- 1Fishery Products Technology Study Program, Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, Indonesia
- 2Aquaculture Study Program, Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, Indonesia
- 3Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, Indonesia
- 4Clinical Nutrition, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 5Biological Sciences, State Islamic University of Sunan Kalijaga (UIN Sunan Kalijaga), Yogyakarta, Indonesia
- 6Nutrition Science Department, Faculty of Medicine, Diponegoro University, Semarang, Indonesia
- 7Medical Programme, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
- 8Faculty of Medicine, Sam Ratulangi University, Manado, Indonesia
- 9Department of Chemistry, Carleton University, Ottawa, ON, Canada
Introduction
Child undernutrition remains an important global health problem. Undernutrition increases the susceptibility to illness and fatality and is related to 45% of child deaths (1). Undernourished children also have severe short-term (e.g., delayed cognitive development), medium-term (e.g., lower school achievement), and long-term implications (e.g., lower earnings and higher probability of adult non-communicable chronic diseases) (2). Child undernutrition is often a consequence of inadequate intake of vitamins and minerals such as vitamin A, iron, iodine, and zinc but also poor quality or insufficient proteins (3, 4). The manifestations are low weight for height (or wasting) and low height for age (or stunting). Furthermore, the incidence of stunting is greatly influenced by early life undernutrition since growth faltering frequently starts while a child is still in the womb and lasts for at least the first 2 years after birth (5, 6). In addition to these determinants, the availability of health services is important (7). The rates of stunting or chronic protein energy malnutrition are increasing in certain parts of the world. One of the proposed solutions is producing food or supplements rich in nutrition from the conversion of food by-products high in good-quality proteins.
Fish is a healthy food with a high nutritional value which makes it extremely important for the human food chain (8). Mass quantities of fish waste are produced annually during fish processing, where fish waste, including fish scales, is discarded (9). To date, fish waste is partly used for the production of fishmeal, fertilizers, and fish oil with low profitability or utilized as a raw material for direct feeding in aquaculture, while the rest are thrown away (10). Fish scales are frequently regarded as abandoned waste from the aquaculture industry, including fish canning, fileting, salting, and smoking processes (11). An estimated 7.2–12 million tons of fish waste are thrown away globally each year, with the 5 most utilized species being Oreochromis niloticus, Sardinella brasiliensis, Pogonias cromis, Labeo rohita, and Leporinus elongatus (11). The fish scale yields various functional applications originating from its valuable components such as hydroxyapatite, collagen, and chitin (10, 12). Furthermore, the collagen in fish scales can be utilized into bioactive peptides with various health benefits (13, 14). Many efforts and research are being carried out to exploit the potential of the fish scale, starting from the potential in the fields of nutrition and food to medicine (15, 16). However, the development of functional food from the fish scale to contribute to nutritional problem solutions is currently underdeveloped.
Fish-derived peptides exhibit various biological activities such as an angiotensin-I-converting enzyme (ACE) inhibitory activity, antioxidant, antimicrobial as well as anticancer activity, and immunostimulant activity (17). Peptides, in addition to their nutritional characteristics as sources of amino acids, are known to also have beneficial health effects, as they can present the ability to interact directly with human metabolism routes, acting as health promoters and in the mitigation of the aging process (18). Previous studies have identified many types of bioactive peptides derived from the fish scale. Four types of bioactive peptides from the sea bream (Sparus aurata) scale have been showing antihypertensive activity with various efficacies. Other bioactive peptides from different fish scales yielded antioxidant activities (10, 19). Regulation of oxidative stress and immunity plays an important role in the growth and physiological metabolism. Eventually, it could lead to the prevention of malnutrition, especially undernutrition conditions including stunting. A preclinical study by Sabrina et al. (20) showed that bioactive peptides could improve nutritional status biomarkers such as serum protein, hemoglobin, and IGF-1 levels. Stunting is a condition in which a child has a below-average height, which is two standard deviations lower than their age on the standard growth chart (21, 22). With its abundance in protein and bioactive peptides, fish scales showed interesting potential as a nutraceutical that could act to fulfill the unmet needs of the stunting population.
