
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 15 November 2022
Sec. Nutrition and Food Science Technology
Volume 9 - 2022 | https://doi.org/10.3389/fnut.2022.1055651
Broccoli (Brassica oleracea L. var. Italic) is rich in nutrition. However, it is susceptible to yellowing after harvest, leading to nutritional and economic losses. In this study, diacetyl, a natural food additive compound, was selected to inhibit the yellowing of broccoli florets and maintain the nutrient quality during storage time. It was found that 20 μl L–1 diacetyl treatment for 12 h could significantly delay the yellowing and decrease the weight loss and lignin content of broccoli florets. Meanwhile, diacetyl could maintain higher contents of chlorophyll, vitamin C and flavonoids and suppress the transcript levels of chlorophyll degradation–related genes in broccoli florets. Moreover, accumulations of reactive oxygen species (ROS) were inhibited by diacetyl treatment. Under diacetyl treatment, the generation of ethylene was prevented by inhibiting the activities and related-gene expressions of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase. Based on our findings, exogenous diacetyl could be employed as a novel bioactive molecule for retarding the yellowing and maintaining the quality of postharvest broccoli.
Broccoli (Brassica oleracea L. var. Italic) is favored by consumers as it is rich in vitamin C, soluble fibers, and nutraceutical compounds (1). However, it tends to rapidly senesce after harvest. Senescence often causes quality deterioration, such as yellowing, water loss, increasing in lignin content, and decreasing of vitamin C, flavonoids, and other nutrients, eventually leading to losses of the commercial value (2).
Several studies have been devoted to investigating the mechanism of broccoli senescence. Ethylene was reported to play an essential role in regulating the postharvest quality of broccoli (3). Wounding could induce ethylene synthase in broccoli florets (4). 1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) were crucial enzymes in the ethylene synthesis pathway, which played a critical role in regulating the quality of broccoli (5). Lowering the gene expression and activities of ACS and ACO could retard the floret yellowing of broccoli (6). The inhibition of BoACO2 expression in broccoli decreased the biosynthesis of ethylene and kept it green. Previous studies showed that the BoACS1, BoACS2 and BoACS3, which encoded ACC synthase, were differentially expressed in the senescence course of broccoli florets (7). Meanwhile, reactive oxygen species (ROS) were also sharply accumulated by harvesting broccoli and up-regulated by ethylene (8, 9). Excessive ROS accumulations induced by biotic or abiotic stresses caused oxidative stress damage to broccoli, which accelerated chlorophyll degradation and then led to the loss of quality. The antioxidant system of plant was gradually enhanced to resist oxidative stress with the senescence processing (10, 11). In previous study, the broccoli displayed yellowing during senescence with the decreasing chlorophyll content, which was triggered by ROS and ethylene (11). Thus, lower ethylene and ROS contents were beneficial to maintaining the broccoli quality.
Series of enzymes were reported to regulate the chlorophyll degradation. Firstly, chlorophyll degradation is the transformation of chlorophyll b into chlorophyll a, which is catalyzed by CBR (chlorophyll b reductase) and HCAR (7-hydroxymethyl chlorophyll a reductase) (2). The CBR enzyme is encoded by the genes of NYC1 (NON-YELLOW COLORING 1) and NOL (NYC-LIKE) (12). During the early chlorophyll-degrading process, it is confirmed that pheophytinase (PPH, encoded by PPHs genes) took part in removing pheophytin and forming pheophorbide. Then pheophorbide a oxygenase (PAO, encoded by PAO gene) accelerates the unfolding of the porphyrin macrocycle, which further promotes the chlorophyll degradation (13). Moreover, SGR (Stay Green) gene, the upstream of the PAO pathway, was also involved in the degradation of chlorophyll (14).
Various physical and chemical techniques have been used to maintain the quality of broccoli. Physical techniques include low temperature (15), modified atmosphere packaging (16), ultraviolet irradiation (17), and so on. But these methods require a huge expense. Some chemical compound was also used in postharvest quality maintenance. For example, 1-methylcyclopropene (1-MCP) is a chemical agent for retarding yellowing of broccoli effectively (18). Although some chemosynthetic preservatives are effective and the residue is low enough, the food safety issue is still concerned by consumers. Some natural food additives are reported to be used for preserving fruit and vegetables. For example, chitosan oligosaccharides can alleviate the calyx senescence of mandarin fruits by decreasing the abscisic acid content (19, 20). Folic acid can inhibit the senescence of broccoli by improving the antioxidant capacity (21). Therefore, the development of safe and natural preservatives is the hotspot of future research (22).
Diacetyl, also known as 2,3-butanedione, is naturally present in bay leaves, honey, wine, and balsamic vinegar (23, 24). It was also an aroma component of ripe fruit, such as jalapeno peppers, sweet peppers (25), and lucuma (Pouteria lucuma) fruit (26). The US Food and Drug Administration (FDA) believes that the ingestion of diacetyl in food is generally recognized as safe (GRAS)1. Diacetyl is also a widely used food additive in China (GB1886.51-2015). Recent studies have shown that diacetyl can act as an anti-microbial organic composition applied in mandarins, grapes, apples and strawberries (27, 28). Moreover, diacetyl can inhibit abiotic stress-induced senescence in Arabidopsis according to the latest study (29). It implies that diacetyl can be used in vegetables senescence during storage. Little information is available on the influence of diacetyl on the postharvest quality of fruit and vegetables during storage.
The objective of this study was to study the effect of diacetyl on inhibiting the yellowing of broccoli florets and maintaining the nutrient quality during storage time. Thus, the postharvest broccoli florets were treated with various concentrations of diacetyl during storage time.
Diacetyl (PubChem CID:650) was obtained from Macklin Biochemical Co., Ltd. (Shanghai, China).
Broccoli (Brassica oleracea L. var. Italica, cv. You-xiu) was purchased from Aolaifeng Market, Tai’an, Shandong, China, and transported to our laboratory as soon as possible. The broccoli heads with tight florets and uniform size, maturity, color, and free from diseases and mechanical damage were chosen for the subsequent study. The selected broccoli heads were washed with tap water, drained, and dried with paper towel. All operations of measurement and fumigation with diacetyl were carried out in the ventilation equipment to ensure that the experimental operators were not exposed to the volatile.
Experiment 1 was carried out to investigate the effect of diacetyl on the visual quality of broccoli florets and determine the suitable concentration to inhibit the senescence. The broccoli heads were cut carefully with a sharp knife into florets with length of 7–8 cm and approximately 15 g each. Then the florets were randomly divided into six groups, about 350 g per group. The selected 350 g broccoli florets as one replicate (three replicates for each concentration) were put in a container with a total volume of 5 L (LocknLock Co., Ltd.), sealed, and then fumigated at 25 ± 1°C for 12 h at concentrations of 0, 1, 5, 10, 20, and 40 μl L–1 (volume of liquid diacetyl/volume of the container) diacetyl. The concentration in the control group was 0 μl L–1. After fumigation, the container was opened, ventilated, and then covered with the lid again but not sealed. The florets were kept at 25 ± 1°C for 4 d. The changes in quality were evaluated visually and recorded using images daily.
Experiment 2 was designed to investigate the effect of diacetyl on the objective qualities of florets and explore the mechanism. Twenty-one boxes of broccoli florets were prepared and fumigated in the same way as in experiment 1 but with only 0 and 20 μl L–1 (the selected optimum concentration of diacetyl from Expt. 1). At shelf life of 0, 1, 2, 3, and 4 d after fumigation, broccoli florets were randomly selected every day from each treatment and three independent biological replicates were set. After measuring the color changes, chlorophyll content and ethylene production for each floret, the remaining florets were frozen with liquid nitrogen, ground into powder, and finally stored at –80°C for further physiology and biochemical analysis.
