
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 09 June 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.804880
This article is part of the Research Topic Saturated Fat: Metabolism, Nutrition and Health Impact View all 6 articles
 Tinglan Yuan1,4
Tinglan Yuan1,4 Lei Wang1
Lei Wang1 Jun Jin1
Jun Jin1 Lijuan Mi2
Lijuan Mi2 Jinzhu Pang2
Jinzhu Pang2 Zhengdong Liu3
Zhengdong Liu3 Jinyan Gong4
Jinyan Gong4 Cong Sun5
Cong Sun5 Jufang Li2*
Jufang Li2* Wei Wei1*
Wei Wei1* Qingzhe Jin1
Qingzhe Jin1 Xingguo Wang1
Xingguo Wang1Human breastmilk, the ideal food for healthy infants, naturally contains a high concentration of medium-chain fatty acids (MCFAs, about 15% of total fatty acids). MCFAs are an important energy source for infants due to their unique digestive and metabolic properties. MCFA-enriched oils are widely used in an infant formula, especially the formula produced for preterm infants. Recently, there has been a growing interest in the triglyceride structure of MCFAs in human milk, their metabolism, and their effects on infant health. This study summarized the MCFA composition and structure in both human milk and infant formula. Recent studies on the nutritional effects of MCFAs on infant gut microbiota have been reviewed. Special attention was given to the MCFAs digestion and metabolism in the infants. This paper aims to provide insights into the optimization of formulations to fulfill infant nutritional requirements.
Dietary nutrition is vital for the metabolic outcome and development of infants. Human milk is the optimal source of nutrition for infants. Fat is an important component of milk, supplying ∼50% of energy for infants (1). Human milk fat contains approximately 50 kinds of fatty acids (FAs), mostly in the form of triacylglycerols (TAGs), which are more than 98% of the fat (2). Human milk is a natural source of medium-chain fatty acids (MCFAs), comprising approximately 10–35% of the total FAs (3), and half of the TAG molecules in human milk contain MCFAs (4). MCFAs have great importance for infants with an immature digestive system (5).
By definition, MCFAs generally refer to saturated FAs, with a chain length of 6–12 carbons, naturally occurring in some vegetable oils (coconut and palm kernel oils) and milk fat (5, 6). Typical medium-chain TAG (MCT) is mainly composed of caprylic acid (8:0) and capric acid (10:0). In milk fat, MCFAs are generally regarded as saturated FAs, with a chain length of 8–14 (7–10). During lactation, the mammary epithelial cells are the primary site for de novo FA synthesis, and the presence of an acyl thioester-hydrolase limits FA synthesis to more than 16 (11, 12); therefore, the main FAs synthesized de novo in mammary glands, such as 8:0,10:0, 12:0, and 14:0, are known as MCFAs on the basis of the source of milk FAs, which was adopted in this review.
MCFA-enriched oils, such as coconut oil and palm kernel oil or MCT, are often added to infant formulas as a source of MCFAs to facilitate fat absorption and the growth of infants, especially in formulas designed for low birth weight or preterm infants (13, 14). MCFAs are an important source of energy because their properties during the processes of digestion, absorption, and metabolism are different from those of long-chain fatty acids (LCFAs) (6, 15). It has been well documented that MCFAs are more easily absorbed and oxidized for energy than LCFAs since they are primarily absorbed directly to the liver via the portal vein and rapidly transferred independently of the carnitine shuttle system to the mitochondria (6). The addition of MCT to infant formula could facilitate better absorption of lipids (16). MCFAs also display antiviral and antibacterial activities and have a functional impact on the modulation of the gut microbiota during early infancy (17, 18), which is related to the maturation of the immune system and general health (19).
As an important energy source, the role of MCFAs in lipid metabolism in infants deserves more attention. However, a recent updated systematic review has concluded that an MCT formula did not improve preterm infant growth or have fewer adverse effects (20). Knowledge of human milk and infant formulas has greatly increased, which will help to understand better the health benefits of different diets. In this paper, we focus on the advances and controversies in the MCFA nutrition of infants. This work is intended to provide a holistic review of MCFAs, from structural characteristics to metabolic effects in infants.
Human milk fat was considered the reference standard of an infant formula. The MCFAs in human milk have been well studied as important saturated fatty acids, and their content was influenced by multiple factors (21). The MCFAs in an infant formula depend on the oil species supplied. The difference in MCFA between human milk and the infant formula was seldom compared. In this study, MCFA content in human milk fat and the infant formula fat were compared with emphasis on the differences between TAG structures.
Medium-chain fatty acids (8:0–14:0) account for 7–23% of the total FAs in human milk, and 12:0 and 14:0 are predominant, accounting for approximately 5% and 6%, followed by 10:0 and 8:0 (Table 1). It was found that the MCFA composition of human milk changes throughout lactations, with a lower amount in colostrum than in transitional and mature milk (22–27), which may be attributed to the immature metabolism of the mammary gland or the biosynthetic capacity in early lactation (12). A pooled data analysis suggested that MCFAs are comparable between preterm and term milk (26). Besides physiological factors, maternal dietary, and sociodemographic and environmental factors are associated with the MCFA composition of human milk (21). It has been suggested that rich n-3 LCFAs or high-carbohydrate and low-fat diet could stimulate the synthesis of de novo FAs in the cytoplasm of the mammary glands (1, 11), resulting in a higher content of MCFAs in human milk (9, 28, 29). Actually, lower levels of MCFAs were found in the human milk of obese mothers (BMI of over 30) relative to the overweight group (BMI between 25 and 30), which may have been caused by the high fat intake (30). Additionally, when mothers or infants suffered cold-like symptoms, the proportions of 10:0 and 12:0 of FAs in human milk were significantly lower (10).
