- 1National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 3Graduate School, China Academy of Chinese Medical Sciences, Beijing, China
- 4Department of Cardiovascular Medicine, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Objective: To evaluate the association between dietary inflammatory index (DII) and Atherosclerotic cardiovascular disease (ASCVD) among U.S. adults.
Methods: We collected data from National Health and Nutrition Examination Survey (NHANES) between 1999 and 2018. Adults who reported complete information to diagnose ASCVD and calculate DII were included. We used three models to differentially adjust the covariates, including age, sex, race or ethnicity, education level, smoking status, poverty, insurance, body mass index, hyperlipemia, hypertension, and diabetes. Logistic regression was used to estimate the Odds Ratio (OR) and 95% confidence interval (95% CI) for ASCVD grouped by DII deciles. We additionally conducted spline smoothing with the generalized additive model (GAM) and the log-likelihood ratio to examine the non-linear relationship between DII and ASCVD. If exists, the segmented linear regression will be used to detect the cutoff point. The subgroup analyses were stratified by various atherosclerotic cardiovascular diseases (i.e., CHD, angina, heart attack, and stroke) and sex.
Results: A total of 48,733 participants (mean age, 47.13 ± 0.19 years) with 51.91% women were enrolled, of which 5,011 were diagnosed with ASCVD. In the crude model, participants in the five highest deciles (D6, 7, 8, 9, and 10) of DII score had a significantly higher risk of having ASCVD compared to those in the first decile. In the fully adjusted model, those in the tenth decile [OR = 1.47, 95% CI = (1.18,1.84)] of DII had a significantly increased risk of ASCVD compared to the first decile. Notably, when DII is above 3, the ASCVD risk increased by 41% for each one increase in DII [OR = 1.41, 95% CI = (1.15,1.73)]. This relationship was more pronounced in females.
Conclusion: Our study revealed a positive and non-linearly association between DII and ASCVD in U.S. adults. This relationship was more pronounced in females. The findings provide a reference for future research and diet recommendations.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains to be the leading cause of morbidity and mortality despite tremendous advances in traditional risk factor management. Therefore, great effort has been put into identifying and managing residual risk factors (1, 2). Growing evidence suggests that inflammation is a novel and effective target to reduce residual risk factors for ASCVD (3, 4). However, since interference with inflammatory pathways can compromise host defenses, optimizing the net benefit remains a challenge (4). Only canakinumab (5) and colchicine (6–8) have been validated to treat ASCVD by targeting inflammation without unacceptable side effects.
Diet is a critical modifiable target for ASCVD prevention and management (9) and the fundamental mechanism has been associated with potential pro- or anti-inflammatory properties of the dietary pattern or dietary components (10–12). Pro-inflammatory foods include processed meats, refined starches, and foods and beverages with added sugars. In contrast, anti-inflammatory foods include dark vegetables, whole grains, fruits, tea, coffee, and wine (11). Obviously, the inflammatory properties of individual foods are not adequate to assess the inflammatory level of various dietary patterns. To quantify the underlying inflammatory level of various diet patterns, in 2009 and 2014, Hébert and colleagues developed and improved the dietary inflammatory index (DII), a literature-derived and population-based scoring system, (13, 14), which has been validated in many studies with a variety of inflammatory markers (10).
Accumulating evidence from diverse populations suggested a positive association between DII and cardiovascular diseases (CVD), such as a 10-year risk for ASCVD, CVD factors, cardiovascular mortality, and CVD risk burden (15–20). That is, the higher the dietary inflammation, the more harmful to people. A secondary analysis of the PREDIMED (Prevención con Dieta Mediterránea) study (21) prospectively examined the relationship between DII and the incidence of CVD (i.e., myocardial infarction, stroke, or cardiovascular death), including 7,169 high-risk participants with a median follow-up of 4.8 years. The results show a direct prospective association between increased diet-associated inflammation and the risk of CVD. As a continuous variable, the HR for each additional standard deviation of the DII was 1.22 (95% CI = 1.06–1.40). In fact, some pro-inflammatory components such as vitamin B12, energy, protein, cholesterol, and carbohydrates are essential to sustain life (14). Some essential ingredients have a non-linear effect on health (22) and DII is non-linear associated with some biomarkers and diseases (23–25). Maybe there is a threshold between DII scores and ASCVD, below which could be considered safe, rather than less is healthier. However, whether a cutoff point exists and its value remains unclear. Furthermore, the association between DII and ASCVD in U.S. adults is still unclear, and whether this relationship persists across genders and various atherosclerotic cardiovascular diseases is controversial (26, 27).
The NHANES1 is an ongoing cross-sectional study aiming to reflect the health of the U.S. population (28). Here we aimed to use the NHANES database (1999–2018) to assess the association between DII and ASCVD, identified the cutoff point if exits, and detected the subgroups population most likely to benefit from controlling DII.
2. Materials and methods
This cross-sectional study was conducted and reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (29).
2.1. Study design and population
National Health and Nutrition Examination Survey is a multistage, nationally representative cross-sectional study (28). The U.S. National Center for Health Statistics (NCHS) conducts a complex, stratified, multistage probability-cluster sampling design to select participants from the U.S. deinstitutionalized civilians (30, 31). Information was collected through in-home interviews and mobile examination center (MEC) visits and released by CDC in 2-year cycles from 1999 (32).
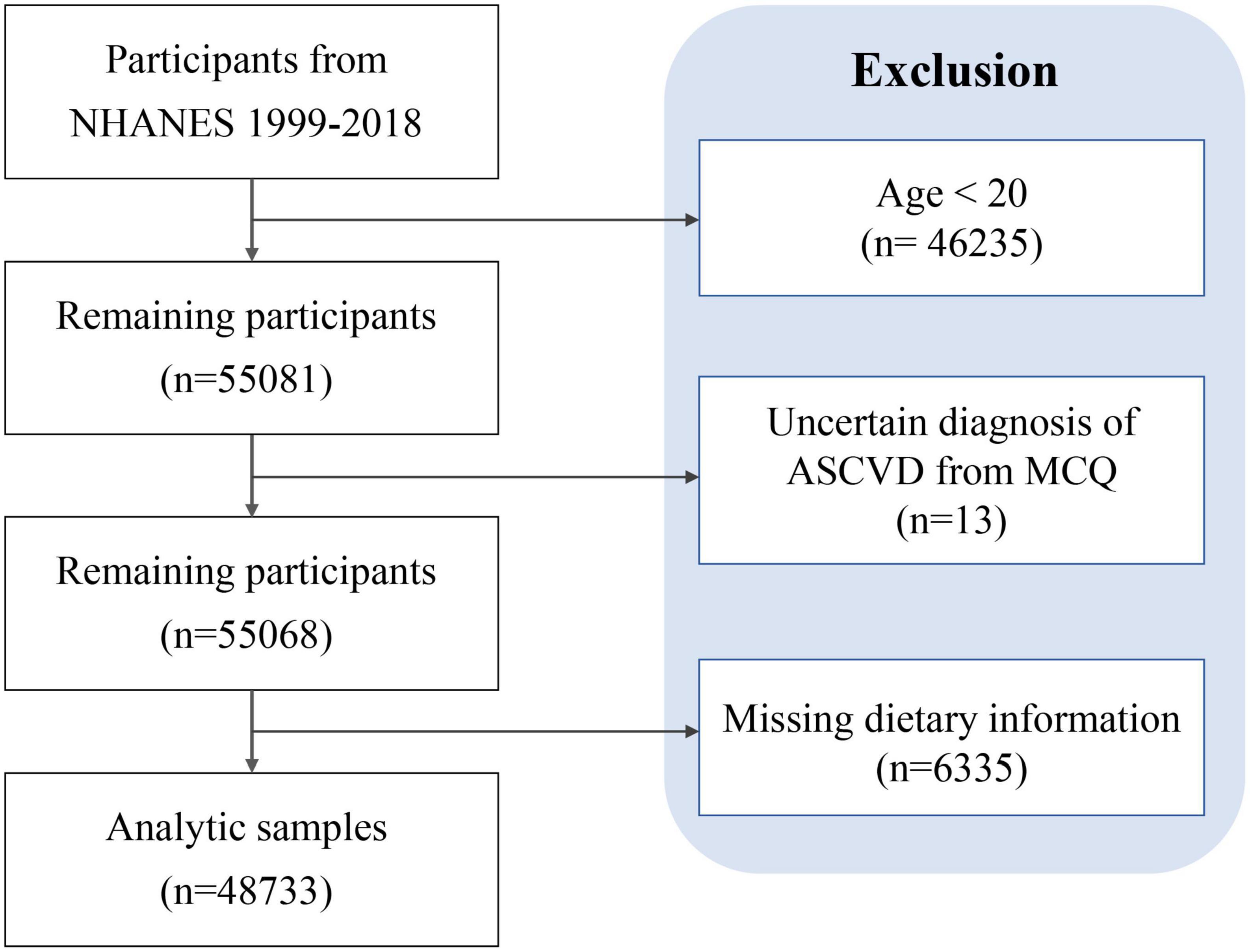
This study included participants in the NHANES from 1999 through 2018 who reported complete information on first-day total nutrient intakes and ASCVD diagnoses related questionnaire. We excluded participants under 20 years of age.