The color of broccoli florets was assayed using the method proposed by Xu et al. (21). It was measured with a digital colorimeter (CR-400, Konica Minolta, Japan) and the a* (red, +or green, −), b* (yellow, +or blue, −) values were determined daily. The sampled broccoli florets from each replicate were randomly selected and tested at 5 equidistant points. The contents of chlorophyll a and b were determined according to the method of Sun et al. (30). Three samples of the fresh broccoli florets were collected at each time point (0 d, 1 d, 2 d, 3 d, 4 d), and 0.5 g of fresh broccoli florets were taken. Then 30 ml of ethanol (95%) was added to extract for 22 h (normal temperature and avoid light). The resulting supernatant was collected and used as a blank control. The absorbance at 470 nm, 665 nm, and 649 nm wavelength was measured to calculate the concentrations of chlorophyll a and b using the following equation:
Ca = 13.95 × A665nm-6.88 × A649nm
Cb = 24.96 × A649nm-7.32 × A665nm
Ca and Cb are the concentrations of chlorophyll a and b, respectively.
The weight loss was assayed as described by Xu et al. (21).
The content of vitamin C was measured according to the method reported by Sohail et al. (31). Frozen broccoli floret powder (10 g) was extracted with 20 ml of meta-phosphoric acid–acetic acid solution. Then 5 ml of solution was added to 2 ml of the ground extract, and the mixture was filtered through a cheesecloth. Samples were titrated in 2,6-dichloroindophenol dye solution until a light pink color developed and kept for 5 s. The content of vitamin C was represented as g kg–1 of broccoli.
Flavonoid content was determined according to the NaNO2–Al (NO3)3 colorimetric method (32). Frozen broccoli floret powder (1 g) was mixed with 30 ml of 70 % ethanol, extracted for 1.5 h at 65°C, and then centrifuged at 10,000 × g for 20 min at 25°C. The supernatant extract (1 ml) was added to 70 % ethanol (1 ml) and 0.3 ml of 5 % NaNO2, mixed thoroughly and placed for 6 min. Then 0.3 ml of 10 % Al (NO3)3 was added to the mixture. The mixture was placed at 25°C for 6 min. Subsequently, 2 ml of 4 % NaOH was added and reacted for 10 min. The absorbance was measured at 510 nm. Rutin was used as a standard to calculate the flavonoid content. The concentration of flavonoid was represented as g kg–1.
The lignin content was determined as described by Yu et al. (33). Frozen broccoli floret powder (2 g) was weighed, added to a 15-ml centrifuge tube containing 5 ml of precooled 95 % ethanol, and centrifuged at 10,000 × g for 15 min. The sediment was washed with 95 % ethanol and ethanol–hexane, collected, and completely dried at 60°C. The dried sediment was mixed with 1 ml of 25 % acetylacetonate in acetic acid and reacted at 70°C for 30 min. Then 1 ml of 2 mol L–1 NaOH was added to the aforementioned mixture to end the reaction, followed by the addition of 0.1 ml of 7.5 mol L–1 hydroxylamine hydrochloride and 2 ml of glacial acetic acid, and centrifugation at 12,000 × g for 10 min. The absorbance of supernatant was measured at 280 nm. The lignin content was represented as g kg–1 based on fresh weight.
The ethylene generation rate was determined according to Zaharah et al. (34). One hundred gram of fresh broccoli florets were sealed in a 5-L container and stored at 25°C for 12 h. Furthermore, 1 ml of headspace gas was injected into a gas chromatography (7820A, Agilent Technologies, Inc., the United States of America). The ethylene level was calculated according to the linear relationship between the peak area and ethylene concentration.
The ACC synthase activity was measured according to the method described by Zaharah et al. (34). Briefly, 10 ml of extraction buffer (containing 1 mmol L–1 ethylene diamine tetraacetic acid (EDTA), 1 mmol L–1 phenylmethylsulfonyl fluoride (PMSF), 4 mmol L–1 dithiothreitol (DTT), 3 % polyvinylpolypyrrolidone (PVPP), and 10 μmol L–1 pyridoxal phosphate) was mixed with 2 g of frozen powder of broccoli flower buds, swirled and shocked immediately, and then centrifuged at 4°C and 12,000 × g for 30 min. The supernatant was collected to obtain the enzyme extract.
The enzyme extract (0.5 ml) and reaction buffer (1.5 ml) were added to a 10-ml sample bottle (with a rubber stopper) and placed at 30°C for 1 h. Then 0.1 ml of 25 mmol L–1 HgCl2 solution was injected to terminate the reaction, and the mixture was placed in an ice bath for 10 min. Subsequently, 0.2 ml of precooled 5 % NaClO–saturated NaOH solution was added, shaken quickly for 5 s, and incubated in the ice bath for 5 min. The gas (1 ml) was extracted from the headspace, and the amount of ethylene generation was determined by gas chromatography.
1-aminocyclopropane-1-carboxylic acid oxidase activity was measured according to the protocol of Zaharah et al. (34). A volume of 10 ml of extraction buffer (containing 10% glycerin, 5% PVPP, 5 mmol L–1 DTT, 30 mmol L–1 sodium ascorbate, and 0.1 mmol L–1 FeSO4) was mixed with 2 g of frozen powder of broccoli flower buds. The remaining measurement steps were the same as that in Section 2.5.1.
The enzyme extract (0.5 ml) and reaction buffer (1.5 ml) were added to a 20-ml sample bottle (with a rubber stopper). Subsequently, 1 ml of NaHCO3 was injected into the sample bottle and incubated at 30°C for 30 min. Then 1 ml of the gas was extracted from the headspace to determine the ethylene release.
The contents of H2O2 and O2– were assayed according to the protocols of Hu et al. (35) and Jin et al. (36).
The activities of CAT and POD were determined based on the method proposed by Hu et al. (35). One gram of frozen broccoli powder was added to 5 ml of 0.1 mol L–1 PBS (pH = 7) containing 0.05 g PVPP, swirled and shocked immediately and centrifuged at 4 °C and 12,000 × g for 15 min, the supernatant was used to analyze the enzyme activity. The CAT activity was assayed by recording the decrease every 30 s for 3 min at 240 nm, the reaction system consisted of 2 ml of PBS, 0.8 ml of 0.3 % H2O2 and 0.5 ml of supernatant. The POD activity was measured according to the oxidation of guaiacol by hydrogen peroxide. The reaction liquid included 50 ml of PBS, 19 μL of 30 % H2O2 and 28 μl of guaiacol. The absorbance value of the mixture was recorded every 30 s for 3 min at 470 nm. The results were expressed in U kg–1.
Total RNA of broccoli florets was extracted using an Omini Plant RNA Kit (Cowin Biosciences, Beijing, China) and cDNA was obtained using a HifiScript cDNA Synthesis Kit (Cowin Biosciences, Beijing, China), respectively, following the manufacturers’ instructions. The concentrations of total RNA and cDNA were determined using the BioPhotometer D30 (Eppendorf AG, Germany).
The primers of RT-qPCR used in this study were reported in published articles (2, 5, 37–39). All primers used (Supplementary Table 1) in this study were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The Ultra SYBR Mixture Kit (Cowin Biosciences, Beijing, China) was used in RT-qPCR assays.
In this study, all experimental designs were fully performed with three biological replicates. The SPSS software was used to conduct the significant difference by the least significant difference test (P < 0.05, P < 0.01). The data were expressed as means ± standard deviations.