Therefore, this article aims to interpret the latest findings about the potential application of fish scales as a functional food that has functional compounds and peptides, which may have the potential to overcome undernutrition as a nutraceutical.
Fish scale
Fish scales are composed of type I collagen and hydroxyapatite (16). The identification of the fish scale's major components revealed that moisture and protein share the majority of fish scale weight. Maktoof et al. (9) analyzed the scales of Cyprinus carpio fish, finding that between 22.1 and 23.9% of the scales' weight consists of protein with a low lipid and carbohydrate content. The proportion of protein tends to increase in association with the increase in weight and length of the fish (9). Due to its nutritional value, especially its high protein, some researchers were able to develop nutritional food and meals from fish scales (15).
Fish scales are the source of many valuable products. Fish scales consist of a type I collagen multilayer with orderly orientation, adequate mechanical strength, transparency, and good biocompatibility (23). Fish scales collagen gained advantages because it is considered a safer collagen source compared to other animal-derived scaffolds due to the absence of zoonotic infections and religious issues (16). Alongside collagen, gelatin is also a component of interest in fish scales. Gelatin belongs to a class of protein fractions derived from collagen by thermal hydrolysis which involves breaking hydrogen bonds between polypeptide chains of collagen molecules. Due to its characteristics, gelatin has the most significant application in the food industry field, pharmaceutical, and cosmetics industries (24). Gelatin supplementation can enhance joint and bone health (25). Fish-based gelatin also encourages tissue regeneration, raising bone marrow density and offering an alternate benefit for patients with osteoporosis (26).
Aside from collagen, the fish scale also contains hydroxyapatite with various utilization values. Hydroxyapatite is the hydroxylated representative of phosphate minerals known as apatites [Ca10(PO4)6(OH)2]. Hydroxyapatite from fish scales has emerged as an alternative to substitute synthetic and bovine hydroxyapatite, due to the similarity of chemical properties that simple and inexpensive methods can achieve (Figure 1). Results from studies have shown that hydroxyapatite from fish scales demonstrated no cytotoxicity, increased mineralization in vitro, and tolerable biocompatibility in murine models (27). Hydroxyapatite constituent from fish scale was also developed as a calcium-binding peptide which promotes calcium cellular uptake (28). Those pieces of evidence suggested a significant role of hydroxyapatite in bone metabolism.
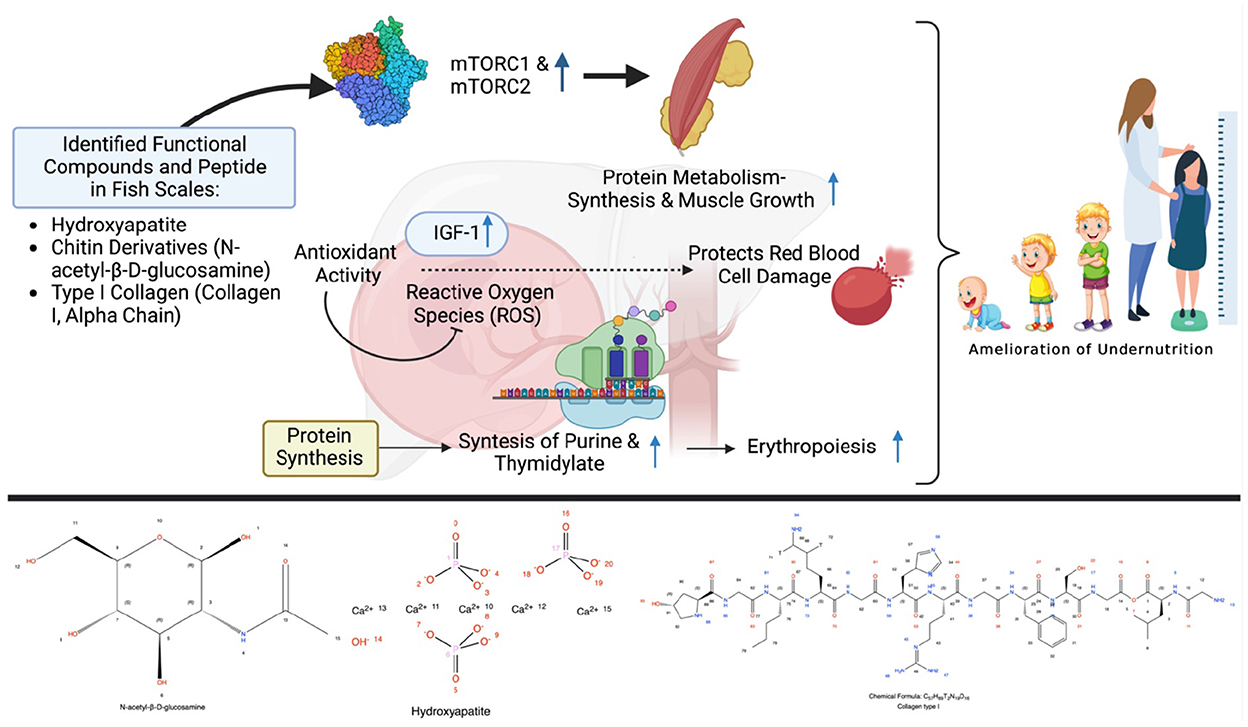
Figure 1. Possible scheme to alleviate stunting via the modulation of the metabolism by fish scale peptide supplementation. Created with BioRender.com premium license by Fahrul Nurkolis.
