
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 10 November 2022
Sec. Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1060436
This article is part of the Research Topic Diet and Gastrointestinal Cancer: Advances in Clinical and Experimental Medicine View all 9 articles
Increasing evidence demonstrated that the ketogenic diet (KD) played a positive effect on cancer treatment. However, no systematic review and bibliometric analysis were conducted in this field. This study aimed to explore the current status, and reveal the potential trends and hotspots to provide a reference for future research. Publications were extracted from the Web of Science Core Collection. CiteSpace (5.6.R3) software and the website of bibliometrics were used for visual analysis. A total of 500 publications with 334 articles and 166 reviews were included, with the timespan of 2012 to 2021. The United States was the most productive country. Majority of the top 10 institutions were from the United States, and Harvard University was the top-contributing institution. The most prolific author and the co-cited author was Thomas N Seyfried from Boston College. The highest cited reference was published in PLoS ONE, authored by Abdelwahab Mohammed G, with 161 citations. Glioma and breast cancer were the most common types of cancer in this field, while hepatocellular carcinoma and pancreatic cancer were the new hotspots. The anti-tumor mechanism of KD mainly focused on regulating metabolism, decanoic acid, oxidative stress, fatty acid oxidation, and cell apoptosis. Additionally, the presence of “chemotherapy” and “radiotherapy” in the keywords indicated that KD combined with anti-tumor research was a topic, while “immunotherapy” has became a recent frontiers. Notably, as a metabolic therapy, KD was deserved more attention in the treatment of hepatocellular carcinoma and pancreatic cancer, and KD combined with immunotherapy was the new hotspot and frontier. Additionally, more molecular studies and high-quality uniformly, randomized, controlled clinical trials are urgently warranted to evaluate the effect of KD in multiple cancers.
Cancer is currently the second leading cause of death worldwide, and also placed a heavy burden on health care systems as one of the major public health problems that were difficult to administrate (1). The GLOBOCAN 2020 estimated to account for 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020 (2). Nowadays, numerous studies have found that diet was closely related to the occurrence and development of cancer (3, 4). Ketogenic diet (KD) consists of a low-carbohydrate combined with high-fat dietary pattern that was first used for the treatment of intractable epilepsy in children (5). Recently, studies has confirmed that KD also maintained an obvious therapeutic effects in a variety of neurological disorders, including, but not limited Alzheimer's and Parkinson's disease, autism, and multiple sclerosis (6–9). Notably, an increasing number of studies were dedicated to the effects of KD on cancer (5, 10), and it has been reported that KD exerted an anti-tumor role by reducing glucose (11), inflammatory response, and oxidative stress (12), inhibiting the mTOR signaling pathway (13), and regulating immune (14).
Bibliometric analysis has been widely used to explore developmental trends and hotspots through quantitative analysis of publications (15). To our knowledge, although the number of publications on KD in the field of cancer were increased steadily, there was no systematic review and bibliometric analysis was performed. Therefore, we visualized the publications with vivid information to (1) identify the most productive, contributors, the key topics, and highly frequent keywords; and (2) to reveal the current research trends of KD on cancer, as well as explore the potential hotspots to shed the light for future research.
All included data in this study were retrieved from the Web of Science Core Collection (WOSCC) with the following search strings: “Topic = (ketogenic diet) OR (ketogenic diets) OR (ketone diet) OR {[(low carb diet) OR (low carbohydrate diet) OR (carbohydrate-restricted diet)] AND (high fat diet)} and [(neoplasia) OR (neoplasm) OR (neoplasms) OR (carcinoma) OR (tumors) OR (cancer) OR (cancers) OR (malignancy)],” and the retrieved publications were published from January 1, 2012 to December 31, 2021. The detailed search strategies and results were also demonstrated in Table 1. Only original articles and reviews were included; other types, such as editorial materials, letters, and meeting abstracts were excluded. However, there were no geographical restrictions.
The publication retrieval and data collection were completed on September 2, 2022 to reduce the deviation caused by daily updating of the WOSCC. All the retrieved publications were screened, respectively, by two researchers (RRL and HQC) to ensure their relevance to the topic of this study after the initial data search. Furthermore, the annual number of publications, country/region outputs, journals, citations per paper (CPP), and H-index were also retrieved from the WOSCC. Additionally, impact factors (IF) and quartiles for journal categories were obtained from the Journal Citation Reports 2021 (JCR). Any disagreements were resolved at the discretion of a third senior reviewer (YW).
The website of bibliometrics (http://bibliometric.com/) was applied to generate the visualization cooperation map of the countries/regions, thereby the countries that cooperate most closely with each other were identified. CiteSpace is widely used for visual analysis of publications, which is an excellent choice to identify the current research hotspots and frontiers (16, 17). All data retrieved from WOSCC were exported to CiteSpace software in the mode of “full record and cited references,” which is used to analyze various important bibliometric parameters, including analysis of countries, journals, institutions, authors, references, and a timeline view of cited-references. In addition, keywords co-occurrence clusters and bursts were also captured.
A total of 912 publications in the field of KD on cancer research were retrieved from WOSCC, with the time span of January 1, 2012 to December 31, 2021. Publications that were not articles or reviews were excluded (n = 67). Additionally, publications that were unrelated to the research topic (n = 345) were also excluded after a careful review of the titles and abstracts by two authors (RRL and CXY). Ultimately, a total of 500 publications were included for final visual analysis, and the specific flowchart of publications screening was shown in Figure 1.