The broccoli florets gradually lost green color and then decayed, leading to the development of off-odors. On day 4, the florets of the control obviously yellowed and decayed. Whereas, 5, 10, 20, and 40 μl L–1 diacetyl treatments maintained greener color and better quality compared with the control. The results showed that the anti-yellowing effect was increased with higher concentrations of diacetyl (Figure 1A). Thus, we selected 20 μl L–1 diacetyl treatment for the further research (Figures 1B–D). It was found that a* and b* values of broccoli florets showed an increasing trend in four days (Figures 1C,D). The b* values of diacetyl treatment were lower than that in control, indicating a decreased yellowing degree with diacetyl treatment. The a* value of the control increased obviously, while the value of the florets under diacetyl treatment changed a little during shelf life and was lower than the control at the end of four days. The values of a* and b* (Figures 1C,D) were consistent with the visual color (Figure 1B). Our study suggested that suitable concentrations of diacetyl treatment could maintain the visual quality of broccoli during ambient storage.
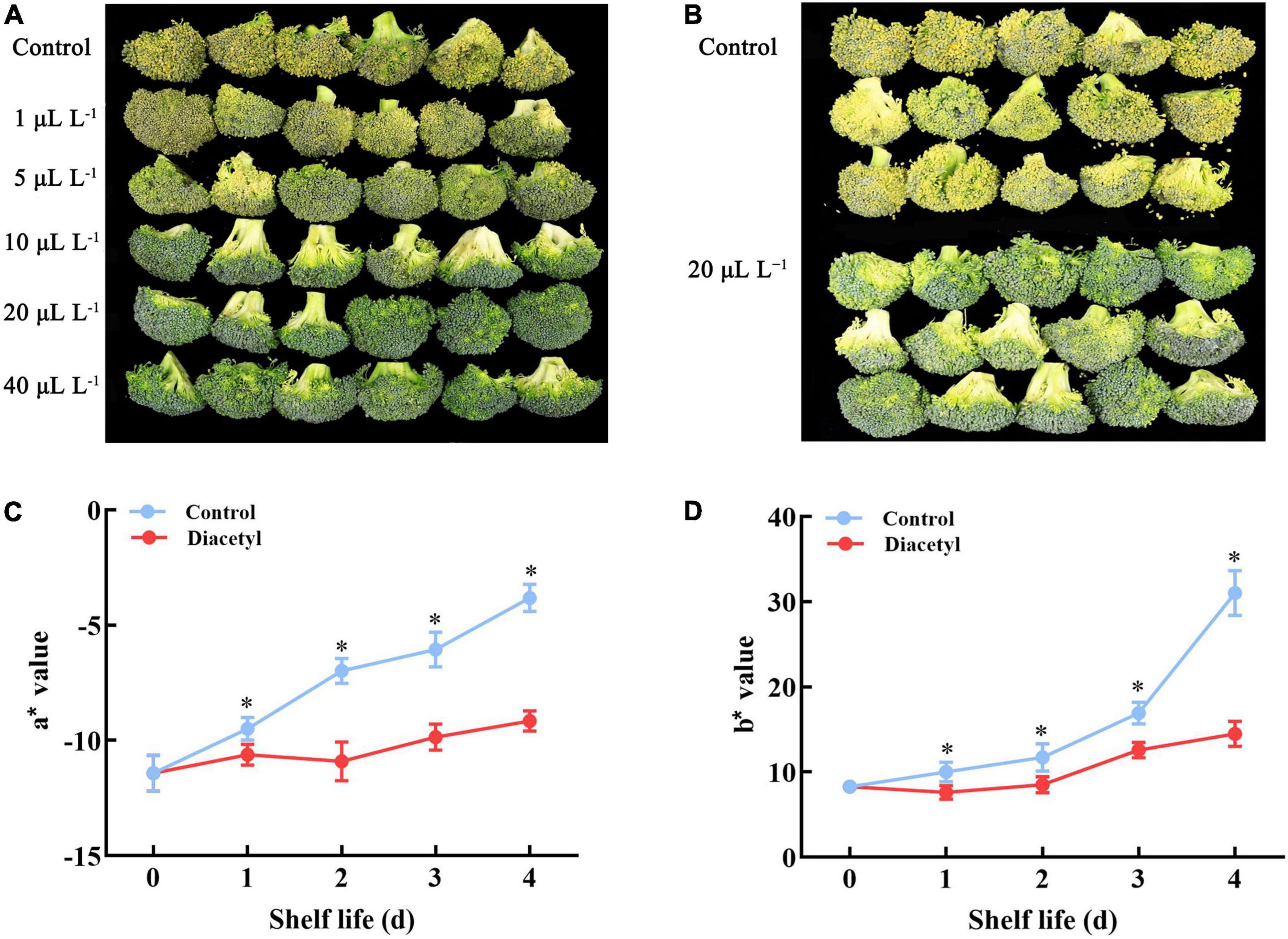
Figure 1. Diacetyl delayed broccoli floret yellowing during shelf life. Appearance of broccoli florets treated with different concentrations of diacetyl (A), appearance of broccoli florets treated with 20 μl L–1 diacetyl (B), a* (C) and b* (D) values of broccoli florets treated with 20 μl L–1 diacetyl. Data are the average of three replicates ± SD. Asterisk (*) indicates a significant difference among the control and diacetyl treatment groups at the same time at P < 0.05.
Then the quality of broccoli florets was studied during shelf life. Firstly, weight loss of broccoli florets was measured with or without the diacetyl treatment. It was found that broccoli florets showed a slower weight loss under diacetyl treatment compared to the control (Figure 2A). Broccoli is rich in vitamin C and flavonoid (2), thus the contents of vitamin C and flavonoid were also analyzed. As demonstrated in Figures 2B,C, diacetyl-treated florets showed higher contents of vitamin C and flavonoid than the control during shelf life. Meanwhile, texture is one of the important quality indices of fruit and vegetables. The lignin content is closely correlated with textural changes. As a component of the cell wall, the lignin content can reflect the senescence degree (40). According to Figure 2D, accumulation of lignin increased slowly in the diacetyl-treated broccoli florets. Therefore, these results suggested that diacetyl treatment could maintain the quality of broccoli florets during shelf life.