Medium-chain fatty acids account for a small portion of human milk; however, recent studies have shown that approximately half of the TAG molecule species in human milk contain at least one MCFA (31, 32). Table 1 shows the content of the TAGs, containing MCFAs (8:0-14:0) in human milk collected in four countries, summarized from eight publications (4, 24, 31–36). The content varies from 16.95% to 47.11% of total TAG. Among these TAGs, only a few TAGs composed of three MCFAs (0.80–1.13%.) were detected. MCFAs are naturally present in human milk as medium- and long-chain triacylglycerols (MLCTs). Particularly, it was recognized that the MCFAs mainly existed together with 16:0 and 18 FAs (18:0, 18:1 or 18:2), such as 12:0/16:0/18:1 (LaPO), 12:0/18:1/18:2 (LaOL), and 12:0/18:1/18:1 (LaOO) (37).
Human milk fat is well-known as the best nutrition for infants and is generally considered the reference standard for the development of an infant formula. Our group has recently analyzed the FA and TAG composition of 180 commercial infant formulas on the Chinese market (38, 39). As for MCFAs, we have found that almost all of the formulas contained higher amounts of 8:0 than mature human milk. Infant formulas supplied with coconut oil generally contained more 12:0 than human milk (38, 40).
The main TAGs containing MCFAs were 12:0/12:0/12:0 (LaLaLa) and 12:0/12:0/14:0 (LaLaM) in the plant oil-based infant formulas. Short- and medium-chain TAGs, such as 4:0/12:0/16:0 (BuLaP), 4:0/14:0/16:0 (BuMP), and 6:0/14:0/16:0 (CoMP), were predominant in the cow milk or goat milk-based infant formulas (39). These compounds are very different from those naturally present in human milk (37). The significant differences in TAG molecular species containing MCFAs between human milk and infant formulas should be given more attention in the future, as they could lead to altered lipid metabolism and physiological health status.
The digestion of lipids begins in the stomach, where the FAs are released by gastric lipase, which is present from the 11th week of gestation and reaches adult activity levels at birth (41). It has been shown that the gastric hydrolysis of fat is higher in infants fed with human milk than those fed with a formula, including the MCT formula, although the 8:0 and 10:0 were less abundant in human milk (approximately 2%) than those in an infant formula (approximately 24%) (42, 43). Moreover, a crossover study showed that MCFAs in the MCT formula (42% MCT) and the LCT formula (7% MCT) fed to 12 preterm infants were hydrolyzed to the same extent (44), suggesting that MCT supplementation has no improvement on gastric lipolysis, possibly because of the stimulation of lipase secretion by LCFAs (44, 45). Still, short-chain FAs and MCFAs could be absorbed directly through the stomach wall and enter the portal vein, so even MCT molecules could be partially absorbed (44, 46), which provides infants with a readily available energy source.
Ingested fat is digested and absorbed primarily in the small intestine, where TAGs are mainly hydrolyzed by pancreatic lipase to form the primary products 2-monoacylglycerol and free FAs (47). Subsequently, these products and bile salts form mixed micelles and reach the enterocytes, where they are absorbed, resynthesized as TAGs, and packaged as chylomicrons. Alternatively, short-chain FAs and MCFAs can leave the intestine and are directly and rapidly released into the portal circulation (Figure 1; 48).