Trained interviewers use a computer-assisted personal interview (CAPI) system to ask for demographic information and characteristics during in-home interviews and MEC. Among them, age, gender, ethnicity, education, poverty, and smoking status were derived from demographic data. Body mass index (BMI) and blood pressure (BP) were extracted from examination data. Insurance information was from questionnaires in which people reporting medicare coverage were asked to show their Medicare cards.
2.2. Dietary inflammatory index
The DII was calculated from a 24-hour recall of dietary data on day one (16, 33, 34), including the types and amounts of foods and beverages consumed during the 24-hour period preceding the interview (midnight to midnight) collected in the MEC. Data are subsequently used to estimate intakes of energy, nutrients, and other food components from those foods and beverages validated by the Nutrition Methodology Working Group (35).
We calculate DII according to Hébert’s scheme (14), which evaluated the inflammatory effects of 45 nutrients and was widely used in assessing dietary inflammation (10, 14, 16, 26, 34). In this study, 28 of the 45 food parameters available in NHANES were used to calculate DII, including carbohydrates, protein, total fat, alcohol, fiber, cholesterol, saturated fat, MUFA, PUFA, n-3 fatty acids, n-6 fatty acids, niacin, vitamin A, thiamin (vitamin B1), riboflavin (vitamin B2), vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, Fe, Mg, zinc, selenium, folic acid, beta-carotene, caffeine, and energy. Previous studies have shown that using fewer than 30 food parameters for DII calculations does not affect the predictive power of DII (16, 34, 36, 37). Data for the calculation of DII are presented in Supplementary Table 1. The calculation steps are as follows. Z score = (daily intake of a certain dietary ingredient or nutrient–global daily mean intake)/the standard deviation of the global mean per capita daily intake of this dietary ingredient or nutrient (14). Then converted the Z-score to a percentile scale, doubled the resulting percentile value, and subtracted “1” to achieve a symmetrical distribution centered on “0”. Finally, multiplied by the total inflammation score for each dietary component, the inflammatory index of each dietary ingredient or nutrient was calculated to obtain an individual DII (14, 33, 37). DII scores range from negative to positive, with lower scores indicating greater anti-inflammatory effects and higher scores implying stronger pro-inflammatory effects of the diet (14).
2.3. Outcome definitions
Our outcome is whether diagnosed with ASCVD. According to the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults (9), ASCVD was defined as having at least one diagnosis of coronary heart disease, angina, heart attack, and stroke; hard criteria was defined as heart attack and stroke. We described the diagnosis methods based on NHANES in Supplementary Table 2.
2.4. Covariates
Covariates about individual characteristics included age, sex, race or ethnicity, BMI, education level, smoking status, poverty income ratio (PIR), and insurance. All detailed measurement procedures are available at www.cdc.gov/nchs/nhanes/publicly available. Physical activity data were analyzed according to the World Health Organization guidelines (38) and converted to metabolic equivalent (MET) minutes of moderate to vigorous physical activity per week (39). The diseases in the covariates included hypertension, hyperlipidemia, and diabetes, and their diagnosis basis was listed in Supplementary Table 2.
2.5. Statistical analysis
We performed analysis using appropriate NHANES sampling weights and took into account complex multistage cluster survey designs. The characteristics of participants were compared by chi-square test or t-test. The baseline information was presented as unweighted frequencies and weighted percentages for categorical variables; and for continuous variables, were shown as weighted mean and standard deviations (SD).
We constructed three models: Model 1 was the crude model without adjustment for any covariates. We adjusted age, sex, race or ethnicity, education level, smoking status, poverty, and insurance in addition to covariates in model 2. In model 3, we further adjusted BMI, hyperlipemia, hypertension, and diabetes in addition to covariates adjusted in model 2. The Independent variable DII was divided into ten deciles. The lowest 10% of DII score is deciles 1 (D1), followed by deciles 2 (D2), and the highest is deciles 10 (D10). Logistic regression was used to estimate the Odds Ratio (OR) and 95% confidence interval (95% CI) for ASCVD grouped by DII ranges. We further examined the non-linear relationship between DII and ASCVD using spline smoothing regression with the generalized additive model (GAM) (40, 41). Subsequently, we identified the inflection point by piecewise regression, which uses a separate line segment to fit each interval. A log-likelihood ratio test comparing the one-line model with the piecewise regression model was used to determine whether a threshold existed. We calculated OR through piecewise multivariable logistic regression if there was a threshold effect; otherwise, we directly used multivariable logistic regression. All analysis was based on weighted data.
In addition, we conducted a subgroup analysis stratified by the individual component of ASCVD (i.e., CHD, angina, heart attack, and stroke) and sex to assess whether there was a sex difference in the association of DII with ASCVD. The sensitivity analysis was conducted by using the second decile as the reference to perform the segmented linear regression of three models and further adjusted physical activity.
All analyses were conducted by R software (4.2.0) and Empower2 following CDC guidelines.3 P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of participants
A total of 48,733 participants were enrolled in this study after screening according to the established protocol, of which 5,011 (10.28%) participants were diagnosed with ASCVD (Figure 1 and Table 1). As shown in Table 1, the mean age was 47.13 ± 0.19 years old, and 51.91% were female. The mean DII score was 1.38 ± 0.02, ranging from −5.28 to 5.79. Compared with those not diagnosed with ASCVD, people diagnosed with ASCVD are more likely to be older, male, Non-Hispanic White, obese, less educated, former smokers, poverty, and have insurance.
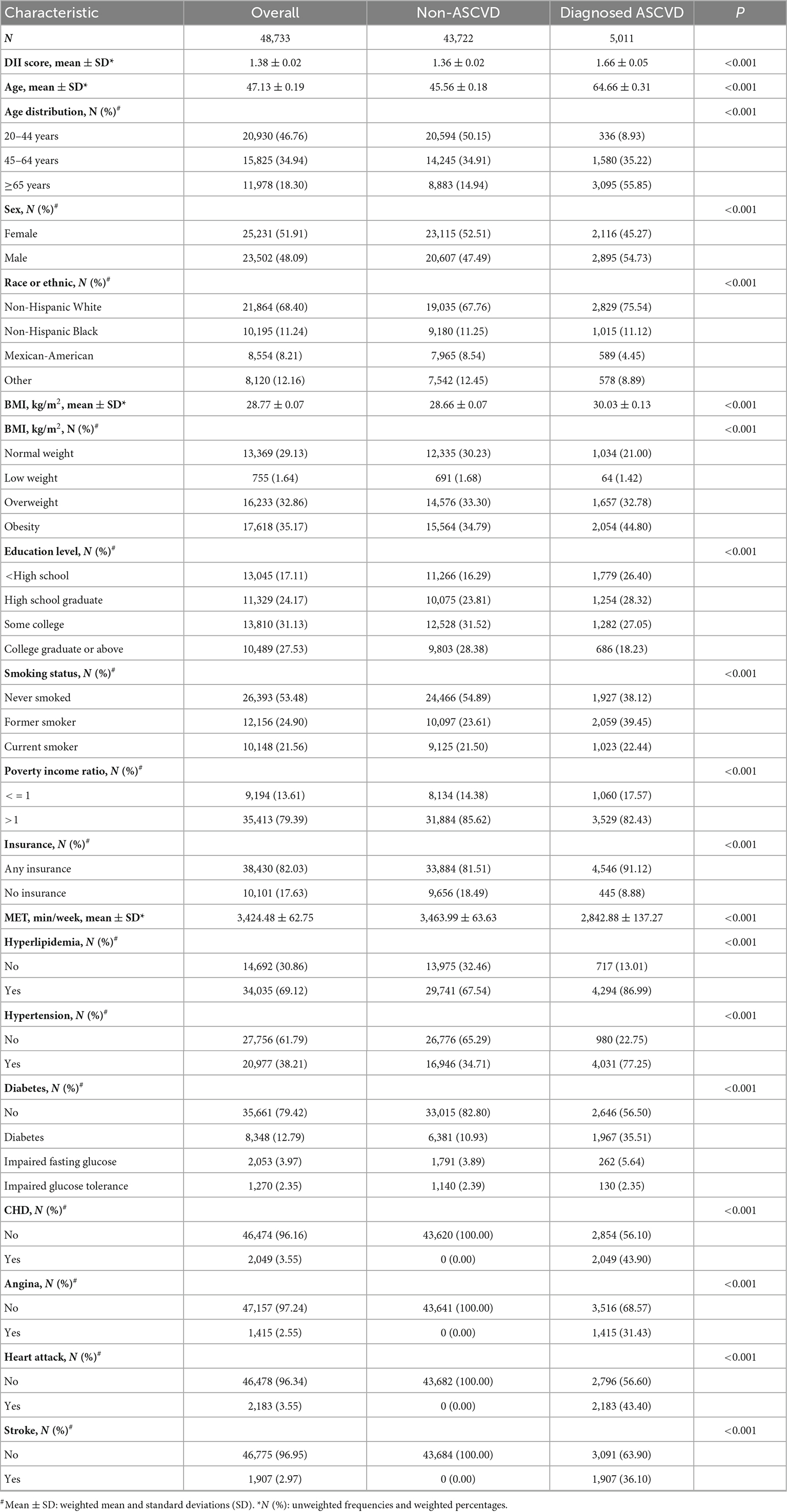
Table 1. Baseline characteristics of participants, from National Health and Nutrition Examination Survey (NHANES) 1999–2018.