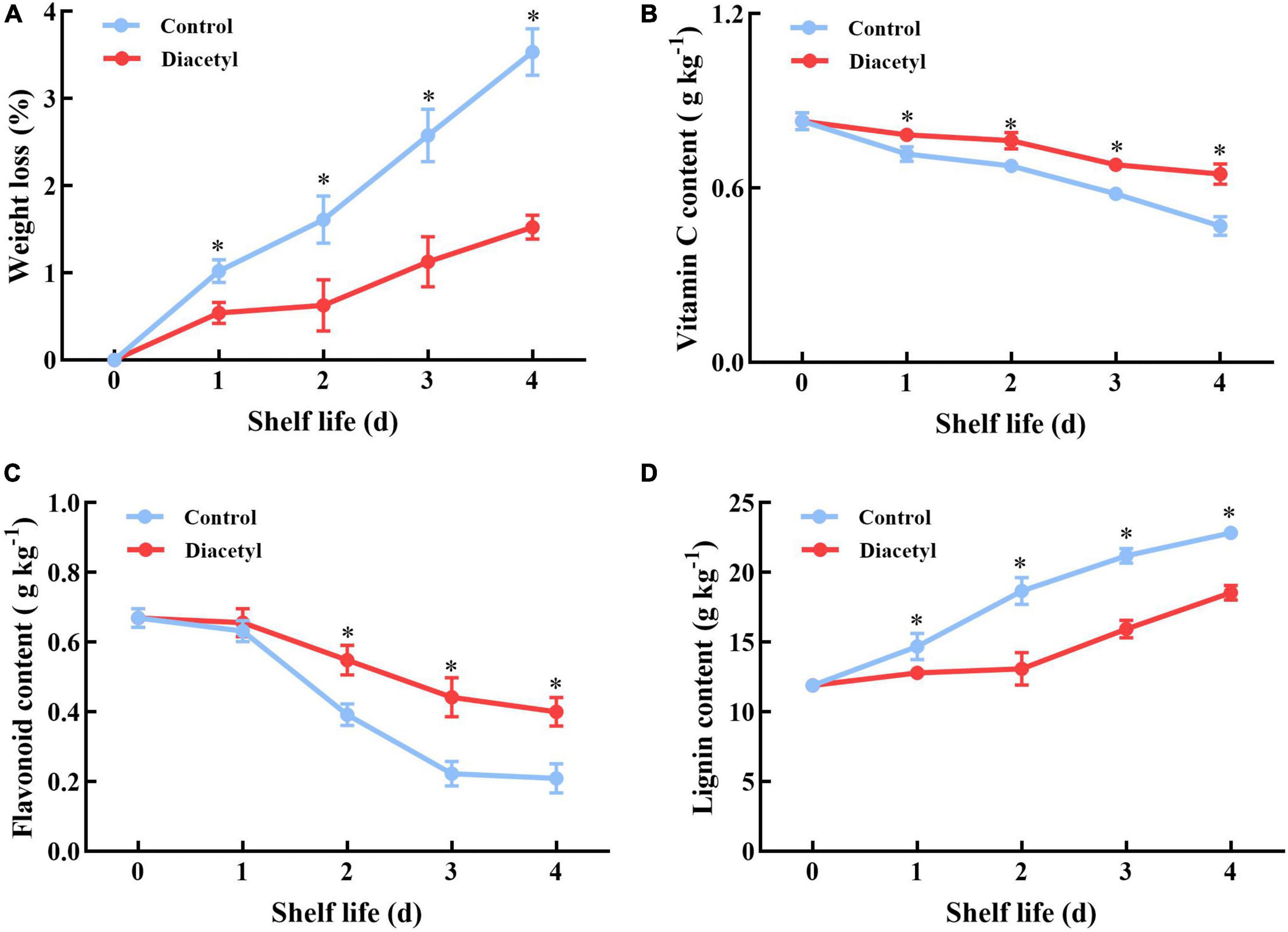
Figure 2. Diacetyl reduced the weight loss (A) and inhibited the decrease in the contents of vitamin C (B) and flavonoid (C) and the increase in the lignin content (D) in broccoli florets during shelf life. Data are the average of three replicates ± SD. Asterisk (*) indicates a significant difference among the control and diacetyl treatment groups at the same time at P < 0.05.
The levels of chlorophyll a and b presented an overall downward trend during broccoli florets shelf life, whereas diacetyl-treated plants maintained a higher chlorophyll content than the control (Figures 3A,B). It indicated that diacetyl could inhibit the degradation of chlorophyll in broccoli florets. These results were consistent with the differences in a* and b* values between the control and treated florets.
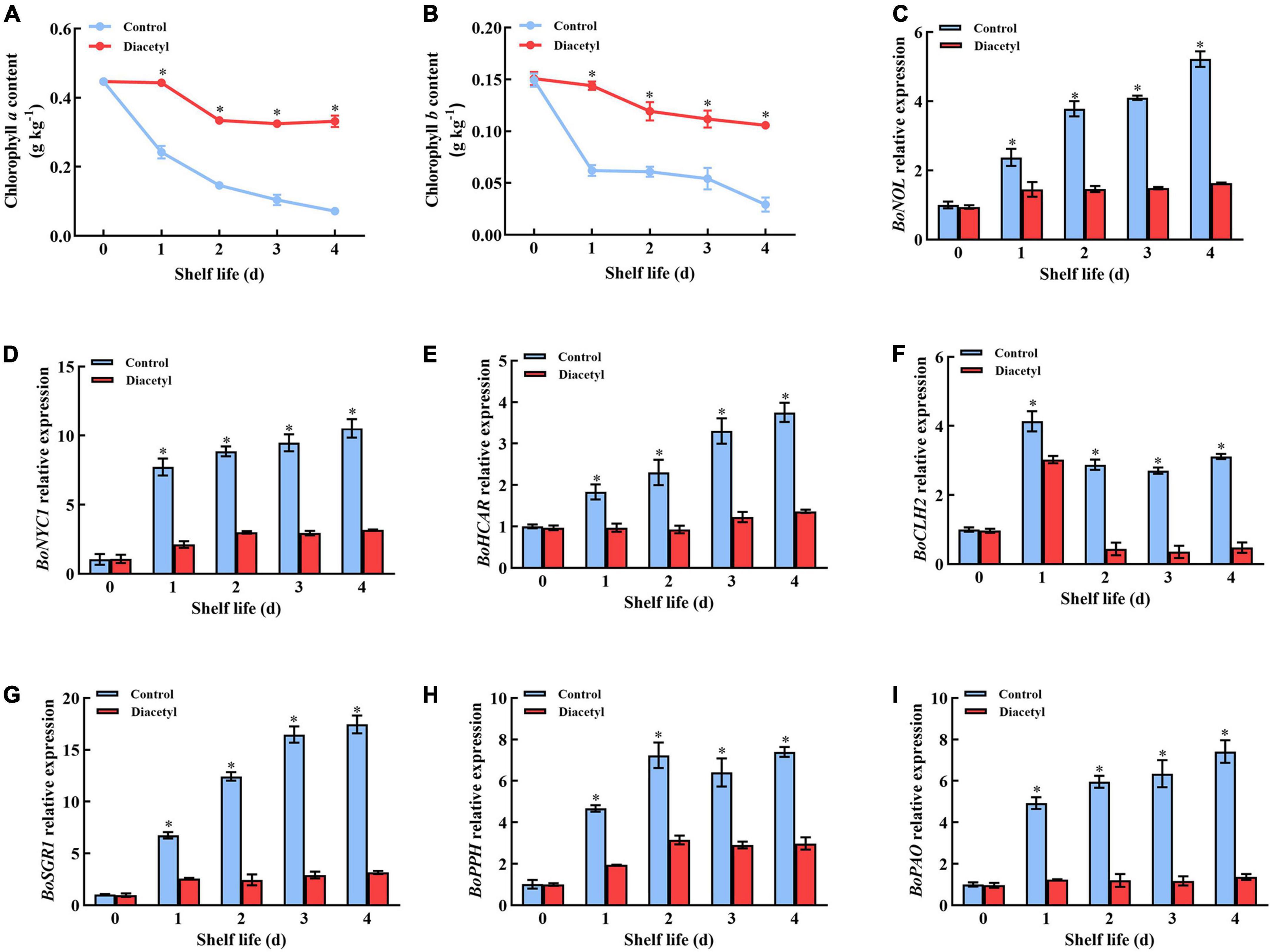
Figure 3. Diacetyl treatment inhibited the decrease in the chlorophyll content and the expression of chlorophyll degradation–related genes broccoli florets during shelf life. Chlorophyll a content (A), chlorophyll b content (B), and the relative expression level of BoNOL (C), BoNYC1 (D), BoHCAR (E), BoCLH2 (F), BoSGR1 (G), BoPPH (H), and BoPAO (I). Data are the average of three replicates ± SD. Asterisk (*) indicates a significant difference among the control and diacetyl treatment groups at the same time at P < 0.05.
Then various chlorophyll degradation-related genes were selected to perform further exploration. The transcript levels of BoNOL, BoNYC1, BoHCAR, BoCLH2, BoSGR1, BoPPH, and BoPAO genes showed an upward trend during shelf life in both control and treated broccoli florets (Figures 3C–I). However, the transcript levels of all these genes were always lower after diacetyl treatment. The results were consistent with the chlorophyll content (Figures 3A,B). It suggested that diacetyl inhibited chlorophyll degradation by suppressing the expression of chlorophyll degradation-related genes in broccoli florets.