Chitin can also be found in fish scales. Chitin is a very attractive item owing to its biological properties and therapeutic feature via antibacterial and antifungal activities. Chitin is a long-chain odorless or tasteless amino polysaccharide of white or off-white color in its pure state, composed of N-acetyl-β-D-glucosamine units and monomers (Figure 1). The utilization of chitin derivatives is numerous, ranging from medical, pharmaceutical, food, and cosmetic industries, to nutraceuticals, bioremediation, gene therapy, and cosmetics (10). Chitin has many beneficial properties as an antioxidant, prebiotic, dietary fiber, and hypocholesterolemic agent (29). Incorporating chitin into a protein-based meal was also shown to improve growth, increase fatty acid production, and modulate gut microbiota (30).
Fish scale supports growth and prevents malnutrition through various mechanisms
Food that are rich in protein show many health benefits which are influenced by the presence of bioactive peptides (31). The antioxidative, anti-inflammatory, anticancer, antimicrobial, immunomodulatory, and antihypertensive properties of bioactive peptides derived from dietary proteins are only a few of their important roles in the living body (17). Fish scale, which is a potential source of bioactive peptides, can be utilized to synthesize chitin and chitosan, which have antioxidant, antimicrobial, and antiviral properties (32, 33). These properties may contribute to the incidence of growth retardation since it involves immune dysfunction, antioxidant, and metabolic (hormonal) system (34). Antioxidant was shown to enhance the activity of insulin-like growth factor-1 (IGF-1; Figure 1) and its receptors (35), while growth hormones also reduce oxidative stress (36). An improvement in immunity will result in a good cellular metabolism through the activation of rapamycin (mTORC1 and mTORC2; Figure 1) which promote protein synthesis, glycolysis, mitochondrial functions, and lipid synthesis (37). Bioactive peptides also upregulate calcium uptake, which is associated with healthy bone growth (38). Collagen peptides from the fish scale also showed immunomodulatory activity by protecting cells from cytotoxicity and inflammation (39, 40). Collagen peptides made from fish scales contain a unique amino acid composition with a high concentration of proline, hydroxyproline, and glycine (41). Due to its ability to control cellular redox equilibrium, proline, a non-essential amino acid, plays an important role in protein structure or function and the regulation of illnesses through extensive metabolic networks (42). Collagen contains 57% of the total amino acids, mostly glycine, proline, and hydroxyproline, which is necessary to preserve the strength and regular structure of connective tissue, including bones, skin, cartilage, and blood vessels (43).