A total of 500 non-duplicate publications, including 334 articles and 166 reviews, were included for bibliometric analysis from 2012 to 2021. The growth of annual publication outputs and annual citations was shown in Figure 2. The number of publications related to KD and cancer had increased steadily in the last decade, whereas there were still some fluctuations between this year. Notably, the number of publications in this field peaked in 2020, with 103 publications, more than five times that of 2012 (n = 20) (Figure 2A). As shown in Figure 2B, the number of annual citations had increased significantly over the last decade, reaching the highest point in 2021 (citations = 4,753).
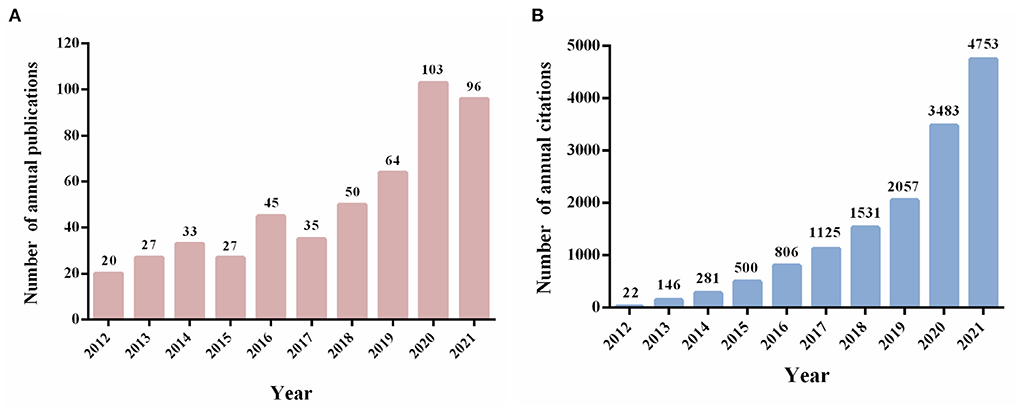
Figure 2. Trends in annual publication outputs and the number of citations in the field of KD on cancer from 2012 to 2021. (A) Trends of annual publication outputs. (B) Trends of annual citations.
The distribution of all 500 publications covered 57 countries/regions. The top 10 most productive countries/regions were listed in Table 2. The United States was the most prolific country (46.20% of 500, with 231 papers), with the highest number of citations (11,969) and the highest centrality (0.89); followed by Germany (12.80%, with 64 papers), Italy (10.20%, with 51 papers), China (8.00%, with 40 papers), and England (7.60%, with 38 papers). Additionally, the top 10 annual national publication outputs were also identified. The most prolific country of the annual publications was also the United States. Notably, the number of publications in China increased significantly in 2021, surpassing Germany and Italy (Figure 3A). As shown in Figure 3B, the United States and Canada possessed the closest international collaborative in this field, followed by Germany, China, Brazil, and France. The visual analysis of the international cooperation network suggested that 57 countries had established cooperation, with 136 links among one another. Nonetheless, the cooperation was largely centered on the United States, with less cooperation among the remaining countries/regions (Figure 3C).
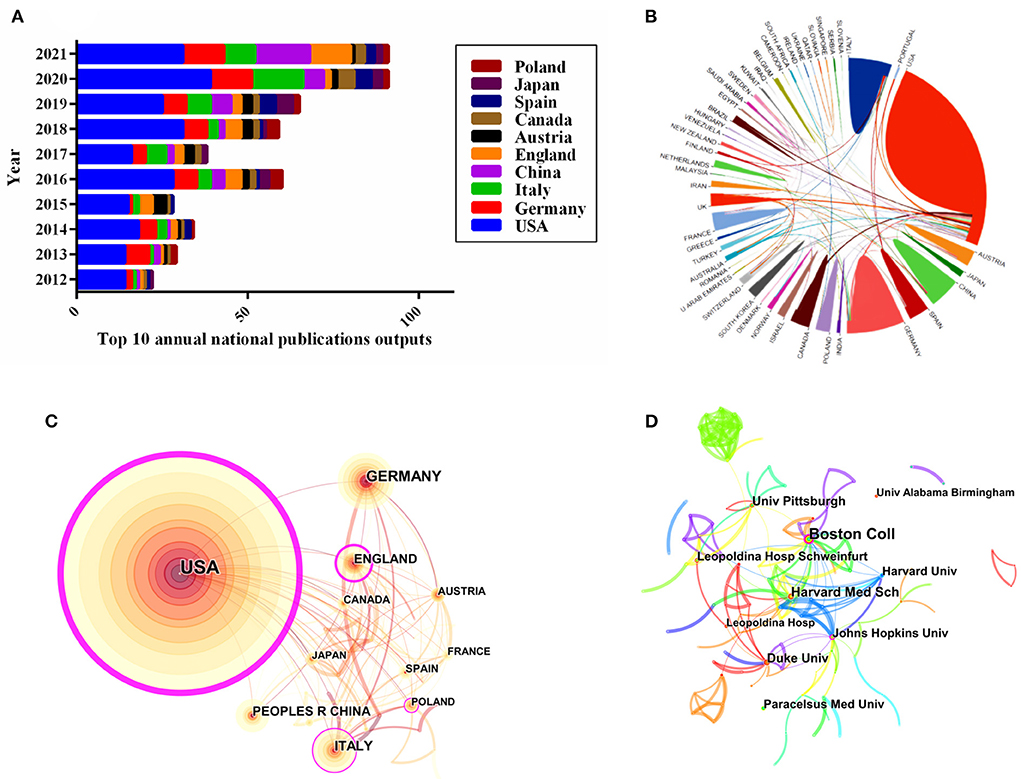
Figure 3. Visualization map of countries/regions and institutions in the field of KD on cancer from 2012 to 2021. (A) Number of annual publications and growth trends of the top 10 countries/regions. (B) The international network cooperation between the countries, exported the results from the website of bibliometrics (http://bibliometric.com). (C) Citespace network map of KD in cancer research involving 57 countries. (D) Collaboration network analysis of institutions.
