- 1Key Lab of Meat Processing of Sichuan Province, Chengdu University, Chengdu, China
- 2College of Animal Science and Technology, Yangzhou University, Yangzhou, China
Meat spoilage (MS) is a complex microbial ecological process involving multiple specific microbial interactions. MS is detrimental to people's health and leads to the waste of meat products which caused huge losses during production, storage, transportation, and marketing. A thorough understanding of microorganisms related to MS and their controlling approaches is a necessary prerequisite for delaying the occurrence of MS and developing new methods and strategies for meat product preservation. This mini-review summarizes the diversity of spoilage microorganisms in livestock, poultry, and fish meat, and the approaches to inhibit MS. This would facilitate the targeted development of technologies against MS, to extend meat's shelf life, and effectively diminish food waste and economic losses.
Introduction
According to a report by the Food and Agriculture Organization of the United Nations, one-third of food produced for human consumption is either spoiled or wasted (1). MS is defined as a change in color and the production of off-flavors, mucus, and exudates that result in unacceptable sensory and organoleptic properties. Parlapani confirms that the deterioration is caused by specific spoilage organisms that dominate and form metabolites that alter the organoleptic properties of the meat, making it unfit for consumption (2). Although the causes for meat deterioration vary, bacteria direct the process more than other factors such as endogenous enzymes. Meat is generally considered sterile before slaughter, but the environment during slaughter is not sterile, so some degree of microbial contamination may occur, leading to meat corruption (3). The sources of microbial contamination in this process can be summarized as both endogenous and exogenous. The microbiological quality of post-slaughter meat depends to a large extent on the type of meat, processing, distribution, and storage conditions. Contaminated slaughter equipment, personnel and environmental factors (e.g. water, air, and soil) can be cross-contaminated with spoilage-associated bacteria (4). After storage, various intrinsic and extrinsic factors affect the process of microbial MS, including oxygen demand, pH, temperature, and competing organisms (5). The diversity of these ecophysiological factors affects the dynamics of microbial growth, including microbial succession and microbiota composition, ultimately affecting the type and rate of MS. Several strategies have been proposed to preserve fresh products to overcome MS, including the addition of ingredients such as food preservatives, essential oils and storage under refrigerated conditions, and aeration packaging (6, 7). Therefore, understanding the sources of spoilage microorganisms in meat, the diversity of microorganisms and measures to retard spoilage, and achieving accurate and effective inhibition of spoilage microorganisms is one of the common goals of meat industry sessions and academia.
Spoilage microbial diversity
Major types of spoilage microorganisms in livestock meat
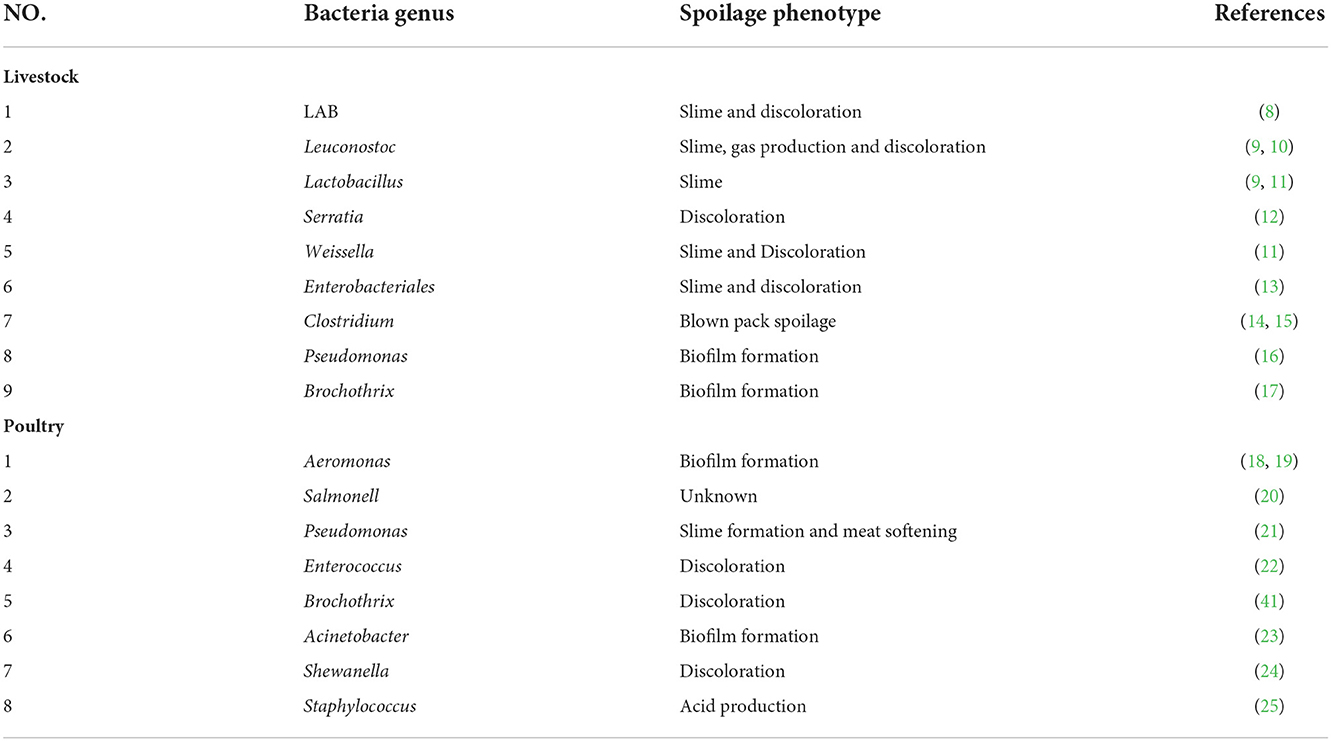
Not all bacteria cause spoilage of food, there is only an initial small group of microorganisms in meat, referred to as specific spoilage organisms (SSO) (8). In meat products, SSO metabolizes available substrates during storage, leading to changes in meat quality and odor (9). This section summarizes the common microorganisms and spoilage phenotypes associated with the spoilage of livestock meat (Table 1). A study showed that the dominant bacteria in the meatballs of the blown pack spoilage (BPS) group packed with 71.85% CO2 were Klebsiella (46.05%) and Escherichia (39.96%). Klebsiella pneumoniae was the main strain causing BPS in meatballs due to its ability to pack swelling (26). Wang et al. (27) revealed that Proteobacteria, Firmicutes, Pseudomonas spp., Acinetobacter spp., Pantoea spp., Brochothrix spp., and Raoultella spp. were the main pathogenic and spoilage bacteria in chilled pork by culture-dependent and non-culture-dependent methods (27). The microbial composition of pork stored at−2°C and 4°C showed a high degree of similarity, with Pseudomonads and Brochothrix being the dominant taxa. Acinetobacter spp., Myroides spp., and Kurthia spp. were markers for spoiled pork