3.2. Relationship between DII and ASCVD
We conducted a weighted multivariable logistic regression analysis to examine the association between DII and ASCVD (Table 2). In the crude model (model 1), participants in the five highest deciles of DII score (D6, 7, 8, 9, and 10) had a significantly higher risk of having ASCVD compared to those in decile 1 (D1). The adjustment for demo socioeconomic covariates (age, sex, race or ethnicity, education level, smoking status, poverty, and insurance) in model 2 attenuated the association [D9 vs. D1, OR = 1.30, 95% CI = (1.06,1.59); D10 vs. D1, OR = 1.67, 95% CI = (1.34,2.08)]. Additional adjustment for comorbidities (hyperlipemia, hypertension, and diabetes) and BMI in model 3 only slightly attenuated the association [D10 vs. D1, OR = 1.47, 95% CI = (1.18,1.84)].
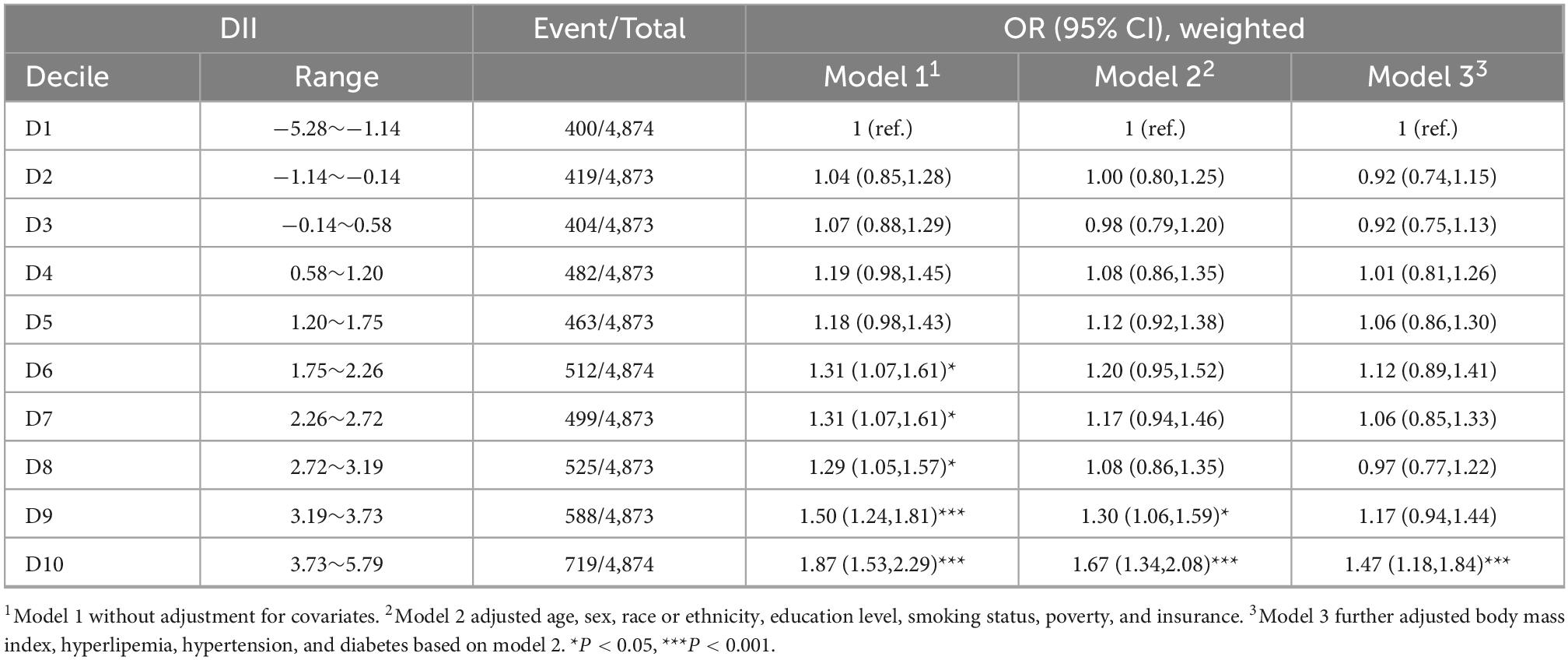
Table 2. Association between dietary inflammatory index (DII) and atherosclerotic cardiovascular disease (ASCVD).
The above multivariable logistic regression suggests a non-linear relationship between DII and ASCVD, we thereby performed an adjusted spline smoothing regression to detect the dose-response and identify the cutoff point (Figure 2 and Supplementary Table 3). Figure 2 shows that the distribution of DII scores is skewed, and the curve representing the relationship between DII and ASCVD is divided into three segments, which the log-likelihood ratio test (Supplementary Table 3) also verified the existence of inflection points. Therefore, we divided the curve into three segments and further conducted a piecewise multivariable logistic regression analysis to explore the threshold effect (Table 3). As we can see, when DII is less than −2, although the curve shows an upward trend, there is no significant relationship between DII and ASCVD [OR = 0.86, 95% CI = (0.53,1.39), P = 0.53, N = 2,194]. There is a midly elevated trend between DII and ASCVD when DII ranges from −2 to 3 [OR = 1.05, 95% CI = (1.00,1.10), P = 0.04, N = 34,863]. Notably, when DII was greater than 3, the percentage of people diagnosed with ASCVD increased rapidly as DII increased [OR = 1.41, 95% CI = (1.15,1.73), P = 0.001, N = 11,676].
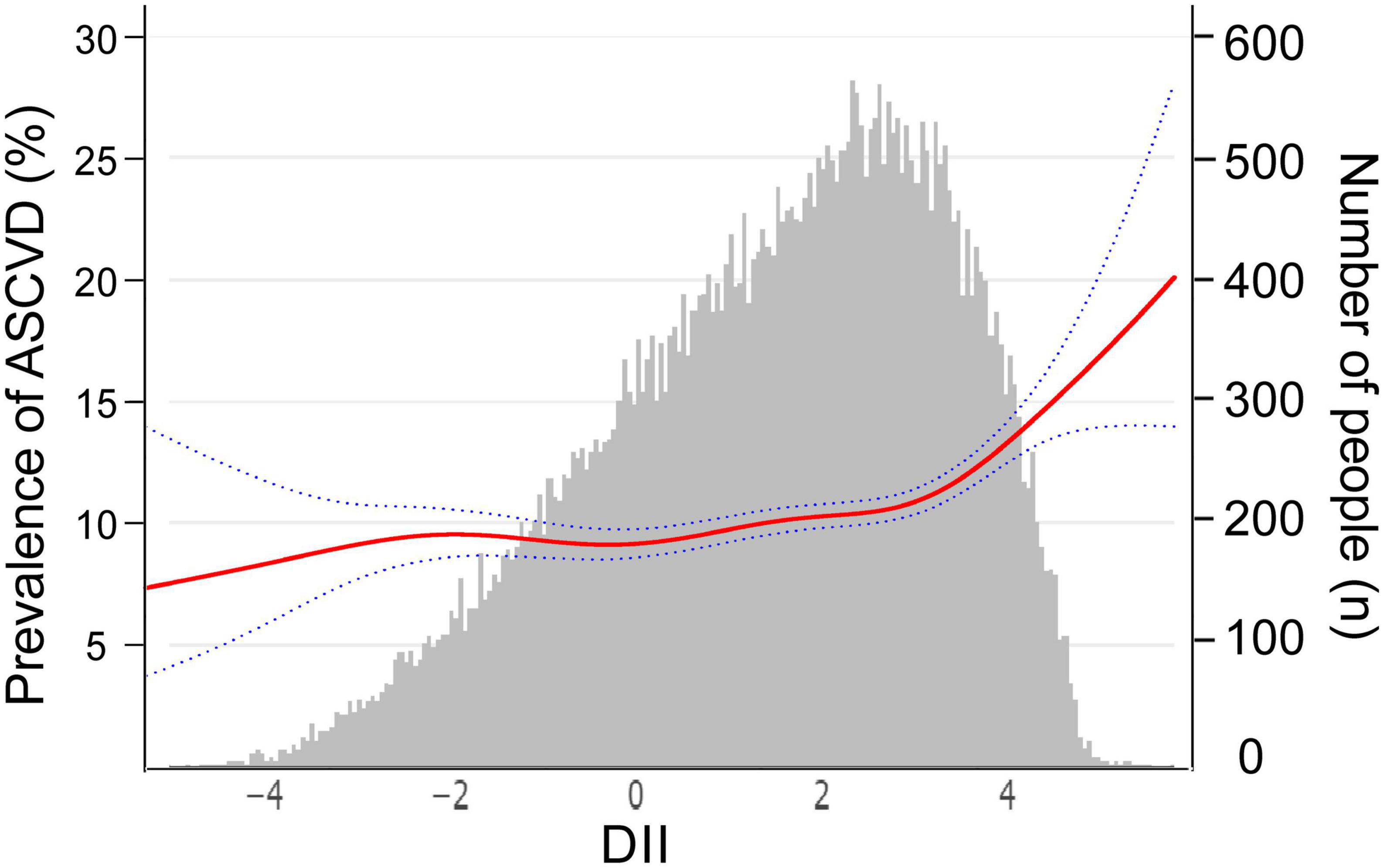
Figure 2. The relationship between dietary inflammatory index (DII) and atherosclerotic cardiovascular disease (ASCVD), weighted. The red curve represents the non-linear relationship between DII and ASCVD, and the dark blue dotted lines represent the 95% of confidence interval from the fit, corresponding to the vertical coordinate on the left. The gray histogram shows the DII distribution of participants, corresponding to the vertical coordinate on the right. We adjusted age, sex, race or ethnicity, body mass index, education level, smoking status, poverty, insurance, hyperlipemia, hypertension, and diabetes.
