactobacillus casein) (25). Therefore, we speculate that different patterns of consumption of probiotics may induce different effects. In addition, considering the adverse effects of glucose-lowering drugs, it is necessary to determine whether probiotics can be used instead of hypoglycaemic drugs to alleviate type 2 diabetes.
Previous studies of glucose-lowering drugs and probiotics are usually based on indirect comparisons, which are usually made using the same control, such as a placebo, by comparing probiotics and placebo, hypoglycemic drugs and placebo, to further compare the efficacy of probiotics and hypoglycemic drugs. To date, no study that involves head-to-head comparisons of the effects of GLP-1RA, DPP4i, SGLT2i, and TZD on glycemia, lipid profile, and blood pressure metabolism has been conducted. Previous meta-analyses only analyzed that the effects of glucose-lowering drugs or probiotic consumption on a few indicators of blood glucose, blood pressure and lipid profiles, sucn as only blood glucose, or blood pressure, or lipid. However, blood glucose indexes [FBS or glycated hemoglobin (HbA1c), insulin, and HMOA-IR), blood lipid indexes (total cholesterol (TC), triglycerides (TG), HDL-C, low-density lipoprotein cholesterol (LDL-C)], and blood pressure indexes [systolic blood pressure (SBP), and diastolic blood pressure (DBP)], were analyzed simultaneously in this study (26, 27). In addition, some of the parameters of the studies, including the classes of the glucose-lowering drugs and probiotics treatment patterns, and subject characteristics (patients' ages, BMI, country [influences diet and genetics], disease duration, and duration of intervention) varied; thus, the analyses yielded inconsistent results. To the best of our knowledge, there has been no comparative study of the efficacies of probiotics supplementation and glucose-lowering drugs for the treatment of type 2 diabetes.
Hence, the aim of this systematic review and meta-analysis was to evaluate and compare the effects of probiotics and glucose-lowering agents, including TZD, GLP-1RA, DPP4i, SGLT2i, on glycemia, lipid profile, and blood pressure in patients with type 2 diabetes. Furthermore, we conducted subgroup analyses to explore the associations between treatment effects and study characteristics, such as treatment patterns, patients' ages, BMI, country, and duration of intervention, to determine whether probiotics can be used instead of hypoglycaemic drugs for the treatment of type 2 diabetes.
The meta-analysis was conducted based on the Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) guidelines (28). Three independent investigators (T.T.L, X.Q.X, and L.W) searched the PubMed, Embase, Web of Science, and Cochrane Library databases for relevant literatures published between Jan 2015 and April 2021. First of all, investigators conduct a preliminary screening based on the title and abstract of the literature, then the literature is screened again by reading the full text. If there were differences, they can decide whether to include them through discussion. If necessary, a fourth investigator (Q.P.W) can help solve them. The main keywords used were as follows: randomized controlled trials, type 2 diabetes, probiotic, glucose-lowering drugs, blood glucose, blood lipids, and blood pressure. The search strategy was conducted using Medical Subject Heading (MeSH) terms combined with keywords and Boolean operators (e.g., AND, OR, NOT). The details of the search strategy are outlined in Supplementary Table 1.
Eligible studies were selected according to the “participants, intervention, comparison, outcome, and study design” format (Supplementary Table 2).
The inclusion criteria were as follows: (1) studies that included adult participants with type 2 diabetes; (2) studies in which the interventions were probiotic supplementation or administration of glucose-lowering drugs; (3) studies that involved comparison of probiotic supplementation or glucose-lowering drug interventions with appropriate placebos; (4) studies that reported one or more of the following outcomes: FBS, HbA1c, insulin, HOMA-IR index, TC, TG, LDL-C, HDL-C, SBP, DBP, diarrhea, hypoglycaemia, or a combination of these; (5) randomized controlled trials (RCT); (6) studies published in English.
The exclusion criteria were as follows: (1) studies that included participants with gestational diabetes, prediabetes, or type 1 diabetes; (2) studies that did not involve probiotic supplementation or glucose-lowering drug interventions; (3) studies with no placebo control group; (4) studies in which the baseline outcomes or outcome changes were not reported, or studies with incomplete information on outcomes; (5) studies that were not RCTs; (6) animal studies; (7) reviews or meeting papers; (8) non-English studies.
Data extraction was performed by two investigators (T.T.L and J.M) independently. Data extracted from each article included the following items: the name of the first author, publication year, sample size, country of study, participant characteristics (sex, age, weight, BMI), disease duration, study design, dose and kinds of placebo, use of probiotic supplementation or glucose-lowering drugs, duration of intervention, and outcome information (including the baseline and endpoint data or data regarding changes in FBS, HbA1c, insulin, HOMA-IR index, TC, TG, LDL-C, HDL-C, SBP, DBP, diarrhea, or hypoglycaemia).
The qualities of the included studies and their risks of bias were independently evaluated by two researchers using the Cochrane Handbook for Systematic Reviews of Interventions tool (29). Risk of bias was assessed in seven aspects, namely: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. These items could be scored as “low risk,” “high risk,” or “unclear.” The qualities and risks of bias of the included studies were analyzed using the Review Manager 5.3 software.
Before statistical analyses, the measurement units of outcomes must be consistent in each study. FBS levels were recorded in mg/dL, which can be converted to mmol/L to reflect glucose concentrations and back to mg/dL when necessary. Insulin levels were collated in mIU/dL, which can be converted to pmol/L and back to mIU/dL when necessary. The lipid indices (TC, TG, HDL-C, and LDL-C) were collated in mg/dL, which can be converted mmol/L and back to mg/dL as appropriate.
A meta-analysis was performed using the STATA software package, version 15.1 (StataCorp, College Station, TX) to analyse the effects of probiotic supplementation vs. those of glucose-lowering drugs. To estimate the effect size of each study, the means and standard deviations (SD) of the changes in outcomes from baseline to the endpoint were calculated and compared between treatment and placebo groups. The means and SDs of the changes were estimated according to the following formula (30):
Forest plots for probiotic and glucose-lowering drug groups were constructed using the STATA software package. A random effects model was used to evaluate the pooled effect of outcomes. The I2 statistic was used to represent the heterogeneity of the included studies, which ranged from 0 to 100%; proportions > 75% were considered to have high heterogeneity (31, 32). P < 0.05 indicated statistical significance (33).
Subgroup analyses were performed to investigate whether there were any significant differences between the patterns of consumption of probiotics and the classes of glucose-lowering drugs. Meta-regression analyses were also performed to determine whether participant characteristics, including age, BMI, country, and duration of intervention, were associated with the treatment effects (34). Qualitative analysis of publication bias was performed using visual funnel plots, whereas quantitative analysis was performed using Egger's tests (35). A sensitivity analysis was conducted to evaluate whether the included studies of each study could influence the overall results of the meta-analysis. The PRISMA checklist was used as a guide for checking the quality of our meta-analysis (Supplementary Table 3).
Initially, 6,883 articles published between Jan 2015 and April 2021 were identified from the literature search. A total of 4,081 articles were excluded after reading their titles, and 470 articles were retrieved after reading their abstracts and full-text articles. Finally, 25 articles that satisfied the inclusion criteria were included in the meta-analysis (Figure 1).
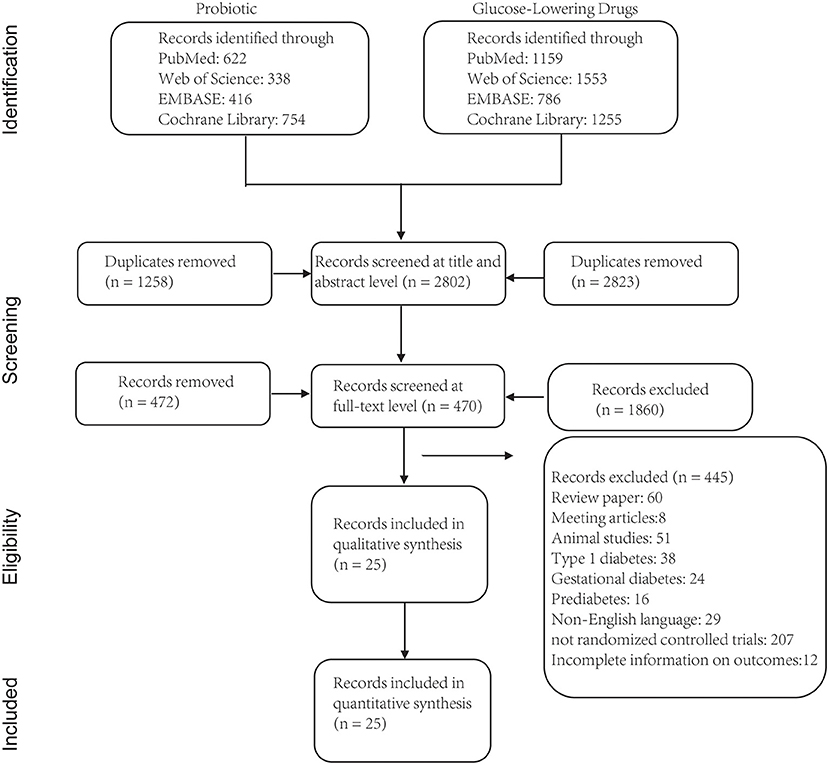
Figure 1. Flow chart depicting the literature search and selection strategy (based on PRISMA guideline).