The top 10 most prolific institutions were shown in Table 3. The leading top 5 institutions of publications were Harvard University with 29 papers, followed by Boston College (18), Pennsylvania Commonwealth System of Higher Education (17), University of California System (17), and Johns Hopkins University (16), respectively. Majority of the top 10 institutions were from the United States. with Harvard University having the highest H-index (20) and the National Institutes of Health ranking the highest number of citations per publication (CPP, 113.15). To further explore the potential relationship between institutions, a visual mapping of institutions was generated by using Citespace. Based on Figure 3D, there were 284 institutions and 335 links, indicating that the distribution of research teams among the institutions was relatively scattered, which shed light on more disciplinary collaboration was urgently needed.
The retrieved publications in this study were published in 285 journals, and the top 10 productive journals were shown in Table 4. Nutrients was ranked the first in the number of publications (4.80%, with 24 records), followed by PLoS ONE (3.60%, with 18 records), Frontiers in Nutritio (2.40%, with 12 records), International Journal of Molecular Sciences and Nutrition and Cancer-An International Journal (1.8%, with 9 records). Notably, the journal of Cell Metabolism had both the highest IF (31.372) and CPP (127.50) among the top 10 prolific journals in this field. Further, half of the productive journals had their category quartile in Q1 (Table 4).
The visual map of authors consisted of 314 nodes by using Citespace. The top 10 most prolific authors were shown in Table 5. Thomas N Seyfried was the most prolific author, with 12 publications, followed by Rainer J Klement (11), Barbara Kofler (9), Adrienne C Scheck (6), and Reinhart A Sweeney (6), respectively. The collaboration of 105 authors was demonstrated in Figure 4A. Furthermore, the network visualization of co-cited authors was shown in Figure 4B. The largest nodes were related to the authors with the highest number of collaborations, including Thomas N Seyfried (140 citations), Rainer J Klement (133 citations), Warburg O (118 citations), Nebeling LC (96 citations), and Rieger J (94 citations) (Table 5). Interestingly, Thomas N Seyfried and Rainer J Klement were the authors with the highest number of publications and co-citations, which indicated that they may be core researchers in the field of KD and cancer research.
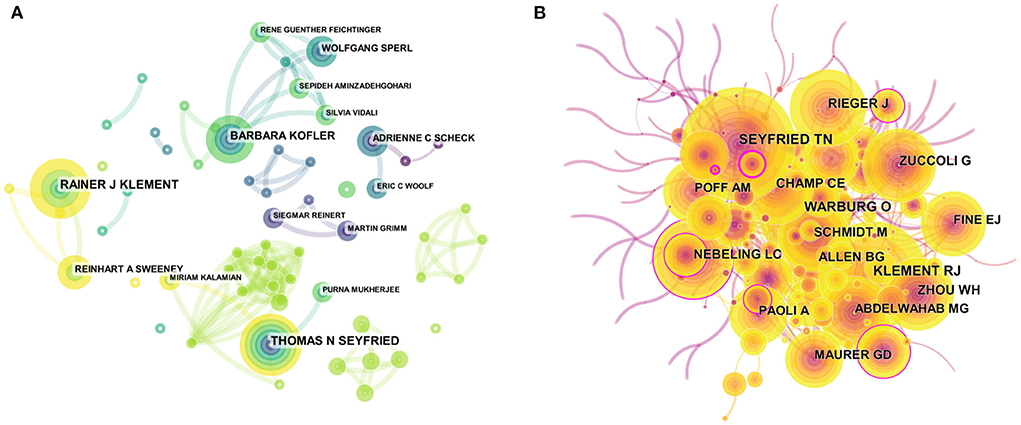
Figure 4. Visualization map of authors/cited-authors. (A) Visualization map of authors. (B) Visualization map of cited-authors. Nodes indicate authors, and the lines between nodes indicate collaborative relationships. The larger the node area, the greater the number of publications by that author or cited author.
The visual map of cited-references consisted of 507 nodes and 2,674 links (Figure 5A). The characteristics of the top 10 highest cited-references were shown in Table 6. Based on Table 6, 5 of the top 10 highest cited-references were related to basic and clinical research on glioma, which indicated that research on glioma has been a hotspot in this field. Among them, entitled “The Ketogenic Diet Is an Effective Adjuvant to Radiation Therapy for the Treatment of Malignant Glioma” was published by Abdelwahab, MG, with the highest frequency of citations, suggesting that KD could significantly enhance the anti-tumor effect of radiotherapy, and pointed out that KD-induced cellular metabolism may be the current adjuvant treatment standard for malignant gliomas (18). In addition, the article entitled “Ketogenic Diets Enhance Oxidative Stress and Radio-Chemo-Therapy Responses in Lung Cancer Xenografts” published in Clinical Cancer Research (with IF 13.801), revealed that KD can improve the chemoradiotherapy response of lung cancer by modulating oxidative stress and inhibiting the proliferation of cancer cells (19).