The accumulations of ROS showed increasing trends in broccoli over time. Whereas, the levels of H2O2 and O2– were lower than that in control with the diacetyl treatment (Figures 4A,B). Then the activities of POD and CAT were determined. It was found that the activities of POD and CAT were higher in diacetyl treated broccoli, although all showed an overall increasing trend (Figures 4C,D). It suggested that diacetyl treatment could improve the antioxidant capacity and scavenge the accumulation of ROS during shelf life.
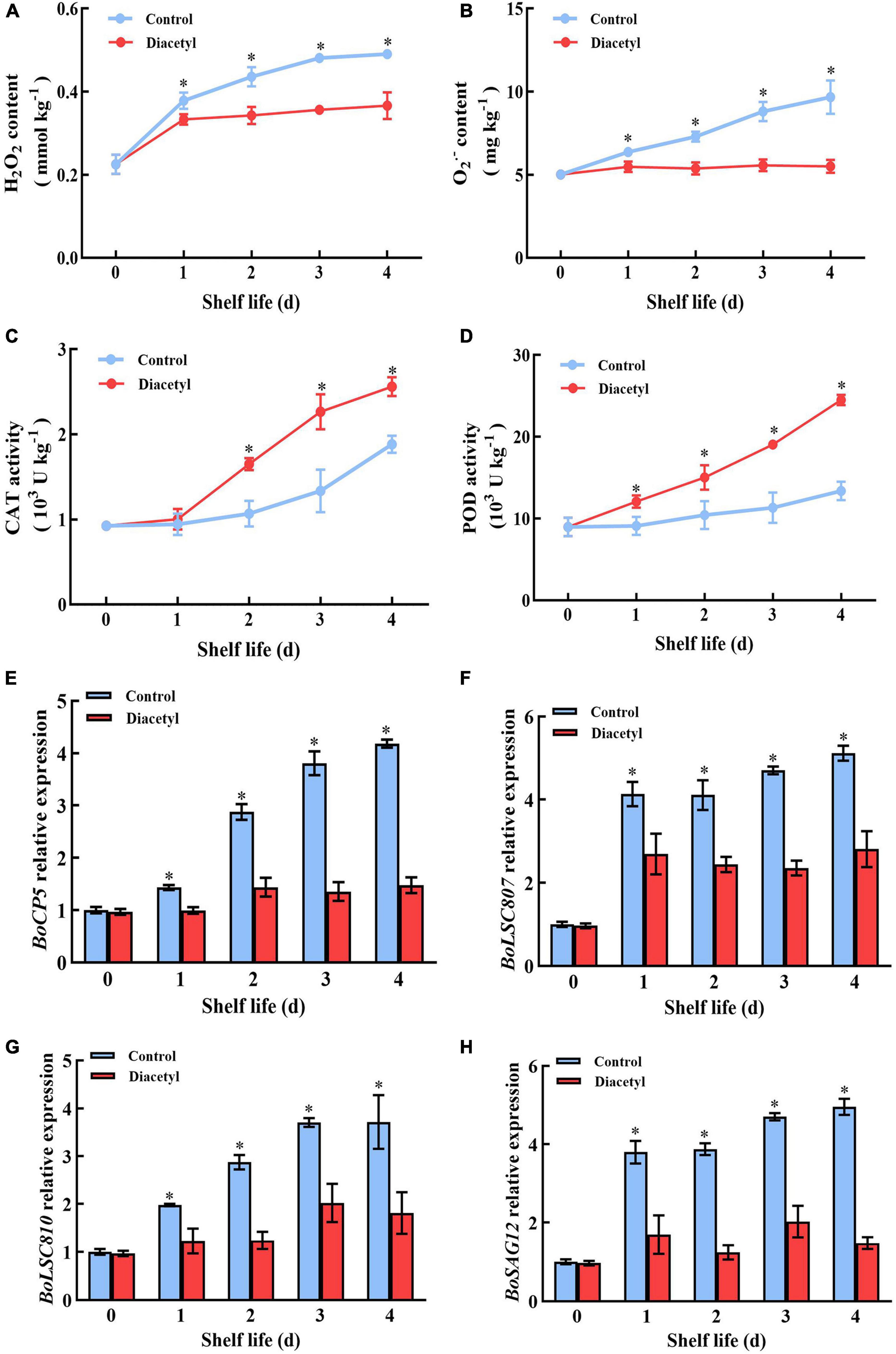
Figure 4. Diacetyl treatment inhibited the accumulation of ROS and the transcription level of senescence-associated genes in broccoli florets. H2O2 content (A), O2– content (B) and the enzyme activities of POD (C) and CAT (D). Relative expression of BoCP5 (E), BoLSC807 (F), BoLSC810 (G) and BoSAG12 (H). Data are the average of three replicates ± SD. Asterisk (*) indicates a significant difference among the control and diacetyl treatment groups at the same time at P < 0.05.
Excessive accumulations of ROS could aggravate the expression of senescence associated genes (41, 42). Thus, the transcript levels of several broccoli senescence-marker genes, including BoCP5, BoLSC807, BoLSC810 and BoSAG12 (5, 43), were detected. All of them were induced in the control groups during shelf life (Figures 4E–H), while these senescence-associated genes did not show significant difference with those in the diacetyl-treated group. These results exhibited that diacetyl could inhibit the postharvest senescence of broccoli through decreasing ROS accumulation.
It was reported that ethylene played an essential role in regulating the senescence of broccoli during postharvest (3). Thus, the contents of ethylene were measured after diacetyl treatment. It showed that the ethylene generation rate in the control was much higher than that in the treated florets (Figure 5A). This result indicated that diacetyl could inhibit the generation of ethylene in broccoli florets. Then the activities of ACS and ACO were detected in broccoli. The ACS and ACO activities under diacetyl treatment were lower than that in the control after 1 d (Figures 5B,C). As shown in Figures 5D-I, the gene expression of BoACO1, BoACO2, BoACO3 BoACS1, BoACS2, and BoACS3 was also obviously suppressed by diacetyl treatment. These results demonstrated that lower ethylene generation in broccoli florets with diacetyl treatment could be ascribed to lower ACS and ACO transcript levels and enzyme activities. Therefore, suppressing ethylene production is crucial to maintaining broccoli quality by diacetyl treatment.
