- 1Center for Non-Communicable Disease Management, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, Beijing, China
- 2State Key Laboratory of Genetic Engineering, Human Phenome Institute, Institute of Biostatistics, School of Life Sciences, Fudan University, Shanghai, China
- 3Shanghai Center for Mathematical Sciences, Fudan University, Shanghai, China
Objectives: Although homocysteine (Hcy) increases the risk of cardiovascular diseases, its effects on obesity and musculoskeletal diseases remain unclear. We performed a Mendelian randomization study to estimate the associations between Hcy and B vitamin concentrations and their effects on obesity and musculoskeletal-relevant diseases in the general population.
Methods: We selected independent single nucleotide polymorphisms of Hcy (n = 44,147), vitamin B12 (n = 45,576), vitamin B6 (n = 1864), and folate (n = 37,465) at the genome-wide significance level as instruments and applied them to the studies of summary-level data for fat and musculoskeletal phenotypes from the UK Biobank study (n = 331,117), the FinnGen consortium (n = 218,792), and other consortia. Two-sample Mendelian randomization (MR) approaches were utilized in this study. The inverse variance weighting (IVW) was adopted as the main analysis. MR-PRESSO, MR-Egger, the weighted median estimate, bidirectional MR, and multivariable MR were performed as sensitivity methods.
Results: Higher Hcy concentrations were robustly associated with an increased risk of knee osteoarthritis [odds ratio (OR) 1.119; 95% confidence interval (CI) 1.032–1.214; P = 0.007], hospital-diagnosed osteoarthritis (OR 1.178; 95% CI 1.012–1.37; P = 0.034), osteoporosis with pathological fracture (OR 1.597; 95% CI 1.036–2.46; P = 0.034), and soft tissue disorder (OR 1.069; 95% CI 1.001–1.141; P = 0.045) via an inverse variance weighting method and other MR approaches. Higher vitamin B12 levels were robustly associated with decreased body fat percentage and its subtypes (all P < 0.05). Bidirectional analyses showed no reverse causation. Multivariable MR analyses and other sensitivity analyses showed directionally similar results.
Conclusions: There exist significant causal effects of vitamin B12 in the serum and Hcy in the blood on fat and musculoskeletal diseases, respectively. These findings may have an important insight into the pathogenesis of obesity and musculoskeletal diseases and other possible future therapies.
Introduction
Musculoskeletal diseases are common and have an increased global prevalence (1). In addition to being a significant contributor to the global illness burden, they are also considered driving forces behind the Bone and Joint Decade (2). Recent evidence suggests that metabolic complications, such as lean mass loss in people with obesity and osteoporosis, can increase the risk of musculoskeletal diseases (3). However, novel bone and nerve regeneration techniques have been developed (4, 5). Low lean mass and quality with elevated fat mass were not only correlated with poor physical function (6) but also could lead to an elevation in the risk for serious musculoskeletal diseases (7, 8). To some degree, fat mass, lean mass, and musculoskeletal diseases—these three compositions—have very strong physiological connections (6–8).
B vitamins, including vitamins B6 and B12, and folate, play a crucial role in the metabolism of homocysteine (Hcy) (Figure 1). A lack of these B vitamins can result in a higher concentration of total Hcy, which has been considered a risk factor and affects cardiovascular diseases (9–11). Previous research has suggested that Hcy levels and folate concentrations impact the increased risk of obesity (12–14). However, the mechanisms concerning these observations remain unclear. There is a great chance that the elevated Hcy levels might affect the development of obesity via regulating body fat storage in the process of epigenetic regulation because a strong association exists between Hcy metabolism and DNA methylation and amino acid residues on histones (15–17). Hcy-thiolactone, a product of an error-correcting reaction in protein biosynthesis, is generated in the human body when Hcy is selected in place of methionine by methionyl-tRNA synthetase. It is a chemically reactive thioester metabolite that modifies protein lysine residues in a process called N-homocysteinylation. The modification causes protein damage/aggregation, a hallmark of many diseases, including fat and musculoskeletal diseases (18). Additionally, emerging evidence also indicates the possible role of Hcy in bone and lean mass (19–21). In vitro studies have indicated that Hcy could elevate the activity and differentiation of osteoclasts, reduce the quality of lean mass, and then produce the apoptosis of human bone marrow stromal cells by enhancing reactive oxygen species (21). Moreover, Hcy also can decrease bone blood flow, which potentially comes to terms with mechanical bone properties (20). Furthermore, Hcy influences bone tissue formation by interrupting the development of collagen cross-links and decreasing bone blood flow (19, 20). In humans, rigorously high Hcy because of cystathionine-β-synthase deficiency leads to connective tissue abnormalities in most body systems, especially the bones and vasculature (18). High Hcy because of methylenetetrahydrofolate reductase has also been correlated with bone abnormalities in humans (22). Similar connective tissue abnormalities have been found in mice (23). It has been recognized that collagen cross-linking defects might be involved (23).
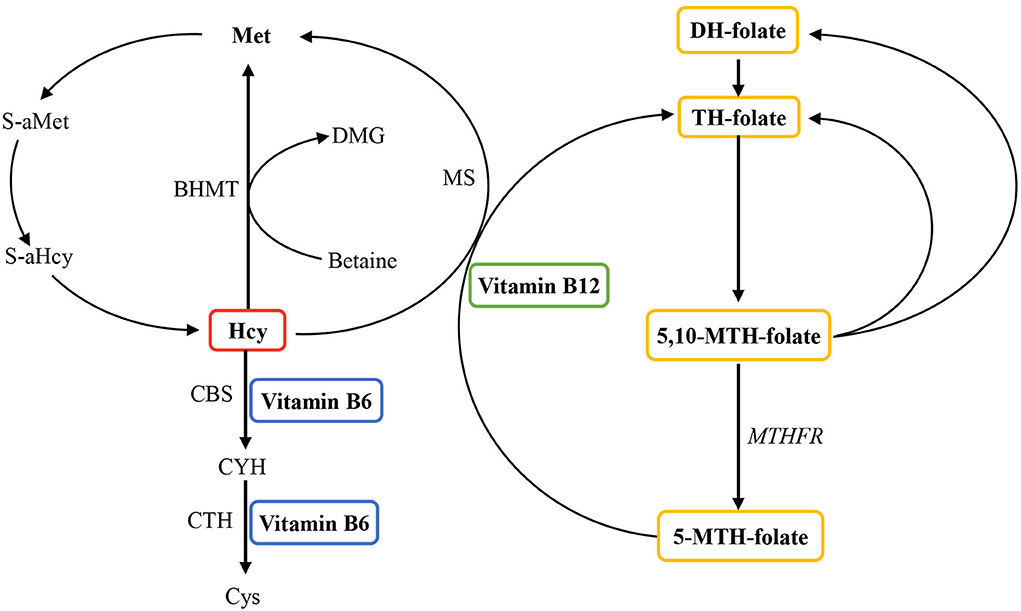
Figure 1. Overview of the role of vitamin B6, vitamin B12, and folate in homocysteine metabolism. Homocysteine is reconverted to methionine by acquiring a methyl group from 5-methyltetrahydrofolate, the positive form of betaine or folate in the remethylation pathway. Irreversible removal of homocysteine happens across the transsulfuration pathway in which homocysteine condenses with serine to form cystathionine. BHMT, betaine homocysteine methyltransferase; CBS, cystathionine-β-synthase; CYH, cystathionine; CTH, cystathionine-gamma-ligase; Cys, cysteine; DH, dihydo; DMG, dimethylglycine; Hcy, homocysteine; Met, methionine; MS, methionine synthase (encoded by the MTR gene); MTH, methylenetetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; S-aMet, S-adenosylmethionine; S-aHcy, S-adenosylhomocysteine; TH, tetrahydro.
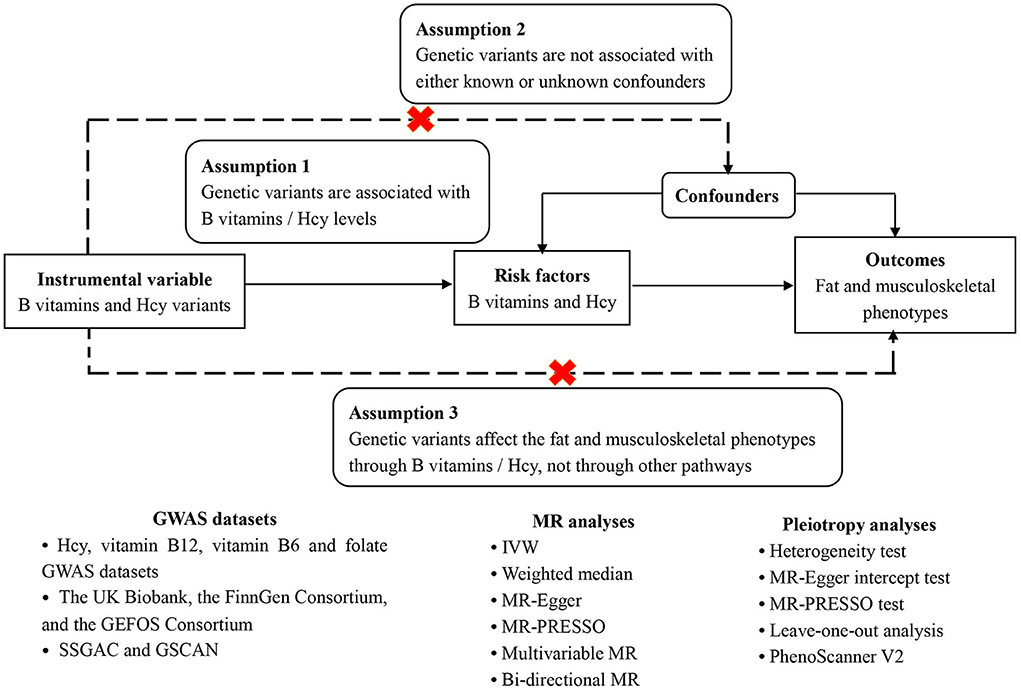
The aforementioned evidence shows the biological plausibility that B vitamins and Hcy could contribute to fat and lean mass changes, which would then elevate the risk of musculoskeletal-relevant diseases. However, there exist inconsistent findings about the associations between B vitamins and Hcy with body composition and musculoskeletal diseases in observational studies (12–14, 24–35), necessitating the inference of the causal associations of B vitamins and Hcy with fat percentage, lean mass, and musculoskeletal diseases to offer accurate causal relationships between them. Nowadays, employing the genetic variants as instruments for exposure (e.g., Hcy and vitamin B12), the Mendelian randomization (MR) study could improve causal inference via diminishing residual confounding and reverse causation (36). Here, we conducted an MR study to explore the associations of genetically predicted Hcy, vitamins B6 and B12, and folate concentrations with fat and musculoskeletally relevant phenotypes. The specific schematic overview of this MR study design can be found in Figure 2.