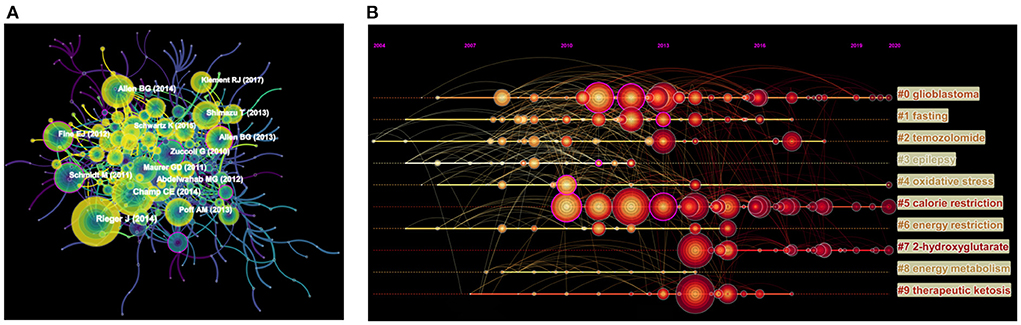
Figure 5. (A) Network visualization map of cited-references in the field of KD on cancer research. The nodes represent cited-references, and the lines between the nodes represent cited-references relationships. (B) Timeline view of co-cited references in the field of KD on cancer research. The cluster with warmer color and larger nodes contained more publications, indicating that this clustering issue was the hotspot in this field.
The timeline view of the cited-references in the field of KD on cancer research was generated by using Citespace (Figure 5B), with the bold timeline indicating that the clustering topic was a hotspot during this period. As shown in Figure 5B, 9 cluster labels were extracted from the cited-references using the log-likelihood rate method, among which glioblastoma (cluster #0) and temozolomide (cluster #2) were closely related; while fasting (cluster #1), oxidative stress (cluster #4), and 2-hydroxyglutarate (cluster #7) may reveal the potential mechanism of KD in cancer treatment.
A total of 21 keywords were identified as occurring more than 25 times (Figure 6A). Notably, “metabolism,” “ketone body,” and “caloric restriction” were the keywords that appeared most frequently in this field over the past 10 years, except for the topic terms of “ketogenic diet” and “cancer.” In addition, a visual map of keywords co-occurrence clusters mainly included but not limited to the following aspects: cluster #0 named “decanoic acid,” was the largest cluster, followed by “oxidation,” “c-reactive protein,” “hyperthermia,” “metabolomics” and “immunotherapy” (Figure 6B).
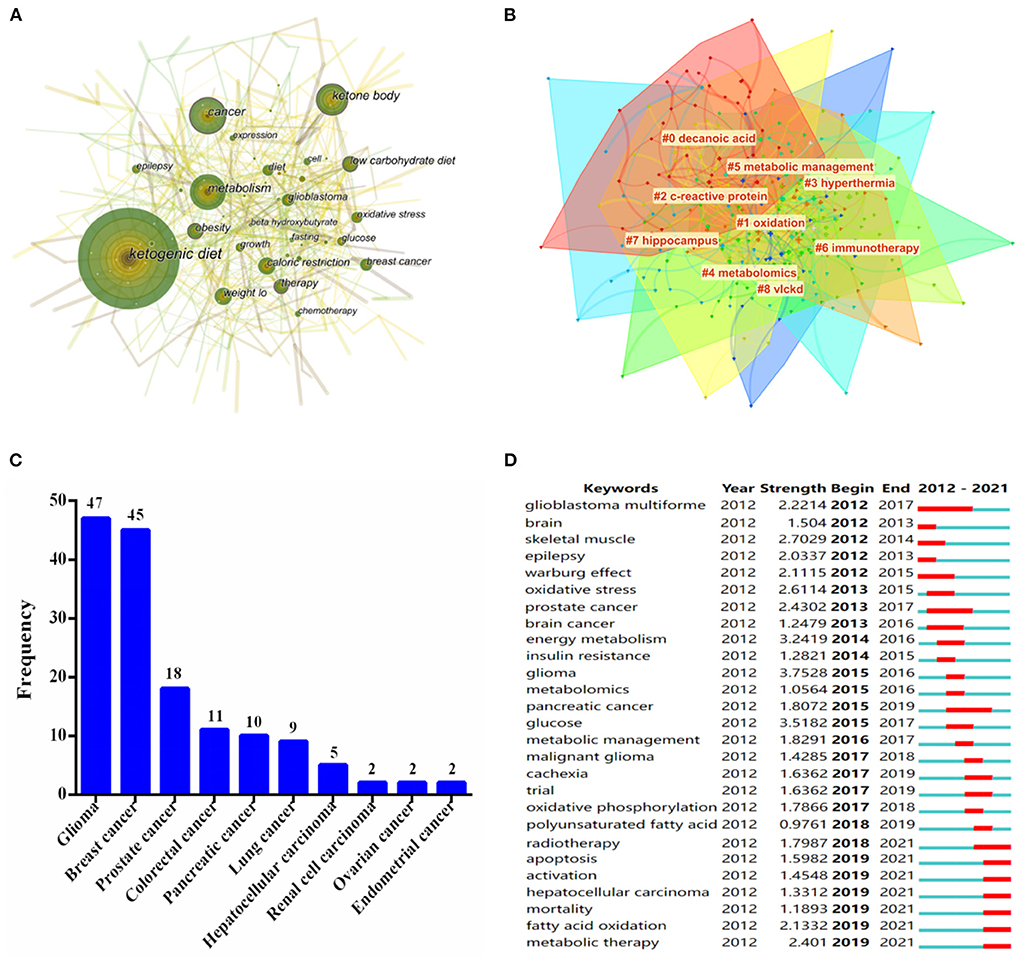
Figure 6. (A) Map of keywords occurrence in the field of KD on cancer research from 2012 to 2021. (B) The cluster of keywords in the field of KD on cancer research. The different colors mean different clusters. (C) Top 10 cancer types in the field of KD on cancer research. (D) Keywords with periods of the strongest burst from 2012 to 2021 among the top 25 burst keywords in publications in the field of KD on cancer research.