meat stored at 25°C (28). The current research results show that the abnormal growth of lactic acid bacteria, Micrococcaceae, Enterobacteriaceae, yeast, and mold plays a key role in the formation of dry cured ham odor defects, while the key putrefactive microorganisms of different types of ham are different (29, 30). In Mianning ham, the dominant bacterial genus was Clostridium_sensu_stricto_2 (92.01%), and the dominant fungal genus was Aspergillus (84.27%) (31). The number of Enterobacteriaceae and Enterococcus in deteriorated ham was significantly higher than that in normal ham. High water content and low salt content lead to abnormal growth of Enterobacteriaceae and Enterococcus in deteriorated ham, leading to the deterioration of Jinhua ham (32, 33). C. farmei CDC 2991–81, B. cereus ATCC 14579, and E. faecalis ATCC 19433 were the main spoilage microorganisms of Jinhua ham (34). C. sestertheticum was detected as the most abundant Clostridium spp. in vacuum packaging beef and other raw meats, associated with BPS (35). Li et al. (36) reported that total viable bacteria (8.75 log CFU/cm2) and Lactobacillus (3.20 log CFU/cm2) counts were higher on meat surfaces dry-aged for 19 days (36). The microbial communities of all samples evaluated in dry-aged beef contain Enterobacteriaceae and Pseudomonas, which are considered to be the major spoilers in dry-aged beef (13). In another study, beef and lamb samples from Europe, North and South America, and Oceania were investigated and Psychrophilic Clostridium spp. was found to be the most prevalent Clostridium (37).
Major types of spoilage microorganisms in poultry meat
There has been a steady increase in consumption and demand for poultry meat globally. Among poultry products, processed chicken meat is the most consumed (about 75% of total poultry meat), followed by turkey (about 25%) and duck meat (38). The bacterial community in poultry meat include pathogenic species such as Salmonella and Campylobacter (18). This section summarizes the common microorganisms and spoilage phenotypes associated with the spoilage of poultry meat (Table 1). When defining the dominant spoilage bacteria in the spoilage process of meat products based on the number of bacteria, Pseudomonas spp., Bacillus spp., Crude Typhimurium spp., Schwartzella spp., Aeromonas spp. are usually considered to be the dominant communities in cold meat and poultry packed under aerobic conditions (19). Poultry meat spoils quickly, even under refrigerated conditions. Wang et al. detected a significant increase of Clostridium perfringens over time in almost poultry samples stored aerobically under different refrigeration conditions. Pseudomonas fluorescens, Aeromonas salmonicida, and Serratia liquefaciens cause spoilage of poultry meat stored at 8°C for 4 days (20). Several new enterococci or lactic acid bacteria were also identified in poultry products, such as Viikkiensis enterococcus, Seigonensis enterococcus, and Heterofermentative lactic acid bacteria (39, 40).
With the development of MS studies, it is more appropriate to determine the dominant spoilage organism by determining the spoilage capacity of bacterial isolates grown in situ. The main common Pseudomonas in poultry meat is Pseudomonas fragilis, Pseudomonas lundengensis, and Pseudomonas fluorescens. Pseudomonas fragilis, Pseudomonas fluorescens, and Pseudomonas aeruginosa produced slime on meat and its products during storage (41). Extracellular enzymes secreted by Pseudomonas aeruginosa have strong protease activity against myogenic fibronectin and myxomatosis protein. This helps bacteria penetrate the meat to obtain new sources of nutrients, increasing the formation of mucus and softening the meat (21). In addition, Serratia spp., Micrococcus spp., Serratia spp., and Brucella spp. were also associated with slime production and softening during MS (42).
Major types of spoilage microorganisms in fish meat
The increase in the global population has led to an increase in the consumption of fish and meat in various countries. It becomes highly susceptible to spoilage through a series of chemical reactions, under the action of microorganisms and enzymes due to its high water content and high pH (43). Similar to other meat, not all microorganisms in fish meat have the potential for corruption, except SSO. This section summarizes the common spoilage microorganisms associated with the spoilage of fish meat (Table 2).