The antimicrobial activity derived from the fish scale may also play a role in preventing malnutrition, which is supported by a systematic and meta-analysis study that found that antibiotics – which treated infections and might modulate intestinal microbiota – promoted growth in children (44). Diarrhea, water supply, sanitation, and hygiene practices were significantly associated with the incidence of malnutrition (45). Preventing infection and diarrhea through the use of antimicrobial agents against Shigella, Vibrio, Salmonella, Campylobacter, and many others is genuinely recommended (46). A considerable amount of micronutrients, such as calcium, iron, magnesium, and phosphorus, were identified in fish scales (47). Calcium and magnesium had a significant contribution to bone and muscle health (48). Next to that, multiple micronutrient supplementations had shown good results by improving growth and reducing the risk of anemia in infants (49). Overall, this strategy may give a significant contribution to preventing anemia (a risk factor for stunting) in teenage girls or pregnant mothers (50) while also potentially resolving the dual-occurrence of anemia and stunting in children (51). Hemoglobin levels were positively correlated with growth hormone levels [e.g., insulin-like growth factor I (IGF-1)] which emphasized the role of hemoglobin in preventing growth retardation (52, 53). These facts highlight the fish scale as a wonderful source of both collagen and bioactive peptides which is rich in amino acid and micronutrients, supports growth, and prevents malnutrition through various mechanisms (Figure 1).
Nutraceutical products and developments based on fish scale bioactive peptides
The processing and utilization of fish scales into a food product of health value (nutraceuticals) are a challenge for researchers. This opinion article attempts to interpret the latest findings about the potential application of fish scales as a functional food that has the potential to overcome undernutrition. However, we also aim to stimulate researchers in the exploration of bioactive peptides derived from fish scales. Therefore, there is a need for further research that focuses on this research topic. Unutilized fish scales may affect the realization of Sustainable Development Goals Number 14 (Life Below Water) since their waste can cause environmental pollution. Therefore, fish scales can be developed as functional food products through various technologies and methods, which may also reduce fish scale waste. Isolation, encapsulation, nanotechnology, and possibly fermentation are some of the alternative methods that can be used to achieve the purpose of utilizing fish skin bioactive peptides. More interestingly, fish scales have collagen composed of bioactive peptides. Supplementation of food products containing bioactive peptides in rats was shown to improve nutritional status biomarkers such as serum protein, hemoglobin, and IGF-1 levels (20). Collagen contained in fish scales will undergo a hydrolysis reaction to produce gelatin. Fish scale gelatin is a class of biopolymers containing abundant and potential bioactive amino acids and peptides, which can be utilized in savory products such as fish scale crispy (15), cookies (54), and protein hydrolysate (28). The natural characteristics of fish gelatin indicate that this fish scale gelatin product can be used as an ingredient in making jelly or agar-agar, both types of food are favored by children. This will be an added value in the intervention of malnourished children or will lead to stunting. Therefore, in addition, to being able to overcome environmental problems, the use of fish scales can also overcome nutritional problems.