To our interest, the application of KD in various types of cancers over the past decade was summarized by further analyzing of the keywords. As shown in Figure 6C, glioma had become a research hotspot in this field, with a frequency of 47, followed by breast cancer (45), prostate cancer (18), colorectal cancer (11), and pancreatic cancer (10), respectively. This finding indicated that the earliest application of KD in the treatment of glioma, while it was also crucial in the treatment of breast cancer or other types of cancers.
Specifically, keywords burst detection was executed to acquire a brisk sight of future research trends in the field of KD on cancer (Figure 6D). The red line indicated a sudden burst of keywords during the relevant period, whereas the blue line means unpopularity. Figure 6D demonstrate that glioblastoma multiforme was the earliest research hotspot in the field of KD on cancer, spanning the years 2012 to 2017, with a burst strength of 2.2214. When focusing on the keywords after 2019, the strongest burst keywords were “apoptosis,” “activation,” “hepatocellular carcinoma,” “mortality,” “fatty acid oxidation,” and “metabolic therapy,” respectively.
This study is the first bibliometric and visual analysis for the research of KD on cancer. In this study, a total of 500 publications were included, with 334 articles and 166 reviews, spanning the period from 2012 to 2021. Publications on KD and cancer research showed an upward trend over the last decade, especially in the last 2 years, with the numbers of publications remaining around 100, indicating that this field is receiving increasing attention from the researchers. A total of 57 countries/regions and 314 authors contributed to this field.
The most productive country was the United States, with the highest number of citations, centrality, and annual publications. Meanwhile, most of the top 10 institutions were from the United States, with the leading institutions were Harvard University, Boston College, Pennsylvania Commonwealth System of Higher Education, and the University of California System, respectively, indicating that the United States was the main driver of the highest academic reputation in this field. Additionally, Germany, Italy, the People's Republic of China, and England excited a certain influence in this field, which was confirmed by the following characteristics: number of publications, number of citations, and the H-index values. Notably, the number of publications from China increased significantly in 2021, surpassing Germany and Italy, which indicated that Chinese scholars have paid more attention to this field in recent years.
All publications were published in 285 journals, with the leading journals including Nutrients, PLoS ONE, and Frontiers in Nutrition. Interestingly, Thomas N Seyfried from Boston College was the most prolific author and the most co-cited author, suggesting that the author had a high level of achievement in this field. Additionally, among the top 10 highly cited references in the field of KD on cancer research, we found that more than half of them reported on KD for malignant glioma. The highest cited reference was published in PLoS ONE, and authored by Abdelwahab Mohammed G with 161 citations, which reported that KD enhanced the anti-tumor effect of radiotherapy in malignant gliomas by elevating the level of β-hydroxybutyrate (18). Furthermore, “malignant glioma” was the most common type of cancer in this field, which may be closely related to the first use of KD in the treatment of brain glioma.
The hotspots and frontiers in this field could be revealed by the highest cited references, analysis of keyword burst and co-occurrence clusters. By generalizing the analysis, we can draw the following points in this field. First, the hotspots and trends of KD in cancer types. The keywords “hippocampus,” “glioblastoma” and “temozolomide” from the clusters of keywords and co-cited references indicated that the research of KD on cancer was mainly focused on glioma. As well-known, KD was first used in epilepsy treatment for effective symptoms control (20). Meanwhile, one in four patients undergoing epilepsy surgery has brain tumors, indicating a strong connection was existed between the brain tumors and epilepsy (21). In addition, keywords analysis demonstrated that KD has high attention on the research of breast cancer and prostate cancer. A recent clinical study demonstrated that KD therapy played a beneficial role by reducing TNF- α, and insulin, and increasing IL-10 in breast cancer patients (22). Besides, Kaiser et al. concluded that KD could inhibit the growth of prostate cancer through regulation of the insulin resistance signaling pathways (23). Notably, the burst keywords of “hepatocellular carcinoma” and “pancreatic cancer” indicated that the treatment of KD in the two types of cancer were the new hotspots. Wang et al. reported that KD could inhibit the progression of HCC by increasing the expression of hydroxymethylglutaryl-CoA synthase 2 in hepatocytes and regulating lipid metabolism (24). Meanwhile, KD exerted anti-HCC effects and increased serum β-hydroxybutyrate levels by inducing histone hyperacetylation (25). Summarily, liver is the glucose and lipid metabolic center that produces ketone bodies, KD has a broad application prospect in the treatment of HCC (26). In addition, several recent studies have demonstrated that KD effectively inhibited the proliferation of pancreatic by reprogramming cell metabolism, epigenome, and the gut microbiome (27, 28). These results suggested that glioma and breast cancer were the most studied cancer types in this field, while hepatocellular carcinoma and pancreatic cancer were the new hotspots and trends.