Materials and methods
This is a two-sample MR study employing the publicly available Genome-Wide Association Study (GWAS) data, which should satisfy three assumptions (Figure 2). Firstly, the genetic variants have to be independently associated with the exposures (B vitamins and Hcy), here regarded as the genome-wide significant level (P < 5 × 10−8) with r2 < 0.01. Secondly, other confounders should not affect the association between the genetic variants and the outcomes. Thirdly, the genetic variants influence the outcomes only through their impact on the exposures, i.e., the exclusion restriction assumption (37, 38). This assumption was evaluated by horizontal pleiotropy, in which the variants influence the outcomes directly or via other identified causes of outcomes that influence the exposures (39). In addition, bi-directional MR analyses and a multivariable MR model (40) were further employed to test whether B vitamins and Hcy remain considered the true causal factors of fat and musculoskeletal phenotypes, given several confounders. Figure 2 shows the overview diagram of this study's design.
Ethical approval
This MR study applies public or published data. No original data were required for this study. Ethical approval and informed consent from each subject for every included study in the research can be described in the original publications.
Genetic predictor of exposures
We selected SNPs that are associated with serum B vitamins or blood homocysteine according to the meta-analyses of GWAS on serum vitamin B12 (n = 45,576 subjects) (41), folate (n = 37,465 subjects) (41), vitamin B6 (n = 1,864 subjects) (42), and blood Hcy (n = 44,147 subjects) (43) in European ancestry at the genome-wide significant level (P < 5 × 10−8) as a genetic predictor of exposures. Based on the 1,000 Genomes European Reference Panel, linkage disequilibrium among SNPs associated with each exposure was evaluated using the PLINK clumping technique. As a result, distinct SNPs without linkage disequilibrium (r2 < 0.01) were employed as the instruments (Supplementary Table 1). Therefore, there are 14 independent SNPs for Hcy, 14 for vitamin B12, two for folate, and one SNP for vitamin B6 (Supplementary Table 1). Overall, the SNPs explained 6.0% of the variance for vitamin B12 (41) and Hcy (43), as well as 1.3 and 1.0% of the variance for vitamin B6 (42) and folate (41), respectively.
Genetic associations with outcomes
Complete GWAS summary data for the outcomes, namely fat percentage and its subtypes (from the UK Biobank) (44), musculoskeletally relevant phenotypes including musculoskeletal system diseases, soft tissue disorders, osteoporosis with pathological fracture, knee osteoarthritis, hip osteoarthritis, and hospital-diagnosed osteoarthritis (data from the UK Biobank and FinnGen Consortium) (44, 45), and lean mass and its subtypes (from the GEFOS Consortium) (46, 47) were acquired from the publicly available online datasets or public GWAS summary data repositories. Detailed descriptions of data sources are exhibited in Table 1. The specific descriptions of diseases and phenotypes can be found in Nealelab (http://www.nealelab.is/uk-biobank), the FinnGen Consortium (https://www.finngen.fi/en/access_results), and the GEFOS Consortium (http://www.gefos.org).
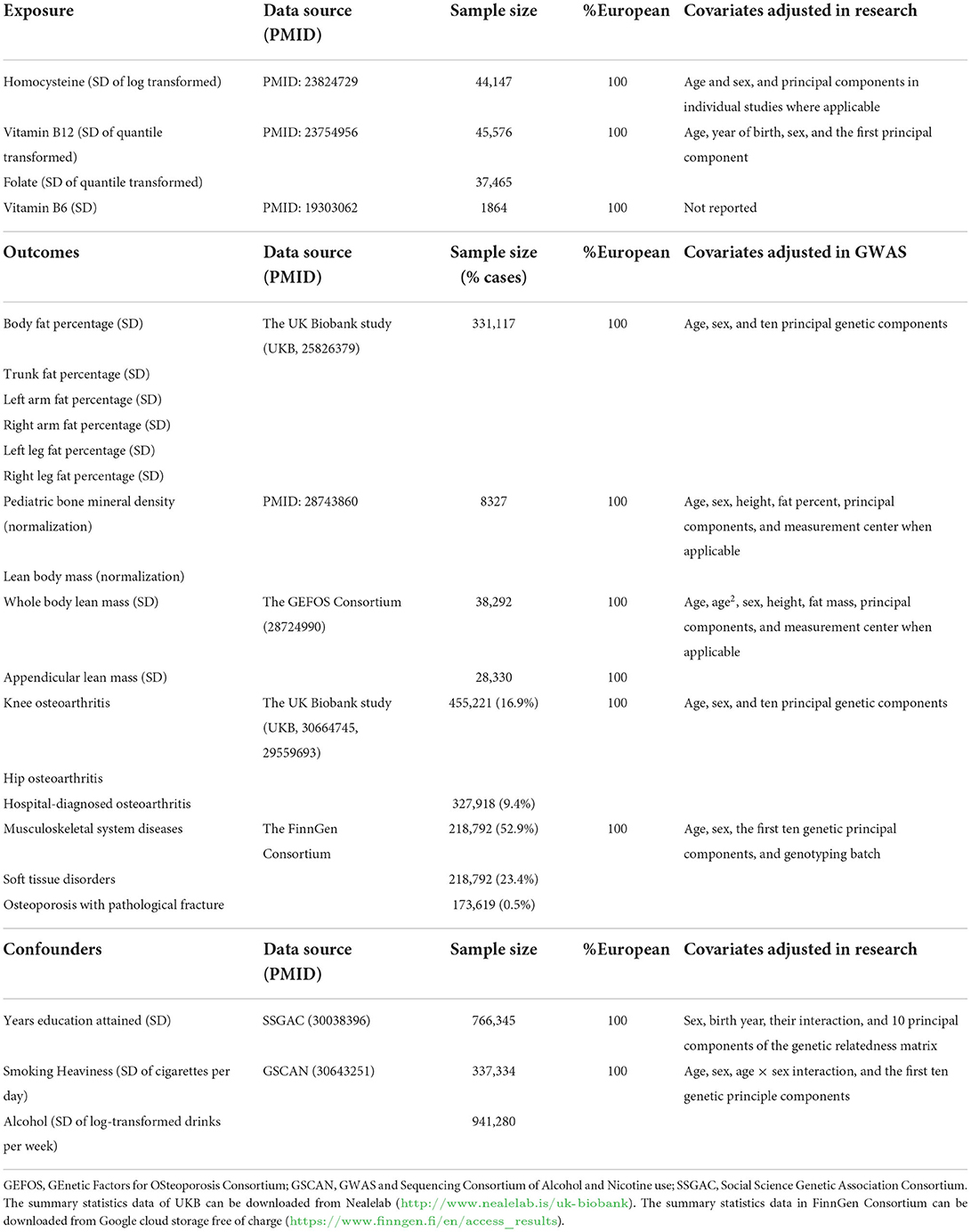
Table 1. Information on the genome-wide association studies utilized in this Mendelian randomization study.
Investigating possible sources of horizontal pleiotropy
We also evaluated the possible association between the genetic instruments and sources of horizontal pleiotropy that could contribute to the violation of the Instrument Strength is Independent of Direct Effect (InSIDE) assumption, which means some risk factors predicted by the instruments are the potential causes of both the exposures and outcomes. These included the degree of education (SSGAC) (48) and the smoking and alcohol use rate (GSCAN) (49). The details of these variables are also presented in Table 1.
Exposures
As the exposures increased, B vitamins were measured at the serum level, and Hcy was measured at the blood level. Both B vitamins and Hcy were transformed into one standard deviation (SD) unit.
Outcomes
The dichotomous outcomes were attributable to musculoskeletal system diseases, soft tissue disorders, osteoporosis with pathological fractures, knee osteoarthritis, hip osteoarthritis, and hospital-diagnosed osteoarthritis. The continued outcomes were lean mass and fat percentage in various body regions (normally transformed or SD transformed).