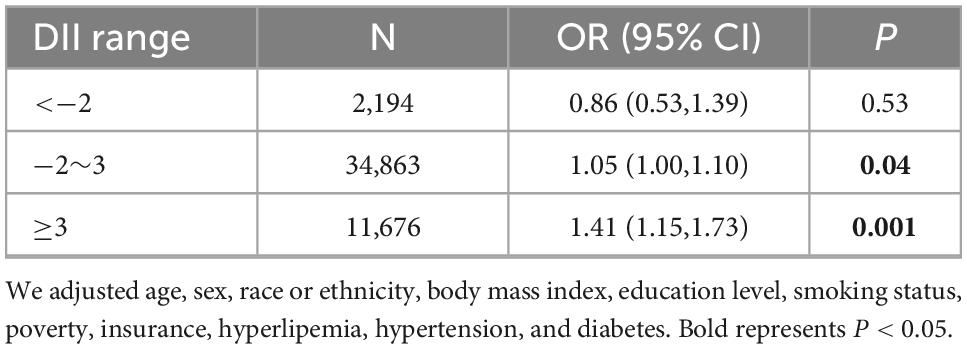
Table 3. Threshold effect analysis of the association between dietary inflammatory index (DII) and atherosclerotic cardiovascular disease (ASCVD), weighted.
3.3. Subgroup analysis
In the all-adjusted model, DII is not significantly associated with CHD, angina pectoris, or heart attack (Table 4, Supplementary Table 3, and Supplementary Figure 1); only for the outcome of stroke, when DII scores more than −1.5, each point related 11% [−1.5 ≤ DII < 3, OR = 1.11, 95% CI = (1.02,1.20), P = 0.01] or 44% [DII ≥ 3, OR = 1.44, 95% CI = (1.15,1.81)] increasing risk. The association between DII and hard criteria (i.e., heart attack and stroke) shows a similar trend to DII and ASCVD. There is no significant relationship between DII and hard criteria [OR = 0.81, 95% CI = (0.42,1.58), P = 0.53] when DII is less than −2. When DII ranges from −2 to 3, the prevalence of hard criteria increased slowly with DII [OR = 1.08, 95% CI = (1.03,1.14), P = 0.004]. Notably, the percentage of people diagnosed with a heart attack or stroke increased rapidly with increasing DII when DII was greater than 3 [OR = 1.28, 95% CI = (1.06,1.53), P = 0.01].
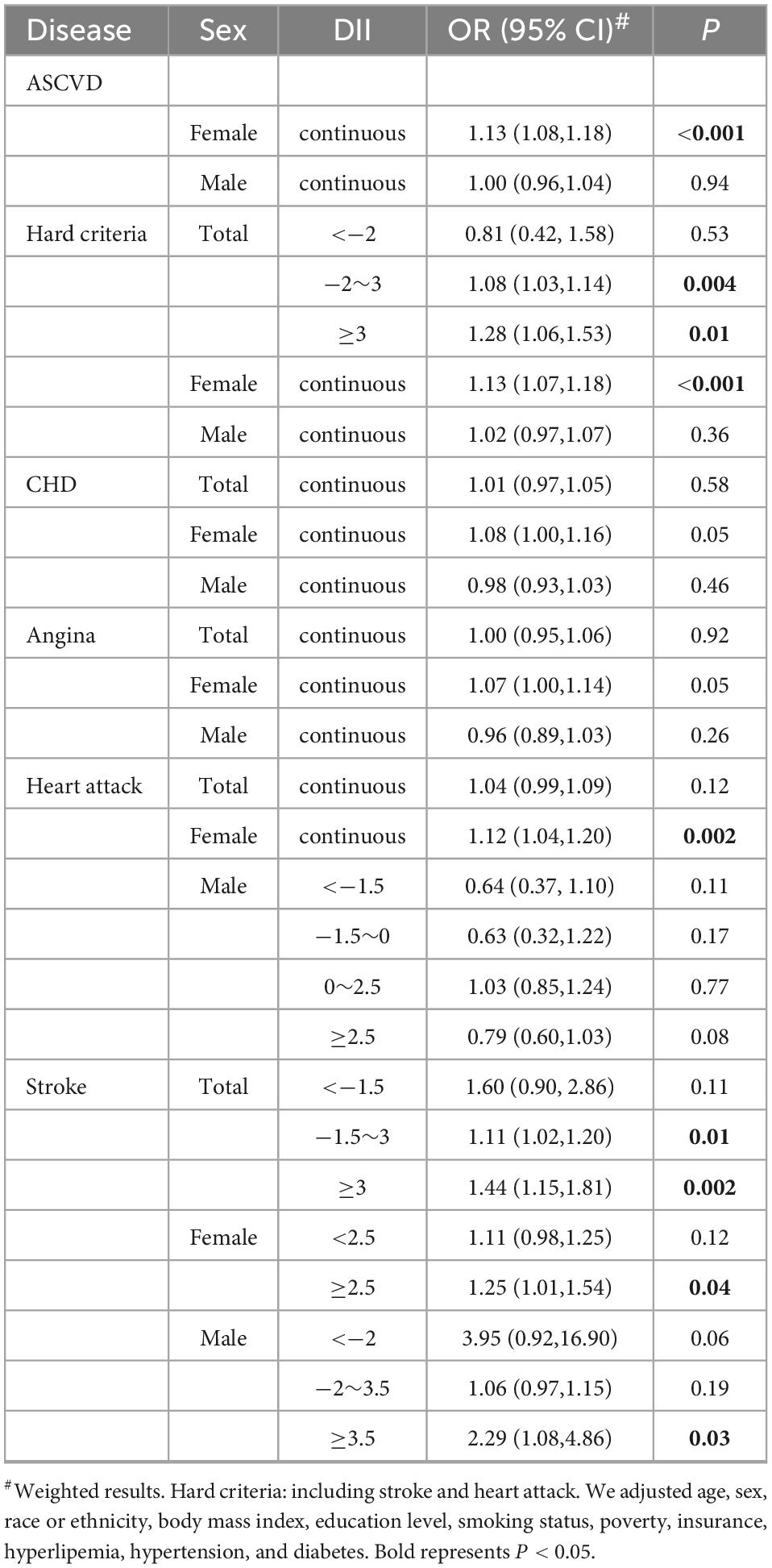
Table 4. Subgroup analysis of the association between dietary inflammatory index (DII) and atherosclerotic cardiovascular disease (ASCVD) stratified by sex and diseases.
We further conducted the smooth curve fittings of subgroup analysis stratification by atherosclerotic cardiovascular diseases and gender (Figure 3) and presented their linear or piecewise multivariable logistic regression (Table 4) according to the log-likelihood ratio (Supplementary Table 3). In the stratified analysis by sex (Figure 3A and Table 4), the overall prevalence of ASCVD in males was higher than that of females. In females, the probability of diagnosed ASCVD was proportional to DII [OR = 1.13, 95% CI = (1.08,1.18)]. While in males, there was no significant association between DII and ASCVD [OR = 1.00, 95% CI = (0.96,1.04)].