The study and participant characteristics are summarized in Tables 1, 2. Of the 25 included studies, 14 were intervention studies (842 participants) that involved the administration of probiotics (single probiotics, multi-strain probiotics, and probiotics with co-supplements) (24, 25, 36–46, 50), whereas 11 were intervention trials (2001 participants) of glucose-lowering drugs (TZD, GLP-1 RA, SGLT-2i, and DPP-4i) (5–12, 47–49). The studies were conducted in Australia (n = 1), India (n = 2), Iran (n = 6), Ukraine (n = 1), Saudi Arabia (n = 1), Malaysia (n = 1), Brazil (n = 1), Sweden (n = 1), Denmark (n = 1), Turkey (n = 1), Netherlands (n = 2), Parkland (n = 1), Japan (n = 1), and China (n = 1); four other studies were carried out simultaneously in several countries. Regarding participants with type 2 diabetes, those included in seven of the studies were aged ≤ 55 years old, whereas those included in seven studies were aged > 55 years old. Eight studies included participants with a mean BMI ≥ 30 kg/m2, whereas 11 included those with mean BMI < 30 kg/m2. In addition, the disease duration of the participants ranged from 3 to 19 years. The duration of the probiotic interventions ranged from 6 to 12 weeks, whereas the duration of the glucose-lowering drug interventions ranged from 24 to 78 weeks.
Most of the included studies had a low risk of random sequence generation. Allocation concealment was not clearly mentioned in two of the articles. One study had a high risk of blinding of participants and personnel, whereas blinding of outcome assessment was unclear in one study. Nine studies had a high risk of incomplete outcome data, whereas selective reporting and other risks were unclear in several studies. The overall quality assessment of the included studies is shown in Figure 2.
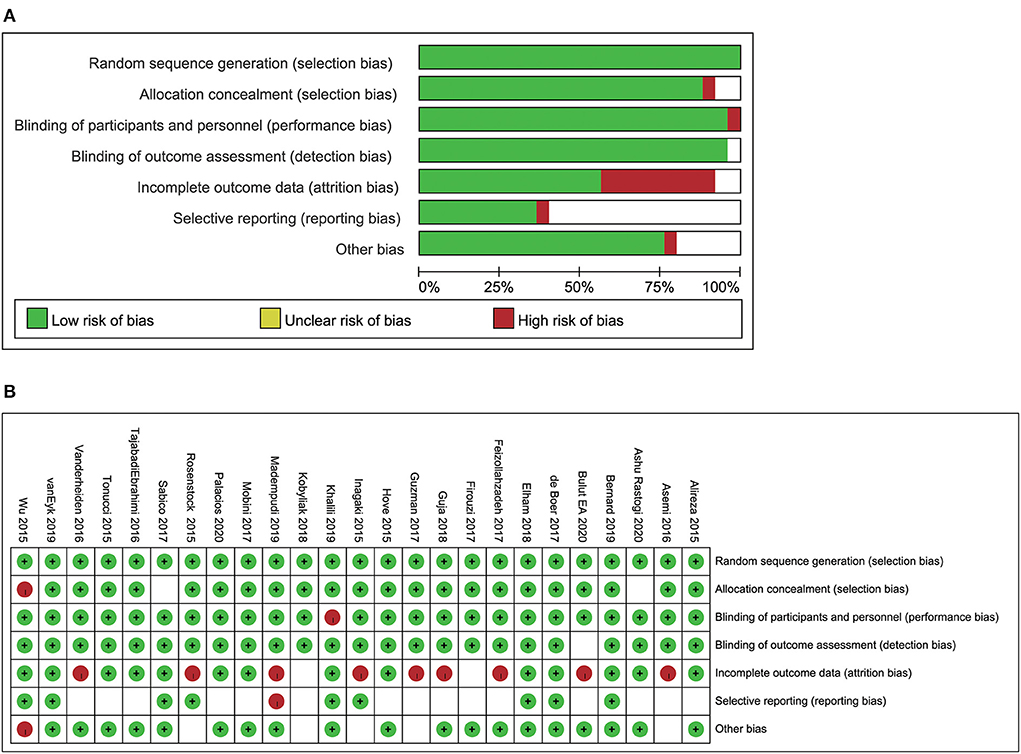
Figure 2. Risk of bias analysis (A) the analysis of the individual studies included in the systermatic review and meta-analysis. (B) The summary of the risk of bias analysis.
FBS data were included 13 probiotics intervention studies and eight glucose-lowering drug intervention studies. Overall, the probiotic groups showed significant reduction in FBS compared with the placebo groups (standardized mean difference (SMD): −0.87 mg/dL; 95% CI: −1.42, −0.32 mg/dL; I2 = 92.5%, p=0.000). However, the glucose-lowering drug groups showed more significant decrease in FBS compared with the placebo groups (SMD: −2.73 mg/dL; 95% CI: −4.22, −1.24 mg/dL; I2 = 99.3%, p = 0.000) (Figure 3A). The test for subgroup differences showed that single species probiotics and multispecies probiotics significantly lowered FBS. In addition, we observed a significant reduction in FBS in groups treated with TZD, GLP-1 RA, and SGLT-2i; however, there was no significant difference between the DPP-4i and placebo groups.
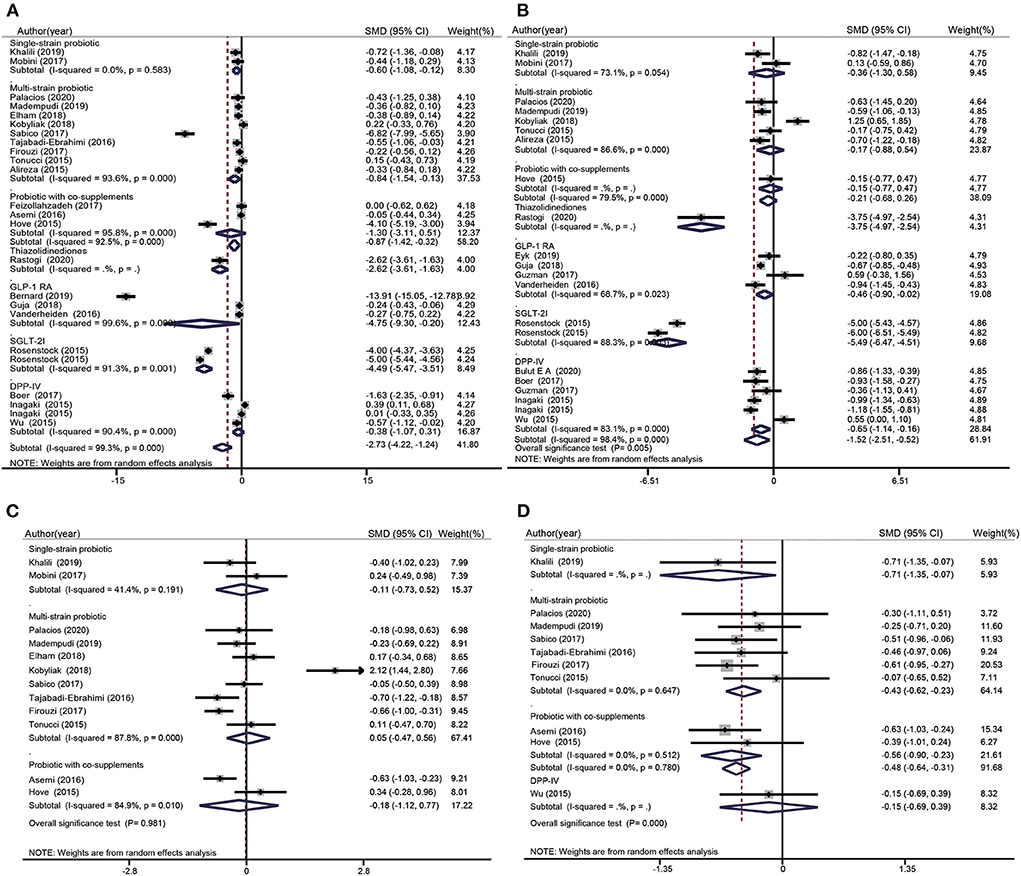
Figure 3. Forest plots for the effect of probiotics supplementation and glucose-lowering drugs on FBS (mg/dL) (A), HbA1c (%) (B), Insulin (mU/mL) (C), and HOMA-IR (D), compared to placebo in pooled analysis. For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
HbA1c data were included in seven probiotics intervention studies and in 11 glucose-lowering drug intervention studies (Figure 3B). The overall effect of probiotic supplementation on HbA1c was not significant (SMD: −0.21%; 95% CI: −0.68%, 0.26%; I2 = 79.5%, p = 0.000). There was a significant decrease in HbA1c levels in the glucose-lowering drug groups compared with the control groups (SMD: −1.52%; 95% CI: −2.51%, −0.52%, I2= 98.4%, p = 0.000). The test for subgroup differences showed that supplementation with single species probiotics, multispecies probiotics, and probiotics with co-supplements had no significant effects on HbA1c, whereas participants treated with TZD, GLP-1 RA, SGLT-2i, and DPP-4i showed significant reduction in HbA1c levels compared with the placebo groups. This reduction was particularly notable in the comparison of SGLT-2i groups and placebo groups (−5.49%; 95% CI: −6.47%, −4.51%; I2 = 88.3%, p = 0.003).