Harmonizing allele
The genetic information from the exposure GWAS and the outcome GWAS was merged based on the alignment of the effect alleles and the corresponding effect directions. We also employed the effect of allele frequency to ensure that palindromic genetic instruments are properly harmonized. For SNPs not available in outcome datasets, we employed proxy SNPs with r2 > 0.8 for the specific B vitamins or Hcy-associated SNPs. Absent SNPs without befitting proxies for outcome in the GWAS summary statistics were excluded from the subsequent analyses.
Statistical analysis
We applied inverse variance weighting (IVW) with multiplicative random effects (50) as the main analysis (Figures 3, 4). In addition, we performed three sensitivity approaches, comprising a weighted median estimate (51), the MR-Egger (52), and the MR-PRESSO (53) method for vitamin B12 and Hcy (Supplementary Tables 5–7). The IVW stands for the weighted regression slope of the effect of SNP outcome on SNP exposure, assuming the intercept is restricted to zero (50). The weighted median allows us to provide an unbiased estimate when 50% of SNPs are invalid instruments (51). The MR-Egger can assess the horizontal pleiotropy by virtue of the P value for its intercept and assess after adjusting for pleiotropic effects under the InSIDE assumption, but it may get wider confidence intervals (CIs) with less statistical power (52). Using the InSIDE assumption, MR-PRESSO offers not only another statistical method for testing biases because of pleiotropy (the global test) but as well as provides a corrected estimate by means of outlier removal but also a distortion test to detect whether the results with or without outliers get similar estimates (53). By finding phenotypes correlated with the employed SNPs for vitamin B12 and Hcy in a website database (PhenoScanner V2, Supplementary Table 3, searching in April 2022) (54), a total of 1 SNP (rs602662) for vitamin B12 and 4 SNPs (rs1047891, rs548987, rs2251468, and rs838133) for Hcy correlated with blood lipids or other phenotypes tended to exert pleiotropic effects (Supplementary Table 3) (55). An additional sensitivity analysis that excluded these five SNPs was conducted to ensure the robustness of the main analysis (Figure 5). We also performed Cochran's Q statistic to explore the degree of heterogeneity (56) among the SNPs in each analysis (Supplementary Tables 5, 6). Power was calculated using an online approach (Supplementary Table 4) instead of providing supporting evidence of the strength of genetic instruments like F-statistics (57). The odds ratios (ORs) for dichotomous outcomes and betas for continuous outcomes with their corresponding 95% CI were scaled to one SD elevation in genetically predicted B vitamins and Hcy.
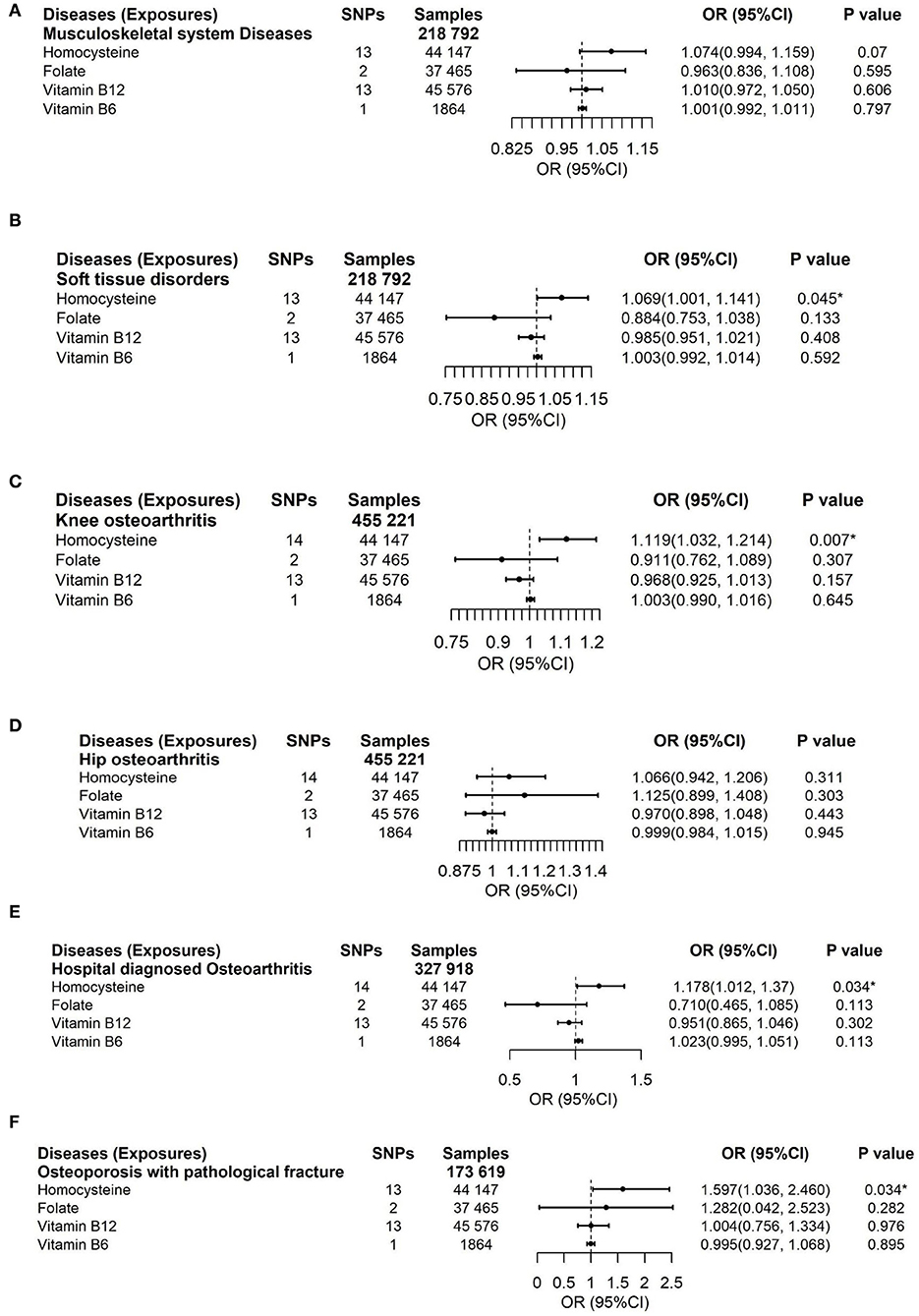
Figure 3. Effects of genetically predicted circulating homocysteine, vitamin B6, folate, and vitamin B12 on musculoskeletal system diseases (A), soft tissue disorders (B), knee osteoarthritis (C), hip osteoarthritis (D), hospital-diagnosed osteoarthritis (E), and osteoporosis with pathological fracture (F) via IVW method. CI, confidence interval. The summary statistics data of UKB can be downloaded from Nealelab (http://www.nealelab.is/uk-biobank). The summary statistics data in FinnGen Consortium can be downloaded from Google cloud storage free of charge (https://www.finngen.fi/en/access_results). *P value reached the significant level; SNP, single nucleotide polymorphism.
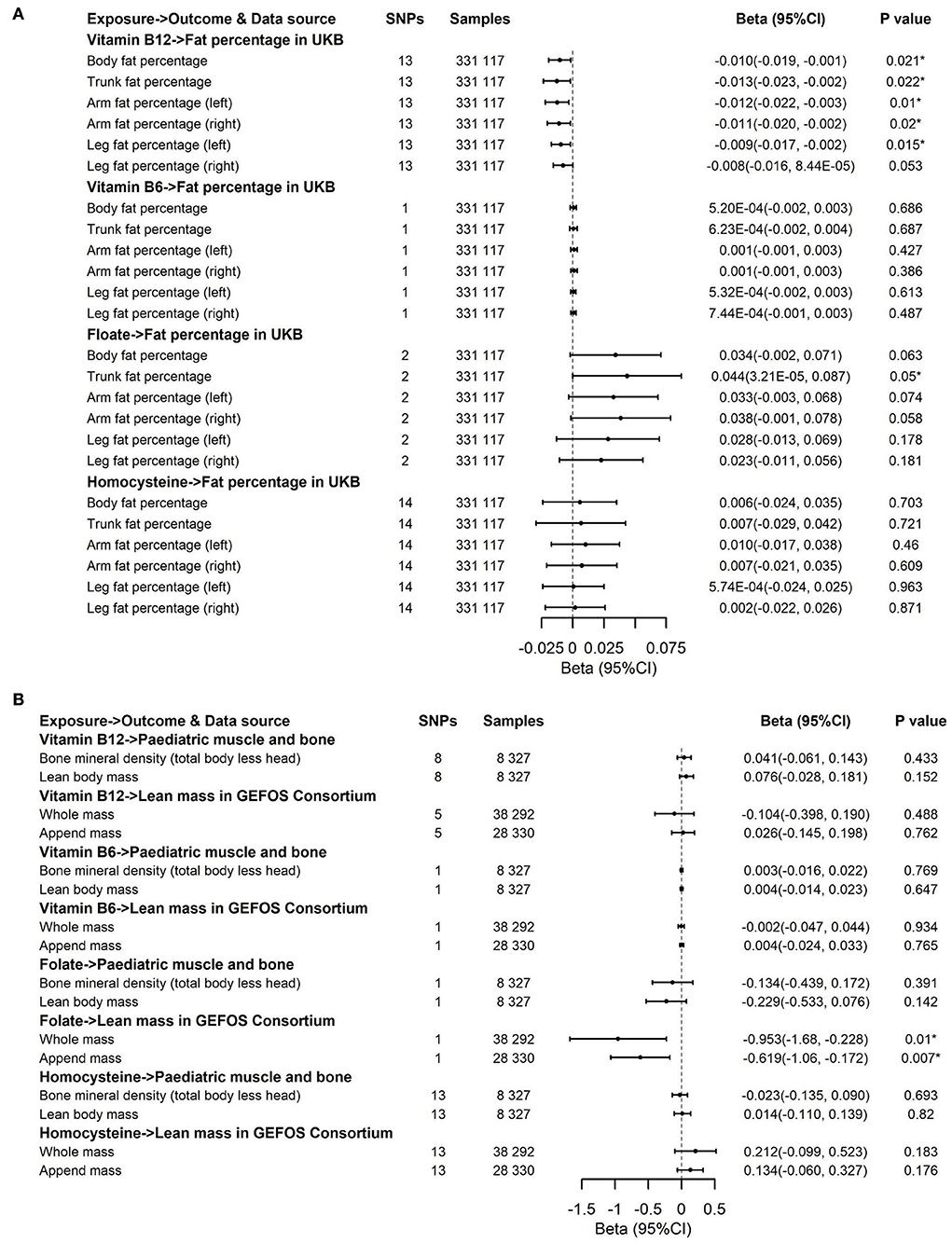
Figure 4. Effects of genetically predicted circulating homocysteine, vitamin B6, folate, and vitamin B12 on fat percentage (A) and lean mass (B) via IVW method. CI, confidence interval; GEFOS, GEnetic Factors for OSteoporosis Consortium. The summary statistics data of UKB can be downloaded from Nealelab (http://www.nealelab.is/uk-biobank). The summary statistics data in FinnGen Consortium can be downloaded from Google cloud storage free of charge (https://www.finngen.fi/en/access_results). *P value reached the significant level; SNP, single nucleotide polymorphism.