Møretrø et al. (44) found higher levels of Pseudomonas spp. and Salmonella spp. on industrially processed salmon filets with the methods of bacterial enumeration 16S rRNA analysis from seven processing plants. Salmonella spp. and Photobacterium spp. were found on salmon at the slaughter stage (44). The main microbiota of air-packaged (AP) and vacuum-packaged (VP) carp filets during storage were systematically identified by Zhang et al. (52) The results showed that Pseudomonas aeruginosa was the only microbiota found in spoiled AP carp, while Karnococcus were found mainly in VP samples (52). Characterization of some specific H2S-producing spoilage organisms isolated from raw tuna and swordfish by Serio et al. (45). Among them, Shewanella spp. can form biogenic amines, showing great corruption potential. Pseudomonas and Shewanella are two spoilage microorganisms of frozen fish meat preserved aerobically, while CO2-resistant Photobacterium phosphoreum is the main flora of fish meat packed under altered atmosphere conditions (46). Pseudomonas can inhibit each other in seafood matrices. Boziaris et al. (53) observed that Pseudomonas fluorescens outcompeted Pseudomonas spp. at increased storage temperatures and that Pseudomonas spp. could cause spoilage bacteria in raw salmon under aerobic conditions (53). Brochothrix thermosphacta produces caramel off-flavors (2,3-butanedione) in seafood under aerobic conditions. The genus Psychrobacter is a gram-negative, psychrophilic and aerobic bacterium found mainly in seafood and meat. Members of this category include Acinetobacter, Photosynthetic bacteria (Psb) cibatius, Psb. maritimus and Psb. proteolyticus are found in a variety of seafood, such as mackerel, anglerfish, lobster, oysters, and Atlantic cod (47). Psychrobacter species, especially Psb. immobilis, are able to break down lipids and hydrolyze amino acids, thus causing a slight ichthyological and musty odor.
Common control approaches
Packaging methods
Factors affecting the growth of microorganisms in meat include intrinsic factors (natural and added ingredients, pH, redox potential, and water activity), as well as extrinsic factors (storage temperature and packaging methods).
Modified atmospheric packaging (MAP) reported extending the shelf life of frozen meat (54). Luong et al. (55) showed that for fresh turkey sausage, a 2% (w/w) lactic acid formulation in combination with MAP (50% CO2-50% N2) significantly reduced acidification, off-flavors and prevented discoloration of the sausage from red to dark gray or brown. In pork sausages, MAP (70% O2-30% CO2) slightly reduces off-flavor perception (55). The decrease in the quality of meat during storage depends not only on the number of bacteria but also on the activity of bacterial metabolism. Proteases produce free amino acids which can be further metabolized by bacteria, resulting in off-flavors and mucus associated with spoilage. Previous studies have shown that a gas mixture of 30% CO2 and 70% N2 for MAP can extend the shelf life of frozen chicken (56). Meat stored under this MAP has a lower number of Pseudomonas spp. and is less likely to spoil than when stored in the air (57). Therefore, MAP may affect the growth as well as the metabolism of bacteria. Different packaging conditions affect the shelf life of carp and the growth of microorganisms. The shelf life of air-packed (AP) and vacuum-packed (VP) filets at 4°C is 8 days and 12 days, respectively, with the highest number of Pseudomonas aeruginosa in the AP sample and a relatively high level of lactic acid bacteria (LAB) in the VP sample. VP delays the increase in biogenic amine content compared to AP (52).
Dohlen et al. (58) studied the effect of novel antimicrobial packaging materials containing poly-[2-(tertbutylamino) methylstyrene] (poly-TBAMS) on the growth of typical spoilage and pathogenic bacteria present in meat. The results showed that gram-positive bacteria were more susceptible to poly(TBAMS) foil than gram-negative bacteria, and an increase of the antimicrobial activity with an increasing amount of poly(TBAMS) in the base polymer (58). Amna et al. (59) developed a new antimicrobial hybrid packaging pad consisting of biodegradable polyurethane. This type of packaging material was found to show effective antibacterial activity against Staphylococcus aureus and Salmonella typhimurium (59). Zeinab et al. (60) found that TiO2 nanocomposites and irradiation at 3kGy maintained chemical, microbiological, and sensory properties for longer periods and extended the shelf life of fish filets in cold storage (60). It was shown that antibacterial polyvinyl alcohol films containing TiO2 nanoparticles inhibited Shewanella spp., Pseudomonas putida, and Aeromonas hydrophila, and prolonged the shelf life of macroscopic rotenone by 1–2 days (61).
Addition of antibacterial substances
Recently, the harmful effects associated with synthetic preservatives have led to a search for new alternatives in natural products. Commercially available polyphenols reduce primary and secondary lipid peroxidation levels, inhibit lipoxygenase activity, improve meat color stability, minimize degradation of salt-soluble myogenic fibrin and sulfhydryl groups, and retard bacterial growth (62). Essential oils (EOs) are secondary metabolites obtained from plants of Asteraceae, Lamiaceae, Lauraceae, Myrtaceae, Rutaceae, Umbelliferae, Zingiberaceae families, among others. Composed of a complex mixture of low molecular weight volatile compounds (63). These valuable substances can be obtained from different parts of the plant, such as bark, flowers, fruits, leaves, roots, and stems (64). This section summarizes the effects of common plant EOs on spoilage microorganisms in meat products and fish meat (Table 3).