Insulin data were included in 10 probiotics intervention studies and none of the glucose-lowering drug intervention studies. However, the data in the 10 studies only referred to the effect of probiotic supplementation on insulin (Figure 3C). Overall, the difference between the probiotic and placebo groups were not significant (SMD: −0.02 mU/L; 95% CI: −0.39, 0.36 mU/L; I2 = 83.6%, p = 0.000). The test for subgroup differences showed that there was no significant reduction in insulin after supplementation with single species probiotics, multispecies probiotics, and probiotics with co-supplements.
HOMA-IR data were pooled from seven probiotics intervention studies and one glucose-lowering drug intervention study (Figure 3D). Overall, probiotic supplementation significantly decreased the HOMA-IR index compared with the placebo (SMD: −0.48; 95% CI: −0.64, −0.31; I2= 0.0%; p = 0.780), regardless of whether the probiotic used was single species, multispecies, or used with a co-supplement. Nevertheless, participants treated with glucose-lowering drugs (DPP-4i) showed no significant difference in the HOMA-IR index compared with control groups (SMD: −0.15; 95% CI: −0.69, 0.39).
TC data were included in nine probiotics intervention studies and in six glucose-lowering drug intervention studies (Figure 4A). Overall, there was no significant difference between the TC levels of participants treated with probiotics (SMD: −0.14 mg/dL; 95% CI: −0.29, 0.01 mg/dL; I2 = 0.0%; p = 0.941) or glucose-lowering drugs (SMD: −1.36 mg/dL; 95% CI: −3.24, 0.52 mg/dL; I2 = 99.0%; p = 0.000) and those of the placebo groups. The test for subgroup differences showed that multispecies probiotics significantly reduced TC levels (SMD: −0.19 mg/dL; 95% CI: −0.36, −0.01 mg/dL; I2= 0.00%; p = 0.871), whereas other treatment methods (single species probiotics, probiotics with co-supplements, TZD, GLP-1 RA, and DPP-4i) did not significantly decrease TC levels.
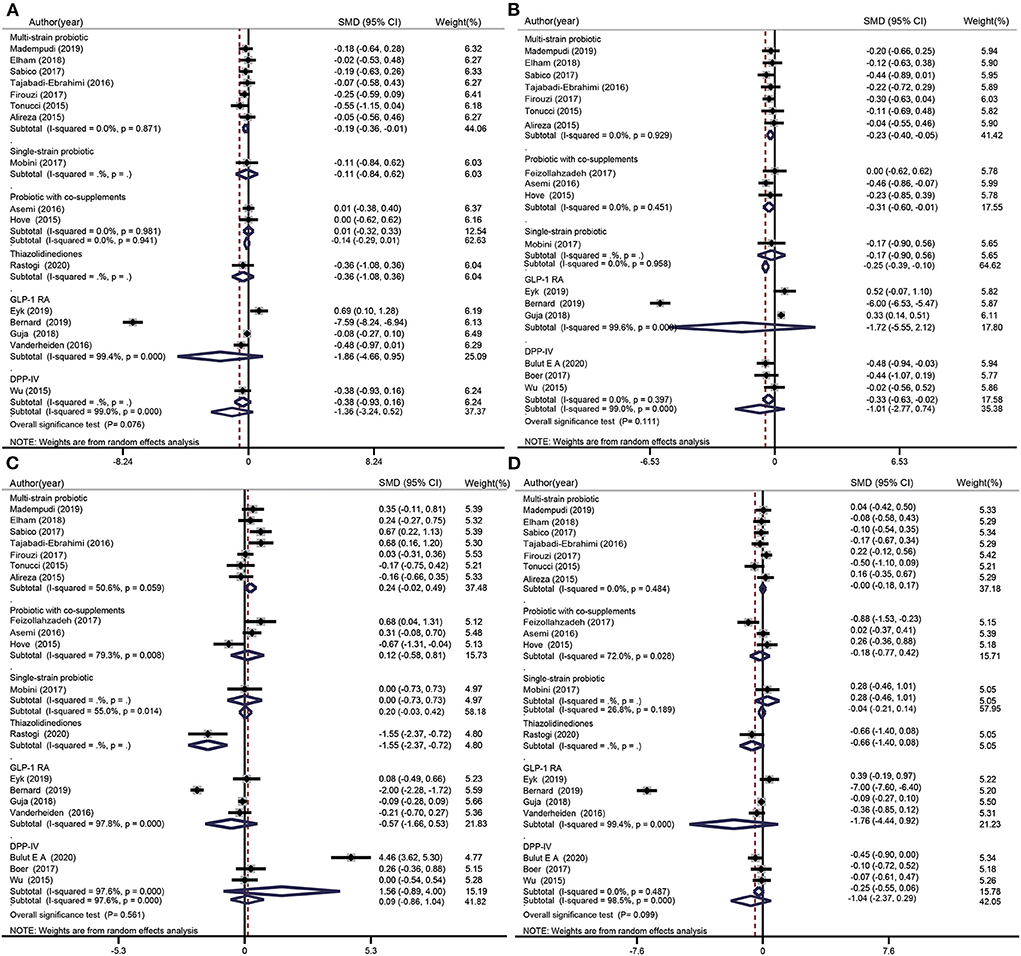
Figure 4. Forest plots for the effect of probiotics supplementation and glucose-lowering drugs on TC (mg/dL) (A), TG (mg/dL) (B), HDL-C (mg/dL) (C), and LDL-C(mg/dL) (D) compared to placebo in pooled analysis. For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
TG data were included in 11 probiotics intervention studies and six glucose-lowering drug intervention studies (Figure 4B). Overall, the difference between the TG levels of the probiotics and placebo groups was −0.25 mg/dL (95% CI: −0.39, −0.10 mg/dL; I2 = 0.0%, p = 0.958). However, there was no significant difference in TG level after treatment with glucose-lowering drugs (SMD: −1.01 mg/dL; 95% CI: −2.77, 0.74 mg/dL, I2 = 99.0%, p = 0.000). The test for subgroup differences showed a significant reduction in TG level when multispecies probiotics, probiotics with co-supplements, or DPP-4i were used, whereas single species probiotics and GLP-1 RA had no effect on TG levels.
HDL-C data were included in 11 probiotic intervention studies and in eight glucose-lowering drug intervention studies (Figure 4C). Overall, there was no significant increase in HDL-C after use of probiotics (SMD: 0.20 mg/dL; 95% CI: −0.03, 0.42 mg/dL; I2 = 55.0%, p = 0.014) or glucose-lowering drugs (SMD: 0.09 mg/dL; 95% CI: −0.86, 1.04 mg/dL; I2 = 97.6%, p = 0.000). The test for subgroup differences showed a significant decrease in HDL-C when TZDs were used (SMD: −1.55 mg/dL; 95% CI: −2.37, −0.72 mg/dL), whereas other treatment methods (single species probiotics, multispecies probiotics, probiotics with co-supplements, GLP-1 RA, and DPP-4i) induced no significant difference in HDL-C levels compared with the placebo.