Second, the hotspots and trends of the anti-tumor mechanism of KD. Through the analysis of keywords and cited-references, we found that the anti-tumor mechanism of KD mainly focused on regulation of metabolism, decanoic acid, fatty acid oxidation, oxidative stress, and apoptosis. Robust evidence showed that KD executed anti-tumor effect by regulating metabolism, which is manifested by a decrease in glucose concentration and an increase in the concentration of ketone body and 3-hydroxybutyric acid (29, 30). Ketone body and 3-hydroxybutyric acid were confirmed to play an important role in promoting tumor immunity by inhibiting the expression of NLRP3 inflammasome (31). Cancer cells were mainly metabolized using aerobic glycolysis (Warburg effect), nevertheless KD as a carbohydrate-restricted dietary pattern, can effectively inhibit glucose/insulin signaling, mitochondrial metabolism and inflammation reaction, thereby hindering the development and deterioration of cancers (32, 33). Notably, the latest study found that KD could promote the production of decanoic acid, and inhibit the AMPA receptor, given that to inhibit the growth of cancer (34). Unfortunately, oxidative stress may aggravate both the damage of tissue and the progression of cancer via promoting the formation of reactive lipid fragments (35). Nevertheless, oxidative stress was significantly inhibited by KD therapy to contribute the effects of anti-inflammation and anti-tumor properties (36). Meanwhile, the metabolism regulation of KD to exert anti-tumor effects has been widely researched, while elucidating the anti-tumor effects of KD from the perspective of decanoic acid and fatty acid oxidation is a current hotspot.
Third, the hotspots and trends of KD synergy with anti-tumor. Based on the analysis of keywords, several keywords related to anti-tumor treatment, such as chemotherapy, radiotherapy, and temozolomide were identified, indicating that KD combined with anti-tumor therapy is a hotspot in this field. Further study confirmed that breast cancer patients in the KD combined with chemotherapy group had a higher quality of life and physical activity scores at 6 weeks compared to the chemotherapy group, while lower levels of lactate and ALP in serum, suggesting that KD may significant beneficial for the patients with breast cancer (37). Simultaneously, accumulating evidence suggested that KD therapy can improve chemotherapy sensitivity and reduce target lesions in different cancers, such as lung cancer, pancreatic cancer, and gastric cancer (38–41). In addition, radiotherapy is the mainstay of anti-tumor treatment, which can significantly improve the survival rate of cancer patients. Study had reported that KD preserved fat free and skeletal muscle mass and effectively improved global quality of life in breast cancer patients undergoing curative radiotherapy (13); it also partially counteracted the adverse effects of radiotherapy on body composition in head and neck cancer patients (42). Importantly, the cluster of keywords showed that immunotherapy is the new hotspot in this field recently. Ferrere G confirmed that KD enhanced the anti-neoplastic effects of PD-1 blockade (43). Further, the latest experiment reinforced theory that KD partly stimulated anti-tumor immunity and strengthened checkpoint blockade immune-therapy via AMPK mediated decreasing PD-L1 protein abundance (14). To the best of our knowledge, most of studies had focused on animal experiments in this field, whereas the robust clinical evidence was still inadequacy. Hence, more high-quality randomized clinical trials to evaluate the combined effects of KD and anti-neoplastic therapy, especially the combination of KD and immunotherapy, are urgently warranted, which is the current research trend in this field. Notably, although KD was regarded as a tolerable method during the clinical management of cancers, some adverse effects have also been reported, such as causing constipation, fatigue, and micronutrient deficiencies in the patients with cancers (44, 45). Therefore, more attention should be paid to the potential adverse reactions of KD in the treatment of anti-tumor in the future clinical researches.
This is the first bibliometric and visual analysis in the field of KD on Cancer, and this study identified the latest research trends and hotspots in this field. Hence, a perspective to the developing trend of KD on cancer was presented, which may help researchers to explore new directions for future research. However, some limitations should be illustrated. First, the data were only extracted from the WOSCC, and no other databases (such as PubMed and Embase) were used, which may lead to under-extraction of publications. However, WOSCC has collected more than 20,000 authoritative and influential academic journals. Therefore, the results and findings of this study were robust. Second, only studies published in English were retrieved from the database, which may lead to source bias. In addition, although we have clearly defined the research topic during the search, it is still not guaranteed that every document is completely relevant to the topic, which leads to the results of incomplete inclusion of core keywords. However, incorrect extraction and omission of publications could be prevented by topic searches. Therefore, this study can still highlight the general situation and research trends in this field.