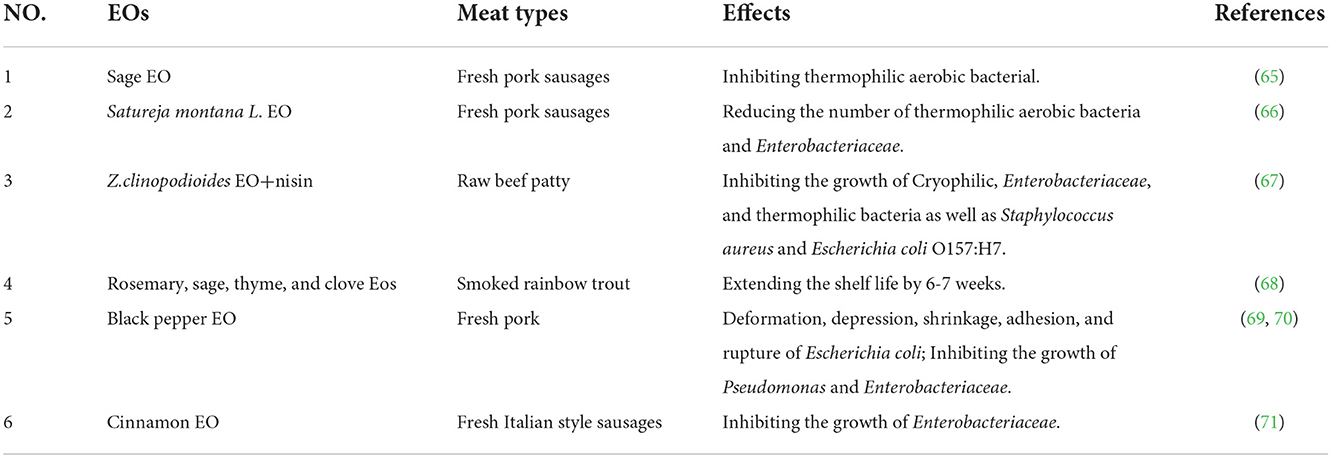
Thymol and carvacrol have inhibitory effects on Bacillus cereus, Pseudomonas aeruginosa, and Staphylococcus aureus (72). In contrast, Guimarães et al. (73) observed that the free terpenes commonly found in essential oils have strong antibacterial activity against gram-negative bacteria (73). Sage EO as a preservative was demonstrated in meat products used as fresh pork sausages, suppressing aerobic thermophilic bacterial counts at the end of storage (4.8–7.3%) (65). Sojic et al. (66) evaluated the antibacterial potential of Satureja montana L. EO in fresh pork sausage. Compared with the control group, adding Satureya montana L.EO can improve the microbial stability of the product, and reduce the total number of thermophilic aerobic bacteria (4.9-10.9%) and Enterobacteriaceae (7.1-19.6%) in sausage (66). Shahbazi et al. (67) found that both essential oils and lactobacillus peptides significantly (p < 0.05) affected the growth of Cryophilic, Enterobacteriaceae, and thermophilic bacteria as well as Staphylococcus aureus and Escherichia coli O157:H7 in raw beef patties, with the fastest decrease in the number of tested microorganisms in samples treated with 0.2% essential oil + 500 IU/g lactobacillus peptide (67).
For fish meat, MAP conditions favor the growth of anaerobic bacteria, which can produce toxins. Therefore, often in combination with other modalities (MAP, edible coatings and films, non-thermal sterilization, etc.) to enhance the effectiveness of natural preservatives (74). Yuan et al. (75) found that the total volatile alkaline nitrogen and total aerobic colony values of black spot shrimp treated with chitosan coating in combination with pomegranate peel extract (PPE) were lower than those of shrimp treated with chitosan coating or PPE alone, indicating a synergistic effect between chitosan coating and PPE (75). Emird et al. (68) found that rosemary, sage, thyme, and clove essential oils as natural antioxidants can be used with vacuum packaging to extend the shelf life of smoked rainbow trout by 6–7 weeks (68). In a previous study, the effects of nanoemulsions based on commercial oils (sunflower, canola, corn, olive, soybean, and hazelnut oils) on the fatty acid compositions of farmed sea bass stored at 2 ± 2°C was investigated. The results showed hazelnut group gave the highest polyunsaturated fatty acid content, followed by canola and soybean at the end of the storage period. These oils can be recommended for nanoemulsions as a preservative for fish (76).
Plasma sterilization
Reactive oxygen species (ROS) in atmospheric pressure cold plasma (APCP) act on gram-positive and gram-negative bacteria through different microbicidal mechanisms, and intracellular ROS levels increase in Listeria monocytogenes and Staphylococcus aureus with prolonged exposure to APCP, but with little damage to the cell wall (77). Exposure of Listeria monocytogenes and Staphylococcus aureus to APCP causes cell shrinkage, but little damage to the cell wall. In addition, intracellular ROS levels of Listeria monocytogenes and Staphylococcus aureus have been shown to increase with the exposure time of APCP (78). Dielectric barrier discharge (DBD) plasma is a source of plasma that generates ROS that can penetrate cell membranes and cause apoptosis through intracellular DNA damage. Previous studies have shown that the levels of Listeria monocytogenes in inoculated meat and meat products were reduced by 0.59–6.52 Log CFU/g after DBD treatment (79).
Bacteriophage sterilization
Phages are considered promising new bioretention agents because they can efficiently and specifically lyse targeted bacteria. A cocktail of three phages effectively inhibited the growth of S. hiva in catfish filets and significantly improved the pH, total volatile basic nitrogen, and organoleptic value indices of the filets (80).
Low-dose irradiation
Low-dose irradiation is considered a common technique for keeping fish meat fresh. Dogruyol et al. reported that sous-vide filets could be irradiated (5.0 kGy) to extend their shelf life up to 8 weeks during refrigerated storage without any damage to the organoleptic and physicochemical properties of the filets (81).
Conclusion
Microbial contamination of meat is the domain cause of losses during production, storage, and distribution, accounting for approximately 21% of total food losses. We review recent advances in research on microbial diversity causing spoilage of livestock, poultry, and fish meat and summarize measures to prevent MS. However, to achieve more accurate and effective control of spoilage microorganisms in meat, it is necessary to obtain more comprehensive and accurate information on the composition of microbial communities and the dynamic processes of their metabolism. By revealing specific interactions between various spoilage phenotypes during MS, we would achieve controllable product quality during the production, transportation, marketing, and storage of meat.
Author contributions
YZ and LC conceived and wrote the original draft. WW, LC, ML, JZ, RZ, LJ, ZZ, and DC reviewed, edited, and revised the manuscript. All authors approved the finalversion.