LDL-C data were included in 11 probiotic intervention studies and in eight glucose-lowering drug intervention studies (Figure 4D). Overall, no significant changes in LDL-C were observed when probiotics (SMD: −0.04 mg/dL; 95% CI: −0.21, 0.14 mg/dL; I2 = 26.8%, p = 0.189) or glucose-lowering drugs were used (SMD: −1.04 mg/dL; 95% CI: −2.37, 0.29 mg/dL; I2 = 98.5%; p = 0.000). The test for subgroup differences showed no significant difference between the treatment groups (single species probiotics, multiple species probiotics, probiotics with co-supplements, TZD, GLP-1 RA, and DPP-4i) and control groups.
SBP data were included in five probiotic intervention studies and in five glucose-lowering drug intervention studies (Figure 5A). Overall, the SBP of the probiotics group was significantly lower than that of the control group (SMD: −3.26 mmHg; 95% CI: −6.44, −0.08 mmHg; I2 = 23.6%, p = 0.044). However, glucose-lowering drugs did not induce any significant change in SBP (SMD: −1.23 mmHg; 95% CI: −2.96, 0.49 mmHg; I2 = 98.9%, p = 0.000). The test for subgroup differences showed that multispecies probiotics significantly decreased SBP (SMD: −5.61 mmHg; 95% CI: −9.78, −1.45 mmHg; I2 = 0.00%, p = 0.421). Nevertheless, there were no significant differences between the other treatment groups (single species probiotics, probiotics with co-supplements, GLP-1 RA, and DPP-4i) and the control groups.
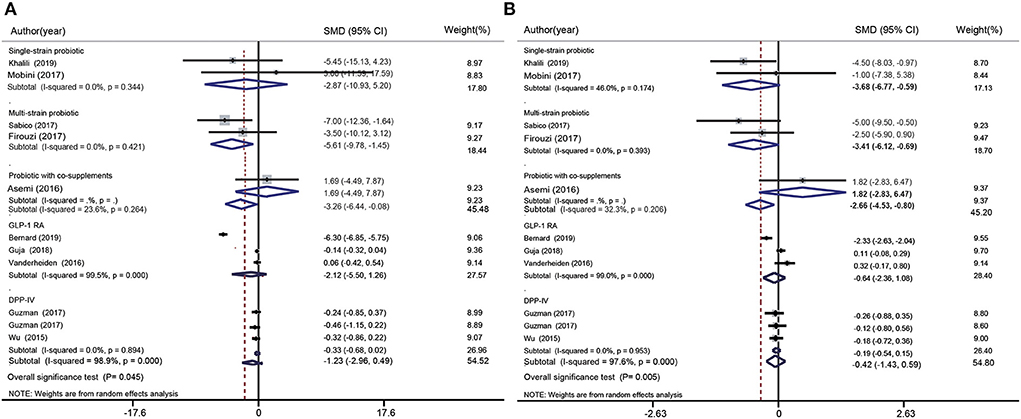
Figure 5. Forest plot for the effect of probiotics supplementation and glucose-lowering drugs on SBP (mmHg) (A) and DBP (mmHg) (B) compared to placebo. For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
DBP data was included in five probiotic intervention studies and in five glucose-lowering drug intervention studies (Figure 5B). Overall, probiotics significantly reduced DBP (SMD: −2.66 mmHg; 95% CI: −4.53, −0.80 mmHg; I2 = 32.3%, p = 0.206). However, no significant changes were observed when glucose-lowering drugs were used (GLP-1 RA and DPP-4i). The test for subgroup differences showed that single species probiotics (SMD: −3.68 mmHg; 95% CI: −6.77, −0.59 mmHg; I2 = 46.0%, p = 0.174) and multispecies probiotics (SMD: −3.41 mmHg; 95% CI: −6.12, −0.69 mmHg; I2 = 0.0%, p = 0.393) significantly reduced DBP.
The effect of glucose-lowering drugs on diarrhea was reported in one article (Supplementary Figure 1A). It indicated that there was no significant difference between the GLP-1 RA group (SMD: 1.81; 95% CI: 0.83, 3.94) and the control group in terms of diarrhea.
The effect of glucose-lowering drugs on hypoglycaemia was reported in two articles; one was for a GLP-1 RA intervention study and the other was for a DPP-4i intervention study (Supplementary Figure 1B). Overall, glucose-lowering drugs significantly increased hypoglycaemia (SMD: 4.70; 95% CI: 1.54, 14.36; I2 = 0.0%; p = 0.571). The test for subgroup differences also showed that GLP-1 RA significantly increased hypoglycaemia (SMD: 5.23; 95% CI: 1.56, 17.48). However, there was no significant difference hypoglycaemia after DPP-4i was used (SMD: 1.97; 95% CI: 0.08, 46.33).
The subgroup analyses of the studies included in the meta-analysis was stratified according to the four glycaemic indices and based on four specific factors, namely: age, BMI, country, and duration of intervention (Supplementary Figures 2–5). Notably, the subgroup analyses showed that the effects of probiotic supplementation and glucose-lowering drugs on FBS and HbA1c were more significantly decreased among participants aged > 55 years old or with BMI ≥ 30 kg/m2 at baseline (P = 0.000, P = 0.002, and P = 0.000, respectively). Moreover, we observed that participants of Eastern descent had more decreased HbA1c levels than their Western counterparts (P = 0.005). The effects of probiotics and glucose-lowering drugs on the HOMA-IR index were more significantly reduced among participants aged ≤ 55 years old, with BMI ≥ 30 kg/m2 at baseline, or of Eastern descent (P = 0.000, P = 0.005, and P = 0.000). Additionally, we noted that the duration of probiotic interventions was generally <12 weeks, whereas the duration of glucose-lowering drug interventions was more than 12 weeks.
The subgroup analyses showed that the effects of probiotic supplementation and glucose-lowering drugs on TC and TG levels were more significantly decreased among participants of Eastern descent (P = 0.041 and P = 0.001, respectively). Moreover, we found that participants with BMI < 30 kg/m2 at baseline had more decreased TG levels than those with BMI > 30 kg/m2 (P = 0.005). However, there was no significant difference between the HDL-C level, LDL-C level, and age, BMI, country, and duration of intervention of the participants in the probiotics and glucose-lowering drugs groups (Supplementary Figures 6–9).
The effects of probiotic and glucose-lowering drugs on SBP were more decreased in participants of Eastern descents than in those of Western descent (p = 0.030). However, there was no significant difference between the probiotics and glucose-lowering drugs groups in terms of DBP indicators, age, BMI, geographic area, and duration of intervention (Supplementary Figures 10, 11).
Funnel plots (Supplementary Figures 12, 14, 16) and Egger's test (quantitative) (Supplementary Figures 13, 15, 17) were used to assess the publication biases of the included studies. There was a significant difference between the publication biases for insulin in probiotic intervention studies and glucose-lowering drug intervention studies (P = 0.039). However, there were no significant differences in the publication biases for FBS (P = 0.075), HbA1c (P = 0.991), and the HOMA-IR index (P = 0.160) in the two types of studies. The probiotics and glucose-lowering drug intervention studies for the control of blood lipids and blood pressure showed no significant publication biases for TC, TG, HDL-C, LDL-C, SDP, and DBP (P = 0.325, P = 0.227, P = 0.136, P = 0.348, P = 0.414 and P = 0.919, respectively).
Meta-regression analysis was performed to assess the sources of heterogeneity in the studies. Univariate meta-regression analysis showed that participants' age, BMI at baseline, countries, and duration of intervention were not associated with change in FBS, HbA1c, insulin, HOMA-IR index, TC, TG, HDL-C, LDL-C, SBP, and DBP levels (p ≥ 0.05) (Table 3). Furthermore, sensitivity analysis was performed to detect the publication bias for insulin. We observed that no study may influence the pooled results or total effect size. Meanwhile, the results of the sensitivity analysis of FBS, HbA1c, the HOMA-IR index, TC, TG, HDL-C, LDL-C, SBP, and DBP also suggested that no study may affect the pooled results or total effect size (Supplementary Figures 18–20).