This study provided a general overview of the main research hotspots and frontiers of KD on cancer research. Summarially, glioma and breast cancer were the most studied cancer types in this field, while hepatocellular carcinoma and pancreatic cancer gained more attention recently. In addition, KD may exert anti-tumor effects by regulating metabolism, oxidative stress, and fatty acid oxidation. Meanwhile, current clinical studies mainly focused on KD combined with chemoradiotherapy for glioma and breast cancer, and the findings demonstrated that KD could effectively improve the anti-tumor effects, reduce adverse effects, and enhance the quality of life. However, more molecular studies and corresponding large-sample RCTs are urgently needed to assess the clinical application of KD as a metabolic therapy, and the potential adverse effects of KD in cancer treatment should be closely concerned. Meanwhile, the development of diversified ketogenic products to improve patient compliance is also a hotspot for future research due to the difficulty of long-term adherence to KD in cancer patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RL, YW, JF, and YL designed the study. RL, QH, and CY were responsible for data collection and drafted the manuscript. RL, QH, CY, CW, and NL were responsible for investigation, figures, and tables construction. YW, JF, and YL revised and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Traditional Chinese Medicine Research Program of Zhejiang Province (No. 2018ZQ020); Medical and Health Platform Program of Zhejiang Province (No. 2021KY556).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lawrence WR, McGee-Avila JK, Vo JB, Luo Q, Chen Y, Inoue-Choi M, et al. Trends in cancer mortality among black individuals in the US From 1999 to 2019. JAMA Oncol. (2022) 8:1184–9. doi: 10.1001/jamaoncol.2022.1472
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. (2022) 22:452–66. doi: 10.1038/s41568-022-00485-y
4. Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. (2021) 375:n2213. doi: 10.1136/bmj.n2213
5. Mohammadifard N, Haghighatdoost F, Rahimlou M, Rodrigues AP, Gaskarei MK, Okhovat P, et al. The effect of ketogenic diet on shared risk factors of cardiovascular disease and cancer. Nutrients. (2022) 14:3499. doi: 10.3390/nu14173499
6. Phillips MC, Deprez LM, Mortimer G, Murtagh DK, McCoy S, Mylchreest R, et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer's disease. Alzheimers Res Ther. (2021) 13:51. doi: 10.1186/s13195-021-00783-x
7. Phillips MC, Murtagh DK, Gilbertson LJ, Asztely FJ, Lynch CD. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. (2018) 33:1306–14. doi: 10.1002/mds.27390
8. Li Q, Liang J, Fu N, Han Y, Qin J. A ketogenic diet and the treatment of autism spectrum disorder. Front Pediatr. (2021) 9:650624. doi: 10.3389/fped.2021.650624
9. De Marchi F, Collo A, Scognamiglio A, Cavaletto M, Cionci NB, Biroli G, et al. Study protocol on the safety and feasibility of a normocaloric ketogenic diet in people with amyotrophic lateral sclerosis. Nutrition. (2022) 94:111525. doi: 10.1016/j.nut.2021.111525
10. Xiang Y, Wang M, Miao H. Ketogenic diet: new avenues to overcome colorectal cancer. Signal Transduct Target Ther. (2022) 7:262. doi: 10.1038/s41392-022-01113-9
11. Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, et al. A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma. Clin Cancer Res. (2016) 22:2482–95. doi: 10.1158/1078-0432.CCR-15-0916
12. Lorenzo PM, Sajoux I, Izquierdo AG, Gomez-Arbelaez D, Zulet MA, Abete I, et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin Nutr. (2022) 41:1566–77. doi: 10.1016/j.clnu.2022.05.007
13. Klement R J, Champ C E. Calories, carbohydrates, and cancer therapy with radiation: exploiting the five R's through dietary manipulation. Cancer Metastasis Rev. (2014) 33:217–29. doi: 10.1007/s10555-014-9495-3
14. Dai X, Bu X, Gao Y, Guo J, Hu J, Jiang C, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. (2021) 81:2317–31. doi: 10.1016/j.molcel.2021.03.037
15. Gu C, Wang Z, Pan Y, Zhu S, Gu Z. Tungsten-based nanomaterials in the biomedical field: a bibliometric analysis of research progress and prospects. Adv Mater. (2022) 2022:e2204397. doi: 10.1002/adma.202204397
16. Sabe M, Pillinger T, Kaiser S, Chen C, Taipale H, Tanskanen A, et al. Half a century of research on antipsychotics and schizophrenia: a scientometric study of hotspots, nodes, bursts, and trends. Neurosci Biobehav Rev. (2022) 136:104608. doi: 10.1016/j.neubiorev.2022.104608
17. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
18. Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. (2012) 7:e36197. doi: 10.1371/journal.pone.0036197
19. Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. (2013) 19:3905–13. doi: 10.1158/1078-0432.CCR-12-0287
20. Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. (2013) 36:32–40. doi: 10.1016/j.tins.2012.11.005
21. Blumcke I, Aronica E, Urbach H, Alexopoulos A, Gonzalez-Martinez JA, A. neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol. (2014) 128:39–54. doi: 10.1007/s00401-014-1288-9