Funding
This work was supported by the National Modern Agricultural Industrial Technology System, Sichuan Innovation Team Construction Project (SCSZTD-2022-08-07), Liangshan Science and Technology Program (22ZDYF0249, 21CGZH0001), and the Natural Science Foundation of Jiangsu Province (BK20200932).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gustavsson J, Cederberg C, Sonesson U, Van Otterdijk R, Meybeck A. Global Food Losses and Food Waste. Rome: Food and Agriculture Organization of the United Nations (2011).
2. Paludetti LF, Kelly AL, O'Brien B, Jordan K. Gleeson, D. The effect of different precooling rates and cold storage on milk microbiological quality and composition. J Dairy Sci. (2018) 101:1921–9. doi: 10.3168/jds.2017-13668
3. Holman BWB, Kerry JP, Hopkins DL. Meat packaging solutions to current industry challenges: a review. Meat Sci. (2018) 144:159–68. doi: 10.1016/j.meatsci.2018.04.026
4. Wambui J, Lamuka P, Karuri E, Matofari J, Njage PMK. Microbial contamination level profiles attributed to contamination of beef carcasses, personnel, and equipment: case of small and medium enterprise slaughterhouses. J Food Prot. (2018) 81:684–91. doi: 10.4315/0362-028X.JFP-17-402
5. Fletcher B, Mullane K, Platts P, Todd E, Power A, Roberts J, et al. Advances in meat spoilage detection: a short focus on rapid methods and technologies. Cyta J Food. (2018) 16:1037–44. doi: 10.1080/19476337.2018.1525432
6. Pateiro M, Munekata PES. Sant'Ana AS, Dominguez R, Rodriguez-Lazaro D, Lorenzo JM. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int J Food Microbiol. (2021) 337:108966. doi: 10.1016/j.ijfoodmicro.2020.108966
7. Wang GY, Ma F, Zeng LY, Bai Y, Wang HH, Xu XL, et al. Modified atmosphere packaging decreased Pseudomonas fragi cell metabolism and extracellular proteolytic activities on meat. Food Microbiol. (2018) 76:443–9. doi: 10.1016/j.fm.2018.07.007
8. Fu LL, Wang C, Liu NN, Ma AJ, Wang YB. Quorum sensing system-regulated genes affect the spoilage potential of Shewanella baltica. Food Res Int. (2018) 107:1–9. doi: 10.1016/j.foodres.2018.01.067
9. Andreevskaya M, Jaaskelainen E, Johansson P, Ylinen A, Paulin L, Bjorkroth J, et al. Food spoilage-associated leuconostoc, lactococcus, and lactobacillus species display different survival strategies in response to competition. Appl Environ Microbiol. (2018) 84:e00554–18. doi: 10.1128/AEM.00554-18
10. Candeliere F, Raimondi S, Spampinato G, Tay MYF, Amaretti A, Schlundt J, et al. Draft genome sequences of 12 Leuconostoc carnosum strains isolated from cooked ham packaged in a modified atmosphere and from fresh sausages. Microbiol Resour Announce. (2020) 9:e01247–19. doi: 10.1128/MRA.01247-19
11. Poirier S, Coeuret G, Champomier-Vergès MC, Chaillou S. Draft genome sequences of nine strains of Brochothrix thermosphacta, Carnobacterium divergens, Lactobacillus algidus, Lactobacillus fuchuensis, Lactococcus piscium, Leuconostoc gelidum subsp. gasicomitatum, Pseudomonas lundensis, and Weissella virid. Genome Announc. (2018) 6:e00479–18. doi: 10.1128/genomeA.00479-18
12. Li LY, Figeys D. Proteomics and Metaproteomics add functional, taxonomic and biomass dimensions to modeling the ecosystem at the mucosal-luminal interface. Mol Cell Proteomics. (2020) 19:1409–17. doi: 10.1074/mcp.R120.002051
13. Ribeiro FA, Lau SK, Furbeck RA, Herrera NJ, Henriott ML, Bland NA, et al. Ultimate pH effects on dry-aged beef quality. Meat Sci. (2021) 172:108365. doi: 10.1016/j.meatsci.2020.108365
14. Bolton DJ, Carroll JJ, Walsh D, A. four year survey of blown pack spoilage Clostridium estertheticum and Clostridium gasigenes on beef primal cuts. Lett Appl Microbiol. (2015) 61:153–7. doi: 10.1111/lam.12431
15. Wambui J, Cernela N, Corti S, Stephan R. Comparative Genome analysis and phenotypic characterization of Clostridium gasigenesCGAS001 isolated from chilled vacuum-packed lamb meat. Front Microbiol. (2020) 11:2048. doi: 10.3389/fmicb.2020.02048
16. Stanborough T, Fegan N, Powell SM, Singh T, Tamplin M, Chandry PS. Genomic and metabolic characterization of spoilage-associated Pseudomonas species. Int J Food Microbiol. (2018) 268:61–72. doi: 10.1016/j.ijfoodmicro.2018.01.005
17. Zwirzitz B, Wetzels SU, Dixon ED, Fleischmann S, Selberherr E, Thalguter S, et al. Co-Occurrence of Listeria spp. and spoilage associated microbiota during meat processing due to cross-contamination events. Front Microbiol. (2021) 12:632935. doi: 10.3389/fmicb.2021.632935
18. Wang GY, Jia K, Xu XL, Zhou GH. Bacterial community and spoilage profiles shift in response to packaging in yellow-feather broiler, a highly popular meat in Asia. Front Microbiol. (2017) 8:2588. doi: 10.3389/fmicb.2017.02588
19. Odeyemi OA, Alegbeleye OO, Strateva M, Stratev D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr Rev Food Sci Food Saf. (2020) 19:311–31. doi: 10.1111/1541-4337.12526
20. Wang GY, Wang HH, Han YW, Xing T, Ye KP, Xu XL, et al. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. (2017) 63:139–46. doi: 10.1016/j.fm.2016.11.015
21. Katiyo W, de Kock HL, Coorey R, Buys EM. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT- Food Sci Technol. (2020) 128:109468. doi: 10.1016/j.lwt.2020.109468