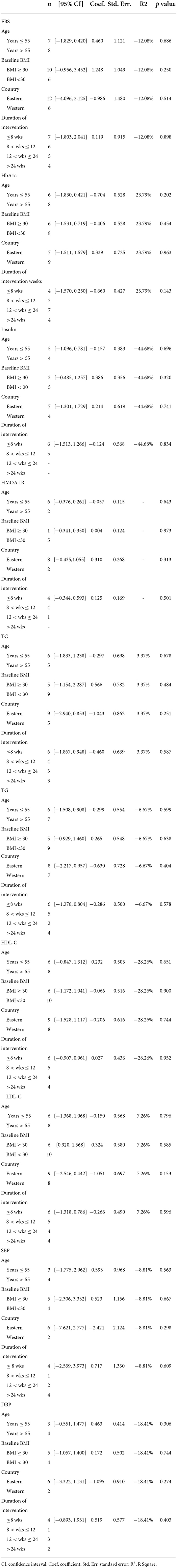
Table 3. Results of meta-regression analyses with age, BMI, country, and duration of intervention in all indexes (FBS, HbA1c, Insulin, HOMA-IR, TC, TG, HDL-C, LDL-C, SBP, and DBP) as independent variables.
In this systematic review and meta-analysis, we compared the effects of probiotic supplementation and glucose-lowering drugs on the blood glucose, lipids, and blood pressure of patients with type 2 diabetes. We found that except for DPP-4i, glucose-lowering drugs caused a significantly greater reduction in FBS than probiotics. The results also indicated that glucose-lowering drugs reduced HbA1c level more than probiotics; SGLT-2i in particular induced the greatest decrease in HbA1c. We noted that probiotic supplementation reduced the HOMA-IR index, and that multispecies probiotics were associated with reduction in TC and TG levels; however, DPP-4i only decreased TG levels. TZD was associated with decrease in HDL-C, whereas probiotic supplementation was associated with higher decrease in SBP and DBP. The results indicated that GLP-1 RA increases the risk of hypoglycaemia.
Our results showed that except for DPP-4i, glucose-lowering drugs reduce FBS better than probiotics. A previous study indicated that administration of SGLT-2i and GLP-1RA can reduce blood glucose levels (51). However, the efficacy of DPP-4i for the management of the complications of type 2 diabetes remains unclear. Previous study have reported that different metabolic organs regulate diabetic hyperglycemia differently, and glucose-lowering drugs regulate blood glucose by targeting corresponding organs (Figure 6). In the present study, GLP-1RA appeared to be the most effective glucose-lowering drug for FBS reduction, followed by SGLT-2i and TZD; however, there was no significant difference between DPP-4i and placebo. A recent meta-analysis showed that the FBS-lowering efficacy of SGLT-2i was significantly greater than those of GLP-1RA and TZD (52), a finding that is inconsistent with our results. Meanwhile, our results showed that single species probiotics and multispecies probiotics lowered FBS better than placebo and DPP-4i. Several researchers have suggested that probiotic supplementation could regulate gut microbiota, improve the patient's glycaemic, lipid, and blood pressure metabolic profiles, and play an important role in type 2 diabetes (53–55). Thus, probiotics and DPP-4i may be considered for patients with relatively less severe hyperglycaemia.
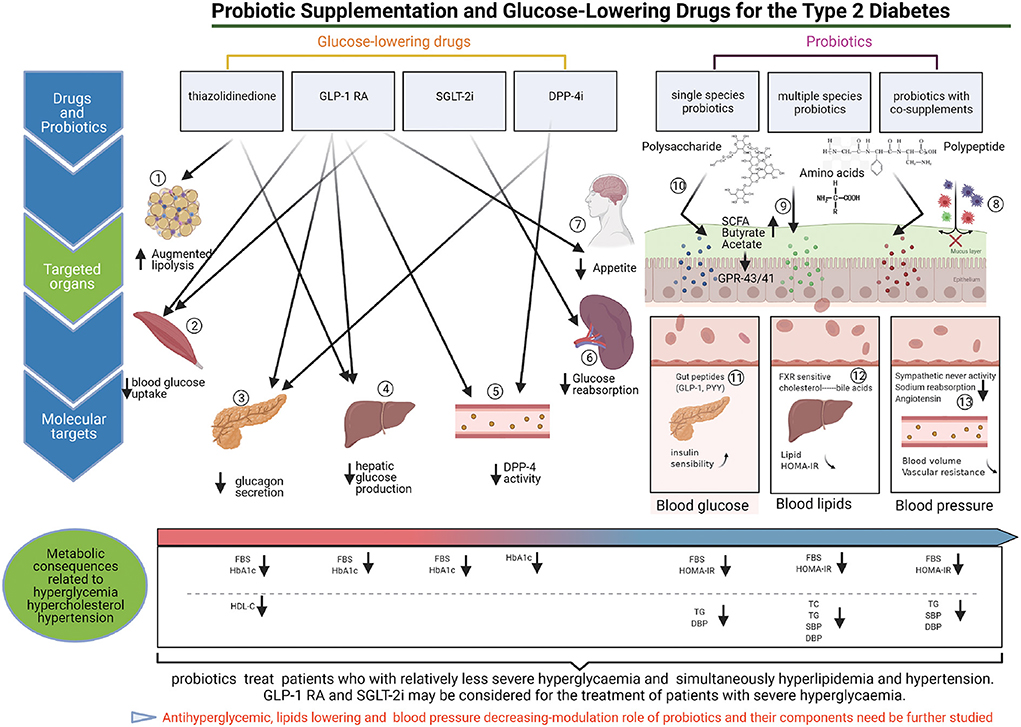
Figure 6. The targeted regulation mechanisms of probiotics and glucose-lowering drugs on antidiabetic. (1) TZDs can inhibit lipolysis in adipose tissue, and decrease hyperglycemia. (2) TZDs and GLP1-RAs can promote blood glucose uptake by the skeletal muscle contributes to reduce blood glucose. (3) GLP1-RA can promote the secretion of insulin from pancreas, meanwhile, GLP1-RA and DPP-4i can inhibit glucagon secretion by pancreas, and contribute to reduce the blood glucose. (4) TZDs, and GLP1-RA can inhibit endogenous hepatic glucose production, and reduce hyperglycemia. (5) GLP-1RA can inhibit DPP-4 enzymatic activity in circulation participates in decrease blood hyperglycemia. (6) SGLT2i (Sodium-Glucose cotransporter 2 inhibitors) blocks glucose reabsorption in kidneys, and reduce the blood glucose. (7) GLP1-RA can inhibit appetite by the brain, contribute to decrease blood glucose (8–13).
It is well known that HbA1c is the most important biomarker of hyperglycaemia (13). We found that glucose-lowering drugs significantly decreased HbA1c level. We also noted that SGLT-2i induced the highest reduction of HbA1c. There was no significant difference between probiotics and placebo regarding HbA1c reduction. Monami et al. (56) indicated that compared with placebo, SGLT-2i are more effective for controlling HbA1c, a result that confirms the findings of the present study. In addition, we found that probiotic supplementation decreased the HOMA-IR index, regardless of whether the intervention was done using single species probiotics, multispecies probiotics, or probiotics with co-supplements; however, no significant effects on insulin were observed.
Interestingly, the results of the subgroup analysis indicated that participants aged > 55 years old showed higher reduction in FBS and HbA1c than those aged ≤ 55 years old, a finding that is inconsistent with that of our previous study (30). This variation may be attributed to the glucose-lowering drug intervention studies included in the present meta-analysis. Gan et al. (27) reported that the older people had longer durations of intervention and severe islet cell dysfunction, which may reduce the effects of antihyperglycemic drugs. Thus, it is still necessary to consider patients' ages when assessing the efficacies of glucose-lowering drugs in patients with type 2 diabetes, especially the elderly. Moreover, subjects with BMI ≥ 30 kg/m2 showed a significant decrease in FBS and the HOMA-IR index compared with those with lower BMI. Matteo et al. reported that lower BMI was related to better glycaemic control (57). However, several previous studies indicated that the efficacies of glucose-lowering drugs (58) and probiotics supplementation (59) appeared to increase as a function of baseline BMI. It is possible that weight loss induced by glucose-lowering drugs and probiotic supplementation plays an important role in glycaemic control in obese patients with type 2 diabetes. In addition, our results showed that subjects from Eastern countries had a higher reduction in FBS, HbA1c, and HOMA-IR than those from Western countries. Previous meta-meta-analyses have shown that blood glucose reduction is greater in Asian-dominant groups than in Caucasian-dominant groups (60, 61), a finding that confirms our results. The present study showed that the overall duration of intervention with glucose-lowering drugs (≥12 weeks) was longer than that of probiotics supplementation (≤ 12 weeks). These results illustrate that for probiotics, a longer duration of intervention may be required to induce gut microbiota changes and beneficial effects on glucose metabolism.