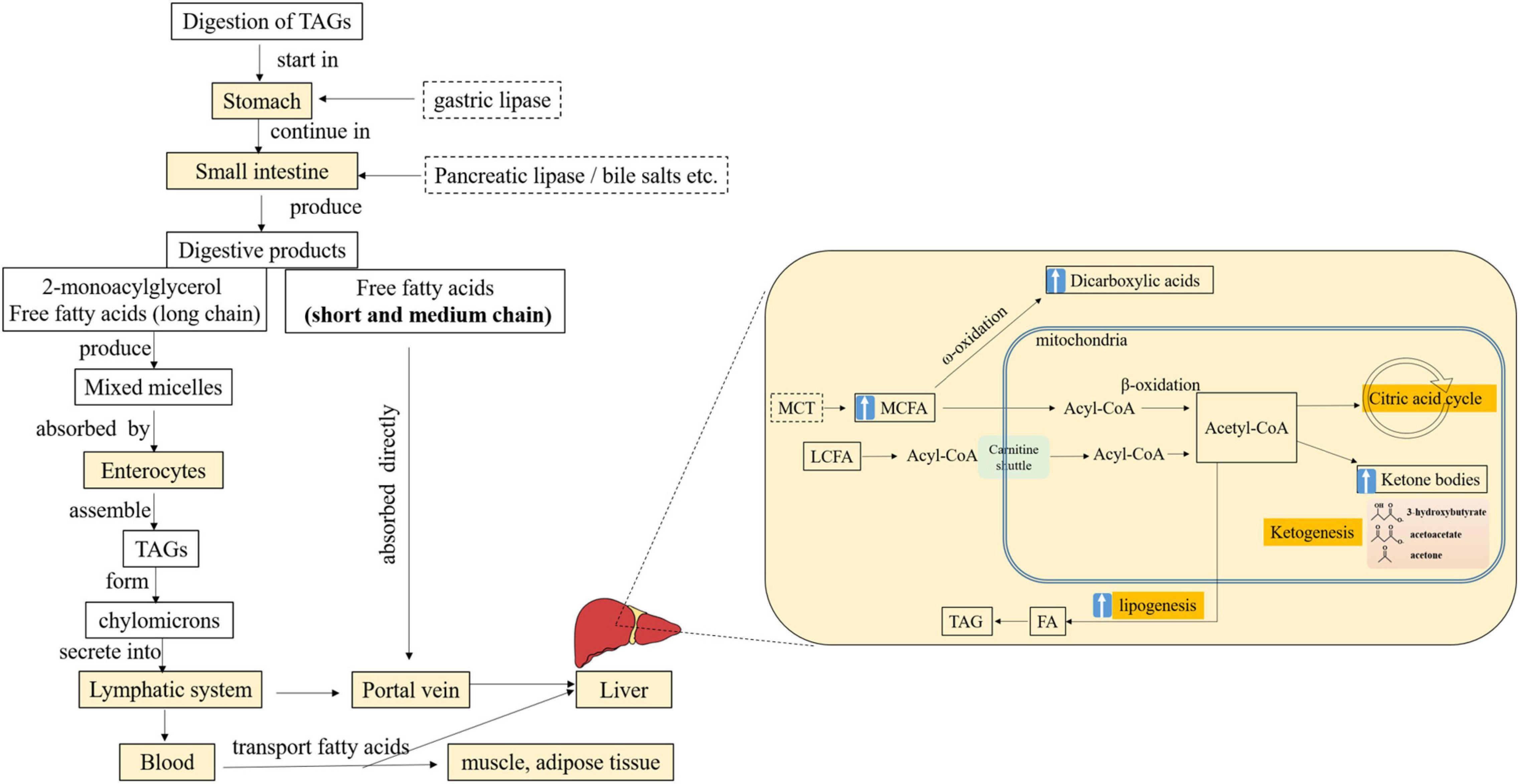
Figure 1. Simplified schematic of digestion, absorption, and transport of MCFAs from TAGs and the metabolism of MCFAs in the liver. FA, fatty acid; MCFA, medium-chain fatty acid; MCT, medium-chain triacylglycerol; LCFA, long-chain fatty acid; TAG, triacylglycerol; “↑,” enhanced. Adopted from (48).
With MCFAs being absorbed easier and faster than LCFAs, it is expected that adding MCTs to formulas would promote the higher absorption of fat (49). Some studies have significantly improved fat absorption by infants fed the MCT formula compared with the LCT formula (50–52). However, other studies have indicated that the fat absorption in infants was similar irrespective of the MCT levels (45, 53). Additionally, a lower fat absorption from formulas than human milk was reported to be common (54), which may be partly affected by the different structures of TAGs containing MCFAs (MLCT vs. MCT/LCT). Previous animal studies have demonstrated that structured lipids MLCT from esterification of MCT and fish oil had a higher lymphatic absorption than the mixture of MCT/LCT (55, 56). The differences in hydrolysis products and FAs released from different TAGs may influence the production of some hormones that control the secretion of pancreatic enzymes and lipid digestion, causing different digestive activities (57). Still, the exact mechanism is not fully understood.
MCFAs as the intestinal energy sources can also improve the growth performance of infants by improving intestinal function. The protective effect of MCFAs and MCT on the intestinal barrier and gut health has been supported in suckling piglets as an in vivo mammalian neonate model (58, 59). Studies have demonstrated that MCFAs in milk, especially 8:0, 10:0, and 12:0, have antimicrobial effects against several bacteria, such as Clostridium, Salmonella, and Helicobacter pylori, which might enhance resistance against intestinal pathogens (17, 60, 61). A recent study has determined that dietary supplementation with MCTs reduced the colonization of Candida in preterm infants (62). Nevertheless, there are no reports of how the MLCT structure in human milk being different from an infant formula would impact the establishment of the microbiota or gut-associated function in infants.
The metabolic fate of MCFAs is chiefly catabolism by the liver, where the major pathway for FAs is β-oxidation in mitochondria. LCFAs cross the mitochondrial membrane with the aid of carnitine, whereas MCFAs can enter independent of the carnitine transport system and undergo preferential oxidation by the tricarboxylic acid cycle (6), and the oxidation is not subject to inhibition by malonyl CoA (Figure 1; 48). Thus, the rapid and almost complete oxidation of MCT has been suggested.