22. Khodabakhshi A, Akbari ME, Mirzaei HR, Seyfried TN, Kalamian M, Davoodi SH. Effects of Ketogenic metabolic therapy on patients with breast cancer: a randomized controlled clinical trial. Clin Nutr. (2021) 40:751–8. doi: 10.1016/j.clnu.2020.06.028
23. Kaiser A, Haskins C, Siddiqui MM, Hussain A, D'Adamo C. The evolving role of diet in prostate cancer risk and progression. Curr Opin Oncol. (2019) 31:222–9. doi: 10.1097/CCO.0000000000000519
24. Wang YH, Suk FM, Liao YJ. Loss of HMGCS2 enhances lipogenesis and attenuates the protective effect of the ketogenic diet in liver cancer. Cancers. (2020) 12:1797. doi: 10.3390/cancers12071797
25. Feng S, Wang H, Liu J, et al. Multi-dimensional roles of ketone bodies in cancer biology: opportunities for cancer therapy. Pharmacol Res. (2019) 150:104500. doi: 10.1016/j.phrs.2019.104500
26. Lan Y, Jin C, Kumar P, Yu X, Lenahan C, Sheng J. et al. Ketogenic diets and hepatocellular carcinoma. Front Oncol. (2022) 12:879205. doi: 10.3389/fonc.2022.879205
27. Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: cellular mechanisms and clinical perspectives. Nutrients. (2021) 13. doi: 10.3390/nu13093202
28. Zhang Y, Zhang T, Yang W, Chen H, Geng X, Li G. Beneficial diets and pancreatic cancer: molecular mechanisms and clinical practice. Front Oncol. (2021) 11:630972. doi: 10.3389/fonc.2021.630972
29. Licha D, Vidali S, Aminzadeh-Gohari S, Alka O, Breitkreuz L, Kohlbache O. Untargeted metabolomics reveals molecular effects of ketogenic diet on healthy and tumor xenograft mouse models. Int J Mol Sci. (2019) 20:3873. doi: 10.3390/ijms20163873
30. Dabek A, Wojtala M, Pirola L, Pirola L, Balcerczy K. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients. (2020) 12:788. doi: 10.3390/nu12030788
31. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. (2017) 25:262–84. doi: 10.1016/j.cmet.2016.12.022
32. Lane J, Brown NI, Williams S, Plaisance E, Fontaine K. Ketogenic diet for cancer: critical assessment and research recommendations. Nutrients. (2021) 13. doi: 10.3390/nu13103562
33. Barrea L, Caprio M, Tuccinardi D, Moriconi E, Renzo L, Muscogiuri G. Could ketogenic diet “starve” cancer? Emerging evidence. Crit Rev Food Sci Nutr. (2022) 62:1800–21. doi: 10.1080/10408398.2020.1847030
34. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak J. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. (2016) 139:431–43. doi: 10.1093/brain/awv325
35. Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. (2017) 30:187–92. doi: 10.1097/WCO.0000000000000432
36. Jin Z, Berthiaume JM Li Q, Henry F, Huang Z, Sadhukhan S. Catabolism of (2E)-4-hydroxy-2-nonenal via ω- and ω-1-oxidation stimulated by ketogenic diet. J Biol Chem. (2014) 289:32327–38. doi: 10.1074/jbc.M114.602458
37. Khodabakhshi A, Seyfried T N, Kalamian M, et al. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutr J. (2020) 19:87. doi: 10.1186/s12937-020-00596-y
38. Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: the university of iowa experience of two phase 1 clinical trials. Radiat Res. (2017) 187:743–54. doi: 10.1667/RR14668.1
39. Iyikesici MS. Feasibility study of metabolically supported chemotherapy with weekly carboplatin/paclitaxel combined with ketogenic diet, hyperthermia and hyperbaric oxygen therapy in metastatic non-small cell lung cancer. Int J Hyperthermia. (2019) 36:446–55. doi: 10.1080/02656736.2019.1589584
40. Iyikesici MS. Survival outcomes of metabolically supported chemotherapy combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy in advanced gastric cancer. Niger J Clin Pract. (2020) 23:734–40. doi: 10.25000/acem.650341
41. Iyikesici MS. Long-term survival outcomes of metabolically supported chemotherapy with gemcitabine-based or FOLFIRINOX regimen combined with ketogenic diet, hyperthermia, and hyperbaric oxygen therapy in metastatic pancreatic cancer. Complement Med Res. (2020) 27:31–9. doi: 10.1159/000502135
42. Klement RJ. Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: V Final results of the KETOCOMP study for head and neck cancer patients. Strahlenther Onkol. (2022) 2022:1–13. doi: 10.1007/s00066-022-01941-2
43. Ferrere G, Tidjani AM, Liu P, Goubet AG, Fidelle M, Kepp O. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.145207
44. Champ CE, Palmer JD, Volek JS, Wasik MW, Andrews DW, Evans JJ. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. (2014) 117:125–31. doi: 10.1007/s11060-014-1362-0
Keywords: bibliometric analysis, ketogenic diet, cancer, mechanism, immunotherapy, clinical trials
Citation: Li R, Huang Q, Ye C, Wu C, Luo N, Lu Y, Fang J and Wang Y (2022) Bibliometric and visual analysis in the field of ketogenic diet on cancer from 2012 to 2021. Front. Nutr. 9:1060436. doi: 10.3389/fnut.2022.1060436
Received: 03 October 2022; Accepted: 27 October 2022;
Published: 10 November 2022.
Edited by:
Pei-hao Yin, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Jing Zhuang, Weifang Traditional Chinese Hospital, ChinaCopyright © 2022 Li, Huang, Ye, Wu, Luo, Lu, Fang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Lu, bHV5aUB6amNjLm9yZy5jbg==; Jianqiao Fang, ZmFuZ2ppYW5xaWFvNzUzMkAxNjMuY29t; Yun Wang, d2FuZ3l1bjYxNjg5NzU2QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.