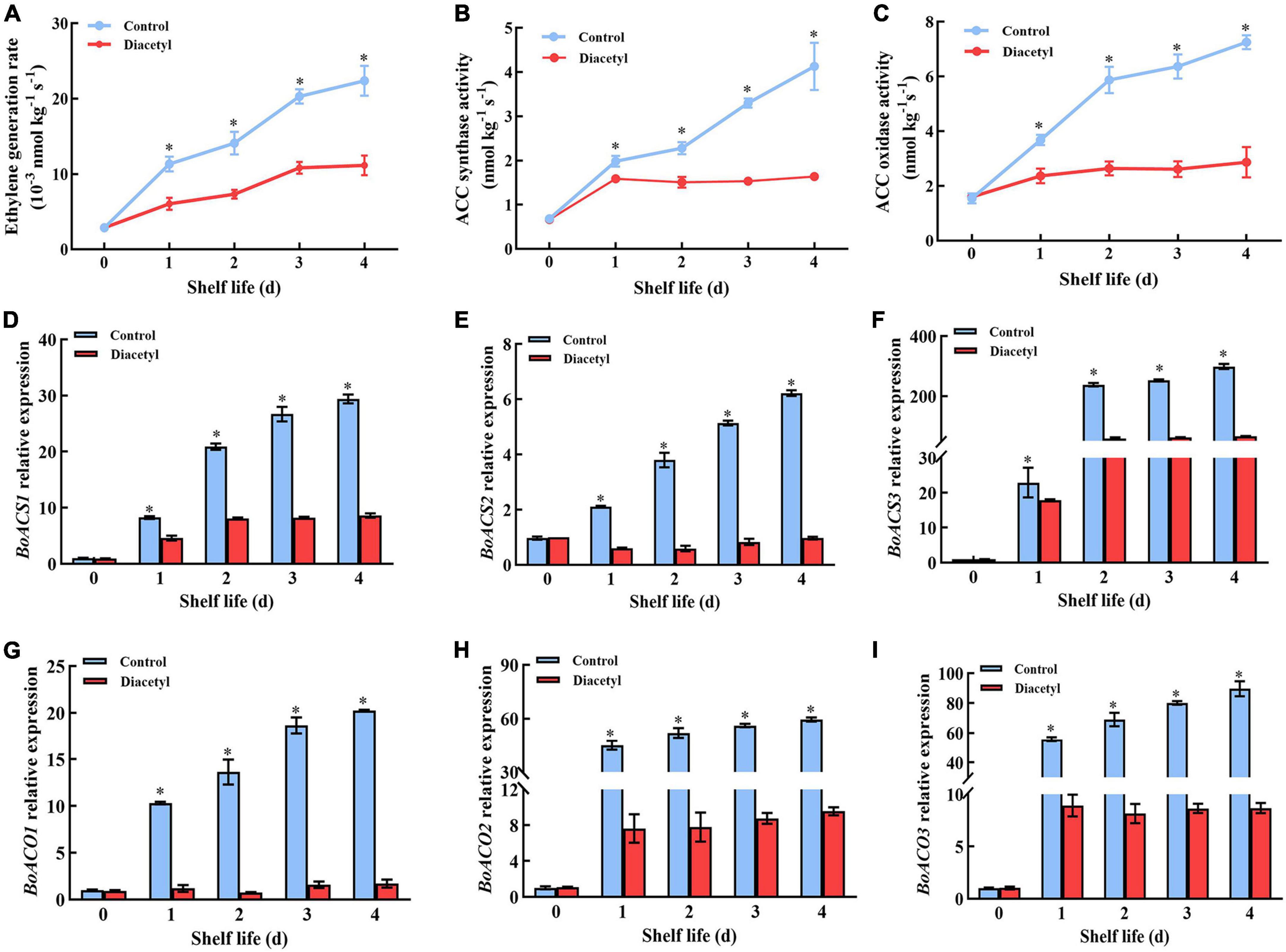
Figure 5. Diacetyl treatment inhibited the ethylene generation of broccoli florets during shelf life. Ethylene generation rate (A), ACS activity (B), ACO activity (C) and the transcription levels of BoACS1 (D), BoACS2 (E), BoACS3 (F), BoACO1 (G), BoACO2 (H), and BoACO3 (I). Data are the average of three replicates ± SD. Asterisk (*) indicates a significant difference among the control and diacetyl treatment groups at P < 0.05.
After harvest, the disruption of water and nutrient supply to broccoli heads can boost senescence, resulting in chlorophyll degradation and loss of nutritional value (21). The harvested broccoli florets usually turn yellow in two days at 25°C. Our study indicated that diacetyl could inhibit the yellowing of broccoli florets (Figure 1) and delayed the nutritional loss of vitamin C, flavonoid and the generation of lignin at suitable concentrations (Figure 2). Excessive ROS accumulation could accelerate the quality loss of broccoli. Scavenging ROS overproduction and maintaining ROS homeostasis were considered as a strategy to maintain the quality of postharvest broccoli. For example, 24-epibrassinolide was able to alleviate yellowing of broccoli by enhancing the antioxidant capacity (11). In this study, diacetyl treatment improved the enzyme activities of POD and CAT to prevent the contents of H2O2 and O2–. Therefore, diacetyl treatment alleviated yellowing of broccoli via improving the antioxidant capacity and maintaining the ROS equilibrium.
Broccoli florets faded at the beginning of yellowing because of the degradation of chlorophyll (21). Some genes, including BoCLH2, BoPPH, BoPAO, BoNYC1, BoNOL, BoHCAR and BoSGR1, have been reported to be involved in the regulation of chlorophyll degradation during broccoli senescence (37, 44). Previous studies showed that ethylene induced the expression of BoPPH and SGR1, which were accompanied by the yellowing of broccoli (44). Likewise, the gene expression of BoPPH and BoSGR1 were induced by broccoli senescence in this study. However, it was suppressed after diacetyl treatment, which could be relevant to the suppression of ethylene production. In our research, the expressions of BoNYC1 and BoNOL, which strikingly increased in untreated broccoli florets, were inhibited by diacetyl treatment during shelf life. Thus, the transcript levels of BoNYC1 and BoNOL were attenuated by diacetyl to retard chlorophyll degradation. HCAR could interact with other chlorophyll catabolic enzymes, such as SGR1/NYE1, NYC1 and NOL, during leaf senescence in Arabidopsis (45). Meanwhile, Jara et al. (2) suggested that HCAR played a protective role due to its contribution to the stability of photosystem II, which prevented the release of chlorophyll. Thus, diacetyl could inhibit the expression of BoHCAR, which might be associated with BoSGR1, BoNYC1, and BoNOL.
Previous studies concluded that ethylene accumulation was relevant to the quality loss of broccoli. Ethanol vapor and phytosulfokine α treatment could inhibit the senescence of broccoli by repressing the ethylene synthesis-related genes’ transcription and enzyme activities (6, 46). Similarly, the diacetyl treatment reduced the activities of ACS and ACO through inhibiting the expression of ethylene synthesis genes, such as BoACS1, BoACS2, BoACS3, BoACO1, BoACO2, and BoACO3, thereby further reducing ethylene generation in this study (Figure 5). Moreover, some studies have shown that ethylene could regulate the transcription levels of genes encoding major chlorophyll degradation and ROS metabolism enzymes (47, 48). For example, ethylene insensitive 3 (EIN3), a positive regulator of ethylene signaling, accelerated chlorophyll degradation by physically binding to NOL, NYC1 and PAO promoters to induce their expression. Moreover, ORE1 was a direct target of EIN3, and was induced by ethylene. It could also activate the expression of ACS2 and subsequently promote the ethylene production (49, 50). Thus, we demonstrated that diacetyl treatment suppressed the synthesis of ethylene and then inhibited ROS accumulation, ultimately repressed the transcription level of chlorophyll degradation gene (Figures 3-5). We emphasized that the suppression of ethylene generation by diacetyl treatment was the principal factor for delaying the quality loss of broccoli.
Diacetyl, which naturally exists in some foods, is popular as a food-flavoring additive (24). According to centuries of human acquaint with diacetyl in fermented foods, FDA believes that there is no apparent health concerns about diacetyl used in food (see text footnote 1). The daily intake of diacetyl is 3,300 μg/person per day in Europe and 8,000 μg/person per day in the United States (World Health Organization). The diacetyl content in yogurt is 200–3,000 mg kg–1 (51). The dose of diacetyl used in this study was 20 μl L–1, about 281 mg kg–1 fresh broccoli, and the residual value of diacetyl in broccoli must be lower than the used dose. Therefore, the residual dose in broccoli should be safe for human beings. In summary, diacetyl has the potential to be used for maintaining the quality of broccoli based on its efficacy and the availability of safe management measures during and after fumigation treatment.