A recent meta-analysis indicated that different glucose-lowering agents can have varying impacts on the lipid profile. It has been reported that TZD significantly increase LDL-C and HDL-C levels while reducing TG (14, 62). It has been reported that DPP-4i can decrease TC (63). However, in the present study, there was no significant difference between the effects of glucose-lowering drugs (including TZD, GLP-1 RA, and DPP-4i) and placebos on all lipid indicators. In addition, it has been reported that the TC and TG levels of patients who consume multispecies probiotics are reduced, a result which is consistent with that of the present meta-analysis (30). Dong et al. (64) reported that intake of probiotics decreases LDL-C levels, but has no significant effect on TC, TG, and HDL-C levels. Wu et al. (65) reported that probiotic supplementation can lower TC and LDL-C levels, but has no significant effects on TG and HDL-C levels. The differences in these results may be due to differences in the patients' ages, BMI, race, and duration of intervention in the included studies. Our results also showed that DPP-4i significantly lowered TG level, whereas TZD reduced HDL-C level; however, further studies are needed to verify this finding. Furthermore, we found that participants of Eastern descent and those with baseline BMI <30 kg/m2 showed significantly higher reductions in TC and TG levels than participants of Western descent and those with BMI ≥ 30 kg/m2, respectively.
The use of lipid lowering medication like statin, and anti-hypertension medication like ACEI can influence lipid profiles and blood pressure much more than glucose-lowering drugs and probiotics. The use of those medication is quite common on T2D patients. Recently, the findings of several studies suggested that ACE inhibitors are highly associated with decrease in blood pressure (66–68). This may be attributed to the production of bioactive peptides that inhibit ACE and lead to a decrease in blood pressure. A previous study demonstrated that DPP-4i can induce reduction of blood pressure (SBP and DBP) (69). SGLT-2i have also been reported to significantly reduce blood pressure (70). However, the results of the present study indicate that glucose-lowering drugs have no significant effect on SBP and DBP, whereas probiotics decrease SBP and DBP, regardless of whether they are single species or multispecies probiotics, a finding which is similar to those of numerous studies (26, 71). However, no significant reduction blood pressure was observed in other studies after the intake of probiotics (25, 64). These differences in results may be due to differences in the races of participants. Our subgroup analysis showed that participants from Eastern countries had a higher decrease in blood pressure than those from Western countries. Further evidence is needed to confirm these findings.
Hypoglycaemia is a serious adverse reaction that cannot be ignored in patients with type 2 diabetes. It can influence a patient's quality of life and is associated with the highest incidence of cardiovascular disease (72, 73). In general, GLP-1 RA drugs do not induce hypoglycaemia unless they are combined with sulfonylureas (74). DPP-4i seldom induce hypoglycaemia in theory because they can regulate the secretion of insulin and glucagon (75). In the present study however, semaglutide, a long-acting GLP-1 analog (6), was associated with an increased risk for hypoglycaemia compared with a placebo. This indicates that the safety of semaglutide needs to be studied further. Linagliptin, a new DPP-4i, has no significant effect on the risk for hypoglycaemia. Thus, DPP-4i may be considered safe for patients with type 2 diabetes. In addition, since the effects of GLP-1 RA and DPP-4i on hypoglycaemia were assessed in only one of the included studies, the difference between the risks for hypoglycaemia associated with glucose-lowering drugs and placebos needs to be clarified.
To our knowledge, this is the first meta-analysis in which the hypoglycaemic effects of probiotic supplementation and those of glucose-lowering drugs were compared. However, this study has several limitations. First, the protocol of our meta-analysis was not registered in PROSPERO. Second, due to the paucity of studies that satisfied the inclusion and exclusion criteria, TZD and SGLT-2i intervention studies were few. This may make the results unreliable. Therefore, future RCTs in which the efficacies of these agents are directly compared are needed. Third, the subgroup analysis of dose that is necessary to evaluate the effect of probiotic supplementation on indicators associated with glucose, lipids, and blood pressure metabolism. At the same time, subgroup analysis is necessary with the sample size and female/male numbers, due to there were different numbers in included studies. Besides, dietary and physical activity also plays an important role in effects of glucose-lowering drugs and probiotics, so these confounders should take it into consideration in furture study. Forth, other traditional hypoglycemic drugs, like metformin, sulfonylureas, alpha-glucosidase inhibitors, and insulin should be also included in furture study. Finally, due to differences in study baseline characteristics, such as age, BMI, disease duration, country, and duration of intervention, there was high heterogeneity in some indicators. As these factors may influence the overall pooled results, larger trials are needed to support the results of this meta-analysis.
In the summary, the results of this meta-analysis suggest some relevant recommendations for the use of probiotic supplementation and glucose-lowering drugs for the treatment of patients with type 2 diabetes. GLP-1 RA may be optimal for the reduction of FBS, followed by SGLT-2i and TZD. Administration of SGLT2i may be a good treatment option for reduction of HbA1c level. TZD, DPP-4i, and GLP-1 RA can also lower HbA1c level. However, probiotic supplementation, especially multispecies probiotics, might be a good choice for reduction of glycemia indexes (FBS, HbA1c and HOMA-IR), lipid profile indicators (TC and TG) and blood pressure (SBP and DBP). Thus, the effect of probiotics in reduction of glycaemic indexes (FBS, HbA1c) is much milder than glucose-lowering drugs, but more effective in improving other metabolic index (lipid profiles and hypertension). So probiotics is fit for less severe T2D patients. T2D patients with high severity should still consider glucose-lowering drugs as primary treatment. Results of this review might be able to give an additional contribute to the field working onto alternative strategies aimed to reduce the use of drugs.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
TL, XX, LW, LL, LY, and HG designed the research and wrote the paper. ZD, XZ, XC, JZ, YD, and QW conducted the research, analyzed the data, and performed the statistical analysis. All authors had equal responsibility for the final content of the paper, read, and agreed to the published version of the manuscript.
This study was supported by research grants from the GDAS Special Project of Capacity Building for Innovation-driven Development (2019GDASYL-0201001), the Guangdong Province Academy of Sciences Special Project for Capacity Building of Innovation Driven Development (2020GDASYL-20200301002), and National Key Research and Development Program (Application demonstration of Key Technologies for Food Safety Emergency Assurance) (2019YFC160630501).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.825897/full#supplementary-material
Supplementary Figure 1. Forest plot for the effect of probiotics supplementation and glucose-lowering drugs on diarrhea (A) and hypoglycemia (B) compared to placebo. For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 2. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of FBS levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 3. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of HbA1c levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 4. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of insulin levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 5. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of HOMA-IR levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 6. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of TC levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 7. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of TG levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 8. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of HDL-C levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 9. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of LDL-C levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 10. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of SBP levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 11. Subgroup analysis of the subject ages, BMI, country, and duration of intervention on the effect of DBP levels of probiotics supplementation and glucose-lowering drugs. Ages (A), BMI (B), country (C), and duration of intervention (D). For each study, the solid black diamonds represent the point estimate of the intervention effect. The horizontal line joins the lower and upper limits of the 95% CI of this effect. The open diamonds represent the subgroup and overall SMD determined with a random-effects model.
Supplementary Figure 12. Begg's funnel plot for publication bias in trials on the effect of probiotics and glucose-lowering drugs on glycemia indexes [FBS (A), HbA1c (B), Insulin (C), HOMA-IR (D)].
Supplementary Figure 13. Egger's test in quantitative for publication bias in trials on the effect of probiotics and glucose-lowering drugs on glycemia indexes [FBS (A), HbA1c (B), Insulin (C), HOMA-IR (D)].
Supplementary Figure 14. Begg's funnel plot for publication bias in trials on the effect of probiotics and glucose-lowering drugs on lipid indexes [TC (A), TG (B), HDL-C (C), LDL-C (D)].
Supplementary Figure 15. Egger's test in quantitative for publication bias in trials on the effect of probiotics and glucose-lowering drugs on lipid indexes [TC (A), TG (B), HDL-C (C), LDL-C (D)].
Supplementary Figure 16. Begg's funnel plot for publication bias in trials on the effect of probiotics and glucose-lowering drugs on blood pressure indexes [SBP (A), DBP (B)].
Supplementary Figure 17. Egger's test in quantitative for publication bias in trials on the effect of probiotics and glucose-lowering drugs on blood pressure indexes [SBP (A), DBP (B)].