Nevertheless, there might be a limit to the amount of MCT being completely oxidized by preterm infants. For example, in infants fed a formula containing 40% fat as MCT, an average of no more than 47% of the administered 8:0 was oxidized (63). Furthermore, Whyte et al. (64) found no significant differences in the rate of energy expenditure and energy storage between the MCT formula and the LCT formula, which meant that MCT was not oxidized, and portions undergo the same metabolic fate as LCT. Additionally, infants fed a commercial formula might store up to 12% of the MCFAs (8:0–10:0) in subcutaneous fat (65). Hence, incorporating high-level MCT into a formula will not necessarily improve the neonate’s ability to consume or metabolize energy (66).
Another consequence of MCFAs (MLCT or MCT) being easily oxidized is the effects on body fat accumulation and obesity, which have been well demonstrated in animal and clinical trials (67–69). MCFAs may enhance mitochondrial function, lipid oxidation, and thermogenesis by modulating cellular signaling and regulating key circulating metabolites and hormones (70). In a rodent model, consumption of MCFAs in early life has been shown to prevent excessive fat accumulation and insulin sensitivity in adulthood (71). While limited in infants, the evidence points to a positive impact of MCFAs on obesity.
Acetyl CoA can also be converted into ketone bodies, such as acetoacetate, 3-hydroxybutyrate (β-HB), and acetone, in the liver mitochondria (Figure1). Extrahepatic tissues, including the brain, can use ketone bodies as fuel. For newborns and older infants, ketones are an essential and important source of energy for the brain (72). MCFAs, in particular, are ketogenic and the ideal ketone precursors. A higher level of plasma β-HB was observed in the preterm infants fed with the MCT formula compared with a control formula without MCT (53). However, a significantly higher level of ketone bodies (β-HB and acetoacetate) was seen in breastfed infants than in formula-fed infants, although the content of MCFAs in infant formula was not reported (73). The relationship between TAG structure (MLCT or MCT) and the level of ketone bodies remains to be further studied.
Besides an increased metabolic rate, MCFAs are associated with increased lipogenesis in the liver (74, 75). MCTs may cause accelerated MCFA oxidation and promote the production of acetyl CoA, which can be a substrate providing carbons for chain elongation and FA synthesis (Figure 1). In preterm infants fed the MCT formula (38%), greater lipogenesis was observed, which might partly explain the incomplete oxidation (76). Increased lipogenesis may increase hepatic fat accumulation and metabolic burden, as well as interfere with the metabolism of other FAs in infants. It was observed that the levels of plasma phospholipid 22:6 n-3 were significantly lower in preterm infants fed the high MCT (46% of 8:0 and 10:0) formula than that low MCT formula (4.8% of 8:0 and 10:0, similar content of 18:2 and 18:3) (77). Recently, in 47 clinically stable preterm infants, Billeaud et al. (78) have observed that human milk supplemented with a fortifier containing MCFAs (8:0 and 10:0, 12.5%) increased the levels of plasma n-9 monounsaturated FA significantly.
Although the impact of MLCT on infant health remains unclear, numerous clinical studies have demonstrated that, compared with a physical mixture of MCT and LCT, MLCT exerted some favorable effects on nutrition status in surgical patients, in particular lipid metabolism and liver function (79, 80). Hence, we can speculate that the MLCT in human milk may be more beneficial for lipid metabolism and development in infants, which must be verified in future studies.
Milk fat is naturally rich in MCFAs, which are very important to the growth and development of infants. Although the consensus is that MCFAs have a unique advantage in absorption and metabolism in infants, the benefits of MCT on the growth performance of infants are not clearly shown in clinical trials. It is necessary to determine how the TAG structure of MCFAs influences lipid digestion and absorption and causes the observed outcome. Moreover, the effects of different MCFAs (e.g., 12:0 and 14:0) on lipid metabolism warrant further investigation. Additionally, the distinct metabolic effects on infants resulting from the differences in the composition of TAGs containing MCFAs between an infant formula and human milk have not been fully addressed. The molecular species of TAGs containing MCFAs in natural fats, their metabolic processing, and the potential health benefits for infants are of great interest. We present a summary of recent studies on MCFA composition in human milk and an appraisal of the function and roles of MCFAs on long-term metabolism in infants, which will be conducive to the development of infant formulas.
WW and JL designed the review. TY wrote the original manuscript. WW, LW, JJ, LM, JP, ZL, JG, CS, QJ, and XW reviewed and edited the manuscript. All authors read, discussed, and agreed to the published version of the manuscript.
This work was supported by the Science and Technology Program of Inner Mongolia “Study on maternal and infant nutrition in different regions and development of a new generation of infant dairy products,” and the Key Scientific and Technological project in Henan Province (No. 202102110288).
LM, JP, and JL were employed by Inner Mongolia Mengniu Dairy (Group) Co., Ltd. ZL was employed by Yashili International Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
FA, fatty acids; LCFAs, long-chain fatty acids; MCFAs, medium-chain fatty acids; MCT, medium-chain triacylglycerol; MLCTs, medium- and long-chain triacylglycerols; TAGs, triacylglycerols.
2. Liu Z, Rochfort S, Cocks B. Milk lipidomics: what we know and what we don’t. Prog Lipid Res. (2018) 71:70–85. doi: 10.1016/j.plipres.2018.06.002
3. Wei W, Jin Q, Wang X. Human milk fat substitutes: past achievements and current trends. Prog Lipid Res. (2019) 74:69–86.