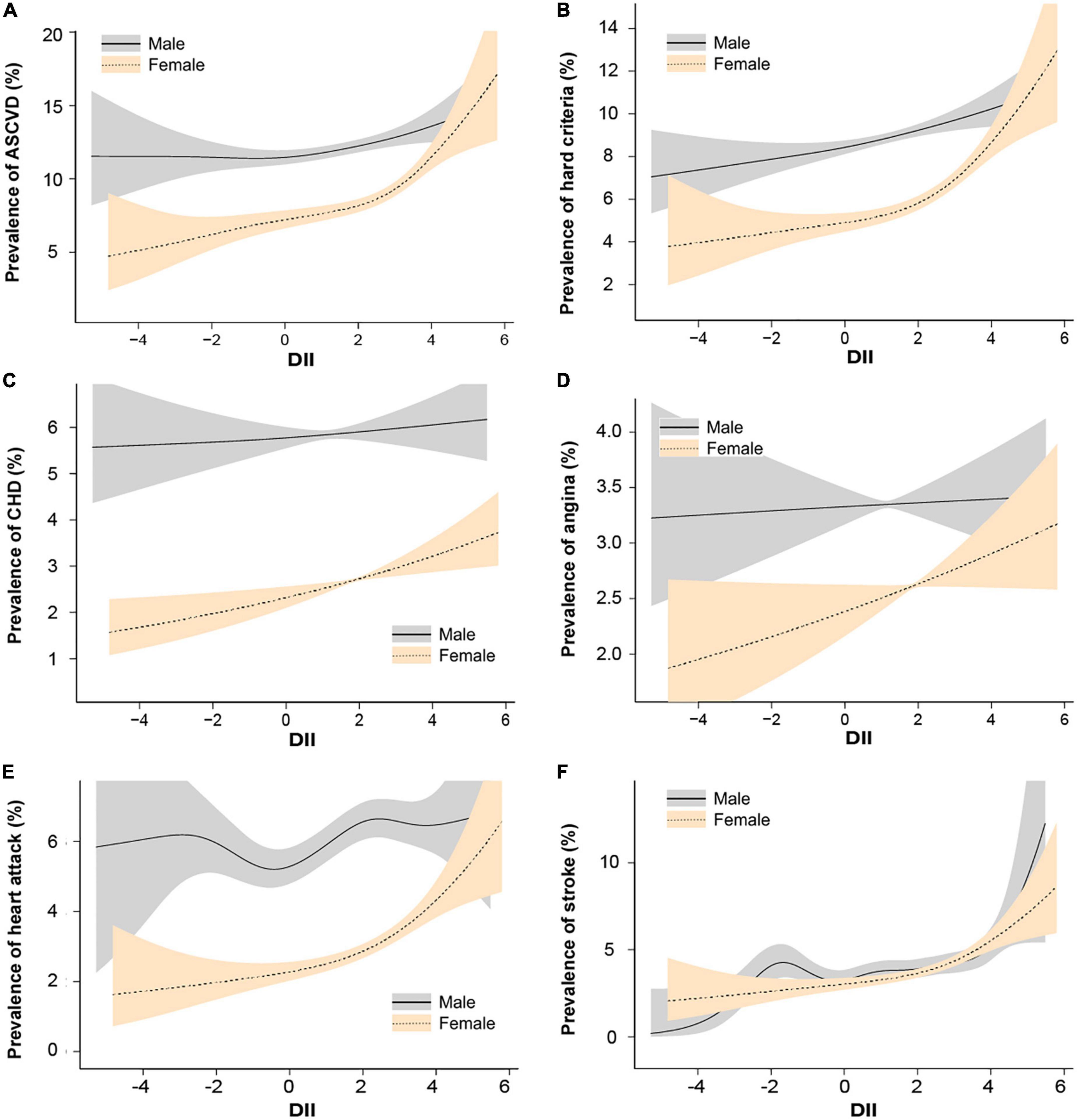
Figure 3. Spline smoothing of dietary inflammatory index (DII) and atherosclerotic cardiovascular diseases stratified by sex, weighted. We adjusted age, sex, race or ethnicity, body mass index, education level, smoking status, poverty, insurance, hyperlipemia, hypertension, and diabetes. (A) Atherosclerotic cardiovascular disease. (B) Hard criteria (including heart attack and stroke). (C) Coronary heart disease. (D) Angina. (E) Heart attack. (F) Stroke. The solid line represents males and the gray shading represents the 95% confidence interval for males. The need line represents females and the orange shading represents the 95% confidence interval for females.
In other subgroups exploring the association with DII, female in heart attack [OR = 1.12, 95% CI = (1.04,1.20)] and stroke [DII ≥ 2.5, OR = 1.25, 95% CI = (1.01,1.54)] showed a significant relationship, while female in CHD [OR = 1.08, 95% CI = (1.00,1.16), P = 0.05] and angina [OR = 1.07, 95% CI = (1.00,1.14), P = 0.05] only shows a borderline significant.
3.4. Sensitivity analysis
Given the large confidence interval of the first decile, we used the second decile as the reference for segmented linear regression (Supplementary Table 4). The sensitivity analysis supports the above results. Furthermore, the multiple logistic regressions with or without adjusting physical activity between DII and ASCVD (Supplementary Table 5) and in subgroup analysis (Supplementary Table 6) are consistent.
4. Discussion
An unhealthy diet is a well-established risk factor for ASCVD (42), one of the fundamental mechanisms is that an unhealthy diet contributes to chronic low-grade inflammation in the gut and the whole body (43). Using a nationally representative cross-sectional study of U.S. adults, the current research involving 48,733 participants demonstrated a significant association between DII and ASCVD. Notably, we observed a non-linear association between DII and ASCVD, with an abruptly increased risk of having ASCVD when the DII score is higher than 3. Additionally, we found that the association of DII with ASCVD was stronger in women than in men.
Studies on DII began in 2004 (44). In 2009, Hébert et al. formulated the first version of DII to provide a pooled measure of diet-related inflammation (13) and revised it in 2014 (14). Subsequently, multiple studies validated the ability of DII in predicting chronic inflammatory disease (10, 45). The DII is universally applicable because it involves the six most commonly inflammatory markers [interleukin(IL)-1β, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and C-reactive protein] (44). We can calculate the DII scores from any dietary assessment tool based on nutrient intake data (44). Furthermore, the DII score is positively related to the glycemic index (GI) (46) and negatively correlated with the Healthy Eating Index-2010 (HEI-2010), the Alternative Healthy Eating Index (AHEI), and the Dietary Approaches to Stop Hypertension Index (DASH) (21, 47–49). That is, an anti-inflammatory diet is healthier than a pro-inflammatory diet evaluated by other diet indexes, such as HEI-2010, AHEI, DASH, and GI scores, which are consistent with the meaning of the DII score. Besides, DII has the added advantage of quantifying the diet’s inflammatory potential.
Our finding that higher DII was associated with an increased risk of having ASCVD was consistent with previous studies. A meta-analysis (27), including 14 observational studies, suggested that the highest versus lowest DII quartile was associated with a 35% [OR = 1.35, 95% CI = (1.11, 1.63), I2 = 36%] increase in CVD risk, and for each one-point increase in the DII score, the risk increased by 8% [OR = 1.08, 95% CI = (1.00, 1.16), I2 = 71%]. The SUN (Seguimiento Universidad de Navarra) Cohort (15) enrolling 18,794 Spanish university graduates and with 8.9 years of follow-up, found that participants in the highest quartile of DII scores had approximately twice the risk of CVD compared with the lowest quartile [hazard ratio (HR) = 2.03, 95% CI = (1.06–3.88), N = 18,794].
Contrary to our findings, an Australian women cohort indicated no association between DII and risk of total CVD, ischemic heart disease, myocardial infarction, cerebrovascular disease, or stroke (50). Another sub-analysis of the MASHAD (Mashhad stroke and heart atherosclerotic disorder) cohort study also found no statistically significant association between the DII and total CVD, myocardium infarction (MI), stable angina (SA), or unstable angina (UA) in middle-aged Iranians (51). However, this study included 4,672 participants, of whom only 124 developed CVD; the sample size might influence the results. Moreover, Catherine et al. (10) explained that race is one of the factors affecting the relationship between DII and CVD; it is more potent in Europe, North America, and Japan than in Australia and Iran.
Notably, our study goes beyond the previous reports (52) by finding a non-linear relationship between DII and ASCVD incidence. When DII was less than −2, no significant association between DII and ASCVD was observed; However, when DII was 3 or greater, one increase of DII was associated with a 41% increase in ASCVD prevalence. This non-linear association observed in the present study indicated that a certain amount of food or elements with pro-inflammatory properties such as vitamin B12, energy, protein, and cholesterol, would not increase the risk of ASCVD probably due to the fact that these elements are essential for basic life activities (14). Only an excessive amount of food or elements with pro-inflammatory properties much greater than needed would increase the risk of ASCVD. This threshold effect has been observed in the relationship between DII score and serum Klotho (23), sex hormones (24), and depression (25).
The potential mechanism of the observed association between DII and ASCVD could be explained by inflammation. Accumulating evidence suggests that ASCVD is a chronic inflammatory disease (53). Shivappa et al. (54) analyzed 532 participants in the HELENA-CSS (Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study) and found that a diet with higher DII scores was related to increased levels of TNF-α, IL-1, IL-2, IFN-γ, and vascular cell adhesion molecule. In the AUSMED heart trial (AUStralian MEDiterranean Diet Heart Trial) (55), after 6 months of dietary intervention, improvement in DII scores was associated with a reduction in IL-6 among Australians diagnosed with CHD (n = 65). Juliana et al. (56) included 329 adolescents from the LabMed Physical Activity Study, and reported that the DII score is positively correlated with component C4 and IL-6. The increased levels of inflammatory cytokines may subsequently induce the migration of inflammatory cells into vascular tissues or mediate leukocyte adhesion to the vascular endothelium by increasing the expression of cell adhesion molecules (10, 57, 58).
Our analysis indicated DII was associated with stroke but irrelevant to CHD, angina, or heart attack in the adjusted model. A previous study conducted by Wirth et al. among U.S. adults (52) partly supported our findings. They found that compared to participants in the lowest quartile of DII, those in DII the highest quartile were more likely to have a stroke and heart attack, but no significant relevance was observed for CHD or angina (52). However, this study does not adjust for hyperlipemia, hypertension, or diabetes, which may lead to biased results because hyperlipemia, hypertension, and diabetes are independent risk factors for ASCVD (42) and could be influenced by a pro-inflammation diet (16, 18, 52). In line with our result, several studies have shown a positive association between DII and stroke in Korean (59), Japanese (60), and U.S. (52), while studies in French (61) and Australian women (50) show no relevance. The association between DII and MI risk is also disputable; studies in Iran (51), Australian women (50), and Korean (59) are consistent with ours, while the studies among French (61) and Italian (62) reported a positive association between DII and MI (61, 62). A possible explanation for this could be that the underlying mechanisms of inflammation and its effects on subtypes of ASCVD vary by sex and country (43, 59, 63, 64). In addition, in NHANES survey, the diagnosed of angina and CHD was according to questionnaire and some case without further details. The inaccurate diagnosis may also account for the negative results in the CHD and angina subgroups in the current study. Furthermore, the subgroup of hard criteria including heart attack and stroke shows a similar trend compared with ASCVD, which supports the above speculation.