22. Al-Nehlawi A, Saldo J, Vega LF, Guri S. Effect of high carbon dioxide atmosphere packaging and soluble gas stabilization pre-treatment on the shelf-life and quality of chicken drumsticks. Meat Sci. (2013) 94:1–8. doi: 10.1016/j.meatsci.2012.12.008
23. Casaburi A, Piombino P, Nychas GJ, Villani F, Ercolini D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. (2015) 45:83–102. doi: 10.1016/j.fm.2014.02.002
24. Rossaint S, Klausmann S, Kreyenschmidt J. Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poult Sci. (2015) 94:96–103. doi: 10.3382/ps/peu001
25. Zhang QQ, Han YQ, Cao JX, Xu XL, Zhou GH, Zhang WY. The spoilage of air-packaged broiler meat during storage at normal and fluctuating storage temperatures. Poult Sci. (2012) 91:208–14. doi: 10.3382/ps.2011-01519
26. Chen YF, Bassey AP, Bai Y, Teng S, Zhou GH, Ye KP. Synergistic effect of static magnetic field and modified atmosphere packaging in controlling blown pack spoilage in meatballs. Foods. (2022) 11:10. doi: 10.3390/foods11101374
27. Wang XH, Deng YH, Sun JS, Ding Y, Liu Y, Tian T. Unraveling characterizations of bacterial community and spoilage profiles shift in chilled pork during refrigerated storage. Food Sci Technol. (2022) 42:e80321. doi: 10.1590/fst.80321
28. Zhao F, Wei ZQ, Zhou GH, Kristiansen K, Wang C. Effects of different storage temperatures on bacterial communities and functional potential in pork meat. Foods. (2022) 11:2307. doi: 10.3390/foods11152307
29. Liao RY, Xia Q, Zhou CY, Geng F, Wang Y, Sun YY, et al. LC-MS/MS-based metabolomics and sensory evaluation characterize metabolites and texture of normal and spoiled dry-cured hams. Food Chem. (2022) 371:131156. doi: 10.1016/j.foodchem.2021.131156
30. Zhou CY, Xia Q, Du LH, He J, Sun YY, Dang YL, et al. Recent developments in off-odor formation mechanism and the potential regulation by starter cultures in dry-cured ham. Crit Rev Food Sci Nutr. (2022) 04:1–15. doi: 10.1080/10408398.2022.2057418
31. Zhu YL, Wang W, Zhang YL Li M, Zhang JM Ji LL, et al. Characterization of quality properties in spoiled mianning ham. Foods. (2022) 11:1713. doi: 10.3390/foods11121713
32. Zhou CY, Pan DD, Cao JX, Zhou GH, A. comprehensive review on molecular mechanism of defective dry-cured ham with excessive pastiness, adhesiveness, and bitterness by proteomics insights. Compr Rev Food Sci Food Saf. (2021) 20:3838–57. doi: 10.1111/1541-4337.12779
33. Zhou CY, Zhan G, Pan DD, Zhou GH, Wang Y, He J, et al. Charactering the spoilage mechanism of “three sticks” of Jinhua ham. Food Sci Hum Wellness. (2022) 11: 1322–30. doi: 10.1016/j.fshw.2022.04.021
34. Zhan G, Pan DD, Zhou CY, Wang Y, He J, Zhang J, et al. Characterizing bacterial strains of spoiled Jinhua ham and evaluating the effect of antimicrobial agents on these isolated bacterial strains. LWT Food Sci Technol. (2021) 136:2. doi: 10.1016/j.lwt.2020.110351
35. Yang XQ, Youssef MK, Gill CO, Badoni M, Lopez-Campos O. Effects of meat pH on growth of 11 species of psychrotolerant clostridia on vacuum packaged beef and blown pack spoilage of the product. Food Microbiol. (2014) 39:13–8. doi: 10.1016/j.fm.2013.10.008
36. Li X, Babol J, Bredie WLP, Nielsen B, Tomankova J, Lundstrom K, et al. comparative study of beef quality after ageing longissimus muscle using a dry ageing bag, traditional dry ageing or vacuum package ageing. Meat Sci. (2014) 97:433–42. doi: 10.1016/j.meatsci.2014.03.014
37. Wambui J, Puntener S, Corti S, Cernela N, Stephan R. Detection of Psychrophilic Clostridium spp. Causing ‘Blown Pack' Spoilage in Meat Juice Samples from Chilled Vacuum-Packed Beef and Lamb Meat Imported from Different Countries to Switzerland. J Food Prot. (2020) 83:56–9. doi: 10.4315/0362-028X.JFP-19-321
38. La consommation de produits carnés en 2021 (Consommation Produits Carnés). (2021). Available online at: http://www.franceagrimer.fr/
39. Rahkila R, Johansson P, Sade E, Bjorkroth J. Identification of enterococci from broiler products and a broiler processing plant and description of Enterococcus viikkiensis sp. nov Appl Environ Microbiol. (2011) 77:1196–203. doi: 10.1128/AEM.02412-10
40. Harada T, Dang VC, Nguyen DP, Nguyen TAD, Sakamoto M, Ohkuma M, et al. Enterococcus saigonensis sp. nov, isolated from retail chicken meat and liver. Int J Syst Evol Microbiol. (2016) 66:3779–85. doi: 10.1099/ijsem.0.001264
41. Rouger A, Tresse O, Zagorec M. Bacterial contaminants of poultry meat: sources, species, and dynamics. Microorganisms. (2017) 5:50. doi: 10.3390/microorganisms5030050
42. Pellissery AJ, Vinayamohan PG, Amalaradjou MAR, Venkitanarayanan K. Chapter 17- Spoilage bacteria and meat quality. Meat Q Anal. (2020) 17:307–34. doi: 10.1016/B978-0-12-819233-7.00017-3
43. Elbashir S, Parveen S, Schwarz J, Rippen T, Jahncke M, DePaola A. Seafood pathogens and information on antimicrobial resistance: a review. Food Microbiol. (2018) 70:85–93. doi: 10.1016/j.fm.2017.09.011
44. Moretro T, Moen B, Heir E, Hansen AA, Langsrud S. Contamination of salmon fillets and processing plants with spoilage bacteria. Int J Food Microbiol. (2016) 237:98–108. doi: 10.1016/j.ijfoodmicro.2016.08.016
45. Serio A, Fusella GC, Paparella A. Spoilage potential of H2S producing bacteria in seafood. Ital J Food Sci. (2011) 23:201–3.