Conclusion
As explained previously, there is the potential for processing fish scales into a functional food product rich in bioactive peptides, which can not only overcome environmental problems, but this can also overcome nutritional problems, especially to overcome undernutrition (Figure 1). Natural processes in the body are modulated almost exclusively by the interaction of certain amino acid sequences, either as peptides or as subsections of proteins or polypeptides. In connection with growth, proteins and peptides are involved in the modulation of cell proliferation, cell migration, inflammation, metabolism (hormonal), and protein synthesis and regulation. Research on the therapeutic peptide or bioactive analogs of specific interactive sequences derived from fish scales has opened the door to a diverse new field of pharmaceutical ingredients and functional foods for the food industry. These facts form the basis that fish scales have the potential to be a source of collagen and bioactive peptides rich in amino acids and micronutrients, support growth, and prevent malnutrition through various mechanisms. It is suitable to be applied in nutritional interventions in children with stunting. Further clinical trials related to these benefits are expected to be conducted by many researchers.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We thank Professor Hardinsyah, Ph.D. [President of the Federation of Asian Nutrition Societies (FANS) who has reviewed and provided suggestions, as well as input on the draft of this opinion article]. We also thank NM, who has given her views on the nutrition programs that are important to do now and in the future. Not to forget, we expressed our gratitude to the three institutions, namely the Sam Ratulangi University, the Hasanuddin University, and the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization Children: reducing mortality. (2016). www.who.int/mediacentre/factsheets/fs178/en/
2. Leroy JL, Frongillo EA. Perspective: What does stunting really mean? A critical review of the evidence. Advances in Nutr. (2019) 10:196–204. doi: 10.1093/advances/nmy101
3. Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. (2012) 61:8–17. doi: 10.1159/000345165
4. Jee KO. Protein energy malnutrition: An overview. Int J Hom Sci. (2021) 5:368–73. doi: 10.33545/26164485.2021.v5.i1f.341
5. Black RE, Heidkamp R. Causes of Stunting and Preventive Dietary Interventions in Pregnancy and Early Childhood. Nestle Nutr Inst Workshop Ser. (2018) 89:105–13. doi: 10.1159/000486496
6. de Onis M, Branca F. Childhood stunting: a global perspective. Matern Child Nutr. (2016) 12:12–26. doi: 10.1111/mcn.12231
7. Sambo J, Bauhofer AFL, Boene SS, Djedje M, Júnior A, Pilale A, et al. Readiness of Mozambique Health Facilities to Address Undernutrition and Diarrhea in Children under Five: Indicators from 2018 and 2021 Survey Data. Healthcare (Basel). (2022) 10:1200. doi: 10.3390/healthcare10071200
8. Fauzan MR, Dahlan CK, Taslim NA, Syam A. The effect of giving fish extract (Pujimin Plus) on intake of protein and hemoglobin hypoalbuminemic patients. Enferm Clin. (2020) 30:452–5. doi: 10.1016/j.enfcli.2020.03.009
9. Maktoof AA, Elherarlla RJ, Ethaib S. (2020). Identifying the nutritional composition of fish waste, bones, scales, and fins In: IOP Conference Series: Materials Science and Engineering IOP Publishing, p. (12013). doi: 10.1088/1757-899X/871/1/012013
10. Coppola, D., Lauritano, C., Palma Esposito, F., Riccio, G., Rizzo, C., de Pascale, D, et al. Fish waste: From problem to valuable resource. Marine Drugs. (2021) 19:116. doi: 10.3390/md19020116