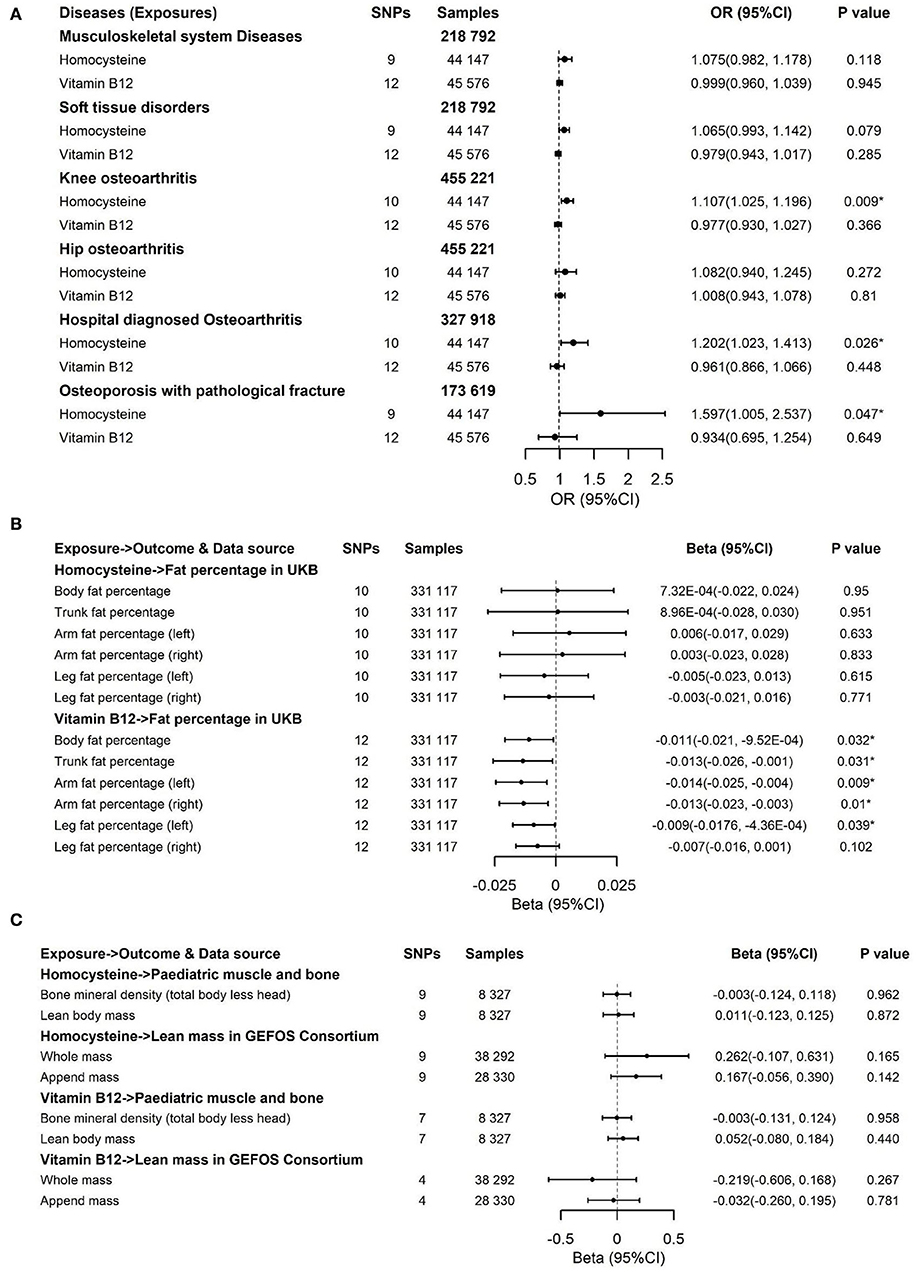
Figure 5. Effects of genetically predicted circulating homocysteine and vitamin B12 on musculoskeletal system diseases, soft tissue disorders, knee osteoarthritis, hip osteoarthritis, hospital-diagnosed osteoarthritis, and osteoporosis with pathological fracture (A), fat percentage (B) and lean mass (C) via IVW method in the sensitivity analysis with exclusion of 4 pleiotropic SNPs for homocysteine and one pleotropic SNP for vitamin B12. CI, confidence interval; GEFOS, GEnetic Factors for OSteoporosis Consortium; The summary statistics data of UKB can be downloaded from Nealelab (http://www.nealelab.is/uk-biobank); The summary statistics data in FinnGen Consortium can be downloaded from Google cloud storage free of charge (https://www.finngen.fi/en/access_results). *P value reached the significant level; SNP, single nucleotide polymorphism; IVW, inverse variance weighted method.
Because overlapping samples between two datasets would bias the evaluated causal effect, we carried out linkage disequilibrium score regression (LDSC) to estimate sample overlap by using the LD Hub (http://ldsc.broadinstitute.org/) (58). For the significant results that Hcy or B vitamins affect fat or musculoskeletal phenotypes, reverse causation analyses were conducted to rule out the possibility that fat and musculoskeletal phenotypes causally affected Hcy or B vitamins by applying fat and musculoskeletal phenotypes' associated SNPs as IVs (Supplementary Table 10). Moreover, multivariable MR was performed to account for the possibility that genetic instruments may have violated the InSIDE assumption via conditioning on the possible confounders. This method admits the direct effects of multiple variables on an outcome to be jointly decided. The effects of Hcy and B vitamins on fat and musculoskeletal phenotypes were evaluated in analyses that adjusted for potential confounders, including educational attainments, smoking, and alcohol usage (Supplementary Tables 8, 9). We also employed a leave-one-out analysis to ensure the robustness of the significant main findings (Figures 6, 7).
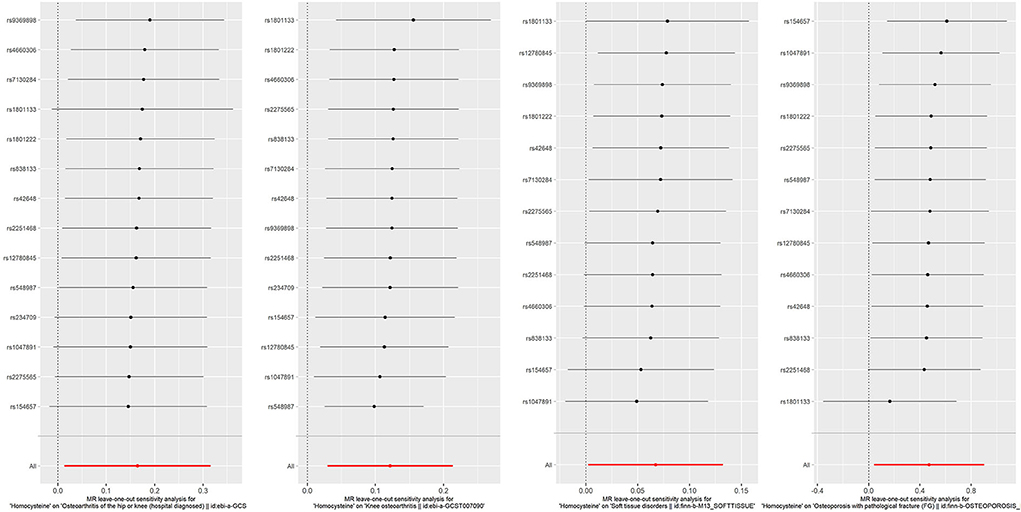
Figure 6. Forest plot for leave-one-out analysis of the significant IVW results of homocysteine with soft tissue disorders, knee osteoarthritis, hospital-diagnosed osteoarthritis, and osteoporosis with pathological fracture, with each point denoting the causal effect by IVW after removing the specific SNP on the left side. IVW, inverse variance weighted method.