Supplementary Figure 18. The sensitivity analysis of individual studies was performed by evaluating each study on the changes overall results of the meta-analysis in glycemia indicators [FBS (A), HbA1c (B), Insulin (C), HOMA-IR (D)].
Supplementary Figure 19. The sensitivity analysis of individual studies was performed by evaluating each study on the changes overall results of the meta-analysis in blood lipids indicators [TC (A), TG (B), HDL-C (C), LDL-C (D)].
Supplementary Figure 20. The sensitivity analysis of individual studies was performed by evaluating each study on the changes overall results of the meta-analysis in blood pressure indicators [SBP (A), DBP (B)].
Supplementary Table 1. The search terms for each database.
Supplementary Table 2. PICOS criteria for inclusion and exclusion of studies.
Supplementary Table 3. PRISMA 2015 Checklist. This checklist has been adapted for use with systematic review protocol submissions to Food reviews international.
1. Cho NH, Shaw JE, Karuranga S, Huang Y, Fernandes JR, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pr. (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023
2. Whang A, Nagpal R, Yadav H. Bi-directional drug-microbiome interactions of anti-diabetics - ScienceDirect. EBioMedicine. (2019) 39:591–602. doi: 10.1016/j.ebiom.2018.11.046
3. Ridker PM. C-Reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American Women. Circulation. (2003) 107:391–7. doi: 10.1161/01.CIR.0000055014.62083.05
4. Auro K, Kristiansson K, Zethelius B, Berne C, Lannfelt L, Taskinen MR, et al. USF1 gene variants contribute to metabolic traits in men in a longitudinal 32-year follow-up study. Diabetologia. (2008) 51:464–72. doi: 10.1007/s00125-007-0892-9
5. Rastogi A, Dunbar RL, Thacker HP, Bhatt J, Parmar DV. Abrogation of postprandial triglyceridemia with dual PPAR α/γ agonist in type 2 diabetes mellitus: a randomized, placebo-controlled study. Acta Diabetol. (2020) 57:809–18. doi: 10.1007/s00592-020-01487-8
6. Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endo. (2019) 7:356–67. doi: 10.1016/S2213-8587(19)30066-X
7. Eyk H, Paiman E, Bizino MB, Heer P, Jazet IM. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol. (2019) 18:87. doi: 10.1186/s12933-019-0890-5
8. Guja C, Frías J, Somogyi A, Jabbour S, Hui W, Hardy E, et al. Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: The Duration-7 randomized study. Diabetes Obes Metab. (2018) 20:1602–14. doi: 10.1111/dom.13266
9. Boer SD, Heerspink H, Orozco LJ, Roon AV, Kamphuisen PW, Smit AJ, et al. Effect of linagliptin on pulse wave velocity in early type 2 diabetes: a randomized, double-blind, controlled 26-week trial (RELEASE). Diabetes Obes Metab. (2017) 19:1147–54. doi: 10.1111/dom.12925
10. Guzman CB, Zhang XM, Liu R, Regev A, Shankar S, Garhyan P, et al. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab. (2017) 19:1521–8. doi: 10.1111/dom.12958
11. Wu W, Ying L, Xiong C, Lin D, Gu X. Effect of Linagliptin on glycemic control in chinese patients with newly-diagnosed, drug-naïve type 2 diabetes mellitus: a randomized controlled trial. Medical Sci Monitor Int Medical Exp Clini Res. (2015) 21:2678–84. doi: 10.12659/MSM.894026
12. Rosenstock J, Jelaska A, Zeller C, Kim G, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. (2015) 17:936–48. doi: 10.1111/dom.12503
13. Chen M, Xie CG, Gao H, Zheng H, Chen Q, Fang JQ. Comparative effectiveness of sodium-glucose co-transporter 2 inhibitors for controlling hyperglycaemia in patients with type 2 diabetes: protocol for a systematic review and network meta-analysis. BMJ Open. (2016) 6:e010252. doi: 10.1136/bmjopen-2015-010252
14. Monami M, Vitale V, Ambrosio ML, Bartoli N, Toffanello G, Ragghianti B, et al. Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: meta-analysis of placebo-controlled trials. Adv Ther. (2012) 29:736–46. doi: 10.1007/s12325-012-0045-5
15. Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. (2009) 11:611–22. doi: 10.1111/j.1463-1326.2009.01056.x
16. Ming J, Xia L, Cao J, Zou D. Sitagliptin/Metformin Versus Insulin Glargine Combined With Metformin in Obese Subjects With Newly Diagnosed Type 2 Diabetes. Medicine. (2016) 95:e2961. doi: 10.1097/MD.0000000000002961
17. Jeon HJ, Ku EJ, Oh TK. Dapagliflozin improves blood glucose in diabetes on triple oral hypoglycemic agents having inadequate glucose control. Diabetes Res Clin Pr. (2018) 142:188–94. doi: 10.1016/j.diabres.2018.05.013
18. Lin CH, Lin CC, Shibu MA, Liu CS, Kuo CH, Tsai FJ, et al. Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Brit J Nutr. (2014) 111:598–605. doi: 10.1017/S0007114513002791
19. Vajihe A, Fatemeh H. Effects of probiotic supplementation in patients with type 2 diabetes: systematic review and meta-analysis. Nutr Rev. (2016) 74:774–84. doi: 10.1093/nutrit/nuw039
20. Niibo M, Shirouchi B, Umegatani M, Morita Y, Ogawa A, Sakai F, et al. Probiotic Lactobacillus gasseri SBT2055 improves insulin secretion in a diabetic rat model. J Dairy Sci. (2019) 102:997–1006. doi: 10.3168/jds.2018-15203
21. Li C, Nie SP, Ding Q, Zhu KX, Wang ZJ, Xiong T, et al. Cholesterol-lowering effect of Lactobacillus plantarum NCU116 in a hyperlipidaemic rat model. J Funct Foods. (2014) 8:340–7. doi: 10.1016/j.jff.2014.03.031
22. Gómezuzmán M, Toral M, Romero M, Jiménez R, Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. (2016) 59:2326–36. doi: 10.1002/mnfr.201500290
23. Brunkwall L, Orho-Melander M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia. (2017) 60:943–51. doi: 10.1007/s00125-017-4278-3
24. Elham R, Amir J, Sadat EH, Parvin M, Maryam J, Abbas Y. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syn: Cli Res Rev. (2018) 13:175–82. doi: 10.1016/j.dsx.2018.08.008
25. Khalili L, Alipoor B, Jafarabadi MA, Faraji I, Sani MA. The effects of lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. (2018) 23:68–77. doi: 10.29252/.23.1.68
26. Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure a systematic review and meta-analysis of randomized, controlled trials. Hypertension. (2014) 64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469
27. Gan S, Dawed AY, Donnelly LA, Nair A, Pearson ER. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. (2020) 43:1948–57. doi: 10.2337/dc19-2419
28. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. PRISMA statement - preferred reporting items for systematic reviews and meta-analyses. Chin J Integr Med. (2009) 7:889–96. doi: 10.3736/jcim20090918
29. Huoponen S, Blom M. A systematic review of the cost-effectiveness of biologics for the treatment of inflammatory bowel diseases. Val Health the J Int Soc Phar & Outcomes Res. (2015) 10:e0145087. doi: 10.1371/journal.pone.0145087
30. Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: an update of meta-analysis. Crit Rev Food Sci. (2021) 61:1670–88. doi: 10.1080/10408398.2020.1764488
31. Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. Brit Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Dan J, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. (2012) 31:3805–20. doi: 10.1002/sim.5453
33. Chang YP, Yang W, Tou C, Gause-Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug-nave Asian patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes/Metab Res and Rev. (2012) 28:268–75. doi: 10.1002/dmrr.1306
35. Stuck AE, Rubenstein LZ, Wieland D, Vandenbroucke JP, Irwig L, Macaskill P, et al. Bias in meta-analysis detected by a simple, graphical test. Test had 10% false positive rate. BMJ Clin Res. (1998) 315:629–34.