Overall, our study established that diacetyl decreased the postharvest quality decline, suppressed the degradation of chlorophyll, and improved the antioxidant capacity in broccoli, which was accompanied by lower ethylene generation. Several studies illustrated that ethylene had complex interactions with abscisic acid (ABA) and jasmonic acid (JA) during plant senescence (9). However, it is still unclear whether diacetyl could regulate the ethylene synthesis through ABA and JA in our research. Meanwhile, some questions need to be further explored. such as “how diacetyl affected the expression of ethylene synthesis–related genes,” “whether exogenous diacetyl treatment could stimulate endogenous resistance to stress factors and enhance the anti-senescence ability of plants” and “whether exogenous diacetyl can act as a signaling molecule to stimulate endogenous diacetyl and then enhance the anti-senescence ability of plants.” Based on our studies, we believed that diacetyl could be a novel and valuable molecular tool to explore the mechanism of senescence of postharvest fruit and vegetables in future.
Appropriate concentrations of diacetyl could inhibit the yellowing and maintain the nutritional quality of broccoli florets. Diacetyl treatment prevented the excessive accumulation of ROS and improved the antioxidant capacity. Furthermore, diacetyl treatment suppressed the expression of ethylene synthesis–related genes, decreased the ethylene synthesis–related enzyme activities, which led to reduced ethylene generation. Therefore, diacetyl treatment could be considered as a meaningful strategy for alleviating senescence and maintaining the quality of postharvest broccoli, it also has a potential to be used as a molecular tool to explore plant senescence.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
XL: investigation, formal analysis, visualization, writing—original draft, and writing—review and editing. ZM: formal analysis and writing—review and editing. AM and SZ: writing—review and editing. QW: project administration, supervision, conceptualization, funding acquisition, resources, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was supported mainly by the grants from the Tai Shan Industry Leading Talents Project, Shandong Province (LJNY201702).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1055651/full#supplementary-material
1. Gu YJ, Guo QH, Zhang L, Chen Z, Han Y, Gu Z. Physiological and biochemical metabolism of germinating broccoli seeds and sprouts. J Agric Food Chem. (2012) 60:209–13. doi: 10.1021/jf203599v
2. Jara AMR, Gómez-Lobato ME, Civello PM, Martínez GA. Expression of BoNOL and BoHCAR genes during postharvest senescence of broccoli heads. J Sci Food Agric. (2021) 101:1629–35. doi: 10.1002/jsfa.10783
3. Gómez-Lobato ME, Mansilla SA, Civello PM, Martínez GA. Expression of stay-green encoding gene (BoSGR) during postharvest senescence of broccoli. Postharvest Biol Technol. (2014) 95:88–94. doi: 10.1016/j.postharvbio.2014.04.010
4. Kato M, Kamo T, Wang R, Nishikawa F, Hyodo H, Ikoma Y, et al. Wound-induced ethylene synthesis in stem tissue of harvested broccoli and its effect on senescence and ethylene synthesis in broccoli florets. Postharvest Biol Technol. (2002) 24:69–78. doi: 10.1016/S0925-521400111-9
5. Chen YT, Chen LFO, Shaw JF. Senescence-associated genes in harvested broccoli florets. Plant Sci. (2008) 175:137–44. doi: 10.1016/j.plantsci.2008.03.007
6. Aghdam MS, Koupaei-Alikhani M. Khademian raheleh. Delaying broccoli floret yellowing by phytosulfokine α application during cold storage. Front Nutr. (2021) 8:609217. doi: 10.3389/fnut.2021.609217
7. Gapper NE, Coupe SA, McKenzie MJ, Scott RW, Christey MC, Lill RE, et al. Senescence-associated down-regulation of 1-aminocyclopropane-1-carboxylate (ACC) oxidase delays harvest-induced senescence in broccoli. Funct Plant Biol. (2005) 32:891–901. doi: 10.1071/FP05076
8. Wilkinson S, DaviesW J. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. (2010) 33:510–25. doi: 10.1111/j.1365-3040.2009.02052.x
9. Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR. Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci. (2017) 8:475. doi: 10.3389/fpls.2017.00475
10. Li SP, Hu KD, Hu LY, Li YH, Jiang AM, Xiao F, et al. Hydrogen sulfide alleviates postharvest senescence of broccoli by modulating antioxidant defense and senescence-related gene expression. J Agric Food Chem. (2014) 62:1119–29. doi: 10.1021/jf4047122
11. Fang HX, Zhou Q, Cheng SC, Zhou X, Wei BD, Zhao YB, et al. 24- Epibrassinolide alleviates postharvest yellowing of broccoli via improving its antioxidant capacity. Food Chem. (2021) 365:130529. doi: 10.1016/j.foodchem.2021.130529
12. Jara AMR, Gómez-Lobato ME, Civello PM, Martínez GA. Effects of hormonal and physical treatments on the expression of a putative chlorophyll b reductase gene (BoNYC1) during postharvest senescence of broccoli. Postharvest Biol Technol. (2019) 147:107–12. doi: 10.1016/j.postharvbio.2018.09.010
13. Hörtensteiner S. Update on the biochemistry of chlorophyll breakdown. Plant Mol Biol. (2013) 82:505–17. doi: 10.1007/s11103-012-9940-z
14. Alós E, Roca M, Iglesias DJ, Mínguez-Mosquera MI, Damasceno CM, Thannhauser TW, et al. An evaluation of the basis and consequences of a stay-green mutation in the navel negra citrus mutant using transcriptomic and proteomic profiling and metabolite analysis. Plant Physiol. (2008) 147:1300–15. doi: 10.1104/pp.108.119917
15. Lemoine ML, Chaves AR, Martínez GA. Influence of combined hot air and UV-C treatment on the antioxidant system of minimally processed broccoli (Brassica oleracea L. var. Italica). LWT Food Sci Technol. (2010) 43:1313–9. doi: 10.1016/j.lwt.2010.05.011
16. Sabir FK. Postharvest quality response of broccoli florets to combined application of 1-methylcyclopropene and modified atmosphere packaging. Agr Food Sci. (2012) 21:421–9. doi: 10.23986/afsci.6387
17. Duarte-Sierra A, Nadeau F, Angers P, Michaud D, Arul J. UV-C hormesis in broccoli florets: preservation, phyto-compounds, and gene expression. Postharvest Biol Technol. (2019) 157:110965. doi: 10.1016/j.postharvbio.2019.110965
18. Xu F, Wang HF, Tang YC, Dong SQ, Qiao X, Chen XH, et al. Effect of 1-methylcyclopropene on senescence and sugar metabolism in harvested broccoli florets. Postharvest Biol Technol. (2016) 116:45–9. doi: 10.1016/j.postharvbio.2016.01.004
19. Deng LL, Yin BF, Yao SX, Wang WH, Zeng KF. Postharvest application of oligochitosan and chitosan reduces calyx alterations of citrus fruit induced by ethephon degreening treatment. J Agric Food Chem. (2016) 64:7394–403. doi: 10.1021/acs.jafc.6b02534
20. Bose SK, Howlader P, Wang WX, Yin H. Oligosaccharide is a promising natural preservative for improving postharvest preservation of fruit: a review. Food Chem. (2021) 341:128178. doi: 10.1016/j.foodchem.2020.128178