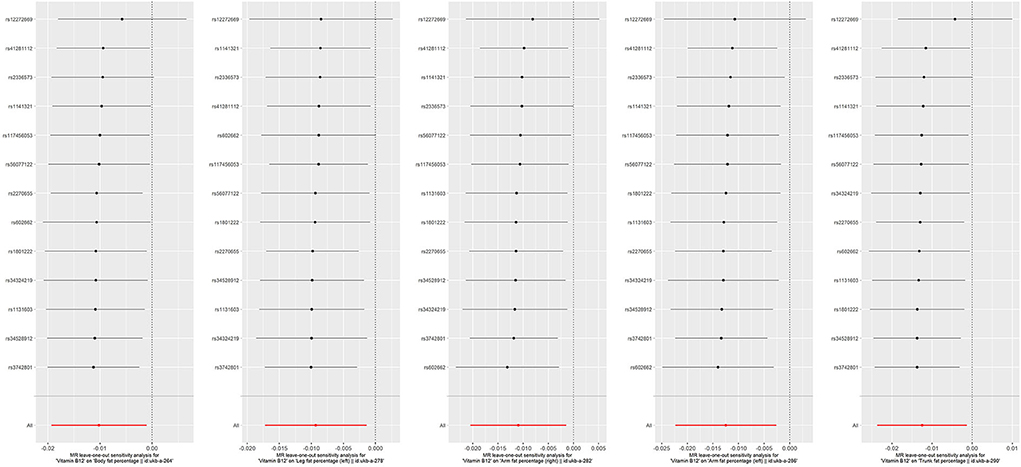
Figure 7. Forest plot for leave-one-out analysis of the significant IVW results of vitamin B12 with fat percentage, with each point denoting the causal effect by IVW after removing the specific SNP on the left side. IVW, inverse variance weighted method.
All analyses were conducted via R Version 4.1.0 using the R packages (“TwoSampleMR”) (59), “MRPRESSO” (53), and “MendelianRandomization” (60). Estimates with P < 0.05 in both the IVW and MR-PRESSO models or significant results after Bonferroni correction (0.05/N, where N is 4 exposures × 16 outcomes in this study) were considered as robust associations to control the type I error under multiple testing; estimates with P values above the corrected significance threshold but < 0.05 in the IVW or MR-PRESSO model and the same direction through all analyses were deemed as suggestive associations (61, 62).
Results
To deal with the concern over bias in the evaluated causal effect because of overlapping samples in the exposure GWAS and outcome GWAS, LDSC was employed to acquire approximately zero intercepts of genetic covariance <10−3 in pairs of exposure-outcome GWAS (P > 0.1 by z-test in all pairs, data not shown), suggesting approximately no sample overlap in pairs of two datasets. For the dichotomous outcomes, including musculoskeletal system diseases, soft tissue disorders, osteoporosis with pathological fracture, knee osteoarthritis, hip osteoarthritis, and hospital-diagnosed osteoarthritis, we noticed that there was a significant association between increased genetically predicted Hcy levels and a higher risk of soft tissue disorders, osteoporosis with pathological fracture, knee osteoarthritis, and hospital-diagnosed osteoarthritis (Figure 3). For 1-SD elevations in genetically predicted Hcy concentrations, the ORs were 1.069 (95% CI, 1.001, 1.141; P = 0.045) for soft tissue disorders, 1.119 (95% CI, 1.032, 1.214; P = 0.007) for knee osteoarthritis, 1.178 (95% CI, 1.012, 1.37; P = 0.034) for hospital-diagnosed osteoarthritis, and 1.597 (95% CI, 1.036, 2.46; P = 0.034) for osteoporosis with pathological fractures. Results were kept directionally consistent in the weighted median model, MR-Egger, and MR-PRESSO approaches (Supplementary Tables 5, 7). We observed no heterogeneity based on Cochran's Q statistic and no pleiotropy based on MR-Egger and MR-PRESSO approaches for B vitamins and Hcy in the analysis of these outcomes (Supplementary Tables 5–7). Multivariable Mendelian randomization, leave-one-out analysis, and reverse Mendelian randomization analysis also confirmed these significant results (Supplementary Tables 8, 10; Figure 6). Therefore, the main results of the analyses of various MR approaches conducted by IVW were relatively robust. Genetically predicted B vitamin levels were not associated with musculoskeletally relevant diseases (Figure 3). In the sensitivity analyses with the exclusion of 1 SNP for vitamin B12 and 4 SNPs for Hcy, the associations remained consistent (Figure 5A), except for a little change in the magnitude of the effect. For 1-SD elevations in genetically predicted Hcy concentrations, the ORs were 1.065 (95% CI, 0.993, 1.142; P = 0.079) for soft tissue disorders, 1.107 (95% CI, 1.025, 1.196; P = 0.009) for knee osteoarthritis, 1.202 (95% CI, 1.023, 1.413; P = 0.026) for hospital-diagnosed osteoarthritis, and 1.597 (95% CI, 1.005, 2.537; P = 0.047) for osteoporosis with pathological fracture, which was similar with the ones before excluding the four SNPs except for soft tissue disorders. Therefore, the associations of Hcy with musculoskeletally relevant diseases were relatively robust according to all those sensitivity analyses. Overall, the positive results of enhanced Hcy levels resulting in an increased risk of musculoskeletal-relevant diseases were believable for this study.
The associations of genetically predicted Hcy and B vitamin concentrations with fat percentage and lean mass are presented in Figures 4, 5B,C. There were significant associations between increased genetically predicted vitamin B12 levels and lower body fat percentage (Beta, −0.01; 95% CI, −0.019, −0.001; P = 0.021) and its subtypes (Trunk fat percentage: Beta, −0.013; 95% CI, −0.023, −0.002; P = 0.022; Left arm fat percentage: Beta, −0.012; 95% CI, −0.022, −0.003; P = 0.01; Right arm fat percentage: Beta, −0.011; 95% CI, −0.02, −0.002; P = 0.02; Left leg fat percentage: Beta, −0.009; 95% CI, −0.017, −0.002; P = 0.015; Right leg fat percentage: Beta, −0.008; 95% CI, −0.016, 8.44 × 10−5; P = 0.053) as well as between higher genetically predicted folate concentrations and decreased whole body lean mass (Beta, −0.953; 95% CI, −1.68, −0.228; P = 0.01) and appended lean mass (Beta, −0.619; 95% CI, −1.06, −0.172; P = 0.007) (Figure 4). Because too few SNPs were used as instruments for folate, the robustness of significant results about the associations of folate with whole-body lean mass and appended, lean mass needs further study. The results of associations between vitamin B12 and fat percentage remained consistent and significant in the weighted median model (MR-Egger and MR-PRESSO) (Supplementary Tables 6, 7). Multivariable Mendelian randomization, leave-one-out analysis, and reverse Mendelian randomization analysis also confirmed these significant results (Supplementary Tables 9, 10; Figure 7). In addition, no heterogeneity and no pleiotropy were observed based on Cochran's Q, MR-Egger, and MR-PRESSO analyses (Supplementary Tables 6, 7). In the sensitivity analysis, we excluded 1 SNP for vitamin B12 to test its direction and significance with fat percentage and other outcomes. As a result, vitamin B12 was also significantly associated with body fat mass and its subtypes (Figure 5B). Genetically predicted Hcy concentrations and vitamin B6 levels were not associated with fat percentage and lean mass (Figures 4, 5B,C, Supplementary Tables 5, 7).
For the confounders, including the degree of education, smoking, and alcohol use rate, we observed that the effect alleles for the B vitamins and Hcy instruments (harmonized to elevated B vitamin levels and Hcy concentrations) had no effect on educational attainment, smoking, or alcohol use (Supplementary Table 2), which indicated that the associations between exposure and outcomes were unlikely to be impacted by these confounders.
Discussion
Hcy is an intermediate outcome in the course of the metabolism of the amino acid methionine. Some nutritional factors, including vitamins B6 and B12, and folate, play a primary role in the metabolism of Hcy. Insufficiencies of either of these vitamins can affect Hcy metabolism, leading to an elevation of Hcy concentrations (12, 63) (Figure 1). Additionally, a deficiency of methionine intake would also impair Hcy metabolism. Therefore, it is up to dietary levels to either elevate or reduce blood Hcy. To date, the literature that has estimated the association of obesity with the risk of hyperhomocysteinemia in the general population is restricted and scarce (12, 26, 28, 35), and the outcomes in different population settings are controversial (13, 33). The latest study found a negative association between general obesity, measured by body mass index, and the risk of hyperhomocysteinemia. It implicated that central obesity, defined by waist circumference, was positively associated with hyperhomocysteinemia (35). Body composition examination (i.e., fat percentage, lean body mass) could better predict chronic cardiovascular diseases than body mass index or waist circumference alone (64). Previously, we researched the harm of different types of obesity and developed some genetic statistics to detect genetic variation in complex diseases (14, 65–70). Assessing human body composition accurately should be considered to further explore the relationships between fat percentage, lean body mass, Hcy, and B vitamin concentrations.