4. Yuan T, Wei W, Zhang X, Wang L, Dai X, Ren C, et al. Medium- and long-chain triacylglycerols composition in preterm and full-term human milk across different lactation stages. LWT Food Sci Technol. (2021) 142:110907.
6. Bach AC, Babayan VK. Medium-chain triglycerides: an update. Am J Clin Nutr. (1982) 36:950–62. doi: 10.1093/ajcn/36.5.950
7. Haddad I, Mozzon M, Frega NG. Trends in fatty acids positional distribution in human colostrum, transitional, and mature milk. Eur Food Res Technol. (2012) 235:325–32.
8. Granot E, Ishay-Gigi K, Malaach L, Flidel-Rimon O. Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants? J Matern Fetal Neonatal Med. (2016) 29:832–5. doi: 10.3109/14767058.2015.1020785
9. Novak EM, Innis SM. Impact of maternal dietary n-3 and n-6 fatty acids on milk medium-chain fatty acids and the implications for neonatal liver metabolism. Endocrinol Metab. (2011) 301:E807–17. doi: 10.1152/ajpendo.00225.2011
10. Gardner AS, Rahman IA, Lai C, Hepworth A, Trengove N, Hartmann PE, et al. Changes in fatty acid composition of human milk in response to cold-like symptoms in the lactating mother and infant. Nutrients. (2017) 9:1034. doi: 10.3390/nu9091034
11. Hachey DL, Silber GH, Wong WW, Garza C. Human lactation II: endogenous fatty acid synthesis by the mammary gland. Pediatr Res. (1989) 25:63–8. doi: 10.1203/00006450-198901000-00015
12. Neville MC, Picciano MF. Regulation of milk lipid secretion and composition. Annu Rev Nutr. (1997) 17:159–84. doi: 10.1146/annurev.nutr.17.1.159
13. Tantibhedhyangkul P, Hashim SA. Medium-chian triglyceride feeding in premature infants: effects on fat and nitrogen absorption. Pediatrics. (1975) 55:359–70.
14. Łoś-Rycharska E, Kieraszewicz Z, Czerwionka-Szaflarska M. Medium chain triglycerides (MCT) formulas in paediatric and allergological practice. Gastroenterol Rev. (2016) 11:226–31. doi: 10.5114/pg.2016.61374
15. Bach AC, Ingenbleek Y, Frey A. The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res. (1996) 37:708–26.
16. Jensen C, Buist NRM, Wilson T. Absorption of individual fatty acids from long chain or medium chain triglycerides in very small infants. Am J Clin Nutr. (1986) 43:745–51. doi: 10.1093/ajcn/43.5.745
17. Sprong RC, Hulstein MF, Van der Meer R. Bactericidal activities of milk lipids. Antimicrob Agents Chemother. (2001) 45:1298–301. doi: 10.1128/AAC.45.4.1298-1301.2001
18. Nejrup RG, Licht TR, Hellgren LI. Fatty acid composition and phospholipid types used in infant formulas modifies the establishment of human gut bacteria in germ-free mice. Sci Rep. (2017) 7:3975. doi: 10.1038/s41598-017-04298-0
19. Martin MA, Sela DA. Infant gut microbiota: developmental influences and health outcomes. In: Clancy KBH, Hinde K, Rutherford JN editors. Buildinges: Primate Development in Proximate and Ultimate Perspective. (New York, NY: Springer New York) (2013). p. 233–56.
20. Perretta L, Ouldibbat L, Hagadorn JI, Brumberg HL. High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants. Cochrane Database Syst Rev. (2021) 2:CD002777.
21. Miliku K, Duan QL, Moraes TJ, Becker AB, Mandhane PJ, Turvey SE, et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD cohort study. Am J Clin Nutr. (2019) 110:1370–83. doi: 10.1093/ajcn/nqz229
22. Qi C, Sun J, Xia Y, Yu R, Wei W, Xiang J, et al. Fatty acid profile and the sn-2 position distribution in triacylglycerols of breast milk during different lactation stages. J Agric Food Chem. (2018) 66:3118–26. doi: 10.1021/acs.jafc.8b01085
23. Zou XQ, Guo Z, Huang JH, Jin QZ, Cheong LZ, Wang XG, et al. Human milk fat globules from different stages of lactation: a lipid composition analysis and microstructure characterization. J Agric Food Chem. (2012) 60:7158–67. doi: 10.1021/jf3013597
24. Zhao P, Zhang S, Liu L, Pang X, Yang Y, Lu J, et al. Differences in the triacylglycerol and fatty acid compositions of human colostrum and mature milk. J Agric Food Chem. (2018) 66:4571–9. doi: 10.1021/acs.jafc.8b00868
25. Moltó-Puigmartí C, Castellote AI, Carbonell-Estrany X, López-Sabater MC. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin Nutr. (2011) 30:116–23. doi: 10.1016/j.clnu.2010.07.013
26. Floris LM, Stahl B, Abrahamse-Berkeveld M, Teller IC. Human milk fatty acid profile across lactational stages after term and preterm delivery: a pooled data analysis. ProstagLeukotr Ess. (2019) 156:102023. doi: 10.1016/j.plefa.2019.102023
27. Wang L, Li X, Hussain M, Liu L, Zhang Y, Zhang H. Effect of lactation stages and dietary intake on the fatty acid composition of human milk (A study in northeast China). Int Dairy J. (2020) 101:104580.