46. Murhekar S, Wright MH, Greene AC, Brownlie JC, Cock IE. Inhibition of Shewanella spp. growth by Syzygium australe and Syzygium luehmannii extracts: natural methods for the prevention of fish spoilage. J Food Sci Tech Mys. (2017) 54:3314–26. doi: 10.1007/s13197-017-2782-6
47. Bekaert K, Heyndrickx M, Herman L, Devlieghere F. Vlaemynck G. Molecular identification of the microbiota of peeled and unpeeled brown shrimp (Crangon crangon) during storage on ice and at 75 degrees C. Food Microbiol. (2013) 36:123–34. doi: 10.1016/j.fm.2013.04.009
48. Bekaert K, Devriese L, Maes S, Robbens J. Characterization of the dominant bacterial communities during storage of Norway lobster and Norway lobster tails (Nephrops norvegicus) based on 16S rDNA analysis by PCR-DGGE. Food Microbiol. (2015) 46:132–8. doi: 10.1016/j.fm.2014.06.022
49. Garcia MR, Vilas C, Herrera JR, Bernardez M, Balsa-Canto E, Alonso AA. Quality and shelf-life prediction for retail fresh hake (Merluccius merluccius). Int J Food Microbiol. (2015) 208:65–74. doi: 10.1016/j.ijfoodmicro.2015.05.012
50. Madigan TL, Bott NJ, Torok VA, Percy NJ, Carragher JF, Lopes MAD, et al. A microbial spoilage profile of half shell Pacific oysters (Crassostrea gigas) and Sydney rock oysters (Saccostrea glomerata). Food Microbiol. (2014) 38:219–27. doi: 10.1016/j.fm.2013.09.005
51. Leroi F, Cornet J, Chevalier F, Cardinal M, Coeuret G, Chaillou S, et al. Selection of bioprotective cultures for preventing cold-smoked salmon spoilage. Int J Food Microbiol. (2015) 213:79–87. doi: 10.1016/j.ijfoodmicro.2015.05.005
52. Zhang YM Li Q, Li DP, Liu XC, Luo YK. Changes in the microbial communities of air-packaged and vacuum-packaged common carp (Cyprinus carpio) stored at 4 °C. Food Microbiol. (2015) 52:197–204. doi: 10.1016/j.fm.2015.08.003
53. Mace S, Cornet J, Chevalier F, Cardinal M, Pilet MF, Dousset X, et al. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR-TTGE. Food Microbiol. (2012) 30:164–72. doi: 10.1016/j.fm.2011.10.013
54. Meredith H, Valdramidis V, Rotabakk BT, Sivertsvik M, McDowell D, Bolton DJ. Effect of different modified atmospheric packaging (MAP) gaseous combinations on Campylobacter and the shelf-life of chilled poultry fillets. Food Microbiol. (2014) 44:196–203. doi: 10.1016/j.fm.2014.06.005
55. Luong NDM, Jeuge S, Coroller L, Feurer C, Desmonts MH, Moriceau N, et al. Spoilage of fresh turkey and pork sausages: influence of potassium lactate and modified atmosphere packaging. Food Res Int. (2020) 137:109501. doi: 10.1016/j.foodres.2020.109501
56. Zhang XX, Wang HH Li N, Li M, Xu XL. High CO2-modified atmosphere packaging for extension of shelf-life of chilled yellow-feather broiler meat: A special breed in Asia. LWT-Food Sci Technol. (2015) 64:1123–9. doi: 10.1016/j.lwt.2015.07.039
57. Remenant B, Jaffres E, Dousset X, Pilet MF, Zagorec M. Bacterial spoilers of food: behavior, fitness and functional properties. Food Microbiol. (2015) 45:45–53. doi: 10.1016/j.fm.2014.03.009
58. Dohlen S, Braun C, Brodkorb F, Fischer B, Ilg Y, Kalbfleisch K, et al. Effect of different packaging materials containing poly-[2-(tert-butylamino) methylstyrene] on the growth of spoilage and pathogenic bacteria on fresh meat. Int J Food Microbiol. (2017) 257:91–100. doi: 10.1016/j.ijfoodmicro.2017.06.007
59. Amna T, Yang J, Ryu KS, Hwang IH. Electrospun antimicrobial hybrid mats: innovative packaging material for meat and meat-products. J Food Sci Technol. (2015) 52:4600–6. doi: 10.1007/s13197-014-1508-2
60. Hashemabad ZN, Shabanpour B, Azizi H, Ojagh SM, Alireza A. Effects of TiO2 Nanocomposite packaging and gamma irradiation on the shelf-life of rainbow trout stored at (+4 degrees C). Turk J Fish Aquat Sc. (2018) 18:1387–97. doi: 10.4194/1303-2712-v18_12_07
61. Tang ZP, Chen CW, Xie J. Development of antimicrobial active films based on poly(vinyl alcohol) containing nano-TiO2 and its application in Macrobrachium rosenbergii packaging. J Food Process Preserv. (2018) 42:e13702. doi: 10.1111/jfpp.13702
62. Papuc C, Goran GV, Predescu CN, Nicorescu V, Stefan G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: classification, structures, sources, and action mechanisms. Compr Rev Food Sci Food Saf. (2017) 16:1243–68. doi: 10.1111/1541-4337.12298
63. Winska K, Maczka W, Lyczko J, Grabarczyk M, Czubaszek A, Szumny A. Essential oils as antimicrobial agents - myth or real alternative? Molecules. (2019) 24:2130. doi: 10.3390/molecules24112130