Recently, Rohit et al. reviewed the literature on DII studies and pointed out that the association between ASCVD and DII is potentially sex-specific (43). Since 1984, the annual CVD mortality rate has remained greater for women than for men, and the absolute numbers of individuals living with and dying of CVD in the United States are larger for women than for men (65). In line with previous studies, our results show that sex (stronger in females than males) seems to be the key factor affecting the relationship between DII and ASCVD (10, 52). In females, elevated DII was significantly associated with the prevalence of ASCVD, heart attack, and stroke, and there was a borderline significant association with the prevalence of CHD and angina. In males, however, only in stroke were significant. Contrary to our findings, the KoGES_HEXA (Korean Genome and Epidemiology Study Health Examination) cohort showed that a pro-inflammatory diet significantly increased the risk of CVD in males, while there was no significant in females (59). In another Korean survey, the relation between DII and the 10-Year Risk for ASCVD also found that male in the highest quartile has a greater risk than those in the lowest quartile (HR = 1.34, N = 4,185), while there is no significant difference in female (26). We can hypothesize that inflammation has a differential effect in predicting chronic disease outcomes between sexes in agreement with the current literature. A cohort study using data from the Multiethnic Cohort Study in Hawaii and California, demonstrated that for the highest vs. lowest quintile of the DII in men and women were 1.15 [95% CI = (1.09,1.21)] and 1.22 [95% CI = (1.14,1.28)] for all-cause mortality, 1.13 [95% CI = (1.03,1.23)] and 1.29 [95% CI = (1.17,1.42)] for CVD, and 1.10 [95% CI = (1.00,1.21)] and 1.13 [95% CI = (1.02,1.26)] for cancer mortality, which supported a stronger association in women (66). In a recent meta-analysis, an increased risk of CVD (either risk of incident disease or mortality) with higher DII scores was significant only in women and in studies conducted in Europe and North America, but not in men and studies conducted in Australia (27). In other chronic disease studies, both the Women’s Health Initiative and the Iowa Women’s Health Study found that women who consumed the most pro-inflammatory diets had an 20% increased risk of colorectal cancer compared with women who consumed the most anti-inflammatory diets (67, 68). A case-control study conducted in Korea showed that higher DII scores were associated with an increased incidence of colorectal cancer. This association was stronger in women [OR = 2.50, 95% CI = (1.64, 3.82)] than men [OR = 1.72, 95% CI = (1.30, 2.28)] (69). The reasons for the inconsistent results could be as follows: First, it has been discussed above that nationality is an essential factor influencing the relationship between DII and ASCVD. Secondly, in our study, the prevalence of various atherosclerotic diseases was higher in males than in females in almost all DII ranges. Vulnerable plaque rupture and thrombosis are the leading causes of stroke and MI (70). Plaques from females tend to be more stable (71), while those from males are more inflamed and have additional unstable features (72, 73). Third, this may also be indirectly due to sex differences in CRP, GI, and sex hormones in DII. However, the specific mechanism is still unknown to our knowledge, which may be a promising direction for the further research.
There are several studies performed in women versus men of DII versus GI, CRP, and sex hormones. Intervention studies showed that a low GI diet (74, 75) lowered plasma CRP in short-term and long-term studies in overweight and obese adults. High GI foods, which are characteristically highly refined carbohydrates and/or carbohydrates with little fiber, are one of the major dietary factors affecting inflammation. However, the relationship between inflammation and GI is more notable in females. A cross-sectional study reported that a strong positive relationship between dietary GI (76) and plasma CRP in healthy, middle-aged women. In another cross-sectional study (46), increased inflammatory potential of diet, as represented by higher DII scores, was associated with increased GI scores. Moreover, the correlation coefficient between DII score and GI score was higher in women than in men (0.32 vs. 0.27), implying that the association of DII with GI was stronger in women. The DII has been positively associated with inflammatory biomarkers such as CRP (i.e., a pro-inflammatory diet leads to a higher inflammation) (77, 78). Furthermore, the study shows that the DII was significantly associated with inflammatory biomarkers in post-menopausal women (79). A cross-sectional study using data of the National Integrated Project for Prospective Observation of Non-communicable Disease and its Trends in the Aged 2010, indicated that DII score was positively associated with hs-CRP. However, this relationship was only stable among women (Men: standardized β = 0.05, P = 0.14; Women: standardized β = 0.06, P = 0.02) (80), which was consistent with our findings. Sex hormones and sex hormone binding globulin (SHBG) are easily affected by inflammation. A cross-sectional study included 2,092 female participants (age ≥ 20) from the 2013–2016 National Health and Nutrition Examination Survey demonstrated that a pro-inflammatory diet caused decreased SHBG in adult women (34). Karelis et al. found that vegetarians presented higher concentrations of SHBG in both pre- and post-menopausal women due to higher levels of fiber intake (81). Another cross-sectional study showed that energy-adjusted DII was positively associated with testosterone (P = 0.035), free testosterone (P = 0.026) and testosterone/estradiol (P = 0.065) in post-menopausal women (24). Thus, the association between DII and sex hormones is apparent in women, and no studies have explored this relationship in adult men, possibly due to the fact that hormonal changes in women are more affected by inflammation.
Our study had several strengths. First, to our knowledge, this is the first study to explore the relationship between DII and ASCVD in U.S. adults. The large sample size and complex sampling from the general population make it possible to extrapolate to the U.S. population. Second, compared with previous studies, we additionally explored the non-linear relation of DII with ASCVD and the potential cutoff point (2.2) for the relation. Third, the curve fitting and segmented linear regression results were consistent, indicating stable and reliable results. Finally, our subgroup analysis identified the populations and diseases affected most by DII. Overall, the current CVD guidelines have not considered DII as a diet recommendation (42). We demonstrated that higher DII was associated with an increased risk of ASCVD, which provides a reference and cutoff value for future research and diet recommendations.
In interpreting these results, several limitations should be taken into account. First, the cross-sectional study can only examine correlations but difficult to make a causal inference or investigate the temporal relationships (82). Subsequent large cohort studies or randomized controlled trials are necessary to confirm the findings. Second, ASCVD was defined based on diagnostic information, which may miss patients unaware of their disease. The diagnosed of CHD and angina was according to questionnaire and some case without further details, which may lead to an inaccurate diagnosis. In addition, due to the limited information collection in NHANES, the current study was unable to include peripheral vascular diseases in ASCVD. These may lead to bias. Further studies should consider the spectrum of diseases caused by atherosclerosis to clarify the relationship between DII and ASCVD further. Third, the DII was calculated based on a single 24-hour dietary recall, which is subject to some chance and recall bias. Nevertheless, this method is currently widely used and validated (16, 33, 34). Forth, due to the limited information in the NHANES database, carbohydrates were jointly taken into account without separating the complex from simple carbohydrates in calculating the DII score (83, 84). However, this study accounts for fiber, a good summary measure for complex carbohydrates (20). Perhaps the next generation of DII calculations could consider separating simple and complex carbohydrates. That is necessary, especially in women whose BMI might be more sensitive than in men to the carbohydrates’ components or ratio.
5. Conclusion
DII was positively and non-linearly associated with ASCVD in U.S. adults. This relationship was more pronounced in females. The findings could provide a reference for future research hypotheses and need to be further studied.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
HX, JQJ, JL, and JZ designed this study. RL and XC cleaned the data. JZ and JJ performed the analysis and revised the draft. WT normalized the pictures. XW and QL re-checked the data. JZ wrote the original draft. HX, JQJ, and JL reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the China Academy of Chinese Medical Sciences Innovation Fund (CACMS Innovation Fund, CI2021A00917), Chinese Academy of Traditional Chinese Medicine Science and Technology Major Achievement Guidance Project (ZZ13-ZD-03), Central Public Welfare Research Institutes of China Academy of Chinese Medical Sciences (ZZ13-YQ-017-C1), and the Fundamental Research Funds for the Central Universities, Beijing University of Chinese Medicine (2022-JYB-XJSJJ-053).
Acknowledgments
The authors thank Jing Zhang for his work on the nhanesR package.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1044329/full#supplementary-material
Footnotes
- ^ https://www.cdc.gov/nchs/nhanes/index.htm
- ^ http://www.empowerstats.net/analysis/
- ^ www.cdc.gov/nchs/nhanes/
References
1. Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. (2016) 118:531–4. doi: 10.1161/CIRCRESAHA.116.308334
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt M, Callaway C, Carson A, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528.
3. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. doi: 10.1038/s41586-021-03392-8
4. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. (2021) 20:589–610. doi: 10.1038/s41573-021-00198-1
5. Ridker PM, Everett BM, Thuren T, MacFadyen J, Chang W, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
6. Nidorf SM, Eikelboom JW, Budgeon CA, Thompson P. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. (2013) 61:404–10. doi: 10.1016/j.jacc.2012.10.027
7. Tardif JC, Kouz S, Waters DD, Bertrand O, Diaz R, Maggioni A, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381:2497–505. doi: 10.1056/NEJMoa1912388
8. Nidorf SM, Fiolet AT, Mosterd A, Eikelboom J, Schut A, Opstal T, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383:1838–47. doi: 10.1056/NEJMoa2021372
9. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz C, Blum C, Eckel R, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a
10. Phillips CM, Chen LW, Heude B. Dietary inflammatory index and non-communicable disease risk: a narrative review. Nutrients. (2019) 11:8. doi: 10.3390/nu11081873
11. Kirkpatrick CF, Maki KC. Dietary influences on atherosclerotic cardiovascular disease risk. Curr Atheroscler Rep. (2021) 23:62. doi: 10.1007/s11883-021-00954-z
12. Minihane AM, Vinoy S, Russell WR, Baka A, Roche H, Tuohy K, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. (2015) 114:999–1012. doi: 10.1017/S0007114515002093
13. Cavicchia PP, Steck SE, Hurley TG, Hussey J, Ma Y, Ockene I, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. (2009) 139:2365–72. doi: 10.3945/jn.109.114025
14. Shivappa N, Steck SE, Hurley TG, Hussey J, Hébert J. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
15. Ramallal R, Toledo E, Martínez-González MA, Hernández-Hernández A, Shivappa N, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS One. (2015) 10:e0135221. doi: 10.1371/journal.pone.0135221
16. Mazidi M, Shivappa N, Wirth MD, Hebert J, Mikhailidis D, Kengne A, et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis. (2018) 276:23–7. doi: 10.1016/j.atherosclerosis.2018.02.020
17. Yuan S, Song C, Zhang R, He J, Dou K. Dietary inflammation index and its association with long-term all-cause and cardiovascular mortality in the general US population by baseline glycemic status. Nutrients. (2022) 14:13. doi: 10.3390/nu14132556
18. Tyrovolas S, Koyanagi A, Kotsakis GA, Panagiotakos D, Shivappa N, Wirth M, et al. Dietary inflammatory potential is linked to cardiovascular disease risk burden in the US adult population. Int J Cardiol. (2017) 240:409–13. doi: 10.1016/j.ijcard.2017.04.104
19. Zhang J, Feng Y, Yang X, Li Y, Wu Y, Yuan L, et al. Dose-response association of the dietary inflammatory potential with all-cause and cause-specific mortality. Adv Nutr. (2022) 13:1834–45. doi: 10.1093/advances/nmac049
20. Puddu PE, Shivappa N, Menotti A, Hébert J, Tolonen H, Kafatos A, et al. Energy-adjusted dietary inflammatory index scores predict long-term cardiovascular disease mortality and other causes of death in an ecological analysis of the seven countries study. Eur J Prev Cardiol. (2020). doi: 10.1177/2047487320903866 [Epub ahead of print].
21. Garcia-Arellano A, Ramallal R, Ruiz-Canela M, Salas-Salvadó J, Corella D, Shivappa N, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients. (2015) 7:4124–38. doi: 10.3390/nu7064124
22. Yu X, Cao L, Yu X. Elevated cord serum manganese level is associated with a neonatal high ponderal index. Environ Res. (2013) 121:79–83. doi: 10.1016/j.envres.2012.11.002
23. Zhang C, Zhang Z, Li J, Deng L, Geng J, Jin K, et al. Association between dietary inflammatory index and serum klotho concentration among adults in the united states. BMC Geriatr. (2022) 22:528. doi: 10.1186/s12877-022-03228-8
24. Chen WY, Fu YP, Zhong W, Zhou M. The association between dietary inflammatory index and sex hormones among postmenopausal women in the US. Front Endocrinol. (2021) 12:771565. doi: 10.3389/fendo.2021.771565
25. Wirth MD, Shivappa N, Burch JB, Hurley T, Hébert J. The dietary inflammatory index, shift work, and depression: results from NHANES. Health Psychol. (2017) 36:760–9. doi: 10.1037/hea0000514
26. Lee YN, Kang P. Relationship between the 10-year risk for atherosclerotic cardiovascular disease and the dietary inflammatory index among korean adults based on the seventh korea national health and nutrition examination survey (KNHANES). Biomed Res Int. (2020) 2020:8196798. doi: 10.1155/2020/8196798
27. Shivappa N, Godos J, Hébert JR, Wirth M, Piuri G, Speciani A, et al. Dietary inflammatory index and cardiovascular risk and mortality-a meta-analysis. Nutrients. (2018) 10:2. doi: 10.3390/nu10020200
28. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. (2021) 384:2219–28. doi: 10.1056/NEJMsa2032271
29. Von Elm E, Altman DG, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
30. Akinbami LJ, Chen TC, Davy O, Ogden C, Fink S, Clark J, et al. National health and nutrition examination survey, 2017-march 2020 prepandemic file: sample design, estimation, and analytic guidelines. Vital Health Stat 1. (2022) 190:1–36. doi: 10.15620/cdc:115434
31. Johnson CL, Paulose-Ram R, Ogden CL, Carroll M, Kruszon-Moran D, Dohrmann S, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. (2013) 161:1–24.
32. Wang L, Li X, Wang Z, Bancks M, Carnethon M, Greenland P, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. (2021) 326:1–13. doi: 10.1001/jama.2021.9883
33. Liu Z, Liu H, Deng Q, Sun C, He W, Zheng W, et al. Association between dietary inflammatory index and heart failure: results from NHANES (1999-2018). Front Cardiovasc Med. (2021) 8:702489. doi: 10.3389/fcvm.2021.702489
34. Liu N, Feng Y, Luo X, Ma X, Ma F. Association between dietary inflammatory index and sex hormone binding globulin and sex hormone in U.S. Adult females. Front Public Health. (2022) 10:802945. doi: 10.3389/fpubh.2022.802945
35. National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-94. Hyattsville, MD: US Department of Health and Human Services, Public Health Service, Centers (1994).
36. Zhang C, Bian H, Chen Z, Tian B, Wang H, Tu X, et al. The association between dietary inflammatory index and sex hormones among men in the united states. J Urol. (2021) 206:97–103. doi: 10.1097/JU.0000000000001703
37. Qin Z, Liu N, Liao R, Jiang L, Su B. The association between dietary inflammatory potential and sex hormones in male children and adolescents aged 6-19 years. Front Endocrinol. (2021) 12:722941. doi: 10.3389/fendo.2021.722941
38. Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health. (2006) 14:66–70. doi: 10.1007/s10389-006-0024-x
39. Xu F, Earp JE, Adami A, Weidauer L, Greene G. The relationship of physical activity and dietary quality and diabetes prevalence in US adults: findings from NHANES 2011-2018. Nutrients. (2022) 14:16. doi: 10.3390/nu14163324
40. Mohammad NS, Nazli R, Zafar H, Fatima S. Effects of lipid based multiple micronutrients supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pak J Med Sci. (2022) 38:219–26. doi: 10.12669/pjms.38.1.4396
41. Zhang J, Cao J, Zhang H, Jiang C, Lin T, Zhou Z, et al. Plasma copper and the risk of first stroke in hypertensive patients: a nested case-control study. Am J Clin Nutr. (2019) 110:212–20. doi: 10.1093/ajcn/nqz099
42. Arnett DK, Blumenthal RS, Albert MA, Buroker A, Goldberger Z, Hahn E, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 74:1376–414.