28. Nasser R, Stephen AM, Goh YK, Clandinin MT. The effect of a controlled manipulation of maternal dietary fat intake on medium and long chain fatty acids in human breast milk in Saskatoon. Canada Int Breastfeed J. (2010) 5:3. doi: 10.1186/1746-4358-5-3
29. Rocquelin G, Tapsoba S, Dop MC, Mbemba F, Traissac P, Martinprével Y. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers’ nutritional status: impact on infants’ EFA supply. Eur J Clin Nutr. (1998) 52:164–71. doi: 10.1038/sj.ejcn.1600529
30. Marín MC, Sanjurjo A, Rodrigo MA, de Alaniz MJT. Long-chain polyunsaturated fatty acids in breast milk in La Plata, Argentina: relationship with maternal nutritional status. ProstagLeukotr Ess. (2005) 73:355–60. doi: 10.1016/j.plefa.2005.07.005
31. Kallio H, Nylund M, Boström P, Yang B. Triacylglycerol regioisomers in human milk resolved with an algorithmic novel electrospray ionization tandem mass spectrometry method. Food Chem. (2017) 233:351–60. doi: 10.1016/j.foodchem.2017.04.122
32. Yuan T, Qi C, Dai X, Xia Y, Sun C, Sun J, et al. Triacylglycerol composition of breast milk during different lactation stages. J Agric Food Chem. (2019) 67:2272–8. doi: 10.1021/acs.jafc.8b06554
33. Tu A, Ma Q, Bai H, Du Z. A comparative study of triacylglycerol composition in Chinese human milk within different lactation stages and imported infant formula by SFC coupled with Q-TOF-MS. Food Chem. (2017) 221:555–67. doi: 10.1016/j.foodchem.2016.11.139
34. Gastaldi D, Medana C, Giancotti V, Aigotti R, Dal Bello F, Baiocchi C. HPLC-APCI analysis of triacylglycerols in milk fat from different sources. Eur J Lipid Sci Technol. (2011) 113:197–207.
35. Zou XQ, Huang JH, Jin QZ, Guo Z, Liu YF, Cheong LZ, et al. Model for human milk fat substitute evaluation based on triacylglycerol composition profile. J Agric Food Chem. (2013) 61:167–75. doi: 10.1021/jf304094p
36. Chen YJ, Zhou XH, Han B, Yu Z, Yi HX, Jiang SL, et al. Regioisomeric and enantiomeric analysis of primary triglycerides in human milk by silver ion and chiral HPLC atmospheric pressure chemical ionization-MS. J Dairy Sci. (2020) 103:7761–74. doi: 10.3168/jds.2019-17353
37. Yuan T, Zhang H, Wang X, Yu R, Zhou Q, Wei W, et al. Triacylglycerol containing medium-chain fatty acids (MCFA-TAG): the gap between human milk and infant formulas. Int Dairy J. (2019) 99:104545.
38. Sun C, Zou X, Yao Y, Jin J, Xia Y, Huang J, et al. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: a comparative study based on fat source and stage. Int Dairy J. (2016) 63:42–51. doi: 10.1016/j.foodchem.2017.09.005
39. Sun C, Wei W, Zou X, Huang J, Jin Q, Wang X. Evaluation of triacylglycerol composition in commercial infant formulas on the Chinese market: a comparative study based on fat source and stage. Food Chem. (2018) 252:154–62. doi: 10.1016/j.foodchem.2018.01.072
40. Fabritius M, Linderborg KM, Tarvainen M, Kalpio M, Zhang Y, Yang B. Direct inlet negative ion chemical ionization tandem mass spectrometric analysis of triacylglycerol regioisomers in human milk and infant formulas. Food Chem. (2020) 328:126991. doi: 10.1016/j.foodchem.2020.126991
41. Bourlieu C, Ménard O, Bouzerzour K, Mandalari G, Macierzanka A, Mackie AR, et al. Specificity of infant digestive conditions: some clues for developing relevant in vitro models. Crit Rev Food Sci Nutr. (2014) 54:1427–57. doi: 10.1080/10408398.2011.640757
42. Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, et al. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res. (1996) 40:429–37. doi: 10.1203/00006450-199609000-00011
43. Roman C, Carriere F, Villeneuve P, Pina M, Millet V, Simeoni U, et al. Quantitative and qualitative study of gastric lipolysis in premature infants: do MCT-enriched infant formulas improve fat digestion? Pediatric Res. (2007) 61:83–8. doi: 10.1203/01.pdr.0000250199.24107.fb
44. Hamosh M, Bitman J, Liao TH, Mehta NR, Buczek RJ, Wood DL, et al. Gastric lipolysis and fat absorption in preterm infants: effect of medium-chain triglyceride or long-chain triglyceride-containing formulas. Pediatrics. (1989) 83:86–92.
45. Hamosh M, Mehta NR, Fink CS, Coleman J, Hamosh P. Fat absorption in premature infants: medium-chain triglycerides and long-chain triglycerides are absorbed from formula at similar rates. J Pediatr Gastroenterol Nutr. (1991) 13:143–9.