21. Xu DY, Zuo JH, Fang YL, Yan ZC, Shi JY, Gao LP, et al. Effect of folic acid on the postharvest physiology of broccoli during storage. Food Chem. (2021) 339:127981. doi: 10.1016/j.foodchem.2020.127981
22. Romanazzi G, Lichter A, Gabler FM, Smilanick JL. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol. (2012) 63:141–7. doi: 10.1016/j.postharvbio.2011.06.013
23. Bartowsky EJ, Henschke PA. The ‘buttery’attribute of wine-diacetyl-disirability, spoilage and beyond. Int J Food Microbiol. (2004) 96:235–52. doi: 10.1016/j.ijfoodmicro.2004.05.013
24. Hernandez-Valdes JA, Solopova A, Kuipers OP. Development of Lactococcus lactis biosensors for detection of diacetyl. Front Microbiol. (2020) 11:1032. doi: 10.3389/fmicb.2020.01032
25. Selahle KM, Sivakumar D, Jifon J, Soundy P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. (2015) 173:951–6. doi: 10.1016/j.foodchem.2014.10.034
26. Inga M, García JM, Aguilar-Galvez A, Campos D, Osorio C. Chemical characterization of odour-active volatile compounds during lucuma (Pouteria lucuma) fruit ripening. J Food. (2019) 17:494–500. doi: 10.1080/19476337.2019.1593248
27. Calvo H, Mendiara I, Arias E, Gracia AP, Blanco D, Venturini ME. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol Technol. (2020) 166:111208. doi: 10.1016/j.postharvbio.2020.111208
28. Li GJ, Chen Y, Zhang ZQ, Li BQ, Chen T, Tian SP. 2,3-Butanedione suppresses gray mold of postharvest fruit by activating the autophagy of Botrytis cinerea. Postharvest Biol Technol. (2022) 193:112057. doi: 10.1016/j.postharvbio.2022.112057
29. Singh SK, Sun YZ, Yang Y, Zuo ZW, Wu XX, Shao CY, et al. Bacterial diacetyl suppresses abiotic stress-induced senescence in Arabidopsis. J Integr Plant Biol. (2022) 64:1135–9. doi: 10.1111/jipb.13260
30. Sun B, Yan HZ, Liu N, Wei J, Wang QM. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. (2012) 131:519–26. doi: 10.1016/j.foodchem.2011.09.016
31. Sohail M, Wills RBH, Bowyer MC, Pristijono P. Multiple amino acids inhibit postharvest senescence of broccoli. Horticulturae. (2021) 7:71. doi: 10.3390/horticulturae7040071
32. Zhou FH, Jiang AL, Feng K, Gu ST, Xu DY, Hu WZ. Effect of methyl jasmonate on wound healing and resistance in fresh-cut cubes. Postharvest Biol Technol. (2019) 157:110958. doi: 10.1016/j.postharvbio.2019.110958
33. Yu XY, Bi Y, Yan L, Liu X, Wang Y, Shen KP, et al. Activation of phenylpropanoid pathway and PR of potato tuber against Fusarium sulphureum by fungal elicitor from Trichothecium roseum. World J Microb Biot. (2016) 32:142. doi: 10.1007/s11274-016-2108-2
34. Zaharah SS, Singh Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol Technol. (2011) 62:258–66. doi: 10.1016/j.postharvbio.2011.06.007
35. Hu LY, Hu SL, Wu J, Li YH, Zheng JL, Wei ZJ, et al. Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. J. Agric. Food Chem. (2012) 60:8684–93. doi: 10.1021/jf300728h
36. Jin T, Dai CW, Xu Y, Chen Y, Xu QH, Wu ZW. Applying cold atmospheric plasma to preserve the postharvest qualities of winter Jujube (Ziziphus jujuba mill. cv. Dongzao) during cold storage. Front Nutr. (2022) 9:934841. doi: 10.3389/fnut.2022.934841
37. Aiamla-or S, Nakajima T, Shigyo M, Yamauchi N. Pheophytinase activity and gene expression of chlorophyll-degrading enzymes relating to UV-B treatment in postharvest broccoli (Brassica oleracea L. Italica group) florets. Postharvest Biol Technol. (2012) 63:60–6. doi: 10.1016/j.postharvbio.2011.08.003
38. Cai HJ, Feng L, Zhao YB, Zhou Q, Wei BD, Zhou X, et al. 24-Epibrassinolide treatment regulates broccoli yellowing during shelf life. Postharvest Biol Technol. (2019) 154:87–95. doi: 10.1016/j.postharvbio.2019.04.019
39. Bárcena A, Bahima J, Casajús V, Martínez G, Lauff D, Guiamet J, et al. The degradation of chloroplast components during postharvest senescence of broccoli florets is delayed by low-intensity visible light pulses. Postharvest Biol Technol. (2020) 168:111249. doi: 10.1016/j.postharvbio.2020.111249
40. Zhu N, Yang YF, Ji MB, Wu D, Chen KS. Label-free visualization of lignin deposition in loquats using complementary stimulated and spontaneous raman microscopy. Hort Res. (2019) 6:72. doi: 10.1038/s41438-019-0153-3
41. Khanna-Chopra R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma. (2012) 249:469–81. doi: 10.1007/s00709-011-0308-z
42. Shi XY, Xu SS, Mu DS, Sadeghnezhad E, Li Q, Ma ZH, et al. Exogenous melatonin delays dark-induced grape leaf senescence by regulation of antioxidant system and senescence associated genes (SAGs). Plants. (2019) 8:366. doi: 10.3390/plants8100366
43. Page T, Griffiths G, Buchanan-Wollaston V. Molecular and biochemical char-acterization of postharvest senescence in broccoli. Plant Physiol. (2001) 125:718–27. doi: 10.1104/pp.125.2.718
44. Luo F, Fang HX, Wei BD, Cheng SC, Zhou Q, Zhou X, et al. Advance in yellowing mechanism and the regulation technology of post-harvested broccoli. Food Qual Saf. (2020) 4:107–13. doi: 10.1093/fqsafe/fyaa020
45. Sakuraba Y, Kim YS, Yoo SC, Hörtensteiner S, Paek NC. 7-Hydroxymethyl chlorophyll a reductase functions in metabolic channeling of chlorophyll breakdown intermediates during leaf senescence. Biochem Bioph Res. (2013) 430:32–7. doi: 10.1016/j.bbrc.2012.11.050
46. Asoda T, Terai H, Kato M, Suzuki Y. Effects of postharvest ethanol vapor treatment on ethylene responsiveness in broccoli. Postharvest Biol Technol. (2009) 52:216–20. doi: 10.1016/j.postharvbio.2008.09.015
47. Yang C, Li W, Cao JD, Meng FW, Yu YQ, Huang JK, et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. (2017) 89:338–53. doi: 10.1111/tpj.13388
48. Zhang DY, Wu SD, Li N, Gao J, Liu SH, Zhu S, et al. Chemical induction of the leaf senescence and powdery mildew resistance involves ethylene-mediated chlorophyll degradation and ROS metabolism in cucumber. Hortic Res. (2022) 9:101. doi: 10.1093/hr/uhac101
49. Qiu K, Li ZP, Yang Z. EIN3 and ORE1 accelerate degreening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Genet. (2015) 11:e1005399. doi: 10.1371/journal.pgen.1005399
50. Woo HR, Kim HJ, Lim PO, Nam HG. Leaf senescence: systems and dynamics aspects. Anuu Rev Plant Biol. (2019) 70:347–76. doi: 10.1146/annurev-arplant-050718-095859
Keywords: broccoli, yellowing, ethylene biosynthesis, maintain quality, diacetyl
Citation: Li X, Meng Z, Malik AU, Zhang S and Wang Q (2022) Maintaining the quality of postharvest broccoli by inhibiting ethylene accumulation using diacetyl. Front. Nutr. 9:1055651. doi: 10.3389/fnut.2022.1055651
Received: 28 September 2022; Accepted: 31 October 2022;
Published: 15 November 2022.
Edited by:
Zhaojun Wei, Hefei University of Technology, ChinaReviewed by:
Zhenlin Han, University of Hawai‘i at Mānoa, United StatesCopyright © 2022 Li, Meng, Malik, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Song Zhang, emhhbmdzb25nNTg2MTkyN0BzZGF1LmVkdS5jbg==; Qingguo Wang, d3FneXl5QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.