43. Hariharan R, Odjidja EN, Scott D, Shivappa N, Hébert J, Hodge A, et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes Rev. (2022) 23:e13349. doi: 10.1111/obr.13349
44. Hébert JR, Shivappa N, Wirth MD, Hussey J, Hurley T. Perspective: the dietary inflammatory index (DII)-lessons learned, improvements made, and future directions. Adv Nutr. (2019) 10:185–95. doi: 10.1093/advances/nmy071
45. Marx W, Veronese N, Kelly JT, Smith L, Hockey M, Collins S, et al. The dietary inflammatory index and human health: an umbrella review of meta-analyses of observational studies. Adv Nutr. (2021) 12:1681–90. doi: 10.1093/advances/nmab037
46. Kim Y, Chen J, Wirth MD, Shivappa N, Hebert J. Lower dietary inflammatory index scores are associated with lower glycemic index scores among college students. Nutrients. (2018) 10:2. doi: 10.3390/nu10020182
47. Wirth MD, Hébert JR, Shivappa N, Hand G, Hurley T, Drenowatz C, et al. Anti-inflammatory dietary inflammatory index scores are associated with healthier scores on other dietary indices. Nutr Res. (2016) 36:214–9. doi: 10.1016/j.nutres.2015.11.009
48. Hodge AM, Bassett JK, Shivappa N, Hébert J, English D, Giles G, et al. Dietary inflammatory index, mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control. (2016) 27:907–17. doi: 10.1007/s10552-016-0770-1
49. Mayr HL, Thomas CJ, Tierney AC, Kucianski T, George E, Ruiz-Canela M, et al. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in dietary inflammatory index scores in patients with coronary heart disease: the AUSMED heart trial. Nutr Res. (2018) 55:94–107. doi: 10.1016/j.nutres.2018.04.006
50. Vissers LE, Waller MA, Van Der Schouw YT, Hebert J, Shivappa N, Schoenaker D, et al. The relationship between the dietary inflammatory index and risk of total cardiovascular disease, ischemic heart disease and cerebrovascular disease: Findings from an Australian population-based prospective cohort study of women. Atherosclerosis. (2016) 253:164–70. doi: 10.1016/j.atherosclerosis.2016.07.929
51. Asadi Z, Yaghooti-Khorasani M, Ghazizadeh H, Sadabadi F, Mosa-Farkhany E, Darroudi S, et al. Association between dietary inflammatory index and risk of cardiovascular disease in the mashhad stroke and heart atherosclerotic disorder study population. IUBMB Life. (2020) 72:706–15. doi: 10.1002/iub.2172
52. Wirth MD, Shivappa N, Hurley TG, Hébert J. Association between previously diagnosed circulatory conditions and a dietary inflammatory index. Nutr Res. (2016) 36:227–33. doi: 10.1016/j.nutres.2015.11.016
53. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
54. Shivappa N, Hebert JR, Marcos A, Diaz L, Gomez S, Nova E, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. (2017) 61:10. doi: 10.1002/mnfr.201600707
55. Mayr HL, Itsiopoulos C, Tierney AC, Ruiz-Canela M, Hebert J, Shivappa N, et al. Improvement in dietary inflammatory index score after 6-month dietary intervention is associated with reduction in interleukin-6 in patients with coronary heart disease: the AUSMED heart trial. Nutr Res. (2018) 55:108–21. doi: 10.1016/j.nutres.2018.04.007
56. Almeida-De-Souza J, Santos R, Barros R, Abreu S, Moreira C, Lopes L, et al. Dietary inflammatory index and inflammatory biomarkers in adolescents from LabMed physical activity study. Eur J Clin Nutr. (2018) 72:710–9. doi: 10.1038/s41430-017-0013-x
57. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. (2004) 109:Ii2–10. doi: 10.1161/01.CIR.0000129535.04194.38
58. Sokol A, Wirth MD, Manczuk M, Shivappa N, Zatonska K, Hurley T, et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr Res. (2016) 36:1298–303. doi: 10.1016/j.nutres.2016.04.004
59. Khan I, Kwon M, Shivappa N, Hébert J, Kim M. Positive association of dietary inflammatory index with incidence of cardiovascular disease: findings from a korean population-based prospective study. Nutrients. (2020) 12:2. doi: 10.3390/nu12020588
60. Okada E, Shirakawa T, Shivappa N, Wakai K, Suzuki K, Date C, et al. Dietary inflammatory index is associated with risk of all-cause and cardiovascular disease mortality but not with cancer mortality in middle-aged and older Japanese adults. J Nutr. (2019) 149:1451–9. doi: 10.1093/jn/nxz085
61. Neufcourt L, Assmann KE, Fezeu LK, Touvier M, Graffouillère L, Shivappa N, et al. Prospective association between the dietary inflammatory index and cardiovascular diseases in the supplémentation en vitamines et minéraux antioxydants (SU.VI.MAX) cohort. J Am Heart Assoc. (2016) 5:e002735. doi: 10.1161/JAHA.115.002735
62. Shivappa N, Tavani A, Hébert JR, Rosato V, La Vecchia C. Dietary inflammatory index and acute myocardial infarction in a large Italian case-control study. Eur J Public Health. (2018) 28:161–6. doi: 10.1093/eurpub/ckx058
63. Albert MA. Inflammatory biomarkers, race/ethnicity and cardiovascular disease. Nutr Rev. (2007) 65:S234–8. doi: 10.1111/j.1753-4887.2007.tb00369.x
64. Park YM, Choi MK, Lee SS, Shivappa N, Han K, Steck S, et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin Nutr. (2019) 38:682–8. doi: 10.1016/j.clnu.2018.04.002
65. Mehta LS, Beckie TM, Devon HA, Grines C, Krumholz H, Johnson M, et al. Acute myocardial infarction in women: a scientific statement from the american heart association. Circulation. (2016) 133:916–47. doi: 10.1161/CIR.0000000000000351
66. Park SY, Kang M, Wilkens LR, Shvetsov Y, Harmon B, Shivappa N, et al. The dietary inflammatory index and all-cause, cardiovascular disease, and cancer mortality in the multiethnic cohort study. Nutrients. (2018) 10:12. doi: 10.3390/nu10121844
67. Shivappa N, Prizment AE, Blair CK, Jacobs D Jr., Steck S, Hébert J, et al. Dietary inflammatory index and risk of colorectal cancer in the iowa women’s health study. Cancer Epidemiol Bio Prev. (2014) 23:2383–92. doi: 10.1158/1055-9965.EPI-14-0537
68. Tabung FK, Steck SE, Ma Y, Liese A, Zhang J, Caan B, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the women’s health initiative. Cancer Causes Control. (2015) 26:399–408. doi: 10.1007/s10552-014-0515-y
69. Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary inflammatory index and risk of colorectal cancer: a case-control study in Korea. Nutrients. (2016) 8:8. doi: 10.3390/nu8080469
70. Stefanadis C, Antoniou CK, Tsiachris D, Pietri P. Coronary atherosclerotic vulnerable plaque: current perspectives. J Am Heart Assoc. (2017) 6:3. doi: 10.1161/JAHA.117.005543
71. Hartman RJ, Owsiany K, Ma L. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation. (2021) 143:713–26. doi: 10.1161/CIRCULATIONAHA.120.051231
72. Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. (2020) 126:1297–319. doi: 10.1161/CIRCRESAHA.120.315930
73. Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, et al. Carotid plaque morphology is significantly associated with sex. Age, and history of neurological symptoms. Stroke. (2015) 46:3213–9. doi: 10.1161/STROKEAHA.115.010558
74. Gögebakan O, Kohl A, Osterhoff MA, van Baak M, Jebb S, Papadaki A, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the diet, obesity, and genes (DiOGenes) study: a randomized, controlled trial. Circulation. (2011) 124:2829–38. doi: 10.1161/CIRCULATIONAHA.111.033274
75. Wolever TM, Gibbs AL, Mehling C, Chiasson J, Connelly P, Josse R, et al. The canadian trial of carbohydrates in diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. (2008) 87:114–25. doi: 10.1093/ajcn/87.1.114
76. Levitan EB, Cook NR, Stampfer MJ, Ridker P, Rexrode K, Buring J, et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism. (2008) 57:437–43. doi: 10.1016/j.metabol.2007.11.002
77. Wirth MD, Shivappa N, Davis L, Hurley T, Ortaglia A, Drayton R, et al. Construct validation of the dietary inflammatory index among African Americans. J Nutr Health Aging. (2017) 21:487–91. doi: 10.1007/s12603-016-0775-1
78. Shivappa N, Steck SE, Hurley TG, Hussey J, Ma Y, Ockene I, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. (2014) 17:1825–33. doi: 10.1017/S1368980013002565
79. Tabung FK, Steck SE, Zhang J, Ma Y, Liese A, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. (2015) 25:398–405. doi: 10.1016/j.annepidem.2015.03.009
80. Yang Y, Hozawa A, Kogure M, Narita A, Hirata T, Nakamura T, et al. Dietary inflammatory index positively associated with high-sensitivity c-reactive protein level in japanese from NIPPON DATA2010. J Epidemiol. (2020) 30:98–107. doi: 10.2188/jea.JE20180156
81. Karelis AD, Fex A, Filion ME, Adlercreutz H, Aubertin-Leheudre M. Comparison of sex hormonal and metabolic profiles between omnivores and vegetarians in pre- and post-menopausal women. Br J Nutr. (2010) 104:222–6. doi: 10.1017/S0007114510000619
82. Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. (2020) 158:S65–71. doi: 10.1016/j.chest.2020.03.012
83. Sun M, Wang X, Wang L, Hu W, Yang Y, Yao N, et al. The mediating role of dietary inflammatory index in the association between eating breakfast and obesity: a cross-sectional study. Nutrients. (2022) 14:20. doi: 10.3390/nu14204378
Keywords: dietary inflammatory index, atherosclerotic cardiovascular disease, NHANES, diet, inflammation
Citation: Zhang J, Jia J, Lai R, Wang X, Chen X, Tian W, Liu Q, Li J, Ju J and Xu H (2023) Association between dietary inflammatory index and atherosclerosis cardiovascular disease in U.S. adults. Front. Nutr. 9:1044329. doi: 10.3389/fnut.2022.1044329
Received: 14 September 2022; Accepted: 13 December 2022;
Published: 05 January 2023.
Edited by:
Xiaohua Liang, Children’s Hospital of Chongqing Medical University, ChinaReviewed by:
Ichiro Sakuma, Hokko Memorial Hospital, JapanPaolo Emilio Puddu, Université de Caen Normandie, France
Copyright © 2023 Zhang, Jia, Lai, Wang, Chen, Tian, Liu, Li, Ju and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingen Li, ✉ bGlqaW5nZW4xOTg5MDhAMTI2LmNvbQ==; Jianqing Ju, ✉ anVqaWFucWluZ0AxNjMuY29t; Hao Xu, ✉ eHVoYW90Y21AaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Jie Zhang
Jie Zhang Jundi Jia
Jundi Jia Runmin Lai
Runmin Lai Xinyi Wang
Xinyi Wang Xuanye Chen
Xuanye Chen Wende Tian
Wende Tian Qiyu Liu
Qiyu Liu Jingen Li
Jingen Li Jianqing Ju
Jianqing Ju Hao Xu
Hao Xu