46. Jensen RG, Jensen GL. Specialty lipids for infant nutrition. I. Milks and formulas. J Pediatr GastroenterolNutr. (1992) 15:232–45. doi: 10.1097/00005176-199210000-00002
47. Mattson FH, Volpenhein RA. The digestion and absorption of triglycerides. J Biolog Chem. (1964) 239:2772–7.
48. Harvey RA, Ferrier DR. Lippincott’s Illustrated Reviews: Biochemistry. Philadelphia, PA: Lippincott Williams & Wilkins (2011).
49. Guillot E, Lemarchal P, Dhorne T, Rerat A. Intestinal absorption of medium chain fatty acids: in vivo studies in pigs devoid of exocrine pancreatic secretion. Br J Nutr. (1994) 72:545–53. doi: 10.1079/bjn19940058
50. Huston RK, Reynolds JW, Jensen C, Buist NR. Nutrient and mineral retention and vitamin D absorption in low-birth-weight infants: effect of medium-chain triglycerides. Pediatrics. (1983) 72:44–8.
51. Roy CC, Ste-Marie M, Chartrand L, Weber A, Bard H, Doray B. Correction of the malabsorption of the preterm infant with a medium-chain triglyceride formula. J Pediatr. (1975) 86:446–50. doi: 10.1016/s0022-3476(75)80983-8
52. Okamoto E, Muttart CR, Zucker CL, Heird WC. Use of medium-chain triglycerides in feeding the low-birth-weight infant. Am J Dis Child. (1982) 135:428–31. doi: 10.1001/archpedi.1982.03970410046011
53. Wu PYK, Edmond J, Morrow J, Auestad N, Ponder D, Benson J. Gastrointestinal tolerance, fat absorption, plasma ketone and urinary dicarboxylic acid levels in low-birth-weight infants fed different amounts of medium-chain triglycerides in formula. J Pediatr Gastroenterol Nutr. (1993) 17:145–52. doi: 10.1097/00005176-199308000-00004
54. Innis SM. Dietary triacylglycerol structure and its role in infant nutrition. Adv Nutr. (2011) 2:275–83. doi: 10.3945/an.111.000448
55. Jensen G, McGarvey N, Taraszewski R, Wixson S, Seidner D, Pai T, et al. Lymphatic absorption of enterally fed structured triacylglycerol vs physical mix in a canine model. Am J Clin Nutr. (1994) 60:518–24. doi: 10.1093/ajcn/60.4.518
56. Tso P, Lee T, Demichele SJ. Lymphatic absorption of structured triglycerides vs. physical mix in a rat model of fat malabsorption. Am J Physiol. (1999) 277:G333–40. doi: 10.1152/ajpgi.1999.277.2.G333
57. Borovicka J, Schwizer W, Mettraux C, Kreiss C, Remy B, Asal K, et al. Regulation of gastric and pancreatic lipase secretion by CCK and cholinergic mechanisms in humans. Am J Physiol. (1997) 273:G374–80. doi: 10.1152/ajpgi.1997.273.2.G374
58. Zentek J, Buchheit-Renko S, Ferrara F, Vahjen W, Van Kessel AG, Pieper R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim Health Res Rev. (2011) 12:83–93. doi: 10.1017/S1466252311000089
59. De Keyser K, Dierick N, Kanto U, Hongsapak T, Buyens G, Kuterna L, et al. Medium-chain glycerides affect gut morphology, immune- and goblet cells in post-weaning piglets: in vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J Anim Physiol Anim Nutr. (2019) 103:221–30.
60. Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA, Wertz PW. Antibacterial activity of sphingoid bases and fatty acids against gram-positive and gram-negative bacteria. Antimicrob Agents Ch. (2012) 56:1157–61. doi: 10.1128/AAC.05151-11
61. Sun CQ, O’Connor CJ, Roberton AM. Antibacterial actions of fatty acids and monoglycerides against Helicobacter pylori. FEMS Immunol Med Microbiol. (2003) 36:9–17. doi: 10.1016/S0928-8244(03)00008-7
62. Arsenault AB, Gunsalus KTW, Laforce-Nesbitt SS, Przystac L, DeAngelis EJ, Hurley ME, et al. Dietary supplementation with medium-chain triglycerides reduces candida gastrointestinal colonization in preterm infants. Pediatr Infect Dis J. (2019) 38:164–8. doi: 10.1097/INF.0000000000002042
63. Sulkers EJ, Lafeber HN, Sauer PJ. Quantitation of oxidation of medium-chain triglycerides in preterm infants. Pediatr Res. (1989) 26:294–7. doi: 10.1203/00006450-198910000-00003
64. Whyte RK, Campbell D, Stanhope R, Bayley HS, Sinclair JC. Energy balance in low birth weight infants fed formula of high or low medium-chain triglyceride content. J Pediatr. (1986) 108:964–71. doi: 10.1016/s0022-3476(86)80941-6
65. Sarda P, Lepage G, Roy C, Chessex P. Storage of medium-chain triglycerides in adipose tissue of orally fed infants. Am J Clin Nutr. (1987) 45:399–405. doi: 10.1093/ajcn/45.2.399