11. Qin D, Bi S, You X, Wang M, Cong X, Yuan C, et al. Development and application of fish scale wastes as versatile natural biomaterials. Chem. Eng. J, (2022) 428:131102. doi: 10.1016/j.cej.2021.131102
12. Wang X. Chapter 20 - Natural bioactive compounds from fish. in Natural Bioactive Compounds. Sinha RP, Häder D-PBT-NBC (eds). Academic Press. (2021) 393–408. doi: 10.1016/B978-0-12-820655-3.00020-3
13. Nuñez SM, Guzmán F, Valencia P, Almonacid S, Cárdenas C. Collagen as a source of bioactive peptides: A bioinformatics approach. Electr J Biotechnol. (2020) 48:101–8. doi: 10.1016/j.ejbt.2020.09.009
14. Ahmed M, Verma AK, Patel R. Collagen extraction and recent biological activities of collagen peptides derived from sea-food waste: A review. Sustain Chem Pharm. (2020) 18:100315. doi: 10.1016/j.scp.2020.100315
15. Sreelakshmi KR, Raj R, Remya S, Minimol VA, Mohan CO, Ninan G, et al. Nutritional crispy from fish scales for human diet. J Aquat Food Product Technol. (2022) 31:763–74. doi: 10.1080/10498850.2022.2103760
16. Yamaura K, Mifune Y, Inui A, Nishimoto H, Mukohara S, Yoshikawa T, et al. Novel therapy using a fish scale collagen scaffold for rotator cuff healing in rat models. J Shoulder Elbow Surg. (2022) 31:2629–37. doi: 10.1016/j.jse.2022.06.024
17. Zaky AA, Simal-Gandara J, Eun JB, Shim JH, Abd El-Aty AM. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: a review. Front Nutr. (2022) 8:815640. doi: 10.3389/fnut.2021.815640
18. Tacias-Pascacio VG, Castaneda-Valbuena D, Morellon-Sterling R, Tavano O, Berenguer-Murcia Á, Vela-Gutiérrez G, et al. Bioactive peptides from fisheries residues: A review of use of papain in proteolysis reactions. Int J Biol Macromol. (2021) 184:415–28. doi: 10.1016/j.ijbiomac.2021.06.076
19. Sierra-Lopera LM, Zapata-Montoya JE. Optimization of enzymatic hydrolysis of red tilapia scales (Oreochromis sp) to obtain bioactive peptides. Biotechnol Rep. (2021) 30:e00611. doi: 10.1016/j.btre.2021.e00611
20. Sabrina N, Rizal M, Nurkolis F, Hardinsyah H, Tanner MJ, Gunawan WB, et al. Bioactive peptides identification and nutritional status ameliorating properties on malnourished rats of combined eel and soy-based tempe flour. Front Nutr. (2022) 9:963065. doi: 10.3389/fnut.2022.963065
21. Prendergast AJ, Humphrey JH. The stunting syndrome in developing countries. Paediatr Int Child Health. (2014) 34:250–65. doi: 10.1179/2046905514Y.0000000158
22. Beal T, Tumilowicz A, Sutrisna A, Izwardy D, Neufeld LM. A review of child stunting determinants in Indonesia. Matern Child Nutr. (2018) 14:e12617. doi: 10.1111/mcn.12617
23. Li KY, Pan HA, Chen KH, Kuo TL, Chou CH, Liang YJ, et al. Fish-scale collagen membrane seeded with corneal endothelial cells as alternative graft for endothelial keratoplasty transplantation. ACS Biomater Sci Eng. (2019) 6:2570–7. doi: 10.1021/acsbiomaterials.9b00562
24. Ideia P, Pinto J, Ferreira R, Figueiredo L, Spínola V, Castilho PC, et al. Fish processing industry residues: A review of valuable products extraction and characterization methods. Waste Biomass Valorizat. (2020) 11:3223–46. doi: 10.1007/s12649-019-00739-1
25. Schauss AG, Stenehjem J, Park J, Endres JR, Clewell A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: a randomized, double-blind, placebo-controlled trial. J Agric Food Chem. (2012) 60:4096–101. doi: 10.1021/jf205295u
26. Lv LC, Huang QY, Ding W, Xiao XH, Zhang HY, Xiong LX. Fish gelatin: The novel potential applications. J Funct Foods. (2019) 63:103581. doi: 10.1016/j.jff.2019.103581