36. Palacios T, Vitetta L, Coulson S, Madigan CD, Caterson ID. Targeting the intestinal microbiota to prevent type 2 diabetes and enhance the effect of metformin on glycaemia: a randomised controlled pilot study. Nutrients. (2020) 12:2041. doi: 10.3390/nu12072041
37. Madempudi RS, Ahire JJ, Neelamraju J, Tripathi A, Nanal S. Efficacy of UB0316, a multi-strain probiotic formulation in patients with type 2 diabetes mellitus: a double blind, randomized, placebo controlled study. PLoS ONE. (2019) 14:e0225168. doi: 10.1371/journal.pone.0225168
38. Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab Synd. (2018) 12:617–24. doi: 10.1016/j.dsx.2018.04.015
39. Sabico S, Al-Mashharawi A, Al-Daghri NM, Yakout S, Alnaami AM, Alokail MS, et al. Effects of a multi-strain probiotic supplement for 12weeks in circulating endotoxin levels and cardiometabolic profiles of medication nave T2DM patients: a randomized clinical trial. J Transl Med. (2017) 15:249. doi: 10.1186/s12967-017-1354-x
40. Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type ii diabetes mellitus. Probiotics Antimicro. (2017) 9:41–7. doi: 10.1007/s12602-016-9233-y
41. Firouzi S, Majid HA, Ismail A, Kamaru Dd In NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. (2017) 56:1535–50. doi: 10.1007/s00394-016-1199-8
42. Tonucci LB, Santos K, Oliveira L, Ribeiro S, Martino H. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr. (2017) 36:85–92. doi: 10.1016/j.clnu.2015.11.011
43. Alireza O, Akbar T, Majid M, Nazila F, Laleh P, Zahra BG, et al. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iranian J Public Health. (2015) 44:228–37.
44. Mobini R, Tremaroli V, Sthlman M, Karlsson F, Levin M, Ljungberg M, et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. (2016) 19:579–89. doi: 10.1111/dom.12861
45. Asemi Z, Alizadeh SA, Ahmad K, Goli M, Esmaillzadeh A. Effects of beta-carotene fortified synbiotic food on metabolic control of patients with type 2 diabetes mellitus: a double-blind randomized cross-over controlled clinical trial. Clin Nutr. (2016) 35:819–25. doi: 10.1016/j.clnu.2015.07.009
46. Hove KD, Brøns C, Færch K, Lund SS, Rossing P, Vaag A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind placebo-controlled study. Eur J Endoc. (2015) 172:11–20. doi: 10.1530/EJE-14-0554
47. Bulut EA, Alak Z, Dokuzlar O, Kocyigit SE, Isik AT. Cognitive and metabolic outcomes of vildagliptin addition to the therapy in patients with type 2 diabetes mellitus: 26 week follow-up study. Arch Gerontol Geriat. (2020) 88:104013. doi: 10.1016/j.archger.2020.104013
48. Vanderheiden A, Harrison L, Warshauer J, Li X, Adams-Huet B, Lingvay I. Effect of adding liraglutide vs placebo to a high-dose lnsulin regimen in patients with type 2 diabetes: a randomized clinical trial. Jama Intern Med. (2016) 176:939–47. doi: 10.1001/jamainternmed.2016.1540
49. Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endo. (2015) 3:191–7. doi: 10.1016/S2213-8587(14)70251-7
50. Tajabadi-Ebrahimi M, Sharifi N., arrokhian AF, Raygan F, Karamali F, Razzaghi R, et al. A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocr Diab. (2017) 125:21–7. doi: 10.1055/s-0042-105441
51. Lajara R. Combination therapy with SGLT-2 inhibitors and GLP-1 receptor agonists as complementary agents that address multi-organ defects in type 2 diabetes. Postgrad Med. (2019) 131:1–11. doi: 10.1080/00325481.2019.1670017
52. Yoon JH, Min SH, Ahn CH, Cho YM, Hahn S. Comparison of non-insulin antidiabetic agents as an add-on drug to insulin therapy in type 2 diabetes: a network meta-analysis. Sci Rep. (2018) 8:4095. doi: 10.1038/s41598-018-22443-1
53. De Lzenne NM, Cani PD, Everard A, Neyrinck AM, Bin De, Ls LB. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia. (2015) 58:2206–17. doi: 10.1007/s00125-015-3712-7
54. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. P Natl Acad Sci Usa. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
55. Kootte RS, Vrieze A, Holleman F, Dallinga-Thie GM, Zoetendal EG, de Vos WM, et al. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. (2012) 14:112–20. doi: 10.1111/j.1463-1326.2011.01483.x
56. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. (2014) 16:457–66. doi: 10.1111/dom.12244
57. Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. (2011) 273:57–64. doi: 10.1185/03007995.2011.602964
58. Brown E, Wilding J, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes Rev. (2019) 20:816–28. doi: 10.1111/obr.12841
59. Mcternan PG, Kusminski CM, Kumar S. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. (2006) 17:4–12. doi: 10.1104/pp.92.4.891
60. Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c-lowering efficacy of glucagon-like peptide-1 analogues between Asians and non-Asians: a systematic review and meta-analysis. Diabetes Obes Metab. (2015) 16:900–9. doi: 10.1111/dom.12293
61. Yang L, Zhang L, He H, Zhang M, An Z. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in east asians with type 2 diabetes: a systematic review and meta-analysis. Diabetes Ther. (2019) 10:1921–34. doi: 10.1007/s13300-019-0674-7
62. Derosa G, Salvadeo SA. Pioglitazone and rosiglitazone: effects of treatment with a thiazolidinedione on lipids and non conventional cardiovascular risk factors. Curr Clin Pharmacol. (2008) 3:77–84. doi: 10.2174/157488408784293688
63. Horton ES, Foley JE, Shen SG, Baron MA. Efficacy and tolerability of initial combination therapy with nateglinide and metformin in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. (2004) 20:883–9. doi: 10.1185/030079903125003881
64. Dong Y, Xu M, Chen L, Bhoc Hh Ibhoya A. Probiotic foods and supplements interventions for metabolic syndromes: a systematic review and meta-analysis of recent clinical trials. Ann Nutr Metab. (2019) 74:224–41. doi: 10.1159/000499028
65. Wu Y, Zhang Q, Ren Y, Zhongbao R, Danilo NG. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS ONE. (2017) 12:e0178868. doi: 10.1371/journal.pone.0178868
66. Padwal R, Laupacis A. Antihypertensive therapy and incidence of type 2 diabetes: a systematic review. Diabetes Care. (2005) 28:762. doi: 10.2337/diacare.28.3.762-a
67. Scheen AJ. Prevention of type 2 diabetes mellitus through inhibition of the renin-angiotensin system. Drugs. (2004) 64:2537–65. doi: 10.2165/00003495-200464220-00004
68. Suzuki K, Nakagawa O, Aizawa Y. Improved Early-Phase Insulin Response after Candesartan Treatment in Hypertensive Patients with Impaired Glucose Tolerance. Clin Exp Hypertension. (2009) 30:309–14. doi: 10.1080/10641960802269927
69. Levetan C. Oral antidiabetic agents in type 2 diabetes. Curr Med Res Opin. (2007) 23:945–52. doi: 10.1185/030079907X178766
70. Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. (2012) 44:375–93. doi: 10.3109/07853890.2011.560181
71. Dong JY, Szeto I, Makinen K, Gao Q, Wang J, Qin LQ, et al. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. British J Nutr. (2013) 110:1188–94. doi: 10.1017/S0007114513001712
72. Qian D, Zhang T, Tan X, Zheng P, Bing S. Comparison of antidiabetic drugs added to sulfonylurea monotherapy in patients with type 2 diabetes mellitus: a network meta-analysis. PLoS ONE. (2018) 13:e0202563. doi: 10.1371/journal.pone.0202563
73. Huang ES, Laiteerapong N, Liu JY, John PM, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus the diabetes and aging study. Jama Intern Med. (2013) 174:251–8. doi: 10.1001/jamainternmed.2013.12956
74. Meier, Juris J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. (2012) 8:728–42. doi: 10.1038/nrendo.2012.140
Keywords: probiotics, glucose-lowering drugs, glycemic, lipids, type 2 diabetes, meta-analysis
Citation: Liang T, Xie X, Wu L, Li L, Yang L, Gao H, Deng Z, Zhang X, Chen X, Zhang J, Ding Y and Wu Q (2022) Comparative analysis of the efficacies of probiotic supplementation and glucose-lowering drugs for the treatment of type 2 diabetes: A systematic review and meta-analysis. Front. Nutr. 9:825897. doi: 10.3389/fnut.2022.825897
Received: 06 January 2022; Accepted: 20 June 2022;
Published: 18 July 2022.
Edited by:
Fariba Ahmadizar, University Medical Center Utrecht, NetherlandsReviewed by:
Ruolin Li, Erasmus Medical Center, NetherlandsCopyright © 2022 Liang, Xie, Wu, Li, Yang, Gao, Deng, Zhang, Chen, Zhang, Ding and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingping Wu, d3VxcDIwM0AxNjMuY29t; Yu Ding, ZGluZ3l1QGpudS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.