64. Nikmaram N, Budaraju S, Barba FJ, Lorenzo JM, Cox RB, Mallikarjunan K, et al. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. (2018) 145:245–55. doi: 10.1016/j.meatsci.2018.06.031
65. Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. (2012) 3:12. doi: 10.3389/fmicb.2012.00012
66. Sojic B, Pavlic B, Tomovic V, Ikonic P, Zekovic Z, Kocic-Tanackov S, et al. Essential oil versus supercritical fluid extracts of winter savory (Satureja montana L) – Assessment of the oxidative, microbiological and sensory quality of fresh pork sausages. Food Chem. (2019) 287:280–6. doi: 10.1016/j.foodchem.2018.12.137
67. Shahbazi Y, Shavisi N, Mohebi E. Effects of Ziziphora clinopodioides essential oil and nisin, both separately and in combination, to extend shelf life and control Escherichia coli o157:h7 and staphylococcus aureus in raw beef patty during refrigerated storage. J Food Saf. (2016) 36:227–36. doi: 10.1111/jfs.12235
68. Coban OE, Patir B, Yilmaz O. Protective effect of essential oils on the shelf life of smoked and vacuum packed rainbow trout (Oncorhynchus mykiss W.1792) fillets. J Food Sci Technol. (2014) 51:2741–7. doi: 10.1007/s13197-012-0795-8
69. Zhang J, Ye KP, Zhang X, Pan DD, Sun YY, Cao JX. Antibacterial activity and mechanism of action of black pepper essential oil on meat-borne Escherichia coli. Front Microbiol. (2017) 7:2094. doi: 10.3389/fmicb.2016.02094
70. Zhang J, Wang Y, Pan DD, Cao JX, Shao XF, Chen YJ, et al. Effect of black pepper essential oil on the quality of fresh pork during storage. Meat Sci. (2016) 117:130–6. doi: 10.1016/j.meatsci.2016.03.002
71. Zhang X, Wang HH, Li X, Sun YY, Pan DD, Wang Y, et al. Effect of cinnamon essential oil on the microbiological and physiochemical characters of fresh Italian style sausage during storage. Anim Sci J. (2019) 90:435–44. doi: 10.1111/asj.13171
72. Yousefi M, Khorshidian N, Hosseini H. Potential application of essential oils for mitigation of listeria monocytogenes in meat and poultry products. Front Nutr. (2020) 7:577287. doi: 10.3389/fnut.2020.577287
73. Guimaraes AC, Meireles LM, Lemos MF, Guimaraes MCC, Endringer DC, Fronza M, et al. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. (2019) 24:2471. doi: 10.3390/molecules24132471
74. García-Soto B, Aubourg SP, Calo-Mata P. Extension of the shelf life of chilled hake (Merluccius merluccius) by a novel icing medium containing natural organic acids. Food Control. (2013) 34:356–63. doi: 10.1016/j.foodcont.2013.05.007
75. Yuan GF, Lv H, Tang WY, Zhang XJ. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control. (2016) 59:818–23. doi: 10.1016/j.foodcont.2015.07.011
76. Ozogul Y, Durmus M, Ucar Y, Kosker AR, Ozogul F. The combined impact of nanoemulsion based on commercial oils and vacuum packing on the fatty acid profiles of sea bass fillets. J Food Process Preserv. (2017) 41:e13222. doi: 10.1111/jfpp.13222
77. Gao YW, Francis K, Zhang XH. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res Int. (2022) 157:111246. doi: 10.1016/j.foodres.2022.111246
78. Han L, Patil S, Boehm D, Milosavljevic V, Cullen PJ, Bourke P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl Environ Microbiol. (2016) 82:450–8. doi: 10.1128/AEM.02660-15
79. Kim HJ, Yong HI, Park S, Choe W, Jo C. Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr Appl Phys. (2013) 13:1953–1953. doi: 10.1016/j.cap.2013.04.021
80. Yang ZQ, Tao XY, Zhang H, Rao SQ, Gao L, Pan ZM, et al. Isolation and characterization of virulent phages infecting Shewanella baltica and Shewanella putrefaciens, and their application for biopreservation of chilled channel catfish (Ictalurus punctatus). Int J Food Microbiol. (2019) 292:107–17. doi: 10.1016/j.ijfoodmicro.2018.12.020
Keywords: livestock, poultry, fish meat, microbial spoilage, bacterial community
Citation: Zhu Y, Wang W, Li M, Zhang J, Ji L, Zhao Z, Zhang R, Cai D and Chen L (2022) Microbial diversity of meat products under spoilage and its controlling approaches. Front. Nutr. 9:1078201. doi: 10.3389/fnut.2022.1078201
Received: 24 October 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Tong Xing, Nanjing Agricultural University, ChinaReviewed by:
Huhu Wang, Nanjing Agricultural University, ChinaJinxuan Cao, Beijing Technology and Business University, China
Copyright © 2022 Zhu, Wang, Li, Zhang, Ji, Zhao, Zhang, Cai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demin Cai, ZGVtaW5jYWlAeXp1LmVkdS5jbg==; Lin Chen, Y2hlbmxpbjExMjIyQDE2My5jb20=
 Yanli Zhu
Yanli Zhu Wei Wang1
Wei Wang1 Zhiping Zhao
Zhiping Zhao Rui Zhang
Rui Zhang Demin Cai
Demin Cai