In traditional observational studies, it is difficult to examine the causal associations between exposures and disease outcomes because of methodological limitations, undetectable confounding factors, and reverse causality (71). MR design uses genetic variants as instrumental variables, which are associated with exposures as proxies for a risk factor for outcomes. It then provides causal inferences about these exposures and outcomes (72). Due to the genetic variants of the offspring being inherited randomly from their parents, generally, confounding factors are scarcely possible to influence these instrumental variables. Thus, the studies based on MR designs are less subject to reverse causality and confounding factors (36).
In the present study, we have searched the genetic variants of exposures (SNPs-Hcy and SNPs-B vitamins) and outcomes (SNPs-fat percentage, SNPs-lean mass, and SNPs-musculoskeletally relevant diseases) from distinct large-scale GWAS data sources and performed the corresponding MR analysis to estimate the causal impacts of Hcy and B vitamins on the changes of fat percentage and lean mass and the risk for musculoskeletally relevant diseases. The results of our study suggested that the increased, genetically predicted vitamin B12 levels were associated with a reduced fat percentage and its subtypes. The higher genetically predicted folate concentrations affected the decreased and appended whole-body lean mass. Moreover, genetically predicted Hcy elevation could influence the elevated risk of soft tissue disorders, osteoporosis with pathological fracture, knee osteoarthritis, and hospital-diagnosed osteoarthritis.
Several mechanisms have been established to clarify the relationships between Hcy concentrations and the pathogenesis of obesity and musculoskeletal metabolism. A changed cysteine and Hcy have a corresponding reaction to the ingested methionine in obesity, in which case both could elevate in response to dietary methionine (73). This may help explain why human obesity consistently impacts the increased concentrations of Hcy and cysteine, as already explored in the existing studies (74–76). In addition, studies stated that Hcy inhibits lipolysis in adipocytes by activating the AMPK pathway (74, 77), and total plasma Hcy and cysteine can have pro-oxidant effects by promoting reactive oxygen species and concomitant endothelial dysfunction in combination with improving low-density lipoprotein oxidation, thus favoring atherogenesis and osteoarthritis (76, 77). As we know, lean mass is a vital indicator of longevity in older adults (78), and under its plasticity and regular remodeling, when the circumstances of the elevation of intramuscular lipid deposits along with obesity and insulin resistance arise, the alterations of structure and the altered capacity for glucose homeostasis would impair muscle integrity, causing persistent atrophy and lipid accumulation in lean mass, which are also risk factors for osteoporosis (3). Furthermore, Hcy could lead to impaired muscle function using the following processes: (i) decreased oxidative defense and production of the elevated reactive oxygen species, (ii) suppression of nitric oxide signaling, (iii) inflammation and its related changes, and (iv) increased stress on the endoplasmic reticulum (79). Previously, we conducted a meta-analysis implementing MR design, confirming that genetically predicted Hcy is positively associated with obesity (14), where we stated that Hcy concentrations substantially affect the process of adjusting the relationship of DNA methylation with amino acid residues upon histones. Taken together, the above evidence indicates a pathogenic role for Hcy in musculoskeletal-relevant diseases.
It has been found that folate and vitamin B12 could affect the serum concentrations of Hcy and that co-supplementation of folate and vitamin B12 was capable of reducing serum Hcy concentrations (24). Several clinical studies have explored the effects of decreasing Hcy interventions via co-supplementation of folate and vitamin B12 on bone metabolism. One scholar conducted a double-blind, randomized controlled study to assess the association of the combined treatment of applying folate and vitamin B12 with the incidence of stroke and osteoporotic fractures in Japanese. The findings suggested that the individuals who had obtained co-supplementation of folate and vitamin B12 not only appeared to have a significant decrease in plasma Hcy concentrations but also had efficiency in reducing the risk of osteoporotic fractures (25). Moreover, the Hcy levels of osteoporosis patients treated with a novel medication aimed at treating osteoporosis for 1 year revealed significant reductions over time compared to the basal Hcy concentrations (27). Additionally, pre-clinical studies have reported a mechanistic physiological relationship whereby vitamin B12 deficiency results in elevated adipogenesis and cholesterol biosynthesis in human cells and rats, respectively (31, 80). The increase of genetically predicted vitamin B12 was associated with this study's reduction in fat percentage, supporting the hypothesis that there is a putative correlation between lower B12 concentrations and obesity.
To further validate the results in this study and find more explanations for possible pathways, we conducted Reactome enrichment analyses on the genes associated with vitamin B12 and Hcy, respectively, to validate the role vitamin B12 and Hcy might play in the pathogenesis and development of fat obesity and musculoskeletally relevant diseases. Figures 8A,B depict the results of enrichment analyses of the vitamin B12 and Hcy gene sets, respectively. From Figure 8A, we observed that the genes associated with vitamin B12 were enriched in mitochondrial fatty acid beta-oxidation, which was active in the liver. A previous study revealed that inhibiting hepatic fatty acid oxidation (FAO) resulted in a systemic hormetic response that protected mice from obesity and glucose intolerance induced by a high-fat diet, which implied vitamin B12 metabolism might relate to FAO and thereby have an association with obesity (81). More importantly, a study in adult Wistar rats fed a vitamin B12-restricted diet during the maternal or postnatal period predicted higher visceral adiposity and resulted in an alteration in the metabolism of lipids in the offspring. Vitamin-restricted offspring also had higher activities of hepatic fatty acid synthase and acetyl-CoA-carboxylase and higher plasma cortisol levels (82, 83). Another study found that restriction of maternal B12, folate, and methionine at the conception stage in sheep models showed increased resistance to insulin and elevated blood pressure in their offspring (83, 84). The aforementioned findings suggest that vitamin B12 may influence body fat mainly through FAO and insulin-related pathways. In Figure 8B, except that Hcy-related genes were enriched in vitamin metabolism for vitamin B6 and B12 and were involved in the metabolism of Hcy, these genes were also enriched in the vitamin D metabolic pathway, which played a crucial role in the musculoskeletal systems (85, 86). The potential pathways by which Hcy and vitamin B12 are involved in the fat, and musculoskeletal systems require further investigation.
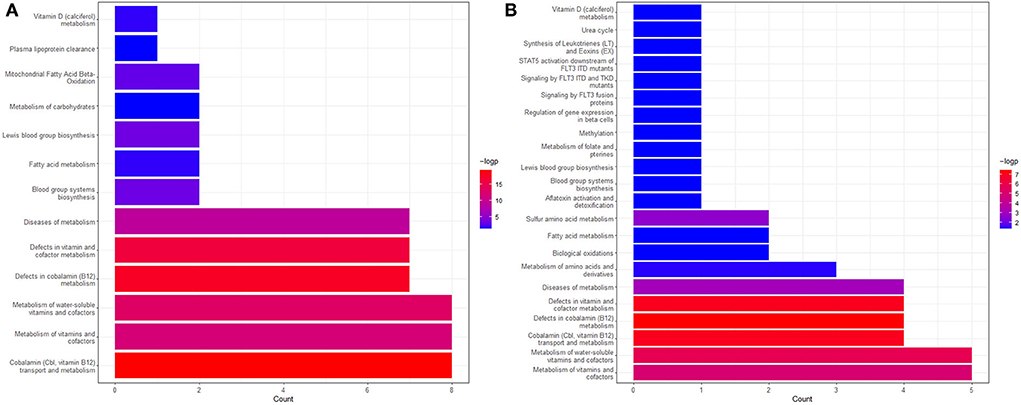
Figure 8. The Reactome enrichment analyses genes associated with vitamin B12 (A) and Hcy (B). The pathways with adjusted P < 0.05 are left in the figure. More information can be found on the website (https://reactome.org/).
The strengths of this study lie in the application of Mendelian randomization, which could diminish residual confounding and reduce the likelihood of false negatives in large GWAS. To our knowledge, this is the first study employing the MR approach to evaluate the causal associations of genetically predicted Hcy and B vitamin concentrations with fat and musculoskeletally relevant phenotypes via several independent data sources. Additionally, combining various non-overlapping data sources and employing more SNPs associated with Hcy and B vitamins could guarantee the sample size, explain the phenotypic variance, respectively, and ensure statistical power, even though certain weak associations have been overlooked. The concerns about vitamin B12 and Hcy may not be independent variables for interfering with causality. Actually, no matter whether it is vitamin B12 or Hcy, the three conditions (Figure 2) for conducting an MR study are satisfied. To avoid horizontal pleiotropy because of the interlink between vitamin B12 and Hcy, we used strict screening criteria for selecting SNPs as instrumental variables. In other words, these instruments could infer the causal effects of vitamin B12 on fat and musculoskeletal diseases and Hcy on fat and musculoskeletal diseases, respectively. We confined the population in this study to subjects of European ancestry to diminish population structure bias, which impeded the generalization of our results to other populations. Another limitation is the possibility of pleiotropy. Nonetheless, our sensitivity analyses produced directionally consistent findings with the same significance in different sensitivity approaches (weighted median method, MR-Egger, MR-PRESSO, reverse Mendelian randomization). We also tested the confounding factors, including education, smoking, and alcohol use, which might confuse the associations between exposures and outcomes. As a result, these confounders cannot confuse the associations (multivariable MR). Moreover, the results of MR-Egger and MR-PRESSO showed no pleiotropic effects in most analyses, so pleiotropy and confounding did not bias our results. Furthermore, several Hcy-associated SNPs or vitamin B12-associated SNPs affecting the genetic predisposition to blood pressure or lipid traits were excluded. The magnitude of our findings did not change significantly, confirming our results' robustness. Moreover, dietary intake was not considered, and the variation in each of the exposures that SNPs explained was small. Unhealthy lifestyles were also major factors that need further consideration. Moreover, 1 SNP for vitamin B6 and 2 SNPs for folate may provide insufficient power to infer the associations. As the involved population encompasses distinct demographic or clinical characteristics and the complex relationship between B vitamins and Hcy is described, more detailed and comprehensive studies about causal effects are urgently needed to further confirm the associations and degrees of Hcy and B vitamins with the risk of fat and musculoskeletally relevant diseases.