27. Granito RN, Renno ACM, Yamamura H, de Almeida MC, Ruiz PLM, Ribeiro DA. Hydroxyapatite from fish for bone tissue engineering: A promising approach. Int J Molec Cell Med. (2018) 7:80-90. doi: 10.22088/IJMCM.BUMS.7.2.80
28. Lin Y, Cai X, Wu X, Lin S, Wang S. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake. J Funct Foods. (2020) 65:103717. doi: 10.1016/j.jff.2019.103717
29. Harkin C, Mehlmer N, Woortman DV, Brück TB, Brück WM. Nutritional and additive uses of chitin and chitosan in the food industry. In: Sustainable Agriculture Reviews. Crini, G., Lichtfouse, E. (eds) Cham: Springer. (2019). vol 36. doi: 10.1007/978-3-030-16581-9_1
30. Khempaka S, Chitsatchapong C, Molee W. Effect of chitin and protein constituents in shrimp head meal on growth performance, nutrient digestibility, intestinal microbial populations, volatile fatty acids, and ammonia production in broilers. J Appl. Poultry Res. (2011) 20:1–11. doi: 10.3382/japr.2010-00162
31. Chakrabarti S, Guha S, Majumder K. Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients. (2018) 10:1738. doi: 10.3390/nu10111738
32. Takarina ND, Fanani AA. Characterization of chitin and chitosan synthesized from red snapper (Lutjanus sp.) scale's waste. In: AIP Conference Proceedings. (2017). Vol. 1862, p. 030108. doi: 10.1063/1.4991212
33. Mutalipassi M, Esposito R, Ruocco N, Viel T, Costantini M, Zupo V, et al. Bioactive compounds of nutraceutical value from fishery and aquaculture discards. Foods. (2021) 10:1495. doi: 10.3390/foods10071495
34. Qi M, Tan B, Wang J, Liao S, Li J, Liu Y, et al. Post-natal growth retardation associated with impaired gut hormone profiles, immune and antioxidant function in pigs. Front Endocrinol. (2019) 10:660. doi: 10.3389/fendo.2019.00660
35. Masodsai K, Lin YY, Chaunchaiyakul R, Su CT, Lee SD, Yang AL, et al. Twelve-week protocatechuic acid administration improves insulin-induced and insulin-like growth factor-1-induced vasorelaxation and antioxidant activities in aging spontaneously hypertensive rats. Nutrients. (2019) 11:699. doi: 10.3390/nu11030699
36. Mohammadjafari H, Arazi H, Nemati N, Bagherpoor T, Suzuki K. Acute effects of resistance exercise and the use of GH or IGF-1 hormones on oxidative stress and antioxidant markers in bodybuilders. Antioxidants (Basel). (2019) 8:587. doi: 10.3390/antiox8120587
37. Linke M, Fritsch SD, Sukhbaatar N, Hengstschläger M, Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett. (2017) 591:3089–103. doi: 10.1002/1873-3468.12711
38. Liu FR, Wang L, Wang R, Chen ZX. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide–calcium complex. J Agric Food Chem. (2013) 61:7537–44. doi: 10.1021/jf401868z
39. Subhan F, Kang HY, Lim Y, Ikram M, Baek SY, Jin S, et al. Fish scale collagen peptides protect against CoCl2/TNF-α-induced cytotoxicity and inflammation via inhibition of ROS, MAPK, and NF-κB pathways in HaCaT cells. Oxid Med Cell Longev. (2017) 2017:9703609. doi: 10.1155/2017/9703609
40. Fatma N, Taslim NA, Nurilmala M. The protein and albumin contents in some species of marine and brackishwater fish of South Sulawesi, Indonesia. Aquac Aquar Conserv Legislat. (2020) 13:1976–85. Available online at: http://www.bioflux.com.ro/docs/2020.1976-1985.pdf
41. Hu Z, Yang P, Zhou C, Li S, Hong P. Marine collagen peptides from the skin of nile tilapia (oreochromis niloticus): characterization and wound healing evaluation. Mar Drugs. (2017) 15:102. doi: 10.3390/md15040102
42. Vettore LA, Westbrook RL, Tennant DA. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids. (2021) 53:1779–1788. doi: 10.1007/s00726-021-03051-2
43. Li P, Wu G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids. (2018) 50:29–38. doi: 10.1007/s00726-017-2490-6