66. Borum PR. Medium-chain triglycerides in formula for preterm neonates: implications for hepatic and extrahepatic metabolism. J Pediatr. (1992) 120:S139–45. doi: 10.1016/s0022-3476(05)81248-x
67. Lee YY, Tang TK, Chan ES, Phuah ET, Lai OM, Tan CP, et al. Medium chain triglyceride and medium-and long chain triglyceride: metabolism, production, health impacts and its applications – a review. Crit Rev Food Sci Nutr. (2021) 62:4169–85. doi: 10.1080/10408398.2021.1873729
68. Matualatupauw JC, Bohl M, Gregersen S, Hermansen K, Afman LA. Dietary medium-chain saturated fatty acids induce gene expression of energy metabolism-related pathways in adipose tissue of abdominally obese subjects. Int J Obes (Lond). (2017) 41:1348–54. doi: 10.1038/ijo.2017.120
69. Bueno NB, de Melo IV, Florencio TT, Sawaya AL. Dietary medium-chain triacylglycerols versus long-chain triacylglycerols for body composition in adults: systematic review and meta-analysis of randomized controlled trials. J Am Coll Nutr. (2015) 34:175–83. doi: 10.1080/07315724.2013.879844
70. Huang L, Gao L, Chen C. Role of medium-chain fatty acids in healthy metabolism: a clinical perspective. Trends Endocrinol Metab. (2021) 32:351–66. doi: 10.1016/j.tem.2021.03.002
71. van de Heijning BJM, Oosting A, Kegler D, van der Beek EM. An increased dietary supply of medium-chain fatty acids during early weaning in rodents prevents excessive fat accumulation in adulthood. Nutrients. (2017) 9:631. doi: 10.3390/nu9060631
72. Cunnane SC, Crawford MA. Energetic and nutritional constraints on infant brain development: implications for brain expansion during human evolution. J Hum Evol. (2014) 77:88–98. doi: 10.1016/j.jhevol.2014.05.001
73. Lucas A, Boyes S, Bloom SR, Aynsley-Green A. Metabolic and endocrine responses to a milk feed in six-day-old term infants: differences between breast and cow’s milk formula feeding. Acta Paediatr Scand. (1981) 70:195–200. doi: 10.1111/j.1651-2227.1981.tb05541.x
74. Chamma CM, Bargut TC, Mandarim-de-Lacerda CA, Aguila MB. A rich medium-chain triacylglycerol diet benefits adiposity but has adverse effects on the markers of hepatic lipogenesis and beta-oxidation. Food Funct. (2017) 8:778–87. doi: 10.1039/c6fo01663d
75. Hwang SG, Yano H, Kawashima R. Influence of dietary medium- and long-chain triglycerides on fat deposition and lipogenic enzyme activities in rats. J Am College Nutr. (1993) 12:643–50. doi: 10.1080/07315724.1993.10718355
76. Sulkers EJ, Lafeber HN, van Goudoever JB, Kalhan SC, Beaufrère B, Sauer PJ. Decreased glucose oxidation in preterm infants fed a formula containing medium-chain triglycerides. Pediatr Res. (1993) 33:101–5. doi: 10.1203/00006450-199302000-00002
77. Carnielli VP, Rossi K, Badon T, Gregori B, Verlato G, Orzali A, et al. Medium-chain triacylglycerols in formulas for preterm infants: effect on plasma lipids, circulating concentrations of medium-chain fatty acids, and essential fatty acids. Am J Clin Nutr. (1996) 64:152–8. doi: 10.1093/ajcn/64.2.152
78. Billeaud C, Boue-Vaysse C, Couedelo L, Steenhout P, Jaeger J, Cruz-Hernandez C, et al. Effects on fatty acid metabolism of a new powdered human milk fortifier containing medium-chain triacylglycerols and docosahexaenoic acid in preterm infants. Nutrients. (2018) 10:690.
79. Wu GH, Zaniolo O, Schuster H, Schlotzer E, Pradelli L. Structured triglycerides versus physical mixtures of medium- and long-chain triglycerides for parenteral nutrition in surgical or critically ill adult patients: systematic review and meta-analysis. Clin Nutr. (2017) 36:150–61. doi: 10.1016/j.clnu.2016.01.004
Keywords: medium-chain fatty acids, human milk fat, infant formula, lipid metabolism, medium-chain triacylglycerols
Citation: Yuan T, Wang L, Jin J, Mi L, Pang J, Liu Z, Gong J, Sun C, Li J, Wei W, Jin Q and Wang X (2022) Role Medium-Chain Fatty Acids in the Lipid Metabolism of Infants. Front. Nutr. 9:804880. doi: 10.3389/fnut.2022.804880
Received: 29 October 2021; Accepted: 16 May 2022;
Published: 09 June 2022.
Edited by:
Fabian Dayrit, Ateneo de Manila University, PhilippinesReviewed by:
Xuefang Wen, Jiangxi Academy of Sciences, ChinaCopyright © 2022 Yuan, Wang, Jin, Mi, Pang, Liu, Gong, Sun, Li, Wei, Jin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wei, d2Vpd0BqaWFuZ25hbi5lZHUuY24=; Jufang Li, bGlqdWZhbmdAbWVuZ25pdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.