Conclusions
In summary, the present study indicated causalities between genetically predicted Hcy increase and the elevated risk of soft tissue disorders, osteoporosis with pathological fracture, knee osteoarthritis, and hospital-diagnosed osteoarthritis, as well as genetically predicted vitamin B12 enhancement and the decrease of fat percentage and its subtypes, revealing that disordered expressions of Hcy and vitamin B12 might play a role in the pathogenesis and development of fat obesity and musculoskeletally relevant diseases, respectively. The supplementation of vitamin B12 and lowering Hcy therapy could be potential options for preventing and treating obesity and musculoskeletal-related diseases. As for folate, further evidence is needed to confirm its relationship with lean mass because of the limited instruments for folate employed in this study.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The authors thank the UK Biobank study, and the summary statistics data of UKB can be download from Nealelab (http://www.nealelab.is/uk-biobank). The authors thank the FinnGen consortium, and the summary statistics data in FinnGen Consortium can be downloaded from Google cloud storage free of charge (https://www.finngen.fi/en/access_results). The authors thank SSGAC for supplying the summary statistics of years of education attainment, which have been downloaded from https://www.thessgac.org/home. The authors thank GSCAN for supplying the summary statistics of smoking and alcohol use phenotypes, which have been downloaded from https://conservancy.umn.edu/handle/11299/201564.
Author contributions
Study concept and design and drafting of the manuscript: LF and Y-QH. Acquisition of data and critical revision of the manuscript for important intellectual content: LF, Y-QH, and YW. Analysis and interpretation of data: LF. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by grants to LF and Y-QH from the National Natural Science Foundation of China (Grants No. 82204063, 11971117, and 11571082).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1048122/full#supplementary-material
References
1. Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. (2006) 7:239–50. doi: 10.1111/j.1467-789X.2006.00251.x
2. Brooks PM. Musculoskeletal medicine: the challenge of the bone and joint decade. APLAR J Rheumatol. (2004) 7:272–77. doi: 10.1111/j.1479-8077.2004.00102.x
3. Collins KH, Herzog W, MacDonald GZ, Reimer RA, Rios JL, Smith IC, et al. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol. (2018) 9:112. doi: 10.3389/fphys.2018.00112
4. Tong W, Zeng Y, Chow DHK, Yeung W, Xu J, Deng Y, et al. Wnt16 attenuates osteoarthritis progression through a PCP/JNK-mTORC1-PTHrP cascade. Ann Rheum Dis. (2019) 78:551–61. doi: 10.1136/annrheumdis-2018-214200
5. Yao Z, Yuan W, Xu J, Tong W, Mi J, Ho PC, et al. Magnesium-encapsulated injectable hydrogel and 3D-engineered polycaprolactone conduit facilitate peripheral nerve regeneration. Adv Sci. (2022) 9:e2202102. doi: 10.1002/advs.202202102
6. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. (2008) 18:388–95. doi: 10.1016/j.numecd.2007.10.002
7. Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. (2000) 904:437–48. doi: 10.1111/j.1749-6632.2000.tb06498.x
8. Brady SR, Mamuaya BB, Cicuttini F, Wluka AE, Wang Y, Hussain SM, et al. Body composition is associated with multisite lower body musculoskeletal pain in a community-based study. J Pain. (2015) 16:700–6. doi: 10.1016/j.jpain.2015.04.006
9. Han L, Liu Y, Wang C, Tang L, Feng X, Astell-Burt T, et al. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: a population-based study and systematic review. Clin Nutr. (2017) 36:1215–30. doi: 10.1016/j.clnu.2016.11.011
10. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. (2017) 14:78. doi: 10.1186/s12986-017-0233-z
11. Rudreshkumar KJ, Majumdar V, Nagaraja D, Christopher R. Relevance of plasma levels of free homocysteine and methionine as risk predictors for ischemic stroke in the young. Clin Nutr. (2018) 37:1715–21. doi: 10.1016/j.clnu.2017.07.005
12. Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am J Clin Nutr. (2001) 73:613–21. doi: 10.1093/ajcn/73.3.613
13. Al-Bayyari N, Hamadneh J, Hailat R, Hamadneh S. Total homocysteine is positively correlated with body mass index, waist-to-hip ratio, and fat mass among overweight reproductive women: a cross-sectional study. Nutr Res. (2017) 48:9–15. doi: 10.1016/j.nutres.2017.10.008
14. Fu L, Li YN, Luo D, Deng S, Hu YQ. Plausible relationship between homocysteine and obesity risk via MTHFR gene: a meta-analysis of 38,317 individuals implementing Mendelian randomization. Diabetes Metab Syndr Obes. (2019) 12:1201–12. doi: 10.2147/dmso.s205379
15. Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. (2005) 15:490–5. doi: 10.1016/j.gde.2005.08.002
16. Terruzzi I, Senesi P, Fermo I, Lattuada G, Luzi L. Are genetic variants of the methyl group metabolism enzymes risk factors predisposing to obesity? J Endocrinol Invest. (2007) 30:747–53. doi: 10.1007/bf03350812
17. Williams KT, Schalinske KL. New insights into the regulation of methyl group and homocysteine metabolism. J Nutr. (2007) 137:311–4. doi: 10.1093/jn/137.2.311
18. Jakubowski H. Homocysteine modification in protein structure/function and human disease. Physiol Rev. (2019) 99:555–604. doi: 10.1152/physrev.00003.2018
19. Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH. Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. (1996) 1315:159–62. doi: 10.1016/0925-4439(95)00119-0
20. Tyagi N, Kandel M, Munjal C, Qipshidze N, Vacek JC, Pushpakumar SB, et al. Homocysteine mediated decrease in bone blood flow and remodeling: role of folic acid. J Orthop Res. (2011) 29:1511–6. doi: 10.1002/jor.21415
21. Wang P, Liu L, Lei SF. Causal effects of homocysteine levels on the changes of bone mineral density and risk for bone fracture: a two-sample mendelian randomization study. Clin Nutr. (2021) 40:1588–95. doi: 10.1016/j.clnu.2021.02.045
22. McLean RR, Karasik D, Selhub J, Tucker KL, Ordovas JM, Russo GT, et al. Association of a common polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene with bone phenotypes depends on plasma folate status. J Bone Miner Res. (2004) 19:410–8. doi: 10.1359/jbmr.0301261
23. Gupta S, Kühnisch J, Mustafa A, Lhotak S, Schlachterman A, Slifker MJ, et al. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. (2009) 23:883–93. doi: 10.1096/fj.08-120584
24. Collaboration HLT. Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. Homocysteine Lowering Trialists' Collaboration. BMJ. (1998) 316:894–8.
25. Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA. (2005) 293:1082–8. doi: 10.1001/jama.293.9.1082
26. Elshorbagy AK, Nurk E, Gjesdal CG, Tell GS, Ueland PM, Nygård O, et al. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: does cysteine link amino acid and lipid metabolism? Am J Clin Nutr. (2008) 88:738–46. doi: 10.1093/ajcn/88.3.738
27. Bayhan I, Uygur D, Ugurlu N, Ozaksit G. Strontium ranelate decreases plasma homocysteine levels in postmenopausal osteoporotic women. Rheumatol Int. (2009) 29:263–6. doi: 10.1007/s00296-008-0693-6
28. Nakazato M, Maeda T, Takamura N, Wada M, Yamasaki H, Johnston KE, et al. Relation of body mass index to blood folate and total homocysteine concentrations in Japanese adults. Eur J Nutr. (2011) 50:581–5. doi: 10.1007/s00394-010-0165-0
29. Rumbak I, ZiŽić V, Sokolić L, Cvijetić S, KajfeŽ R, Colić Barić I. Bone mineral density is not associated with homocysteine level, folate and vitamin B12 status. Arch Gynecol Obstet. (2012) 285:991–1000. doi: 10.1007/s00404-011-2079-3
30. Kim JI, Moon JH, Chung HW, Kong MH, Kim HJ. Association between Homocysteine and Bone Mineral Density according to Age and Sex in Healthy Adults. J Bone Metab. (2016) 23:129–34. doi: 10.11005/jbm.2016.23.3.129
31. Allin KH, Friedrich N, Pietzner M, Grarup N, Thuesen BH, Linneberg A, et al. Genetic determinants of serum vitamin B12 and their relation to body mass index. Eur J Epidemiol. (2017) 32:125–34. doi: 10.1007/s10654-016-0215-x
32. Wang C-S, Wong T-C, Duong TV, Su C-T, Chen H-H, Chen T-H, et al. Hyperhomocysteinemia associated with low muscle mass, muscle function in elderly hemodialysis patients: an analysis of multiple dialysis centers. Biomed Res Int. (2019) 2019:9276097. doi: 10.1155/2019/9276097
33. Wang Y, Jiang Y, Wang N, Zhu M, Liu X, Wang R, et al. Central but not general obesity is positively associated with the risk of hyperhomocysteinemia in middle-aged women. Nutrients. (2019) 11:1614. doi: 10.3390/nu11071614
34. Oliai Araghi S, Braun KVE, van der Velde N, van Dijk SC, van Schoor NM, Zillikens MC, et al. B-vitamins and body composition: integrating observational and experimental evidence from the B-PROOF study. Eur J Nutr. (2020) 59:1253–62. doi: 10.1007/s00394-019-01985-8
35. Xiang Y, Zhao Q, Wang N, Yu Y, Wang R, Zhang Y, et al. Association of obesity with the risk of hyperhomocysteinemia among the Chinese Community residents: a prospective cohort study in < city>Shanghai < /city>, China. Nutrients. (2021) 13:3648. doi: 10.3390/nu13103648
36. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
37. Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. (2016) 45:908–15. doi: 10.1093/ije/dyw127
38. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
39. Yang Q, Sanderson E, Tilling K, Borges MC, Lawlor DA. Exploring and mitigating potential bias when genetic instrumental variables are associated with multiple non-exposure traits in Mendelian randomization. medRxiv. (2019) 19009605. doi: 10.1101/19009605
40. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
41. Grarup N, Sulem P, Sandholt CH, Thorleifsson G, Ahluwalia TS, Steinthorsdottir V, et al. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PLoS Genet. (2013) 9:e1003530. doi: 10.1371/journal.pgen.1003530
42. Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. (2009) 84:477–82. doi: 10.1016/j.ajhg.2009.02.011
43. van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, et al. Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr. (2013) 98:668–76. doi: 10.3945/ajcn.112.044545
44. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
45. FinnGen Documentation of R4 Release. (2020). Available online at: https://finngen.gitbook.io/documentation/ (accessed April 1, 2022).
46. Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, et al. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat Commun. (2017) 8:121. doi: 10.1038/s41467-017-00108-3
47. Zillikens MC, Demissie S, Hsu Y-H, Yerges-Armstrong LM, Chou W-C, Stolk L, et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nat Commun. (2017) 8:80. doi: 10.1038/s41467-017-00031-7
48. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 11 million individuals. Nat Genet. (2018) 50:1112–21. doi: 10.1038/s41588-018-0147-3
49. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 12 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5
50. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J, et al. Framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
51. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
52. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
53. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–98. doi: 10.1038/s41588-018-0099-7
54. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–53. doi: 10.1093/bioinformatics/btz469
55. Ripatti P, Rämö JT, Mars NJ, Fu Y, Lin J, Söderlund S, et al. Polygenic hyperlipidemias and coronary artery disease risk. Circ Genom Precis Med. (2020) 13:e002725. doi: 10.1161/circgen.119.002725
56. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
57. Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. (2013) 42:1497–501. doi: 10.1093/ije/dyt179
58. Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. (2017) 33:272–79. doi: 10.1093/bioinformatics/btw613
59. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
60. Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–39. doi: 10.1093/ije/dyx034
61. Si S, Tewara MA Li Y, Li W, Chen X, Yuan T, et al. Causal pathways from body components and regional fat to extensive metabolic phenotypes: a mendelian randomization study. Obesity. (2020) 28:1536–49. doi: 10.1002/oby.22857
62. Yuan S, Xiong Y, Larsson SC. An atlas on risk factors for multiple sclerosis: a Mendelian randomization study. J Neurol. (2021) 268:114–24. doi: 10.1007/s00415-020-10119-8
63. Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA. (1993) 270:2693–8. doi: 10.1001/jama.1993.03510220049033
64. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. (2018) 61:142–50. doi: 10.1016/j.pcad.2018.07.003
65. Fu L, Zhang M, Hu YQ, Zhao X, Cheng H, Hou D, et al. Gene-gene interactions and associations of six hypertension related single nucleotide polymorphisms with obesity risk in a Chinese children population. Gene. (2018) 679:320–27. doi: 10.1016/j.gene.2018.09.019
66. Fu L, Li YN, Luo D, Deng S, Wu B, Hu YQ. Evidence on the causal link between homocysteine and hypertension from a meta-analysis of 40 173 individuals implementing Mendelian randomization. J Clin Hypertens. (2019) 21:1879–94. doi: 10.1111/jch.13737
67. Fu L, Wang Y, Li T, Hu YQ. A novel approach integrating hierarchical clustering and weighted combination for association study of multiple phenotypes and a genetic variant. Front Genet. (2021) 12:654804. doi: 10.3389/fgene.2021.654804
68. Fu L, Cheng H, Zhao X, Hou D, Xie X, Mi J. Distinct causal effects of body fat distribution on cardiometabolic traits among children: findings from the BCAMS study. Nutr Metab Cardiovasc Dis. (2022) 32:1753–65. doi: 10.1016/j.numecd.2022.03.030
69. Fu L, Wang Y, Li T, Yang S, Hu YQ. A Novel hierarchical clustering approach for joint analysis of multiple phenotypes uncovers obesity variants based on ARIC. Front Genet. (2022) 13:791920. doi: 10.3389/fgene.2022.791920
70. Fu L, Wang Y, Hu Y-Q. Association between homocysteine and nonalcoholic fatty liver disease: Mendelian randomisation study. Eur J Clin Invest. (2022) e13895. doi: 10.1111/eci.13895. [Epub ahead of print].
71. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
72. Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet. (2017) 13:e1006944. doi: 10.1371/journal.pgen.1006944
73. Elshorbagy AK, Graham I, Refsum H. Body mass index determines the response of plasma sulfur amino acids to methionine loading. Biochimie. (2020) 173:107–13. doi: 10.1016/j.biochi.2020.03.001
74. Wang Z, Pini M, Yao T, Zhou Z, Sun C, Fantuzzi G, et al. Homocysteine suppresses lipolysis in adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol Metab. (2011) 301:E703–E12. doi: 10.1152/ajpendo.00050.2011
75. Elshorbagy AK, Kozich V, Smith AD, Refsum H. Cysteine and obesity: consistency of the evidence across epidemiologic, animal and cellular studies. Curr Opin Clin Nutr Metab Care. (2012) 15:49–57. doi: 10.1097/MCO.0b013e32834d199f
76. Lima A, Ferin R, Bourbon M, Baptista J, Pavão ML. Hypercysteinemia, a potential risk factor for central obesity and related disorders in Azores, Portugal. J Nutr Metab. (2019) 2019:1826780. doi: 10.1155/2019/1826780
77. Ma CH, Chiua YC, Wu CH, Jou IM, Tu YK, Hung CH, et al. Homocysteine causes dysfunction of chondrocytes and oxidative stress through repression of SIRT1/AMPK pathway: a possible link between hyperhomocysteinemia and osteoarthritis. Redox Biol. (2018) 15:504–12. doi: 10.1016/j.redox.2018.01.010
78. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. (2014) 127:547–53. doi: 10.1016/j.amjmed.2014.02.007
79. Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. (2013) 14:15074–91. doi: 10.3390/ijms140715074
80. Pasiakos SM, Lieberman HR, Fulgoni VL. Higher-protein diets are associated with higher HDL cholesterol and lower BMI and waist circumference in US adults. J Nutr. (2015) 145:605–14. doi: 10.3945/jn.114.205203
81. Lee J, Choi J, Selen Alpergin ES, Zhao L, Hartung T, Scafidi S, et al. Loss of hepatic mitochondrial long-chain fatty acid oxidation confers resistance to diet-induced obesity and glucose intolerance. Cell Rep. (2017) 20:655–67. doi: 10.1016/j.celrep.2017.06.080
82. Kumar KA, Lalitha A, Pavithra D, Padmavathi IJ, Ganeshan M, Rao KR, et al. Maternal dietary folate and/or vitamin B12 restrictions alter body composition (adiposity) and lipid metabolism in Wistar rat offspring. J Nutr Biochem. (2013) 24:25–31. doi: 10.1016/j.jnutbio.2012.01.004
83. Boachie J, Adaikalakoteswari A, Samavat J, Saravanan P. Low Vitamin B12 and lipid metabolism: evidence from pre-clinical and clinical studies. Nutrients. (2020) 12: 1925. doi: 10.3390/nu12071925
84. Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci USA. (2007) 104:19351–6. doi: 10.1073/pnas.0707258104
85. Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. (2013) 34:33–83. doi: 10.1210/er.2012-1012
Keywords: homocysteine, musculoskeletal diseases, obesity, B vitamins, Mendelian randomization
Citation: Fu L, Wang Y and Hu Y-Q (2022) Causal effects of B vitamins and homocysteine on obesity and musculoskeletal diseases: A Mendelian randomization study. Front. Nutr. 9:1048122. doi: 10.3389/fnut.2022.1048122
Received: 19 September 2022; Accepted: 24 October 2022;
Published: 24 November 2022.
Edited by:
Ning Zhang, Stanford University, United StatesReviewed by:
Meiling Jin, City of Hope National Medical Center, United StatesWenxue Tong, The Chinese University of Hong Kong, China
Ni Su, Stanford University, United States
Copyright © 2022 Fu, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liwan Fu, bGl3YW5mZWxpeEBmb3htYWlsLmNvbQ==; Yue-Qing Hu, eXVlaHVAZnVkYW4uZWR1LmNu
 Liwan Fu1*
Liwan Fu1* Yue-Qing Hu
Yue-Qing Hu