44. Gough EK, Moodie EE, Prendergast AJ, Johnson SM, Humphrey JH, Stoltzfus RJ, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. (2014) 348:g2267. doi: 10.1136/bmj.g2267
45. Soboksa NE, Gari SR, Hailu AB, Mengistie Alemu B. Childhood malnutrition and the association with diarrhea, water supply, sanitation, and hygiene practices in kersa and omo nada districts of jimma zone, Ethiopia. Environ Health Insights. (2021) 15:1178630221999635. doi: 10.1177/1178630221999635
46. Cohen R, Raymond J, Gendrel D. Antimicrobial treatment of diarrhea/acute gastroenteritis in children. Arch Pediatr. (2017) 24:S26–9. doi: 10.1016/S0929-693X(17)30515-8
47. Begum M, Mun MZUAM, Satter MA. Nutritional profiling of selected fish's scales: An approach to determine its prospective use as a biomaterial. Int J Fish Aquat Stud. (2021) 9:26–31. Available online at: https://www.fisheriesjournal.com/archives/2021/vol9issue3/PartA/9-2-42-318.pdf
48. Capozzi A, Scambia G, Lello S. Calcium, vitamin D, vitamin K2, and magnesium supplementation and skeletal health. Maturitas. (2020) 140:55–63. doi: 10.1016/j.maturitas.2020.05.020
49. Albelbeisi A, Shariff ZM, Mun CY, Rahman HA, Abed Y. Multiple micronutrient supplementation improves growth and reduces the risk of anemia among infants in Gaza Strip, Palestine: a prospective randomized community trial. Nutr J. (2020) 19:1–11. doi: 10.1186/s12937-020-00652-7
50. Tampy ST. The associations between anemia, stunting, low birthweight, and cognitive ability in indonesian children: an analysis from indonesian family life survey. J Maternal Child Health. (2020) 5:402–412. doi: 10.26911/thejmch.2020.05.04.07
51. Gosdin L, Martorell R, Bartolini RM, Mehta R, Srikantiah S, Young MF, et al. The co-occurrence of anaemia and stunting in young children. Matern Child Nutr. (2018) 14:e12597. doi: 10.1111/mcn.12597
52. Zhang T, Ban B, Zhang M, Ji B, Sun H, Sun B, et al. Association between hemoglobin and growth hormone peak in chinese children and adolescents with short stature: a cross-sectional study. Int J Gen Med. (2021) 14:497–504. doi: 10.2147/IJGM.S292920
53. Zhao Q, Zhang M, Ji B, Chu Y, Pan H, Yan W, et al. Relationship between hemoglobin and insulin-like growth factor-1 in children and adolescents with idiopathic short stature. BMC Endocr Disord. (2020) 20:119. doi: 10.1186/s12902-020-00600-w
Keywords: fish scale, bioactive peptides, nutraceutical, stunting, growth, undernutrition, bioactive compounds, supplementation
Citation: Salindeho N, Mokolensang JF, Manu L, Taslim NA, Nurkolis F, Gunawan WB, Yusuf M, Mayulu N and Tsopmo A (2022) Fish scale rich in functional compounds and peptides: A potential nutraceutical to overcome undernutrition. Front. Nutr. 9:1072370. doi: 10.3389/fnut.2022.1072370
Received: 17 October 2022; Accepted: 21 November 2022;
Published: 09 December 2022.
Edited by:
Leila Ktari, Institut National des Sciences et Technologies de la Mer, TunisiaReviewed by:
Muhammad Faisal Manzoor, Foshan University, ChinaAbdunnur Abdunnur, Mulawarman University, Indonesia
Copyright © 2022 Salindeho, Mokolensang, Manu, Taslim, Nurkolis, Gunawan, Yusuf, Mayulu and Tsopmo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Netty Salindeho, bmV0dHlzYWxpbmRlaG8wMzEyQHVuc3JhdC5hYy5pZA==
†These authors share senior authorship
 Netty Salindeho
Netty Salindeho Jeffrie F. Mokolensang2
Jeffrie F. Mokolensang2 Nurpudji Astuti Taslim
Nurpudji Astuti Taslim Fahrul Nurkolis
Fahrul Nurkolis William Ben Gunawan
William Ben Gunawan Muhammad Yusuf
Muhammad Yusuf Nelly Mayulu
Nelly Mayulu Apollinaire Tsopmo
Apollinaire Tsopmo